- 1Office of National Central Cancer Registry, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Cancer Prevention, Institute of Cancer and Basic Medicine (IBMC), The Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Chinese Academy of Sciences, Hangzhou, China
- 3Department of Head and Neck Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 4Cancer Institute, The Fourth Hospital of Hebei Medical University, Shijiazhuang, China
- 5Henan Cancer Prevention and Control Office, The Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, China
- 6Office of Yunnan Cancer Center, Yunnan Cancer Hospital, Kunming, China
- 7Office of Cancer Prevention and Treatment, Hubei Cancer Hospital, Wuhan, China
- 8Heilongjiang Cancer Center, Institute of Cancer Prevention and Treatment, Harbin Medical University, Harbin, China
- 9Department of Cancer Prevention and Control, Hunan Cancer Hospital and the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China
- 10Scientific Research Education Department, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital and Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, China
- 11Information Management and Big Data Center, The Tumor Hospital Affiliated to Xinjiang Medical University, Ürümqi, China
- 12Science and Education Department, The Fifth People's Hospital of Qinghai, Xining, China
- 13Medical Department, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital and Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, China
- 14Human Resources Office, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Background: Thyroid cancer (TC), was the fastest-rising tumor of all malignancies in the world and China, predominantly differentiated thyroid cancer (DTC). However, evidence on TC stage distribution and influencing factors of late-stage were limited in China.
Methods: We carried out a retrospective study and enrolled TC patients who were first diagnosed and hospitalized in 8 hospitals in China in 2017. Logistic regression was used to evaluate associations between influencing factors and DTC stage. We extracted eligible primary DTC records newly diagnosed in 2017 from the USA's Surveillance, Epidemiology, and End Results (SEER) database. We compared clinicopathological features and surgical treatment between our DTC records and those from the SEER database.
Results: A total of 1970 eligible patients were included, with 1861 DTC patients with known stage. Among patients ≥45 years old, males (OR = 1.76, 95%CI 1.17–2.65) and those with new rural cooperative medical scheme insurance (NCMS) (OR = 1.99, 95%CI 1.38–2.88) had higher risks of late-stage DTC (stage III-IV). Compared with SEER database, over-diagnosis is more common in China [more DTC patients with onset age< 45 years old (50.3 vs. 40.7%, P < 0.001), with early-stage (81.2 vs. 76.0%, P < 0.001), and with tumors<2cm (74.9 vs. 63.7%, P < 0.001)]. Compared with the USA, TC treatment is more conservative in China. The proportion of lobectomy in our database was significantly higher than that in the SEER database (41.3 vs. 17.0%, P < 0.001).
Conclusions: Unique risk factors are found to be associated with late-stage DTC in China. The differences in the aspect of clinicopathological features and surgical approaches between China and the USA indicate that potential over-diagnosis and over-surgery exist, and disparities on surgery extent may need further consideration. The findings provided references for other countries with similar patterns.
Background
Thyroid cancer (TC) is the most common endocrine and head and neck malignancy worldwide. According to Global Cancer Statistics 2020, 586,202 new cases and 44,000 deaths occurred worldwide in 2020 (1). TC incidence has been rising at the fastest speed, predominantly differentiated thyroid carcinoma (DTC) (2, 3), and has already become a huge health concern in the current era. The incidence rate of TC has been increasing rapidly in China in the past decades. According to the national report released by the National Cancer Center in China, TC became the fastest-growing malignant tumor in China (4–7). Despite the slow progression for TC, gaps in 5-year survival exist between China and developed countries such as the USA (84.30 vs. 98.16%) (8, 9). Compared with advanced TC patients, early-stage patients are more likely to be cured and have a better prognosis (10–12). The stage information in the USA was collected in the Surveillance, Epidemiology, and End Results (SEER) database. Identifying stage distribution and influencing factors is beneficial to narrow the survival gap and improve the prognosis of TC. However, so far, evidence on TC stage distribution and influencing factors of late-stage were limited in China.
Because of the increasing prevalence of excessive BMI around the world, the impact of obesity on cancer risk has been extensively studied. Epidemiological evidence confirmed that BMI was independently associated with an increased incidence of DTC (13). However, the possible associations between BMI and aggressive clinicopathologic features of thyroid cancer have remained controversial. Several studies showed that Obesity was associated with several poor clinicopathologic prognostic features: extrathyroidal extension, multifocality, and advanced tumor/node/metastasis stage (14). On the contrary, others showed that there was no independent association between obesity and more aggressive clinicopathological features of TC (15). Previous related studies in China were limited to small samples from one or a small number of hospitals, and evidence at the population-based level was not available. Therefore, based on the multicenter, large-scale, population-based study in China, we further explored the associations between BMI and aggressive clinicopathological characteristics.
What's more, the reasons for the differences in TC disease burden may lie in the differences in cancer screening policies and clinical guidelines between China and the USA, which can be reflected by comparing clinicopathological features and therapeutic schedules between the two countries. However, few multicentered and large-scale studies focus on stage distribution, clinicopathological characteristics and surgical treatment of TC in China. To fill the evidence gap, the National Cancer Center in China initiated a multicenter hospital-based retrospective survey in 2017. We aimed (1) to ascertain DTC stage distribution at diagnosis and investigate relevant risk factors for late-stage DTC patients, and (2) to compare clinicopathological disparities and surgical treatments of DTC between our data and SEER data.
Methods
Study design and participants
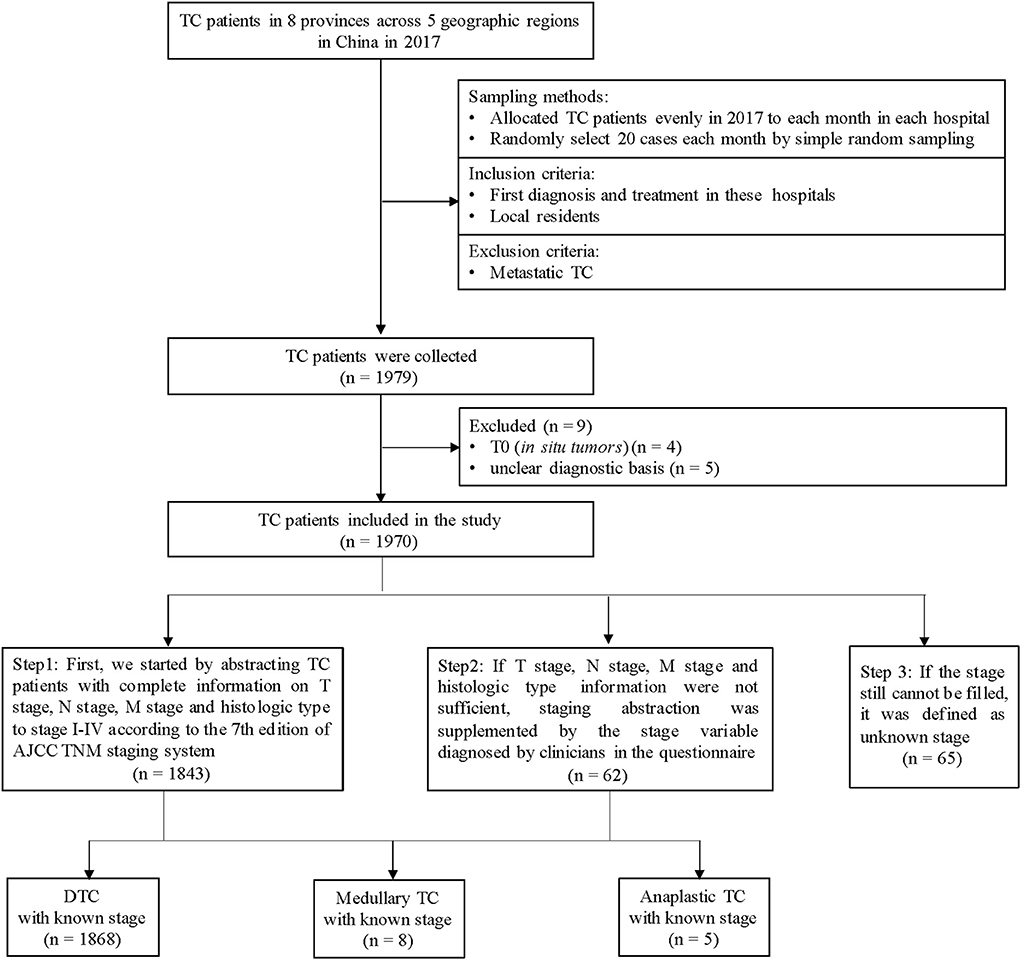
We carried out a retrospective investigation and included TC patients from 8 grade A tertiary hospitals in 8 provinces (Henan, Hubei, Shenzhen, Hunan, Heilongjiang, Xinjiang, Yunnan and Qinghai) in China. Inclusion criteria: (a) newly diagnosed as TC in 2017; (b) hospitalized between January to December in 2017; (c) resident of the province where the hospital was located. We excluded patients with other primary tumors that metastasized to the thyroid. The study flowchart was shown in Figure 1. Considering the unknown risks in the research process, the total target sample size of TC was determined as 1,920 cases (240 cases in each hospital). We allocated sample sizes evenly to each month, which was 20 cases per month for each hospital. We sorted all eligible TC cases according to discharge time in each month and selected the cases via simple random sampling.
The third edition of the International Classification of Diseases for Oncology (ICD-O-3) topography was used for identifying TC (C73.9). Trained investigators extracted information using a case report form from electronic medical records, including (1) demographic characteristics; (2) clinicopathological characteristics; and (3) therapeutic information.
Staging
First, we started by abstracting TC patients with complete information on T stage, N stage, M stage and histologic type to stage I-IV according to the 7th edition of the American Joint Committee on Cancer (AJCC) Tumor-Node-Metastasis (TNM) staging system (16). If the above information (T, N, M and histologic type) were not sufficient, staging abstraction was supplemented by the stage variable diagnosed by clinicians in the questionnaire. If the stage still cannot be filled, it was defined as an unknown stage (Figure 1). Stage I and II were defined as early-stage, and stage III and IV were defined as late-stage.
SEER database in the USA
SEER*Stat was used to generate a case listing. We extracted eligible primary TC records diagnosed in 2017 from the SEER database, collecting information including age, sex, histologic type ICD-O-3, derived SEER combined T, derived SEER combined N, derived SEER combined M, derived SEER combined stage (the 7th edition AJCC), tumor size summary, focality, surgery primary site, reason no cancer-directed surgery. A total of 13,053 TC cases in 2017 were downloaded from the SEER database. In order to be comparable with the subjects included in this study, we only included DTC patients with a known stage in the SEER database (n = 11,006).
Statistical analysis
We used the Chi-square test or Fisher's exact test to compare the stage distribution, clinicopathological characteristics and therapeutic information among different groups. We estimated adjusted odds ratios (ORs) for late-stage DTC patients using logistic regression. In model 1, only sex at diagnosis was adjusted. In model 2, area and medical insurance were added. NCMS is a new community-based rural health insurance system now covering over 98% of rural residents in China, first implemented in 2003. In model 3, multiple factors including BMI, smoking, drinking, history of thyroid diseases and family history of TC were included by the enter method. In model 4, the main model, we used the stepwise method to identify significant variables. No collinear among variables was found in logistic regression. Data management, programming, and analyses were carried out using SAS 9.4. All tests of significance were two-tailed, and P < 0.05 was considered statistically significant.
Results
The risk factors of late-stage DTC patients
Characteristics of DTC patients by stage distribution
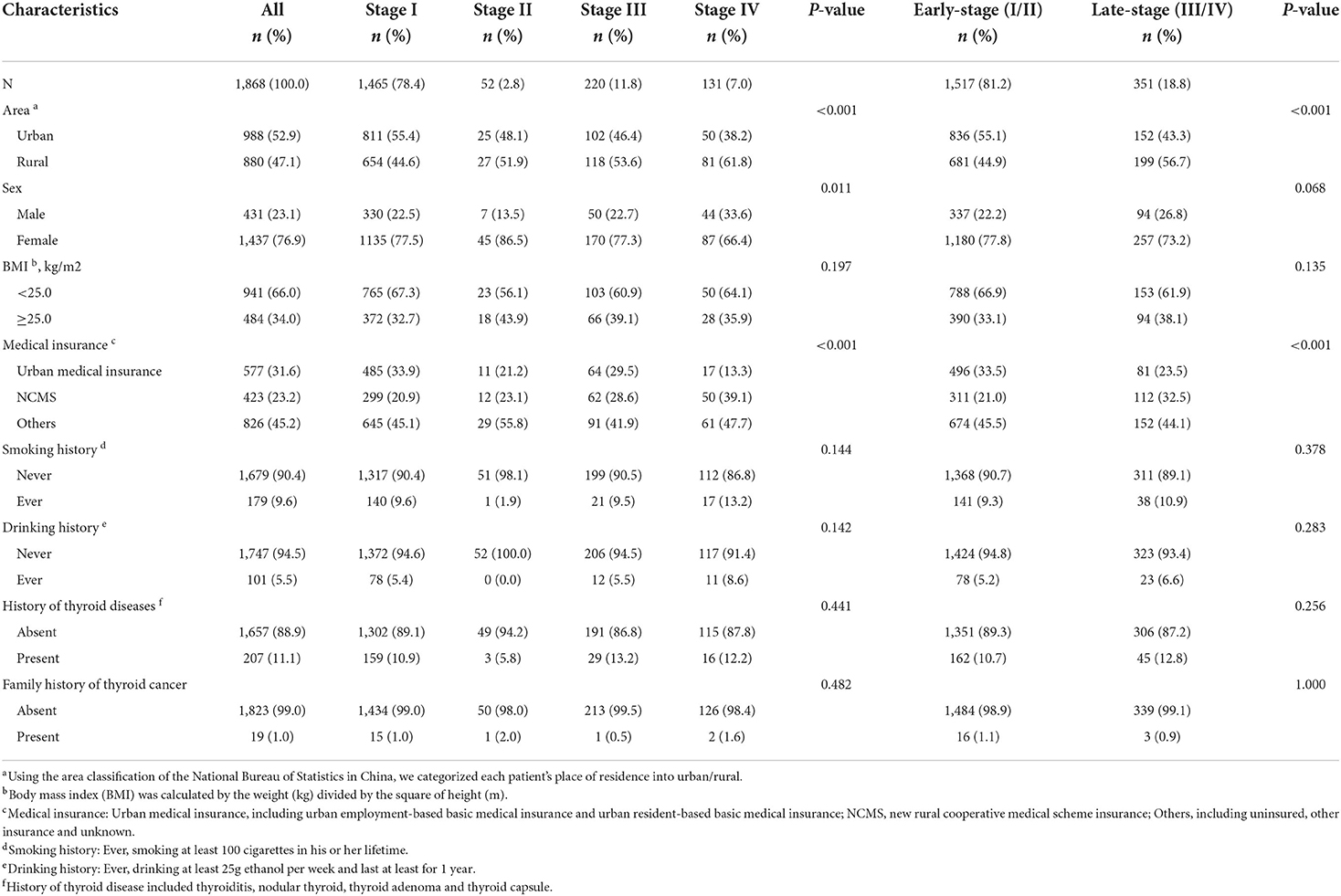
A total of 1,970 eligible patients were included, with a mean age of 44.3 ± 11.5 years, of which 96.7% (n = 1905) had known stage (Figure 1; Supplementary Table S1). 94.8% of TC with the known stage was DTC (n = 1868), and the characteristics of DTC patients were shown in Table 1. The proportion of DTC patients in urban areas, with urban insurance, was higher than patients living in rural areas and those with NCMS (52.9 vs. 47.1%, 31.6 vs. 23.2%). The female/male sex ratio was 3.3. As shown in Table 1, the proportion of early-stage and late-stage of DTC were 81.2 and 18.8%. Significant differences in stage distribution were found among different regions (P < 0.001). The characteristics of DTC patients by stage distribution, by age were presented in Supplementary Table S2.
Multivariate analysis for late-stage DTC patients
Considering only 1.0% of DTC patients aged <45y were stage II, the multivariate analysis for late-stage was performed in DTC patients aged ≥45y (Table 2). The sex-adjusted ORs between late-stage and rural area, NCMS, BMI ≥ 25.0, smoking, drinking, history of thyroid diseases and family history of TC were 1.33 (1.02–1.74), 2.08 (1.44–2.99), 0.93 (0.67–1.30), 0.81 (0.50–1.34), 1.06 (0.57–2.00), 1.10 (0.74–1.65) and 1.21 (0.27–5.50), respectively. Male patients (1.76, 95%CI 1.17–2.65, P = 0.006) and those with new rural cooperative medical scheme insurance (NCMS) (OR = 1.99, 95%CI 1.38–2.88, P < 0.001) had a higher risk of late-stage DTC, after adjusting for area, BMI, smoking history, drinking history, history of thyroid disease and family history of TC.
The association between BMI and aggressive clinicopathological features of DTC patients
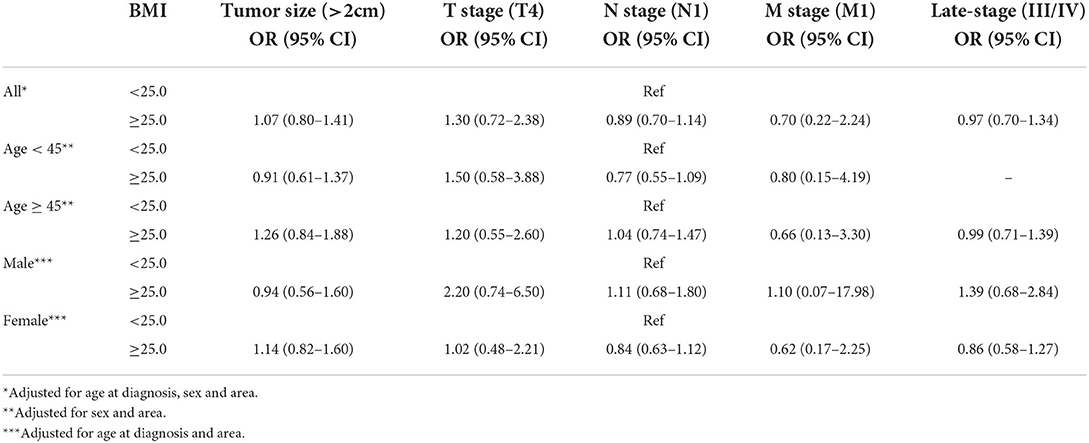
As shown in Supplementary Table S3 and Table 3, compared with underweight and normal DTC patients, we found no evidences that overweight and obese was associated with tumor size (OR = 1.07, 95%CI 0.80–1.41, P = 0.655), pathologic T4 stage (OR = 1.30, 95%CI 0.72–2.38, P = 0.387), lymph node metastasis (OR = 0.89, 95%CI 0.70–1.14, P = 0.366), distant metastasis (OR = 0.70, 95%CI 0.22–2.24, P = 0.552) and late-stage (OR = 0.97, 95%CI 0.70–1.34, P = 0.838). Similar patterns were shown in the subgroup analysis stratified by age and sex.
Comparations of clinicopathological features of DTC patients between the study and SEER database
As shown in Supplementary Table S4, compared with the 7th edition TNM staging system, we found the 8th staging downstages many DTC patients. 78.4 and 2.8% of DTC patients were distributed in stage I and stage II by the 7th edition TNM staging system. In contrast, 92.9 and 4.9% of DTC patients were distributed in stage I and stage II with the 8th edition staging. The dominant early-stage features were more obvious when we tried the 8th edition TNM staging (increased by 16.6%, P < 0.001) (Supplementary Table S4).
As shown in Table 4, compared with the SEER database, our study had more early-stage DTC patients (81.2 vs. 76.0%, P < 0.001). The proportion of stage I in the study and SEER database in the USA were 78.4 and 69.0%, respectively. Besides, compared with the SEER database, we have higher proportion of DTC with 2 cm or smaller (P < 0.001), more TC patients aged < 45y (P < 0.001), higher proportion of lymph node metastasis (P < 0.001) and more unifocal tumors (P < 0.001). There were younger aged and < 2cm tumors in early-stage DTC patients in China than in the USA (P < 0.001) (Figure 2).
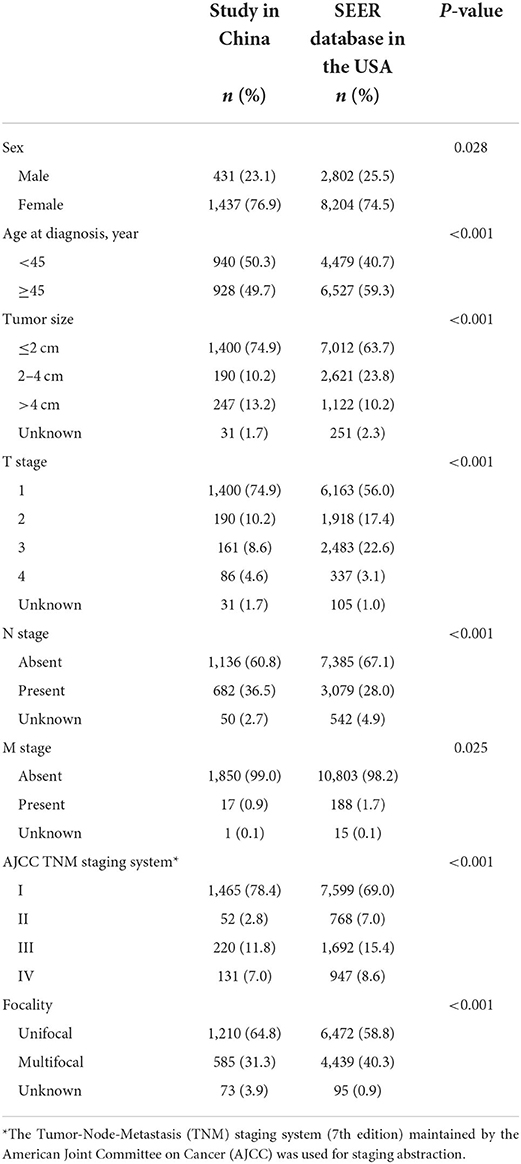
Table 4. Comparisons of clinicopathological features of DTC patients between the study in China and SEER database in the USA.
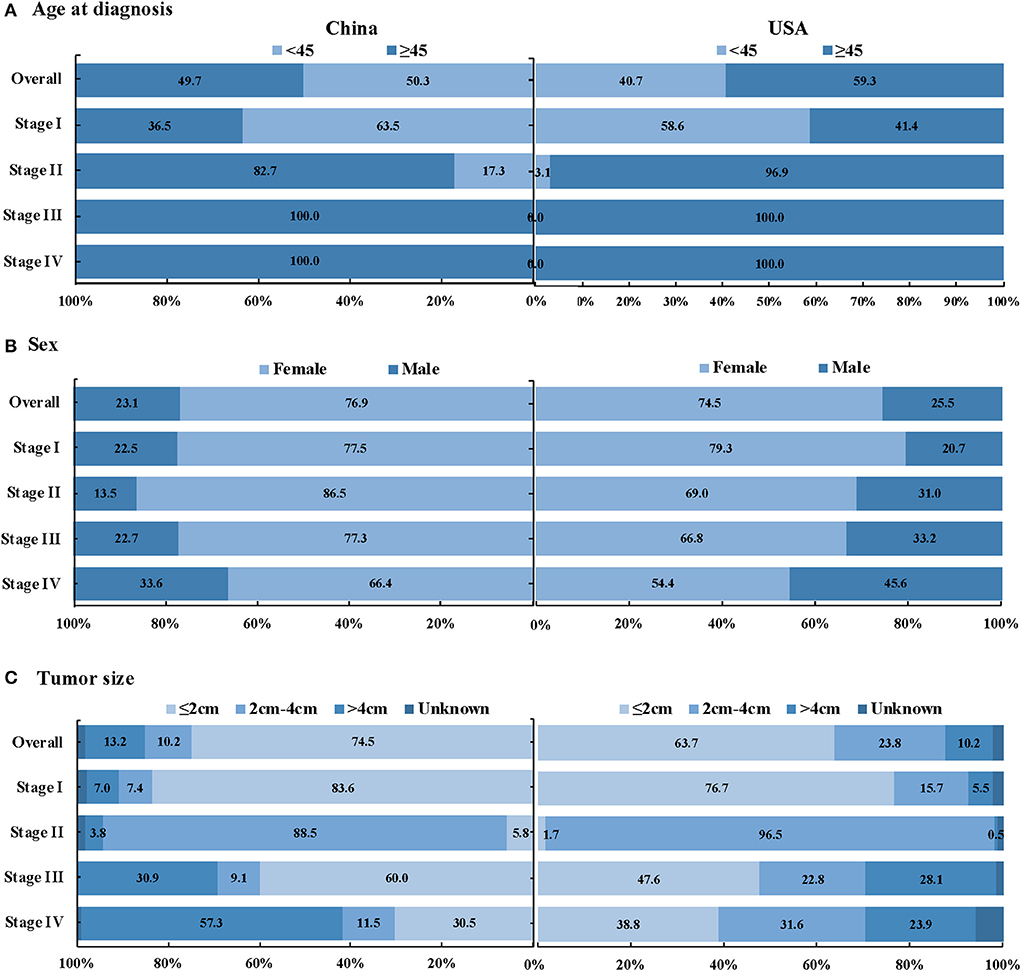
Figure 2. Stage distribution of DTC patients between the study in China and SEER database in the USA, by age at diagnosis, sex and tumor size (A) Age at diagnosis, (B) Sex, and (C) Tumor size.
Comparations of surgery and extent of surgery of DTC patients between the study and SEER database
The surgery rate of DTC patients of the study in China was slightly higher than that of SEER in the USA (99.9 vs. 97.2%, P < 0.001) (Table 5). There was no significant difference in the choice of surgery among stages in the study (P = 0.964), and almost all hospitalized patients underwent surgery in China. We found a significant difference of surgical treatment among stages in the USA (P < 0.001), and the surgical rate of stage IV was the lowest (90.4%). The surgical rate of stage IV was significantly higher than that in the USA (100.0 vs. 90.4%, P < 0.001). Besides, significant differences in surgery and extent of surgery were found among different regions in China (all P < 0.001)
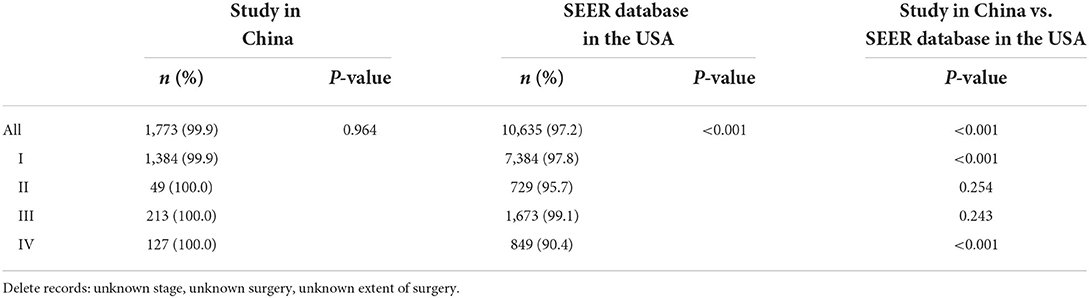
Table 5. Comparisons on surgery of DTC patients between the study in China and SEER database in the USA.
In Table 6, total thyroidectomy was more common than lobectomy both in the study and the SEER database (58.7 vs. 41.3% in the study; 83.0 vs. 17.0% in the SEER database). Significant differences were shown in surgical extensiveness (thyroid lobectomy vs. total thyroidectomy). With the aggravation of stage, the proportion of total thyroidectomy increased (P < 0.001). The proportion of total thyroidectomy at stage I, II, III and IV were 51.6, 55.1, 4.0, and 95.3% in our study, respectively (P < 0.001), and the corresponding proportion of total thyroidectomy at each stage were 79.6, 83.1, 91.3, and 96.2%, respectively (P < 0.001).

Table 6. Comparisons on extent of surgery of DTC patients between the study in China and SEER database in the USA.
Besides, we found the proportion of total thyroidectomy in our study was significantly lower than that in the SEER database (58.7 vs. 80.7%, P < 0.001). Similar findings were observed in stage I-III (P < 0.001). No significant difference was observed in the extent of surgery in stage IV between China and the USA (P = 0.604).
Discussion
To our knowledge, this was the first large-scale, multicentered study collecting updated and comprehensive clinicopathological and surgery information on DTC in China. Findings from the present study showed that the prevalence of late-stage DTC was 18.5%. Male, patients with NCMS had an increased risk of late-stage. Compared with the SEER database, more early-stage and young-aged DTC patients with smaller sizes were detected in China. The proportion of lobectomy in our database was significantly higher than that in the SEER database. Our findings gave us a better understanding of clinicopathological characteristics of hospitalized DTC patients in China and provided informative evidence for decision-making in DTC prevention and control.
Stage information is crucial for prognosis and survival (10–12). Compared with stage I DTC, the 10-year disease-specific survival of stage IV patients decreased by almost 27.9% (11). Over 80% of TC cases were diagnosed as early-stage in China, exceeding the USA (73.4%). The increasing use of ultrasonography for TC screening in routine check-ups in China might be one of the reasons for this phenomenon. However, it was not recommended in the USA (17). The proportion of early-stage in this study was also higher than that in South Korea in 2008 (53.8%) (18). However, the proportion of early-stage in the study (81.2%) was lower than that in a previous single-center analysis in northeast China (99.6%) and in eastern China (83.91%), and lower than that in a population-based cohort study in East Asia (84.4%) (19, 20). This difference could be due to this time trend analysis only covering one hospital, which may cause bias. Our study is the first large-scale, multicenter study in China, which could truly reflect the staging of DTC patients in grade A tertiary specialized hospitals in China.
In this multicentered study, the female/male sex ratio of DTC was 3.3, while male patients aged ≥45y had a higher risk of advanced DTC than females. According to the National Cancer Registry of China, the incidence rate of TC in women was 3.04 times that of men in 2015 (6). A similar female/male ratio (three-fold) was found in Global Cancer Statistics 2020 (1). The interaction between reproductive hormones in women, particularly estrogen, and the thyroid may lead to the generation of thyroid nodules (21, 22). However, autopsy evidence indicated that the actual incidence of thyroid nodules might not be much different between women and men (23). Since perinatal care and gynecological and obstetric clinics can provide women with more access to medical services, they may have additional opportunities to receive thyroid examinations (22, 24). This can harm both females who are subject to over-detection, and males who may be at risk of under-detection. In the study, although the numbers of males were fewer than females, male patients aged ≥45y had a higher risk of advanced DTC than females, in line with the existing evidence that DTC presented more advanced stage, higher mortality and recurrence rates in males than in females (25–27).
We found that access to NCMS was a risk factor for advanced DTC. Patients living in urban areas have a higher detection rate, but patients with NCMS were more likely to be diagnosed as late-stage (28). Considering the medical insurance system in China, this risk factor largely reflected the disease difference between urban and rural areas (29). Similar results were found in the latest study covering five major cancers in China, which showed a persistent diagnostic disparity that patients with NCMS had a higher risk of late-stage diagnosis compared with patients with urban insurance (OR = 1.4) (30). This phenomenon may be related to urban-rural inequities in medical utilization. Patients with urban medical insurance have better socioeconomic conditions and more access to medical resources, screening and other healthcare services (31, 32). Besides, compared with rural residents with NCMS, urban dwellers with urban medical insurance might also have a higher awareness of cancer prevention and regular doctor appointment in China (33, 34). Geographic disparities were also found in a prior report (30, 31, 35). Urban-rural disparities reflected that the current allocation of medical resources in China may be inequitable (36). This inequity should be considered in future TC prevention and control policy-making.
More young-aged, early-staged and small-sized TC patients were detected in our database compared with the SEER database. A population-based study proved that the incidence of young/middle-aged TC was on the rise in high-resource countries (37). Studies in South Korea and USA showed that the increased detection rate of early-stage and microcarcinoma may be related to the over-diagnosis caused by ultrasound screening (21, 22, 38–40). After TC screening was stopped, the proportion of localized stage of TC in South Korea declined gradually from 2005 to 2016 (39, 41). Previous studies indicated that the estimated proportion of TC cases attributable to overdiagnosis accounted for approximately 83% in women in China between 2008 and 2012 (5, 21). The high proportion of early-stage and small size tumors in our study also implied a warning signal of TC overdiagnosis caused by inappropriate ultrasonography screening for asymptomatic persons in China (5, 25, 42, 43). However, the acceleration of China's urbanization, the increasing access to routine physical examination, the improvement of diagnostic sensitivity and accuracy and the improvement of people's health awareness can also contribute to the specific clinicopathological characteristics of TC patients in this study (5, 24, 38, 44).
With the international attention to over-diagnosis and accumulation of evidence, many updated international recommendations recommend against screening for TC in asymptomatic adults using either neck palpation or ultrasound (Supplementary Table S5) (17, 45). Korea also called to stop TC screening in 2014 in order to turn the epidemic tide. The operation rate decreased significantly after TC screening was stopped in Korea (39). And the 5-year relative survival rate increased from 94.2% in 1993–1995 to 100.3% in 2011–2015 (46). Experience with TC screening in South Korea could serve as a cautionary tale for China. To date, there is no national TC screening plan in China. Only two provincial and municipal recommendations (Shanghai and Chongqing) on screening and prevention of common malignant tumors for residents, recommend thyroid-related examinations (clinical neck physical examination or ultrasonography) regularly for the general population. The wide application of ultrasound screening in China leads to the high detection of early-stage and small tumors (42). Evidence on the effectiveness of TC screening was at least insufficient if not disapproval. More considerations are needed for the necessity of TC screening in China. Considering the good prognosis of TC, large-scale and prospective studies are still needed to evaluate its long-term cost-effectiveness.
Subjects in this study were inpatients in oncology specialist hospitals, while patients in the SEER database were collected in cancer registries, including inpatients and outpatients, which may lead to a slightly higher surgery rate in China than in the USA. Before 2015, surgery for TC was the most preferred treatment. With the accumulation of evidence (47, 48), several international associations advocated active surveillance (AS) for small and low-risk TC instead of immediate surgery (Supplementary Table S6) (49, 50). The ATA 2015 Guidelines also represented a cost-effective strategy regarding AS and the extent of surgery (51). In accordance with international guidelines, the Chinese Society of Clinical Oncology also recommended active surveillance for low-risk papillary thyroid microcarcinoma patients in 2016 (52).
We found that more DTC patients with early-stage took lobectomy treatment in our database than in the SEER database, indicating that the choice of operation in China might be more conservative than that in the USA, which is in line with the updated guidelines. The high proportion of low-risk tumors with early-stage and small size in this study may result in more conservative surgery. Prior studies indicated that total thyroidectomy for the treatment of PTC measuring 1.0–4.0 cm does not result in a clinically significant improvement in disease-specific survival compared with lobectomy (53, 54). The health-related quality of life of DTC patients is not associated with the extent of surgery (55). With the accumulation of evidence, the extent of surgery of TC is constantly updated. We summarized global updated guidelines on surgery and the extent of surgery among DTC patients (Supplementary Table S6). The updated ATA guidelines promote surgical de-escalation by targeting low-risk patients (56). The global trend toward more partial thyroidectomy with a significant increase in the proportion of lobectomy (56). The number of unilateral thyroidectomies without neck dissection increased 2.56 times between 2007 and 2011 in Korea (57). There was a significant increase in the proportion of lobectomy over the last 10 years in France (2010–2019) (58). In addition, we observed increasing conservative surgery in the SEER database from 2015 to 2018 (Supplementary Figure S1). In recent years, surgery management in China gradually evolved from aggressive treatment to a more conservative approach. A Time trend analysis of TC surgery between 2008 and 2017 in a single institutional database of 15,000 Chinese patients showed that TC patients receiving total thyroidectomy decreased from 71 to 41% (59). A previous study showed that 53% of DTC with low to intermediate risk of recurrence took lobectomy in 2018–2019 (55). The proportion of lobectomy in early-stage DTC patients in our study was similar to previous studies. The decrease in total thyroidectomy indicated the benefits of evidence-based guidelines in accelerating changes to clinical practice to reduce overtreatment. Considering disparities in economic level, medical care level and surgical accessibility among regions, significant differences in stage and surgery extent among different regions.
Besides, we found no aggressive association between higher BMI and clinicopathological characteristics in our study. Similar to our results, previous studies have shown that a higher BMI is not associated with more aggressive tumor features (late-stage, lymph node metastasis, distant metastasis, etc.) (15, 60). In addition, a prior study showed that subjects in the underweight group had a significantly greater risk for distant metastasis (OR = 34.5) and macroPTCs (OR = 3.99) when compared with the normal-weight group (61). Paes et al. even indicated that a higher BMI was associated with a lower risk of tumor invasion and nodal metastases (62). On the contrary, several studies suggested that obesity was significantly related to larger tumor size, lymph node metastasis and advanced stage of PTC (aggressive features) (14, 63, 64). The causes remain uncertain. Biological factors, such as thyroid-stimulating hormone serum levels, have been considered contributors to increasing PTC aggressiveness in obese patients. TSH level has a positive correlation with vascular endothelial growth factor expression, and vascular endothelial growth factor can stimulate angiogenesis and increase vascular permeability (65). Cheng et al. found that leptin mediated the migration of PTC by activating the PI3K/AKT and MAPK pathways (66). Experimental models have shown that adipokines such as leptin and hepatocyte growth factor may regulate cancer cell proliferation and invasiveness (67).
Strengths and limitations
This is a multicentered retrospective hospital-based study. Eight participating hospitals are distributed in 5 geographic regions of China, which enhances the representativeness of the study sample. What's more, since all TC patients are inpatients, detailed diagnostic and treatment information can be collected. However, several limitations of our study should be acknowledged. (1) Compared with the population-based SEER database, the sample size of our hospital-based database was limited, which cannot adequately represent current care in China. Even though, our multi-center hospital-based design took into consideration geographical variation, population density and socioeconomic status, which is the best available data at present. The extrapolation and interpretation of the differences should be cautious. (2) Information on height and weight was not available in individual hospitals, and the missing rate of BMI was relatively high (24.2%). This reduced the statistical power of tests, which might be the reason for the negative results between BMI and DTC stage. (3) Outpatients and non-hospitalized patients were not included, and the results may not be generalizable to the total population diagnosed and treated with TC during this period. (4) Affected by the geographical location, social and cultural background and other factors of China and the United States, the tumor biology may be different in the two countries, such as pathological type and stage. Further studies are warranted to confirm these findings and establish potential relationships between obesity and TC biology.
Conclusions
This multicentered epidemiology study depicts clinicopathological features of DTC in China and compared them with the SEER database. Unique risk factors are found to be associated with late-stage DTC in China. The differences in clinicopathological features and surgical approaches between China and the USA indicate that the two countries' screening strategies and clinical practices are different. Overdiagnosis and overtreatment exist. Surgical comparisons provide clinical recommendations for examining physicians and surgeons. More research is needed for DTC prevention and control policy-making in China. These findings are intriguing, provided scientific evidence to clinical practice and research, were informative for screening strategy and treatment guidelines in China and provided references for other countries with similar patterns.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was conducted according to the guidelines of the Helsinki declaration and was approved by the Institutional Review Board of the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, the Chinese Academy of Medical Sciences, and Peking Union Medical College (Approval Number: 2019/146-1930). The patients/participants provided their written informed consent to participate in this study.
Author contributions
JZ, KS, JW, SL, SC, and WW contributed to the conception and design of the study. YHe, DL, SL, YHu, MZ, BS, XL, HL, QZ, MS, LG, YZ, YLi, YLu, JT, YX, HS, HX, YJ, CY, JQ, HZ, RZ, and SZ contributed to data collection and quality control. JZ, KS, and JW drafted the paper and interpreted the results. All authors contributed to data interpretation and rewriting the paper, reviewed and approved the final version, and had full access to all the data and were responsible for the decision to submit the manuscript.
Funding
The National Key Research and Development Program of China, Grant/Award Number: 2018YFC1311704.
Acknowledgments
We gratefully thank the hospitals and staff for their cooperation with regard to providing, sorting, and verifying the data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.974359/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Wiltshire JJ, Drake TM, Uttley L, Balasubramanian SP. Systematic review of trends in the incidence rates of thyroid cancer. Thyroid. (2016) 26:1541–52. doi: 10.1089/thy.2016.0100
3. Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. (2016) 12:646–53. doi: 10.1038/nrendo.2016.110
4. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
5. Li M, Zheng R, Dal Maso L, Zhang S, Wei W, Vaccarella S. Mapping overdiagnosis of thyroid cancer in China. Lancet Diabetes Endocrinol. (2021) 9:330–2. doi: 10.1016/S2213-8587(21)00083-8
6. Du L, Zhao Z, Zheng R, Li H, Zhang S, Li R, et al. Epidemiology of thyroid cancer: incidence and mortality in China, 2015. Front Oncol. (2020) 10:1702. doi: 10.3389/fonc.2020.01702
7. Du L, Li R, Ge M, Wang Y, Li H, Chen W, et al. Incidence and mortality of thyroid cancer in China, 2008-2012. Chin J Cancer Res. (2019) 31:144–51. doi: 10.21147/j.issn.1000-9604.2019.01.09
8. He J, Wei WQ, Zhang SW, Zheng RS, Ma F, Wang N, et al. 2019 China Cancer Registry Annual Report. Beijing: People's Medical Publishing House (2019).
9. Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. (2018) 6:e555–67. doi: 10.1016/S2214-109X(18)30127-X
10. National Cancer Institute. Surveillance Epidemiology and End Results (SEER) Program. Cancer Query System: SEER Survival Statistics. Available online at: https://seer.cancer.gov/statfacts/html/all.html
11. Tam S, Boonsripitayanon M, Amit M, Fellman BM Li Y, Busaidy NL, et al. Survival in differentiated thyroid cancer: comparing the AJCC cancer staging seventh and eighth editions. Thyroid. (2018) 28:1301–10. doi: 10.1089/thy.2017.0572
12. Pontius LN, Oyekunle TO, Thomas SM, Stang MT, Scheri RP, Roman SA, et al. Projecting survival in papillary thyroid cancer: a comparison of the seventh and eighth editions of the American joint commission on cancer/union for international cancer control staging systems in two contemporary national patient cohorts. Thyroid. (2017) 27:1408–16. doi: 10.1089/thy.2017.0306
13. Ma J, Huang M, Wang L, Ye W, Tong Y, Wang H. Obesity and risk of thyroid cancer: evidence from a meta-analysis of 21 observational studies. Med Sci Monit. (2015) 21:283–91. doi: 10.12659/MSM.892035
14. Cui N, Sun Q, Chen L. A meta-analysis of the influence of body mass index on the clinicopathologic progression of papillary thyroid carcinoma. Medicine (Baltimore). (2021) 100:e26882. doi: 10.1097/MD.0000000000026882
15. Grani G, Lamartina L, Montesano T, Ronga G, Maggisano V, Falcone R, et al. Lack of association between obesity and aggressiveness of diferentiated thyroid cancer. J Endocrinol Invest. (2019) 42:85–90. doi: 10.1007/s40618-018-0889-x
16. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, et al. Cancer staging manual 7th edition. New York: Springer (2010).
17. Lin JS, Bowles EJA, Williams SB, Morrison CC. Screening for thyroid cancer: updated evidence report and systematic review for the us preventive services task force. JAMA. (2017) 317:1888–903. doi: 10.1001/jama.2017.0562
18. Park S, Oh CM, Cho H, Lee JY, Jung KW, Jun JK, et al. Association between screening and the thyroid cancer “epidemic” in South Korea: evidence from a nationwide study. BMJ. (2016) 355:i5745. doi: 10.1136/bmj.i5745
19. Xiang J, Wu Y, Li DS, Shen Q, Wang ZY, Sun TQ, et al. New clinical features of thyroid cancer in eastern China. J Visc Surg. (2010) 147:e53–6. doi: 10.1016/j.jviscsurg.2010.02.007
20. Shah BR, Griffiths R, Hall SF. Thyroid cancer incidence among Asian immigrants to Ontario, Canada: A population-based cohort study. Cancer. (2017) 123:3320–5. doi: 10.1002/cncr.30746
21. Li M, Dal Maso L, Vaccarella S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol. (2020) 8:468–70. doi: 10.1016/S2213-8587(20)30115-7
22. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA. (2017) 317:1338–48. doi: 10.1001/jama.2017.2719
23. LeClair K, Bell KJL, Furuya-Kanamori L, Doi SA, Francis DO, Davies L. Evaluation of gender inequity in thyroid cancer diagnosis: differences by sex in us thyroid cancer incidence compared with a meta-analysis of subclinical thyroid cancer rates at autopsy. JAMA Intern Med. (2021) 181:1351–8. doi: 10.1001/jamainternmed.2021.4804
24. Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. (2016) 375:614–7. doi: 10.1056/NEJMp1604412
25. Machens A, Hauptmann S, Dralle H. Disparities between male and female patients with thyroid cancers: sex difference or gender divide? Clin Endocrinol (Oxf). (2006) 65:500–5. doi: 10.1111/j.1365-2265.2006.02623.x
26. Jonklaas J, Nogueras-Gonzalez G, Munsell M, Litofsky D, Ain KB, Bigos ST, et al. The impact of age and gender on papillary thyroid cancer survival. J Clin Endocrinol Metab. (2012) 97:E878–87. doi: 10.1210/jc.2011-2864
27. Zahedi A, Bondaz L, Rajaraman M, Leslie WD, Jefford C, Young JE, et al. Risk for Thyroid cancer recurrence is higher in men than in women independent of disease stage at presentation. Thyroid. (2020) 30:871–7. doi: 10.1089/thy.2018.0775
28. Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. (2008) 9:222–31. doi: 10.1016/S1470-2045(08)70032-9
29. Barber S, Lan Y. Health Insurance Systems in China: A Briefing Note. World Health Report (2010) Background Paper 37. Geneva: World Health Organization.
30. Zeng H, Ran X, An L, Zheng R, Zhang S, Ji JS, et al. Disparities in stage at diagnosis for five common cancers in China: a multicentre, hospital-based, observational study. Lancet Public Health. (2021) 6:e877–87. doi: 10.1016/S2468-2667(21)00157-2
31. Lortet-Tieulent J, Franceschi S, Dal Maso L, Vaccarella S. Thyroid cancer “epidemic” also occurs in low- and middle-income countries. Int J Cancer. (2019) 144:2082–7. doi: 10.1002/ijc.31884
32. Liu Y, Lai F, Long J, Peng S, Wang H, Zhou Q, et al. Screening and the epidemic of thyroid cancer in China: An analysis of national representative inpatient and commercial insurance databases. Int J Cancer. (2021) 148:1106–14. doi: 10.1002/ijc.33298
33. Li H, Zeng H, Zheng R, Zou X, Cao M, Sun D, et al. Association of cancer awareness levels with the risk of cancer in rural China: a population-based cohort study. Cancer. (2020) 126:4563–71. doi: 10.1002/cncr.33029
34. Ma C, Song Z, Zong Q. Urban-rural inequality of opportunity in health care: evidence from China. Int J Environ Res Public Health. (2021) 18:7792. doi: 10.3390/ijerph18157792
35. Kim J, Gosnell JE, Roman SA. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol. (2020) 16:17–29. doi: 10.1038/s41574-019-0263-x
36. Niu J, Qi Y. Regional disparity in China's medical insurance system and its implication on health service use. Sociol Rev China. (2016) 4:43–58.
37. Vaccarella S, Dal Maso L, Laversanne M, Bray F, Plummer M, Franceschi S. The impact of diagnostic changes on the rise in thyroid cancer incidence: a population-based study in selected high-resource countries. Thyroid. (2015) 25:1127–36. doi: 10.1089/thy.2015.0116
38. Ahn HS, Kim HJ, Welch HG. Korea's thyroid-cancer “epidemic”—screening and overdiagnosis. N Engl J Med. (2014) 371:1765–7. doi: 10.1056/NEJMp1409841
39. Ahn HS, Welch HG. South Korea's Thyroid-Cancer “Epidemic”—Turning the Tide. N Engl J Med. (2015) 373:2389–90. doi: 10.1056/NEJMc1507622
41. Oh CM, Lim J, Jung YS, Kim Y, Jung KW, Hong S, et al. Decreasing trends in thyroid cancer incidence in South Korea: What happened in South Korea? Cancer Med. (2021) 10:4087–96. doi: 10.1002/cam4.3926
42. Li Y, Teng D, Ba J, Chen B, Du J, He L, et al. Efficacy and safety of long-term universal salt iodization on thyroid disorders: epidemiological evidence from 31 provinces of Mainland China. Thyroid. (2020) 30:568–79. doi: 10.1089/thy.2019.0067
43. Vaccarella S, Lortet-Tieulent J, Colombet M, Davies L, Stiller CA, Schüz J, et al. Global patterns and trends in incidence and mortality of thyroid cancer in children and adolescents: a population-based study. Lancet Diabetes Endocrinol. (2021) 9:144–52. doi: 10.1016/S2213-8587(20)30401-0
44. Welch HG, Doherty GM. Saving thyroids—overtreatment of small papillary cancers. N Engl J Med. (2018) 379:310–2. doi: 10.1056/NEJMp1804426
45. Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedüs L, et al. AACE/ACE/AME Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules-−2016 update. Endocr Pract. (2016) 22:622–39. doi: 10.4158/EP161208.GL
46. Kweon SS. Updates on Cancer Epidemiology in Korea, 2018. Chonnam Med J. (2018) 54:90–100. doi: 10.4068/cmj.2018.54.2.90
47. Oh HS, Ha J, Kim HI, Kim TH, Kim WG, Lim DJ, et al. Active surveillance of low-risk papillary thyroid microcarcinoma: a multi-center cohort study in Korea. Thyroid. (2018) 28:1587–94. doi: 10.1089/thy.2018.0263
48. Leboulleux S, Tuttle RM, Pacini F, Schlumberger M. Papillary thyroid microcarcinoma: time to shift from surgery to active surveillance? Lancet Diabetes Endocrinol. (2016) 4:933–42. doi: 10.1016/S2213-8587(16)30180-2
49. Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. (2019) 30:1856–83. doi: 10.1093/annonc/mdz400
50. Sugitani I, Ito Y, Takeuchi D, Nakayama H, Masaki C, Shindo H, et al. Indications and strategy for active surveillance of adult low-risk papillary thyroid microcarcinoma: consensus statements from the Japan Association of endocrine surgery task force on management for papillary thyroid microcarcinoma. Thyroid. (2021) 31:183–92. doi: 10.1089/thy.2020.0330
51. White C, Weinstein MC, Fingeret AL, Randolph GW, Miyauchi A, Ito Y, et al. Is less more? A microsimulation model comparing cost-effectiveness of the revised american thyroid association's 2015 to 2009 guidelines for the management of patients with thyroid nodules and differentiated thyroid cancer. Ann Surg. (2020) 271:765–73. doi: 10.1097/SLA.0000000000003074
52. Gao M, Ge M, Ji Q, Cheng R, Lu H, Guan H, et al. 2016 Chinese expert consensus and guidelines for the diagnosis and treatment of papillary thyroid microcarcinoma. Cancer Biol Med. (2017) 14:203–11. doi: 10.20892/j.issn.2095-3941.2017.0051
53. Adam MA, Pura J, Gu L, Dinan MA, Tyler DS, Reed SD, et al. Extent of surgery for papillary thyroid cancer is not associated with survival: an analysis of 61,775 patients. Ann Surg. (2014) 260:601–5. doi: 10.1097/SLA.0000000000000925
54. Gartland RM, Lubitz CC. Impact of extent of surgery on tumor recurrence and survival for papillary thyroid cancer patients. Ann Surg Oncol. (2018) 25:2520–5. doi: 10.1245/s10434-018-6550-2
55. Chen W, Li J, Peng S, Hong S, Xu H, Lin B, et al. Association of total thyroidectomy or thyroid lobectomy with the quality of life in patients with differentiated thyroid cancer with low to intermediate risk of recurrence. JAMA Surg. (2021) e216442. doi: 10.1001/jamasurg.2021.6442
56. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. (2009) 19:1167–214. doi: 10.1089/thy.2009.0110
57. Sung MW, Park B, An SY, Hah JH, Jung YH, Choi HG. Increasing thyroid cancer rate and the extent of thyroid surgery in Korea. PLoS ONE. (2014) 9:e113464. doi: 10.1371/journal.pone.0113464
58. Marciniak C, Lenne X, Clément G, Bruandet A, Lifante JC, Sebag F, et al. Partial Versus Total Thyroidectomy: What Influences Most Surgeons' Decision? Analysis of a Nationwide Cohort of 375,810 Patients Over 10 Years. Ann Surg. (2021) 274:829–35. doi: 10.1097/SLA.0000000000005134
59. Sui C, Liang N, Du R, He Q, Zhang D, Li F, et al. Time trend analysis of thyroid cancer surgery in China: single institutional database analysis of 15,000 patients. Endocrine. (2020) 68:617–28. doi: 10.1007/s12020-020-02230-7
60. Kim JY, Jung EJ, Jeong SH, Jeong CY, Ju YT, Lee YJ, et al. The indices of body size and aggressiveness of papillary thyroid carcinoma. J Korean Surg Soc. (2011) 80:241–4. doi: 10.4174/jkss.2011.80.4.241
61. Feng JW, Yang XH, Wu BQ, Sun DL, Jiang Y, Qu Z. Influence of body mass index on the clinicopathologic features of papillary thyroid carcinoma. Ann Otol Rhinol Laryngol. (2019) 128:625–32. doi: 10.1177/0003489419834314
62. Paes JE, Hua K, Nagy R, Kloos RT, Jarjoura D, Ringel MD. The relationship between body mass index and thyroid cancer pathology features and outcomes: a clinicopathological cohort study. J Clin Endocrinol Metab. (2010) 95:4244–50. doi: 10.1210/jc.2010-0440
63. Kim SK, Woo JW, Park I, Lee JH, Choe JH, Kim JH, et al. Influence of body mass index and body surface area on the behavior of papillary thyroid carcinoma. Thyroid. (2016) 26:656–66. doi: 10.1089/thy.2015.0632
64. Li CL, Dionigi G, Zhao YS, Liang N, Sun H. Influence of body mass index on the clinicopathological features of 13,995 papillary thyroid tumors. J Endocrinol Invest. (2020) 43:1283–99. doi: 10.1007/s40618-020-01216-6
65. Jin L, Lisong T, Honggang J. Relationship between preoperative serum TSH levels and expression of VEGF in papillary thyroid carcinoma. Asia Pac J Clin Oncol. (2014) 10:149–52. doi: 10.1111/ajco.12075
66. Cheng SP, Yin PH, Hsu YC, Chang YC, Huang SY, Lee JJ, et al. Leptin enhances migration of human papillary thyroid cancer cells through the PI3K/AKT and MEK/ERK signaling pathways. Oncol Rep. (2011) 26:1265.
Keywords: thyroid cancer, differentiated thyroid carcinoma, stage, surgery, lobectomy, total thyroidectomy, BMI, China
Citation: Zhu J, Sun K, Wang J, He Y, Li D, Liu S, Huang Y, Zhang M, Song B, Liao X, Liang H, Zhang Q, Shi M, Guo L, Zhou Y, Lin Y, Lu Y, Tuo J, Xia Y, Sun H, Xiao H, Ji Y, Yan C, Qiao J, Zeng H, Zheng R, Zhang S, Liu S, Chang S and Wei W (2022) Clinicopathological and surgical comparisons of differentiated thyroid cancer between China and the USA: A multicentered hospital-based study. Front. Public Health 10:974359. doi: 10.3389/fpubh.2022.974359
Received: 21 June 2022; Accepted: 02 September 2022;
Published: 28 September 2022.
Edited by:
Emese Mezosi, University of Pécs, HungaryReviewed by:
Heng Wan, Southern Medical University, ChinaKevin P. Mollen, University of Pittsburgh, United States
Copyright © 2022 Zhu, Sun, Wang, He, Li, Liu, Huang, Zhang, Song, Liao, Liang, Zhang, Shi, Guo, Zhou, Lin, Lu, Tuo, Xia, Sun, Xiao, Ji, Yan, Qiao, Zeng, Zheng, Zhang, Liu, Chang and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheng Chang, ZHJfY2hhbmcmI3gwMDA0MDsxMjYuY29t; Wenqiang Wei, d2Vpd3EmI3gwMDA0MDtjaWNhbXMuYWMuY24=
†These authors have contributed equally to this work and share first authorship
 Juan Zhu
Juan Zhu Kexin Sun1†
Kexin Sun1† Yunchao Huang
Yunchao Huang He Liang
He Liang Lanwei Guo
Lanwei Guo Yongchun Zhou
Yongchun Zhou Haifan Xiao
Haifan Xiao Hongmei Zeng
Hongmei Zeng Rongshou Zheng
Rongshou Zheng Siwei Zhang
Siwei Zhang Wenqiang Wei
Wenqiang Wei