
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 13 September 2022
Sec. Digital Public Health
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.954816
Background: Comprehensive studies on the prognosis of solitary bone plasmacytoma (SPB) are lacking, especially in elderly patients with SPB. This study aims to establish a novel nomogram and risk stratification system to predict the overall survival (OS) of elderly patients with SPB.
Methods: The data of elderly patients with SPB from 2000 to 2017 were identified in the SEER database. SPB patients were randomly assigned to the training set (n = 825) and validation set (n = 354). The Cox regression analysis was used to determine the independent risk factors for OS in elderly SPB patients. The nomogram was established and assessed by the area under the receiver operating curve (AUC), the consistency index (C-index), and the calibration plot. Patients were divided into low-, medium-, and high-risk groups based on the score of the nomogram. The Kaplan-Meier (K-M) curve was used to verify the differences in overall survival among the three groups.
Result: A total of 1,179 elderly patients with SPB were included in the study. Age at diagnosis, prior cancer before SPB, marital status, radiotherapy, and chemotherapy were independent risk factors of OS. The AUC of the 3, 5, and 8-year OS in the training and validation sets were between 0.707 and 0.860. The C-index and calibration plot also indicated that the nomogram has great predictive accuracy and robustness. After risk stratification, patients in the high-risk group had the worst OS.
Conclusion: A novel nomogram was built to predict the OS of elderly patients with SPB. It will help clinicians formulate more reasonable and personalized treatment strategies.
Solitary plasmacytoma (SP) is a malignant tumor caused by monoclonal proliferation of plasma cells, accounting for about 3–5% of all plasma cell neoplasms (1, 2). SP can be divided into extramedullary plasmacytoma (EMP) and solitary bone plasmacytoma (SBP). SPB accounts for 60–70% of SP, mainly in the red marrow-containing bone, especially vertebrae and femurs (3, 4). SPB patients may experience bone pain, neurological symptoms, and pathological fractures, but lack multiple myelomas (MM) characteristics such as multiple lytic bone lesions, hypercalcemia, and renal insufficiency (5).
Studies have reported that older age, the primary site of the tumor, developing MM, histologic grade, treatment methods, and posttreatment persistent M protein were significant prognostic factors of SPB (6–12). Among them, age plays an important role in the prognosis of SPB patients. Studies have showed that age > 60 years was an important risk factor for the worse OS (13, 14) and progression to MM (14) in SPB patients. Similarly, older age (≥65 years) is significantly associated with the worse OS compared to younger SPB patients (1). Elderly SPB patients are more likely to receive palliative care rather than cure treatment. And older patients are more likely to be intolerant of radical radiation therapy than their younger counterparts (15). Meanwhile, elderly patients are more likely to be frail or have comorbidity. These factors may affect the survival of SPB patients. Therefore, it has a clinically important role in predicting the survival of elderly SPB patients.
Therefore, based on the SEER database, we collect data from a large number of patients to develop a survival prediction nomogram and a risk-stratifying system that can dynamically predict the long-term survival of elderly SPB.
All data in this study were obtained through the SEER*Stat software version 8.3.9. In the SEER database, subjects of SPB were identified by International Classification of Tumor Diseases, Third Edition (ICD-O-3) histology code 9731/3. Elderly Patients (≥60 years old) with SPB between 2000 and 2017 were included in our study. The individualized data we extracted from the SEER database included age at diagnosis, sex, tumor stage, age, race, sex, marital status, year of diagnosis, prior cancer before SPB, and treatment (radiotherapy, chemotherapy, and surgery), vital status, and survival time. Patients with incomplete individualized data were not included in this study. Also, patients diagnosed with SPB on death certificates or at autopsy were excluded from this study. The last follow-up day was December 31, 2018. The flow chart of patient screening is shown in Figure 1.
The elderly patients with SPB were randomly divided into a training set and validation set at a ratio of 7:3. Display and compare variables between training sets and validation sets. Classification variables were presented regarding the number of cases and percentage, and the chi-square test was used to compare groups. The Cox regression analysis was performed in the training set to identify the independent prognostic factors for OS in SPB patients. Candidate variables with a p-value < 0.25 on univariate analysis were included in the multivariable model. Variables of p-value < 0.05 in the multivariate model were considered significantly related to OS. Based on these independent prognostic factors, a nomogram of OS was built. The receiver operating characteristic (ROC) curves and their corresponding AUC were generated to assess the discrimination of the nomogram. The calibration curves were used to measure the degree of agreement between the predicted probabilities of the model and the actual results. A risk stratification system was established. Based on the patient's total risk score, SPB patients were accurately divided into low, medium, and high-risk groups through X-tile software. The Kaplan-Meier curves were used to verify differences in OS among these risk groups.
Statistical analysis was performed in R version 4.1.1 and SPSS statistics 24. A two-sided P < 0.05 was considered statistically significant.
The data extraction complies with the SEER database usage agreement. The data in the SEER database is public and does not require the patient's informed consent. Our study was exempt from review by the Ethics Committee of the Second Affiliated Hospital of Guangxi Medical University. All methods are carried out by relevant guidelines and regulations.
1179 elderly patients with SPB were included in this study. in the whole cohort, 81.1% of patients were white, and 60.3% were male. The median age of SPB was 71.0 years old. 27.1% of patients had prior tumor before SPB Diagnosis. The most common tumor grade was the grade pre-B (97.3%). The most common primary sites of tumor were vertebrae bone (42.7%), followed by pelvis (17.6%), ribs/sternum/clavicle (16.6%), extremities (14.4%), and facial/skull bone (8.7%). As to treatment, 905 (76.8%) received radiotherapy, only 252 (21.4%) patients received surgery, and 236 (20.0%) received chemotherapy (Table 1). The patients were randomly divided into the training set (825 cases) and the validation set (354 cases). The characteristics of SPB patients are shown in Table 1.
In the training set, the median OS of SPB patients was 64 (1–209) months. And the 3, 5, and 8-year OS was 63.9%, 51.1%, and 37.2%, respectively. The Kaplan–Meier survival analyses were used to stratify patients according to their demographics and treatment patterns. Age at diagnosis, marital status, prior cancer, tumor grade, radiotherapy, surgery, and chemotherapy were risk factors for OS. The above factors were included in the multivariate cox regression analysis. The results showed that age at diagnosis, marital status, prior cancer, radiotherapy, and chemotherapy were considered independent prognosis factors (Table 2).
Five independent prognostic factors were included to construct the nomogram for OS (Figure 2). The ROC curves showed that the AUC of the 3, 5, and 8-year OS were 0.743, 0.732, and 0.707 in the training set and 0.714, 0.714, and 0.860 in the validation set (Figure 3). The C-index of the training set and validation set were 0.688 (95% CI: 0.660–0.718) and 0.691 (95% CI: 0.650–0.732), respectively. The calibration curves showed great agreement between the predictions and actual outcomes for 3, 5, and 8-year survival (Figure 4).
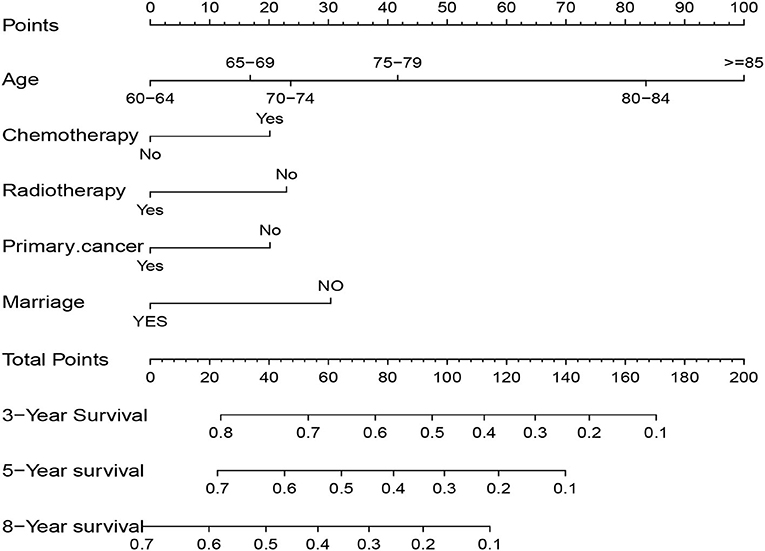
Figure 2. Nomogram to predict 3, 5, and 8-year overall survival in elderly patients with solitary bone plasmacytoma.
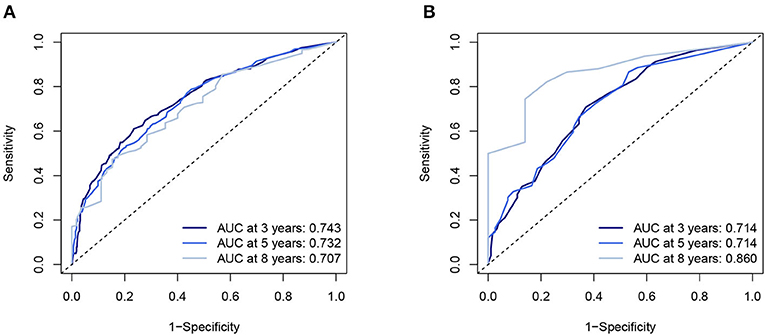
Figure 3. The AUC of nomogram of 3-, 5-, and 8-year in the training set (A) and validation set (B). AUC, Receiver operating curve.
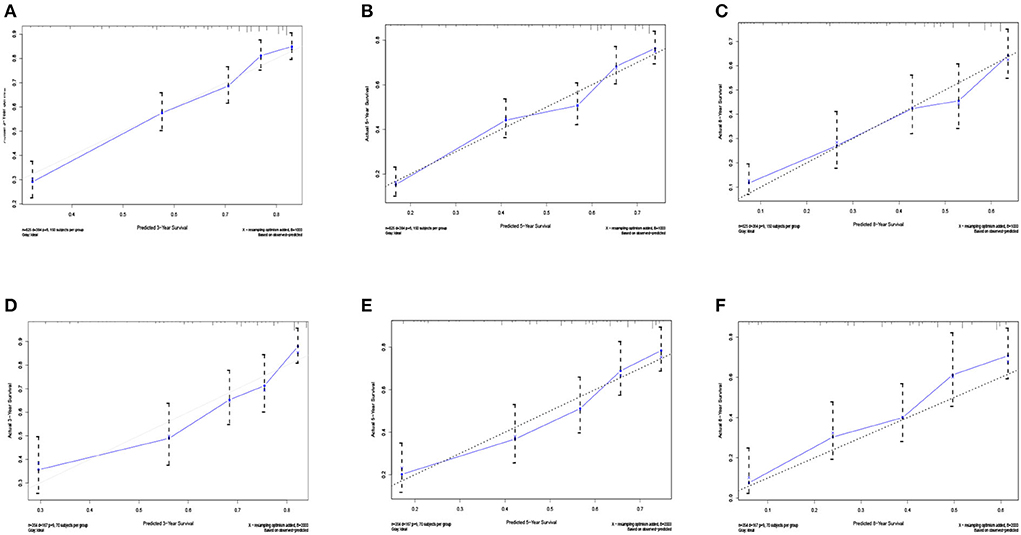
Figure 4. The calibration curves for predictions of overall survival in the training set (A–C) and validation set (D–F) at 3, 5, and 8-year.
The total score of patients was calculated according to the nomogram. The best cut-off point of the total score was determined by X-tile software. The cut-off points are 63 and 106. And we designated a total score of < 63 as the low-risk group, between 63 and 106 as the medium-risk group, and a score > 106 as the high-risk group. The survival curves showed that the survival curves of the three risk groups were significantly different (P < 0.001), whether in the training or validation set. Elderly patients with SPB in the high-risk group have the worst OS, while patients in the low-risk group have the best OS (Figure 5).
We have developed a user-friendly online application to predict the OS of elderly SPB patients, which can be accessed at https://yingyingwu.shinyapps.io/DynNomapp/. Enter the patient's personal and clinical characteristics; we can immediately predict the patient's survival probability. In short, this calculating tool is convenient and friendly to both patients and physicians.
The SEER database covers a great number of cancer cases with complete follow-up. It is often used in combination with nomograms to predict the survival of cancers (16, 17). We included 1,179 elderly SPB patients to analyze clinical and demographic sociology-related prognostic factors. Then we built a nomogram to predict the OS of elderly SPB patients. The nomogram was based on five independent risk factors: age, marital status, prior cancer, radiotherapy, and chemotherapy. And with the increase of age, the immune system's ability continues to decline, which may cause tumor deterioration or serious treatment complications, reducing the patient's survival time. Older patients often have more comorbidities such as diabetes, cardiovascular and cerebrovascular diseases, and other cancers than younger patients. It may directly have a negative impact on survival or affect treatment tolerance (18). Also, in this study, prior cancer before SPB and older age are the negative factors in elderly patients with SPB.
Marital status is a significant prognostic factor for many cancers (19–21). Compared with unmarried or divorced patients, married patients may get better financial, emotional support, and life care from their partners or family members. Therefore, it may be associated with a better prognosis. Similarly, we found that married was a favorable prognostic factor for OS in SPB patients.
Radiotherapy, surgery, and systematic chemotherapy are the commonly used treatments for SPB. The guidelines recommend that the standard treatment of SPB is local radiotherapy (22, 23). Local radiotherapy can provide good local control and survival for SPB patients (11, 24, 25). And our study reaffirmed the benefits of radiotherapy for SPB patients. We found that radiotherapy was the primary treatment, and 78.8% of patients received radiotherapy. Radiotherapy can significantly improve the OS of SPB patients.
Guidelines recommend that surgery may be considered when SPB patients have a structural imbalance or neurological damage caused by tumor (22). Our results suggested that surgery does not affect patient outcomes. Surgery can only relieve symptoms of SPB patients and improve their self-care ability, but it may not stop the progression of the SPB.
Currently, adjuvant chemotherapy is not recommended due to insufficient research support. Several previous multicenter studies have shown that chemotherapy does not benefit the survival of SPB patients (14, 26, 27). A recent survey by Khaled et al. shows that the addition of chemotherapy mainly based on bortezomib/dexamethasone or lenalidomide/dexamethasone improves multiple myeloma-free survival and PFS in patients with SPB (28). However, it was retrospective studies of a small sample, and additional adjuvant chemotherapy did not improve the survival of SPB in most previous studies. More evidence is needed in the future. In our study, 236 (20.0%) patients received chemotherapy. The results showed that chemotherapy is an adverse prognostic factor. The following reasons should be considered: chemotherapy is often used in patients who are intolerant to radiotherapy or have the potential risk of developing MM. The prognosis of those SPB patients is often poor. Besides, we must acknowledge that there is no detailed information about chemotherapy regimens in our data, and we cannot compare the effects of different chemotherapy regimens on survival. It may lead to biased final results.
However, some limitations of our research should be noted. Firstly, it was a retrospective study, and the potential selection bias was unavoidable. Secondly, since detailed treatment data such as chemotherapy and radiotherapy regimens cannot be obtained from the SEER database, the treatment effect cannot be further evaluated. Third, much clinical and pathological information, such as the extent of bone marrow involvement and the M protein in blood/urine cannot be obtained, which may lead to study bias.
Undeniably, it was a large population-based study, and its results were representative. Then, we constructed an effective nomogram to evaluate the OS of elderly SPB patients. The excellent performance of the nomogram has been confirmed by ROC curves, calibration curves, and decision curve analysis.
In summary, the novel nomogram and risk-stratifying system could effectively predict long-term OS in elderly patients with SPB and identify high-risk patients. It is of great significance to improve the prognosis of patients.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
YW and JC designed the main study. JW and SC collected and analyzed the data. YW and XL made contributions to the drafting of the manuscript. JC contributed substantially to the revision of the manuscript. All authors have read and approved the final draft of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Goyal G, Bartley AC, Funni S, Inselman J, Shah ND, Marshall AL, et al. Treatment approaches and outcomes in plasmacytomas: analysis using a national dataset. Leukemia. (2018) 32:1414–20. doi: 10.1038/s41375-018-0099-8
2. Dores GM, Landgren O, McGlynn KA, Curtis RE, Linet MS, Devesa SS. Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: incidence and survival in the United States, 1992-2004. Br J Haematol. (2009) 144:86–94. doi: 10.1111/j.1365-2141.2008.07421.x
3. Caers J, Paiva B, Zamagni E, Leleu X, Bladé J, Kristinsson SY, et al. Diagnosis, treatment, and response assessment in solitary plasmacytoma: updated recommendations from a European Expert Panel. J Hematol Oncol. (2018) 11:10. doi: 10.1186/s13045-017-0549-1
4. Thumallapally N, Meshref A, Mousa M, Terjanian T. Solitary plasmacytoma: population-based analysis of survival trends and effect of various treatment modalities in the USA. BMC Cancer. (2017) 17:13 doi: 10.1186/s12885-016-3015-5
5. Grammatico S, Scalzulli E, Petrucci MT. Solitary plasmacytoma. Mediterr J Hematol Infect Dis. (2017) 9:e2017052. doi: 10.4084/mjhid.2017.052
6. Knobel D, Zouhair A, Tsang RW, Poortmans P, Belkacémi Y, Bolla M, et al. Prognostic factors in solitary plasmacytoma of the bone: a multicenter Rare Cancer Network study. BMC Cancer. (2006) 6:118. doi: 10.1186/1471-2407-6-118
7. Röllig C, Knop S, Bornhäuser M. Multiple myeloma. Lancet (London, England). (2015) 385:2197–208. doi: 10.1016/S0140-6736(14)60493-1
8. Ouyang H, Han S, Jiang L, Zhuang H, Yang S, Liu Y, et al. Reossification and prognosis following radiotherapy with/without surgery for spinal solitary plasmacytoma of the bone: a retrospective study of 39 patients. Spine. (2020) 20:283–91. doi: 10.1016/j.spinee.2019.09.018
9. Shen X, Liu S, Wu C, Wang J, Li J, Chen L. Survival trends and prognostic factors in patients with solitary plasmacytoma of bone: a population-based study. Cancer Med. (2021) 10:462–70. doi: 10.1002/cam4.3533
10. Kilciksiz S, Karakoyun-Celik O, Agaoglu FY, Haydaroglu A. A review for solitary plasmacytoma of bone and extramedullary plasmacytoma. ScientificWorld J. (2012) 2012:895765. doi: 10.1100/2012/895765
11. Barzenje DA, Kolstad A, Ghanima W. Holte H. Long-term outcome of patients with solitary plasmacytoma treated with radiotherapy: A population-based, single-center study with median follow-up of 137 years. Hematol Oncol. (2018) 36:217–23. doi: 10.1002/hon.2415
12. Dimopoulos MA, Moulopoulos LA, Maniatis A, Alexanian R. Solitary plasmacytoma of bone and asymptomatic multiple myeloma. Blood. (2000) 96:2037–44. doi: 10.1182/blood.V96.6.2037.h8002037_2037_2044
13. El-Fattah MA, Aboelmagd M, Elhamouly M. Clinical risk factors of Plasmacytoma mortality: a US population-based study. Br J Haematol. (2017) 179:161–2. doi: 10.1111/bjh.14189
14. Katodritou E, Terpos E, Symeonidis AS, Pouli A, Kelaidi C, Kyrtsonis MC, et al. Clinical features, outcome, and prognostic factors for survival and evolution to multiple myeloma of solitary plasmacytomas: a report of the Greek myeloma study group in 97 patients. Am J Hematol. (2014) 89:803–8. doi: 10.1002/ajh.23745
15. Sharpley FA, Neffa P, Panitsas F, Kothari J, Subesinghe M, Cutter D, et al. Long-term clinical outcomes in a cohort of patients with solitary plasmacytoma treated in the modern era. PLoS ONE. (2019) 14:e0219857. doi: 10.1371/journal.pone.0219857
16. Zhu L, Han X, Liu Z, Leng S, Shan N, Lv X, et al. Survival prediction model for patients with mycosis fungoides/Sezary syndrome. Future oncology (London, England). (2020) 16:2487–98. doi: 10.2217/fon-2020-0502
17. Wang J, Zhou M, Zhou R, Xu J, Chen B. Nomogram for predicting the overall survival of adult patients with primary gastrointestinal diffuse large B cell lymphoma: a SEER- based study. Front Oncol. (2020) 10:1093. doi: 10.3389/fonc.2020.01093
18. Yancik R, Ries LA. Aging and cancer in America. Demographic and epidemiologic perspectives. Hematol Oncol Clin North Am. (2000) 14:17–23. doi: 10.1016/S0889-8588(05)70275-6
19. Celeng C, Takx RAP, Lessmann N, Maurovich-Horvat P, Leiner T, Išgum I, et al. The association between marital status, coronary computed tomography imaging biomarkers, and mortality in a lung cancer screening population. J Thorac Imaging. (2020) 35:204–9. doi: 10.1097/RTI.0000000000000457
20. Huang Z, Hu C, Liu K, Yuan L, Li Y, Zhao C, et al. Risk factors, prognostic factors, and nomograms for bone metastasis in patients with newly diagnosed infiltrating duct carcinoma of the breast: a population-based study. BMC Cancer. (2020) 20:1145. doi: 10.1186/s12885-020-07635-1
21. Osazuwa-Peters N, Christopher KM, Cass LM, Massa ST, Hussaini AS, Behera A, et al. What's Love Got to do with it? marital status and survival of head and neck cancer. Eur J Cancer Care. (2019) 28:e13022. doi: 10.1111/ecc.13022
22. Kumar SK, Callander NS, Adekola K, Anderson L, Baljevic M, Campagnaro E, et al. Multiple Myeloma, Version 3.2021, NCCN Clinical Practice Guidelines in Oncology. JNCCN. (2020) 18:1685–17. doi: 10.6004/jnccn.2020.0057
23. Tsang RW, Campbell BA, Goda JS, Kelsey CR, Kirova YM, Parikh RR, et al. Radiation therapy for solitary plasmacytoma and multiple myeloma: guidelines from the international lymphoma radiation oncology group. Int J Radiat Oncol Biol Phys. (2018) 101:794–808. doi: 10.1016/j.ijrobp.2018.05.009
24. Finsinger P, Grammatico S, Chisini M, Piciocchi A, Foà R, Petrucci MT. Clinical features and prognostic factors in solitary plasmacytoma. Br J Haematol. (2016) 172:554–60. doi: 10.1111/bjh.13870
25. Suh YG, Suh CO, Kim JS, Kim SJ, Pyun HO, Cho J. Radiotherapy for solitary plasmacytoma of bone and soft tissue: outcomes and prognostic factors. Ann Hematol. (2012) 91:1785–93. doi: 10.1007/s00277-012-1510-6
26. Mheidly K, Lamy T, Escoffre M, Hunault M, Benboubker L, Esvan M, et al. Adjuvant chemotherapy in the treatment of solitary bone plasmacytoma. Blood. (2016) 128:4514–4514. doi: 10.1182/blood.V128.22.4514.4514
27. Mignot F, Schernberg A, Arsène-Henry A, Vignon M, Bouscary D, Kirova Y. Solitary plasmacytoma treated by lenalidomide-dexamethasone in combination with radiation therapy: clinical outcomes. Int J Radiat Oncol Biol Phys. (2020) 106:589–96. doi: 10.1016/j.ijrobp.2019.10.043
Keywords: solitary bone plasmacytoma, elderly patients, overall survival, SEER, nomogram, online application
Citation: Wu Y, Wei J, Chen S, Liu X and Cao J (2022) A new prediction model for overall survival of elderly patients with solitary bone plasmacytoma: A population-based study. Front. Public Health 10:954816. doi: 10.3389/fpubh.2022.954816
Received: 27 May 2022; Accepted: 22 August 2022;
Published: 13 September 2022.
Edited by:
Chuanzhao Zhang, Guangdong Provincial People's Hospital, ChinaReviewed by:
Amit Khanal, Wake Forest Baptist Medical Center, United StatesCopyright © 2022 Wu, Wei, Chen, Liu and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaozhu Liu, WGlhb3podWxpdTIwMjFAMTYzLmNvbQ==; Junyi Cao, anVueWlfY2FvQHllYWgubmV0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.