- 1Pediatric Dentistry and Dental Public Health Department, Faculty of Dentistry, Cairo University, Cairo, Egypt
- 2Oral Biology Department, Faculty of Dentistry, Cairo University, Cairo, Egypt
- 3World Health Organization (WHO), Regional Office for the Eastern Mediterranean (EMRO), Cairo, Egypt
Background: Obesity and dental caries are public health problems in Egypt. Factors such as unhealthy diet, poor oral hygiene, and physical inactivity can play a major role in both problems. This study was carried out to illuminate the mutual unhealthy dietary risk factors associated with the incidence of both health conditions.
Methods: Between 1 October 2020 and 1 July 2021, 369 Egyptian children (5–10 years) were examined. Dental status was assessed using decayed, missing/extracted, and filled tooth indices (dmft, deft, and DMFT) for deciduous, mixed, and permanent dentitions, respectively. Moreover, the lifestyle, food habits, and body mass index (BMI) were recorded.
Results: A total of 342 (93.7%) of the included subjects suffered from caries, and only 27(7.3%) were caries-free. Based on BMI percentiles, 247 (66.9%) of the youngsters were overweight/obese, while 122 (33.1%) had normal weight. The mean dmft was 6.9 (±4.6), deft 4.2 (±3.3), and DMFT 0.1 (±1.7). In the primary dentition, a significant positive correlation was detected between dmft and BMI, legumes, sweetened milk and juice, soft drinks, and desserts, while a significant negative correlation was detected between dmft/deft, meat/poultry/fish, fresh fruits, and vegetables. A significant positive correlation was detected between deft and BMI, sweetened milk and juice, ice cream, candies, and crackers. In the permanent dentition, a significant positive correlation was detected between age, soft drinks, sweetened juice, desserts, and DMFT, while a significant negative correlation was detected with fresh fruits and vegetables. BMI was significantly negatively correlated with a healthy lifestyle, meat/poultry/fish consumption, and fresh fruits and vegetables while positively correlated with legumes, ice cream, soft drinks, granulated sugars, desserts, fast food, and caffeinated drinks.
Conclusion: Overweight/obesity was positively correlated with primary dentition dental caries. Desserts (sweetened snacks) and soft drinks could be the common risk factors associated with high caries and overweight/obesity incidence among Egyptian school children; conversely, consumption of fruits and vegetables could hinder both health conditions. Moreover, sweetened juices were associated with primary and permanent dental caries.
Introduction
Dental caries and obesity are considered worldwide growing public health problems that significantly impact children's lives and impose an enormous cost on society (1, 2). The Global Burden of Disease Study 2017 revealed that oral diseases affect 3.5 billion people around the world (3). Dental caries is considered the most common chronic disease affecting 2.43 billion people globally (4). Caries in deciduous teeth affects more than 530 million children worldwide (3). Despite the global spread of the disease, its incidence exhibits geographical diversity across developing and developed countries. It has been asserted that dental caries is decreasing in most industrialized nations due to enhanced prevention programs and expanded access to dental health services. Even so, contradictory results suggest that dental caries is still prevalent among underprivileged communities in many of these nations (5–7). In most developing countries, dental caries levels were low until recent years. An increase was observed due to rising sugar consumption, inadequate fluoride exposure, and limited access to oral healthcare services (5, 6, 8). The Egyptian Ministry of Health and the World Health Organization (WHO) in 2014 conducted an oral health survey in Egypt and revealed that approximately 70% of children had untreated caries (9). In 2022, the comparison between different governorates showed that the highest dental caries prevalence was in children living in Cairo (85%), while for those living in upper Egypt and Deltas was lower (82% and 83.5, respectively) (10).
Children with dental caries have significantly impaired social and psychological functioning. Pain in the teeth, mouth, or jaws, irritation or frustration, difficulty in eating, and sleeping are the most common effects reported by parents in the literature (11–15). Poor dental hygiene greatly impacts the child's growth and cognitive development over time by interfering with the child's nutrition which results in reduced body mass and stature (16–20). Subsequently, the impacts include school absences, inability to concentrate in school, decreased self-esteem, poor social relations, impaired speech development, sleep problems, and inadequate nutrition (21). Family members also suffer when a child has untreated dental caries, including the caretaker's inability to get enough rest, the caretaker missing time at work, and the caretaker's stress and financial hardship due to the time and money required to get the child to the dentist (11, 12, 14, 15).
The WHO in 2003 established global goals for oral health to guide health planners and policymakers in improving the oral health status of their populations (22). Subsequently, the WHO published its global action plan for preventing and controlling non-communicable diseases (NCDs) 2013–2020 (23). The action plan has two goals; the first is to start reducing risk factors for NCDs and underlying social determining factors through developing health-promoting environments; second, to strengthen and direct health systems to prevent and control NCDs and the social factors that cause them through people-centered primary healthcare and universal health coverage. The WHO resolution on Oral Health in 2021 reinforces these goals regarding oral diseases and dental caries. This occurs by encouraging countries to abandon the traditional curative approach in favor of a “preventive promotional approach with risk identification for timely, comprehensive, and inclusive care, taking into account all stakeholders in contributing to the improvement of the population's oral health with a positive impact on overall health” (24, 25).
Obesity is a major public health concern affecting over 650 million people globally (26). According to WHO, Egypt ranks 18th globally in the prevalence of obesity (27). The prevalence of overweight among children and adolescents aged 5–19 has significantly risen in Egypt from 22.6% in 2000 to 36.7% in 2016. Among EMR countries that suffer from a high incidence of overweight among children, Egypt has been ranked third after Kuwait and Qatar. In addition, the prevalence of obesity among children and adolescents aged 5–19 in Egypt has nearly doubled between 2000 and 2016 from 9 to 17.6% (28). The WHO in 2016 reported that more than 340 million children and adolescents between the ages of 5 and 19 are classified overweight or obese (26). Moreover, a report by WHO in 2018 revealed that the worldwide prevalence of obesity in children and adolescents had increased more than 10-fold in the last four decades (29). Despite the WHO efforts, the expanded utilization of unhealthy diets led to an increased prevalence of dental caries and childhood overweight/obesity worldwide in the past decades (30–32).
Dietary sugar specifically becomes a significant public health issue with concerns regarding its contribution to increased obesity prevalence and its negative impact on oral health (33, 34). Sweetened foods and soft drinks are the first choice of children as snacks between meals (35). These food items are rich in carbohydrates and thus result in an increased risk for caries development (36).
Both dental caries and obesity possess common characteristics of being chronic and highly prevalent conditions (5, 37, 38). They are also thought to have the same contributing factors, including genetic, biological, dietary, socioeconomic, cultural, and lifestyle predisposing factors (39, 40). Diet plays an essential role in the development of dental caries and obesity where a high consumption of sugar-sweetened beverages, junk foods, and fermentable carbohydrates influences their incidence (41, 42). Moreover, lifestyle may contribute to the development of obesity and dental caries also by increasing the time spent on social media and watching TV while consuming unhealthy snacks, which can lead to a reduction in physical activity time (43, 44).
Furthermore, both conditions are more prevalent in specific communities with low socioeconomic status in association with the low education level of the parents, unhealthy diet consumption, and the difficulty in acquiring adequate healthcare and services (41, 45, 46). The results of our previous study emphasize this assumption as SES, parental educational, and oral hygiene measures were significantly inversely correlated with primary teeth dental caries, whereas permanent teeth caries revealed non-significant correlations (47). Conversely, low and high socioeconomic status were demonstrated to increase the risk of dental caries, while a middle socioeconomic status had a 20% lesser chance in low- and middle-income countries. It was reported that 44% of the children had access to dental care services, which implies the role of numerous socioeconomic constraints in addition to geographical restrictions (48). Moreover, the relation between obesity and socioeconomic status depends on the stage of the nutrition transition (49). In nutrition, the concept of “transitions” has been used to describe trends in significant population health parameters to provide insight into underlying determinants, positive deviations, and future directions (50). Analysis of Egypt's Demographic and Health Survey data between 1992-1995 and 2005-2008 revealed that the greatest relative increases in the prevalence of obesity occurred among women with no/primary education and in the lowest income quintile (25).
The correlation between dental caries and obesity is a controversial issue. In literature, being overweight has been linked to an increased incidence of dental caries (51) due to the upregulated intake frequency of sugary foods and snacks (52). This correlation was supported by a systematic review that reported a significant relationship between childhood obesity and dental caries (53). On the contrary, being underweight has also been associated with high caries prevalence (54, 55), as the pain resulting from dental caries could make the child eat less food (17, 56). Other studies reported that dental caries was not correlated with obesity (57, 58).
Estimating the burden of the unhealthy diet that could contribute to the incidence of dental caries and obesity is crucial in determining the public health intervention priorities. It also helps educate the public about the negative effects of dental caries and obesity and provides health policy decision makers with information about the scope of health problems. The impact of unhealthy diets in correlation to dental caries and obesity among the Egyptian population is not well–established. Therefore, this study aims to evaluate the impact of unhealthy dietary habits on the incidence of dental caries and overweight/obesity among children aged 5 to 10 years to guide parents to be more conscious of their role in preventing these related health problems. Moreover, the need for high-quality and comparable data is a key component for the healthcare ministry to take essential actions regarding major health problems.
Subjects and methods
The research was conducted following the Research Ethics Committee of Cairo University's Faculty of Dentistry's requirements (Approval: 24,920). The parents or guardians of the children gave their written informed consent to participate in the study. From 1 October 2020 to 1 July 2021, individuals were recruited from the Pedodontic outpatient clinics of Cairo University's Faculty of Dentistry. The criteria for the included children were as follows: age: ranges from 5 to 10 years old; Gender: males and females; Ethnicity: Egyptians. Children with visible disorders, physical or mental abnormalities, diabetes, or other systemic ailments or having orthodontic treatment were not included in the study. Children and parents who refused to participate were also excluded.
The sample size was calculated to be 369 using the basic formula (59). For dental caries, the prevalence in Egypt was estimated at 60% according to the WHO Regional Office for the Eastern Mediterranean (WHO EMRO) report in 2014 (9). Abbass et al. reported 74 % among children aged 3–18 years while reported 93.2 % among children aged 5–10 years (47). In EMR, caries was estimated by 66% (59–73%) for children aged 6–15 years (60). Moreover, 51.6% was reported among different Australian refugees (61). The average of the previously mentioned percentages was utilized in calculations; accordingly, the estimated sample size was 330. For overweight/obesity, Egypt's population of children aged (5–10) years was estimated at 30,000,000 (62), and the prevalence of overweight and obesity in this population was estimated at 40.2 % according to WHO data (63), and the estimated sample size was 369.
Data collection and grouping
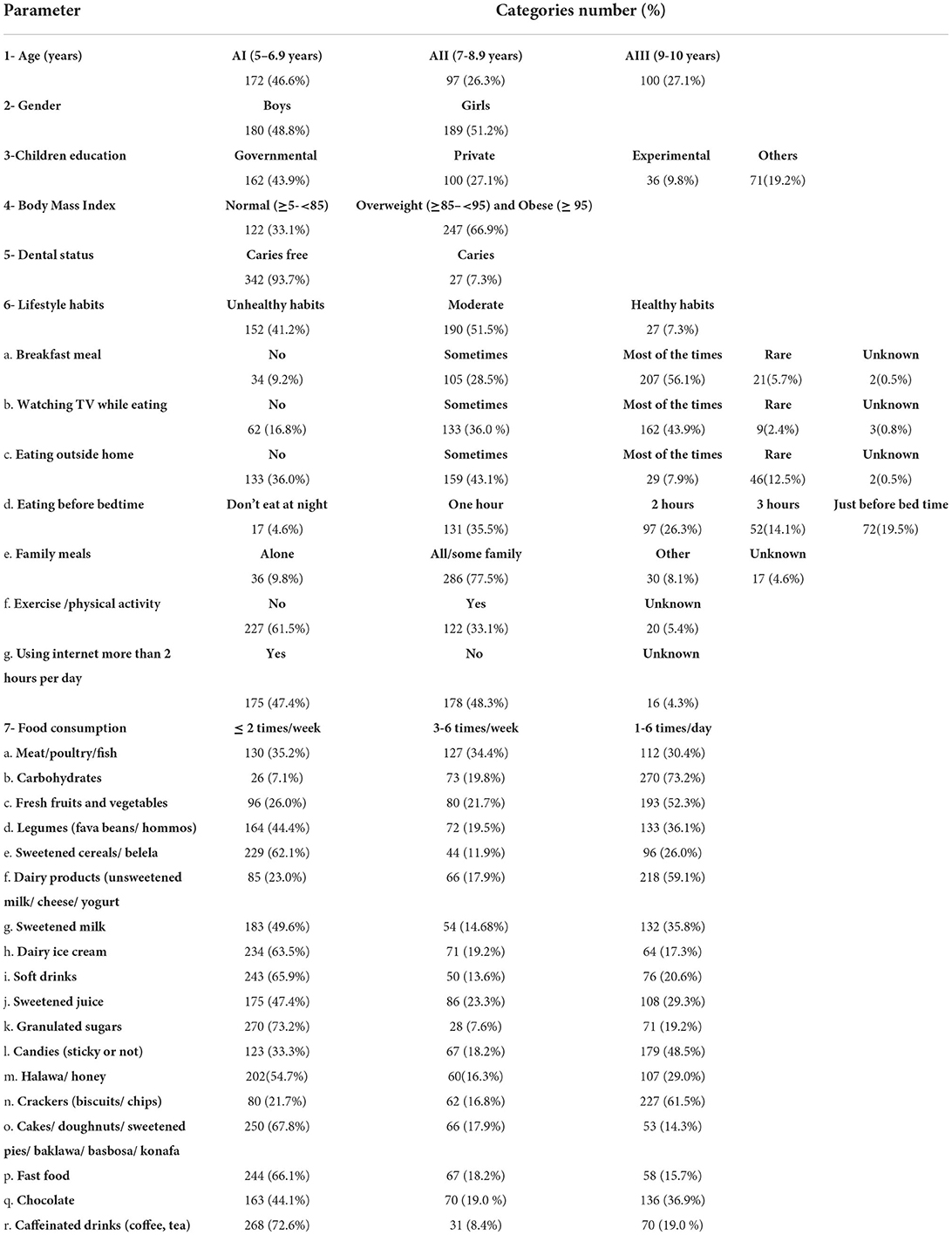
Name, age, gender, address, and type of education (government, experimental, or private) were among the sociodemographic data obtained from children's guardians. The children's lifestyle habits were documented, and the dietary habits were thoroughly reported using a food frequency questionnaire. The first two parts of the questionnaire that included the sociodemographic data and the dentition status were validated in WHO-oral health surveys (64), while the third part that included the lifestyle habits was validated in (65, 66). Finally, the part of the dietary habits was validated by Abbass et al. and the diet history questionnaire (47, 67–69). The assessed 18 dietary elements are included in Table 1.
The authors filled out the questionnaire according to parents' answers on behalf of their children. The frequencies used in the questionnaire were once per month; 1–2 times per week; 3–4 times per week; 5–6 times per week; once per day; 2–3 times per day; 4–5 times per day; and 6 or more times per day. To facilitate the comparison and statistical analysis, these frequencies were merged into the three frequencies displayed: ≤ 2 times/week; 3–6 times/week; 1–6 times/day. According to WHO (1995) (70), body weights were measured using a Beurer scale (Ulm, Germany) with the participants wearing clothes but without shoes. Standing heights were measured to the nearest 0.1 cm utilizing a stadiometer. Body mass index (BMI) was calculated from the measured heights and weights. The obtained BMI values were plotted on the WHO percentile body mass index (BMI/age) charts for boys and girls (71). The children were divided into four categories based on their BMI percentiles: the underweight group (<5th percentile); the normal group (≥ 5th- <85th percentile); the overweight group (≥ 85th- <95th percentile); and the obese group (≥ 95th percentile). Moreover, children were divided into three groups based on their age: group I (5– <7 years old), group II (7– <9 years old), and group III (9, 10).
Furthermore, participants' responses to questions regarding lifestyle habits were marked, and a total score was calculated for each participant as previously described (72–75). Participants with a cumulative score of (0–3) were categorized into unhealthy lifestyle habits, while those with a cumulative score of (4–5) were categorized into moderate lifestyle habits, while participants with a cumulative score of (6–7) were categorized into healthy lifestyle habits.
Oral examination
Examiners were trained and calibrated over 3 days in 3 sessions, with disparities in observations discussed among the examiners for reassessment and consensus (76, 77). Following the WHO guidelines, oral examination was performed on a dental chair in artificial light using a plain mouth mirror and a dental probe (78). During the clinical examination, all of the teeth that were present were considered (77).
Any lesion with a detectably softened floor, undermined enamel, or softened wall in a pit or fissure or on a smooth tooth surface, tooth surface containing a temporary filling requiring further treatment, and tooth surface containing a permanent restoration with an area of decay were all considered carious (either primary or secondary caries). The DMFT index, which measures the number of D (decayed tooth), M (missing tooth), and F (filled tooth), was used to determine the severity of caries in permanent teeth. The dmft index was employed for primary teeth: d (decayed teeth), m (missing teeth), and f (filled tooth). The deft index was employed for mixed dentition: d (decayed tooth indicated for filling), e (decayed tooth advised for extraction), and f (filled tooth) (64). The DMFT, dmft, and deft indices were divided into three categories: caries index zero (no carious teeth), caries index 1–3 (number of carious teeth 1–3), and caries index ≥4 (number of carious teeth ≥ 4) for statistical analysis (79).
Statistical analysis
Data were statistically described as frequencies (number of cases) and percentages. Since data were non-parametric, the Kruskal–Wallis test was used for comparing more than two groups, while the Mann–Whitney test was used for comparing two groups. Spearman's rank correlation was used for the detection of correlation between different variables. Multivariate logistic regression analysis was used to test for the preferential effect of each food item on caries index and obesity after adjusting the effect of age and gender. P-values of < 0.05 were considered statistically significant. All statistical calculations were done using the computer program IBM SPSS (Statistical Package for the Social Science; IBM Corp, Armonk, NY, United States), release 22 for Microsoft Windows.
Results
Population profile
The study included 180 (48.8%) boys and 189 (51.2%) girls. About 172(46.6%) participants fell into the first age group AI (5–6.9 years), 97(26.3%) fell into the second age group AII (7–8.9 years), while 100 (27.1%) fell into the third age group AIII (9–10 years). The majority of participants 162 (43.9%) attended governmental schools. A total of 122 (33.1%) of the participants had normal weight, while 247 (67.0%) were overweight or obese. No underweight participants have been reported in this work; therefore, this category is not presented in the result section. About 342 (93.7%) of the included subjects suffered from dental caries, and only 27 (7.3%) individuals were caries-free (Table 2).
Lifestyle habits
Most of the parents stated that their children had breakfast meal most of the time. In addition, 162 (43.9%) of the parents mentioned that their child would watch TV while eating, 159 (43.1%) of the parents stated that their children sometimes eat outside their home, and 131 (35.5%) of the participants would have their dinner 1 h before bedtime. Most of the parents 286 (77.5%) mentioned that the whole family had their meals together, and 227 (61.5%) acknowledged that their child does not perform any sort of physical exercise. A total of 175 (47.4%) of the participating children used the internet more than 2 h per day, while 178 (48.2%) did not. The majority of the participants 190 (51.5%) were categorized as moderate regarding lifestyle habits (Table 2).
Dietary habits
Most of the participants consumed meat, legumes (fava beans/ hommos), sweetened cereals/ belela, sweetened milk, ice cream, soft drinks, sweetened juice, granulated sugars, desserts (cakes/ doughnuts/ sweetened pies/ baklawa/ basbosa/ konafa), fast food, chocolate, and caffeinated drinks (coffee, tea) less than or equal to 2 times/week, while the majority consumed carbohydrates, fresh fruits, and vegetables, dairy products (unsweetened milk/ cheese/ yogurt), candies, and crackers (biscuits/ chips), 1 to 6 times per day (Table 2).
Mean decayed, missing/extracted, filled teeth (Dmft, deft, and DMFT)
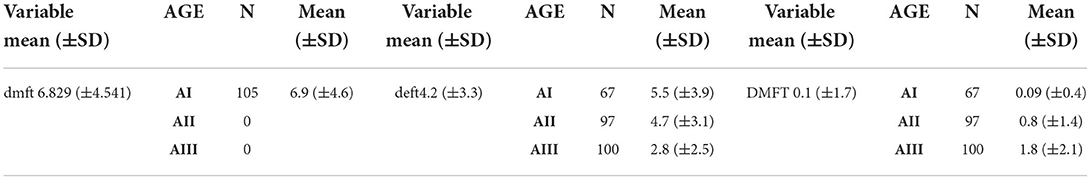
The mean dmft recorded for the participants was 6.8 (±4.5), the mean deft was 4.2 (±3.3), while the mean DMFT was 0.9 (±1.7). The highest mean DMFT 1.8 (±2.0) was detected within AIII age group. In contrast, AI age group showed the highest mean dmft and deft of 6.9 (±4.5) and 5.5 (±3.9), respectively (Table 3).
Correlation between caries indices and different risk factors
Correlation between dmft index and different risk factors
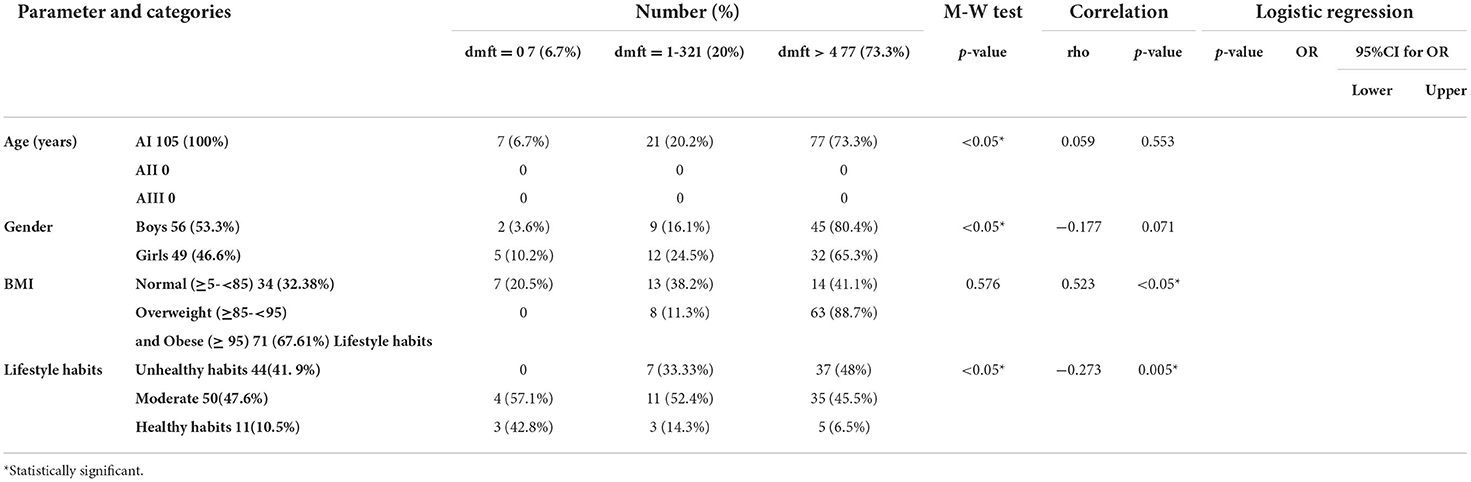
The majority of participants had dmft more than 4. The highest percentage of participants with dmft more than 4 were overweight or obese. The highest percentage of participants with dmft more than 4 were males. Most of the participants with dmft more than 4 had unhealthy life habits. No significant correlation was detected between dmft and gender or age, a statistically significant positive correlation was detected between dmft and BMI, while a statistically significant negative correlation was detected between dmft and healthy lifestyle habits (Table 4).
The highest percentage of participants with dmft more than 4 consumed meat, sweetened cereals/ belela, sweetened milk, dairy ice cream, soft drinks, granulated sugar, halawa/ honey, desserts (cakes/ doughnuts/ sweetened pies/ baklawa/ basbosa/ konafa), fast food, and caffeinated drinks (coffee, tea), less than or equal to 2 times/week, while carbohydrates, fresh fruits and vegetables, legumes (fava beans/ hommos), dairy products unsweetened milk/ cheese/ yogurt, candies (sticky or not), crackers (biscuits/ chips), and chocolate were consumed 1–6 times/day by the majority of participants with dmft more than 4.
A statistically significant positive correlation was detected between dmft and legumes, sweetened milk, soft drinks, sweetened juice, and desserts (cakes/ doughnuts/ sweetened pies/ baklawa/ basbosa/ konafa), while a statistically significant negative correlation was detected between dmft and meat/poultry/fish, fresh fruits and vegetables, and dairy products (unsweetened milk/ cheese/ yogurt).
After adjusting the effect of age and gender, the following items were found to be significantly associated with high dmft: legumes, sweetened milk, soft drinks, sweetened juice, granulated sugars, and desserts (cakes/ doughnuts/ sweetened pies/ baklawa/ basbosa/ konafa). Conversely, meat/poultry/fish, fresh fruits and vegetables, and dairy products, including unsweetened milk/ cheese/ yogurt, were significantly associated with low dmft (Table 5).
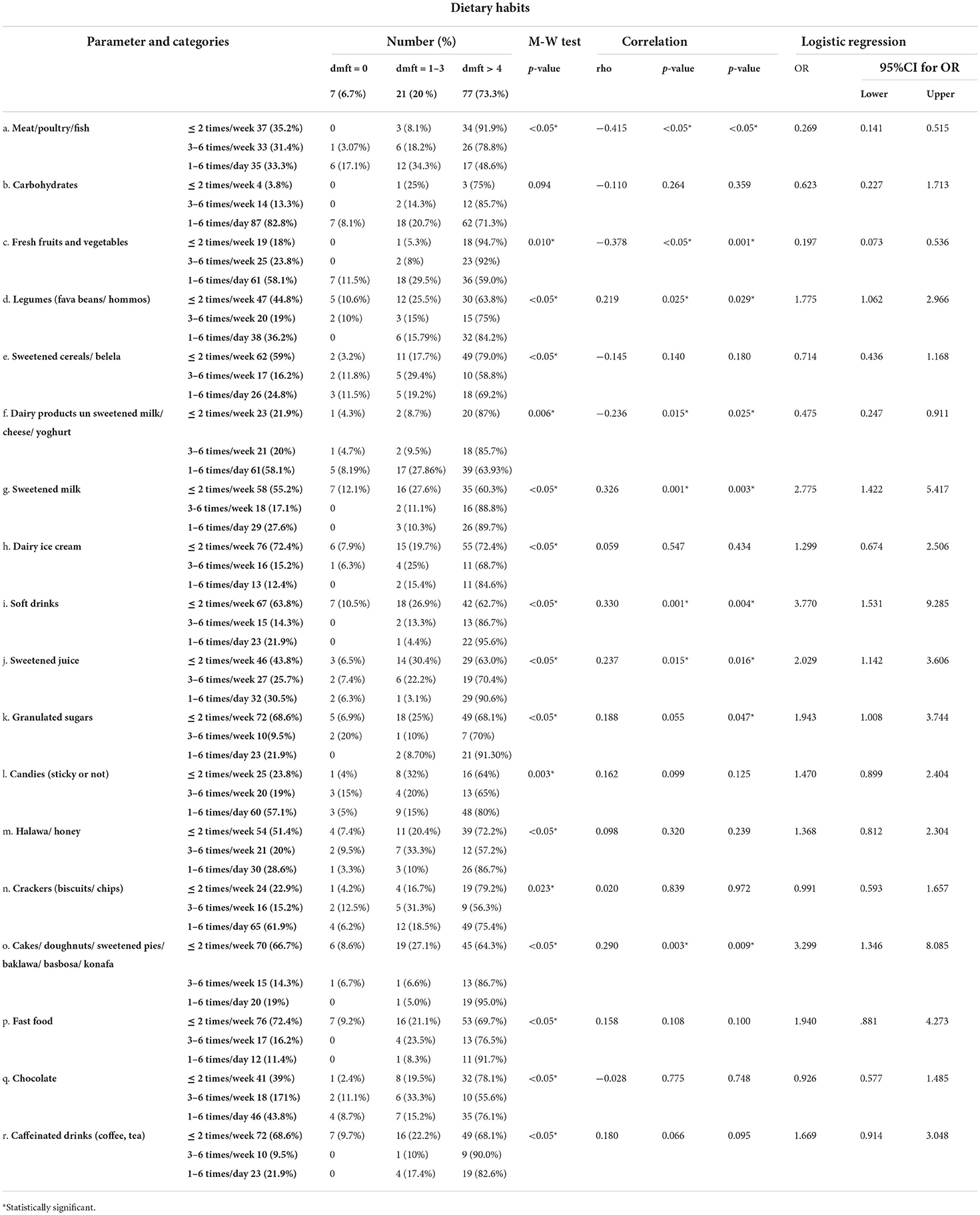
Table 5. Correlation between dmft index and frequency of intake of different food items (Total number 105).
Correlation between deft index and different risk factors
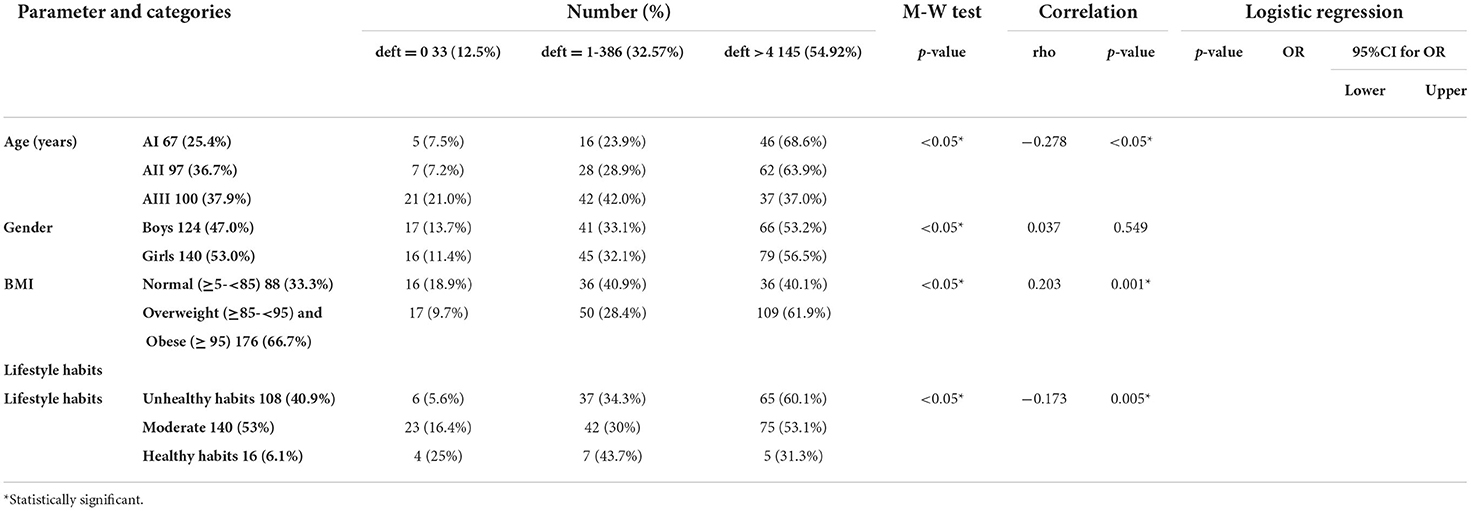
Most of the studied population 145 (54.9%) had a deft index of more than 4. The highest percentage of participants with deft index of more than 4 fell into the AII age group. The highest percentage of participants with deft index of more than 4 were females. The highest percentage of participants with deft index of more than 4 were overweight or obese. A statistically significant negative correlation was detected between age and deft also between deft and healthy lifestyle habits. A statistically significant positive correlation was detected between deft and BMI (Table 6).
The highest percentage of participants with deft index of more than 4 consumed meat/poultry/fish, sweetened cereals/ belela, dairy ice cream, soft drinks, sweetened juice, granulated sugars, halawa/ honey, desserts (cakes/ doughnuts/ sweetened pies/ baklawa/ basbosa/ konafa), fast food, chocolate, and caffeinated drinks less than or equal to 2 times/week, while carbohydrates, fresh fruits and vegetables, dairy products, sweetened milk, candies, and crackers were consumed 1 to 6 times daily.
A statistically significant positive correlation was detected between deft and sweetened milk, dairy ice cream, sweetened juice, candies, and crackers. In contrast, a statistically significant negative correlation was detected between deft and meat/poultry/fish, fresh fruits, and vegetables.
After adjusting the effect of age and gender, the following items were found to be significantly associated with high deft, sweetened milk, dairy ice cream, soft drinks, candies, and crackers. On the contrary, meat/poultry/fish, fresh fruits, and vegetables were associated with low deft (Table 7).
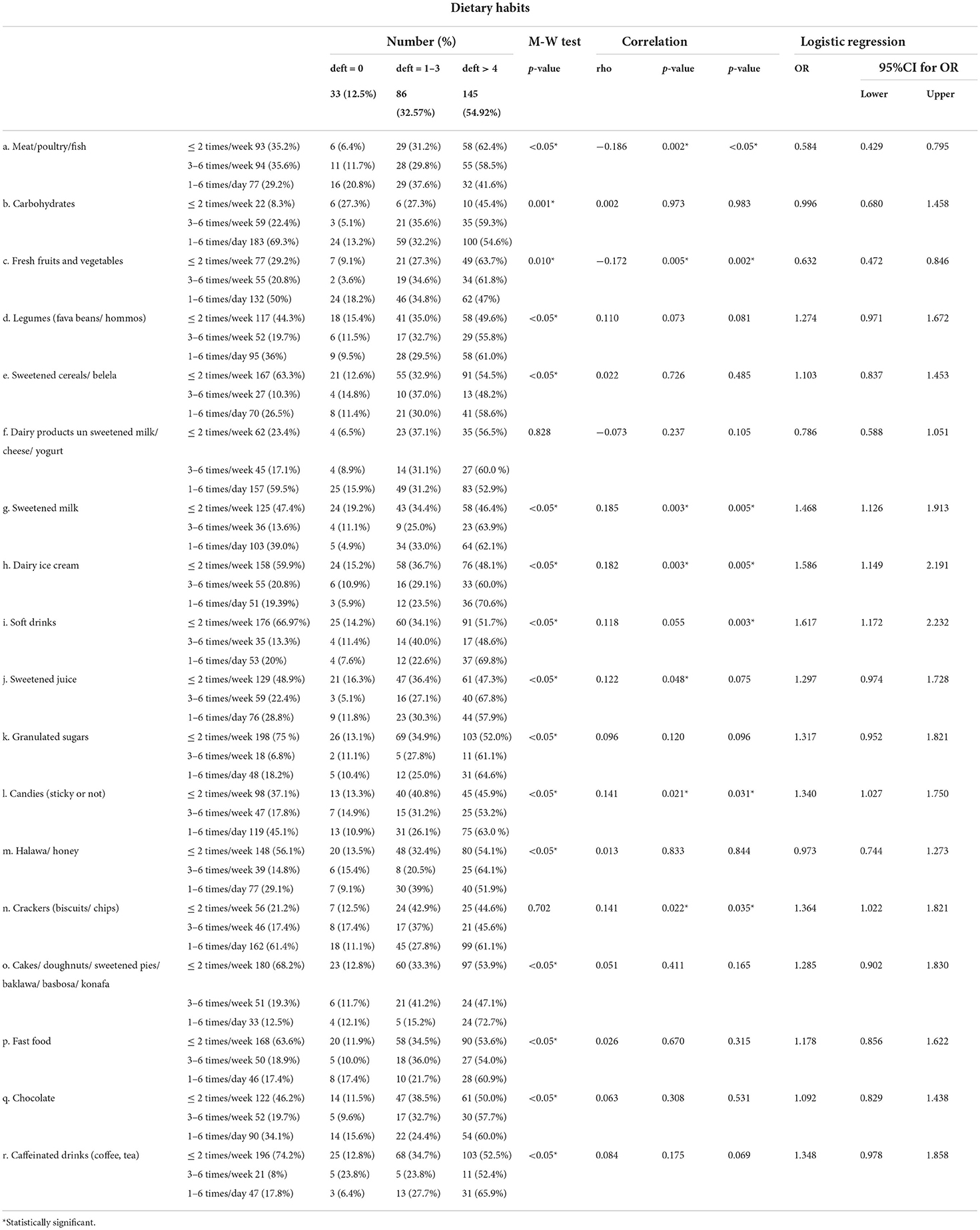
Table 7. Correlation between deft index and frequency of intake of different food items (Total number 264).
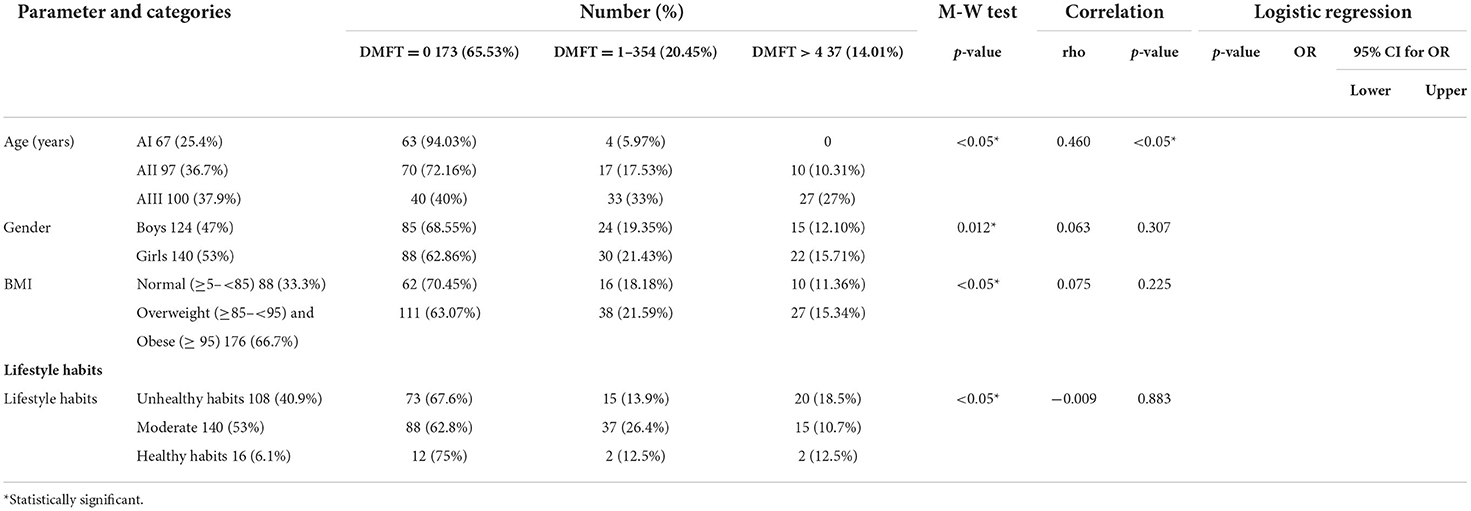
Correlation between dmft index and different risk factors
The majority of participants 173 (65.5%) had DMFT = 0. The highest percentage of participants with DMFT index of more than 4 fell into the AIII age group. The highest percentage of participants with DMFT index of more than 4 were females. The highest percentage of participants with DMFT index of more than 4 were overweight or obese. A statistically significant positive correlation was detected between age and DMFT, while a non-significant positive correlation was detected between gender, BMI, and DMFT (Table 8).
The highest percentage of participants with DMFT more than 4 consumed meat/poultry/fish, legumes, sweetened cereals, dairy ice cream, soft drinks, granulated sugars, desserts (cakes/ doughnuts/ sweetened pies/ baklawa/ basbosa/ konafa), fast food, and caffeinated drinks less than or equal to 2 times/week, while carbohydrates, fresh fruits and vegetables, dairy products, sweetened juice, candies, halawa/ honey, crackers, and chocolate were consumed 1–6 times/day.
A statistically significant positive correlation was detected between DMFT and soft drinks, sweetened juice, and desserts (cakes/ doughnuts/ sweetened pies/ baklawa/ basbosa/ konafa), while a statistically significant negative correlation was detected between DMFT and fresh fruits and vegetables.
After adjusting the effect of age and gender, the following items were found to be significantly associated with high DMFT: soft drinks, sweetened juice, and desserts (cakes/ doughnuts/ sweetened pies/ baklawa/ basbosa/ konafa). In contrast, fresh fruits and vegetables were significantly associated with low DMFT (Table 9).
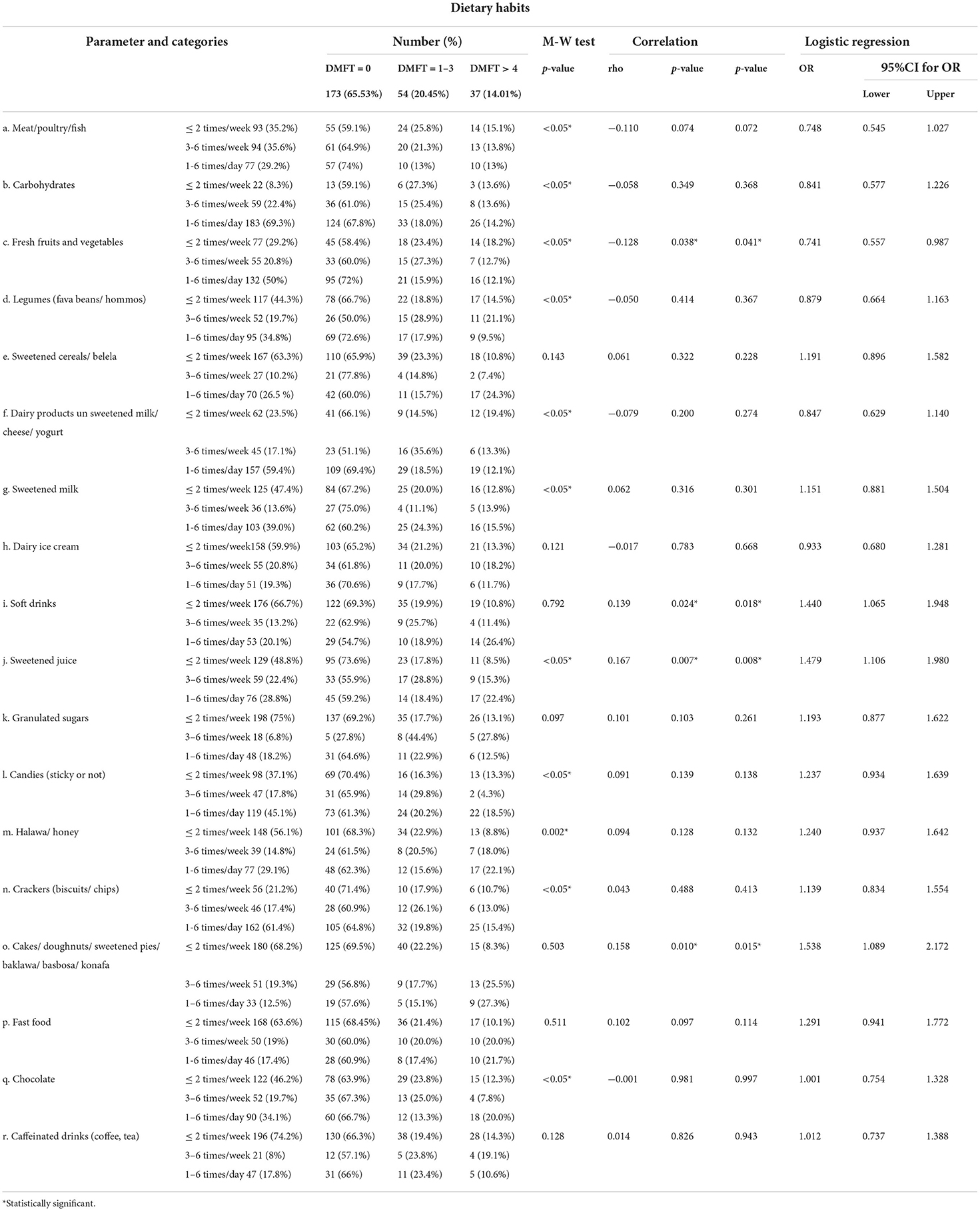
Table 9. Correlation between DMFT index and frequency of intake of different food items (Total number 264).
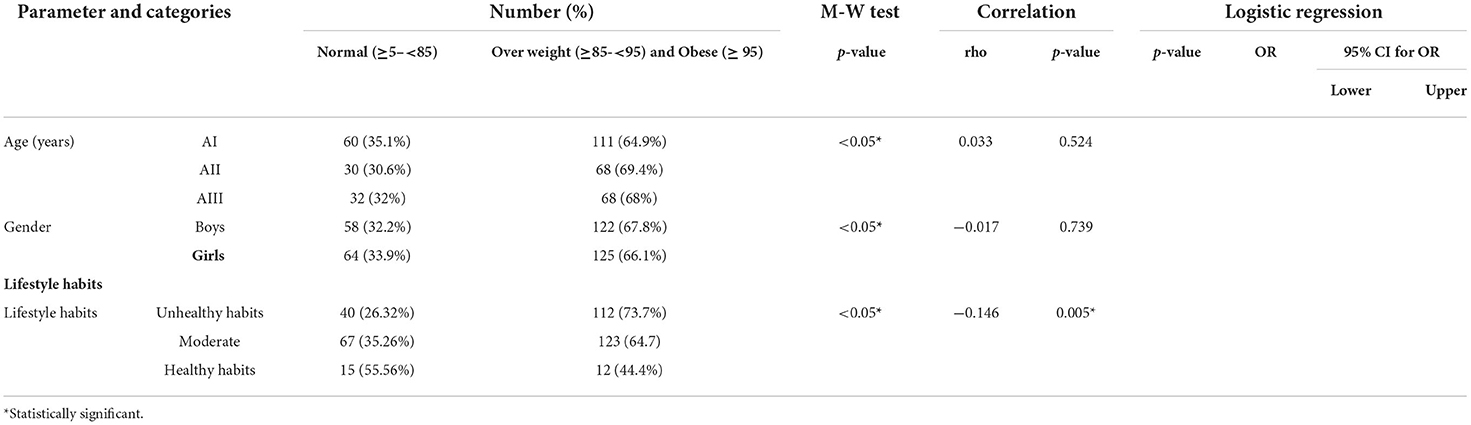
Correlation between BMI and different risk factors
Most of the studied population 247 (66.9%) were overweight (≥ 85– <95) and obese (≥ 95), while 122(33.1%) of the participants had normal BMI (≥ 5– <85). The highest percentage of overweight/obese participants fell into AII age group. The highest percentage of overweight/obese participants were males. Overweight/obese participants had the highest percentages of unhealthy lifestyle habits.
A non-statistically positive correlation was detected between BMI and age. Moreover, a non-statistically negative correlation was detected between BMI and gender. On the contrary, a statistically significant negative correlation was detected between BMI and healthy lifestyle habits (Table 10).
The highest percentage of overweight/obese participants consumed meat/poultry/fish, carbohydrates, fresh fruits and vegetables, dairy products, and chocolate less than or equal to 2 times/week and consumed sweetened milk 3 to 6 times per week. Legumes, sweetened cereals/ belela, dairy ice cream, soft drinks, sweetened juice, granulated sugars, halawa/honey, crackers, desserts (cakes/ doughnuts/ sweetened pies/ baklawa/ basbosa/ konafa), fast food, and caffeinated drinks were consumed 1 to 6 times per day within overweight/obese participants, while candies were nearly equally consumed less than or equal to 2 times/week and 1–6 times/day.
A statistically significant positive correlation was detected between BMI and legumes, dairy ice cream, soft drinks, granulated sugars, desserts (cakes/ doughnuts/ sweetened pies/ baklawa/ basbosa/ konafa), fast food, and caffeinated drinks, while a statistically significant negative correlation was detected between BMI and meat/poultry/fish, fresh fruits, and vegetables.
After adjusting the effect of age and gender, the following items were found to be associated with high BMI: legumes, dairy ice cream, soft drinks, granulated sugars, desserts (cakes/ doughnuts/ sweetened pies/ baklawa/ basbosa/ konafa), fast food, and caffeinated drinks. While meat/poultry/fish, fresh fruits, and vegetables were significantly associated with low BMI (Table 11).
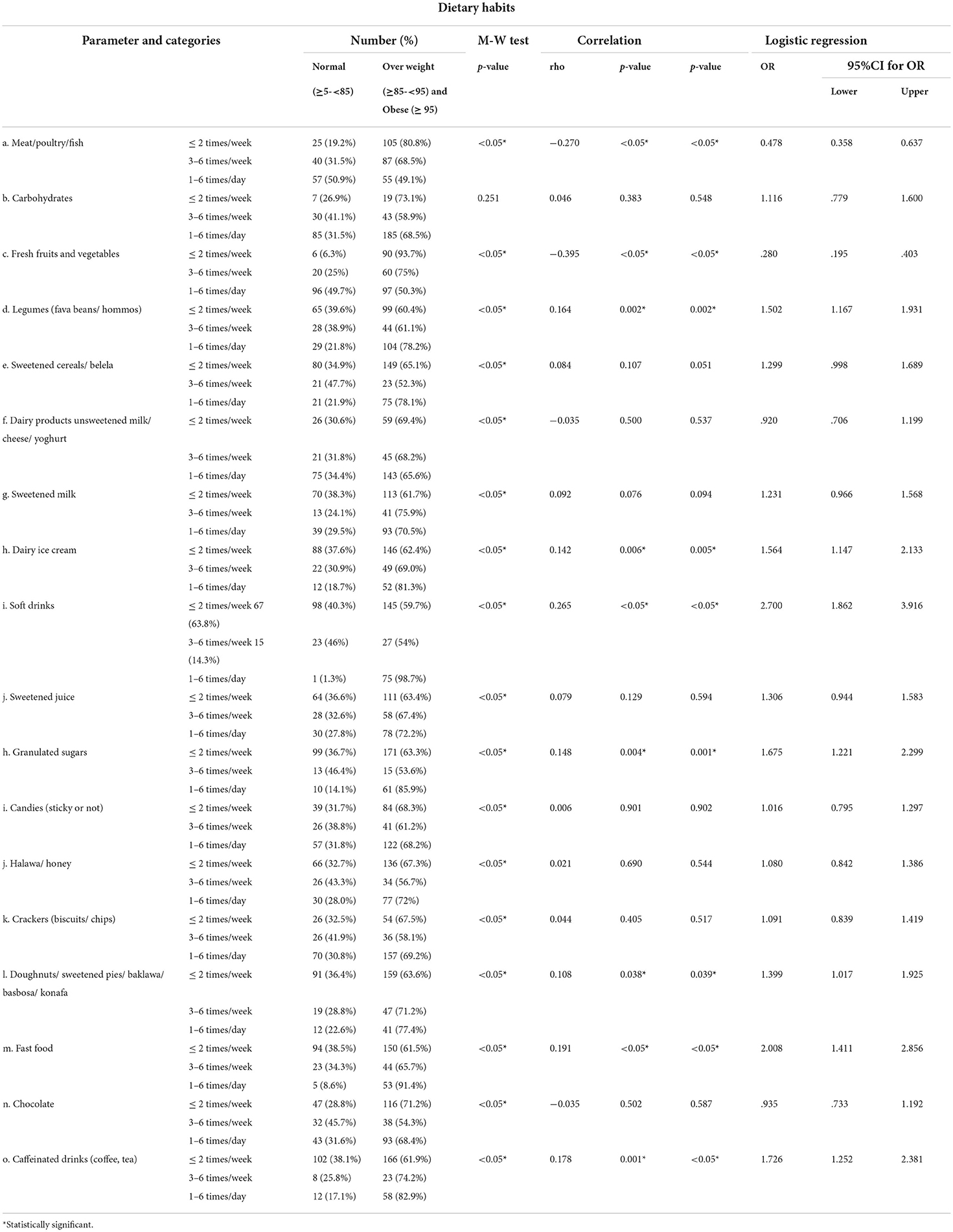
Table 11. Correlation between BMI and frequency of intake of different food items (Total number 369).
Discussion
Dental caries is considered the most prevalent non-communicable chronic disease worldwide, affecting all ages, from infants to old adults. It is a biofilm-mediated, sugar-induced, multifactorial, dynamic disease that leads to a cyclic demineralization and remineralization of dental hard tissues (80). This study examined the correlation between caries prevalence and malnutrition (overweight/obesity) in association with unhealthy food consumption patterns.
The strengths of our study include the collection of data representative to the whole nation since the patients come to the clinics of the Faculty of Dentistry, Cairo University from all the Egyptian governorates, and the high-quality outcome data. An additional strength is the use of a questionnaire validated by the WHO. Despite these strengths, the exposure measurement used in our study has several weaknesses including the sampling technique, where a non-random sampling technique was used as all the participants were recruited from the outpatient clinics of the Faculty of Dentistry, Cairo University. In addition, another major limitation is the cross-sectional study design, as the exposure and outcome are concurrently assessed. Finally, this study did not include questions about participants' oral hygiene and socioeconomic status which can be justified by the inclusion of such questions in a previous study among Egyptian adults (69) and children (47) conducted by our group. As per our previous study, poor oral hygiene was one of the risk factors significantly associated with caries prevalence among Egyptian adults (69) and children (47).
Study participants were recruited from the pediatrics dental clinics, Faculty of Dentistry, Cairo University. Hence, the majority of participants 342 (93.7%) suffered from dental caries, while only 27(7.3%) were caries-free. The highest mean dmft 6.9(± 4.6) and deft 5.5(± 3.9) were detected in an age group ranging from 5 to 6.9 years. This result could be attributed to lack of parental education, which affects their attitude regarding dental healthcare, with subsequent negligence toward maintaining a healthy primary dentition. Parents' ignorance can result in neglecting seeking dental treatment for their children as they regard primary teeth as unimportant and replaceable by permanent teeth (47, 81). On the contrary, the highest mean DMFT 1.8 (± 2.1) was detected in age group ranging from 9 to 10 years. A statistically significant positive correlation was detected between age and DMFT. Early loss of primary teeth due to a lack of parental education, with subsequent early eruption of their permanent successors, subjects permanent teeth to poor oral environment and to multiple caries-promoting factors (81).
The absence of dental services within the primary healthcare centers in Egypt leads to limited or no access to effective dental care (82). According to a WHO-sponsored epidemiological study on the state of oral health in Egypt, dental services were not being used to their full potential, and 40% of the included individuals stated that they had dental problems at the time of the study but chose not to seek dental care. According to the subjects' visitation patterns, roughly 20% had not seen a dentist in more than 2 years, and another 20% had never visited a dentist (9).
Diet and diet-related risks play a significant role in the incidence of obesity and dental caries. These diet factors include dietary habits frequencies, consumption of large amounts of fermentable carbohydrates, junk foods, and highly cariogenic diets (41).
Sugars are the most important and the most studied dietary factor in developing dental caries. In this study, there was a significant positive correlation between both dmft and DMFT with the consumption of desserts (cakes/ doughnuts/ sweetened pies/ baklawa/ basbosa/ konafa). Another significant positive correlation was found between deft and the consumption of dairy ice cream, candies, and crackers. The form (solid or liquid) of the fermentable carbohydrate directly influences the duration of exposure and retention of the food on the teeth. Thereby, prolonged retention of cariogenic components on teeth surfaces causes acid production with subsequent early teeth demineralization and caries initiation and propagation (83).
Previous studies revealed that fluid consumption patterns have changed over the past decade with rising consumption of sugary drinks and less consumption of water and milk (84, 85). In this study, the consumption of sweetened juices was found to be associated with all caries indices: dmft, deft, and DMFT. A recent systematic review (86) declared the need to control, limit, and manage the intake of sweetened juices, as they could degenerate not only an adult's dental health but one's overall wellness. Furthermore, Kim et al. (87) recorded in their study conducted in the United States, that among the studied subjects, 26% of young adults lost one or more teeth in association with massive intake of sweetened juices. Interestingly, frequent and prolonged bottle-feeding practices, as well as high consumption of sweet drinks during weaning, could be associated with the formation of early childhood caries (88). It is important to increase the awareness among parents concerning bottle-feeding practices and weaning diet contents and highlight the potential implication for their children's oral health.
In this study, there was a significant positive correlation between dmft and DMFT with soft drinks. It was reported that children with a high consumption rate of soft drinks have undesirable eating patterns and eat high amounts of sugars from other dietary sources. Such an unhealthy diet pattern affects both the primary and permanent dentition (89–91). In contrast, this disagrees with our previous work (47), which recorded a non-significant correlation between soft drinks and dental caries.
A statistically significant negative correlation was detected between dmft and dairy products (unsweetened milk/ cheese/ yogurt). These findings are in agreement with our previous work (47). Egyptian children who consume milk more frequently possessed lower caries index. Milk has low cariogenic potential as it contains calcium, phosphorus, and casein, which are considered caries protective factors (83, 90).
The inverse significant association between fruit/vegetable consumption and caries in all age groups agrees with a study that recorded that dental caries prevalence was higher in non-vegetarians than vegetarians (92). This association could be explained by a lower tendency to consume sweets between meals in vegetarians than non-vegetarians (93). In addition, the naturally occurring sugar within whole fruits, vegetables, some grains, and dairy products is less likely to be overconsumed and is counteracted by the wide range of bioactive health-enhancing nutrients, antioxidants, fiber, and phytochemicals present in fruits/vegetables that can reduce inflammation and improve endothelial function (94).
It has been authorized that a lower risk for first permanent molars caries was found in children consuming diet rich in red meat, poultry, organs, eggs, and seafood (95). Moreover, there was an inverse significant association between meat/poultry/fish and dmft (p <0.05) in this study. These results are concomitant with a study carried out in Zhejiang Province, exploring the prevalence of dental caries in 6- to 8-year-old children, especially their first permanent molars (95).
Obesity and dental caries are multifactorial in etiology as multiple genetic and environmental factors may impact them. Consequently, these confounding factors, including age, gender, lifestyle, and oral hygiene habits, might control their incidence (96).
Individual body weight relative to population norms was evaluated by calculating BMI using the formula BMI = weight in kilograms/height in meters squared (97). Our study found a positive correlation between increased weight represented through BMI and both dmft and deft. This finding contradicts our previous findings, where no correlation between BMI and all caries indices was reported (47). Overweight–obese children have a higher risk of developing dental caries than normal-weight children (98) as they tend to consume high levels of cariogenic and obesogenic food and drinks (26). These findings are confirmed by our results where a significant correlation was recorded between overweight/obesity and the consumption of soft drinks, fast foods, dairy ice cream, granulated sugars, and desserts (cakes/ doughnuts/ sweetened pies/ baklawa/ basbosa/ konafa).
In this study, 66.9% of the studied population were overweight/obese. This percentage is higher than what has been reported in our previous work in 2020, as the percentage of overweight/obese children in the same age group was 44.4% (99). The small sample size for this age group (5–9 years) in the previous work as well as the change in the dietary habits with the time could explain this discrepancy. In this work, a higher percentage of male participants were overweight/obesity than females, and BMI was significantly positively correlated with soft drinks. These findings support the results of our previous work (99).
A statistically significant positive correlation was detected between BMI and legumes. This result was in disagreement with a cross-sectional study conducted by Papanikolaou et al. (100), which concluded that legume consumption is related to healthier body weights, as legumes are not considered energy-dense food, they are low in dietary fat (101) and rich in dietary fibers (102). Since bread is an important dietary constituent and legumes are usually consumed with bread in the Egyptian diet, this combination might explain the positive correlation between BMI and legumes.
A statistically significant negative correlation was detected between BMI and meat/poultry/fish, fresh fruits, and vegetable consumption. This finding supports previous report that obese children's diet is usually low in fiber (103) and proteins, with protein-deficient malnutrition, while their energy intake comprises high carbohydrates and highly processed foods (104).
The literature reports on the association of dental caries with BMI are conflicting, and three systematic reviews (53, 105, 106) found inconclusive evidence regarding the association between obesity and dental caries. The possible reason (81) for the conflicting reports is that dental caries and BMI are non–linearly related, with more dental caries occurring in individuals with either higher or lower BMI. Thereby, we suggest that methodological factors together with sample demographics, dental examination accuracy, and data analysis methodology affect whether or not the association is detected.
There is a growing evidence that emphasizes the role of physical inactivity in the development of obesity among children and adults (26, 107, 108). This comes in accordance with our results, a statistically significant negative correlation was detected between BMI and healthy lifestyle habits.
There are multiple social and environmental factors linked to the purchase and consumption of sugar-sweetened beverages and junk food. Policies aiming to reduce their consumption should be addressed. First, regulating food advertising and promotion since there is a growing evidence on the relation between food advertising and increased intake of calorie-dense products by adult populations (109) and children (110). Second, labeling of sugar-sweetened beverages and raising awareness about their health effects. A systematic review on the impact of front-of-package labeling on consumption suggested that consumers identify healthier foods more easily from nutrient-specific schemes (111). Third, school interventions and nutrition policies to restrict the availability of sugar-sweetened beverages and provide healthier food in schools (112). Finally, imposing taxes on sugar-sweetened beverages. Higher prices of sugar-sweetened beverages, food taxes, and subsidies may affect their consumption rate and improve health outcomes (113–116). Among EMR, 15 countries have developed either a policy or prepared a draft to reduce the impact of marketing of unhealthy foods and beverages to children in educational facilities and on media. These countries include all high-income countries (GCC), middle-income countries (Egypt, Iran, Jordan, Lebanon, Morocco, Pakistan, and Palestine), and a low-income country (Yemen) (110, 117). Taxes on sugar-sweetened beverages have been implemented in all high-income countries (GCC) and middle-income countries including Egypt, Iran, Morocco, Palestine, and Tunisia. Front-of-pack labeling has been applied in five countries in EMR, Saudi Arabia, UAE, Iran, Morocco, and Tunisia (117).
Taken with this findings, both overweight/obesity and dental caries have mutual determinants and require an inclusive, integrated management approach by multidisciplinary medical teams. BMI calculation should be included in the standard dental evaluation of any pediatric patient, providing a screen for prevention, timely diagnosis, and treatment of the children suffering from dental caries and malnutrition (overweight/obesity). Soft drinks, sweetened juices, and sweetened snacks, which are frequently available at schools, are associated with overweight/obesity and dental caries. Hence, health promotion activities should highlight the importance of reducing the consumption of these unhealthy food items and other sugary foods in homes and schools.
Conclusion
Investigating the dietary habits of the Egyptian school children revealed a positive correlation between desserts (sweetened snacks), soft drinks, and the incidence of dental caries and overweight/obesity; conversely, the consumption of fruits and vegetables was negatively associated. Sweetened juices were positively associated with the three caries indices. Overweight/obesity was significantly positively correlated with primary dentition caries; accordingly, BMI calculation should be included in the routine examination in dental clinics. Despite the forward step taken by the Egyptian government by applying taxes on soft drinks, more taxes need to be applied on other sweetened beverages, including juices and milk. Moreover, legislation regulating the sale of these food items and prohibiting the marketing of these unhealthy food items in school canteens is mandatorily needed. In addition, the results of this study have identified children as a target group of the population to apply interventional and preventive strategies against dental caries and obesity. The government should educate parents about healthy dietary habits, dental health practices, and the benefits of physical activity through different media approaches. Further studies assessing the level of parents' awareness in more detail are needed.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Research Ethics Committee of Cairo University's Faculty of Dentistry's requirements (Approval: 24920). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gill M. Book review: the challenge of oral disease: a call for global action. Br Dent J. (2016) 221:687. doi: 10.1038/sj.bdj.2016.898
2. World Health Organization. Report of the Commission on Ending Childhood Obesity: World Health Organization. (2016). Available online at: https://www.who.int/publications/i/item/9789241510066 (accessed February 15, 2022).
3. James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392:1789–858. doi: 10.1016/S0140-6736(18)32279-7
4. Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (Ylds) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. (2012) 380:2163–96. doi: 10.1016/S0140-6736(12)61729-2
5. Kassebaum N, Bernabé E, Dahiya M, Bhandari B, Murray C, Marcenes W. Global burden of untreated caries: a systematic review and metaregression. J Dent Res. (2015) 94:650–8. doi: 10.1177/0022034515573272
6. Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. The global burden of oral diseases and risks to oral health. Bull World Health Organ. (2005) 83:661–9.
7. Tinanoff N, Baez RJ, Diaz Guillory C, Donly KJ, Feldens CA, McGrath C, et al. Early childhood caries epidemiology, aetiology, risk assessment, societal burden, management, education, and policy: global perspective. Int J Paediatr Dent. (2019) 29:238–48. doi: 10.1111/ipd.12484
8. Lagerweij M, Van Loveren C. Declining caries trends: are we satisfied? Curr Oral Health Rep. (2015) 2:212–7. doi: 10.1007/s40496-015-0064-9
9. Who Emro Egypt Releases Results of Epidemiological Study on Oral Health Status Egypt-Events Egypt. (2014). Available online at: http://www.emro.who.int/egy/egypt-events/results-of-epidemiological-study-on-oral-health-status-released.html (accessed February 15, 2022).
10. Mohamed Ismail AEA. Caries assessment in preschool children in relation to some socioeconomic factors. Adv Dent J. (2022) 4:61–73. doi: 10.21608/adjc.2022.101295.1116
11. Abanto J, Carvalho TS, Mendes FM, Wanderley MT, Bönecker M, Raggio DP. Impact of oral diseases and disorders on oral health-related quality of life of preschool children. Community Dent Oral Epidemiol. (2011) 39:105–14. doi: 10.1111/j.1600-0528.2010.00580.x
12. Cantekin K, Yildirim MD, Cantekin I. Assessing change in quality of life and dental anxiety in young children following dental rehabilitation under general anesthesia. Pediatr Dent. (2014) 36:12E−7.
13. Cunnion D, Spiro A, Jones J, Rich S, Papageorgiou C, Tate A, et al. Pediatric oral health-related quality of life improvement after treatment of early childhood caries: a prospective multisite study. J Dent Child. (2010) 77:4–11.
14. Ramos-Jorge J, Pordeus IA, Ramos-Jorge ML, Marques LS, Paiva SM. Impact of untreated dental caries on quality of life of preschool children: different stages and activity. Community Dent Oral Epidemiol. (2014) 42:311–22. doi: 10.1111/cdoe.12086
15. Sajadi FS, Pishbin L, Azhari SH, Moosazadeh M. Impact of oral and dental health on children's and parents' quality of life based on early childhood oral health impact scale (Ecohis) index. Int J Dent Sci Res. (2015) 3:28–31. doi: 10.12691/ijdsr-3-2-2
16. Acs G, Lodolini G, Kaminsky S, Cisneros GJ. Effect of nursing caries on body weight in a pediatric population. Pediatr Dent. (1992) 14:303.
17. Acs G, Shulman R, Chussid S, Ng M. The effect of dental rehabilitation on the body weight of children with early childhood caries. Pediatr Dent. (1999) 21:109–13.
18. Casamassimo PS, Thikkurissy S, Edelstein BL, Maiorini E. Beyond the Dmft: the human and economic cost of early childhood caries. J Am Dent Assoc. (2009) 140:650–7. doi: 10.14219/jada.archive.2009.0250
19. Sheiham A. Dental caries affects body weight, growth and quality of life in pre-school children. Br Dent J. (2006) 201:625–6. doi: 10.1038/sj.bdj.4814259
20. Thomas CW, Primosch RE. Changes in incremental weight and well-being of children with rampant caries following complete dental rehabilitation. Pediatr Dent. (2002) 24:109–13.
21. Low W, Tan S, Schwartz S. The effect of severe caries on the quality of life in young children. Pediatr Dent. (1999) 21:325–6.
22. Hobdell M, Clarkson J, Petersen P, Johnson N. Global goals for oral health 2020. Int Dent J. (2003) 53:285–8. doi: 10.1111/j.1875-595X.2003.tb00761.x
23. WHO Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. Geneva: World Health Organization (2013).
24. World Health Organization. Oral Health. (2021). Available online at: https://www.who.int/news-room/fact-sheets/detail/oral-health
25. Aitsi-Selmi A, Chandola T, Friel S, Nouraei R, Shipley MJ, Marmot MG. Interaction between education and household wealth on the risk of obesity in women in Egypt. PLoS ONE. (2012) 7:e39507. doi: 10.1371/journal.pone.0039507
26. World Health Organization. Obesity and Overweight. (2021). Available online at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed February 15, 2022).
27. Most Obese Countries in the World Procon Org. (2020). Available online at: https://obesity.procon.org/global-obesity-levels (accessed February 15, 2022).
28. World Health Organization. The Global Health Observatory. Available online at: https://www.who.int/data/gho/data (accessed February 15, 2022).
29. WHO Organization. Taking Action on Childhood Obesity. World Health Organization (2018). Available online at: https://apps.who.int/iris/handle/10665/274792.
30. Petersen PE. The world oral health report 2003: continuous improvement of oral health in the 21st century–the approach of the who global oral health programme. Community Dent Oral Epidemiol. (2003) 31:3–24. doi: 10.1046/j..2003.com122.x
31. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among us children and adolescents, 1999-2010. JAMA. (2012) 307:483–90. doi: 10.1001/jama.2012.40
32. Bagramian RA, Garcia-Godoy F, Volpe AR. The global increase in dental caries. A pending public health crisis. Am J Dent. (2009) 22:3–8.
33. Allison DB, Bassaganya-Riera J, Burlingame B, Brown AW, le Coutre J, Dickson SL, et al. Goals in nutrition science 2015–2020. Front Nutr. (2015) 2:26. doi: 10.3389/fnut.2015.00026
34. Pyne V, Macdonald IA. Update on carbohydrates and health: the relevance of the scientific advisory committee on nutrition report for children. Arch Dis Child. (2016) 101:876–80. doi: 10.1136/archdischild-2015-310200
35. Drewnowski A, Mennella JA, Johnson SL, Bellisle F. Sweetness and food preference. J Nutr. (2012) 142:1142S−8. doi: 10.3945/jn.111.149575
36. Shenkin JD, Heller KE, Warren JJ, Marshall TA. Soft drink consumption and caries risk in children and adolescents. Gen Dent. (2003) 51:30–6.
37. Maffeis C, Tatò L. Long-term effects of childhood obesity on morbidity and mortality. Horm Res Paediatr. (2001) 55(Suppl. 1):42–5. doi: 10.1159/000063462
38. Gilchrist F, Marshman Z, Deery C, Rodd HD. The impact of dental caries on children and young people: what they have to say? Int J Paediatr Dent. (2015) 25:327–38. doi: 10.1111/ipd.12186
39. Spiegel KA, Palmer CA. Childhood dental caries and childhood obesity. Different problems with overlapping causes. Am J Dent. (2012) 25:59–64.
40. Chi DL, Luu M, Chu F. A scoping review of epidemiologic risk factors for pediatric obesity: implications for future childhood obesity and dental caries prevention research. J Public Health Dent. (2017) 77:S8–31. doi: 10.1111/jphd.12221
41. Kottayi S, Bhat SS, Hegde KS, Peedikayil FC, Chandru T, Anil S, et al. cross-sectional study of the prevalence of dental caries among 12-to 15-year-old overweight schoolchildren. Contemp Dent Pract. (2016) 17:750–4. doi: 10.5005/jp-journals-10024-1924
42. da Silva RAB, Barreiros D, Oliveira S, da Silva LAB, Nelson-Filho P, Küchler EC. Association between body mass index and caries experience in Brazilian children and adolescents. J Dent Child. (2016) 83:146–51.
43. El Qomsan MAA, Alasqah MN, Alqahtani FA, Alobaydaa MA, Alharbi MM, Kola Z. Intricate evaluation of association between dental caries and obesity among the children in Al-Kharj City (Saudi Arabia). JCDP. (2017) 18:29–33. doi: 10.5005/jp-journals-10024-1983
44. Lobstein T, Jackson-Leach R, Moodie ML, Hall KD, Gortmaker SL, Swinburn BA, et al. Child and adolescent obesity: part of a bigger picture. Lancet. (2015) 385:2510–20. doi: 10.1016/S0140-6736(14)61746-3
45. Blomquist H, Bergstrøm E. Obesity in 4-year-old children more prevalent in girls and in municipalities with a low socioeconomic level. Acta Paediatr. (2007) 96:113–6. doi: 10.1111/j.1651-2227.2006.00018.x
46. Marshall TA, Eichenberger-Gilmore JM, Broffitt BA, Warren JJ, Levy SM. Dental caries and childhood obesity: roles of diet and socioeconomic status. Community Dent Oral Epidemiol. (2007) 35:449–58. doi: 10.1111/j.1600-0528.2006.00353.x
47. Abbass MM, Mahmoud SA, El Moshy S, Rady D, AbuBakr N, Radwan IA, et al. The prevalence of dental caries among egyptian children and adolescences and its association with age, socioeconomic status, dietary habits and other risk factors. a cross-sectional study. F1000Research. (2019) 8:243. doi: 10.12688/f1000research.17892.1
48. Yousaf M, Aslam T, Saeed S, Sarfraz A, Sarfraz Z, Cherrez-Ojeda I. Individual, family, and socioeconomic contributors to dental caries in children from low-and middle-income countries. Int J Environ Res Public Health Monograph. (2022) 19:7114. doi: 10.3390/ijerph19127114
49. Jaacks LM, Vandevijvere S, Pan A, McGowan CJ, Wallace C, Imamura F, et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. (2019) 7:231–40. doi: 10.1016/S2213-8587(19)30026-9
50. Popkin BM. Nutritional patterns and transitions. Popul Dev Rev. (1993) 19:138–57. doi: 10.2307/2938388
51. Alswat K, Mohamed WS, Wahab MA, Aboelil AA. The association between body mass index and dental caries: cross-sectional study. J Clin Med Res. (2016) 8:147. doi: 10.14740/jocmr2433w
52. Costacurta M, DiRenzo L, Sicuro L, Gratteri S, De Lorenzo A, Docimo R. Dental caries and childhood obesity: analysis of food intakes, lifestyle. Eur J Paediatr Dent. (2014) 15:343–8.
53. Hayden C, Bowler JO, Chambers S, Freeman R, Humphris G, Richards D, et al. Obesity and dental caries in children: a systematic review and meta-analysis. Community Dent Oral Epidemiol. (2013) 41:289–308. doi: 10.1111/cdoe.12014
54. Alkarimi HA, Watt RG, Pikhart H, Sheiham A, Tsakos G. Dental caries and growth in school-age children. Pediatrics. (2014) 133:e616–23. doi: 10.1542/peds.2013-0846
55. Yang F, Zhang Y, Yuan X, Yu J, Chen S, Chen Z, et al. Caries experience and its association with weight status among 8-year-old children in Qingdao, China. J Int Soc Prevent Communit Dent. (2015) 5:52. doi: 10.4103/2231-0762.151978
56. Miller J, Vaughan-Williams E, Furlong R, Harrison L. Dental caries and children's weights. J Epidemiol Community Health. (1982) 36:49–52. doi: 10.1136/jech.36.1.49
57. Peng SM, Wong HM, King NM, McGrath C. Is dental caries experience associated with adiposity status in preschool children? Int J Paediatr Dent. (2014) 24:122–30. doi: 10.1111/ipd.12039
58. de Jong-Lenters M, van Dommelen P, Schuller AA, Verrips EH. Body mass index and dental caries in children aged 5 to 8 years attending a dental paediatric referral practice in the Netherlands. BMC Res Notes. (2015) 8:1–7. doi: 10.1186/s13104-015-1715-6
59. Daniel WW, Cross CL. Biostatistics: A Foundation for Analysis in the Health Sciences. 11th ed. Hoboken, NJ: Wiley (2018).
60. Kale S, Kakodkar P, Shetiya S, Abdulkader R. Prevalence of dental caries among children aged 5–15 years from 9 countries in the eastern mediterranean region: a meta-analysis. East Mediterr Health J. (2020) 26:726–35. doi: 10.26719/emhj.20.050
61. Quach A, Laemmle-Ruff IL, Polizzi T, Paxton GA. Gaps in smiles and services: a cross-sectional study of dental caries in refugee-background children. BMC Oral Health. (2015) 15:1–10. doi: 10.1186/1472-6831-15-10
62. World Bank. World Bank Staff Estimates Based on Age/Sex Distributions of United Nations Population Division's World Population Prospects: 2019 Revision. (2019).
63. World Health Organization. Global Health Observatory Data Repository. Available online at: http://apps.who.int/gho/data/node.main (accessed January 13, 2021).
64. WHO Organization. Oral Health Surveys: Basic Methods. 5th edition ed. Geneva: World Health Organization (2013).
65. Otsuka Y, Kaneita Y, Itani O, Osaki Y, Higuchi S, Kanda H, et al. Association between unhealthy dietary behaviors and sleep disturbances among Japanese adolescents: a nationwide representative survey. Sleep Biol Rhythms. (2019) 17:93–102. doi: 10.1007/s41105-018-0193-3
66. Making Sense of Media. (2019). Available online at: https://www.ofcom.org.uk/__data/assets/pdf_file/0024/134907/children-and-parents-media-use-and-attitudes-2018.pdf (accessed January 16, 2022).
67. Abbass MM, Rady D, Radwan IA, El Moshy S, AbuBakr N, Ramadan M, et al. The occurrence of periodontal diseases and its correlation with different risk factors among a convenient sample of adult Egyptian population: a cross-sectional study. F1000Research. (2019) 8:1740. doi: 10.12688/f1000research.20310.1
68. Diet History Questionnaire, Version, 2,.0 (Dhq-Ii) Snap-Ed. (2016). Available online at: https://snaped.fns.usda.gov/library/materials/diet-history-questionnaire-version-20-dhq-ii (accessed January 16, 2022).
69. Abbass MM, AbuBakr N, Radwan IA, Rady D, El Moshy S, Ramadan M, et al. The potential impact of age, gender, body mass index, socioeconomic status and dietary habits on the prevalence of dental caries among egyptian adults: a cross-sectional study. F1000Research. (2019) 8.
70. WHO Organization. Physical Status: The Use of and Interpretation of Anthropometry, Report of a Who Expert Committee. World Health Organization (1995).
71. World Health Organization. Growth Reference 5–19 Years. World Health Organization (2014). Available online at: https://www.who.int/tools/growth-reference-data-for-5to19-years (accessed January 16, 2022).
72. Tanaka K, Ogata S, Tanaka H, Omura K, Honda C, Hayakawa K. The relationship between body mass index and uric acid: a study on Japanese adult twins. Environ Health Prev Med. (2015) 20:347–53. doi: 10.1007/s12199-015-0473-3
73. Tenjin K, Sekine M, Yamada M, Tatsuse T. Relationship between parental lifestyle and dietary habits of children: a cross-sectional study. J Epidemiol. (2020) 5:253–9. doi: 10.2188/jea.JE20190015
74. Haruyama Y, Fukuda H, Arai T, Muto T. Change in lifestyle through health promotion program without face-to-face intervention in a large-scale Japanese enterprise. J Occup Health. (2013) 55:74–83. doi: 10.1539/joh.12-0145-OA
75. Breslow L, Enstrom JE. Persistence of health habits and their relationship to mortality. Prev Med. (1980) 9:469–83. doi: 10.1016/0091-7435(80)90042-0
76. Mobarak E, Shabayek M, Mulder J, Reda A, Frencken J. Caries experience of Egyptian adolescents: does the atraumatic restorative treatment approach offer a solution? Med Princ Pract. (2011) 20:545–9. doi: 10.1159/000329790
77. Ivančić Jokić N, Bakarčić D, Janković S, Malatestinić G, Dabo J, Majstorović M, et al. Dental caries experience in croatian schoolchildren in Primorsko-Goranska county: a pilot study. Cent Eur J Public Health. (2013) 21:39–42. doi: 10.21101/cejph.a3752
78. World Health Organization. Oral Health Surveys: Basic Methods. 4th ed. Geneva: World Health Organization (1997).
79. Lin HS, Lin JR, Hu SW, Kuo HC, Yang YH. Association of dietary calcium, phosphorus, and magnesium intake with caries status among schoolchildren. Kaohsiung J Med Sci. (2014) 30:206–12. doi: 10.1016/j.kjms.2013.12.002
80. Pitts NB, Zero DT, Marsh PD, Ekstrand K, Weintraub JA, Ramos-Gomez F, et al. Dental caries. Nat Rev Dis Primers. (2017) 3:1–16. doi: 10.1038/nrdp.2017.30
81. Yabao R, Duante C, Velandria F, Lucas M, Kassu A, Nakamori M, et al. Prevalence of dental caries and sugar consumption among 6–12-y-old schoolchildren in La Trinidad, Benguet, Philippines. Eur J Clin Nutr. (2005) 59:1429–38. doi: 10.1038/sj.ejcn.1602258
82. Moussa A, Ibrahim E, Esmat A, Eissa S, Ramzy M. An overview of oral health status, socio-economic and behavioral risk factors, and the pattern of tooth loss in a sample of Egyptian rural population. Bull Natl Res Cent. (2020) 44:1–6. doi: 10.1186/s42269-020-0268-6
83. Moynihan P, Petersen PE. Diet, nutrition and the prevention of dental diseases. Public Health Nutr. (2004) 7:201–26. doi: 10.1079/PHN2003589
84. Lee J, Brearley Messer L. Contemporary fluid intake and dental caries in Australian children. Aust Dent J. (2011) 56:122–31. doi: 10.1111/j.1834-7819.2011.01313.x
85. Feferbaum R, de Abreu LC, Leone C. Fluid intake patterns: an epidemiological study among children and adolescents in Brazil. BMC Public Health. (2012) 12:1–7. doi: 10.1186/1471-2458-12-1005
86. Merugu M, Kodali S, Cherian R, SanjayKumar PK. Effect of sugar sweetened beverages on dental caries among adults: a systematic review. Eur J Mol Clin Med. (2020) 7:2051–65.
87. Kim S, Park S, Lin M. Permanent tooth loss and sugar-sweetened beverage intake in us young adults. J Public Health Dent. (2017) 77:148–54. doi: 10.1111/jphd.12192
88. Rusali R, Najwa Hamali N, Muhammad Razi F, Mustafa N, Asilah Harun N, Azwani Mohd Shukri N. Early childhood feeding practices and its association with early childhood caries. J Food Nutr Res. (2019) 7:801–4. doi: 10.12691/jfnr-7-11-7
89. Sohn W, Burt BA, Sowers MR. Carbonated soft drinks and dental caries in the primary dentition. J Dent Res. (2006) 85:262–6. doi: 10.1177/154405910608500311
90. Lim S, Sohn W, Burt BA, Sandretto AM, Kolker JL, Marshall TA, et al. Cariogenicity of soft drinks, milk and fruit juice in low-income African-American children: a longitudinal study. J Am Dent Assoc. (2008) 139:959–67. doi: 10.14219/jada.archive.2008.0283
91. Ismail AI, Burt BA, Eklund SA. The cariogenicity of soft drinks in the United States. J Am Dent Assoc. (1984) 109:241–5. doi: 10.14219/jada.archive.1984.0346
92. Shah N, Sundaram KR. Impact of socio-demographic variables, oral hygiene practices, oral habits and diet on dental caries experience of indian elderly: a community-based study. Gerodontology. (2004) 21:43–50. doi: 10.1111/j.1741-2358.2004.00010.x
93. Sherfudhin H, Abdullah A, Shaik H, Johansson A. Some aspects of dental health in young adult indian vegetarians: a pilot study. Acta Odontol Scand. (1996) 54:44–8. doi: 10.3109/00016359609003508
94. Hojsak I, Colomb V, Braegger C, Bronsky J, Campoy C, Domellöf M, et al. Espghan committee on nutrition position paper. Intravenous lipid emulsions and risk of hepatotoxicity in infants and children: a systematic review and meta-analysis. J Pediatr Gastroenterol Nutr. (2016) 62:776–92. doi: 10.1097/MPG.0000000000001121
95. Zhu F, Chen Y, Yu Y, Xie Y, Zhu H, Wang H. Caries prevalence of the first permanent molars in 6–8 years old children. PLoS ONE. (2021) 16:e0245345. doi: 10.1371/journal.pone.0245345
96. Araujo D, Marquezin M, Barbosa T, Fonseca F, Fegadolli C, Castelo P. Assessment of quality of life, anxiety, socio-economic factors and caries experience in Brazilian children with overweight and obesity. Int J Dent Hyg. (2017) 15:e156–62. doi: 10.1111/idh.12248
97. Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. (2006) 295:1549–55. doi: 10.1001/jama.295.13.1549
98. Hooley M, Skouteris H, Millar L. The relationship between childhood weight, dental caries and eating practices in children aged 4–8 years in Australia, 2004–2008. Pediatr Obes. (2012) 7:461–70. doi: 10.1111/j.2047-6310.2012.00072.x
99. Al Jawaldeh A, Radwan IA, Rady D, El Moshy S, Abu Bakr N, Abbass MMS. Food consumption patterns among children and adolescents and their correlation with overweight/obesity in Egypt: a cross-sectional study. Piel Zdr Publ. (2020) 10:149–57. doi: 10.17219/pzp/118081
100. Papanikolaou Y, Fulgoni III VL. Bean consumption is associated with greater nutrient intake, reduced systolic blood pressure, lower body weight, and a smaller waist circumference in adults: results from the national health and nutrition examination survey 1999-2002. J Am Coll Nutr. (2008) 27:569–76. doi: 10.1080/07315724.2008.10719740
101. Astrup A, Ryan L, Grunwald GK, Storgaard M, Saris W, Melanson E, et al. The role of dietary fat in body fatness: evidence from a preliminary meta-analysis of ad libitum low-fat dietary intervention studies. Br J Nutr. (2000) 83:S25–32. doi: 10.1017/S0007114500000921
102. Sathe SK, Deshpande S, Salunkhe D, Rackis JJ. Dry beans of phaseolus. A review part 2 chemical composition: carbohydrates, fiber, minerals, vitamins, and lipids. Crit Rev Food Sci Nutr. (1984) 21:41–93. doi: 10.1080/10408398409527396
103. Bailleul-Forestier I, Lopes K, Souames M, Azoguy-Levy S, Frelut ML, Boy-Lefevre ML. Caries experience in a severely obese adolescent population. Int J Paediatr Dent. (2007) 17:358–63. doi: 10.1111/j.1365-263X.2007.00848.x
104. Psoter WJ, Reid BC, Katz RV. Malnutrition and dental caries: a review of the literature. Caries Res. (2005) 39:441–7. doi: 10.1159/000088178
105. Hooley M, Skouteris H, Boganin C, Satur J, Kilpatrick N. Body mass index and dental caries in children and adolescents: a systematic review of literature published 2004 to 2011. Syst Rev. (2012) 1:1–26. doi: 10.1186/2046-4053-1-57
106. Silva AER, Menezes AMB, Demarco FF, Vargas-Ferreira F, Peres MA. Obesity and dental caries: systematic review. Rev Saude Publica. (2013) 47:799–812. doi: 10.1590/S0034-8910.2013047004608
107. El-Kassas G, Ziade F. Exploration of the risk factors of generalized and central obesity among adolescents in North Lebanon. J Environ Public Health. (2017) 2017:2879075. doi: 10.1155/2017/2879075
108. Winkvist A, Hultén B, Kim J-L, Johansson I, Torén K, Brisman J, et al. Dietary intake, leisure time activities and obesity among adolescents in Western Sweden: a cross-sectional study. Nutr J. (2015) 15:1–12. doi: 10.1186/s12937-016-0160-2
109. Mills SDH, Tanner LM, Adams J. Systematic literature review of the effects of food and drink advertising on food and drink-related behaviour, attitudes and beliefs in adult populations. Obes Rev. (2013) 14:303–14. doi: 10.1111/obr.12012
110. Al-Jawaldeh A, Jabbour J. Marketing of food and beverages to children in the eastern mediterranean region: a situational analysis of the regulatory framework. Front Nutr. (2022) 9:868937. doi: 10.3389/fnut.2022.868937
111. Hersey JC, Wohlgenant KC, Arsenault JE, Kosa KM, Muth MK. Effects of front-of-package and shelf nutrition labeling systems on consumers. Nutr Rev. (2013) 71:1–14. doi: 10.1111/nure.12000
112. Levy DT, Friend KB, Wang YC. A review of the literature on policies directed at the youth consumption of sugar sweetened beverages. Adv Nutr. (2011) 2:182S−200. doi: 10.3945/an.111.000356
113. Ruopeng A. Effectiveness of subsidies in promoting healthy food purchases and consumption: a review of field experiments. Public Health Nutr. (2013) 16:1215–28. doi: 10.1017/S1368980012004715
114. Escobar MAC, Veerman JL, Tollman SM, Bertram MY, Hofman KJ. Evidence that a tax on sugar sweetened beverages reduces the obesity rate: a meta-analysis. BMC Public Health. (2013) 13:1–10. doi: 10.1186/1471-2458-13-1072
115. Niebylski ML, Redburn KA, Duhaney T, Campbell NR. Healthy food subsidies and unhealthy food taxation: a systematic review of the evidence. Nutrition. (2015) 31:787–95. doi: 10.1016/j.nut.2014.12.010
116. Thow AM, Jan S, Leeder S, Swinburn B. The effect of fiscal policy on diet, obesity and chronic disease: a systematic review. Bull World Health Organ. (2010) 88:609–14. doi: 10.2471/BLT.09.070987
Keywords: unhealthy diet, dental caries, school children, overweight, obesity
Citation: Mahmoud SA, El Moshy S, Rady D, Radwan IA, Abbass MMS and Al Jawaldeh A (2022) The effect of unhealthy dietary habits on the incidence of dental caries and overweight/obesity among Egyptian school children (A cross-sectional study). Front. Public Health 10:953545. doi: 10.3389/fpubh.2022.953545
Received: 26 May 2022; Accepted: 11 July 2022;
Published: 16 August 2022.
Edited by:
Rafaela Rosário, University of Minho, PortugalReviewed by:
George Kitsaras, The University of Manchester, United KingdomKaren Sokal-Gutierrez, University of California, Berkeley, United States
Copyright © 2022 Mahmoud, El Moshy, Rady, Radwan, Abbass and Al Jawaldeh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marwa M. S. Abbass, bWFyd2EubWFnZHlAZGVudGlzdHJ5LmN1LmVkdS5lZw==
 Sara Ahmed Mahmoud1
Sara Ahmed Mahmoud1 Israa Ahmed Radwan
Israa Ahmed Radwan Marwa M. S. Abbass
Marwa M. S. Abbass Ayoub Al Jawaldeh
Ayoub Al Jawaldeh