
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 12 August 2022
Sec. Public Mental Health
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.951136
This article is part of the Research Topic The COVID-19 Pandemic, Problematic Internet Use, Post-traumatic Stress and Mental Health View all 21 articles
 Valeria Carola1*
Valeria Carola1* Cristina Vincenzo1
Cristina Vincenzo1 Chiara Morale1
Chiara Morale1 Massimiliano Pelli2
Massimiliano Pelli2 Monica Rocco2
Monica Rocco2 Giampaolo Nicolais1*
Giampaolo Nicolais1*Along with physical changes, psychological changes are detectable in patients with COVID-19. In these patients, the stressful experience of intensive care unit (ICU) hospitalization may aggravate psychological conditions. Our study examines the short- and long-term psychological consequences of COVID-19 in ICU patients. COVID-19 patients completed the self-rating questionnaires Kessler 10 Psychological Distress Scale (K10), Perceived Stress Scale-10 (PSS), Impact of Event Scale Revised (IES-R), and Post-traumatic Growth Inventory (PTGI) and were clinically interviewed 1 and 6 months after discharge. Altered behavioral-psychological symptoms and patients' strategies (adaptive vs. maladaptive) for coping with stress during and after hospitalization were coded during clinical interviews. Between 20 and 30% of patients showed moderate symptoms of depression or anxiety and perceived stress 1 and 6 months after discharge. Sleep problems, difficulty concentrating, confusion in placing events, and fear of reinfection were observed in many (6–17%) patients. At 6 months, only 7% of patients showed PTSD symptoms, and 50% showed post-traumatic growth in the “appreciation of life” sub-scale. Finally, 32% of subjects were classified as “maladaptive coping patients,” and 68% as “adaptive coping patients.” Patients who adopted “adaptive” coping strategies showed significantly lower levels of anxious-depressive symptoms and perceived stress when compared to subjects with “maladaptive” strategies at both time points. Coping strategy had no effect on PTSD symptoms or post-traumatic growth at 6 months. These findings clarify the short- and long-term psychological effects of intensive care due to COVID-19 infection and demonstrate that patient characteristics, particularly strategies for coping with stress, seem to play a critical role in psychological outcomes.
The novel coronavirus 2019 (COVID-19) pandemic has generated worldwide alarm. COVID-19 infection causes respiratory disease ranging from mild—or pauci-symptomatic—to fatal (1). Since its outbreak, COVID-19 has infected more than 300 million people and resulted in over 5 million deaths (2). Italy was one of the first countries to be severely impacted by COVID-19; more than 8 million confirmed cases and more than 130 thousand deaths were recorded in Italy as of December 2021 (2, 3).
COVID-19 affects both physical and psychological health. The post-acute phase of disease is often characterized by physical and psychological sequelae ranging from respiratory difficulty, cardiovascular abnormality, and prolonged infirmity to neurological and psychological (including cognitive and behavioral) complications (1). Psychological stressors, such as fear of illness, uncertainty about the future, traumatic memories of severe illness, and social isolation, may foster psychopathological outcomes, which, in turn, may worsen a patient's general medical condition (3, 4). Furthermore, studies suggest that coronaviruses can indirectly induce psychopathological sequelae via an immune response as well as by direct viral infection of the central nervous system. COVID-19 can cross the blood-brain barrier and infect the central nervous system (CNS), resulting in both short- and long-term neurological and neuropsychological sequelae (5–11). There is increasing evidence that while people with mild or moderate COVID-19 infection generally develop only respiratory symptoms, some patients with severe infection also develop neurological conditions like confusion, stroke, and even infectious toxic encephalopathy and viral encephalitis (12).
COVID-19 has a significant emotional impact on patients due to both the characteristics of the virus itself and its psycho-physical-social consequences. COVID-19 survivors often develop psychiatric distress such as insomnia, along with anxious, depressive, and even post-traumatic psychological reactions (11, 13, 14). Younger age, chronic disease, or a history of psychiatric illness may contribute to the development of depressive and anxious symptoms during the pandemic, while more social support, including physical and psychological assistance, is correlated with lower stress levels (13, 15–20).
In 5–11% of cases, COVID-19 infection causes medical complications, chiefly acute respiratory failure, that necessitate hospitalization in an intensive care unit (ICU) (21); treatment of ARF (22) often involves mechanical ventilation. ICU care can have a major impact on the psychological wellbeing of patients (23). The ICU environment can be stressful, with the noise of medical devices, constant lighting, ongoing alarms, and staff working under pressure. Some studies suggest that sounds alone can contribute to sleep and mood disturbances (24, 25). ICU patients have limited freedom of movement, and especially patients in need of ventilation support may have difficulties communicating. During invasive and prolonged medical interventions, patients under sedation often have impaired perception, and they may experience altered mental states including delirium. Such experiences in an ICU may themselves become a specific risk factor for the development of psychopathology and for a reduction in psychological wellbeing and quality of life. Studies suggest that patients are at a heightened risk for experiencing psychological symptoms during and following an ICU stay (26, 27). During the first year of recovery after an ICU stay, approximately one third of survivors experience symptoms of anxiety and depression, and about a fifth experience clinically important symptoms of PTSD (26, 28–33). In a systematic review (28), the median point prevalence of questionnaire-assessed substantial PTSD symptoms was 22%, and the median point prevalence of clinician-diagnosed PTSD was 19% assessed from ≈6 weeks to 7 years, though most studies had PTSD assessments within the first year post-ICU. Pre-ICU psychopathology, greater in-ICU benzodiazepine administration, and post-ICU memories of frightening and/or psychotic experiences in the ICU might predict the onset of post-traumatic syndrome after discharge (28, 34). While it is well known that ICU admission can result in PTSD, literature on the short- and long-term consequences of ICU admission specifically for COVID-19 is lacking.
Traumatic experiences like ICU hospitalization can also lead to positive developments. This phenomenon is known as post-traumatic growth (PTG), defined as the subjective experience of a significantly positive change for an individual following a major life crisis. PTG can follow many different types of traumatic event, such as bereavement (35), combat (36), or cancer (37).
Patients' coping strategies during adaptation to trauma may have a dramatic impact on their general recovery. Adaptive coping strategies, i.e., problem-centered strategies that help the individual openly face and internalize the traumatic event, correlate with better outcomes (38–40). On the other hand, maladaptive coping strategies, aimed at the reduction of tension via the activation of specific defensive mechanisms, are likely to worsen the patient's current conditions and prognosis (40–43).
Literature on the psychopathological sequelae of COVID-19 patients after ICU hospitalization is still largely lacking. Longitudinal data at 3 months after discharge are rare (44–46), and, to our knowledge, longer-term data are non-existent.
We found little evidence of published studies related to PTG in COVID-19 patients (47–49) particularly with samples of COVID-19 patients after discharge from the ICU (49).
Similarly, we found no studies on the use of adaptive vs. maladaptive coping strategies in COVID-19 patients after ICU hospitalization.
We hypothesize, first, that the experience of COVID-19 infection may be a risk factor for the development of anxious, depressive, and PTSD symptoms; second, that post-traumatic growth may occur following recovery from COVID-19; and third, that psychopathological outcomes may be moderated by coping strategies adopted by the patients.
All patients (N = 71) who were infected with COVID-19 and admitted to the ICU of the Sant'Andrea University Hospital in Rome (73.5% men, 26.5% women), between November 2020 and May 2021, were included in this study. Patients completed self-assessment questionnaires and were clinically interviewed 3 months (Timepoint 1, T2) and 5–6 months (Timepoint 2, T2) after discharge. Psychological interviews were included in a general medical screening after discharge. The subjects' mean age was 60 years, and the age range was 34–85 years.
Prior to enrolment, all participants were given a complete description of the study and provided written informed consent. The patients were interviewed and screened for psychological symptoms using questionnaires. The study was approved by the Ethical Committee of the Department of Dynamic and Clinical Psychology, Sapienza, University of Rome (Prot. n. 0000144).
Questionnaires were administered online or, for patients who had difficulty with digital tools, face-to-face before the interviews. At the first time point, 1–3 months after discharge, patients completed the Kessler 10 Psychological Distress Scale (K10) and Perceived Stress Scale (PSS) questionnaires. At the second time point, 5–6 months after discharge, the same patients completed the K10 and PSS questionnaires again and, in addition, the Post-Traumatic Growth Inventory (PTGI) and Impact of Event Scale Revised (IES-R) questionnaires. At the second time point, 10% of patients in the first sample were excluded from the second interview because they had begun psychotherapy.
K10 (50), a 10-item questionnaire, provides a global measure of distress experienced in the previous 4 weeks. We used the validated Italian translation (51). Each item is scored on a 5-point Likert scale: 1 (“never”), 2 (“rarely”), 3 (“some of the time”), 4 (“most of the time”), or 5 (“all of the time”). To be consistent with previous validation studies (50, 52), patients who scored between 20 and 24 were considered mildly distressed, and patients who scored between 27 and 40 were considered highly distressed.
PSS (53), a 10-item questionnaire, measures the degree to which one perceives aspects of one's life as uncontrollable, unpredictable, and overwhelming. Participants are asked to respond to each question on a 5-point Likert scale ranging from 0 (never) to 4 (very often), indicating how often they have felt or thought a certain way within the past month. Scores range from 0 to 40, with higher composite scores indicative of greater perceived stress. Patients were considered to have intermediate perceived stress if they scored between 18 and 26, whereas they were considered to have high perceived stress if they scored between 27 and 40. The PSS possesses adequate internal reliability (53).
PTGI (54) is a 21-item inventory that assesses the positive psychological change that may occur after a traumatic experience. We used the validated Italian translation (55). Participants are asked to respond to each statement on a 6-point Likert scale ranging from 0 (“I did not experience this change as a result of my crisis”) to 5 (“I experienced this change to a very great degree as a result of my crisis”), with intermediate scores of 1 (“a very small degree”), 2 (“a small degree”), 3 (“a moderate degree”), and 4 (“a great degree”). The PTGI assesses patient growth on five sub-scales: relating to others, new possibilities, personal strength, spiritual change, and appreciation of life. Patients' scores were compared to scores obtained by an Italian normative sample (55). Mean of the normative reference sample was for “appreciation of life” 7.66 ± 4.37, for “personal strength” 9.48 ± 5.64, for “relating to others” 14.12 ± 9.13, for “new possibilities” 10.38 ± 7.07, and for “spiritual change,” 3.33 ± 3.36. The test-retest reliability (alpha) of the PTGI is 0.71 and its internal consistency is 0.90 (55).
IES-R (56), a 22-item questionnaire, assesses the magnitude of symptomatic response in the past 7 days to a specific traumatic life event. This version of the IES comprises three dimensions: avoidance, intrusion, and hyperarousal. We used the Italian validation (57). Participants are asked to report their degree of distress during the past 7 days on a 5-point Likert scale: 0 (not at all), 1 (a little bit), 2 (moderately), 3 (quite a bit), or 4 (extremely). Given the timing of PTSD onset assessable 1 month after the traumatic event (58), we assessed the presence of post-traumatic symptoms only at the second time point.
Clinical interviews were conducted within a medical screening process prepared by the ICU and investigated the following areas:
• general anamnestic information
• sleep quality before, during, and after hospitalization
• post-traumatic symptomatology
• spiritual faith and its possible supporting role for the patient
• memories and experiences of hospitalization
• current psychological state.
Frequency tables of reported behavioral-psychological symptoms were developed from the content of the interviews.
Adaptive and maladaptive coping strategies employed by the patient during and after hospitalization were investigated during the clinical interviews. Coping strategies are behaviors implemented by individuals to deal with stressful or traumatic situations. In accordance with the literature (38, 59), we define adaptive coping strategies as problem-centered strategies (such as active coping, planning, and social support). Maladaptive coping strategies are strategies aimed at reducing tension (such as avoidance, denial, and emotional release). Assessment of coping strategies from the content of the interviews was performed independently by four different certified psychologists. Indicators of post-traumatic growth were also assessed from the interviews.
Count data were expressed as frequency and percentage. Measurements were described by the mean and standard deviation. Repeated-measures analyses of variance (RM-ANOVA) were performed to assess the long-term effects of ICU admission due to COVID-19 on anxious-depressive symptoms (from the K10) and stress-related variables (from the PSS). One-way analyses of variance (ANOVAs) were performed to evaluate the impact of coping strategy on anxious-depressive and PTSD symptoms (from the K10 and IES-R), perceived stress levels (from the PSS), and post-traumatic growth (from the PTGI). Significant RM-ANOVAs and ANOVAs (P < 0.05) were followed by post-hoc comparisons using Duncan's test. Statistical analyses were carried out using Statistica, version 12.0 (StatSoft, Tulsa, OK, USA).
To assess the levels of anxious-depressive symptoms and perceived stress in COVID-19 patients at the first time point, the frequencies of scores on the K10 and PSS were evaluated. Twenty-two percent of patients exhibited medium to high levels of anxious-depressive symptoms (sample mean ± SD: 16.85 ± 6.25; Figure 1). A larger percentage (30%) of patients showed medium to high levels of perceived stress (11.48 ± 9.32; Figure 1). Based on questionnaire results and each patient's psychological condition according to the clinical interview, 10% of patients were referred for psychotherapy and excluded from the assessment of psychological symptoms at the second time point.

Figure 1. Frequencies of COVID-19 patients showing high, intermediate, and low symptomatology on the K10 and PSS (anxious-depressive symptoms and perceived stress) at T1.
To evaluate whether levels of anxiety-depression and of perceived stress had changed between the first and second time points, two repeated-measure ANOVAs were performed on the scores from the K10 and the PSS. No significant change in the level of anxious-depressive symptoms was detected between time points (Figure 2). Time had a statistically significant effect on PSS scores; levels of perceived stress were lower at time point 2 than at time point 1 (Figure 2).
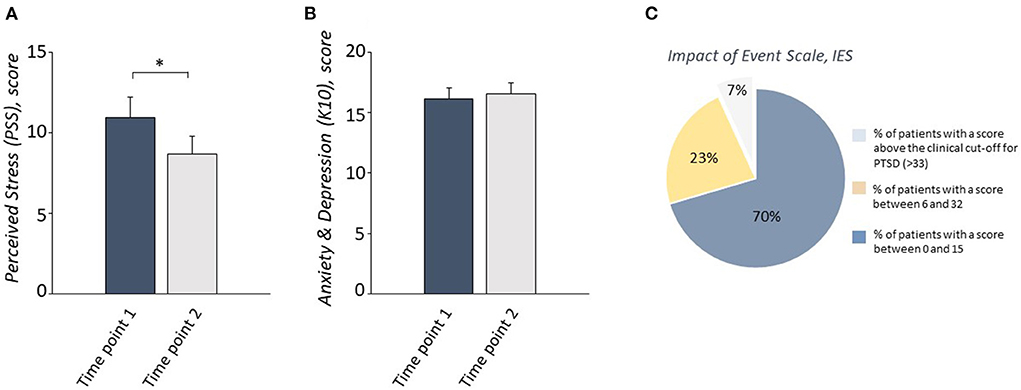
Figure 2. A reduction of the perceived stress levels (A), but no change in anxious-depressive symptoms (B), measured respectively by PSS and K10, was observed in COVID-19 patients between Time point 1 and Time point 2. 70% of patients obtained a score between 0 and 15, 23% obtained a score between 16 and 32, and only 7% of patients scored above the clinical cut-off for PTSD symptoms measured by IES-R at Timepoint 2 (C). *P < 0.05.
The frequency of other behavioral symptoms was also recorded during the clinical interviews at the first time point. Specifically, 17% of patients reported sleep problems, 9% reported active inhibition of ICU memories, 7% reported confusion in temporally placing events, 7% reported short-term memory problems, 14% reported concentration problems, and 6% reported fear of being infected again (Table 1).
In order to detect PTSD symptoms at the second time point, the frequencies of scores on the IES-R questionnaire were recorded. Only 7% of patients showed a score above the clinical cutoff of 33; the majority of the sample showed no symptoms or moderate symptoms (total IES-R = 12.09 + 12.33; Table 2, Figure 2).
The presence of PTG on each of the PTGI sub-scales was evaluated at the second time point (Table 2, Figure 3). The fraction of patients who scored at or below the normative reference sample on each sub-scale was 48% % for “appreciation of life,” 68% for “personal strength,” 66% for “relating to others,” 73% for “new possibilities,” 66% for “spiritual change.”
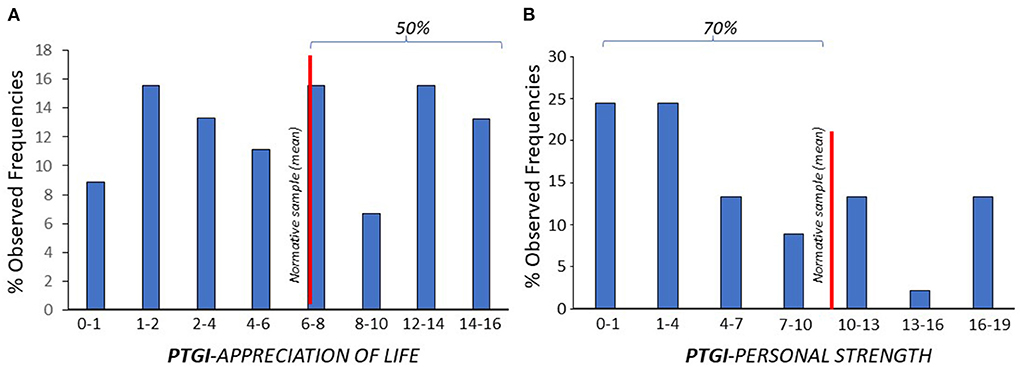
Figure 3. The fraction of patients who scored (% observed frequencies) at or below the normative reference sample (red line) was 48% for “appreciation of life” (A), and 68% for “personal strength” (B) subscales.
Qualitative analysis of clinical interviews at the first time point classified 32% of patients as “maladaptive coping patients” (8M and 3F; age 63.20 ± 11.29) and 68% as “adaptive coping patients” (22M and 5F; age 60.92 ± 10.66). Four ANOVAs were performed to assess whether the coping strategy was associated with anxious-depressive symptoms and levels of perceived stress at each of the two time points. Coping strategy had a statistically significant effect on both scores at both time points; “adaptive coping patients” showed significantly lower levels of anxious-depressive symptoms and perceived stress than “maladaptive coping patients” at both time points (perceived stress, time point 1: F(1, 65) = 11.91, p < 0.001; perceived stress, time point 2: F(1, 37) = 7.85, p = 0.008; anxiety-depression, time point 1: F(1, 65) = 21. 51, p < 0.001; anxiety-depression, time point 2: F(1.37) = 8.90, p = 0.005; Figure 4).
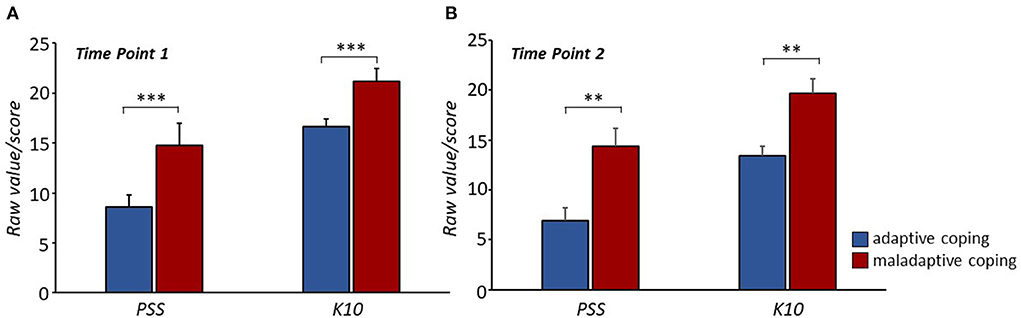
Figure 4. Significant lower perceived stress levels and anxious-depressive symptoms were observed in “adaptive coping patients” compared to “maladaptive coping patients” at both time points (A, B). **P < 0.01, ***P < 0.001.
ANOVAs were performed to assess whether coping strategy was associated with PTSD symptoms and PTG levels at the second time point. Coping strategy showed no significant effect on PTSD or any of the PTG sub-scales.
The perception of post-traumatic growth, along the six dimensions of the PTGI, was investigated through qualitative analysis of the clinical interviews. The subjects' accounts provided an overview of the type of post-traumatic growth present in the sample and its characteristics.
Growth in “relating to others” was one of the clearest trends that emerged in clinical interviews. “Relating to others” refers to the propensity of survivors of a traumatic event to talk to family, friends, or fellow survivors, with whom survivors might feel a sense of closeness and connection. Growth along this dimension underscores the subject's greater sensitivity to interpersonal relationships and thus greater perception of appreciating and valuing them. For example, one of the interviews reported,
(…) He acknowledges a high level of apprehension toward his wife and son after hospitalization and acknowledges that at times this is exacerbated by the lived experience (…) Reports that after discharge he is reconsidering priorities in his life, giving more space to his marital relationship and with his son (…).
“Appreciation of life” relates to how trauma clarifies what is truly important in the survivor's life. Having experienced a traumatic event can lead the survivor to reconsider the value of life, as well as the precarious balance between life and death. For example, one interview reported,
(…) He can't wait to get back to work and wants to devote himself body and soul to patients, especially those in intensive care. He says he knows what it feels like in those conditions and how important even a caress or an extra word is in those places, so he wants to commit himself to this field. He feels that this has been an important life experience that has changed him for the better (…)
“New possibilities” was a prominent theme in the clinical interviews. “New possibilities” refers to the readiness of the survivor to experience new life scenarios following the traumatic event. The traumatic event and the experience of survival become the engine that pushes the survivor to live new realities, seek new experiences, and pursue new interests. For example,
(…) If she had to go back, she would seriously think about being a nurse. She ended up being so motivated that we even talked about the possibility for her to volunteer in hospitals in the future (…). She feels it is her mission to tell everyone about what she went through there and the humanity she found, so that everyone can appreciate what the doctors and nurses at ICU do for us.
“Personal strength” was the area of post-traumatic growth most evident in the clinical interviews. “Personal strength” refers to the development of greater self-efficacy and capacity. The survivor of the traumatic event may feel greater confidence in their own actions and wisdom and therefore may feel more able of dealing with future events. In the interviews, participants reflected on their increased perception that they could cope with future challenges.
(…) He feels that the ICU experience may have improved him because now when he faces a problem, he realizes that he is calmer because he knows that a solution can always be found (…)
“Spiritual change” was not a prominent aspect of the post-traumatic growth experienced by participants. This dimension concerns an individual's connection to nature, to others, and to the world and the individual's understanding and acceptance of him/herself and others.
Despite growing interest in the effects of the COVID-19 pandemic and of infection on mental health (60–62), many unknows remain. This study investigated the short- and long-term psychological effects of COVID-19 infection on patients who experienced acute illness and ICU admission. Among our patients, 22% exhibited moderate anxious and depressive symptoms, and 30% exhibited medium to high levels of perceived stress ≈1 month after discharge from the ICU. Levels of anxious and depressive symptomatology remained stable, while perceived stress levels in this sample decreased by ≈6 months after discharge. These findings are in line with many studies that suggest that anxious and depressive symptoms result from hospitalization due to COVID-19 (13, 43, 63–65). A meta-analysis by Saidi et al. (66) found prevalence levels for symptoms of depression and anxiety at 45 and 47%, respectively, in hospitalized COVID-19 patients. Other studies have reported rates ranging from 18 to 30% within the first 3 months after discharge (44–46). Deng et al. (67) and Moayed et al. (68) found a prevalence of 46.6% for elevated perceived stress in patients infected with COVID-19. SARS and MERS patients admitted to the ICU experienced similar psychological distress that persisted even 6 months after discharge (15).
Our interviews revealed other relevant behavioral symptoms, including sleep disturbances, concentration problems, active inhibition of ICU memories, confusion in the temporal placement of events, short-term memory problems, and fear of re-infection. The presence of sleep-related disorders seems to be in line with data from previous studies (66) in which the prevalence of sleep disorders was 34% among COVID-19 patients. Consistent with our study, Poyraz et al. (45) found that a notable percentage of COVID-19 patients also reported behavioral symptoms after recovery, such as sleep disturbance and difficulty concentrating in 38.8 and 15% of the sample, respectively. A qualitative study (68) of patients admitted to the ICU for other medical causes found an absence of ICU-related memories at 3 months post-discharge, likely because the patients had been sedated. In our study a substantial percentage of COVID-19 patients complained of active memory inhibition in the ICU, even though they had been awake and conscious during hospitalization.
The experience of being hospitalized for COVID-19 has characteristics that make it a risk factor for the development of PTSD (28, 33). However, ICU admission for COVID-19 appears to be associated with relatively low prevalence rates of PTSD. In our sample, only 7% of patients reported PTSD symptoms. In a meta-analysis, Nagarajan et al. (69) found a 16% prevalence of PTSD among patients with COVID-19 infection that led to acute illness. By contrast, for coronaviruses SARS and MERS, the prevalence of post-admission PTSD was 39% (15). For other medical causes, the prevalence of PTSD after ICU admission is 19–22%, higher than but comparable to that for COVID-19 (28).
The potential for psychological growth after trauma is less well studied than the more physiological consequences of trauma (70, 71) and may add an important perspective to current thinking about trauma. PTG can be transformative; in the face of emotionally overwhelming and stressful events, individuals can commit their resources and skills toward overcoming adversity and emerge with a perception of an improvement in themselves (72–74). This process in survivors of intensive care for COVID-19 has been studied little. Our results agree with previous work (47–49) that PTG can be significant in patients who have experienced intensive care for COVID-19, even if it is accompanied by moderate psychological distress. We found significantly higher “appreciation of life” but lower “personal strength” among our sample relative to the Italian normative sample. Less PTG in “personal strength” may make sense given the history of our sample. The feeling of helplessness and the lack of autonomy that ICU patients experienced during wakefulness may have contributed to lower self-efficacy, especially for managing future adverse events after hospitalization. The lower values of “personal strength” may also have been a consequence of “long COVID,” defined as the persistence of fatigue and residual respiratory symptoms after recovery (75, 76). Previous studies have shown positive associations between COVID-19-related concerns and PTG in samples of U.S. civilians and veterans (70, 77). Others have reported high rates of PTG, especially in “appreciation of life” and “relating to others,” in a sample of parents in Portugal during the pandemic (78). An assessment of PTG in hospitalized patients undergoing bone marrow and/or stem cell transplantation and palliative care (79, 80), found, as did our study, that “appreciation of life” was among the areas of most significant growth; the assessment also found no change in “personal strength.” Finally, a study conducted by Holtmaat et al. (81) on cancer patients showed that the most impacted domain was “relating to others.”
To investigate patient response to the experience of hospitalization in the ICU, we further assessed the coping strategies patients used during hospitalization and upon recovery. In our sample, 32% of subjects were classified as “maladaptive coping patients” while 68% were classified as “adaptive coping patients.” Patients who adopted “adaptive coping strategies” showed significantly lower levels of anxious-depressive symptoms and PSS, compared to subjects with “maladaptive strategies.” But coping strategy had no effect on PTSD symptoms or PTG levels. An association between adaptive coping style and lower risk of psychological distress has also been described in students during the COVID-19 pandemic (82). A lack of association between coping strategy and PTSD symptoms has been previously observed, as well (83). Although the relationship between coping strategies and PTG in COVID-19-infected populations is still not well established, our data appear to be at odds with recent research showing that coping strategies can influence PTSD and PTG in other contexts (49, 74, 84–87). Adaptive coping strategies, such as problem solving, were positively associated with high levels of PTG and negatively correlated with PTSD symptoms in military and civilian samples. Together, our findings and the wider literature suggest a complex relationship between coping strategy, PTG, and PTSD symptoms that should be further investigated.
Our sample was small and not representative; the characteristics of the sample itself and of the pandemic more generally did not allow for the selection of an ideal experimental sample. Because of sample size, we did not perform statistical analyses controlling for demographic factors such as age groups, gender, and level of education.
Second, in our study we did not perform an evaluation of risk factors for the development of anxiety, depressive or stress symptoms following COVID-19 infection and ICU hospitalization. In fact, longer-term hospitalization (88), female gender (14, 89–91), perception of low social support (14, 88), previous psychiatric problems (89) and low oxygen saturation (92) are associated with increased psychological distress at discharge.
Third, coping strategy was assessed on a clinical basis by four independent expert clinical evaluators and psychologists, rather than via a standardized questionnaire.
Fourth, the study was limited by specific characteristics of the sample and the absence of a control group. Because our sample included only patients who had been admitted to the ICU for COVID-19, it was not possible to compare them either to patients who had experienced only COVID-19 infection or to patients who had experienced an ICU admission independently of COVID-19.
Finally, the patients in our original sample who were most psychologically compromised at the first time point were referred, for ethical reasons, to psychotherapy services and thus were excluded from the second phase of assessment in which PTSD and PTG were investigated.
Future research should investigate whether the psychological effects of ICU hospitalization for COVID-19 resolve or persist over a longer timescale, especially in patients who used maladaptive coping strategies. Longitudinal studies should be performed at least 1 year after discharge from the ICU. In addition, comparisons should be made between patient groups like ours and (1) COVID-19 patients for whom ICU hospitalization was not necessary and (2) patients admitted to the ICU for other organic causes/pathologies. These controls would help clarify the independent effects of COVID-19 and ICU hospitalization.
This study provided an in-depth look at the short- and long-term psychological effects of the experience of intensive care for severe COVID-19. Results indicated that patient characteristics and patient coping strategies may play a decisive role in psychological outcomes. Moreover, this study showed that survival of COVID-19 together with ICU hospitalization may foster positive psychological growth, as well.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethical Committee of the Department of Dynamic and Clinical Psychology, Sapienza, University of Rome (Prot. n. 0000144). The patients/participants provided their written informed consent to participate in this study.
VC, CV, MR, MP and GN design the study and collected the data. VC, CV, and CM analyzed the data and designed the figures/tables. All authors wrote the manuscript. All authors contributed to the article and approved the submitted version.
The authors would like to thank Fabio Lucidi and Annamaria Speranza for facilitating the establishment of the collaboration necessary to carry out this study, and Valentina Cecchi for helping with the formatting of the manuscript. The professional editorial work of Blue Pencil Science is also acknowledged.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Iodice F, Cassano V, Rossini PM. Direct and indirect neurological, cognitive, and behavioral effects of COVID-19 on the healthy elderly, mild-cognitive-impairment, and Alzheimer's disease populations. Neurol Sci. (2020) 42:455–65. doi: 10.1007/s10072-020-04902-8
2. World Health Organization. WHO coronavirus (COVID-19) dashboard overview data table (2022) [cited 2022 May 2]. Available from: https://covid19.who.int/
3. Supino M, d'Onofrio A, Luongo F, Occhipinti G, Dal Co A. The effects of containment measures in the Italian outbreak of COVID-19. BMC Public Health. (2020) 20:1806. doi: 10.1186/s12889-020-09913-w
4. Brooks S, Webster R, Smith LE, Woodland L, Wessely S, Greenberg N, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. (2020) 395:912–20. doi: 10.1016/S0140-6736(20)30460-8
5. Carvalho-Schneider C, Laurent E, Lemaignen A, Beaufils E, Bourbao-Tournois C, Laribi S, et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. (2021) 27:258–63. doi: 10.1016/j.cmi.2020.09.052
6. Arbour N, Ekandé S, Côté G, Lachance C, Chagnon F, Tardieu M, et al. Persistent infection of human oligodendrocytic and neuroglial cell lines by human coronavirus 229E. J Virol. (1999) 73:3326–37. doi: 10.1128/JVI.73.4.3326-3337.1999
7. Fazzini E, Fleming J, Fahn S. Cerebrospinal fluid antibodies to coronavirus in patients with Parkinson's disease. Mov Disord. (1992) 7:153–8. doi: 10.1002/mds.870070210
8. Murray RS, Cai G-Y, Zhang J-Y, Soike KF, Cabirac GF. Coronavirus infects and causes demyelination in primate central nervous system. Virology. (1992) 188:274–84. doi: 10.1016/0042-6822(92)90757-G
9. Stewart JN, Mounir S, Talbot PJ. Human coronavirus gene expression in the brains of multiple sclerosis patients. Virology. (1992) 191:502–5. doi: 10.1016/0042-6822(92)90220-J
10. Yeh EA, Collins A, Cohen ME, Duffner PK, Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Am Acad Pediatr. (2004) 113:e73–6. doi: 10.1542/peds.113.1.e73
11. Wu Z, McGoogan JM. Characteristic of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA. (2020) 323:1239–42. doi: 10.1001/jama.2020.2648
12. Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. (2020) 89:594–600. doi: 10.1016/j.bbi.2020.07.037
13. Kaseda ET, Levine AJ. Post-traumatic stress disorder: a differential diagnostic consideration for COVID-19 survivors. Clin. Neuropsychol. (2020)34:7–8. doi: 10.1016/j.bbi.2020.03.028
14. Kong X, Zheng K, Tang M, Kong F, Zhou J, Diao L, et al. Prevalence and Factors associated with depression and anxiety of hospitalized patients with COVID-19. MedRxiv. (2020). doi: 10.1101/2020.03.24.20043075
15. Vannorsdall TD, Brigham E, Fawzy A, Raju S, Gorgone A, Pletnikova A, et al. Rates of cognitive dysfuncion, psychiatric distress, and functional decline after COVID-19. J Acad Consult-Liaison Psychiatry. (2022) 63:133–43. doi: 10.1016/j.jaclp.2021.10.006
16. Ahmed MdZ, Ahmed O, Aibao Z, Hanbin S, Siyu L, Ahmad A. Epidemic of COVID-19 in China and associated psychological problems. Asian J Psychiatr. (2020) 51:102092. doi: 10.1016/j.ajp.2020.102092
17. Gao J, Zheng P, Jia Y, Chen H, Mao Y, Chen S, et al. Mental health problems and social media exposure during COVID-19 outbreak. PLoS ONE. (2020) 15:e0231924. doi: 10.1371/journal.pone.0231924
18. González-Sanguino C, Ausín B, Castellanos MA, Saiz J, López-Gómez A, Ugidos C, et al. Mental health consequences during the initial stage of the 2020 Coronavirus pandemic (COVID-19) in Spain. Brain Behav Immun. (2020) 87:172–6. doi: 10.1016/j.bbi.2020.05.040
19. Huang Y., Zhao N. Generalized anxiety disorder, depressive symptoms and sleep quality during COVID-19 outbreak in China: a web-based cross-sectional survey. Psychiatry Res. (2020) 288:112954. doi: 10.1016/j.psychres.2020.112954
20. Moreira PS, Ferreira S, Couto B, Machado-Sousa M, Fernández M, Raposo-Lima C, et al. Protective elements of mental health status during the COVID-19 Outbreak in the Portuguese population. Int J Environ Res Public Health. (2021) 18:1910. doi: 10.3390/ijerph18041910
21. Zhankg Y, Ma ZF. Impact of the COVID-19 pandemic on mental health and quality of life among local residents in Liaoning Province, China: a cross-sectional study. Int J Environ Res Public Health. (2020) 17:2381. doi: 10.3390/ijerph17072381
22. World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) (2020) [cited 2022 May 10]. Available from: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf
23. Grasselli G, Tonetti T, Protti A, Langer T, Girardis M, Bellani G, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med. (2020) 8:1201–8. doi: 10.1016/S2213-2600(20)30370-2
24. Tingey JL, Bentley JA, Hosey MM. COVID-19: understanding and mitigating trauma in ICU survivors. Psychol Trauma. (2020) 12:S100–4. doi: 10.1037/tra0000884
25. Devlin JW, Weinhouse GL. Earplugs, sleep improvement, and delirium: a noisy relationship. Crit Care Med. (2016) 44:1022–3. doi: 10.1097/CCM.0000000000001734
26. Tainter CR, Levine AR, Quraishi SA, Butterly AD, Stahl DL, Eikermann M, et al. Noise levels in surgical ICUs are consistently above recommended standards. Crit Care Med. (2016) 44:147–52. doi: 10.1097/CCM.0000000000001378
27. Bienvenu OJ, Friedman LA, Colantuoni E, Dinglas VD, Sepulveda KA, Mendez-Tellez P, et al. Psychiatric symptoms after acute respiratory distress syndrome: a 5-year longitudinal study. Intensive Care Med. (2018) 44:38–47. doi: 10.1007/s00134-017-5009-4
28. Neufeld KJ, Leoutsakos JS, Yan H, Lin S, Zabinski JS, Dinglas VD, et al. Fatigue symptoms during the first year following ARDS. Chest. (2020) 158:999–1007. doi: 10.1016/j.chest.2020.03.059
29. Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. (2008) 30:421–34. doi: 10.1016/j.genhosppsych.2008.05.006
30. Nikayin S, Rabiee A, Hashem MD, Huang M, Bienvenu OJ, Turnbull AE, et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry. (2016) 43:23–9. doi: 10.1016/j.genhosppsych.2016.08.005
31. Rabiee A, Nikayin S, Hashem MD, Huang M, Dinglas VD, Bienvenu OJ, et al. Depressive symptoms after critical illness: a systematic review and meta-analysis. Crit Care Med. (2016) 44:1744–53. doi: 10.1097/CCM.0000000000001811
32. Jackson JC, Hart RP, Gordon SM, Hopkins RO, Girard TD, Ely EW. Post-traumatic stress disorder and post-traumatic stress symptoms following critical illness in medical intensive care unit patients: assessing the magnitude of the problem. Crit Care. (2007) 11:R27. doi: 10.1186/cc5707
33. Parker AM, Sricharoenchai T, Raparla S, Schneck KW, Bienvenu OJ, Needham DM. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med. (2015) 43:1121–9. doi: 10.1097/CCM.0000000000000882
34. Wade D, Hardy R, Howell D, Mythen M. Identifying clinical and acute psychological risk factors for PTSD after critical care: a systematic review. Minerva Anestesiol. (2013) 79:944–63.
35. Girard TD, Shintani AK, Jackson JC, Gordon SM, Pun BT, Henderson MS, et al. Risk factors for post-traumatic stress disorder symptoms following critical illness requiring mechanical ventilation: a prospective cohort study. Crit Care. (2007) 11:R28. doi: 10.1186/cc5708
36. Tan J, Andriessen K. The experiences of grief and personal growth in university students: a qualitative study. Int J Environ Res Public Health. (2021) 18:1899. doi: 10.3390/ijerph18041899
37. Marotta-Walters S, Choi J, Shaine MD. Posttraumatic growth among combat veterans: a proposed developmental pathway. Psychol Trauma. (2015) 7:356–63. doi: 10.1037/tra0000030
38. Marziliano A, Tuman M, Moyer A. The relationship between post-traumatic stress and post-traumatic growth in cancer patients and survivors: a systematic review and meta-analysis. Psycho-Oncology. (2020) 29:604–16. doi: 10.1002/pon.5314
39. Sica C, Magni C, Ghisi M, Altoè G, Sighinolfi C, Chiri LR, et al. Coping orientation to problems experienced-Nuova Versione Italiana (COPE-NVI): uno strumento per la misura degli stili di coping. Psicoterapia Cognitiva e Comportamentale. (2008) 14:27–53.
40. Stanislawski K. The coping circumplex model: an integrative model of the structure of coping with stress. Front Psychol. (2019) 10:694. doi: 10.3389/fpsyh.2019.00694
41. Gurvich C, Thomas N, Thomas E, Hudaib A-R, Sood L, Fabiatos K, et al. Coping styles and mental health in response to societal changes during the COVID-19 pandemic. Int J Soc Psychiatry. (2021) 67:540–9. doi: 10.1177/0020764020961790
42. Snider KR, Elhai JD, Gray MJ. Coping style use predicts posttraumatic stress and complicated grief symptom severity among college students reporting a traumatic loss. J Counsel Psychol. (2007) 54:344–50. doi: 10.1037/0022-0167.54.3.344
43. Horwitz AG, Czyz EK, Berona J, King CA. Prospective associations of coping styles with depression and suicide risk among psychiatric emergency patients. Behav Ther. (2018) 49:225–36. doi: 10.1016/j.beth.2017.07.010
44. Li Y, Li J, Yang Z, Zhang J, Dong L, Wang F, et al. Gender differences in anxiety, depression, and nursing needs among isolated coronavirus disease 2019 patients. Front Psychol. (2021) 12:615909. doi: 10.3389/fpsyg.2021.615909
45. Tomasoni D, Bai F, Castoldi R, Barbanotti D, Falcinella C, Mulè G, et al. Anxiety and depression symptoms after virological clearance of COVID-19: a cross-sectional study in Milan, Italy. J Med Virol. (2021) 93:1175–9. doi: 10.1002/jmv.26459
46. Poyraz BC, Poyraz CA, Olgun Y, Gürel Ö, Alkan S, özdemir YE, et al. Psychiatric morbidity and protracted symptoms after COVID-19. Psychiatry Res. (2021) 295:113604. doi: 10.1016/j.psychres.2020.113604
47. Vincent A, Beck K, Becker C, Zumbrunn S, Ramin-Wright M, Urben T, et al. Psychological burden in patients with COVID-19 and their relatives 90 days after hospitalization: a prospective observational cohort study. J Psychosom Res. (2021) 147:110526. doi: 10.1016/j.jpsychores.2021.110526
48. Zhang H, Xie F, Yang B, Zhao F, Wang C, Chen X. Psychological experience of COVID-19 patients: a system review and qualitative meta-synthesis. Am J Infect Control. (2022) 50:809–819. doi: 10.1016/j.ajic.2022.01.023
49. Xiao X, Yang X, Zheng W, Wang B, Fu L, Luo D, et al. Depression, anxiey and post-traumatic growth among COVID-19 survivors six-months after discharge. Eur J Psychotraumatol. (2022) 13:1. doi: 10.1080/20008198.2022.2055294
50. Yan S, Yang J, Ye M, Chen S, Xie C, Huang J, et al. Post-traumatic growth and related influencing factors in discharged COVID-19 patients: a cross-sectional study. Front Psychol. (2021) 12:658307. doi: 10.3389/fpsyg.2021.658307
51. Kessler RC, Barker PR, Colpe LJ, Epstein JF, Gfroerer JC, Hiripi E, et al. Screening for serious mental illness in the general population. Arch Gen Psychiatry. (2003) 60:184–9. doi: 10.1001/archpsyc.60.2.184
52. Carrà G, Sciarini P, Segagni-Lusignani G, Clerici M, Montomoli C, Kessler RC. Do they actually work across borders? Evaluation of two measures of psychological distress as screening instruments in a non Ango-Saxon country. Eur Psychiatry. (2010) 26:122–7. doi: 10.1016/j.eurpsy.2010.04.008
53. Andrews G, Slade T. Interpreting scores on the Kessler psychological distress scale (K10). Aust N Z J Public health. (2001) 25:494–7. doi: 10.1111/j.1467-842x.2001.tb00310.x
54. Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In Spacapan S, Oskamp S, editors. The Social Psychology of Health. Newbury Park, CA: Sage Publications, Inc. (PSS) (1988). p. 31–67.
55. Tedeschi RG, Calhoun LG. The posttraumatic growth inventory: measuring the positive legacy of trauma. J Trauma Stress. (1996) 9:455–71. doi: 10.1007/BF02103658
56. Prati G, Pietrantoni L. Italian adaptation and confirmatory factor analysis of the full and the short form of the posttraumatic growth inventory. J Loss Trauma. (2014) 19:12–22. doi: 10.1080/15325024.2012.734203
57. Weiss DS. The impact of event scale: revised. In: Wilson JP, So-kum Tang C, editors. Cross-Cultural Assessment of Psychological Trauma and PTSD. Boston, MA: Springer Editor (2007). p. 219–38.
59. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Washington, DC: American Psychiatric Pub. (2013). 1168 p.
60. Lazarus R, Folkma S. Stress, Appraisal, and Coping. New York, NY: Springer Publishing Company (1984). 462 p.
61. Salari N, Hosseinian-Far A, Jalali R, Vaisi-Raygani A, Rasoulpoor S, Mohammadi M, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. (2020) 16:57. doi: 10.1186/s12992-020-00589-w
62. Bendau A, Kunas SL, Wyka S, Petzold MB, Plag J, Asselmann E, et al. Longitudinal changes of anxiety and depressive symptoms during the COVID-19 pandemic in Germany: the role of pre-existing anxiety, depressive, and other mental disorders. J Anxiety Disord. (2021) 79:102377. doi: 10.1016/j.janxdis.2021.102377
63. Gallagher MW, Zvolensky MJ, Long LJ, Rogers AH, Garey L. The impact of Covid-19 experiences and associated stress on anxiety, depression, and functional impairment in American adults. Cogn Ther Res. (2020) 44:1043–51. doi: 10.1007/s10608-020-10143-y
64. Parker C, Shalev D, Hsu I, Shenoy A, Cheung S, Nash S, et al. depression, anxiety, and acute stress disorder among patients hospitalized with COVID-19: a prospective cohort study. J Acad Consult Liaison Psychiatry. (2021) 62:211–9. doi: 10.1016/j.psym.2020.10.001
65. Kim JW, Stewart R, Kang SJ, Jung SI, Kim SW, Kim JM. Telephone based interventions for psychological problems in hospital isolated patients with COVID-19. Clin Psychopharmacol Neurosci. (2020) 18:616–20. doi: 10.9758/cpn.2020.18.4.616
66. Saidi I, Koumeka PP, Ait Batahar S, Amro L. Factors associated with anxiety and depression among patients with Covid-19. Respir Med. (2021) 186:106512. doi: 10.1016/j.rmed.2021.106512
67. Deng J, Zhou F, Hou W, Silver Z, Wong CY, Chang O, et al. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: a meta-analysis Ann N Y Acad Sci. (2021) 1486:90–111. doi: 10.1111/nyas.14506
68. Moayed MS, Vahedian-Azimi A, Mirmomeni G, Rahimi-Bashar F, Goharimoghadam K, Pourhoseingholi MA, et al. Depression, anxiety, and stress among patients with COVID-19: a cross-sectional study. Adv Exp Med Biol. (2021) 1321:229–36. doi: 10.1007/978-3-030-59261-5_19
69. Chahraoui K, Laurent A, Bioy A, Quenot J-P. Psychological experience of patients 3 months after a stay in the intensive care unit: a descriptive and qualitative study. J Crit Care. (2015) 30:59–605. doi: 10.1016/j.jcrc.2015.02.016
70. Nagarajan R, Krishnamoorthy Y, Basavarachar V, Dakshinamoorthy R. Prevalence of post-traumatic stress disorder among survivors of severe COVID-19 infections: a systematic review and meta-analysis. J Affect Disord. (2022) 299:52–9. doi: 10.1016/j.jad.2021.11.040
71. Na PJ, Tsai J, Southwick SM, Pietrzak RH. Factors associated with post-traumatic growth in response to the COVID-19 pandemic: results from a national sample of U.S. military veterans. Soc Sci Med. (2021) 289:114409. doi: 10.1016/j.socscimed.2021.114409
72. Maia A, Sousa B, Correia-Santos P, Morgado D. Posttraumatic stress symptoms and posttraumatic growth in a community sample exposed to stressful events: a (not so) curvilinear relationship. Traumatology. (2021) 28:98–108 doi: 10.1037/trm0000314
73. Tamiolaki A, Kalaitzaki AE. “That which does not kill us, makes us stronger”: COVID-19 and posttraumatic growth. Psychiatry Res. (2020) 289:113044. doi: 10.1016/j.psychres.2020.113044
74. Tedeschi RG, Calhoun LG. Posttraumatic growth: conceptual foundations and empirical evidence. Psychol. Inquiry. (2004) 15:1–18. doi: 10.1207/s15327965pli1501_01
75. Gökalp SZ, Koç H, Özteke Kozan HI. Coping and post-traumatic growth among COVID-19 patients: a qualitative study. J Adult Dev. (2022) 1:12. doi: 10.1007/s10804-022-09398-4
76. Callard F, Perego E. How and why patients made Long Covid. Soc Sci Med. (2021) 268:113426. doi: 10.1016/j.socscimed.2020.113426
77. Raveendran AV, Jayadevan R, Sashidhran S. Long COVID: an overview. diabetes & metabolic syndrome. Clin Res Rev. (2021) 15:869–75. doi: 10.1016/j.dsx.2021.04.007
78. Hyun S, Wong GTF, Levy-Carrick NG, Charmaraman L, Cozier Y, Yip T, et al. Psychosocial correlates of posttraumatic growth among U.S. young adults during the COVID-19 pandemic. Psychiatry Res. (2021) 302:114035 doi: 10.1016/j.psychres.2021.114035
79. Stallard P, Pereira A, Barros L. Post-traumatic growth during the COVID-19 pandemic in carers of children in Portugal and the UK: cross-sectional online survey. BJPsych Open. (2021) 7:E37. doi: 10.1192/bjo.2021.1
80. Bernard M, Poncin E, Althaus B, Borasio GD. Posttraumatic growth in palliative care patients and its associations with psychological distress and quality of life. Palliat Support Care. (2022) 1:8. doi: 10.1017/S1478951521002066
81. Ager NF. Quality of life, and spiritual well-being in outpatients undergoing bone marrow transplantation: a pilot study. Oncol Nurs Forum. (2016) 43:772–80. doi: 10.1188/16.ONF.772.780
82. Holtmaat K, Van der Spek N, Cujipers P, Leemans CR, Verdonck-de Leeuw IM. Posttraumatic growth among head and neck cancer survivors with psychological distress. Psycho-Oncology. (2017) 26:96–101. doi: 10.1002/pon.4106
83. Li N, Fan L, Wang Y, Wang J, Huang Y. Risk factors of psychological distress during the COVID-19 pandemic: the roles of coping style and emotional regulation. J Affect Disord. (2022) 299:326–34. doi: 10.1016/j.jad.2021.12.026
84. Peters J, Bellet BW, Jones PJ, Wu GWY, Wang L, McNally RJ. Posttraumatic stress or posttraumatic growth? Using network analysis to explore the relationships between coping styles and trauma outcomes. J Anxiety Disord. (2021) 78:102359. doi: 10.1016/j.janxdis.2021.102359
85. Whealin JM, Pitts B, Tsai J, Rivera C, Fogle BM, Southwick SM, et al. Dynamic interplay between PTSD symptoms and posttraumatic growth in older military veterans. J Affect Disord. (2020) 269:185–91. doi: 10.1016/j.jad.2020.03.020
86. Wild ND, Paivio SC. Psychological adjustment, coping, and emotion regulation as predictors of posttraumatic growth. J Aggress Maltreat Trauma. (2004) 8:97–122. doi: 10.1300/j146v08n04_05
87. Xie C-S, Kim Y. Post-traumatic growth during COVID-19: the role of perceived social support, personality, and coping strategies. Healthcare. (2022) 10:224. doi: 10.3390/healthcare10020224
88. Matalon N, Dorman-Ilan S, Hasson-Ohayon I, Hertz-Palmor N, Shani S, Basel D, et al. Trajectories of post-traumatic stress symptoms, anxiety, and depression in hospitalized COVID-19 patients: a one-month follow-up. J Psychosomatic Res. (2021) 143:110399. doi: 10.1016/j.jpsychores.2021.110399
89. Shanbehzadeh S, Tavahomi M, Zanjari N, Ebrahimi-Takamjani I, Amiri-Arimi S. Physical and mental health complications post-COVID-19: scoping review. J Psychosomatic Res. (2021) 147:110525. doi: 10.1016/j.jpsychores.2021.110525
90. Halpin SJ, McIvor C, Whyatt G, Adams A, Harvey O, McLean L, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. (2021) 93:1013–22. doi: 10.1002/jmv.26368
91. Hurissi E, Abu-jabir E, Mohammed A, Mahnashi M, Alharbi S, Alharbi A, et al. Assessment of new-onset depression and anxiety associated with COVID-19. Middle East Curr Psychiatry. (2021) 28:1–6. doi: 10.1186/s43045-021-00112-w
Keywords: SARS-CoV 2, COVID-19, clinical psychology, K10, perceived stress, state anxiety, intensive care unit
Citation: Carola V, Vincenzo C, Morale C, Pelli M, Rocco M and Nicolais G (2022) Psychological health in COVID-19 patients after discharge from an intensive care unit. Front. Public Health 10:951136. doi: 10.3389/fpubh.2022.951136
Received: 23 May 2022; Accepted: 21 July 2022;
Published: 12 August 2022.
Edited by:
Guohua Zhang, Wenzhou Medical University, ChinaReviewed by:
Mariano Supino, Istituto Nazionale di Geofisica e Vulcanologia (INGV), ItalyCopyright © 2022 Carola, Vincenzo, Morale, Pelli, Rocco and Nicolais. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valeria Carola, dmFsZXJpYS5jYXJvbGFAdW5pcm9tYTEuaXQ=; Giampaolo Nicolais, Z2lhbXBhb2xvLm5pY29sYWlzQHVuaXJvbWExLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.