- 1Public Health Emergency Center, Chinese Center for Disease Control and Prevention, Beijing, China
- 2State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, School of Public Health, Xiamen University, Fujian, China
- 3School of Basic Medical Sciences, Cheeloo College of Medicine, Shandong University, Jinan, China
Background: The epidemiological characteristics and transmissibility of Coronavirus Disease 2019 (COVID-19) may undergo changes due to the mutation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) strains. The purpose of this study is to compare the differences in the outbreaks of the different strains with regards to aspects such as epidemiological characteristics, transmissibility, and difficulties in prevention and control.
Methods: COVID-19 data from outbreaks of pre-Delta strains, the Delta variant and Omicron variant, were obtained from the Chinese Center for Disease Control and Prevention (CDC). Case data were collected from China's direct-reporting system, and the data concerning outbreaks were collected by on-site epidemiological investigators and collated by the authors of this paper. Indicators such as the effective reproduction number (Reff), time-dependent reproduction number (Rt), rate of decrease in transmissibility (RDT), and duration from the illness onset date to the diagnosed date (DID)/reported date (DIR) were used to compare differences in transmissibility between pre-Delta strains, Delta variants and Omicron variants. Non-parametric tests (namely the Kruskal-Wallis H and Mean-Whitney U tests) were used to compare differences in epidemiological characteristics and transmissibility between outbreaks of different strains. P < 0.05 indicated that the difference was statistically significant.
Results: Mainland China has maintained a “dynamic zero-out strategy” since the first case was reported, and clusters of outbreaks have occurred intermittently. The strains causing outbreaks in mainland China have gone through three stages: the outbreak of pre-Delta strains, the outbreak of the Delta variant, and outbreaks involving the superposition of Delta and Omicron variant strains. Each outbreak of pre-Delta strains went through two stages: a rising stage and a falling stage, Each outbreak of the Delta variant and Omicron variant went through three stages: a rising stage, a platform stage and a falling stage. The maximum Reff value of Omicron variant outbreaks was highest (median: 6.7; ranged from 5.3 to 8.0) and the differences were statistically significant. The RDT value of outbreaks involving pre-Delta strains was smallest (median: 91.4%; [IQR]: 87.30–94.27%), and the differences were statistically significant. The DID and DIR for all strains was mostly in a range of 0–2 days, with more than 75%. The range of duration for outbreaks of pre-Delta strains was the largest (median: 20 days, ranging from 1 to 61 days), and the differences were statistically significant.
Conclusion: With the evolution of the virus, the transmissibility of the variants has increased. The transmissibility of the Omicron variant is higher than that of both the pre-Delta strains and the Delta variant, and is more difficult to suppress. These findings provide us with get a more clear and precise picture of the transmissibility of the different variants in the real world, in accordance with the findings of previous studies. Reff is more suitable than Rt for assessing the transmissibility of the disease during an epidemic outbreak.
Introduction
The Corona Virus Disease 2019 (COVID-19) has caused serious strains on the worldwide public health systems since the outbreak in late 2019 (1, 2). Over time, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has continued to evolve and mutate, producing a variety of SARS-CoV-2 variants (3–5). The Delta variant (B.1.617.2) was first reported in India in December 2020 and became the main variant in most parts of the world in the second half of 2021. A new Variant of Concern (VOC), the Omicron variant (B.1.1.529), was reported on November 26, 2021 (6), and quickly became the dominant global variant. The continuing mutation of SARS-CoV-2 strains resulted in changes in the epidemiological characteristics and transmissibility of Coronavirus Disease 2019 (COVID-19). Studies have found that the infectivity rate of the Delta variant is 97 or 100% higher than that of pre-Delta strains (7), and the rapid spread of the Omicron variant in Gauteng Province, South Africa confirmed its high infectivity rate (8).
The value of the Reproductive number (R) is a useful indicator of the status of an outbreak (9). A value of R > 1 reflects active community transmission. The basic reproduction number (R0) refers to the estimated number of secondary infections caused by an infected individual in a susceptible population of susceptible people during the infectious cycle. This definition assumes a complete lack of immunity in all individuals, absent any external intervention measures, such as isolation, vaccination, etc. (10). R0 applies to a situation of maximal transmissibility of the disease. When the population is not completely susceptible, or intervention measures are taken, the transmissibility of the disease should be measured by the effective reproduction number (Reff). In addition, there is an indicator derived from Reff, namely the time-dependent reproduction number (Rt), which can be regarded as time-varying estimate of Reff (11) reflecting the instantaneous transmissibility of the case at a certain point in time.
The Delta variant is characterized by stronger infectivity, higher viral load, a shorter incubation period, and rapid onset. Studies have shown that the R0 of the Delta variant is about 7.0, absent any intervention (12), which is much higher than the 2.2–3.77 range seen in the early stages of the epidemics (13–15). Data from South Africa indicates that the positivity rate of the Omicron variant increased from 1 to 30% within 2 weeks, and subsequent cases also increased exponentially (8). At present, with regards to the local mainland China outbreaks, there is a lack of comprehensive research utilizing first-hand incidence data, nor is there any comparative analysis of the epidemiological characteristics and transmissibility of the different strains. The purpose of this study is to review the aggregated data concerning all outbreaks in China since the first Wuhan-related wave, using multiple indicators to clarify the differences in epidemiological characteristics and transmissibility of different strains. The research also compares Reff and Rt, concluding that Reff is more suitable than Rt in evaluating the transmissibility of the disease. The goal is to develop more effective prevention and control strategies, and provide better assessment of the outbreak trajectories of other variants that may occur in the future.
Methods
Data collection
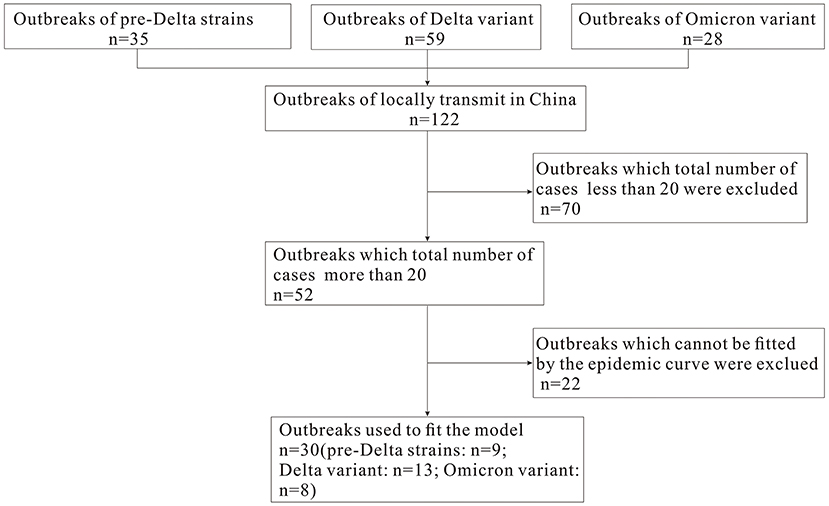
In this study, we define an “outbreak” as an event that involving more than one local case, and where the virus strain is confirmed through epidemiological investigation and genome sequencing to be unrelated to previous outbreaks in other regions. The COVID-19 data concerning pre-Delta strains, and Delta and Omicron variants of SARS-CoV-2 from March 28, 2020 to March 1, 2022 were collected to analyze the epidemiological characteristics of COVID-19. Based on epidemic curves and the total number of cases during each outbreak (excluding outbreaks that totaled <20 cases, and which did not fit the epidemic curve), we selected nine pre-Delta strain outbreaks, 13 Delta variant outbreaks and eight Omicron variant outbreaks in order to calculate the Reff and Rt values (Figure 1). Daily incidence data for each outbreak were gathered from the Chinese Center for Disease Control and Prevention (CDC). Case data came from China's direct-reporting system network, and outbreak data was collected by the on-site epidemiological investigators and collated by the authors of this paper. The demographics were collected from the Statistical Yearbook of the locations where the outbreak occurred.
Estimating the reproductive number (R)
Estimating the Reff
In scenarios where there are interventions during the prediction of outbreak trends, the Reff can often replace the R0 as an indicator of virus transmissibility (16).
The equation is as follows:
The susceptible-exposed-recovered (SEIR) propagation dynamics model was used for the calculation of Reff. In the SEIR model, the population is divided into four categories: susceptible (S), exposed (E), infectious (I), and recovered (R).
The equations of the model are as follows:
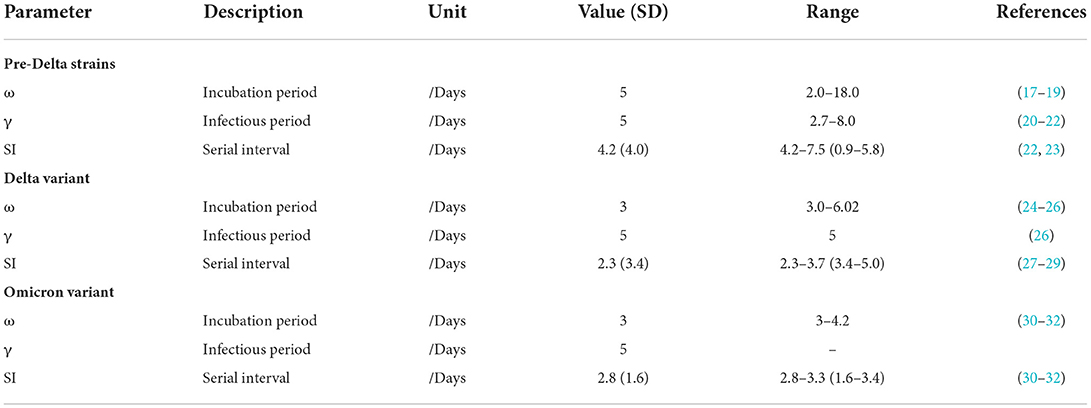
There are four parameters (β, ω, γ, f) in the SEIR model. The parameter values of different strains are shown in Table 1. The total population was set based upon the statistical yearbook data of the location where the outbreak occurred.
Estimating the Rt
Rt and its 95% confidence interval were estimated based on a 5-day moving average using a Bayesian framework based on a time series of the number of new cases of COVID-19 and serial intervals (SI) (Table 1) (10). In Supplementary Figures S1–S3, the gray horizontal dotted line indicates Rt = 1, below which sustained transmission is unlikely as long as intervention measures are maintained.
In this study, we fitted the epidemic curve of the COVID-19 according to the epidemic trend of the disease and the implementation nodes of the prevention and control measures. When comparing the Reff/Rt of different strains, we selected the Reff/Rt value fitted to the rising stage of each epidemic for analysis, because the Reff/Rt at this stage are generally represent the Reff/Rt prior to the implementation of various prevention and control measures (except vaccination), which are the closest to R0, and thus can better restore the transmissibility of the virus itself.
The rate of decrease in transmissibility
Our study also evaluated the effectiveness of outbreak prevention and control measures in each outbreak through the decline range of Reff. Prior to any public health and social measures (PHSMs), the epidemic curve will manifest in the natural rising stage. During this time, the Reff is denoted as Reff1. When PHSMs are implemented to control the outbreak situation, the daily number of new cases decreases, and the Reff is denoted as Reff2 during the falling stage. If the outbreak is difficult to control and the epidemic curve enters the rising period followed by the plateau period and then the falling period, the Reff of the outbreak status in the plateau period is denoted as . The rate of decrease in transmissibility (RDT) is calculated to analyze the decline of transmissibility of each outbreak after effective intervention.
Statistics analysis
The calibration between incidence data and the SEIR model was performed using the least squares method. The coefficient of determination (R2) was used to evaluate the goodness-of-fit. Berkeley Madonna ver. 8.3.18 (developed by Robert Macey and George Oster of the University of California at Berkeley, CA, USA) was used for parameter fitting and model simulation. The “EpiEstim” package in the R software (version 3.6.0, R Core Team, Austria) was used for Rt estimated. SPSS 21.0 (IBM Corp, Armonk, NY, USA) was used for the goodness-of-fit by calculating the coefficient of determination (R2). GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA) was used for figure production. Non-parametric tests (namely Kruskal-Wallis H and Mean-Whitney U-tests) were used to compare differences in the epidemiological characteristics and transmissibility between outbreaks of pre-Delta strains, the Delta variant and the Omicron variant. We calculated the test statistics (H value/U value), determined the P-value, and then made statistical inference, P < 0.05 indicating that the difference was statistically significant.
Results
Epidemiological characteristics of COVID-19 transmitted locally in China
Mainland China has maintained a “dynamic zero-out strategy” since the first case was reported, and during subsequent intermittent clusters of outbreaks. After epidemiological investigation and genome sequencing, the virus strains involved in each outbreak were confirmed to be unrelated to previous outbreaks. The strains causing the outbreaks in mainland China have manifested in three stages: the outbreak of pre-Delta strains, the outbreak of the Delta variant, and the superposition of outbreaks of Delta and Omicron variant strains. During the outbreak period of each strain, there were peaks in the epidemic curve. On May 21, 2021, Guangzhou City reported the first outbreak of the Delta variant in China, and subsequently Delta variant outbreaks occurred in many cities. On December 15, 2021, Tianjin reported the first outbreak of the Omicron variant in China. Since then, domestic outbreaks have exhibited characteristics of multi-point and frequent occurrence. Outbreaks of Delta and Omicron variants have occurred in superposition, and the number of locally confirmed cases has been at a high level. Doses of COVID-19 vaccine in mainland China have gradually increased (including the booster vaccine) (Figure 2).
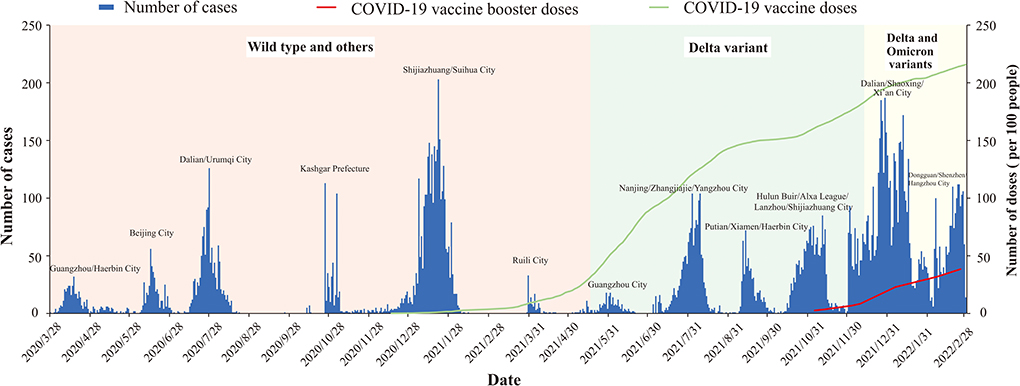
Figure 2. Epidemic curve COVID-19 transmitted locally in China from March 28, 2020 to March 1, 2022.
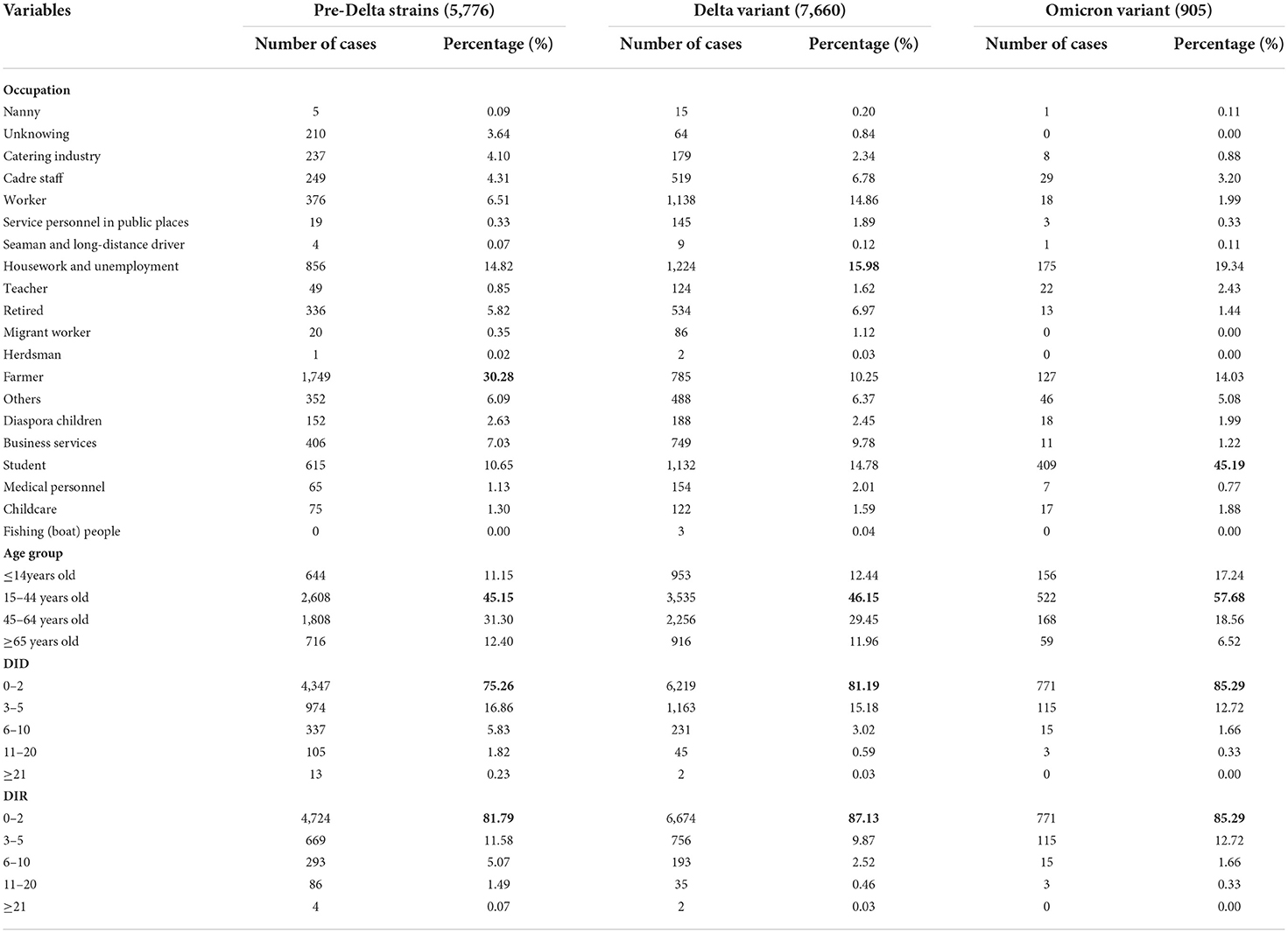
In Table 2, the numbers of cases for each strains is a summation of all outbreaks of this strain. Individuals infected with pre-Delta strains, Delta variants and Omicron variants were predominantly farmers, housekeepers and the unemployed, and students, respectively; Individuals infected with pre-Delta strains, Delta variants and Omicron variants were mainly in the age group of 15–44 years of age (Table 2).
Curve fitting results
Based on the epidemic curve and total number of cases during each outbreak, we selected outbreaks of nine pre-Delta strains, 13 Delta variant outbreaks and eight Omicron variant outbreaks for model fitting. It can be seen from Supplementary Figures S1–S3 that the model fits well with the outbreak data (R2 are >0.40 and the P-values are <0.05).
As can be seen from Supplementary Figures S1–S3. Each outbreak of the pre-Delta strains went through two stages: a rising stage and a falling stage; each outbreak of Delta and Omicron variants went through three stages: a rising stage, a platform stage and a falling stage. The Reff value of each outbreak of pre-Delta strains was below 4, and the Reff value of each outbreak of Delta and Omicron variants was above 4. At the beginning of each outbreak, the Reff and Rt values were >1. After strong intervention measures were applied, both the Reff and Rt values would drop lower than 1, suggesting that outbreaks were under control. However, the timing of the values <1 were significantly different in many outbreaks.
Comparison of transmissibility between different strains
As can be seen from Figure 3A, the maximum Reff values of outbreaks of nine pre-Delta strains ranged from 2.9 to 3.8, with a median of 3.5; the maximum Reff values of 13 Delta variant outbreaks ranged from 4.2 to 6.8, with a median of 5.5; and the maximum Reff values of eight Omicron variant outbreaks ranged from 5.3 to 8.0, with a median of 6.7. The median of the maximum Reff value of Omicron variant outbreaks was highest. The differences between any two groups were statistically significant (H = 20.667, P < 0.05).
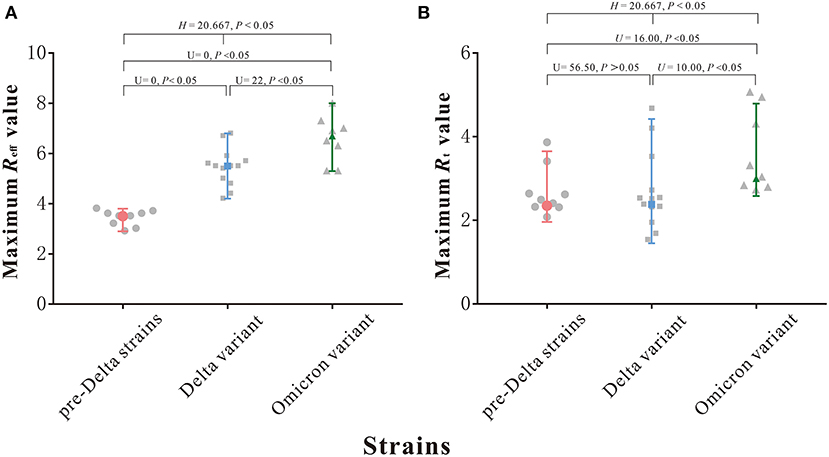
Figure 3. Maximum Reff and Rt value of 9 pre-Delta strains outbreaks and 13 Delta variant outbreaks and 8 Omicron variant outbreaks. (A) Maximum Reff value. (B) Maximum Rt value.
As can be seen from Figure 3B, the maximum Rt values of outbreaks of nine pre-Delta strains ranged from 1.96 to 3.65, with a median of 2.35; the maximum Rt values of 13 Delta variant outbreaks ranged from 1.45 to 4.42, with a median of 2.38; and the maximum Rt values of eight Omicron variant outbreaks ranged from 2.58 to 4.79, with a median of 3.00. The median of the maximum Rt value of Omicron variant outbreaks was highest, but the differences between three groups were statistically significant (H = 8.461, P > 0.05), but though the differences between Delta variant and pre-Delta strains was not statistically significant (U = 56.50, P > 0.05).
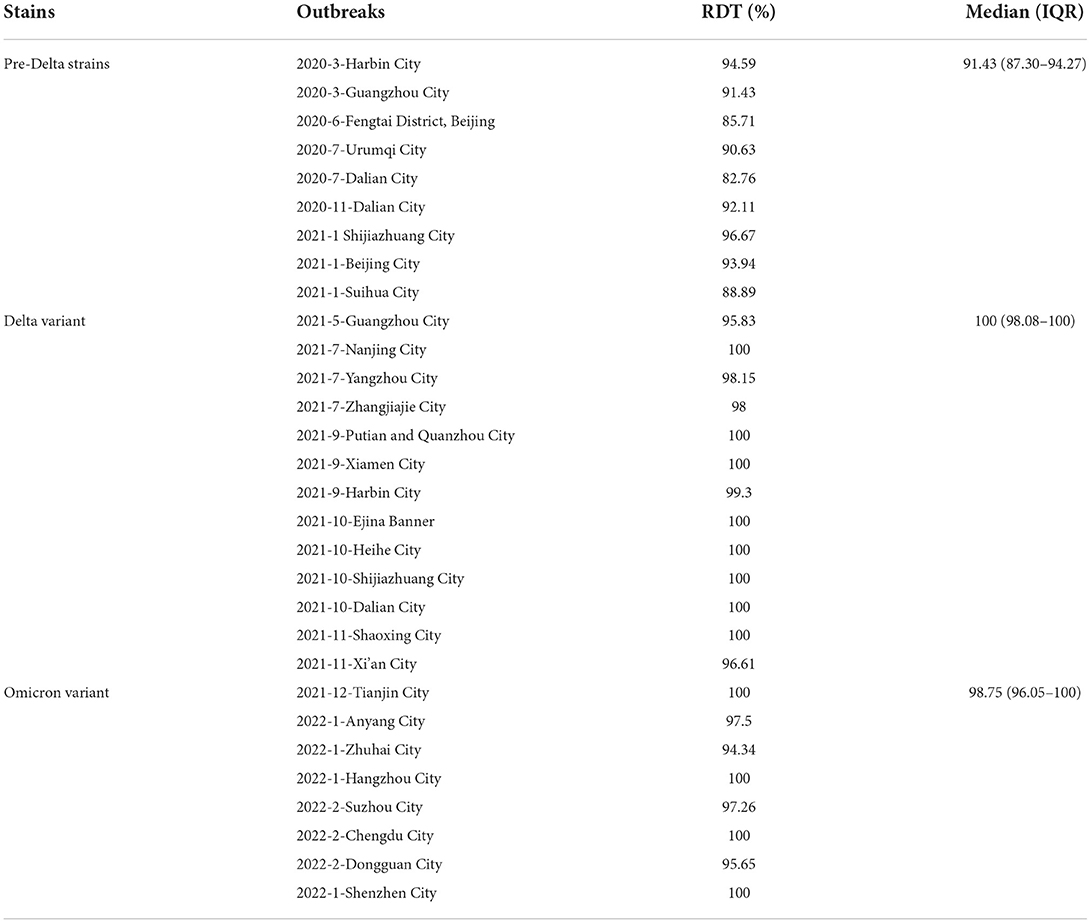
The median of RDT of outbreaks involving pre-Delta strains was 91.4% (inter-quartile range [IQR]: 87.30–94.27%); the RDT median of Delta variant outbreaks was 100% (inter-quartile range [IQR]: 98.08–100%); and the median of RDT of Omicron variant outbreaks was 98.75% (inter-quartile range [IQR]: 96.05–100%). The RDT value of outbreaks due to pre-Delta strains was the smallest (Table 3), and the differences between any two groups were statistically significant (H = 17.998, P < 0.05).
Duration of the illness from onset date to the diagnosis date (DID)/reported date (DIR) of outbreaks
The DID and DIR of all outbreaks included in this study obey a gamma distribution, with durations basically concentrated in a range of 0–5 days. The median DIDof outbreaks due to pre-Delta strains, Delta variant and Omicron variant were all 1 day (inter-quartile range [IQR]: 1–2 days) (Figures 4A,C,E); The median DIR of outbreaks of pre-Delta strains was 0 days (inter-quartile range [IQR]: 0–2 days); The median DIR of Delta variant outbreaks was 1 day (inter-quartile range [IQR]: 0–1 days); The median DIR of Omicron variant outbreaks was 1 day (inter-quartile range [IQR]: 1–2 days) (Figures 4B,D,F). The DID and DIR of all outbreaks were in a range of 0–2 days, accounting for more than 75% (Table 2), which indicated that PHSMs applied were equally effective in case tracing and management. This result could also help to eliminate confounding factors that influenced the transmissibility of different variants.
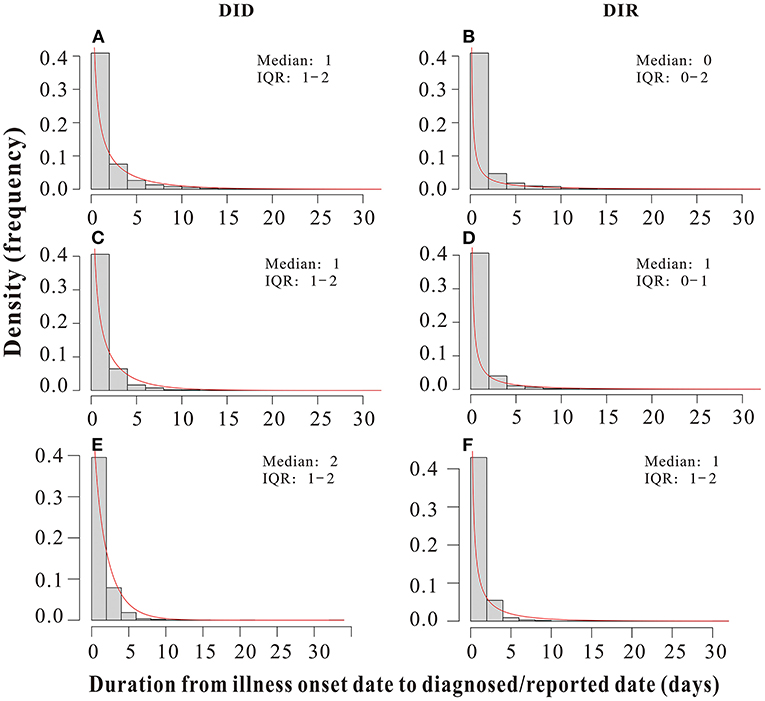
Figure 4. Duration from the illness onset date to the diagnosed date (DID)/reported date (DIR) of all pre-Delta strains, Delta variant and Omicron variant outbreaks in China from March 28, 2020 to March 1, 2022. (A,C,E) DID of all pre-Delta strains, Delta variant and Omicron variant outbreaks, respectively. (B,D,F) DIR of all pre-Delta strains, Delta variant and Omicron variant outbreaks, respectively.
Comparison of duration and total number of cases between outbreaks of different strains
Results showed that the duration of outbreaks of pre-Delta strains ranged from 1 to 61 days (median 20 days); duration of outbreaks of the Delta variant ranged from 1 to 47 days (median 16 days); and the duration of outbreaks of the Omicron variant ranged from 1 to 23 days (median 4 days). The range of the duration of outbreaks of pre-Delta strains was largest, and the differences between any two groups were statistically significant (H = 26.745, P < 0.05) (Figure 5A).
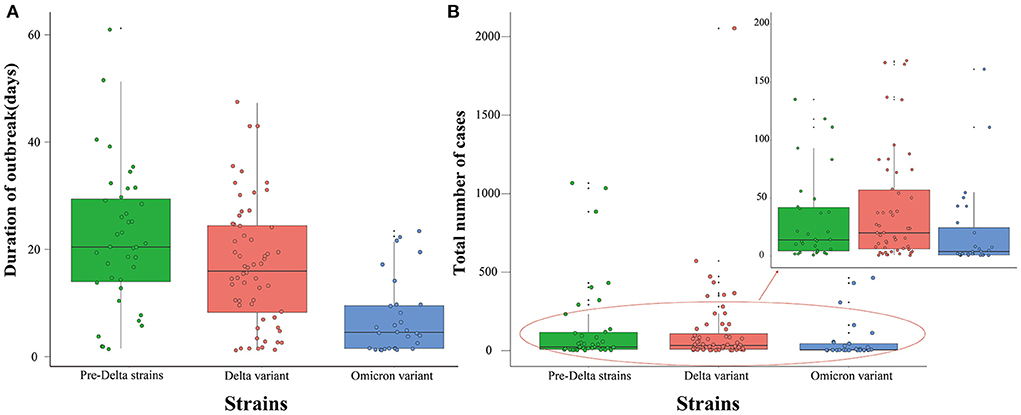
Figure 5. Duration of outbreak and total number of cases of all pre-Delta strains, Delta variant and Omicron variant outbreaks in China from March 28, 2020 to March 1, 2022. (A) Duration of outbreak. (B) Total number of cases.
The total number of cases during outbreaks of pre-Delta strains ranged from 1 to 1,066 persons (median 21 persons); the total number of cases during Delta variant outbreaks ranged from 1 to 2,052 persons (median 31 persons); and the total number of cases during Omicron variant outbreaks ranged from 1 to 462 persons (median 5 persons). The range of the total number of cases during Omicron variant outbreaks was smallest, and the differences between any two groups were statistically significant (H = 9.236, P < 0.05) (Figure 5B).
Discussion
This study is a large-scale sample size study with a long time span, we collected the data of all aggregated outbreaks in China since the first Wuhan-related wave. This also is the first comparative study in China to systematically review all local outbreaks in the country, and to analyze the differences in the characteristics and transmissibility of outbreaks involving different strains across multiple indicators using first-hand incidence data. The results will enable global scholars to deepen their understanding of Delta and Omicron variants, and will also provide theoretical references for the prevention and control of other possible future variants.
The WHO has reported several VOCs (Variants of Concern) (8, 33, 34). Since April 2021, the SARS-CoV-2 Delta variant has become the major variant worldwide, and the global prevalence of the Delta variant is also related to its enhanced interpersonal transmissibility (35). Global reports have documented rapid transmission characteristics of the Delta variant in different countries, such as Korea and France (16, 36). One study also found that the R0 of the Delta strain was 5.08 (37), which was similar to our results, our study showed that the median Reff of Delta variants was 5.5. Currently the Omicron variant is the most prevalent strain in global outbreaks, and shows greater transmissibility than the Delta variant (38). The Omicron variant is estimated to be 100.3% more transmissible than the ancestral SARS-CoV-2 and 36.5% more transmissible than the Delta variant (8), A modeling study in South Africa showed that the mean Reff of the Omicron strain was 7.57 (39), which was higher than our results, this may be related to the fact that this research was carried out during the early period of the Omicron outbreak in South Africa, which was also the early stage of the Omicron variant, and the transmissibility may be different from that of the later period. The Reff value of Delta and Omicron outbreaks was >4, which indicates that the transmissibility of the Delta and Omicron variants has increased, resulting in more breakthrough infections. After a proportion of the population has received the vaccine, one individual can still infect more than 4 people, and the Reff of the Omicron variant can be as high as 8 in this study, which suggests that the transmissibility of the strains increases as the virus mutates, a finding that is in accordance with the results of many studies (40–43).
Many studies have found that the average incubation period, average intergenerational interval, and average sequence interval of the Delta variant are significantly shorter than those of pre-Delta strains. The Delta variant is capable of spreading 4 generations within 10 days, with the fastest intergenerational transmission occurring in >24 h (24, 27). As for the Omicron variant, its estimated mean serial interval of 2.9 days was shorter than that observed for pre-Delta strains and the Delta variant, as found in studies conducted in South Korea (28, 44). The Omicron variant also has a growth advantage over the Delta variant due to its higher transmissibility, immunity evasion, and shorter serial interval (30). In this study, the epidemic curve and curve fitting results seen in outbreaks of the Delta and Omicron variants showed that there is a plateau stage for each epidemic curve, which indicates that Delta and Omicron outbreaks are more difficult to suppress than those of pre-Delta strains. After PHSMs were adopted, the disease continued to spread for about one incubation period. These findings provide us with a clearer and more precise picture of the transmissibility of different variants in the real world.
When both Reff and Rt values are <1, the outbreak is less likely to spread continuously. We found that the values of Reff and Rt fell below 1 following the rising stage of the outbreak. This decline in transmissibility may be related to many factors. Even though the vaccination rate in China is increasing, some studies have found that the vaccines have limited effect on the transmissibility of the virus. A UK community-based study suggests that vaccination alone is not sufficient to prevent all transmission of the Delta variant in the household setting, where exposure is close and prolonged (45). A cohort study also highlights that the effectiveness of vaccines in reducing transmission is minimal in the context of Delta variant circulation (46). Indeed, preclinical studies of adenovirus and mRNA candidate vaccines demonstrated persistent virus in nasal swabs despite preventing COVID-19. This suggests that systemically vaccinated patients, while asymptomatic, may still be become infected and transmit live virus from the upper airway (47).The scope and duration of outbreaks are largely influenced by PHSMs, and an outbreak can be controlled if the isolation scheme is implemented in time, and strict public health strategies are adopted (48). These results indicate that success in suppressing small-scale outbreaks can be mostly attributed to the PHSMs implemented by the Chinese government.
The discovery methods and timing of each outbreak are different, resulting in different entry points for controlling the spread of the epidemic. However, the outbreaks of Delta and Omicron strains have been successfully controlled after the platform period following an incubation period, which shows the effectiveness of our control measures. Our results also show that the RDT of the Delta and Omicron variants was higher than that of pre-Delta strains. The duration of Omicron outbreaks was significantly shorter than those of pre-Delta strains or the Delta variant in this study, since both pre-Delta and Delta strains have been superseded all around the world, Omicron is picking up the pace. We also found that the DID and DIR of the Delta and Omicron variants were most often 0–2 days. This can be attributed to China's increasing experience in fighting the virus. Even in face of variants with increasing transmissibility, China continues to implement the “Dynamic Clearing” policy to suppress the spread of the outbreak. Comparisons between virus variants especially involving different outbreaks, should be taken with caution, since these outbreaks have a base-line differences in the diagnostic methods, which already differ according to the time of the outbreaks and the availability and accuracy of the technology (49). However, in our study, the DID and DIR of all the strains was most often 0–2 days, indicating that base-line differences in diagnostic methods of these outbreaks had no impact on the results.
The R0 value is usually used in public health research to measure the transmissibility of the disease. But in reality, the R0 value is not directly available due to the fact that not all individuals in the population are fully susceptible, and a percentage of the population has been vaccinated, in addition to the imposition of human intervention, the R0 value is therefore not directly available. Thus, the Reff value is then generated to measure the disease transmissibility under interventions, and the Rt value is derived (50, 51). In predictions of the course of the COVID-19 epidemic, we usually judge the change in transmissibility at each stage of the outbreak by observing the rise and fall of the Reff and Rt values, where the Reff and Rt values = 1. At the key point when the Reff and Rt values are <1, this indicates that transmission of the outbreak has been attenuated (51). However, there are situations in which time nodes with Reff and Rt values <1 are different during the same outbreak, which implies that that there are some differences between Reff and Rt values in measuring transmissibility. In describing the dynamics of the spread of the outbreak, the Rt value estimates the number of reproductions over time over a 5-day moving average, which represents the transmission trend of the outbreak over a period of time. However, we can usually use Reff value to quantitatively express how many individuals one infected person can infect, which seems to have more explanatory and epidemiological value from a public health perspective. Some studies have pointed out that the Rt estimation may be biased due to certain complicating factors, including the use of inaccurate data sources in the calculation process and the use of the Serial Interval (SI) instead of the difficult-to-obtain Generation Time (GT) (52). In addition, Rt suffers from the shortcoming of averaging, which is an average of the total population and thus may mask the local variations. In this study, the difference in Reff values between the pre-Delta strains, the Delta variant and the Omicron variant were statistically significant, whereas the difference in Rt between the Delta variant and pre-Delta strains was not statistically significant, and the median Rt values of Delta outbreaks and pre-Delta strains outbreaks were the same. This shows that Reff is more likely to identify and measure the differences in transmissibility between different strains, and further indicates that Reff is more suitable than Rt for assessing the transmissibility of the disease during an outbreak.
Limitations
This study also has certain limitations. Firstly, in explaining the effectiveness of the PHSMs adopted in China, we have not undertaken an intensive analysis of the prevention and control effects of any specific intervention measure, such as closing schools and businesses, physical distancing etc. Secondly, the parameter values required to calculate Reff and Rt were obtained by consulting reference literature, which may be slightly different from the actual parameters of each outbreak. In future research, we can consider using first-hand epidemiological survey data for more in-depth analysis. Finally, the vaccination rate, coverage rate and types of vaccine may affect the transmissibility of the strains, in the future research, if more detailed vaccine data can be obtained, we can then try to conduct more in-depth research to specifically analyze the extent of the impact of vaccines on transmissibility.
Conclusion
The transmissibility of the Omicron variant is greater than that of pre-Delta strains or the Delta variant, and thus the Omicron variant is more difficult to control. At present, China's general policy of “Dynamic Clearing” has been extremely effective in dealing with the Omicron outbreak. There are certain differences between Reff and Rt values in measuring transmissibility. The Reff value is more suitable than the Rt value for evaluating the transmissibility of the disease in epidemic outbreaks.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary materials, further inquiries can be directed to the corresponding author.
Author contributions
YN, LL, TC, and QL made substantial contributions to conception and design and critically revised the manuscript for important intellectual content. NY, XW, QL, and LL collected data. YN, LL, TC, SY, GA, BD, ZL, KL, TY, CL, ZeZ, and TC conceived the experiments. LL, WL, YW, ZhZ, BD, PL, JR, LL, SY, KL, and JH conducted experiments and analyzed results. YN, LL, TC, and QL wrote the manuscript. All authors have read and approved the final manuscript.
Funding
This study was supported, in whole or in part, by the National Key R&D Program of China (2021ZD0113903) and (2021YFC2301604); Bill & Melinda Gates Foundation [INV-005832].
Acknowledgments
We would like to thank the staff members at the hospitals, local health departments, and the CDC at all levels across China for their valuable assistance in coordinating data collection, and David James Moser for his modifications and refinements to the English grammar of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.949594/full#supplementary-material
Supplementary Figure S1. Epidemic curve, Reff value and Rt value of nine pre-Delta strains, (A) Results of curve fitting of the SEIR model with the onset data; (B) The time-dependent reproduction number (Rt) of the ongoing COVID-19 outbreak.
Supplementary Figure S2. Epidemic curve, Reff value and Rt value of 13 Delta variant outbreaks, (A) Results of curve fitting of the SEIR model with the onset data; (B) The time-dependent reproduction number (Rt) of the ongoing COVID-19 outbreak.
Supplementary Figure S3. Epidemic curve, Reff value and Rt value of eight Omicron variant outbreaks, (A) Results of curve fitting of the SEIR model with the onset data; (B) The time-dependent reproduction number (Rt) of the ongoing COVID-19 outbreak.
Supplementary Table S1. The value of R2 of nine pre-Delta strains, 13 Delta variant and eight Omicron variant outbreaks.
References
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel Coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
2. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
3. Feder K, Pearlowitz M, Goode A, Duwell M, Williams T, Chen-Carrington P, et al. Linked clusters of SARS-CoV-2 variant B.1.351 - Maryland, January-February 2021. MMWR Morbidity Mortality Weekly Rep. (2021) 70:627–31. doi: 10.15585/mmwr.mm7017a5
4. Bal A, Destras G, Gaymard A, Stefic K, Marlet J, Eymieux S, et al. Two-step strategy for the identification of SARS-CoV-2 variant of concern 202012/01 and other variants with spike deletion H69-V70, France, August to December 2020. Euro Surveillance. (2021) 26:2100008. doi: 10.2807/1560-7917.ES.2021.26.3.2100008
5. Annavajhala M, Mohri H, Wang P, Nair M, Zucker JE, Sheng Z, et al. Emergence and expansion of SARS-CoV-2 B.1.526 after identification in New York. Nature. (2021) 597:703–708. doi: 10.1038/s41586-021-03908-2
6. Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. (2021) 398:2126–8. doi: 10.1016/S0140-6736(21)02758-6
7. Campbell F, Archer B, Laurenson-Schafer H, Jinnai Y, Konings F, Batra N, et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Euro Surveillance. (2021) 26:2100509. doi: 10.2807/1560-7917.ES.2021.26.24.2100509
8. Yang W, Shaman J. COVID-19 pandemic dynamics in South Africa and epidemiological characteristics of three variants of concern (Beta, Delta, and Omicron). medRxiv. (2022) 29:21268073. doi: 10.1101/2021.12.19.21268073
9. Wallinga J, Lipsitch M. How generation intervals shape the relationship between growth rates and reproductive numbers. P Roy Soc B Biol Sci. (2007) 274:599–604. doi: 10.1098/rspb.2006.3754
10. Delamater P, Street E, Leslie T, Yang Y, Jacobsen K. Complexity of the Basic Reproduction Number (R0). Emerg Infect Dis. (2019) 25:1–4. doi: 10.3201/eid2501.171901
11. Xu C, Dong YQ, Yu XY, Wang HW, Tsamlag L, Zhang SX, et al. Estimation of reproduction numbers of COVID-19 in typical countries and epidemic trends under different prevention and control scenarios. Front Med Prc. (2020) 14:613–22. doi: 10.1007/s11684-020-0787-4
12. Burki T. Lifting of COVID-19 restrictions in the UK and the Delta variant. Lancet Respir Med. (2021) 9:e85. doi: 10.1016/S2213-2600(21)00328-3
13. Sanche S, Lin YT, Xu CG, Romero-Severson E, Hengartner N, Ke RA. High contagiousness and rapid spread of severe acute respiratory syndrome Coronavirus 2. Emerg Infect Dis. (2020) 26:1470–7. doi: 10.3201/eid2607.200282
14. Najafimehr H, Mohamed Ali K, Safari S, Yousefifard M, Hosseini M. Estimation of basic reproduction number for COVID-19 and the reasons for its differences. Int J Clin Prac. (2020) 74:e13518. doi: 10.1111/ijcp.13518
15. Zhou T, Liu Q, Yang Z, Liao J, Yang K, Bai W, et al. Preliminary prediction of the basic reproduction number of the Wuhan novel Coronavirus 2019-nCoV. J Evid Based Med. (2020) 13:3–7. doi: 10.1111/jebm.12376
16. Hwang H, Lim J, Song S, Achangwa C, Sim W, Kim G, et al. Transmission dynamics of the Delta variant of SARS-CoV-2 infections in South Korea. J Infect Dis. (2022) 225:793–799. doi: 10.1093/infdis/jiab586
17. Lauer S, Grantz K, Bi Q, Jones F, Zheng Q, Meredith H, et al. The incubation period of Coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Internal Med. (2020) 172:577–82. doi: 10.7326/M20-0504
18. Deng Y, You C, Liu Y, Qin J, Zhou X. Estimation of incubation period and generation time based on observed length-biased epidemic cohort with censoring for COVID-19 outbreak in China. Biometrics. (2021) 77:929–41. doi: 10.1111/biom.13325
19. Khalili M, Karamouzian M, Nasiri N, Javadi S, Mirzazadeh A, Sharifi H. Epidemiological characteristics of COVID-19: a systematic review and meta-analysis. Epidemiol Infect. (2020) 148:e130. doi: 10.1017/S0950268820001430
20. Linton N, Kobayashi T, Yang Y, Hayashi K, Akhmetzhanov A, Jung S, et al. Incubation period and other epidemiological characteristics of 2019 novel Coronavirus infections with right truncation: a statistical analysis of publicly available case data. J Clin Med. (2020) 9:538. doi: 10.3390/jcm9020538
21. Thompson R. Novel Coronavirus outbreak in Wuhan, China, 2020: intense surveillance is vital for preventing sustained transmission in new locations. J Clin Med. (2020) 9:498. doi: 10.3390/jcm9020498
22. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel Coronavirus-infected pneumonia. N Engl J Med. (2020) 382:1199–207. doi: 10.1056/NEJMoa2001316
23. Alene M, Yismaw L, Assemie M, Ketema D, Gietaneh W, Birhan T. Serial interval and incubation period of COVID-19: a systematic review and meta-analysis. BMC Infect Dis. (2021) 21:257. doi: 10.1186/s12879-021-05950-x
24. Wang Y, Chen R, Hu F, Lan Y, Yang Z, Zhan C, et al. Transmission, viral kinetics and clinical characteristics of the emergent SARS-CoV-2 Delta VOC in Guangzhou, China. EClinicalMedicine. (2021) 40:101129. doi: 10.1016/j.eclinm.2021.101129
25. Li L, Han Z, Qin P, Liu W, Yang Z, Chen Z, et al. Transmission and containment of the SARS-CoV-2 Delta variant of concern in Guangzhou, China: a population-based study. PLoS Neglec Trop Dis. (2022) 16:e0010048. doi: 10.1371/journal.pntd.0010048
26. Liu W, Ye W, Zhao Z, Liu C, Deng B, Luo L, et al. Modelling the emerging COVID-19 epidemic and estimating intervention effectiveness - Taiwan, China, 2021. China CDC Weekly. (2021) 3:716–9. doi: 10.46234/ccdcw2021.177
27. Zhang M, Xiao J, Deng A, Zhang Y, Zhuang Y, Hu T, et al. Transmission dynamics of an outbreak of the COVID-19 Delta variant B.1.617.2 - Guangdong province, China, May-June 2021. China CDC Weekly. (2021) 3:584–6. doi: 10.46234/ccdcw2021.148
28. Ryu S, Kim D, Lim J, Ali S, Cowling B. Serial interval and transmission dynamics during SARS-CoV-2 Delta variant predominance, South Korea. Emerg Infect Dis. (2022) 28:407–10. doi: 10.3201/eid2802.211774
29. Geismar C, Fragaszy E, Nguyen V, Fong W, Shrotri M, Beale S, et al. Household serial interval of COVID-19 and the effect of variant B.1.1.7: analyses from prospective community cohort study (Virus watch). Wellcome Open Res. (2021) 6:224. doi: 10.12688/wellcomeopenres.16974.2
30. Backer J, Eggink D, Andeweg S, Veldhuijzen I, van Maarseveen N, Vermaas K, et al. Shorter serial intervals in SARS-CoV-2 cases with Omicron BA.1 variant compared with Delta variant, the Netherlands, 13 to 26 December 2021. Euro Surveillance. (2022) 27:2200042. doi: 10.2807/1560-7917.ES.2022.27.6.2200042
31. Song J, Lee J, Kim M, Jeong H, Kim M, Kim S, et al. Serial intervals and household transmission of SARS-CoV-2 Omicron variant, South Korea, 2021. Emerg Infect Dis. (2022) 28:756–9. doi: 10.3201/eid2803.212607
32. Lee J, Choe Y, Jeong H, Kim M, Kim S, Yoo H, et al. Importation and transmission of SARS-CoV-2 B.1.1.529 (Omicron) variant of concern in Korea, November 2021. J Korean Med Sci. (2021) 36:e346. doi: 10.3346/jkms.2021.36.e346
33. Boehm E, Kronig I, Neher R, Eckerle I, Vetter P, Kaiser L. Novel SARS-CoV-2 variants: the pandemics within the pandemic. Clin Microbiol Infect. (2021) 27:1109–17. doi: 10.1016/j.cmi.2021.05.022
34. Teyssou E, Delagrèverie H, Visseaux B, Lambert-Niclot S, Brichler S, Ferre V, et al. The Delta SARS-CoV-2 variant has a higher viral load than the Beta and the historical variants in nasopharyngeal samples from newly diagnosed COVID-19. J Infect. (2021) 83:e1–e3. doi: 10.1016/j.jinf.2021.08.027
35. Iacobucci G. COVID-19: are high rates of B.1.617.2 linked to vaccine hesitancy? BMJ. (2021) 373:n1345. doi: 10.1136/bmj.n1345
36. Alizon S, Haim-Boukobza S, Foulongne V, Verdurme L, Trombert-Paolantoni S, Lecorche E, et al. Rapid spread of the SARS-CoV-2 Delta variant in some French regions, June 2021. Euro Surveillance. (2021) 26:2100573. doi: 10.2807/1560-7917.ES.2021.26.28.2100573
37. Liu Y, Rocklov J. The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus. J Travel Med. (2021) 28. doi: 10.1093/jtm/taab124
38. Ingraham N, Ingbar D. The Omicron variant of SARS-CoV-2: understanding the known and living with unknowns. Clin Transl Med. (2021) 11:e685. doi: 10.1002/ctm2.685
39. Nishiura H, Ito K, Anzai A, Kobayashi T, Piantham C, Rodriguez-Morales AJ. Relative reproduction number of SARS-CoV-2 Omicron (B.1.1.529) compared with delta variant in South Africa. J Clin Med. (2022) 11:30. doi: 10.3390/jcm11010030
40. Wall E, Wu M, Harvey R, Kelly G, Warchal S, Sawyer C, et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. (2021) 397:2331–3. doi: 10.1016/S0140-6736(21)01290-3
41. Xia F, Yang X, Cheke R, Xiao Y. Quantifying competitive advantages of mutant strains in a population involving importation and mass vaccination rollout. Infect Dis Model. (2021) 6:988–96. doi: 10.1016/j.idm.2021.08.001
42. McEwen A, Cohen S, Bryson-Cahn C, Liu C, Pergam S, Lynch J, et al. Variants of Concern Are Over represented Among Post vaccination break through Infections of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Washington State. Clin Infect Dis. (2022) 74:1089–1092. doi: 10.1093/cid/ciab581
43. Burki T. Omicron variant and booster COVID-19 vaccines. Lancet Respir Med. (2022) 10:e17. doi: 10.1016/S2213-2600(21)00559-2
44. Hong K, Yum S, Kim J, Chun B. The serial interval of COVID-19 in Korea: 1,567 Pairs of symptomatic cases from contact tracing. J Korean Med Sci. (2020) 35:e435. doi: 10.3346/jkms.2020.35.e435
45. Singanayagam A, Hakki S, Dunning J, Madon K, Crone M, Koycheva A, et al. Community transmission and viral load kinetics of the SARS-CoV-2 Delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis. (2022) 22:183–95. doi: 10.1016/S1473-3099(21)00648-4
46. Wilder-Smith A. What is the vaccine effect on reducing transmission in the context of the SARS-CoV-2 Delta variant? Lancet Infect Dis. (2022) 22:152–3. doi: 10.1016/S1473-3099(21)00690-3
47. Bleier B, Ramanathan M, Lane A. COVID-19 vaccines may not prevent nasal SARS-CoV-2 infection and asymptomatic transmission. Otolaryngol Head Neck Surg. (2021) 164:305–7. doi: 10.1177/0194599820982633
48. Liu Y, Gayle A, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS Coronavirus. J Travel Med. (2020) 27:taaa021. doi: 10.1093/jtm/taaa021
49. Amer R, Samir M, Gaber O, El-Deeb N, Abdelmoaty A, Ahmed A, et al. Diagnostic performance of rapid antigen test for COVID-19 and the effect of viral load, sampling time, subject's clinical and laboratory parameters on test accuracy. J Infect Public Health. (2021) 14:1446–53. doi: 10.1016/j.jiph.2021.06.002
50. Yuan J, Li M, Lv G, Lu Z. Monitoring transmissibility and mortality of COVID-19 in Europe. Int J Infect Dis. (2020) 95:311–5. doi: 10.1016/j.ijid.2020.03.050
51. Choi S, Ki M. Estimating the reproductive number and the outbreak size of COVID-19 in Korea. Epidemiol Health. (2020) 42:e2020011. doi: 10.4178/epih.e2020011
Keywords: SARS-CoV-2, effective reproduction number, variant, transmissibility, Delta variant, Omicron variant
Citation: Niu Y, Luo L, Yang S, Abudurusuli G, Wang X, Zhao Z, Rui J, Li Z, Deng B, Liu W, Zhang Z, Li K, Liu C, Li P, Huang J, Yang T, Wang Y, Chen T and Li Q (2022) Comparison of epidemiological characteristics and transmissibility of different strains of COVID-19 based on the incidence data of all local outbreaks in China as of March 1, 2022. Front. Public Health 10:949594. doi: 10.3389/fpubh.2022.949594
Received: 21 May 2022; Accepted: 29 August 2022;
Published: 15 September 2022.
Edited by:
Ahmed Mostafa, National Research Centre, EgyptReviewed by:
Galal Metwally, Zagazig University, EgyptMohamed Samir, The Pirbright Institute, United Kingdom
Copyright © 2022 Niu, Luo, Yang, Abudurusuli, Wang, Zhao, Rui, Li, Deng, Liu, Zhang, Li, Liu, Li, Huang, Yang, Wang, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qun Li, bGlxdW5AY2hpbmFjZGMuY24=; Tianmu Chen, MTM2OTg2NjVAcXEuY29t
†These authors have contributed equally to this work
 Yan Niu
Yan Niu Li Luo2†
Li Luo2† Guzainuer Abudurusuli
Guzainuer Abudurusuli Zeyu Zhao
Zeyu Zhao Jia Rui
Jia Rui Zhe Zhang
Zhe Zhang Tianmu Chen
Tianmu Chen Qun Li
Qun Li