- 1Gansu Provincial Maternity and Child-Care Hospital, Lanzhou, China
- 2The First Clinical Medical College, Gansu University of Chinese Medicine, Lanzhou, China
- 3School of Nursing, Gansu University of Chinese Medicine, Lanzhou, China
Background: Previous studies have found that exposure to heavy metals increased the incidence of congenital heart defects (CHDs). However, there is a paucity of information about the connection between exposure to titanium and CHDs. This study sought to examine the relationship between prenatal titanium exposure and the risk of CHDs in offspring.
Methods: We looked back on a birth cohort study that was carried out in our hospital between 2010 and 2012. The associations between titanium exposure and the risk of CHDs were analyzed by using logistic regression analysis to investigate titanium concentrations in maternal whole blood and fetal umbilical cord blood.
Results: A total of 97 case groups and 194 control groups were included for a nested case-control study. The [P50 (P25, P75)] of titanium were 371.91 (188.85, 659.15) μg/L and 370.43 (264.86, 459.76) μg/L in serum titanium levels in pregnant women and in umbilical cord serum titanium content in the CHDs group, respectively. There was a moderate positive correlation between the concentration of titanium in pregnant women's blood and that in umbilical cord blood. A higher concentrations of maternal blood titanium level was associated with a greater risk of CHDs (OR 2.706, 95% CI 1.547–4.734), the multiple CHDs (OR 2.382, 95% CI 1.219–4.655), atrial septal defects (OR 2.367, 95% CI 1.215–4.609), and patent ductus arteriosus (OR 2.412, 95% CI 1.336–4.357). Dramatically higher concentrations of umbilical cord blood levels had an increased risk of CHDs and different heart defects.
Conclusion: Titanium can cross the placental barrier and the occurrence of CHDs may be related to titanium exposure.
Introduction
Congenital heart diseases (CHDs) refer to congenital heart structure and function defects during embryonic development. As one of the most common birth defects, the global prevalence rate is about 0.8–1.2% (1). The quality of life for patients who have CHDs is reduced by the disease's high mortality and morbidity rates, which may necessitate several operations to fix problems (2). CHDs is caused by genetic factors, environmental factors alone or both (3). Compared with genetic factors, environmental factors are more recognizable and controllable, so CHDs can be prevented by identifying and avoiding adverse environmental risk factors.
With the development of modern society and the progress of industry level, the living environment and industrial pollution are increasing day by day, which seriously endangers the life and health of pregnant women and fetuses. Among them, heavy metal pollution is one of the main environmental pollution at present. Current studies indicate that maternal exposure to heavy metal during pregnancy is a risk factor for CHDs, including cadmium, arsenic, copper, barium and nickel (4, 5). Titanium (Ti) is one of the most common heavy metal elements. Among its common form, titanium dioxide (TiO2) can be used as coatings, plastics, personal care products (cosmetics, sunscreen) and food in the common additives (6). The European Chemicals Agency (ECHA) classifies TiO2 as a Category 2 carcinogens: suspected human carcinogen inhaled (7).
Titanium can be absorbed by the human body through ingestion, inhalation, skin media and implant dissolution (8). Numerous in vivo and in vitro studies have shown that exposure to titanium induces cytotoxicity, oxidative stress, and inflammation and disrupts DNA and lipid metabolism (9–11). Animal experiments implied titanium and titanium compounds have been known to cause neurotoxicity, reproductive toxicity, skeletal malformations, and cardiopulmonary effects in offspring (12). It was found that prenatal exposure to TiO2 NPs inhibited the expression of Rac1 and Cdc42 proteins in the offspring's hippocampus and reduced the number of axon and dendrite branches, possibly inhibiting the offspring's brain development (13). TiO2 nanoparticles (20 nm; 5 mg/mL, 21 nm; 0.01, 10 and 1,000 g/mL, and 240–280 nm in water; 0.1 g/mL) caused circulatory system abnormalities, premature hatching, and reduced reproduction in zebrafish in prior research (14, 15). Additionally, unborn mice's skeletal development fell behind that of the control group when their mother was exposed to 100 mg/kg nano-TiO2, leading to loss or reduction of ossification and fetal dysplasia. The interference of calcium, zinc and other metabolic processes by nano-tio2 may be the cause of these side effects, either directly or indirectly (16). In addition, following exposure to nano-TiO2, mice's decreased fertility and ovarian damage may be linked to altered production of cytokines related to inflammation or follicular atresia (17).
In vivo tests show that TiO2 will accumulate in the lungs, digestive tract, liver, heart, spleen, kidney and myocardium after inhalation or oral exposure (18). A growing number of epidemiological studies suggested that titanium exposure may be associated with diabetes, colitis, and cardiovascular disease (11, 19, 20). In addition, exposure to titanium was found to be associated with adverse pregnancy outcomes, including fetal distress, preterm birth, low birth weight and neural tube defects (8, 21, 22).
However, few studies have independently assessed the correlation between titanium concentrations in maternal whole blood and umbilical cord blood and the risk of CHDs in offspring. In order to thoroughly investigate the relationship between maternal titanium exposure and the risk of CHDs developing in offspring, we carried out a prospective nested case-control research in Lanzhou, China.
Methods
Study population
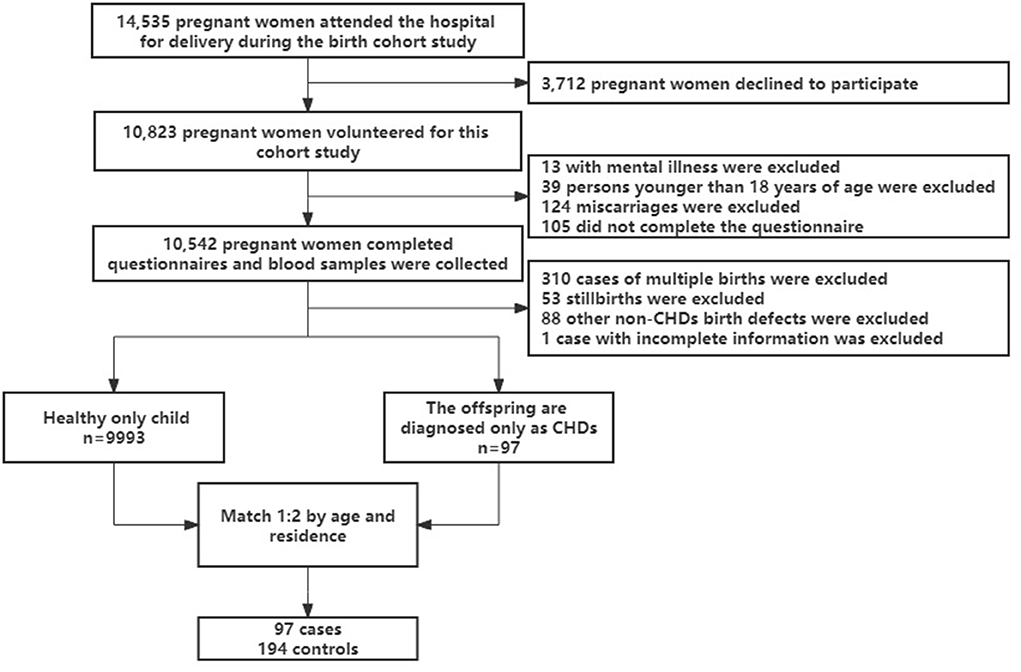
In Lanzhou, China, at the Gansu Provincial Maternity and Child Care Hospital, a birth cohort study was carried out between 2010 and 2012 (23). A total of 14,535 pregnant women delivered at the hospital during this time. Pregnant women who delivered at least 20 weeks pregnant, without mental illness, and aged at least 18 years were eligible, 176 of whom were not eligible for the study (13 with mental illness, 39 under 18 years of age, and 124 miscarried within 20 weeks). Fourteen thousand three hundred fifty-nine eligible women contacted, 3,712 declined to participate and 105 did not complete the questionnaire. Finally, a total of 10,542 people completed the baseline questionnaire and blood sample collection, with a response rate of 73.4%. Excluding multiple births, stillbirths and/or other birth defects (non-CHDs) and incomplete information at baseline, 97 of the only children born to the study subjects were diagnosed with CHDs. In this study, subjects who delivered a healthy only child at baseline were selected as controls, and subjects were randomly selected from the controls for 1:2 matching based on age (±2 years) and place of residence. A total of 291 subjects were included in the study (as showed in Figure 1). The research project was approved by the Medical Ethics Committee of the Gansu Provincial Maternity and Child Care Hospital and Yale University. When eligible women arrived at the hospital for the birth, they were briefed about the study's protocol. A systematic and structured questionnaire was used to conduct in-person interviews in the hospital following the receipt of written consent. The majority of women (84%) were questioned during the first 3 days following birth. Demographic data, reproductive and medical history, smoking habits, alcohol and tea use, employment and housing history, physical activity level, and food were all gathered via the questionnaire. The medical records were mined for data on pregnancy complications and birth outcomes.
Classification of congenital heart defects
Depending on the degree of the problem, each congenital cardiac abnormality was classified as “Isolated,” “Multiple,” or “Syndrome.” Our previous study provided more detailed information on the classification of CHDs (24). All CHDs were divided into three categories based on the clinical phenotype and the ICD-10 codes (Q20–Q28) for the International Classification of Diseases: (1) Congenital abnormalities of the great arteries, such as patent ductus arteriosus (PDA), aortic coarctation, pulmonary artery stenosis, and other congenital malformations of the great arteries (ICD 10: Q25). (2) Congenital septal malformations, such as atrioventricular septal defects (AVSDs), ventricular septal defects (VSDs), atrial septal defects (ASDs), and other congenital cardiac septal malformations (ICD 10: Q21). (3) Other CHDs, such as congenital heart abnormalities, defects in the chambers and connections of the heart, etc.
Blood collection, storage and analysis
The subjects blood samples were collected at the time of delivery in hospital. 3 ml of whole blood from the median elbow vein was collected on an empty stomach in the morning, and 7 ml of Ethylenediaminetetraacetic acid (EDTA) Vacutainer blood was collected 3 mL of cord blood was also taken before placental abruption (cesarean section or vaginal delivery) and collected with 7 mL special Vacutainer of EDTA for anticoagulation, and stored in a refrigerator AT-80 degrees Celsius. All sample information is incorporated into the electronic database of biological samples.
On an electric hot plate set at 175°C for 6 h, 0.2 mL of whole blood is combined with 1 mL HNO3 and 0.2 mL H2O2. Following digestion, deionized water is used to dilute the solution to a final volume of 10 mL. After that, inductively coupled plasma-tandem mass spectrometry is used to detect the amount of titanium in maternal and cord blood (ICP-MS, iCAP RQ; Thermo Fisher, USA). We employed the internal standard approach for quality control and chose the internal standards Sc45, Ge72, Rh103, and Re187. Measurements are made twice on each sample. We tested the standard reference materials during the experiment for every 20 samples, and we verified that the values of the measured reference materials were within the suggested range for each metal. Ti had a 0.22 g/L lower limit of detection (LOD). When the concentration of titanium was below the limit of detection, it was reported as zero.
Statistical analysis
SPSS Statistics 25.0 was used to conduct all statistical analyses (IBM, Chicago, USA). Percentage was used to describe the classification variables of pregnant women, such as BMI, education level, family per capita monthly income, production time and smoking history. The case group and the control group's disparities in maternal traits and demographic data were compared using the chi-square test. Continuous variables, such as the level of titanium element in each biological sample, were not in accordance with normal distribution, so they were represented by median and quartile range. Wilcoxon-Mann-Whitney U test was employed to compare groups. In order to explore the correlation between titanium level and CHDs in offspring, Multivariate conditional Logistic regression was used to analyze the factors of CHDs caused by titanium exposure in pregnancy. P < 0.05 denoted a statistically significant difference and was used to include factors in a multivariate analysis that had P < 0.05 in the univariate analysis.
Since there is no normal reference range for maternal blood titanium level and umbilical cord blood titanium level, Receiver Operating Characteristic (ROC) curve was used to analyze the influence of maternal blood titanium level and umbilical cord blood titanium level on the occurrence of CHDs, with the occurrence of CHDs as the dependent variable, and the maternal blood titanium level and umbilical cord blood titanium level in the original data as the independent variable. We refer to previous research methods (25), the ROC curve was used to obtain cut-off values to classify maternal and umbilical cord blood titanium levels into high and low concentrations; Low concentrations in maternal and umbilical cord blood titanium levels were used as reference; Two-tailed P < 0.05 and 95% CIs excluding 1.00 were considered statistically significant.
Results
Characteristics of participants
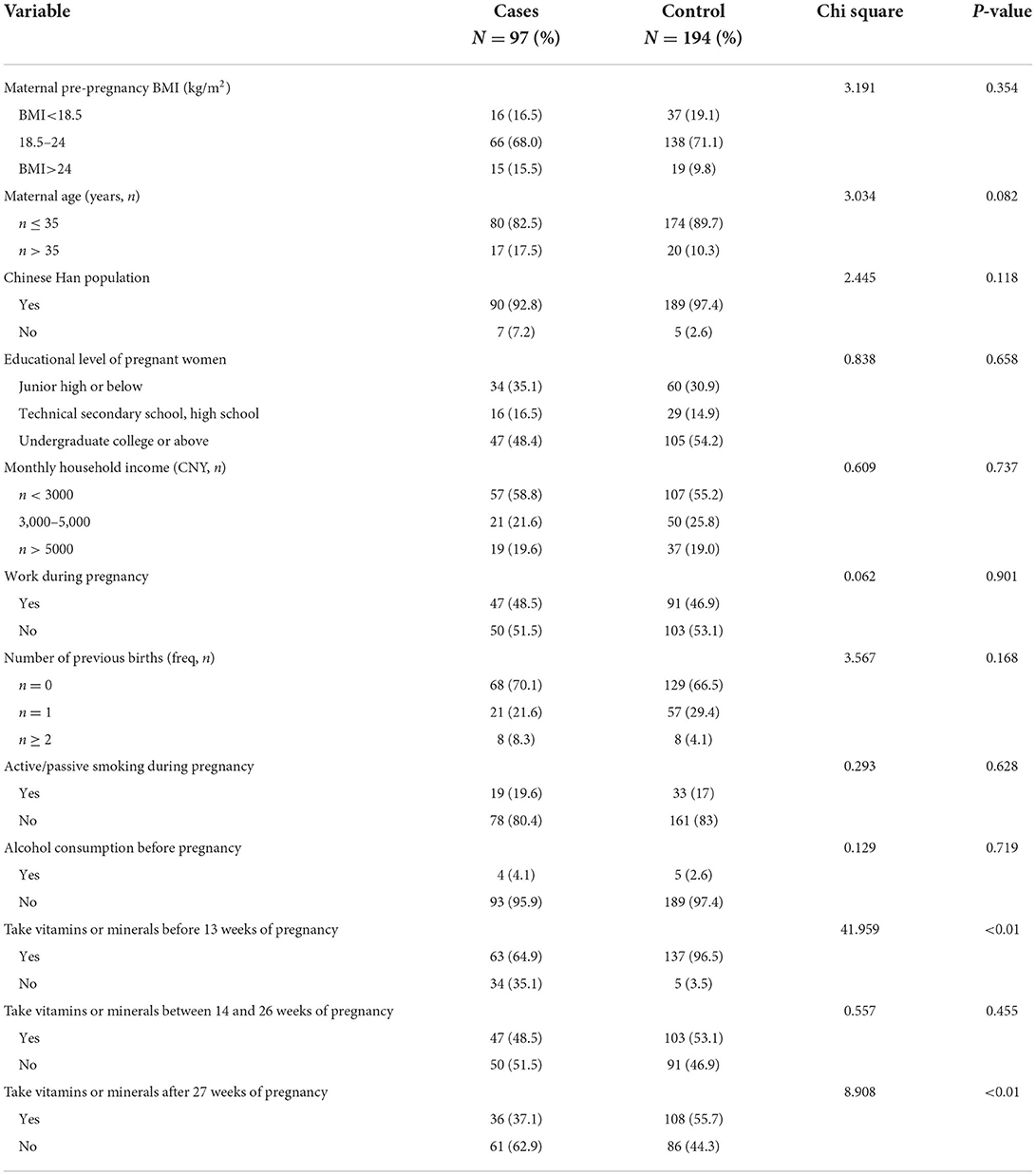
One hundred ninety-four controls and 97 cases were recruited and all biological samples were obtained. The 97 cases of CHDs included The isolated CHDs (43 cases), The multiple CHDs (48 cases), The Syndrome CHDs (6 cases), PDA (70 cases), ASDs (48 cases), VSDs (7 cases) and AVSDs (4 cases). The maternal characteristics of the samples are shown in Table 1. Take vitamin or mineral supplements during the first 13 weeks of pregnancy and after 27 weeks of pregnancy were significantly different between the two groups (P < 0.01, In the control group, 52 women were deficient in vitamin use before 13 weeks of pregnancy), Other baseline data showed no significant difference between the two groups.
Concentration of titanium in blood samples from pregnant women
Compared with the control group, the level of titanium in the blood samples of pregnant women in the case group difference was statistically significant (P < 0.05), as showed in Table 2. The median titanium concentration in pregnant women was 304.65 μg/L, including the case group and the control group. We conducted a statistical analysis on the concentration of titanium in the blood of pregnant women with different CHDs subtypes. There were statistically significant differences between some subtypes in the case group and the control group, such as the isolated CHDs, The multiple CHDs and ASDs (P < 0.05).

Table 2. Comparison of serum titanium levels between pregnant women in case group and control group.
The concentration of titanium in the cord blood sample
The content of titanium in umbilical cord blood samples in the case group was higher than that in the control group, with statistical significance (P < 0.05), as showed in Table 3. The median titanium concentration in the cord blood was 320.07 μg/L, including the case group and the control group. Using the same method, we also found that there were statistically significant differences between some subtypes in the case group and the control group, such as The isolated CHDs, PDA, ASDs and VSDs (P < 0.05).
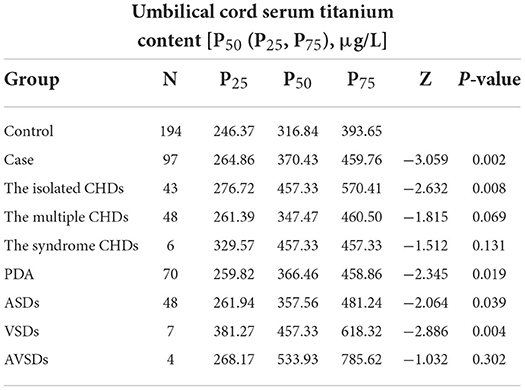
Table 3. Comparison of titanium content in umbilical cord blood between case group and control group.
Relationship between serum titanium content in pregnant women and that in the umbilical cord
The maximum concentration of titanium in the blood of pregnant women in the case group and the control group was 10,366.45 μg/L and 1,384.84 μg/L, respectively, and the minimum concentration was 64.72 μg/L and 84.14 μg/L. The maximum concentration of titanium in the cord blood of the case group and the control group was 2,434.01 μg/L and 1,831.69 μg/L, respectively, and the minimum concentration was 116.93 μg/L and 13.90 μg/L, respectively. As showed in Figure 2, correlation analysis showed that there was a positive correlation between the concentration of titanium in pregnant women's blood and that in umbilical cord blood (r = 0.278).
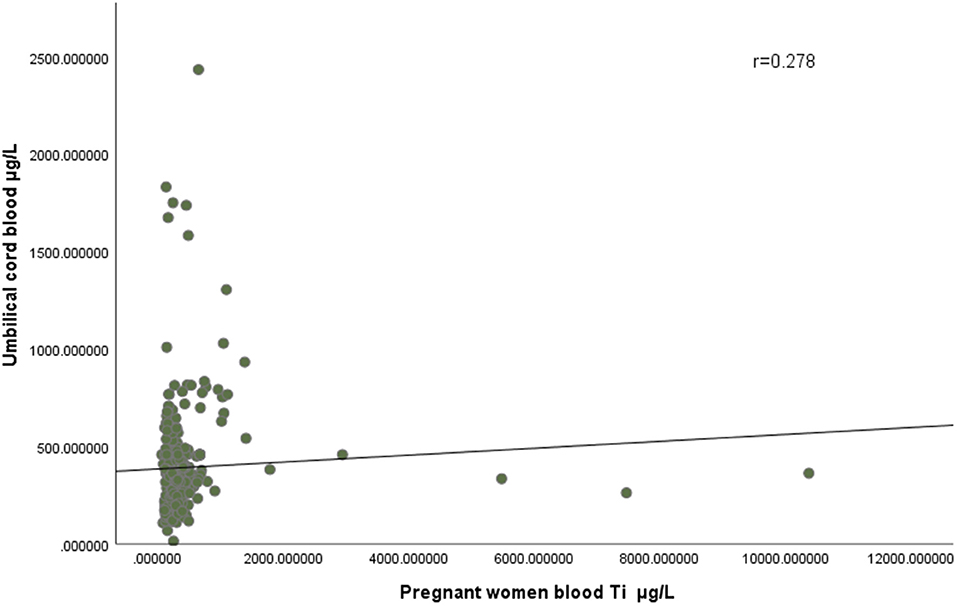
Figure 2. There was a positive correlation between the concentration of titanium in pregnant women's blood and that in umbilical cord blood (r = 0.278).
ROC curve analysis
ROC curve analysis showed that the Area Under Curve (AUC) area of maternal blood titanium level on the occurrence of CHDs was 0.589 (95% CI: 0.513–0.666, P = 0.013), and that of umbilical cord blood titanium level was 0.610 (95% CI: 0.539–0.681, P = 0.002). The cut-off values of titanium levels in maternal blood and umbilical cord blood were 404.22 μg/L and 453.58 μg/L, respectively.
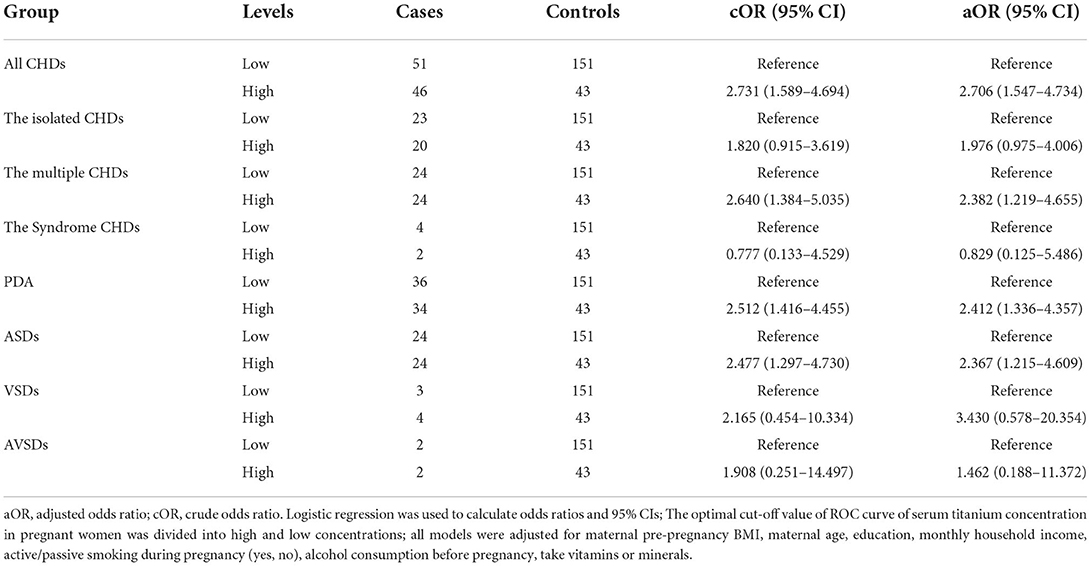
Association between serum titanium levels in pregnant women and CHDs in offspring
The association between different titanium levels and the risk of CHDs was further analyzed by dividing all maternal blood samples into low and high groups using cut-off values. As shown in Table 4, we found an increased risk of CHDs (aOR, 2.706, 95% CI, 1.547–4.734; P < 0.05), The multiple CHDs (aOR, 2.382, 95% CI, 1.219–4.655; P < 0.05), PDA (aOR, 2.412, 95% CI, 1.336–4.357; P < 0.05) and ASDs (aOR, 2.367, 95% CI, 1.215–4.609; P < 0.05) in the high concentrations of maternal blood titanium group in the model adjusted for all potential confounders. All results suggest that maternal blood titanium exposure may increase the risk of CHDs in offspring.
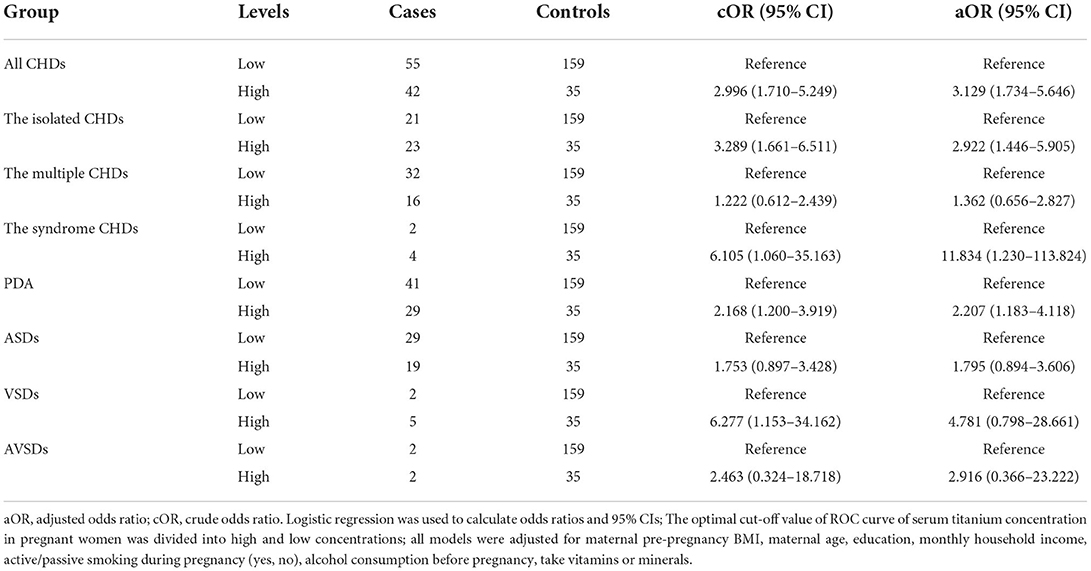
Association between umbilical cord serum titanium content and CHDs in offspring
The association between different titanium levels and the risk of CHDs was further analyzed by dividing all umbilical cord blood samples into low and high groups using cut-off values. As shown in Table 5, we found an increased risk of CHDs (aOR, 3.129, 95% CI, 1.734–5.646; P < 0.05), The isolated CHDs (aOR, 2.922, 95% CI, 1.446–5.905; P < 0.05), The Syndrome CHDs (aOR, 11.834, 95% CI, 1.230–113.824; P < 0.05) and PDA (aOR, 2.207, 95% CI, 1.183–4.118; P < 0.05) in the high concentrations of umbilical cord blood group in the model adjusted for all potential confounders. All the results also indicated that the umbilical cord blood titanium exposure may increase the CHDs risk in offspring.
Discussion
The findings of this study showed that the fetal cord blood titanium concentration in the CHDs group was higher than that in the control group. Our results indicate that increased umbilical cord and maternal blood titanium concentrations are significantly associated with CHDs risk. This is in line with epidemiological data, which suggests that greater levels of titanium in the umbilical cord and maternal blood may be linked to a higher risk of CHDs in offspring, including some major subtypes.
In our study, the median titanium concentration in pregnant women and the cord blood were 304.65 and 320.07 μg/L, respectively, including the case group and the control group. Compared with previous human studies, the value of titanium in our study is higher (26, 27), which probably due to the high titanium exposure of mining around Lanzhou. Widespread variations in blood titanium concentrations may result from changes in living conditions, dietary preferences, exposure to airborne particulate matter, and metal analysis techniques (28).
The placenta is an important organ responsible for the exchange of substances between the fetus and the mother, and is related to the health of the mother and the fetus. Compared to heavy metal exposure through fetal placental tissue (5), We used umbilical cord blood to analyze titanium exposure to give a more intuitive picture of the actual fetal titanium exposure level. A recent study confirmed the transmission of titanium across the placental barrier to the fetus during pregnancy by detecting titanium levels in meconium samples (29), this study also demonstrated that titanium can cross the placental barrier by detecting titanium levels in umbilical cord blood, and our results showed that fetal exposure to titanium increases with maternal exposure. In addition, animal experiments demonstrated that gestation exposure to titanium not only significantly impaired the growth and development of the placenta in mice, but also increased the total peripheral resistance of the placenta and impaired umbilical cord blood reactivity (30, 31). The mechanism of titanium induced placental dysfunction may be related to the reduction of formation of the placental vascular system (32), induction of autophagy (33) and endocrine disorders (12).
When titanium can cross the placental barrier and enter the fetus, it may affect the developing heart. It has been suggested that the RA signal caused by TiO2 NPs may affect the developing heart of the fetus (12). According to studies, prenatal exposure to TiO2 NPs impaired mitochondrial metabolism by reducing the electronic transmission chain (ETC) in the progeny (34). The decreased mitochondrial ETC can also limit the contractility of myocardial cells and harm the progeny's heart function and bioenergetics (35, 36). Pan et al. use of quantitative proteomics approach applying iTRAQ-based mass spectrometry (MS) analysis found that TiO2 antiparticle may cause human embryonic stem cells (hESCs) to lose their pluripotency and disrupt the differentiation of hESCs mesoderm into cardiomyocytes, thus affecting the normal development of cardiomyocytes, and it was also found that this may be due to DNA damage and oxidative stress in hESCs after TiO2 exposure (37). Therefore, minimizing titanium exposure during pregnancy is essential.
Our study has a few limitations. First of all, the questionnaires and samples in this study were collected 10 years ago, representing the exposure level of titanium in the environment at that time, which may have some deviation from the current exposure situation. Second, we only measured titanium concentrations at one point in the third trimester of pregnancy and could not assess changes in titanium levels during pregnancy. Third, the interaction between titanium and other heavy metal was not considered in this study. Further studies are necessary to confirm these findings.
Conclusion
Overall, titanium could cross the placental barrier, and fetal titanium exposure was significantly higher in the CHDs group than in the control group. Titanium during pregnancy was associated with CHDs risk at a high level of exposure. Further research is needed to investigate the underlying mechanism of congenital heart disease caused by titanium exposure during pregnancy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Requests to access the data sets should be directed to MTAzODgxNzE5MUBxcS5jb20=.
Ethics statement
This study was approved by the Institutional Ethics Committee of the Gansu Provincial Maternity and Child-care Hospital, China (2012-5). Written informed consent was obtained from all participants for their participation in this study.
Author contributions
JS, BM, and ZW: conceptualization, formal analysis, software, and writing—original draft. XJ: investigation and formal analysis. YW: writing—review and editing. YL and XM: data curation and validation. XLiu and XLin: methodology. XX: investigation. HC: resources. JQ: project administration. BY: funding acquisition. QL: conceptualization and supervision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported in part by the Gansu Provincial Science and Technology Department Grant (No. 21JR1RA043), the Key Research and Development Program of Gansu Province (No. 20YF8WA095), and the Coronavirus disease prevention and control research program of Lanzhou city (2020-XG-12).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wu WL, He JX, Shao XB. Incidence and mortality trend of congenital heart disease at the global, regional, and national level, 1990-2017. Medicine. (2020) 99:e20593. doi: 10.1097/MD.0000000000020593
2. Bruneau BG. The developmental genetics of congenital heart disease. Nature. (2008) 451:943–8. doi: 10.1038/nature06801
3. Joshi RO, Chellappan S, Kukshal P. Exploring the role of maternal nutritional epigenetics in congenital heart disease. Curr Dev Nutr. (2020) 4:nzaa166. doi: 10.1093/cdn/nzaa166
4. Zhang NN, Liu Z, Tian XX, Chen M, Deng Y, Guo YX, et al. Barium exposure increases the risk of congenital heart defects occurrence in offspring. Clin Toxicol. (2018) 56:132–9. doi: 10.1080/15563650.2017.1343479
5. Zhang NN, Chen M, Li J, Deng Y, Li SL, Guo YX, et al. Metal nickel exposure increase the risk of congenital heart defects occurrence in offspring a case-control study in China. Medicine. (2019) 98:e15352. doi: 10.1097/MD.0000000000015352
6. Musial J, Krakowiak R, Mlynarczyk DT, Goslinski T, Stanisz BJ. Titanium dioxide nanoparticles in food and personal care products-what do we know about their safety? Nanomaterials. (2020) 10:1110. doi: 10.3390/nano10061110
7. Canu IG, Fraize-Frontier S, Michel C, Charles S. Weight of epidemiological evidence for titanium dioxide risk assessment: current state and further needs. J Expo Sci Environ Epidemiol. (2020) 30:430–5. doi: 10.1038/s41370-019-0161-2
8. Jin Y, Li ZY, An H, Pang YM, Li KX, Zhang YL, et al. Environmental titanium exposure and reproductive health: Risk of low birth weight associated with maternal titanium exposure from a nested case-control study in northern China. Ecotoxicol Environ Saf . (2021) 208:111632. doi: 10.1016/j.ecoenv.2020.111632
9. Shabbir S, Kulyar F, Bhutta ZA, Boruah P, Asif MJB. Toxicological consequences of titanium dioxide nanoparticles (TiO 2 NPs) and their jeopardy to human population. Bionanoscience. (2021) 11:621–32. doi: 10.1007/s12668-021-00836-3
10. Fadoju O, Ogunsuyi O, Akanni O, Alabi O, Alimba C, Adaramoye O, et al. Evaluation of cytogenotoxicity and oxidative stress parameters in male Swiss mice co-exposed to titanium dioxide and zinc oxide nanoparticles. Environ Toxicol Pharmacol. (2019) 70:103204. doi: 10.1016/j.etap.2019.103204
11. Zhao L, Zhu YF, Chen ZJ, Xu HD, Zhou JW, Tang SC, et al. Cardiopulmonary effects induced by occupational exposure to titanium dioxide nanoparticles. Nanotoxicology. (2018) 12:169–84. doi: 10.1080/17435390.2018.1425502
12. Wu Y, Chen LM, Chen FR, Zou H, Wang ZY. A key moment for TiO2: Prenatal exposure to TiO2 nanoparticles may inhibit the development of offspring. Ecotoxicol Environ Saf . (2020) 202:110911. doi: 10.1016/j.ecoenv.2020.110911
13. Hong F, Zhou Y, Ji J, Zhuang J, Sheng L, Wang L. Nano-TiO2 inhibits development of the central nervous system and its mechanism in offspring mice. J Agric Food Chem. (2018) 66:11767–74. doi: 10.1021/acs.jafc.8b02952
14. Samaee SM, Rabbani S, Jovanovic B, Mohajeri-Tehrani MR, Haghpanah V. Efficacy of the hatching event in assessing the embryo toxicity of the nano-sized TiO2 particles in zebrafish: a comparison between two different classes of hatching-derived variables. Ecotoxicol Environ Saf. (2015) 116:121–8. doi: 10.1016/j.ecoenv.2015.03.012
15. Wang JX, Zhu XS, Zhang XZ, Zhao Z, Liu H, George R, et al. Disruption of zebrafish (Danio rerio) reproduction upon chronic exposure to TiO2 nanoparticles. Chemosphere. (2011) 83:461–7. doi: 10.1016/j.chemosphere.2010.12.069
16. Hong FS, Zhou YJ, Zhao XY, Sheng L, Wang L. Maternal exposure to nanosized titanium dioxide suppresses embryonic development in mice. Int J Nanomedicine. (2017) 12:6197–204. doi: 10.2147/IJN.S143598
17. Zhao XY, Ze YG, Gao GD, Sang XZ, Li B, Gui SX, et al. Nanosized TiO2-induced reproductive system dysfunction and its mechanism in female mice. PLoS ONE. (2013) 8:e59378. doi: 10.1371/journal.pone.0059378
18. Baranowska-Wojcik E, Szwajgier D, Oleszczuk P, Winiarska-Mieczan A. Effects of titanium dioxide nanoparticles exposure on human health-a review. Biol Trace Elem Res. (2020) 193:118–29. doi: 10.1007/s12011-019-01706-6
19. Yuan Y, Xiao Y, Yu YQ, Liu YY, Feng W, Qiu GK, et al. Associations of multiple plasma metals with incident type 2 diabetes in Chinese adults: the Dongfeng-Tongji cohort. Environ Pollut. (2018) 237:917–25. doi: 10.1016/j.envpol.2018.01.046
20. Ruiz PA, Moron B, Becker HM, Lang S, Atrott K, Spalinger MR, et al. Titanium dioxide nanoparticles exacerbate DSS-induced colitis: role of the NLRP3 inflammasome. Gut. (2017) 66:1216–24. doi: 10.1136/gutjnl-2015-310297
21. Li ZJ, Huo WH, Li ZW, Wang B, Zhang JX, Ren AG. Association between titanium and silver concentrations in maternal hair and risk of neural tube defects in offspring: a case-control study in north China. Reprod Toxicol. (2016) 66:115–21. doi: 10.1016/j.reprotox.2016.10.006
22. Zheng GC, Zhong HX, Guo ZZ, Wu ZC, Zhang HQ, Wang CG, et al. Levels of heavy metals and trace elements in umbilical cord blood and the risk of adverse pregnancy outcomes: a population-based study. Biol Trace Elem Res. (2014) 160:437–44. doi: 10.1007/s12011-014-0057-x
23. Qiu J, He XC, Cui HM, Zhang C, Zhang HH, Dang Y, et al. Passive smoking and preterm birth in urban China. Am J Epidemiol. (2014) 180:94–102. doi: 10.1093/aje/kwu092
24. Mao B, Qiu J, Zhao N, Shao Y, Dai W, He X, et al. Maternal folic acid supplementation and dietary folate intake and congenital heart defects. PLoS ONE. (2017) 12:e0187996. doi: 10.1371/journal.pone.0187996
25. Ren B, Chen X, Lei P, Hou L, Wang H, Zhou Y, et al. The relationship between preoperative systemic immune inflammation index and prognostic nutritional index and the prognosis of patients with alveolar hydatid disease. Front Immunol. (2021) 12:691364. doi: 10.3389/fimmu.2021.691364
26. Li AJ, Zhuang TF, Shi JB, Liang Y, Song MY. Heavy metals in maternal and cord blood in Beijing and their efficiency of placental transfer. J Environ Sci. (2019) 80:99–106. doi: 10.1016/j.jes.2018.11.004
27. Li XY, Li AJ, Zhang WJ, Liu XW, Liang Y, Yao XL, et al. A pilot study of mothers and infants reveals fetal sex differences in the placental transfer efficiency of heavy metals. Ecotoxicol Environ Saf . (2019) 186:109755. doi: 10.1016/j.ecoenv.2019.109755
28. Koller D, Bramhall P, Devoy J, Goenaga-Infante H, Harrington CF, Leese E, et al. Analysis of soluble or titanium dioxide derived titanium levels in human whole blood: consensus from an inter-laboratory comparison. Analyst. (2018) 143:5520–9. doi: 10.1039/C8AN00824H
29. Guillard A, Gaultier E, Cartier C, Devoille L, Noireaux J, Chevalier L, et al. Basal Ti level in the human placenta and meconium and evidence of a materno-foetal transfer of food-grade TiOnanoparticles in an ex vivo placental perfusion model. Part Fibre Toxicol. (2020) 17:51. doi: 10.1186/s12989-020-00381-z
30. Zhang L, Xie XX, Zhou YG, Yu DN, Deng Y, Ouyang JX, et al. Gestational exposure to titanium dioxide nanoparticles impairs the placentation through dysregulation of vascularization, proliferation and apoptosis in mice. Int J Nanomedicine. (2018) 13:777–89. doi: 10.2147/IJN.S152400
31. Abukabda AB, Bowdridge EC, McBride CR, Batchelor TP, Goldsmith WT, Garner KL, et al. Maternal titanium dioxide nanomaterial inhalation exposure compromises placental hemodynamics. Toxicol Appl Pharmacol. (2019) 367:51–61. doi: 10.1016/j.taap.2019.01.024
32. Teng CF, Jia JB, Wang ZP, Sharma VK, Yan B. Size-dependent maternal-fetal transfer and fetal developmental toxicity of ZnO nanoparticles after oral exposures in pregnant mice. Ecotoxicol Environ Saf . (2019) 182:109439. doi: 10.1016/j.ecoenv.2019.109439
33. Mao ZL, Yao MM Li YQ, Fu ZQ Li SS, Zhang LN, et al. miR-96-5p and miR-101-3p as potential intervention targets to rescue TiO2 NP-induced autophagy and migration impairment of human trophoblastic cells. Biomater Sci. (2018) 6:3273–83. doi: 10.1039/C8BM00856F
34. Kunovac A, Hathaway QA, Pinti MV, Goldsmith WT, Durr AJ, Fink GK, et al. ROS promote epigenetic remodeling and cardiac dysfunction in offspring following maternal engineered nanomaterial (ENM) exposure. Part Fibre Toxicol. (2019) 16:24. doi: 10.1186/s12989-019-0310-8
35. Barton GP, de Lange WJ, Ralphe JC, Aiken J, Diffee G. Linking metabolic and contractile dysfunction in aged cardiac myocytes. Physiol. Rep. (2017) 5:e13485. doi: 10.14814/phy2.13485
36. Hathaway QA, Nichols CE, Shepherd DL, Stapleton PA, McLaughlin SL, Stricker JC, et al. Maternal-engineered nanomaterial exposure disrupts progeny cardiac function and bioenergetics. Am J Physiol Heart Circ Physiol. (2017) 312:H446–58. doi: 10.1152/ajpheart.00634.2016
Keywords: congenital heart defects, metal exposure, titanium, pregnancy, maternal blood, umbilical cord blood
Citation: Sun J, Mao B, Wu Z, Jiao X, Wang Y, Lu Y, Ma X, Liu X, Xu X, Cui H, Lin X, Yi B, Qiu J and Liu Q (2022) Relationship between maternal exposure to heavy metal titanium and offspring congenital heart defects in Lanzhou, China: A nested case-control study. Front. Public Health 10:946439. doi: 10.3389/fpubh.2022.946439
Received: 17 May 2022; Accepted: 12 July 2022;
Published: 03 August 2022.
Edited by:
Jing Wei, University of Maryland, United StatesReviewed by:
Qing Chen, The University of Iowa, United StatesCheng-Yang Hu, Anhui Medical University, China
Copyright © 2022 Sun, Mao, Wu, Jiao, Wang, Lu, Ma, Liu, Xu, Cui, Lin, Yi, Qiu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing Liu, MjMwNTQ3MDgxNkBxcS5jb20=
†These authors have contributed equally to this work
 Jianhao Sun
Jianhao Sun Baohong Mao1†
Baohong Mao1† Zhenzhen Wu
Zhenzhen Wu Qing Liu
Qing Liu