
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Public Health, 14 October 2022
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.939095
 Nianzong Hou1,2
Nianzong Hou1,2 Lin Wang3
Lin Wang3 Mingzhe Li4
Mingzhe Li4 Bing Xie2
Bing Xie2 Lu He5
Lu He5 Mingyu Guo2
Mingyu Guo2 Shuo Liu2
Shuo Liu2 Meiyu Wang6
Meiyu Wang6 Rumin Zhang3
Rumin Zhang3 Kai Wang3*
Kai Wang3*Background: Chest computerized tomography (CT) plays an important role in detecting patients with suspected coronavirus disease 2019 (COVID-19), however, there are no systematic summaries on whether the chest CT findings of patients within mainland China are applicable to those found in patients outside.
Methods: Relevant studies were retrieved comprehensively by searching PubMed, Embase, and Cochrane Library databases before 15 April 2022. Quality assessment of diagnostic accuracy studies (QUADAS) was used to evaluate the quality of the included studies, which were divided into two groups according to whether they were in mainland China or outside. Data on diagnostic performance, unilateral or bilateral lung involvement, and typical chest CT imaging appearances were extracted, and then, meta-analyses were performed with R software to compare the CT features of COVID-19 pneumonia between patients from within and outside mainland China.
Results: Of the 8,258 studies screened, 19 studies with 3,400 patients in mainland China and 14 studies with 554 outside mainland China were included. Overall, the risk of quality assessment and publication bias was low. The diagnostic value of chest CT is similar between patients from within and outside mainland China (93, 91%). The pooled incidence of unilateral lung involvement (15, 7%), the crazy-paving sign (31, 21%), mixed ground-glass opacities (GGO) and consolidations (51, 35%), air bronchogram (44, 25%), vascular engorgement (59, 33%), bronchial wall thickening (19, 12%), and septal thickening (39, 26%) in patients from mainland China were significantly higher than those from outside; however, the incidence rates of bilateral lung involvement (75, 84%), GGO (78, 87%), consolidations (45, 58%), nodules (12, 17%), and pleural effusion (9, 15%) were significantly lower.
Conclusion: Considering that the chest CT features of patients in mainland China may not reflect those of the patients abroad, radiologists and clinicians should be familiar with various CT presentations suggestive of COVID-19 in different regions.
The epidemic of coronavirus disease 2019 (COVID-19), originally occurred in Wuhan, Hubei, China, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread worldwide (1, 2). As a public health emergency, COVID-19 is still an ongoing outbreak, and humans have been experiencing a relentless spread of variations of SARS-CoV-2 (3, 4), significantly undermining the domains of health, economy, environment, and society, unfortunately (5).
Given the fact that the transmission ability of COVID-19 is stronger and the incidence of mortality is relatively higher (6, 7), rapid and accurate diagnostic methods show their great significance for the prevention, control, and management of COVID-19. Real-time fluorescence polymerase chain reaction (RT-PCR) remains the current gold standard for COVID-19 diagnosis (8); however, this diagnosis method has some shortcomings: (1) it is very time-consuming to obtain the results after sampling and may lead to experimental errors caused by manual handling (9, 10), (2) there are a high rate of false-negative results (11), where there were even repeated negatives confirmed by other methods for patients when viral load is insufficient (12), (3) not all hospitals and clinics can implement these methods or the supply and quality of the reagents cannot keep up with the demand in time (13, 14), (4) the severity, progression, and the evaluation of patients cannot be judged or traced (11, 15, 16). Compared to several limitations mentioned above, chest computerized tomography (CT), as a routine and powerful tool for diagnosing viral pneumonia, has the advantage of timeliness, celerity, and high stability and sensibility (17, 18). The superiorities of chest CT in the diagnosis of COVID-19 have been approved and can be used as a quick and efficient method to detect COVID-19 (17–20), which is a significant alternative to RT-PCR testing for early diagnosis (17, 19, 21), especially when the results of RT-PCR are postponed or capacities are finite (22) or when the SARS-CoV-2 infection needs to be ruled out rapidly in those patients involved in emergency surgery (23, 24).
Nevertheless, a study by Kim et al. (25) observed that chest CT screening of patients with suspected COVID-19 had a low positive predictive value in a low prevalence region; several reviews and meta-analysis demonstrated that the considerable variation in the prevalence of disease severity and mortality was across different geographic regions (26–28). In a case-control study, Zhang et al. (29) discovered that a few patients with COVID-19, out of Wuhan but, from China lacked typical CT manifestations. All of the studies mentioned above indicated that the chest CT features of patients with COVID-19 may vary across different countries, territories, and regions. Hence, a comprehensive understanding of the suggestive features of chest CT based on specific countries or regions could help us to differentiate COVID-19 pneumonia and screen highly suspicious cases. Furthermore, explicitly stating whether chest CT findings of patients with COVID-19 in mainland China are applicable to those outside mainland China could provide indirect evidence, which contributes to the exchange of opinions and information, defeats the purpose of settling disputes, and adds to the literature on the CT performance of patients with SARS-CoV-2 infection, potentially enhancing the understanding of COVID-19. To the best of our knowledge, the distinctiveness of chest CT features has not been observed between patients with COVID-19 from within and outside mainland China.
We performed this meta-analysis, with the primary objective of quantitatively summarizing the results from published studies to date to compare and assess the differences in the diagnostic value and appearances of chest CT between patients with COVID-19 from within and outside mainland China, to provide a more precise estimate in detecting patients with COVID-19 in different regions. Our secondary objective of the systematic review was an attempt to clarify what causes the differences in chest CT appearances between patients with COVID-19 from within and those outside mainland China.
We searched PubMed, Embase, and Cochrane Library for studies reporting chest CT features of patients with COVID-19 published online before 15 April 2022. Search terms included (2019-nCoV) OR (2019 Novel Coronavirus) OR (COVID-19) OR (SARS-CoV-2) AND (chest CT), which were used as the subject or free words adjusted according to the different characteristics of the databases involved. We also manually searched the references of the studies included to retrieve any eligible studies. In addition, only articles in English were included. Two authors (LW and ML) independently screened the titles and abstracts and then carefully read the full texts to select suitable articles according to the inclusion and exclusion criteria. These included articles were separated into two groups according to patients in or outside mainland China. Meta-analyses of the two groups were performed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (30).
The following inclusion criteria were used to identify all eligible studies: (1) full-text original articles in English, (2) the study population including patients diagnosed with COVID-19, (3) cohort studies, case-control studies, or case studies consisting of at least five patients, (4) at least one of the observational indicators having chest CT features of COVID-19, and (5) the number of corresponding imaging features extractable in this study.
The exclusion criteria were (1) duplicate studies or study populations completely overlapping other studies, (2) full-texts nonaccessible or with a sample size <5, (3) studies that only reported the specificity or sensibility of chest CT, (4) patients that could not be labeled within or outside mainland China, (5) studies on pregnant women, and (6) corresponding outcome parameters or necessary data could not be acquired or separated even by contacting the author.
Two researchers independently extracted the following information from each included article: the first author's full name, countries/regions of patients, study sample size, diagnostic criteria, experimental design method, mean or median age, gender, the application of special CT or not, CT imaging manifestations, and the number of patients with abnormal CT results. If there was a disagreement, it was resolved by discussion or consultation with the third author. The content of the recorded lesion patterns on chest CT mainly included the following aspects: ground glass opacities (GGO), consolidations, GGO mixed consolidations, the crazy-paving sign, linear opacities, nodules, tree-in-bud appearance, air bronchogram, halo sign, adjacent pleural thickening, septal thickening, lymphadenopathy, pericardial effusion, pleural effusion, and vascular engorgement (bilateral or unilateral lungs). Because the included articles in this research were observational studies, we utilized the quality assessment of diagnostic accuracy studies (QUADAS) scale to evaluate study quality by two independent reviewers (BX and LH) (31).
Corresponding data from the two groups (patients in and outside mainland China) in the meta-analyses were pooled using single-arm analyses. Because some related data extracted from the original articles were too volatile, we used the double arcsine method to transform the incidence rates to a normal distribution, and then the transformed data were used in meta-analyses. We conducted the I2 statistics to analyze heterogeneity between the studies, and heterogeneity was considered significant or severe if the value of I2 was >50%, and in this situation, a random-effects model was utilized; otherwise, a fixed effects model was suitable when no statistical heterogeneity was observed. Pooled data for the results of meta-analyses were recalculated using the formula {p = [sin(tp/2)]2}. Publication bias was evaluated using Egger's regression test, and a p-value < 0.10 was statistically significant. Chest CT characteristics in the two groups were compared using the χ2 test or the Fisher's exact test when data were limited. A two-tailed p-value < 0.05 was considered statistically significant. All statistical analyses were performed using the R statistical computing language, version 3.6.1 (The R Foundation for Statistical Computing, Vienna, Austria, https://www.r-project.org).
Totally, 8,258 studies published online before 15 April 2022 were identified along with the search strategy and 3,441 studies remained after the exclusion of duplicates. Then, 3,245 studies were excluded by the title and abstract. After full-text review, 163 studies were excluded for the following reasons: full text not accessible (n = 11), population overlapping with other studies (n = 6), specificity or sensibility of CT alone (n = 24), sample size <5 (n = 18), studies on pregnant women (n = 10), comment, perspective, and correspondence (n = 27), editorial, review, and guideline (n = 35), and lack of extractable data (n = 32). Consequently, the remaining 33 independent studies that satisfy the inclusion and exclusion criteria were used in the current analyses, which had 3,954 patients with 3,449 having one or more abnormal CT imaging features and nearly all CT scans were carried out within 7 days after the onset of symptoms. Of these studies, 14 studies (32–45) with 554 participants reported at least one abnormal chest CT performance in 453 patients outside mainland China (i.e., Japan, USA, Australia, Tunisia, Turkey, Iran, Mexico, Brazil, Hong Kong, Taiwan, etc.) and 19 studies (16, 17, 46–62) with 3,400 patients reported at least one abnormal chest CT performance in 2,996 patients in mainland China. A detailed search procedure is summarized in Figure 1, and the characteristics of the included studies are outlined in Supplementary Table S1.
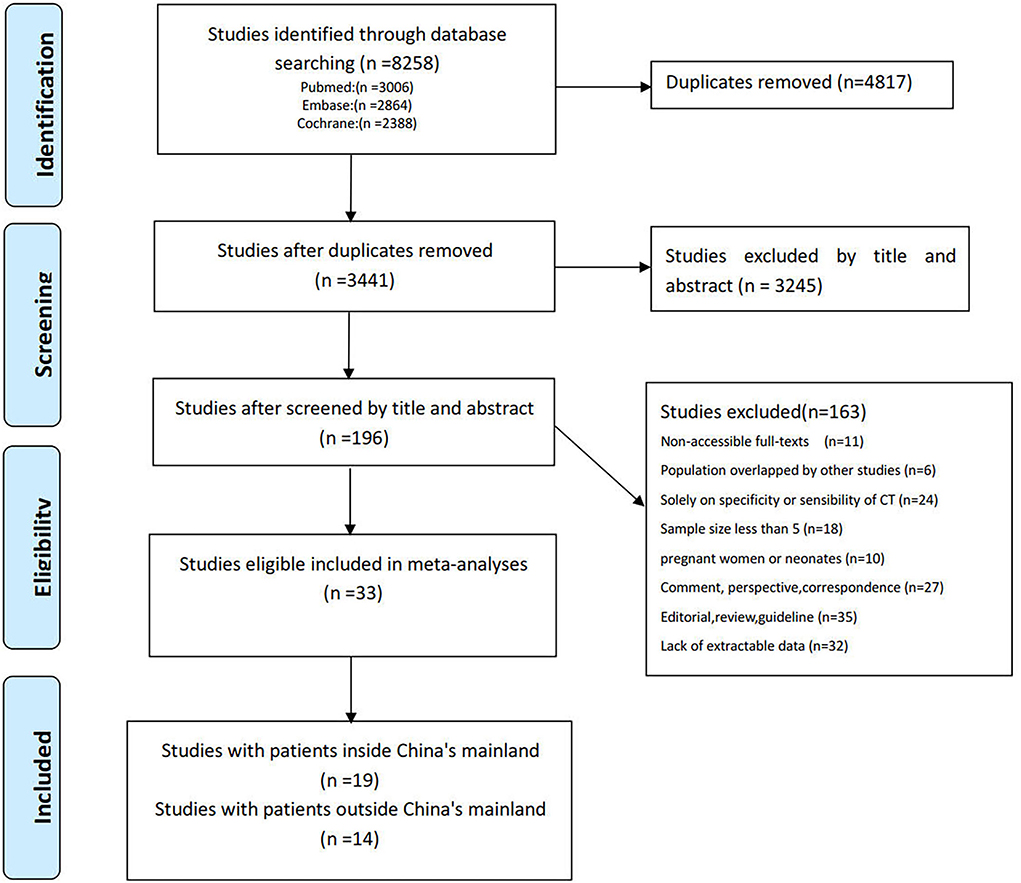
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) flow diagram of the study selection process.
The analysis of the QUADAS scale showed that the reporting quality of the articles included was better (Supplementary Table S2). Most of the included studies had a relatively low risk of bias in patient selection, index tests, reference standards, and patient flow. Meanwhile, the paucity of details in some studies raised a few concerns regarding applicability in those descriptions (Supplementary Figure S1).
There was substantial heterogeneity in most analyses of chest CT for patients with COVID-19 in different countries and regions; therefore, the random effects models were used in most of these meta-analyses. However, air bronchogram and nodule analyses in patients outside mainland China used the fixed-effects models in which heterogeneity was less obvious.
The pooled prevalence of positive chest CT for all patients was 93%, for patients in mainland China was 93%, and for patients outside was 91%, respectively. However, there was no significant difference in the diagnostic value of chest CT between patients from within and those outside mainland China. All results are shown in Figure 2 and Table 1.
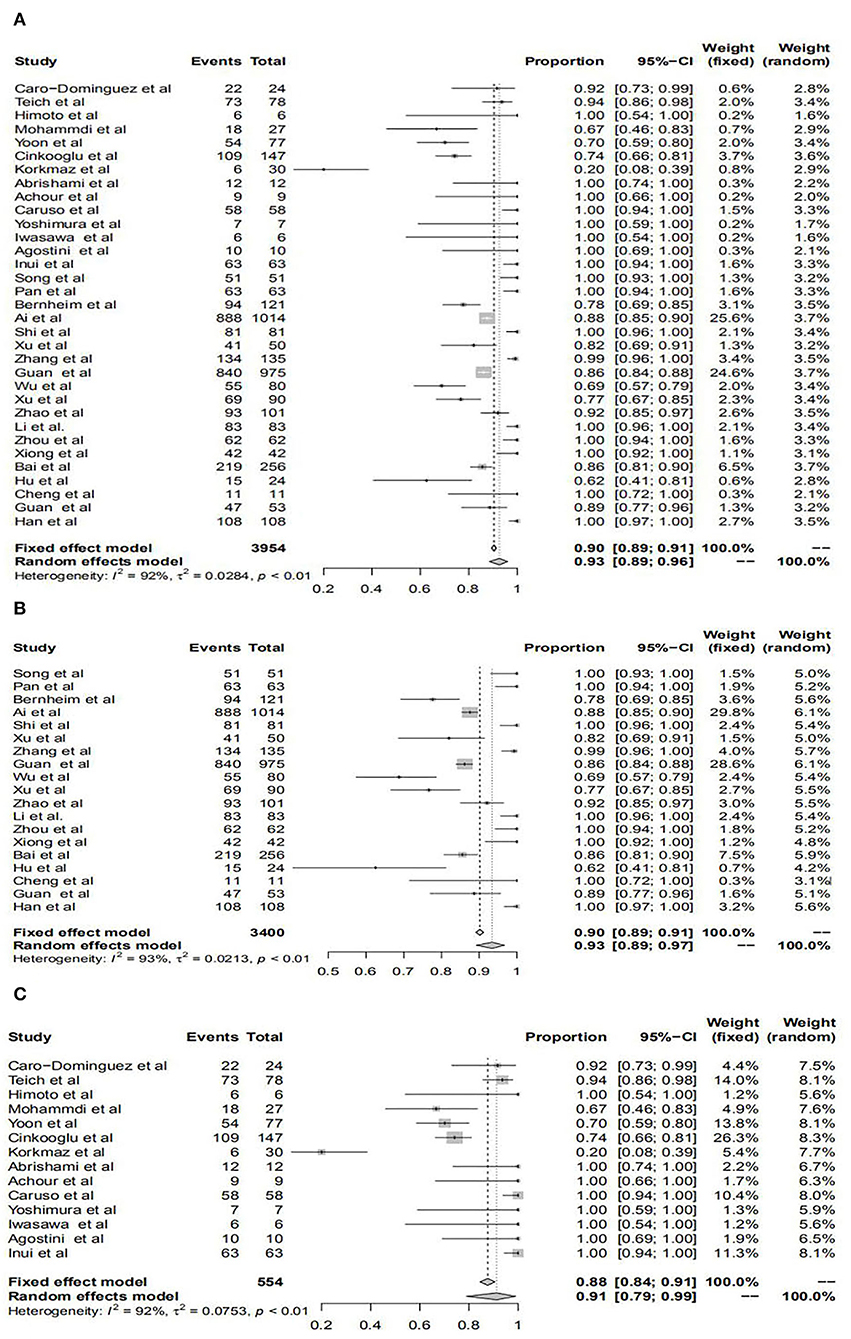
Figure 2. Forest plots of the diagnostic performance of chest computerized tomography (CT) in studies included in the meta-analyses. Forest plots show the prevalence of positive chest CT for all patients (A), patients within mainland China (B), and patients outside mainland China (C).
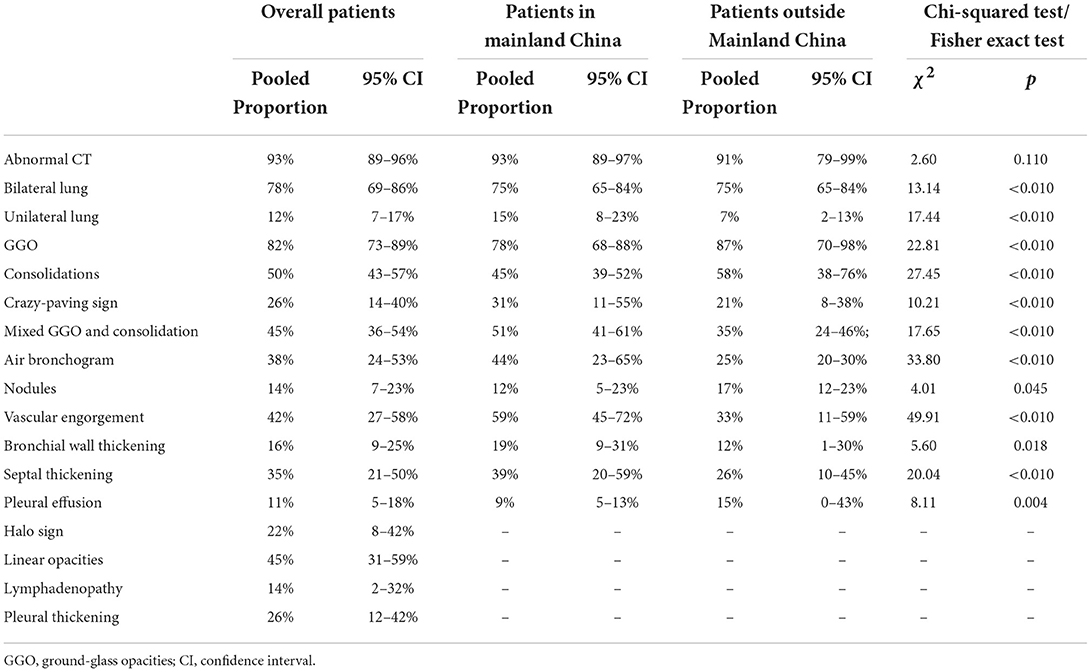
Table 1. Computerized tomography (CT) imaging findings in different analyses and comparisons in patients from within and outside mainland China.
The pooled transformed incidence rates of unilateral and bilateral lung involvement in all patients were 12% and 78%, respectively. For patients in mainland China, we found that the incidence rates of unilateral and bilateral lung involvement were 15 and 75%; however, these data were 7 and 84% for the outside, respectively. In addition, through the χ2 test, it was proven that there were significant differences in the incidence rates of both unilateral and bilateral lung infection manifested by chest CT for patients in and outside mainland China. All results are shown in Figure 3, Supplementary Figure S2, and Table 1.
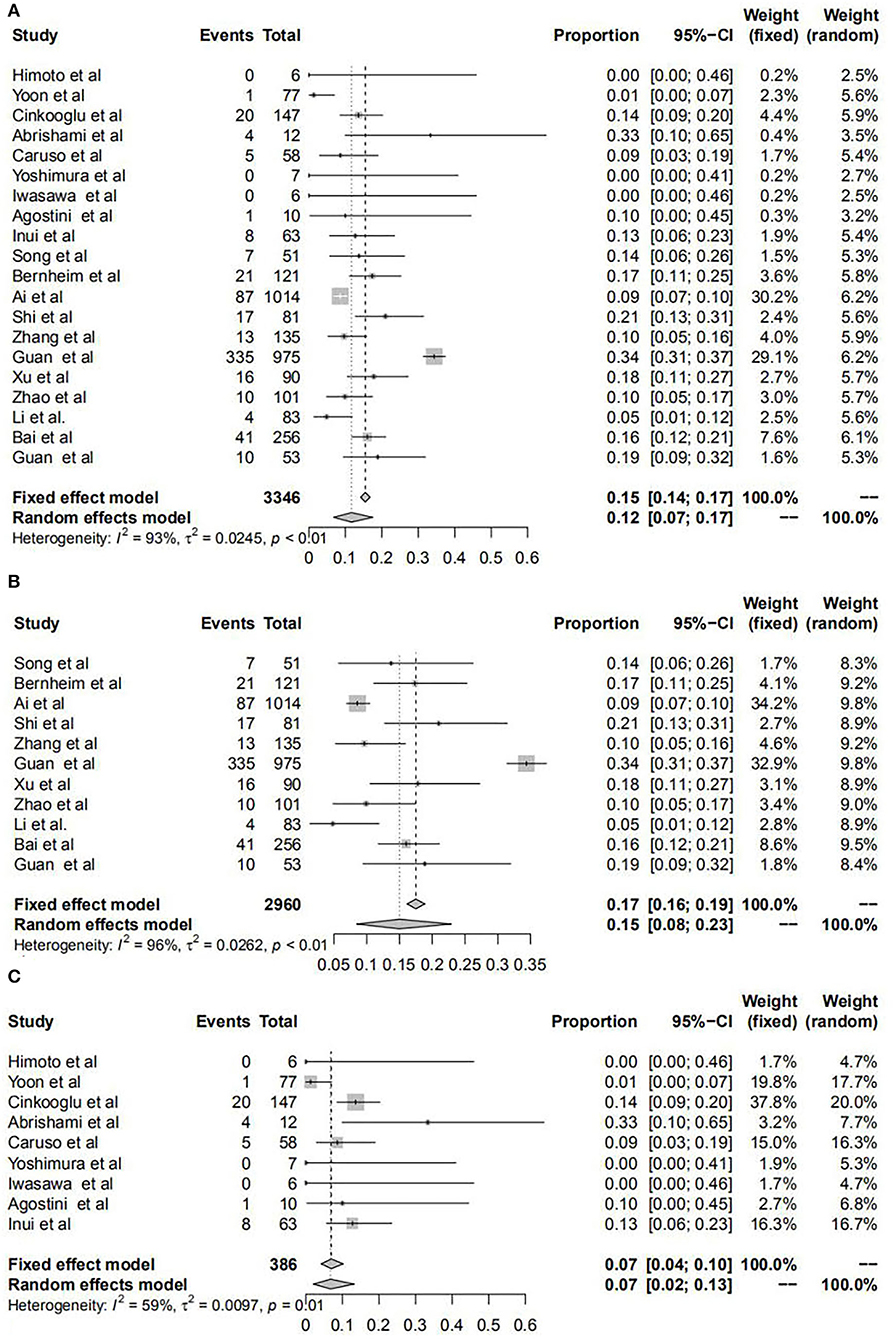
Figure 3. Forest plots of unilateral lung infection manifested by chest CT for patients with COVID-19. Forest plots show the transformed incidence rate of unilateral lung involvement in all patients (A), patients within mainland China (B), and patients outside mainland China (C). COVID-19: coronavirus disease 2019; CI, confidence interval.
We found that typical CT imaging appearances of patients both in and outside mainland China were GGO, consolidations, the crazy-paving sign, mixed GGO and consolidation, air bronchogram, nodules, vascular engorgement, bronchial wall thickening, septal thickening, pleural effusion, halo sign, linear opacities, lymphadenopathy, and pleural thickening. For GGO, the pooled incidence rates were 82% for all patients, 78% for patients in mainland China, and 87% for patients outside mainland China, respectively; for consolidations, the pooled incidence rates were 50, 45, and 58%, respectively; for the crazy-paving sign, the rates were 26, 31, and 21%, respectively; for mixed GGO and consolidation, the rates were 45, 51, and 35%, respectively; for air bronchogram, the rates were 38, 44, and 25%, respectively; for nodules, the rates were 14, 12, and 17%, respectively; for vascular engorgement, the rates were 42, 59, and 33%, respectively; for bronchial wall thickening, the rates were 16, 19, and 12%, respectively; for septal thickening, the rates were 35, 39, and 26%, respectively; for pleural effusion, the rates were 11, 9, and 15%, respectively; and for halo sign, linear opacities, lymphadenopathy, and pleural thickening, the pooled incidence rates of all patients were 22, 45, 14, and 26%, respectively. However, we could not extract the pooled incidence rates for patients in or outside mainland China. All results of subgroup analyses are shown in Supplementary Figures S3–S6 and Table 1.
There were obvious differences in the incidence rates of most typical CT imaging appearances between patients from within and those outside mainland China, and these CT appearances included GGO, consolidation, the crazy-paving sign, mixed GGO and consolidation, air bronchogram, nodules, vascular engorgement, bronchial wall thickening, septal thickening, and pleural effusion. Through the χ2 test, the incidence rates of the crazy-paving sign, mixed GGO and consolidation, air- bronchogram, vascular engorgement, bronchial wall thickening, and septal thickening in patients within mainland China were significantly higher than those outside; however, the incidence rates of GGO, consolidations, nodules, and pleural effusion were significantly lower. All results of subgroup analyses are summarized in Table 1.
The values of p derived from Egger's regression asymmetry test for most observational indicators suggested that the publication bias was not obvious (Table 2). There was a low probability of publication bias in the following subanalyses: abnormal CT, bilateral lungs, unilateral lungs, consolidations, the crazy-paving sign, mixed GGO and consolidation, vascular engorgement, bronchial wall thickening, pleural effusion, halo sign, linear opacities, lymphadenopathy, and pleural thickening of overall patients; abnormal CT, bilateral lung, unilateral lung, consolidations, mixed GGO and consolidation, nodules, and vascular engorgement of patients in mainland China; and GGO, consolidations, the crazy-paving sign, mixed GGO and consolidation, air bronchogram, nodules, vascular engorgement, bronchial wall thickening, septal thickening, and pleural effusion of patients outside mainland China. The publication bias of these subanalyses (halo sign, linear opacities, lymphadenopathy, and pleural thickening of patients either in or outside mainland China) could not be evaluated for fewer included studies in each subgroup.
In our present study, we focused on investigating the disagreement of typical chest CT characteristics between patients with COVID-19 from within and outside mainland China. Above all, our meta-analysis reinforces the high proportion of COVID-19 detected by chest CT. Our results showed that the pooled incidence rates of abnormal chest CT for all patients were 93% (95% confidence interval (CI): 89–96%), which were similar to several other studies, such as 89.76% (95% CI: 84–94%) by Bao et al. (63) and 94% (95%CI: 91–96%) by Kim et al. (25). This study once again suggested that chest CT could be used as a vital diagnostic tool for COVID-19. Although the pooled incidence rate of abnormal chest CT for patients in mainland China was higher than those outside (93 vs. 91%), this difference did not reach a statistical significance, indicating that chest CT for screening of patients with COVID-19 in different regions deserved recognition.
Regarding bilateral or unilateral lung involvement, we discovered several intriguing results. The pooled incidence rates of bilateral lung involvement in all patients were 78% and indicated that COVID-19 infection most commonly affected bilateral lungs, which was consistent with the results of 78.2% by Bao et al. (63) and 73.8% by Zhu et al. (64). For subgroup analyses, we found that the incidence rates of unilateral lung involvement for patients in mainland China were significantly higher than those outside (15 vs. 7%) and the incidence rates of bilateral lung involvement were significantly low (75 vs. 84%). However, there are no studies addressing these differences in the distribution of COVID-19 infection among patients within different regions. Ooi et al. (65) and Das et al. (66) pointed out that unilateral lung involvement was more common in the early stage of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) and that unilateral involvement of pneumonic infiltrates at a later stage is very rare. However, bilateral lung involvement seemed to be a unique imaging characteristic for COVID-19 although these three respiratory viruses all belong to the family of coronaviruses and the CT features are similar. The results of Bao et al. (63) showed that the incidence of bilateral lung infection had risen to 82% from 78% when they excluded studies without mentioning thin-section chest CT, which might mean that a special CT could show lung changes in more detail. In our meta-analysis, 58% of studies (11/19) for patients in mainland China and 21% (3/14) for those outside reported the use of a special CT for COVID-19 diagnosis, respectively. The higher proportion of the use of special CT in mainland China may help recognize and dismiss the false-positive rate of bilateral lung infection. However, several studies revealed that very basic instruments, low radiation doses, or improper practices might not influence the performance of chest CT in the diagnosis of COVID-19. Dai et al. (67) found that the image quality of the shelter hospital CT (CT Ark) had no obvious difference compared to ordinary CT (Brilliance 64) based on subjective observations. Andrea et al. (68) and Niu et al. (69) recognized that a low radiation dose could still provide sufficient diagnostic quality to exclude COVID-19. Bernardo et al. (70) deemed that there was little difference in the distribution and lobar extent of pulmonary lesions on chest CT despite substantial differences in CT usage. Moreover, the CT images included in the articles were read by two or more senior radiologists with a host of experience. Here, the use of a special CT instrument might not be the cause of such differences.
We also demonstrated that all CT features and corresponding pooled incidence rates were in accordance with previous meta-analyses (63, 64); however, large gaps in the incidence rates of CT features between patients from within and outside mainland China were striking and all differences reached statistical significance. These results that CT patterns of patients with COVID-19 from within mainland China may not reflect those outside mainland China should be interpreted with caution, and the evidence provided to elucidate why features differ between those from within and outside mainland China is indirect and even anecdotal. Because few analyses of anatomical–pathological–radiological correlations have been carried out and studies on the formation of imaging features are scarce and superficial, immune damage from inflammatory responses and deep airway and alveolar epithelial injuries from direct virus attack account for these pathological changes in the lungs (71–73). Angiotensin-converting enzyme-2 (ACE2) is used by SARS-CoV-2 as a cell receptor in human lungs, causing interstitial damage first and parenchymal lesions later (71). We speculate that the coexistence of SARS-CoV-2 with different mutation patterns, race and ethnicity, and other atypical cases of pneumonia may contribute to explain why features differ between patients from within and outside mainland China. In our opinion, pathology is the observation of microanatomy and radiology is the observation of gross anatomy; therefore, CT performances will be different according to the change in viral pathologic manifestations. Wabalo et al. (74) reviewed multiple literature studies and concluded that the high mutation rate of SARS-CoV-2 is likely to change the properties of the virus, including its virulence or infectivity, and may have higher viral loads and develop more severe clinical manifestations. Song et al. (75) found that the infection with the B.1.1.7 variant could lead to a more severe inflammatory response and more severe pneumonia, which implied that this variant might have higher pathogenicity than previous wild type and variants. Zhang et al. (29) found that some of these patients with COVID-19 from other cities in China outside Wuhan lacked the typical CT imaging features, and the results can be attributed to the majority of infections being second-generation human-to-human transmissions. Wu et al. (76) found that the proportion of halo signs and reversed halo signs had increased greatly in patients with SARS-CoV-2 Delta pneumonia. McLaren et al. (77) discovered that the new “bullseye sign,” a variant of the reverse halo sign, could alert clinicians to the possibility of COVID-19, which, we reasoned, might be related to the mutation of the virus. Cheng et al. (78) detected that chest CT changes with the Delta COVID-19 variant were milder than pre-existing strains and considered that the pathological features of the mutated virus could change under the pressure of immune surveillance. Although the radiological severity of alpha variant infection was not increased compared to the original virus, Tsakok et al. (79) showed that the CT severity scores applied to angiography is associated with the COVID-19 outcome. Considering the fact that immune damage is one of the main reasons for pulmonary tissue injury (71–73) and that ethnic differences in immune status in genetic polymorphism are associated with immune-mediated diseases. Therefore, it is reasonable to presume that racial disparity is partly responsible for the different features of chest CT between patients from within and outside mainland China (80). The findings of Ahmed et al. (81) directly showed that there were large variations in radiological manifestations between different ethnic groups, such as Egyptians, Saudis, Indians, Bangladeshis, and so on. In a COVID-19 cohort, Smith et al. (82) observed significant differences in the breadth and strength of the humoral immune response in relation to ethnicity, which might reflect differences in genetic factors. The expression of ACE2, which plays a significant role in determining ethnic susceptibility and protecting pulmonary parenchyma from deterioration due to COVID-19, differs among the world's three main racial groups: Africans, Asians, and Caucasians, revealing that Asians have a significantly higher ACE2 expression in various organs, and the Black population shows a reduced molecular expression of ACE2 compared to other races (83, 84). Furthermore, Li et al. (85) reported that the use of ACE inhibitors/angiotensin receptor blockers (ARBs) was associated with a significant reduction in mortality among African-American patients with COVID-19 positive in the hospital, and Pabalan et al. (86) recommended that ACE2 genotypes might be useful for acute lung injury (ALI)/ARDS therapy for patients with COVID-19. Antoon et al. (87) showed that there were differences in the severity of COVID-19 pneumonia by race and ethnicity but additional research is needed to clarify the sources of such disparities. Meanwhile, Wu et al. found that the 3p21.31 locus is the risk haplotype specific to Europeans and South Asians, but the MEF2B variant specific to East Asians confers an eight-fold increase in the risk of COVID-19 severity (88). In addition, the degree of discrepancies within ethnicity and race also influences the COVID-19 clinical manifestation. For example, Upadhyai et al. (89) revealed that asymptomatic patients with COVID-19 in Europe possessed discernibly higher proportions of the Ancestral North Eurasian, which may be associated with the pathways that govern host immunity, such as interferon, interleukin, cytokine signaling. CT appearances of COVID-19 may overlap or interweave with other viral pneumoniae. Garrana et al. (90–92) reported that the prevalence of influenza viruses, parainfluenza virus, adenovirus, respiratory syncytial virus, rhinovirus, human metapneumovirus, etc. was not the same in different regions, which might be another reason concerning the different features between patients within or outside mainland China. Similar to COVID-19, most cases of viral pneumonia involved both the lungs and multiple lung lobes with a predominant distribution in the posterior and peripheral parts of the lungs; however, CT findings for COVID-19 overlap substantially with those of influenza to a greater extent (93). Li et al. (94) suggested that CT is still limited in identifying specific viruses and distinguishing between the viruses and a new method has been created, which can accurately discriminate COVID-19 from other types of pneumonia (95). Cheng et al. (92) found that patients with COVID-19 with influenza A virus had a significantly lower CT score than those with only SARS-CoV-2 infection, indicating that co-infection may alleviate inflammation in the lungs. Moreover, Zhang et al. (96) emphasized that the pulmonary changes in radiological findings did not show any difference in their location or distribution between patients with COVID-19 and those with seasonal influenza. Investigation of SARS-CoV-2 co-infection with other respiratory viruses is essential to provide novel insights into the development of highly sensitive diagnostics in different regions.
Although the CT findings in patients with COVID-19 are different on various days, it is reasonable to assume that chest CT performance within 7 days is relatively constant and can be compared between patients from within and outside mainland China in our research. First, in clinical studies, monitoring the dynamic changes of chest CT in patients with COVID-19 every day would be unrealistic and unnecessary. In a systematic review and meta-analysis on dynamic changes in COVID-19 images, Zhou et al. (97) revealed that, from 0 (negative CT) to 5 days (after firs positive CT), progressive deterioration of the lesions in the lungs, fluctuations of lesions (gradually improved and absorbed or gradually improved and absorbed after reaching the peak), seemingly did not achieve statistical significance. In a longitudinal study, Wang et al. (98) found that the common predominant pattern of chest CT had no changes on illness days 0–5 compared to days 12–17. For instance, the percentages of GGO and consolidation were 62%/23% on illness days 0–5 and 59%/24% on illness days 6–11, respectively. Second, chest CT changes in the course of COVID-19 were divided into two to three stages in most of the literature studies, including early stage, the progressive stage, and/or the severe stage, which are in accordance with the pathophysiological process of COVID-19 (48, 97, 98). Shi et al. (48) described and compared CT findings at different timepoints throughout the COVID-19 course and the grouping method was based on the interval between the onset of symptoms and the first CT scan: scans done before the onset of symptoms were group 1 and ≤ 1 week after the onset of symptoms were group 2. Zhou et al. (53) analyzed the CT features of COVID-19 and the dynamic changes of chest CT imaging features, and the course of the disease was divided into an early phase (≤ 7 days after the onset of symptoms) and an advanced phase (8–14 days after the onset of symptoms). Another study on the imaging features and the evolution of CT in patients with COVID-19 pneumonia showed that the early rapid progressive stage was 1–7 days from the onset of symptoms and the advanced stage with peak levels of abnormalities on CT was 8–14 days (99). Chang et al. (100) analyzed the CT findings of asymptomatic patients with COVID-19 and showed the differences between abnormal CT findings in the early phase (≤ 7 days after the onset of symptoms) and those in the advanced phase (8–14 days after the onset of symptoms).
Chest CT findings in COVID-19 are known to be different according to gender, age, and SARS-CoV-2 mutation (93, 101, 102). The reasons behind these disparities remain unclear; however, genetic, immunological, and social differences may be the essential contributing factors (103–105). However, it might seem unpractical and unnecessary to account for age, gender, and mutations based on the current meta-analysis. First, none of the included studies provided specific information on patient-level age, gender, and mutations, and the parameters could not be justified based solely on the data provided by the included studies. Second, no methodology that we have retrieved could justify these parameters in the type of meta-analysis and the methodology is imperfect and developing. Third, the existing literature found no significant difference in chest CT findings between men and women (106). Although age, gender, and mutations are important factors that evidently influence chest CT performance, last but not at least, the aim of this meta-analysis was to review the different CT features in patients with COVID-19 from within and outside mainland China to help radiologists and clinicians become more familiar with the disease, thus, including patients across all conditions might seem to be well-rounded.
Radiologists could accurately identify patients with COVID-19 positive using standard CT diagnostic procedures; however, there were obvious subjective factors and a lack of objective quantitative standards in the quantitative score of CT images to identify COVID-19 at the moment. Hence, the Radiological Society of North America (RSNA), the British Society of Thoracic Imaging (BSTI), the Dutch Radiological Society (COVID-19 Reporting and Data System, CO-RADS), etc., issued their expert consensus document to standardize COVID-19 CT findings and reports (107–109), among which RSNA is the most accepted structural reporting system (108, 110, 111). There is a bias in sensitivity values in most chest CT studies that do not include RSNA structural reporting system or others. Uysal et al. (112) reported that one-quarter of patients with asymptomatic COVID-19 had a normal chest CT. Kavak et al. (109) emphasized that false-negative patients should not be neglected in the RSNA and BSTI systems. Although the near perfect consistent and reproducible in the positive predictive value were observed in the system of RSNA and CO-RADS′ O′ Neill et al. (108) found that end-users preferred RSNA system for it's reporting language. From the experience of Falaschi et al. (113), the clinical and epidemiological features should be taken into account when using chest CT for the diagnosis of COVID-19. In addition, machine learning plays a pivotal role in the detection of COVID-19 pneumonia. For example, Herath et al. (114) developed an algorithm based on GGO with an accuracy of 92.8% and a precision of 0.931. On the one hand, studies that include the RSNA structural reporting system or machine learning algorithm should be included in this meta-analysis, and data for those that have and those that do not have the RSNA structural reporting system or machine learning algorithm should be presented separately. However, these parameters cannot be represented or extracted in our article. On the other hand, such worries that these data without the RSNA system are probably related to differences in outcome may not be necessary. Although without standards or quantitative scoring and with high subjectivity in these studies included in the manuscript, there are several reasons to think that the “qualitative evaluation” might not influence the differences in chest CT features between patients from within and outside mainland China: (1) though radiologists in the USA had minimal specific training to diagnose COVID-19 and Chinese radiologists practiced in an area with a relatively low prevalence of the disease, these radiologists could distinguish COVID-19 with high specificity and moderate sensitivity, which demonstrated that radiologists in and outside mainland China can identify COVID-19 with a high degree of agreement and high diagnosis accuracy (61). (2) Few literature studies reported that the corresponding standard or score (such as the CO-RADS lexicon) could help radiologists at different levels of experience to accurately distinguish patients with COVID-19 positive (115); however, the CT images included in this article were read by two or more senior radiologists with a host of experience, making the role of “standards” nonsignificant. (3) The sensitivity of manual qualitative analyses of chest CT for the diagnosis of COVID-19 within and outside mainland China is high (from 92 to 96%) (116–118), which is helpful for the early recognition of suspected cases, and the contribution of “standards” to sensitivity is really limited.
Our meta-analysis has several advantages. First, it was the first time to put forward the hierarchy of CT manifestation for patients in different regions, which could guide the clinical work. Second, a wide range of search strategies but strict inclusion criteria were used to minimize the possibility of publication bias. Third, compared to prior studies, we excluded non-English literature to ensure the accuracy of the results. Finally, the included studies were conducted in different countries or regions, which made the results more representative.
Nevertheless, our present meta-analysis inevitably has some disadvantages and limitations. First, all of the included studies were comparative trials or cohort studies that did not provide the powerful statistical power that a randomized controlled trial could do. Second, neither the different degrees of severity for patients with COVID-19 (asymptomatic, mild, moderate, or severe) nor possible comorbidities or chronic diseases could be distinguished from most of the included studies. Third, patients outside mainland China were fewer than those inside, which may bias their estimates. Finally, some of the subgroup analyses for patients within mainland China showed some publication biases, which might affect the accuracy of the results. Future studies into COVID-19 should focus on exploring the mechanism of the significant differences in chest CT in different regions, which could provide more experience and evidence regarding COVID-19 diagnosis and management.
In conclusion, there is no doubt that chest CT plays an important role in the detection of patients with suspected COVID-19; however, the CT patterns of patients in mainland China cannot reflect those of the patients outside mainland China. Given substantial differences in chest CT between patients from within and outside mainland China and the low specificity in differentiating cases of viral pneumonia, radiologists and clinicians should be familiar with various CT manifestations suggestive of COVID-19 in different regions and should be carefully adjudicated.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
NH and KW designed this study. LW, ML, BX, and LH conducted literature searches and screening, literature data extraction, and statistical analysis. MW, MG, and SL checked the extracted data. ML, BX, and MG wrote the first draft. RZ, KW, and NH corrected the manuscript and supervised the conduct of the study. All authors have read and approved the final submitted version.
This work was supported by grants from the Science and Technology Development Project of Medical and Health of Shandong Province (Nos. 202010000131 and 202104070065) and the Key Research and Development Project of Zibo (Policy Guidance Program, No. 2020ZC010048).
We would like to acknowledge Dr. Lefeng Zhang (a radiologist working in the radiology department of Zibo central hospital) for his help in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.939095/full#supplementary-material
Supplementary Figure S1. Methodological evaluation according to Quality Assessment of Diagnostic Accuracy Studies (QUADAS) of the included studies by grouped bar charts, proportion of studies with low, high, or unclear risk of bias (A), and concerns regarding applicability (B).
Supplementary Figure S2. Forest plots of bilateral lung infection manifested by chest computerized tomography (CT) for patients with COVID-19. Forest plots show the transformed incidence rate of bilateral lung involvement in all patients (A), patients in mainland China (B), and patients outside mainland China (C). COVID-19: coronavirus disease 2019; CI, confidence interval.
Supplementary Figure S3. Forest plots of subgroup analyses of typical CT imaging appearances by chest CT for overall patients with COVID-19: GGO (A), consolidation (B), crazy-paving sign (C), mixed GGO and consolidation (D), air bronchogram (E), nodules (F), vascular engorgement (G), bronchial wall thickening (H), septal thickening (I), and pleural effusion (J). COVID-19: coronavirus disease 2019; CI, confidence interval; GGO, ground-glass opacities.
Supplementary Figure S4. Forest plots of subgroup analyses of typical CT imaging appearances by chest CT for patients with COVID-19 within mainland China: GGO (A), consolidation (B), crazy-paving sign (C), mixed GGO and consolidation (D), air bronchogram (E), nodules (F), vascular engorgement (G), bronchial wall thickening (H), septal thickening (I), and pleural effusion (J). COVID-19: coronavirus disease 2019; CI, confidence interval; GGO: ground-glass opacities.
Supplementary Figure S5. Forest plots of subgroup analyses of typical CT imaging appearances by chest CT for patients with COVID-19 outside mainland China: GGO (A), consolidation (B), crazy-paving sign (C), mixed GGO and consolidation (D), air bronchogram (E), nodules (F), vascular engorgement (G), bronchial wall thickening (H), septal thickening (I), and pleural effusion (J). COVID-19: coronavirus disease 2019; CI, confidence interval; GGO: ground-glass opacities.
Supplementary Figure S6. Forest plots of subgroup analyses of typical CT imaging appearances by chest CT for overall patients with COVID-19: halo sign (A), linear opacities (B), lymphadenopathy (C), and pleural thickening (D). COVID-19: coronavirus disease 2019; CI, confidence interval; GGO: ground-glass opacities.
Supplementary Table S1. The characteristics of the included studies.
Supplementary Table S2. Risk of bias assessment of the included studies according to the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) scale.
COVID-19, Coronavirus disease 2019; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; RT-PCR, Reverse transcription-polymerase chain reaction; CT, Computerized tomography; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines; GGO, Ground-glass opacities; QUADAS, Quality assessment of diagnostic accuracy studies; HRCT, Ultra-high-resolution CT; HD-DECT, High-dose dual-energy acquisition CT; LDCT, Low-dose CT; CI, Confidence interval; SARS, Severe acute respiratory syndrome; MERS, Middle East respiratory syndrome; ACE2, Angiotensin-converting enzyme-2; ARBs, Angiotensin receptor blockers; ALI, Acute lung injury; RSNA, the Radiological Society of North America; BSTI, the British Society of Thoracic Imaging; CO-RADS, the Dutch Radiological Society (COVID-19 Reporting and Data System).
1. Zhou T, Guan R, Rosenthal SL, Moerdler S, Guan Z, Sun L. Supporting health-care workers and patients in quarantine wards: evidence from a survey of frontline health-care workers and inpatients with COVID-19 in Wuhan, China. Front Public Health. (2021) 9:705354. doi: 10.3389/fpubh.2021.705354
2. Li RYM, Yue XG, Crabbe MJC. COVID-19 in Wuhan, China: pressing realities and city management. Front Public Health. (2021) 8:596913. doi: 10.3389/fpubh.2020.596913
3. Temsah MH, Aljamaan F, Alenezi S, Alhasan K, Alrabiaah A, Assiri R, et al. SARS-CoV-2 omicron variant: exploring healthcare workers' awareness and perception of vaccine effectiveness: a national survey during the first week of WHO variant alert. Front Public Health. (2022) 10:878159. doi: 10.3389/fpubh.2022.878159
4. Barrera-Avalos C, Luraschi R, Acuña-Castillo C, Vidal M, Mella-Torres A, Inostroza-Molina A, et al. Description of symptoms caused by the infection of the SARS-CoV-2 B. 1621 (Mu) Variant in Patients With Complete CoronaVac Vaccination Scheme: First Case Report From Santiago of Chile. Front Public Health. (2022) 10:797569. doi: 10.3389/fpubh.2022.797569
5. Mofijur M, Fattah IMR, Alam MA, Islam ABMS, Ong HC, Rahman SMA, et al. Impact of COVID-19 on the social, economic, environmental and energy domains: Lessons learnt from a global pandemic. Sustain Prod Consum. (2021) 26:343–59. doi: 10.1016/j.spc.2020.10.016
6. Yesudhas D, Srivastava A, Gromiha MM. COVID-19 outbreak: history, mechanism, transmission, structural studies and therapeutics. Infection. (2021) 49:199–213. doi: 10.1007/s15010-020-01516-2
7. Chang SL, Cliff OM, Zachreson C, Prokopenko M. Simulating transmission scenarios of the delta variant of SARS-CoV-2 in Australia. Front Public Health. (2022) 10:823043. doi: 10.3389/fpubh.2022.823043
8. Rabaan AA, Tirupathi R, Sule AA, Aldali J, Mutair AA, Alhumaid S, et al. Viral dynamics and real-time RT-PCR Ct values correlation with disease severity in COVID-19. Diagnostics (Basel). (2021) 11:1091. doi: 10.3390/diagnostics11061091
9. Ahmadzadeh M, Vahidi H, Mahboubi A, Hajifathaliha F, Nematollahi L, Mohit E. Different respiratory samples for COVID-19 detection by standard and direct quantitative RT-PCR: a literature review. Iran J Pharm Res. (2021) 20:285–99. doi: 10.22037/ijpr.2021.115458.15383
10. Sureka B, Garg PK, Saxena S, Garg MK, Misra S. Role of radiology in RT-PCR negative COVID-19 pneumonia: Review and recommendations. J Family Med Prim Care. (2021) 10:1814–7. doi: 10.4103/jfmpc.jfmpc_2108_20
11. Li Y, Yao L, Li J, Chen L, Song Y, Cai Z, et al. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J Med Virol. (2020) 92:903–8. doi: 10.1002/jmv.25786
12. Beneš J, DŽupová O, Poláková A, Sojková N. Repeatedly negative PCR results in patients with COVID-19 symptoms: Do they have SARS-CoV-2 infection or not? Epidemiol Mikrobiol Imunol. (2021) 70:3–9.
13. Alsharif W, Qurashi A. Effectiveness of COVID-19 diagnosis and management tools: a review. Radiography (Lond). (2021) 27:682–7. doi: 10.1016/j.radi.2020.09.010
14. Wernike K, Keller M, Conraths FJ, Mettenleiter TC, Groschup MH, Beer M. Pitfalls in SARS-CoV-2 PCR diagnostics. Transbound Emerg Dis. (2021) 68:253–7. doi: 10.1111/tbed.13684
15. Karimi F, Saleh M, Vaezi AA, Qorbani M, Avanaki FA. Clinical and laboratory findings and PCR results in severe and non-severe COVID19 patients based on CURB-65 and WHO severity indices. Virol J. (2021) 18:189. doi: 10.1186/s12985-021-01658-1
16. Wu J, Wu X, Zeng W, Guo D, Fang Z, Chen L, et al. Chest CT Findings in patients with coronavirus disease 2019 and its relationship with clinical features. Invest Radiol. (2020) 55:257–61. doi: 10.1097/RLI.0000000000000670
17. Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. (2020) 296:E32–40. doi: 10.1148/radiol.2020200642
18. Gu J, Yang L, Li T, Liu Y, Zhang J, Ning K, et al. Temporal relationship between serial RT-PCR results and serial chest CT imaging, and serial CT changes in coronavirus 2019 (COVID-19) pneumonia: a descriptive study of 155 cases in China. Eur Radiol. (2021) 31:1175–84. doi: 10.1007/s00330-020-07268-9
19. Özkarafakili MA, Özkurt H, Bardakçi MI, Akilli IK, Yanç U, Altuntaş Y, et al. Comparison of chest computed tomography findings of RT-PCR negative and RT-PCR positive cases in COVID-19 patients. Clin Imaging. (2022) 82:7–12. doi: 10.1016/j.clinimag.2021.10.013
20. Waller JV, Allen IE, Lin KK, Diaz MJ, Henry TS, Hope MD. The limited sensitivity of chest computed tomography relative to reverse transcription polymerase chain reaction for severe acute respiratory syndrome coronavirus-2 infection: a systematic review on COVID-19 diagnostics. Invest Radiol. (2020) 55:754–61. doi: 10.1097/RLI.0000000000000700
21. Gross A, Heine G, Schwarz M, Thiemig D, Gläser S, Albrecht T. Structured reporting of chest CT provides high sensitivity and specificity for early diagnosis of COVID-19 in a clinical routine setting. Br J Radiol. (2021) 94:20200574. doi: 10.1259/bjr.20200574
22. Baksh S, Volodko N, Soucie M, Geier SB, Diep A, Rozak K, et al. Extractionless nucleic acid detection: a high capacity solution to COVID-19 testing. Diagn Microbiol Infect Dis. (2021) 101:115458. doi: 10.1016/j.diagmicrobio.2021.115458
23. De Simone B, Chouillard E, Sartelli M, Biffl WL, Di Saverio S, Moore EE, et al. The management of surgical patients in the emergency setting during COVID-19 pandemic: the WSES position paper. World J Emerg Surg. (2021) 16:14. doi: 10.1186/s13017-021-00349-0
24. Griswold D, Gempeler A, Rosseau G, Kaseje N, Johnson WD, Kolias A, et al. Chest computed tomography for the diagnosis of COVID-19 in emergency trauma surgery patients who require urgent care during the pandemic: protocol for an umbrella review. JMIR Res Protoc. (2021) 10:e25207. doi: 10.2196/25207
25. Kim H, Hong H, Yoon SH. Diagnostic performance of CT and reverse transcriptase polymerase chain reaction for coronavirus disease 2019: a meta-analysis. Radiology. (2020) 296:E145–55. doi: 10.1148/radiol.2020201343
26. Alves da. Costa F, Andersen Y, Ferreira-Borges C. Success in vaccination efforts of vulnerable populations in the WHO/European Region: focus on prisons. Front Public Health. (2021) 9:738422. doi: 10.3389/fpubh.2021.738422
27. Patoulias D, Katsimardou A, Stavropoulos K, Imprialos K, Kalogirou MS, Doumas M. Renin-angiotensin system inhibitors and COVID-19: a systematic review and meta-analysis. evidence for significant geographical disparities. Curr Hypertens Rep. (2020) 22:90. doi: 10.1007/s11906-020-01101-w
28. Chua PEY, Shah SU, Gui H, Koh J, Somani J, Pang J. Epidemiological and clinical characteristics of non-severe and severe pediatric and adult COVID-19 patients across different geographical regions in the early phase of pandemic: a systematic review and meta-analysis of observational studies. J Investig Med. (2021) 69:1287–96. doi: 10.1136/jim-2021-001858
29. Zhang H, Du F, Cao XJ, Feng XL, Zhang HP, Wu ZX, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in patients out of Wuhan from China: a case control study. BMC Infect Dis. (2021) 21:207. doi: 10.1186/s12879-021-05897-z
30. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
31. Henningsson AJ, Aase A, Bavelaar H, Flottorp S, Forsberg P., Kirkehei I, et al. Laboratory methods for detection of infectious agents and serological response in humans with tick-borne infections: a systematic review of evaluations based on clinical patient samples. Front Public Health. (2021) 9:580102. doi: 10.3389/fpubh.2021.580102
32. Caro-Dominguez P, Shelmerdine SC, Toso S, Secinaro A, Toma P, Damasio MB, et al. Thoracic imaging of coronavirus disease 2019 (COVID-19) in children: a series of 91 cases. Pediatr Radiol. (2020) 50:1354–68. doi: 10.1007/s00247-020-04747-5
33. Himoto Y, Sakata A, Kirita M, Hiroi T, Kobayashi KI., Kubo K, et al. Diagnostic performance of chest CT to differentiate COVID-19 pneumonia in non-high-epidemic area in Japan. Jpn J Radiol. (2020) 38:400–6. doi: 10.1007/s11604-020-00958-w
34. Mohammadi A, Mohebbi I, Khademvatani K, Pirnejad H, Mirza-Aghazadeh J, Gharebaghi N, et al. Clinical and radiological characteristics of pediatric patients with COVID-19: focus on imaging findings. Jpn J Radiol. (2020) 38:987–92. doi: 10.1007/s11604-020-01003-6
35. Yoon SH, Lee KH, Kim JY, Lee YK, Ko H, Kim KH, et al. Chest Radiographic and CT Findings of the 2019 Novel Coronavirus Disease (COVID-19): Analysis of Nine Patients Treated in Korea. Korean J Radiol. (2020) 21:494–500. doi: 10.3348/kjr.2020.0132
36. Çinkooglu A, Hepdurgun C, Bayraktaroglu S, Ceylan N, Savaş R, CT. imaging features of COVID-19 pneumonia: initial experience from Turkey. Diagn Interv Radiol. (2020) 26:308–14. doi: 10.5152/dir.2020.20307
37. Abrishami A, Samavat S, Behnam B, Arab-Ahmadi M, Nafar M, Sanei Taheri M. Clinical course, imaging features, and outcomes of COVID-19 in kidney transplant recipients. Eur Urol. (2020) 78:281–6. doi: 10.1016/j.eururo.2020.04.064
38. Achour A, Dkhil O, Saad J, Abdelali M, Zrig A, Hmida B, et al. Chest CT-scan finding of asymptomatic COVID-19 pneumonia: a prospective 542 patients' single center study. Pan Afr Med J. (2020) 36:257. doi: 10.11604/pamj.2020.36.257.23632
39. Caruso D, Zerunian M, Polici M, Pucciarelli F, Polidori T, Rucci C, et al. Chest CT Features of COVID-19 in Rome, Italy. Radiology. (2020) 296:E79–85. doi: 10.1148/radiol.2020201237
40. Iwasawa T, Sato M, Yamaya T, Sato Y, Uchida Y, Kitamura H, et al. Ultra-high-resolution computed tomography can demonstrate alveolar collapse in novel coronavirus (COVID-19) pneumonia. Jpn J Radiol. (2020) 38:394–8. doi: 10.1007/s11604-020-00956-y
41. Agostini A, Floridi C, Borgheresi A, Badaloni M, Esposto Pirani P, Terilli F, et al. Proposal of a low-dose, long-pitch, dual-source chest CT protocol on third-generation dual-source CT using a tin filter for spectral shaping at 100 kVp for CoronaVirus Disease 2019 (COVID-19) patients: a feasibility study. Radiol Med. (2020) 125:365–73. doi: 10.1007/s11547-020-01179-x
42. Inui S, Fujikawa A, Jitsu M, Kunishima N, Watanabe S, Suzuki Y, et al. Chest CT Findings in cases from the cruise ship Diamond Princess with coronavirus disease (COVID-19). Radiol Cardiothorac Imaging. (2020) 2:e200110. doi: 10.1148/ryct.2020200110
43. Teich VD, Klajner S, Almeida FAS, Dantas ACB, Laselva CR, Torritesi MG, et al. Epidemiologic and clinical features of patients with COVID-19 in Brazil. Einstein (São Paulo). (2020) 18:eAO6022. doi: 10.31744/einstein_journal/2020AO6022
44. Korkmaz MF, Türe E, Dorum BA, Kiliç ZB. The epidemiological and clinical characteristics of 81 children with COVID-19 in a pandemic hospital in Turkey: an observational cohort study. J Korean Med Sci. (2020) 35:e236. doi: 10.3346/jkms.2020.35.e236
45. Yoshimura Y, Sasaki H, Horiuchi H, Miyata N, Tachikawa N. Clinical characteristics of the coronavirus disease 2019 (COVID-19) outbreak on a cruise ship. J Infect Chemother. (2020) 26:1177–80. doi: 10.1016/j.jiac.2020.06.010
46. Pan Y, Guan H, Zhou S, Wang Y, Li Q, Zhu T, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. (2020) 30:3306–9. doi: 10.1007/s00330-020-06731-x
47. Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. (2020) 295:200463. doi: 10.1148/radiol.2020200463
48. Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. (2020) 20:425–34. doi: 10.1016/S1473-3099(20)30086-4
49. Xu YH, Dong JH, An WM, Lv XY, Yin XP, Zhang JZ, et al. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Infect. (2020) 80:394–400. doi: 10.1016/j.jinf.2020.02.017
50. Xu X, Yu C, Qu J, Zhang L, Jiang S, Huang D, et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. (2020) 47:1275–80. doi: 10.1007/s00259-020-04735-9
51. Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. AJR Am J Roentgenol. (2020) 214:1072–7. doi: 10.2214/AJR.20.22976
52. Li K, Wu J, Wu F, Guo D, Chen L, Fang Z, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. (2020) 55:327–31. doi: 10.1097/RLI.0000000000000672
53. Zhou S, Wang Y, Zhu T, Xia L, CT. Features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol. (2020) 214:1287–94. doi: 10.2214/AJR.20.22975
54. Xiong Y, Sun D, Liu Y, Fan Y, Zhao L, Li X, et al. Clinical and high-resolution CT features of the COVID-19 infection: comparison of the initial and follow-up changes. Invest Radiol. (2020) 55:332–9. doi: 10.1097/RLI.0000000000000674
55. Cheng Z, Lu Y, Cao Q, Qin L, Pan Z, Yan F, et al. Clinical features and chest CT manifestations of coronavirus disease 2019 (COVID-19) in a single-center study in Shanghai, China. AJR Am J Roentgenol. (2020) 215:121–6. doi: 10.2214/AJR.20.22959
56. Guan CS, Lv ZB, Yan S, Du YN, Chen H, Wei LG, et al. Imaging features of coronavirus disease 2019 (COVID-19): evaluation on thin-section CT. Acad Radiol. (2020) 27:609–13. doi: 10.1016/j.acra.2020.03.002
57. Han R, Huang L, Jiang H, Dong J, Peng H, Zhang D. Early Clinical and CT manifestations of coronavirus disease 2019 (COVID-19) pneumonia. AJR Am J Roentgenol. (2020) 215:338–43. doi: 10.2214/AJR.20.22961
58. Song F, Shi N, Shan F, Zhang Z, Shen J, Lu H, et al. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. (2020) 297:E346. doi: 10.1148/radiol.2020209021
59. Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. (2020) 75:1730–41. doi: 10.1111/all.14238
60. Guan WJ Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
61. Bai HX, Hsieh B, Xiong Z, Halsey K, Choi JW, Tran TML, et al. Performance of radiologists in differentiating COVID-19 from non-COVID-19 viral pneumonia at chest CT. Radiology. (2020) 296:E46–54. doi: 10.1148/radiol.2020200823
62. Hu Z, Song C, Xu C, Jin G, Chen Y, Xu X, et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. (2020) 63:706–11. doi: 10.1007/s11427-020-1661-4
63. Bao C, Liu X, Zhang H, Li Y, Liu J. Coronavirus Disease 2019 (COVID-19) CT findings: a systematic review and meta-analysis. J Am Coll Radiol. (2020) 17:701–9. doi: 10.1016/j.jacr.2020.03.006
64. Zhu J, Zhong Z, Li H, Ji P, Pang J, Li B, et al. CT imaging features of 4121 patients with COVID-19: A meta-analysis. J Med Virol. (2020) 92:891–902. doi: 10.1002/jmv.25910
65. Ooi GC, Daqing M. SARS: radiological features. Respirology. (2003) 8:S15–9. doi: 10.1046/j.1440-1843.2003.00519.x
66. Das KM, Lee EY, Langer RD, Larsson SG. Middle east respiratory syndrome coronavirus: what does a radiologist need to know? AJR Am J Roentgenol. (2016) 206:1193–201. doi: 10.2214/AJR.15.15363
67. Dai G, Duan J, Zheng L, He M, Dai Y, Zhang M, et al. Comparison of lung image quality between CT Ark and Brilliance 64 CT during COVID-19. BMC Med Imaging. (2021) 21:192. doi: 10.1186/s12880-021-00720-2
68. Steuwe A, Rademacher C, Valentin B, Köhler MH, Appel E, Keitel V, et al. Dose-optimised chest computed tomography for diagnosis of Coronavirus Disease 2019 (COVID-19)—Evaluation of image quality and diagnostic impact. J Radiol Prot. (2020) 40:877–91. doi: 10.1088/1361-6498/aba16a
69. Niu Y, Huang S, Zhang H, Li S, Li X, Lv Z, et al. Optimization of imaging parameters in chest CT for COVID-19 patients: an experimental phantom study. Quant Imaging Med Surg. (2021) 11:380–91. doi: 10.21037/qims-20-603
70. Bernardo M, Homayounieh F, Cuter MCR, Bellegard LM, Oliveira Junior HM, Buril GO, et al. Chest CT usage in COVID-19 pneumonia: multicenter study on radiation doses and diagnostic quality in Brazil. Radiat Prot Dosimetry. (2021) 197:135–45. doi: 10.1093/rpd/ncab171
71. Delorey TM, Ziegler CGK, Heimberg G, Normand R, Yang Y, Segerstolpe Å, et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature. (2021) 595:107–13. doi: 10.1038/s41586-021-03570-8
72. Leng L, Cao R, Ma J, Mou D, Zhu Y, Li W, et al. Pathological features of COVID-19-associated lung injury: a preliminary proteomics report based on clinical samples. Signal Transduct Target Ther. (2020) 5:240. doi: 10.1038/s41392-020-00355-9
73. Pairo-Castineira E, Clohisey S, Klaric L, Bretherick AD, Rawlik K, Pasko D, et al. Genetic mechanisms of critical illness in COVID-19. Nature. (2021) 591:92–8. doi: 10.1038/s41586-020-03065-y
74. Kelta Wabalo E, Dukessa Dubiwak A, Welde Senbetu M, Sime Gizaw T. Effect of genomic and amino acid sequence mutation on virulence and therapeutic target of severe acute respiratory syndrome coronavirus-2 (SARS COV-2). Infect Drug Resist. (2021) 14:2187–92. doi: 10.2147/IDR.S307374
75. Song Y, Ge Z, Cui S, Tian D, Wan G, Zhu S, et al. COVID-19 cases from the first local outbreak of the SARS-CoV-2 B. 117 variant in China may present more serious clinical features: a prospective, comparative cohort study. Microbiol Spectr. (2021) 9:e0027321. doi: 10.1128/Spectrum.00273-21
76. Wu J, Tang J, Zhang T, Chen YC, Du C. Follow-up CT of “reversed halo sign” in SARS-CoV-2 delta VOC pneumonia: a report of two cases. J Med Virol. (2022) 94:1289–91. doi: 10.1002/jmv.27533
77. McLaren TA, Gruden JF, Green DB. The bullseye sign: A variant of the reverse halo sign in COVID-19 pneumonia. Clin Imaging. (2020) 68:191–6. doi: 10.1016/j.clinimag.2020.07.024
78. Cheng QR, Fan MX, Hao J, Hu XC, Ge XH, Hu ZL, et al. Chest CT features of children infected by B. 16172 (Delta) variant of COVID-19. World J Pediatr. (2022) 18:37–42. doi: 10.1007/s12519-021-00484-3
79. Tsakok MT, Watson RA, Lumley SF, Khan F, Qamhawi Z, Lodge A, et al. Parenchymal involvement on CT pulmonary angiography in SARS-CoV-2 Alpha variant infection and correlation of COVID-19 CT severity score with clinical disease severity and short-term prognosis in a UK cohort. Clin Radiol. (2022) 77:148–55. doi: 10.1016/j.crad.2021.11.002
80. Kopel J, Perisetti A, Roghani A, Aziz M, Gajendran M, Goyal H. Racial and gender-based differences in COVID-19. Front Public Health. (2020) 8:418. doi: 10.3389/fpubh.2020.00418
81. Ahmed RI, Khalil MAF, Mahmoud EE, Nakhla OL, Ali SA, Ahmed MI. Comparison of the clinical and radiological manifestations of male patients with COVID-19 from different ethnicities. Int J Clin Pract. (2021) 75:e14735. doi: 10.1111/ijcp.14735
82. Smith M, Abdesselem HB, Mullins M, Tan TM, Nel AJM, Al-Nesf MAY, et al. Age, Disease severity and ethnicity influence humoral responses in a multi-ethnic COVID-19 cohort. Viruses. (2021) 13:786. doi: 10.3390/v13050786
83. Choudhary S, Sreenivasulu K, Mitra P, Misra S, Sharma P. Role of genetic variants and gene expression in the susceptibility and severity of COVID-19. Ann Lab Med. (2021) 41:129–38. doi: 10.3343/alm.2021.41.2.129
84. Darbani B. The expression and polymorphism of entry machinery for COVID-19 in human: juxtaposing population groups, gender, and different tissues. Int J Environ Res Public Health. (2020) 17:3433. doi: 10.3390/ijerph17103433
85. Li S, Sarangarajan R, Jun T, Kao YH, Wang Z, Hao K, et al. In-hospital use of ACE inhibitors/angiotensin receptor blockers associates with COVID-19 outcomes in African American patients. J Clin Invest. (2021) 131:e151418. doi: 10.1172/JCI151418
86. Pabalan N, Tharabenjasin P, Suntornsaratoon P, Jarjanazi H, Muanprasat C. Ethnic and age-specific acute lung injury/acute respiratory distress syndrome risk associated with angiotensin-converting enzyme insertion/deletion polymorphisms, implications for COVID-19: A meta-analysis. Infect Genet Evol. (2021) 88:104682. doi: 10.1016/j.meegid.2020.104682
87. Antoon JW, Grijalva CG, Thurm C, Richardson T, Spaulding AB, Teufel RJ. 2nd, et al. Factors associated with COVID-19 disease severity in US children and adolescents. J Hosp Med. (2021) 16:603–10. doi: 10.12788/jhm.3689
88. Wu P, Ding L, Li X, Liu S, Cheng F, He Q, et al. Trans-ethnic genome-wide association study of severe COVID-19. Commun Biol. (2021) 4:1034. doi: 10.1038/s42003-021-02549-5
89. Upadhyai P, Suresh G, Parit R, Das R. Genomic and ancestral variation underlies the severity of COVID-19 clinical manifestation in individuals of European descent. Life (Basel). (2021) 11:921. doi: 10.3390/life11090921
90. Garrana SH, Som A, Ndakwah GS, Yeung T, Febbo J, Heeger AP, et al. Comparison of chest CT findings of COVID-19, influenza, and organizing pneumonia: a multireader study. AJR Am J Roentgenol. (2021) 217:1093–102. doi: 10.2214/AJR.21.25640
91. Jing R, Vunnam RR, Schnaubelt E, Vokoun C, Cushman-Vokoun A, Goldner D, et al. Co-infection of COVID-19 and influenza A in a hemodialysis patient: a case report. BMC Infect Dis. (2021) 21:68. doi: 10.1186/s12879-020-05723-y
92. Cheng Y, Ma J, Wang H, Wang X, Hu Z, Li H, et al. Co-infection of influenza A virus and SARS-CoV-2: A retrospective cohort study. J Med Virol. (2021) 93:2947–54. doi: 10.1002/jmv.26817
93. Wang J, Zhu X, Xu Z, Yang G, Mao G, Jia Y, et al. Clinical and CT findings of COVID-19: differences among three age groups. BMC Infect Dis. (2020) 20:434. doi: 10.1186/s12879-020-05154-9
94. Li Y, Xia L. Coronavirus Disease 2019 (COVID-19): Role of chest CT in diagnosis and management. AJR Am J Roentgenol. (2020) 214:1280–6. doi: 10.2214/AJR.20.22954
95. Mortani Barbosa EJ Jr, Georgescu B, Chaganti S, Aleman GB, Cabrero JB, Chabin G, et al. Machine learning automatically detects COVID-19 using chest CTs in a large multicenter cohort. Eur Radiol. (2021) 31:8775–85. doi: 10.1007/s00330-021-07937-3
96. Zhang J, Ding D, Huang X, Zhang J, Chen D, Fu P, et al. Differentiation of COVID-19 from seasonal influenza: A multicenter comparative study. J Med Virol. (2021) 93:1512–9. doi: 10.1002/jmv.26469
97. Zhou X, Pu Y, Zhang D, Xia Y, Guan Y, Liu S, et al. CT findings and dynamic imaging changes of COVID-19 in 2908 patients: a systematic review and meta-analysis. Acta Radiol. (2022) 63:291–310. doi: 10.1177/0284185121992655
98. Wang Y, Dong C, Hu Y, Li C, Ren Q, Zhang X, et al. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. (2020) 296:E55–64. doi: 10.1148/radiol.2020200843
99. Zhou S, Zhu T, Wang Y, Xia L. Imaging features and evolution on CT in 100 COVID-19 pneumonia patients in Wuhan, China. Eur Radiol. (2020) 30:5446–54. doi: 10.1007/s00330-020-06879-6
100. Chang MC, Lee W, Hur J, Park D. Chest computed tomography findings in asymptomatic patients with COVID-19. Respiration. (2020) 99:748–54. doi: 10.1159/000509334
101. Gu Q, Ouyang X, Xie A, Tan X, Liu J, Huang F, et al. A retrospective study of the initial chest CT imaging findings in 50 COVID-19 patients stratified by gender and age. J Xray Sci Technol. (2020) 28:875–84. doi: 10.3233/XST-200709
102. Eftekhari Moghadam AR, Fazelinejad Z, Nashibi R, Pour MB, CT. Characteristics of coronavirus disease 2019 pneumonia and its association with C-reactive protein, erythrocyte sedimentation rate and gender. Adv Biomed Res. (2022) 11:10. doi: 10.4103/abr.abr_33_21
103. Wenham C, Smith J, Morgan R. Gender and COVID-19 working group. COVID-19: the gendered impacts of the outbreak. Lancet. (2020) 395:846–8. doi: 10.1016/S0140-6736(20)30526-2
104. Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. (2020) 8:152. doi: 10.3389/fpubh.2020.00152
105. Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med. (2020) 8:e20. doi: 10.1016/S2213-2600(20)30117-X
106. Moradi B, Ghanaati H, Kazemi MA, Gity M, Hashemi H, Davari-Tanha F, et al. Implications of sex difference in CT scan findings and outcome of patients with COVID-19 pneumonia. Radiol Cardiothorac Imaging. (2020) 2:e200248. doi: 10.1148/ryct.2020200248
107. Som A, Lang M, Yeung T, Carey D, Garrana S, Mendoza DP, et al. Implementation of the Radiological Society of North America expert consensus guidelines on reporting chest CT findings related to COVID-19: a multireader performance study. Radiol Cardiothorac Imaging. (2020) 2:e200276. doi: 10.1148/ryct.2020200276
108. O' Neill SB, Byrne D, Müller NL, Jalal S, Parker W, Nicolaou S, et al. Radiological Society of North America (RSNA) expert consensus statement related to chest CT findings in COVID-19 versus CO-RADS: comparison of reporting system performance among chest radiologists and end-user preference. Can Assoc Radiol J. (2021) 72:806–13. doi: 10.1177/0846537120968919
109. Kavak S, Duymus R, RSNA. and BSTI grading systems of COVID-19 pneumonia: comparison of the diagnostic performance and interobserver agreement. BMC Med Imaging. (2021) 21:143. doi: 10.1186/s12880-021-00668-3
110. Ciccarese F, Coppola F, Spinelli D, Galletta GL, Lucidi V, Paccapelo A, et al. Diagnostic accuracy of North America expert consensus statement on reporting CT findings in patients suspected of having COVID-19 infection: an Italian single-center experience. Radiol Cardiothorac Imaging. (2020) 2:e200312. doi: 10.1148/ryct.2020200312
111. Özer H, Kilinçer A, Uysal E, Yormaz B, Cebeci H, Durmaz MS, et al. Diagnostic performance of Radiological Society of North America structured reporting language for chest computed tomography findings in patients with COVID-19. Jpn J Radiol. (2021) 39:877–88. doi: 10.1007/s11604-021-01128-2
112. Uysal E, Kilinçer A, Cebeci H, Özer H, Demir NA, Öztürk M, et al. Chest CT findings in RT-PCR positive asymptomatic COVID-19 patients. Clin Imaging. (2021) 77:37–42. doi: 10.1016/j.clinimag.2021.01.030
113. Falaschi Z, Danna PSC, Arioli R, Pasché A, Zagaria D, Percivale I, et al. Chest CT accuracy in diagnosing COVID-19 during the peak of the Italian epidemic: a retrospective correlation with RT-PCR testing and analysis of discordant cases. Eur J Radiol. (2020) 130:109192. doi: 10.1016/j.ejrad.2020.109192
114. Herath HMKKMB, Karunasena GMKB, Madhusanka BGDA. Chapter 10-Early detection of COVID-19 pneumonia based on ground-glass opacity (GGO) features of computerized tomography (CT) angiography. Intell Data-Centric Syst 5G IoT Edge Comput Smart Healthcare. (2022) 257–77. doi: 10.1016/B978-0-323-90548-0.00013-9
115. Nair AV, McInnes M, Jacob B, Kumar D, Soman DK, Subair HSV, et al. Diagnostic accuracy and inter-observer agreement with the CO-RADS lexicon for CT chest reporting in COVID-19. Emerg Radiol. (2021) 28:1045–54. doi: 10.1007/s10140-021-01967-6
116. Machnicki S, Patel D, Singh A, Talwar A, Mina B, Oks M, et al. The usefulness of chest CT imaging in patients with suspected or diagnosed COVID-19: a review of literature. Chest. (2021) 160:652–70. doi: 10.1016/j.chest.2021.04.004
117. Li J, Yan R, Zhai Y, Qi X, Lei J. Chest CT findings in patients with coronavirus disease 2019 (COVID-19): a comprehensive review. Diagn Interv Radiol. (2021) 27:621–32. doi: 10.5152/dir.2020.20212
Keywords: chest CT, COVID-19, diagnosis, meta-analysis, SARS-CoV-2
Citation: Hou N, Wang L, Li M, Xie B, He L, Guo M, Liu S, Wang M, Zhang R and Wang K (2022) Do COVID-19 CT features vary between patients from within and outside mainland China? Findings from a meta-analysis. Front. Public Health 10:939095. doi: 10.3389/fpubh.2022.939095
Received: 08 May 2022; Accepted: 25 July 2022;
Published: 14 October 2022.
Edited by:
Ana Afonso, University of São Paulo, BrazilReviewed by:
Halil Özer, Selcuk University, TurkeyCopyright © 2022 Hou, Wang, Li, Xie, He, Guo, Liu, Wang, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai Wang, d2FuZ2thaWljdUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.