- 1Clinical Research Service Center, Affiliated Hospital of Guangdong Medical University, Zhanjiang, China
- 2Guangdong Engineering Research Center of Collaborative Innovation Technology of Clinical Medical Big Data Cloud Service in Medical Consortium of West Guangdong Province, Affiliated Hospital of Guangdong Medical University, Zhanjiang, China
Objective: Multiple myeloma (MM) imposes a heavy burden in China. Understanding the secular trend of MM burden and projecting its future trend could facilitate appropriate public health planning and improve the management of MM.
Methods: Sex-specific incidence and mortality rates of MM in China from 1990 to 2019 were collected from the Global Burden of Disease 2019 study. The secular trend of MM burden was analyzed by joinpoint regression. Age–period–cohort model was used to analyze the effects of age, period, and birth cohort on MM burden and project future trends up to 2044.
Results: From 1990 to 2019, the age-standardized incidence and mortality rates of MM continued to increase in males. For females, the age-standardized rates were stable in MM incidence and decreased in MM mortality. Males had a higher disease burden of MM than females. Age effects were the most significant risk factor for MM incidence and mortality. Moreover, the risk of MM incidence and mortality increased with increasing time period but decreased with birth cohort in males and females. The age-standardized incidence and mortality rates of MM in China is predicted to be continuously increasing over the next 25 years.
Conclusion: The burden of MM in China is expected to continue to increase in the future, with significant sex difference. A comprehensive understanding of the risk characteristics and disease pattern of MM could help develop timely intervention measures to effectively reduce its burden.
Introduction
Multiple myeloma (MM) is a malignant proliferative disease of plasma cells. It is characterized by abnormal proliferation of clonal plasma cells in bone marrow. In most cases, monoclonal immunoglobulin or its fragment (M-protein) is secreted, resulting in damage to related organs or tissues. The common clinical manifestations of MM are bone pain, anemia, renal function damage, hypercalcemia and infection. According to the GLOBOCAN 2020 statistics, 176,404 new cases of MM were reported worldwide in 2020, ranking 21nd among all cancers; MM also led to 117,077 deaths, ranking 17th among leading causes of cancer death (1). In 2020, the estimated numbers of new MM cases and deaths in China were 32,000 and 27,600, ranking the 21nd and 18th leading causes of cancer diagnosis and mortality in China, respectively (1). This finding indicated that China is facing a serious disease burden of MM (1, 2). A comprehensive understanding of MM epidemiology is the basic requirement to promote effective intervention measures and the rational allocation of healthcare resources (2, 3).
The diagnostic criteria and techniques of MM are constantly being updated and improved. Meanwhile, the treatment of MM continues to evolve rapidly with the arrival of multiple new drugs and emerging data from randomized trials to guide therapy (4). The 5-year survival rate of young patients with MM has increased from 74.1% in 2010 to 78.5% in 2016 (5). Early diagnosis and timely treatment could considerably improve the survival of patients with MM. Despite great advances in diagnosis and treatment of MM over the past few decades, knowledge of MM etiology and its potential mechanism remain lacking.
Studies have suggested that ionizing radiation, overweight and obesity, lack of nutrients and unhealthy lifestyle are risk factors for MM, and these factors may vary with chronological age, time period and birth cohort (6–8). However, few studies in China specifically analyzed the trends of MM incidence and mortality rates by age, period and birth cohort (9). Age effects exhibit different risks associated with different age brackets. Period effects refer to the effects of a complex combination of historical events and environmental factors. Birth cohort effects represent the influence of physical and social exposure in early life that may accumulate over time. Therefore, by simultaneously reflecting the net age, period (year of survey) and cohort (year of birth) effects of long-term trends in disease, the age–period–cohort model has an advantage in assessing the disease burden and provide information on the etiology of MM. This study aimed to provide a comprehensive assessment on the secular trend of MM incidence and mortality in China from 1990 to 2019 under the age–period–cohort framework and project their trends in the next 25 years.
Data and Methods
Data Sources
Data on the sex-specific disease burden of MM in China from 1990 to 2019 were obtained from the official website of the Global Burden of Disease (GBD) 2019 Study (http://ghdx.healthdata.org/gbdresults-tool). The GBD 2019 database provides a comprehensive assessment of the incidence rate, mortality, life lost years, and disability adjusted life years of 369 diseases and injuries and 87 risk factors in 204 countries and 21 regions from 1990 to 2019. Details on the data, statistical modeling, and metrics for GBD 2019 have been reported in previous studies (10, 11). The sources of disease death data in China are mainly the national disease surveillance system, maternal and child surveillance system, and national cause of death reporting system. “China” was chosen from the database as the location, “multiple myeloma” for the cause, and “deaths” and “incidence” for measures. In this study, the age-standardized rates (ASRs), the crude rates and the absolute number of MM incidence and mortality were also extracted by sex and age. The ASRs and 95% uncertainty intervals (UIs) were calculated on the basis of GBD 2019 global age-standard population (12).
Joinpoint Analysis
Joinpoint regression model is used to describe the continuous change in the slopes of overall trend through displacement analysis, and it divides a secular trend line into several statistically significant trend section by model fitting. In this model, the average annual percent change (AAPC) and annual percent change (APC) were calculated. The AAPC or APC > 0 denotes an increasing trend, whereas the AAPC or APC < 0 represents a decreasing trend, which only makes sense if their 95% confidence intervals (CIs) upper limit and lower limit have the same sign. Otherwise, the trend was regarded as stable over time. The Joinpoint Regression Program 4.7.0.0 software was used for joinpoint regression analysis.
Age–Period–Cohort Model Analysis
An age–period–cohort model was developed on the basis of Poisson distribution, which could simultaneously estimate the influence of age, period and birth cohort on the trends. The APC model could be expressed as follows:
The age–period–cohort model requires an equal time interval in age, period, and cohort. Otherwise, an information overlap in the adjacent queues could occur. GBD 2019 assumed no newly diagnosed cases prior to age 20 for MM. These settings are in line with the corresponding cause of death model for MM. The sex-specific rates were appropriately recorded into successive 5-year groups (20–24, 25–29… 95+ years), consecutive 5-year period from 1990 to 2019, and correspondingly consecutive 5-year birth cohort groups starting from 1895–1899 to 1995–1999.
As the cohort is obtained by subtracting the corresponding age from the period (cohort = period – age), a collinearity problem exists among the age, period and cohort. When estimating the age, period, and cohort effects independently, the parameters are not identifiable. Intrinsic estimator (IE) is introduced when using the parameter estimation of the APC model to solve this problem (13, 14). The age–period–cohort analysis with the IE method provided estimated coefficients for the age, period and cohort effects. These coefficients were transformed into the exponential value [exp(coef.) = ecoef.], which denotes the relative risk (RR) of a particular age, period or birth cohort relative to the average level of all ages, periods or birth cohorts combined. For example, for the age effect in males, the RR of MM incidence in people aged 85–89 years was 3.29, which indicated that the risk in this age group was 3.29 times greater than the risk for all ages combined. The age–period–cohort analysis was performed using STATA 15.0 software.
Bayesian Age-Period-Cohort (BAPC) Analysis
The ASRs of the MM incidence and death in China from 2020 to 2044 were also predicted. Bayesian age-period-cohort (BAPC) analysis was conducted based on the assumption that the effects of age, period, and cohort adjacent in time are similar, which applies the second-order random walk for smoothing priors of age, period, and cohort effects and to project posterior incidence and mortality rates. In order to avoid any mixing and convergence issues caused by Markov chain Monte Carlo sampling techniques traditionally used in the Bayesian approach. The integrated nested Laplace approximations (INLA) are introduced when using the Bayesian age-period-cohort model to approximate the marginal posterior distributions. Which shows better coverage and precision than other prediction methods (15, 16). The BAPC analysis was performed using R-package BAPC. The population predictions for China were taken from the 2019 revision of the United Nations (UN) World Population Prospects and were used to estimate China's population in 2020 and beyond.
Results
Descriptive Analysis
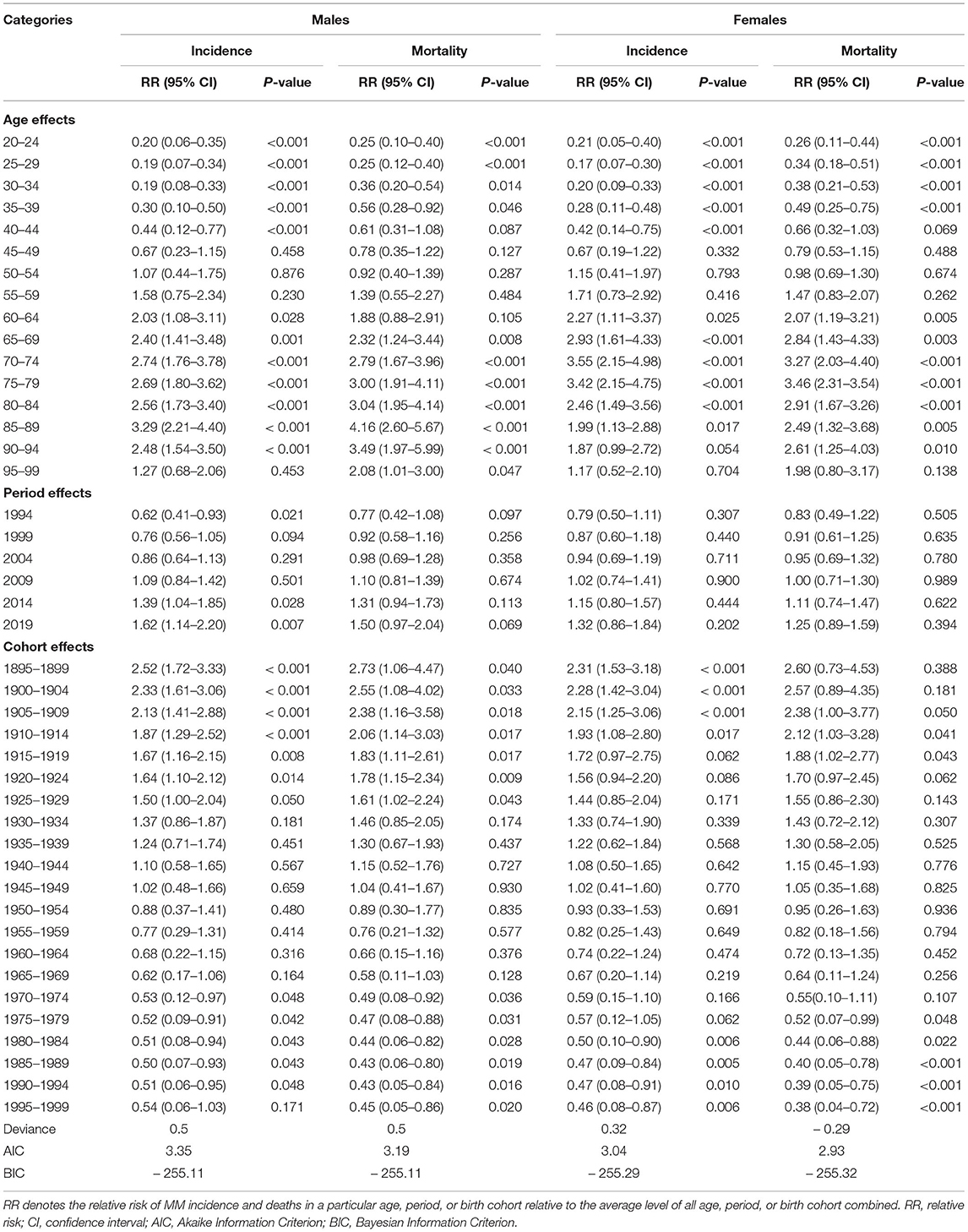
The sex-specific incidence and death rates of MM by age groups in China in 2019 are listed in Table 1. In 2019, the age-standardized incidence rates (ASIRs) of MM in China were 1.16 (95% UI: 0.77–1.54) and 0.72 (95% UI: 0.49–0.94) per 100,000 population in males and females, respectively. The age-standardized mortality rates (ASMRs) of MM in China were 0.83 (95% UI: 0.54–1.08) and 0.55 (95% UI: 0.39–0.71) per 100,000 population in males and females, respectively. The males had higher ASRs of incidence and mortality than the females in all patients and each age group. For males, the highest ASIR and ASMR were observed in the group aged 85–89 years, followed by the groups aged 90–94 years. For females, the highest ASIR and ASMR were observed in the group aged 75–79 years, followed by the groups aged 70–74 years.
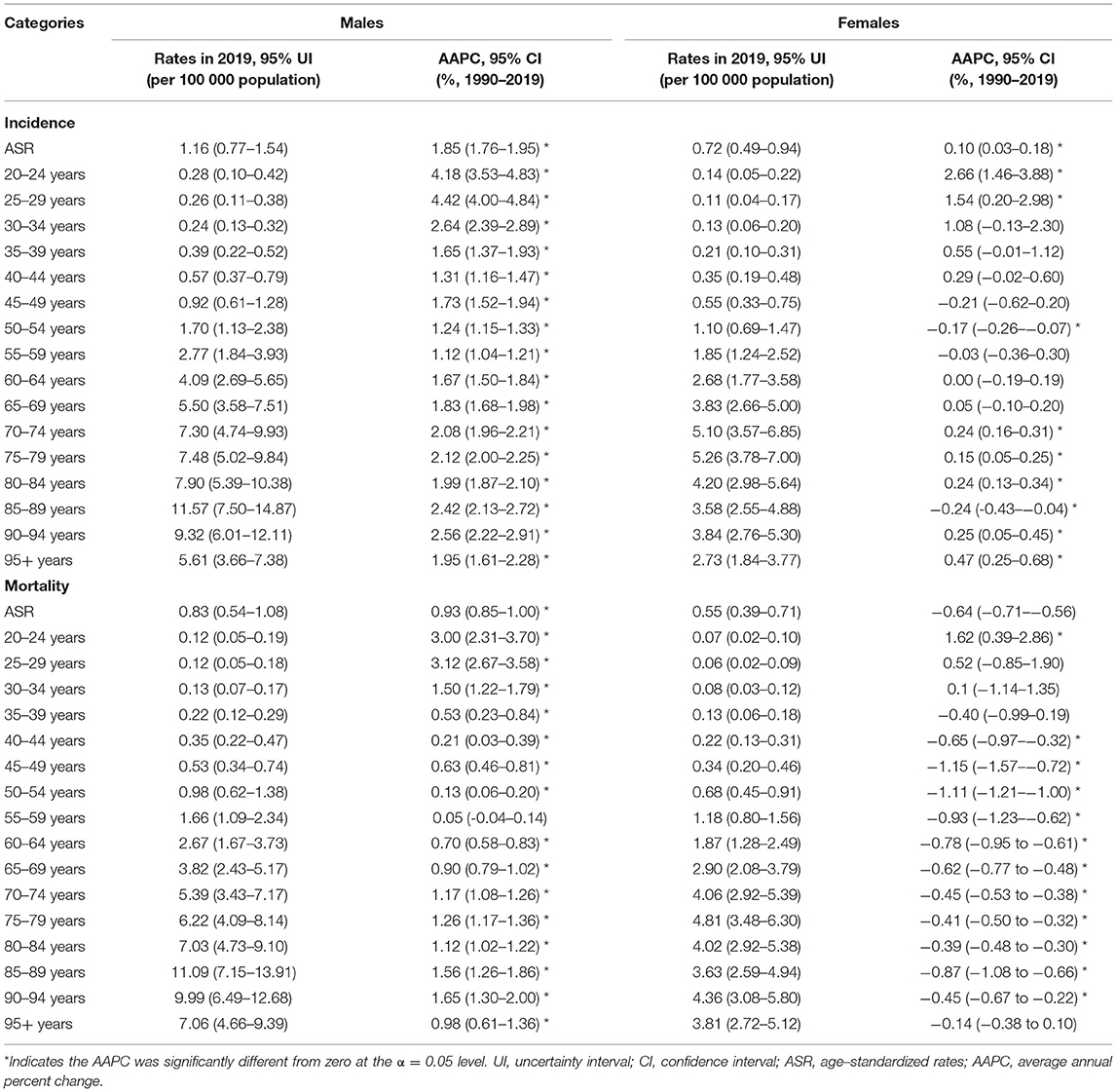
Table 1. The sex-age-specific rates of MM in China in 2019 and their percentage changes from 1990 to 2019.
Jointpoint Regression Analysis
The AAPCs of the age-sex-specific rates of MM incidence and mortality from 1990 to 2019 are shown in Table 1. For males, the ASIRs and ASMRs of MM in China from 1990 to 2019 annually rose by 1.85% (95% CI: 1.76–1.95) and 0.93% (95% CI: 0.85–1.00), respectively. For females, the ASIRs showed an increasing trend, with AAPC of 0.10% (95% CI: 0.03–0.18), whereas the ASMRs presented a decreasing trend, with AAPC of −0.64% (95% CI: from −0.71 to −0.56) during the observation period.
As shown in Figure 1, during 1990–2019, the temporal trend of sex-specific ASIRs of MM contained three turning points and were divided into four sections, while the secular trend of ASMRs contained four turning points and were divided into five sections in both sexes. For males, the ASIRs and ASMRs continuously increased after 1992, with the most significant increase during the period of 2007–2011 (APC = 3.51, 95% CI: 3.04–3.98 for ASIR; APC = 2.51, 95% CI: 2.06–2.96 for ASMR). For females, the ASIRs continuously increased after 2007 (APC = 0.65, 95% CI: 0.53–0.76), whereas the ASMRs showed a significant decrease during the period of 1990–2015, with the most significant decrease during the period of 2004–2007 (APC = −2.07, 95% CI: from −3.35 to −0.78), as shown in Supplementary Table 1.
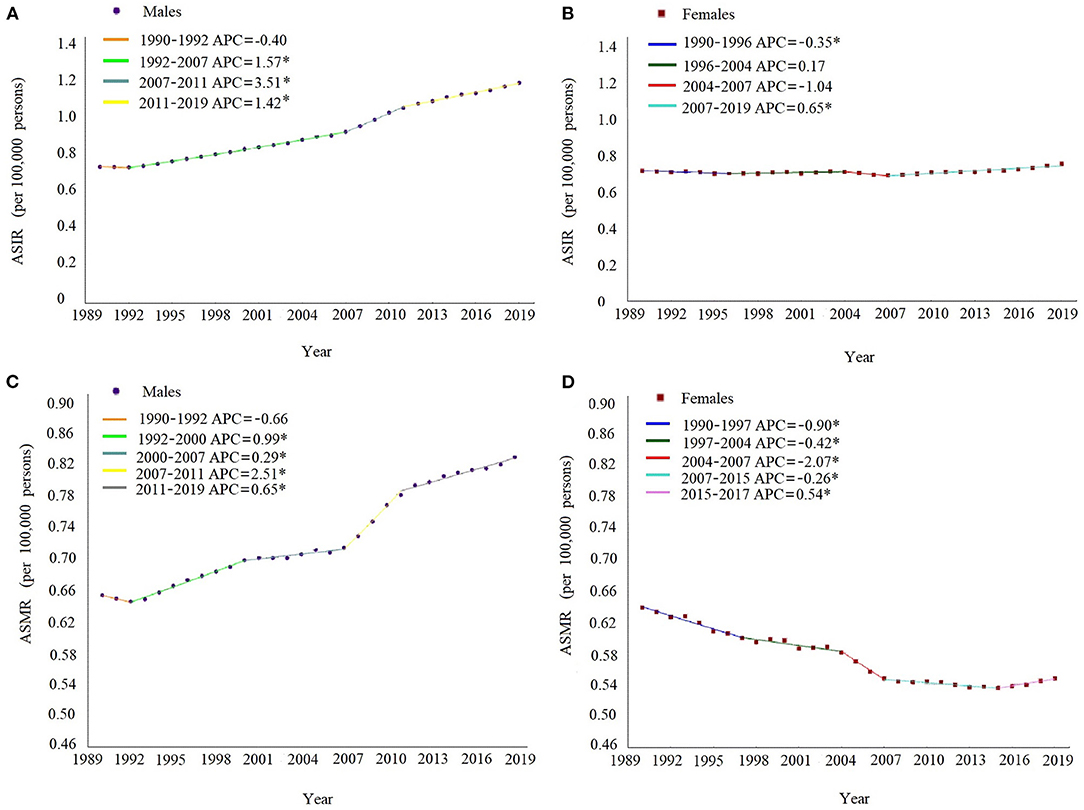
Figure 1. Joinpoint regression analysis in sex-specific age-standardized incidence and death rates of multiple myeloma in China from 1990 to 2019. The ASIR in males (A), ASIR in females (B), ASMR in males (C), and ASMR in females (D). Notes: the annual percent change is statistically significantly different from zero at the a = 0.05 level. ASIR, age-standardized incidence rates. ASMR, age-standardized death rates.
Age–Period–Cohort Analysis
The estimated RRs of MM incidence and mortality due to age, period and cohort effects are shown in Table 2. When the period and cohort effects were controlled, the RRs of age effects on MM incidence sharply increased from 0.20 (95% CI: 0.05–0.35) in the group aged 20–24 years to 3.29 (95% CI: 2.21–4.40) in the group aged 85–89 years for males, and from 0.21 (95% CI: 0.05–0.40) in the group aged 20–24 years to 3.55 (95% CI: 2.15–4.98) in the group aged 70–74 years for females. Then, the RRs continuously decreased with increasing age in males and females. Time period had a significant effect on MM incidence in males, with the RRs increased from 0.62 (95% CI: 0.41–0.93) in 1994 to 1.62 (95% CI: 1.14–2.20) in 2019. For cohort effects, the RRs due to cohort effects continuously decreased from 2.52 (95% CI 1.72–3.33) in the cohort 1895–1899 to 0.54 (95% CI 0.06–1.03) in the cohort 1995–1999 for males, and from 2.31 (95% CI 1.53–3.18) in the cohort 1895–1899 to 0.46 (95% CI 0.08–0.87) in the cohort 1995–1999 for females.
As shown in Figure 2, after adjustment by the period and cohort effects, the RRs due to age effects on MM mortality continuously increased from 0.25 (95% CI: 0.10–0.40) in the group aged 20–24 years to 4.16 (95% CI: 2.60–5.67) in the group aged 85–89 years for males, and from 0.26 (95% CI: 0.11–0.44) in the group aged 20–24 years to 3.46 (95% CI: 2.31–3.54) in the group aged 75–79 years for females. Then, the RRs slowly decreased with increasing age in males and females. However, time period had a non-significant effect on MM mortality in both sexes. For cohort effects, later birth cohorts experienced a relatively low risk of MM-related death compared with earlier cohorts. The RRs due to cohort effects decreased from 2.73 (95% CI: 1.06–4.47) in the cohort 1895–1899 to 0.45 (95% CI: 0.05–0.86) in the cohort 1995–1999 for males, and from 2.60 (95% CI: 0.73–4.53) in the cohort 1895–1899 to 0.38 (95% CI: 0.04–0.72) in the cohort 1995–1999 for females.
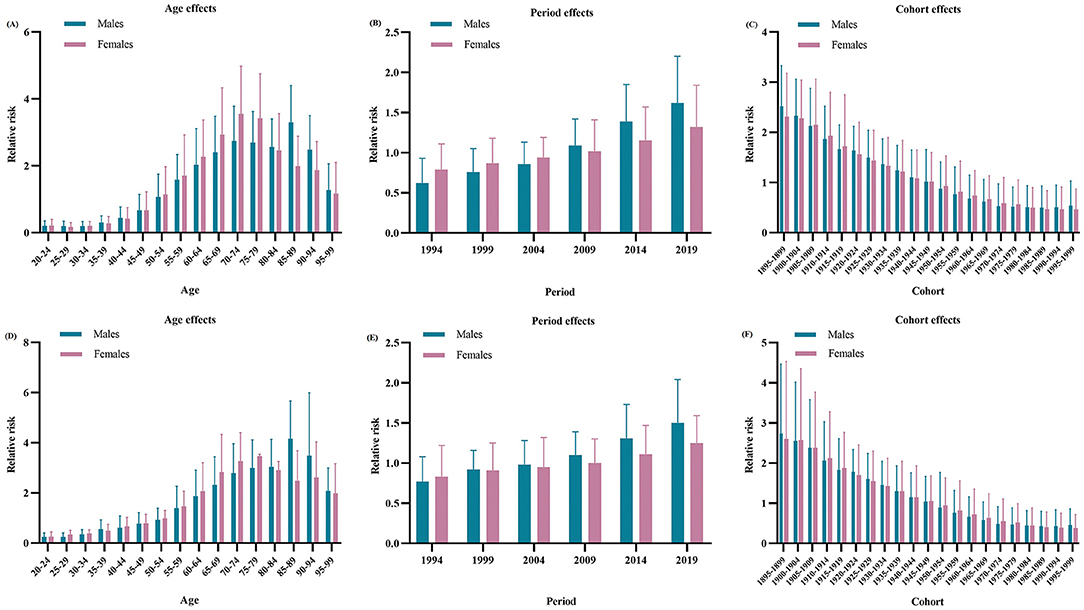
Figure 2. Relative risks of incidence due to age (A), period (B), and cohort (C) effects, and relative risks of deaths due to age (D), period (E), and cohort (F) effects of multiple myeloma in China from 1990 to 2019.
Predictions of MM Burden in the Coming 25 Years in China
As shown in Figure 3, the ASIRs and ASMRs of MM in China will continue to increase over the next 25 years, especially among men. In 2044, the ASIRs of MM in China will reach 2.03 (95% UI: 1.15–3.91) and 1.10 (95% UI: 0.16–2.03) per 100,000 population in males and females, respectively. The ASMRs of MM in China in 2044 will be 1.12 (95% UI: 0.11–2.12) and 0.68 (95% UI: 0.11–1.25) per 100,000 population in males and females, respectively.
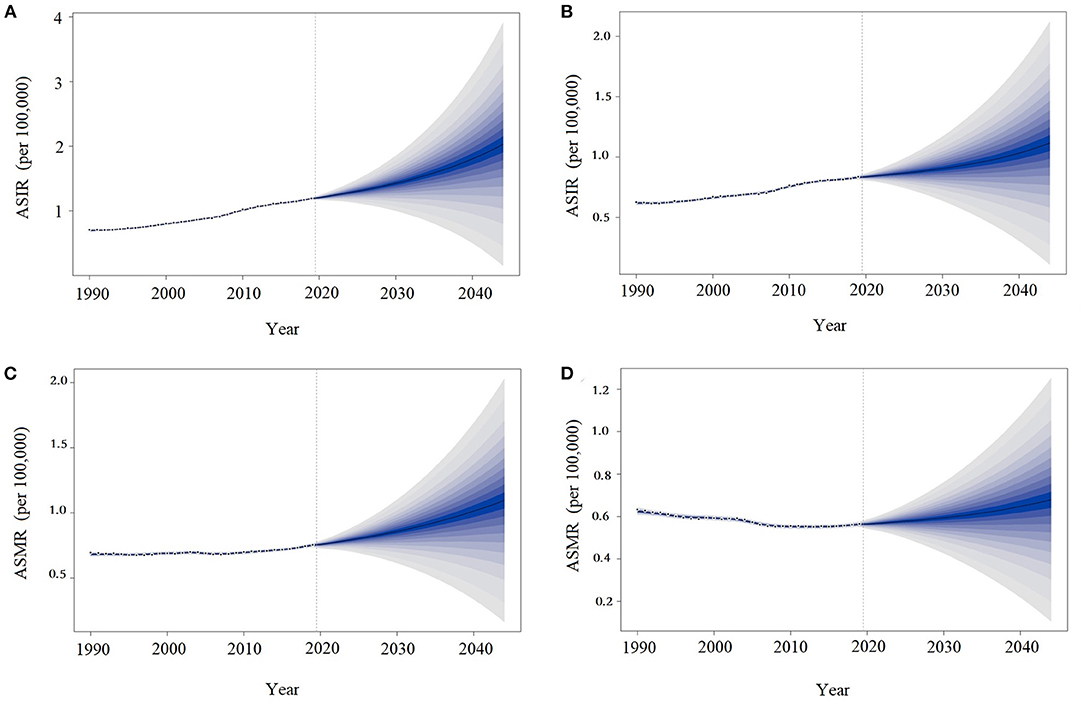
Figure 3. Trends in multiple myeloma incidence and death rates by sex in China: observed (dashed lines) and predicted (solid lines). The ASIR in males (A), ASMR in males (B), ASIR in females (C), and ASMR in females (D). ASIR, age-standardized incidence rates; ASMR, age-standardized death rates.
Discussion
This study showed that the ASIRs and ASMRs of MM in males presented an increasing trend, whereas the ASMRs in females presented a decreasing trend from 1990 to 2019. During the observation period, the incidence rates of MM consistently exceeded the mortality rates, regardless of gender and age group. The burden of MM in males was higher than that in females. Furthermore, age and cohort effects are crucial factors of MM burden, with a high risk of MM incidence and mortality in elderly people and early-born cohort. These findings not only provide a comprehensive and up-to-date overview of temporal trends in MM burden in China over the past 30 years but also produce an important basis for strategy formulation to reduce the MM burden.
Surprising, the incidence rates increased much faster starting from 2007, especially among males, which may be largely due to improved cognition of MM and medical diagnostic techniques (17). Socioeconomic status is well-known to be significantly associated with the burden of diseases (18). The growing economy in China in recent decades has led to enhanced access to health services and more timely and accurate diagnosis of MM (19). The incidence of MM has always been higher than the mortality, may be due to advances in medical technology that have improved the detection rates and the survival rates of MM patients, and not all MM patients die from this cancer (20). For the past decades, new treatments are being developed continually, from the initial monotherapy (e.g., bortezomib, lenalidomide, and thalidomide), to the later modern combination therapy (e.g., combination of chemotherapy and autologous stem-cell transplantation), even individualized treatments for different characteristics, all of which can improve the patients' survival (21, 22). However, the mortality of males has also increased due to the influence by the increasing incidence. Notably, the mortality rates of females have been falling when the incidence of females has remained stable, which may be partly explained by the younger age of onset in female patients than in male patients (4), and age of onset is an important factor of MM burden (23). One study found that younger patients had a better prognosis than older patients for new drug use (24).
The disease burden of MM in China was much greater for males than for females. The significant difference in gender distribution of MM is consistent with previous studies (2). This finding may be due to hormonal differences between males and females, such as androgen being more likely to be a risk factor for MM than estrogen (25). Moreover, it may be partly due to the difference in exposure to risk factors between genders, such as smoking, drinking, obesity and occupational exposure, including farming, nuke industry, chemical industry and medical radiation (26). The trends of males notably showed an increasing trend in incidence and mortality rates, whereas females showed a steady trend in incidence rate and even a decreasing trend in mortality rates. Perhaps, the difference in incidence is due to different living habits, such as diet in males and females. A significant positive correlation was reported for red meat and food of animal origin men like, whereas a significant negative correlation was found for vegetables or cruciferous vegetables women like with risk of MM (27, 28). Meanwhile, the gender difference in mortality rates could be explained in part by the characteristics of the disease itself, in which the male-to-female incidence ratio was 1.5 (4), because mortality is somewhat affected by incidence. Moreover, a population-based study showed that the use of a new drug significantly reduced mortality in the group aged 70–79 years, which is mostly females, but not in the group aged 80 years and above, which is mostly males (24). This finding implied that females have a better prognosis than males.
Age is the most important risk factor for MM burden. The incidence and mortality of MM increase with age, and the risk is the highest among the elderly. Aging creates a favorable condition for the development of various chronic diseases (29). Studies have shown that aging contributes to the decline in stem cell regeneration (30). Therefore, the elderly tends to be accompanied by a decline in physiological and immune functions, and they are more likely to develop cancer (31). Moreover, the elderly have poor tolerance to side effects of antitumor therapy, and they are particularly vulnerable to toxicities during the treatment process, thus affecting the therapeutic effect and leading to poor prognosis (3). The screening of malignant tumors in the elderly should be strengthened to achieve early diagnosis and treatment to reduce MM burden.
The period effects have a significant influence on the MM incidence in males, and the risk of MM incidence increases over time. A mate-analysis showed that new diagnostic techniques in the 21st century have improved diagnostic accuracy and sensitivity of MM that was not available in earlier times (32). Another reason is that with the improvement of the medical system, cancer screening has been gradually strengthened and widely implemented, enabling more people to find the disease in time (17). Moreover, changes in the definition and diagnostic classification of the disease may also explain the rise in MM incidence. For example, from monoclonal gammopathy of undetermined significance to smoldering MM then to MM, which is a more accurate diagnosis of disease. Furthermore, China is undergoing an accelerating aging process, and studies have shown that aging increases the burden of various diseases, including MM. Although China has carried out comprehensive prevention and treatment of cancers for many years, the burden of MM is still serious due to the increase in unhealthy lifestyle, such as smoking, drinking, sedentary disease, lack of exercise and overweight (8). An aging population and the persistence of carcinogens in the environment have also notably contributed to the increased burden of MM (33). In particular, males tend to have higher levels of exposure to these risk factors than females (34).
Concerning the cohort effects, the risk of MM burden decreased with later birth cohort. In the late 19th century and early 20th century, China is experiencing various wars and natural disasters (35, 36), which increased exposure of risk factors for MM, such as ionizing radiation, chemicals, air pollution, and water pollution (8), which may explain the phenomenon that the risk of MM incidence is higher in the early-born cohort. Similarly, due to economic and medical constraints in early times, people born early cannot receive timely diagnosis and treatment, especially those with a rare disease like MM, which may account for a higher risk of death in this population (37). Fortunately, China has experienced rapid social development since the middle and late 20th century, and its socio-economic conditions, sanitation, and medical system have greatly improved (38). Studies have shown that improvements in social and occupational environments have reduced the exposure and dose of MM risk factors like ionizing radiation and medical radiation. With the spread of health knowledge, people's awareness and prevention of MM have been greatly improved (39). Moreover, the nutritional status of later-born cohort significantly improved compared with that of early-born cohort (40). Other studies have shown that health insurance status is associated with the risk of chronic disease morbidity and mortality, including MM (41). Nowadays, the increased coverage rates of three basic medical insurance in China (urban residents' medical insurance, urban workers' medical insurance, and new rural cooperative medical care) has greatly improved the medical security of later-born cohort (42). All these reasons may explain the reduced risk of MM incidence and mortality in the post-birth cohort.
The ASRs of MM incidence and mortality in China was predicted to continuous increase in the next 25 years in both sexes. On the one hand, the life expectancy in Chinese population is on the rise, indicating that the burden of MM will continue to increase with aging in the future (33). On the other hand, Chinese society is undergoing great changes, and some risk factors related to MM, such as unhealthy lifestyle, air and water pollution and occupational exposure, are changing with great uncertainty. Therefore, active and effective measures should be taken to prevent and control the risk factors of MM, such as popularizing tumor screening, reducing environmental pollution, promoting healthy diet and living habits and reducing occupational exposure, to reduce the disease burden of MM.
This study filled the gap in the long-term trend of MM disease burden in China. However, some limitations should be acknowledged. First, the GBD estimates were reconstructed on the basis of a large number of sources with different qualities, leading to potential bias from the actual data (43). Second, although the IE method used in this study has the characteristics of unbiased, efficient, asymptotic and superior estimation, the theoretical basis is complex and could not explain the practical significance of parameter estimation (44, 45). Third, because the GBD 2019 data did not include data on specific related risk factors of MM, speculations could only be conducted on the drivers of the period and cohort effects in which the incidence and mortality trends of MM were observed. Finally, age–period–cohort analysis considers a community as the observed and analyzed unit, possibly resulting in ecological fallacies. Thus, scientific assumptions concerning the causality of these temporal trends were pointed out on the basis of available data and existing literature.
Conclusion
This study provides new evidence for the secular trend of MM incidence and mortality in China. From 1990 to 2019, the burden of MM in China is on the rise in general, and it will continue to increase in the next 25 years. A significant gender difference was observed in MM burden, which was higher in males than in females. Age and cohort effects are crucial factors of MM burden, with a high risk in elderly people and early-born cohort. A comprehensive understanding of the risk characteristics and disease pattern of MM is urgently needed, the publicity and education of relevant knowledge must be strengthened, and targeted interventions to help reduce the disease burden of MM must be urgently taken.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: http://ghdx.healthdata.org/gbdresults-tool.
Ethics Statement
The data in the Global Burden of Disease 2019 study database are publicly available; thus, their use was exempt from review by the Hospital Affiliated Hospital of Guangdong Medical University. The requirement for informed consent was waived.
Author Contributions
YMZ, DDN, and JYW: study design, data collection and analysis support, and critical revision of manuscript. YMZ and DDN: data collection and analysis and drafting the manuscript. ELY, JSH, JW, and XFH: data collection. JYW: data analysis. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was supported by Guangdong Medical University Scientific Research Fund Program (GDMUM201806).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Jingrong Shi from Guangzhou Tianpeng Computer Technology Co., Ltd., for their contribution and assistance in terms of data analysis.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.938770/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Liu J, Liu W, Mi L, Zeng X, Cai C, Ma J, et al. Incidence and mortality of multiple myeloma in China, 2006–2016: an analysis of the global burden of disease study 2016. J Hematol Oncol. (2019) 12:136. doi: 10.1186/s13045-019-0807-5
3. Wildes TM, Rosko A, Tuchman SA. Multiple myeloma in the older adult: better prospects, more challenges. J Clin Oncol. (2014) 32:2531–40. doi: 10.1200/JCO.2014.55.1028
4. Kumar SK, Rajkumar V, Kyle RA, van Duin M, Sonneveld P, Mateos MV, et al. Multiple myeloma. Nat Rev Dis Primers. (2017) 3:17046. doi: 10.1038/nrdp.2017.46
5. Chang-Chan DY, Ríos-Tamayo R, Rodríguez Barranco M, Redondo-Sánchez D, González Y, Marcos-Gragera R, et al. Trends of incidence, mortality and survival of multiple myeloma in Spain. A twenty-three-year population-based study. Clin Transl Oncol. (2021) 23:1429–39. doi: 10.1007/s12094-020-02541-1
6. Larsson SC, Wolk A. Body mass index and risk of multiple myeloma: a meta-analysis. Int J Cancer. (2007) 121:2512–6. doi: 10.1002/ijc.22968
7. Shimizu Y, Kato H, Schull WJ. Mortality among atomic bomb survivors. J Radiat Res. (1991) 32(Suppl:2):12–30. doi: 10.1269/jrr.32.supplement_212
8. Zhou L, Yu Q, Wei G, Wang L, Huang Y, Hu K, et al. Measuring the global, regional, and national burden of multiple myeloma from 1990 to 2019. BMC Cancer. (2021) 21:606. doi: 10.1186/s12885-021-08280-y
9. Huang SY, Yao M, Tang JL, Lee WC, Tsay W, Cheng AL, et al. Epidemiology of multiple myeloma in Taiwan: increasing incidence for the past 25 years and higher prevalence of extramedullary myeloma in patients younger than 55 years. Cancer. (2007) 110:896–905. doi: 10.1002/cncr.22850
10. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/s0140-6736(20)30925-9
11. GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1223–49. doi: 10.1016/s0140-6736(20)30752-2
12. Li S, Huang X, Liu J, Yue S, Hou X, Hu L, et al. Trends in the Incidence and DALYs of urolithiasis From 1990 to 2019: results from the global burden of disease study 2019. Front Public Health. (2022) 10:825541. doi: 10.3389/fpubh.2022.825541
13. Wu J, Lin Z, Liu Z, He H, Bai L, Lyu J. Secular trends in the incidence of eating disorders in China from 1990 to 2017: a joinpoint and age-period-cohort analysis. Psychol Med. (2022) 52:946–56. doi: 10.1017/S0033291720002706
14. Wang Y, Huang X, Li S, Yue S, Liu J, Wu J. Secular trend in the incidence of conduct disorder in china from 1990 to 2019: a joinpoint and age-period-cohort analysis. J Dev Behav Pediatr. (2022) 43:e339–e46. doi: 10.1097/dbp.0000000000001049
15. Knoll M, Furkel J, Debus J, Abdollahi A, Karch A, Stock C. An R package for an integrated evaluation of statistical approaches to cancer incidence projection. BMC Med Res Methodol. (2020) 20:257. doi: 10.1186/s12874-020-01133-5
16. Riebler A, Held L. Projecting the future burden of cancer: Bayesian age-period-cohort analysis with integrated nested Laplace approximations. Biom J. (2017) 59:531–49. doi: 10.1002/bimj.201500263
17. Brigle K, Rogers B. Pathobiology and diagnosis of multiple meloma. Semin Oncol Nurs. (2017) 33:225–36. doi: 10.1016/j.soncn.2017.05.012
18. Fidler MM, Soerjomataram I, Bray F. A global view on cancer incidence and national levels of the human development index. Int J Cancer. (2016) 139:2436–46. doi: 10.1002/ijc.30382
19. Song X, Lan L, Zhou T, Yin J, Meng Q. Economic burden of major diseases in China in 2013. Front Public Health. (2021) 9:649624. doi: 10.3389/fpubh.2021.649624
20. Gerecke C, Fuhrmann S, Strifler S, Schmidt-Hieber M, Einsele H, Knop S. The diagnosis and treatment of multiple myeloma. Dtsch Arztebl Int. (2016) 113:470–6. doi: 10.3238/arztebl.2016.0470
21. Pawlyn C, Davies FE. Toward personalized treatment in multiple myeloma based on molecular characteristics. Blood. (2019) 133:660–75. doi: 10.1182/blood-2018-09-825331
22. Cowan AJ, Green DJ, Kwok M, Lee S, Coffey DG, Holmberg LA, et al. Diagnosis and management of multiple myeloma: a review. Jama. (2022) 327:464–77. doi: 10.1001/jama.2022.0003
23. Larocca A, Dold SM, Zweegman S, Terpos E, Wäsch R, D'Agostino M, et al. Patient-centered practice in elderly myeloma patients: an overview and consensus from the European myeloma network (EMN). Leukemia. (2018) 32:1697–712. doi: 10.1038/s41375-018-0142-9
24. Usui Y, Ito H, Koyanagi Y, Shibata A, Matsuda T, Katanoda K, et al. Changing trend in mortality rate of multiple myeloma after introduction of novel agents: a population-based study. Int J Cancer. (2020) 147:3102–9. doi: 10.1002/ijc.33135
25. Hammes SR, Levin ER. Impact of estrogens in males and androgens in females. J Clin Invest. (2019) 129:1818–26. doi: 10.1172/JCI125755
26. Sinks T. Screenings and clusters: a cancer cluster in a chemical plant. Occup Environ Med. (2014) 71:2–3. doi: 10.1136/oemed-2013-101789
27. Caini S, Masala G, Gnagnarella P, Ermini I, Russell-Edu W, Palli D, et al. Food of animal origin and risk of non-Hodgkin lymphoma and multiple myeloma: a review of the literature and meta-analysis. Crit Rev Oncol Hematol. (2016) 100:16–24. doi: 10.1016/j.critrevonc.2016.02.011
28. Brown LM, Gridley G, Pottern LM, Baris D, Swanso CA, Silverman DT, et al. Diet and nutrition as risk factors for multiple myeloma among blacks and whites in the United States. Cancer Causes Control. (2001) 12:117–25. doi: 10.1023/a:1008937901586
29. He S, Sharpless NE. Senescence in health and disease. Cell. (2017) 169:1000–11. doi: 10.1016/j.cell.2017.05.015
30. Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. (2007) 8:703–13. doi: 10.1038/nrm2241
31. Calcinotto A, Kohli J, Zagato E, Pellegrini L, Demaria M, Alimonti A. Cellular senescence: aging, cancer, and injury. Physiol Rev. (2019) 99:1047–78. doi: 10.1152/physrev.00020.2018
32. Regelink JC, Minnema MC, Terpos E, Kamphuis MH, Raijmakers PG, Pieters-van den Bos IC, et al. Comparison of modern and conventional imaging techniques in establishing multiple myeloma-related bone disease: a systematic review. Br J Haematol. (2013) 162:50–61. doi: 10.1111/bjh.12346
33. GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet. (2017) 390:1260–344. doi: 10.1016/s0140-6736(17)32130-x
34. Zhang J, Mauzerall DL, Zhu T, Liang S, Ezzati M, Remais JV. Environmental health in China: progress towards clean air and safe water. Lancet. (2010) 375:1110–9. doi: 10.1016/s0140-6736(10)60062-1
35. Lonial S, Dhodapkar MV, Rajkumar SV. Smoldering myeloma and the art of war. J Clin Oncol. (2020) 38:2363–5. doi: 10.1200/jco.20.00875
36. Prohaska TR, Peters KE. Impact of natural disasters on health outcomes and cancer among older adults. Gerontologist. (2019) 59:S50–s6. doi: 10.1093/geront/gnz018
37. He J, Song P, Kang Q, Zhang X, Hu J, Yang Y, et al. Overview on social security system of rare diseases in China. Biosci Trends. (2019) 13:314–23. doi: 10.5582/bst.2019.01209
38. Cohen AK, Syme SL. Education: a missed opportunity for public health intervention. Am J Public Health. (2013) 103:997–1001. doi: 10.2105/AJPH.2012.300993
39. Li X, Lu J, Hu S, Cheng KK, De Maeseneer J, Meng Q, et al. The primary health-care system in China. Lancet. (2017) 390:2584–94. doi: 10.1016/s0140-6736(17)33109-4
40. Liang F, Dong XY, Tang GF, Qi KM, Chen W, Sang W, et al. [Influence of prognostic nutritional index and controlling nutritional status on the prognosis of patients with multiple myeloma]. Zhonghua Xue Ye Xue Za Zhi. (2021) 42:332–7. doi: 10.3760/cma.j.issn.0253-2727.2021.04.011
41. Bittoni MA, Wexler R, Spees CK, Clinton SK, Taylor CA. Lack of private health insurance is associated with higher mortality from cancer and other chronic diseases, poor diet quality, and inflammatory biomarkers in the United States. Prev Med. (2015) 81:420–6. doi: 10.1016/j.ypmed.2015.09.016
42. Rosenberg AR, Kroon L, Chen L, Li CI, Jones B. Insurance status and risk of cancer mortality among adolescents and young adults. Cancer. (2015) 121:1279–86. doi: 10.1002/cncr.29187
43. Wu J, Lai T, Han H, Liu J, Wang S, Lyu J. Global, regional and national disability-adjusted life years due to HIV from 1990 to 2019: findings from the global burden of disease study 2019. Trop Med Int Health. (2021) 26:610–20. doi: 10.1111/tmi.13565
44. Wang Y, Huang X, Yue S, Liu J, Li S, Ma H, et al. Secular trends in the incidence of migraine in China from 1990 to 2019: a joinpoint and age-period-cohort analysis. J Pain Res. (2022) 15:137–46. doi: 10.2147/JPR.S337216
Keywords: multiple myeloma, disease burden, secular trend, projection, age-period-cohort model
Citation: Zhao Y, Niu D, Ye E, Huang J, Wang J, Hou X and Wu J (2022) Secular Trends in the Burden of Multiple Myeloma From 1990 to 2019 and Its Projection Until 2044 in China. Front. Public Health 10:938770. doi: 10.3389/fpubh.2022.938770
Received: 08 May 2022; Accepted: 17 June 2022;
Published: 08 July 2022.
Edited by:
Sumaira Mubarik, Wuhan University, ChinaCopyright © 2022 Zhao, Niu, Ye, Huang, Wang, Hou and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiayuan Wu, d3VqaWF5JiN4MDAwNDA7Z2RtdS5lZHUuY24=
†These authors have contributed equally to this work
 Yumei Zhao
Yumei Zhao Dongdong Niu1†
Dongdong Niu1† Jiasheng Huang
Jiasheng Huang Xuefei Hou
Xuefei Hou Jiayuan Wu
Jiayuan Wu