- 1Department of Pediatrics, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children, Sichuan University, Ministry of Education, Chengdu, China
Background and aim: Abdominal tuberculosis (TB) is a common type of extrapulmonary TB with an insidious onset and non-specific symptoms. Adenosine deaminase (ADA) levels increase rapidly in the early stages of abdominal TB. However, it remains unclear whether ADA serves as a diagnostic marker for abdominal TB.
Methods: We performed a systematic literature search for relevant articles published in PubMed, Web of Science, Cochrane Library, and Embase up to April 2022. First, we used the Quality Assessment of Diagnostic Accuracy Studies tool-2 (QUADAS-2), to evaluate the quality of the included articles. Bivariate and hierarchical summary receiver operating characteristic (HSROC) models were then utilized to analyze pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR) and area under the receiver operating characteristic curve (AUROC). In addition, we explored a subgroup analysis for potential heterogeneity and publication bias among the included literature.
Results: Twenty-four articles (3,044 participants, 3,044 samples) which met the eligibility criteria were included in this study. The pooled sensitivity and specificity of ADA for abdominal TB detection were 93% [95% confidence interval (CI): 0.89–0.95] and 95% (95% CI: 0.93–0.96), respectively. PLR and NLR were 18.6 (95% CI: 14.0–24.6) and 0.08 (95% CI: 0.05–0.12), respectively. DOR and AUROC were 236 (95% CI: 134–415) and 0.98 (95% CI: 0.96–0.99), respectively. Furthermore, no heterogeneity or publication bias was found.
Conclusions: Our meta-analysis found ADA to be of excellent diagnostic value for abdominal TB and could be used as an auxiliary diagnostic tool.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier: CRD42022297931.
Introduction
Tuberculosis (TB) is one of the most serious global health conditions, with high prevalence and mortality rates (1); millions of new cases are reported worldwide, and ~1.2 million people die from TB each year, particularly in high-burden countries such as India and Central Africa (1–3). Abdominal TB accounts for 6.1% of all extrapulmonary TB cases, and the incidence of abdominal TB in pulmonary TB patients is 10–30% (4, 5). Mycobacterium tuberculosis (Mtb) can spread through the blood and lymph to the abdominal cavity or via the digestive tract and adjacent organs causing abdominal TB (6, 7), which can be further classified into mesenteric, peritoneal, intestinal, and visceral TB (5, 8–10). Ascites is one of the most common clinical manifestations of abdominal TB (11). Abdominal TB has an insidious course, which can delay diagnosis and treatment, and result in increased disease severity and mortality (6, 12, 13). In order to reduce severity, mortality, and morbidity, it is important to make a timely diagnosis and institute effective treatment.
At present, the golden standard in clinical diagnosis of abdominal TB is still laparoscopic pathological biopsy and/or culture of Mtb with ascites (5). However, the high cost and invasiveness of laparoscopy make it impossible to be used routinely in clinics. Furthermore, adverse events (3%) and mortality (0.04%) have been reported in laparoscopic detection (5). The culture of Mtb has a low positive rate (25 to 36% in ascitic fluid) and takes up to 8 weeks to provide a result (5, 14). Thus, both biopsy and culture are impractical for the early diagnosis in patients with abdominal TB. Additionally, there are other examination methods available in clinics, e.g., blood tests, biochemical examinations, GeneXpert MTB/RIF assay, and imaging methods. Blood tests show signs of chronic inflammation but are non-specific (6). Biochemical examination of ascites may suggest exudation, but this may be indistinguishable from diseases such as cirrhosis, which often coexist with abdominal TB (15). The GeneXpert MTB/RIF assay, a heminested real-time polymerase chain reaction method, has high diagnostic power for pulmonary samples but low sensitivity for extrapulmonary samples (16, 17). Imaging methods, such as ultrasound and computed tomography, can only assist in paracentesis and tissue biopsy but cannot provide a definitive diagnosis (18, 19). Therefore, developing a detection method which is rapid, efficient, and economical would be conducive to both the early detection, and timely and effective treatment of abdominal TB.
Adenosine deaminase (ADA) is an enzyme that degrades immunosuppressive signals due to adenosine and plays an important regulatory role in immune homeostasis (20). While infecting the patient, Mtb can cause an imbalance of host innate and adaptive immune homeostasis resulting in TB (21). Recently, ADA levels were found to be significantly upregulated in a variety of TB cavity effusions, suggesting that ADA could be used as a marker for diagnosing TB (22–26). ADA was reported to have a high value in diagnosing abdominal TB, even in patients with low immunity caused by human immunodeficiency virus (HIV) infection (27, 28). As a test which requires minimally invasive sampling, ADA also has high clinical applicability. However, in some low-burden countries, such as the USA (sensitivity: 58.8%, specificity: 95.4%) and South Korea (sensitivity: 82%, specificity: 79%), ADA diagnostic performance is unsatisfactory (29, 30). In addition, ADA has been reported to have different sensitivities and specificities for diagnosing TB when using different cut-off values (31–33). Therefore, finding an optimal cut-off value could improve the availability of the ADA test for abdominal TB screening. Here, we performed a systematic review and meta-analysis of published ADA results to explore its overall diagnostic value in abdominal TB.
Methods
Literature search
This study was based on PRISMA-DTA statement published in 2018 and registered in PROSPERO (CRD42022297931) (34). Two independent reviewers searched and retrieved original English research articles in PubMed, Web of Science, Cochrane Library, and Embase, since each database's creation until April 2022. The following Medical Subject Heading keywords in the text, title, and abstract of published literature were used to identify relevant articles: “tuberculosis,” “tuberculous,” “abdominal,” “peritonitis,” “ascites,” “peritoneal,” “TBP,” “intestinal,” “mesenteric lymph node,” “mesenteric lymph nodal,” “extra-pulmonary,” “adenosine deaminase,” and “ADA.” References (forward citations) and citation lists (backward citations) of the relevant literature were also consulted to find as many available articles as possible. Two independent reviewers examined all available literature to discuss and resolve any discrepancies.
Study selection
Research articles were included according to the following inclusion criteria: (i) cases of abdominal TB and non-TB controls; (ii) ADA level in ascites as the index test; (iii) clinical diagnosis, bacteriology, or histopathology as reference standards for abdominal TB; and (iv) sensitivity and specificity of ADA as primary outcomes, with more than five participants in each study. Reviews, abstracts, comments, case reports, papers published in a language other than English, and animal experiments were excluded. Two independent reviewers evaluated the articles to eliminate eligibility bias according to the above requirements. Consensus was reached through discussion and scientific persuasion, and qualified articles were included in our study for further processing.
Data extraction
We extracted the following data from each article: country/region, TB burden (World Health Organization adjustment) (35); study design type; abdominal TB category; reference standard; number of participants (abdominal TB and non-TB controls); method; sample type; ADA cut-off values; and ADA sensitivity, specificity, true positive (TP), false positive (FP), false negative (FN), and true negative (TN) rates. Data extraction was independently evaluated and cross-checked by two reviewers, and consensus was reached through discussion.
Quality assessment
In accordance with QUADAS-2, two independent reviewers assessed the quality of the included articles. RevMan (version 5.3) was used for the analysis (36).
Data analysis
The data analyses were conducted using the HSROC, and bivariate models were constructed with the “metandi” package in Stata (version 14.0) (37, 38). Pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), HSROC curve, and area under the summary receiver operating characteristic curve (AUROC) were calculated with a confidence interval (CI) of 95% (39). The underlying factors of potential heterogeneity were grouped and analyzed by meta-regression analysis. Four subgroups were considered: TB-burden country/region (high or low), study design type (case-control or not), disease category (TB ascites or not), and different cut-offs of ADA (≥40 IU/L or not) (40). Publication bias was assessed by Deeks' funnel plot asymmetry test (41). Statistical significance was set at p < 0.05.
Results
Search results
Among 1,156 studies retrieved, 383 were considered duplicates (the same article) and therefore, excluded. Upon preliminary screening, 697 studies were excluded: 238 studies were ineligible based on the patient selection criteria (cirrhosis, abdominal tumor, Crohn's disease, etc.); 184 studies consisted of reviews, abstracts, comments, and case reports; 108 studies were ineligible based on the intervention criteria (interferon-γ, new tuberculosis vaccine, T-SPOT.TB, etc.); 79 studies consisted of non-English publications (Chinese, Spanish, Japanese, etc.); 47 used other detection methods (computed tomography, polymerase chain reaction, immunochromatographic assay, etc.); 37 studies focused on TB mechanisms (signaling pathway, cell death, immune response, etc.); and 4 studies included animal experiments (rats, mice, etc.). The full text of remaining articles was reviewed; 24 of the 76 articles were included in the meta-analysis (29–33, 42–60) (Figure 1).
Main characteristics
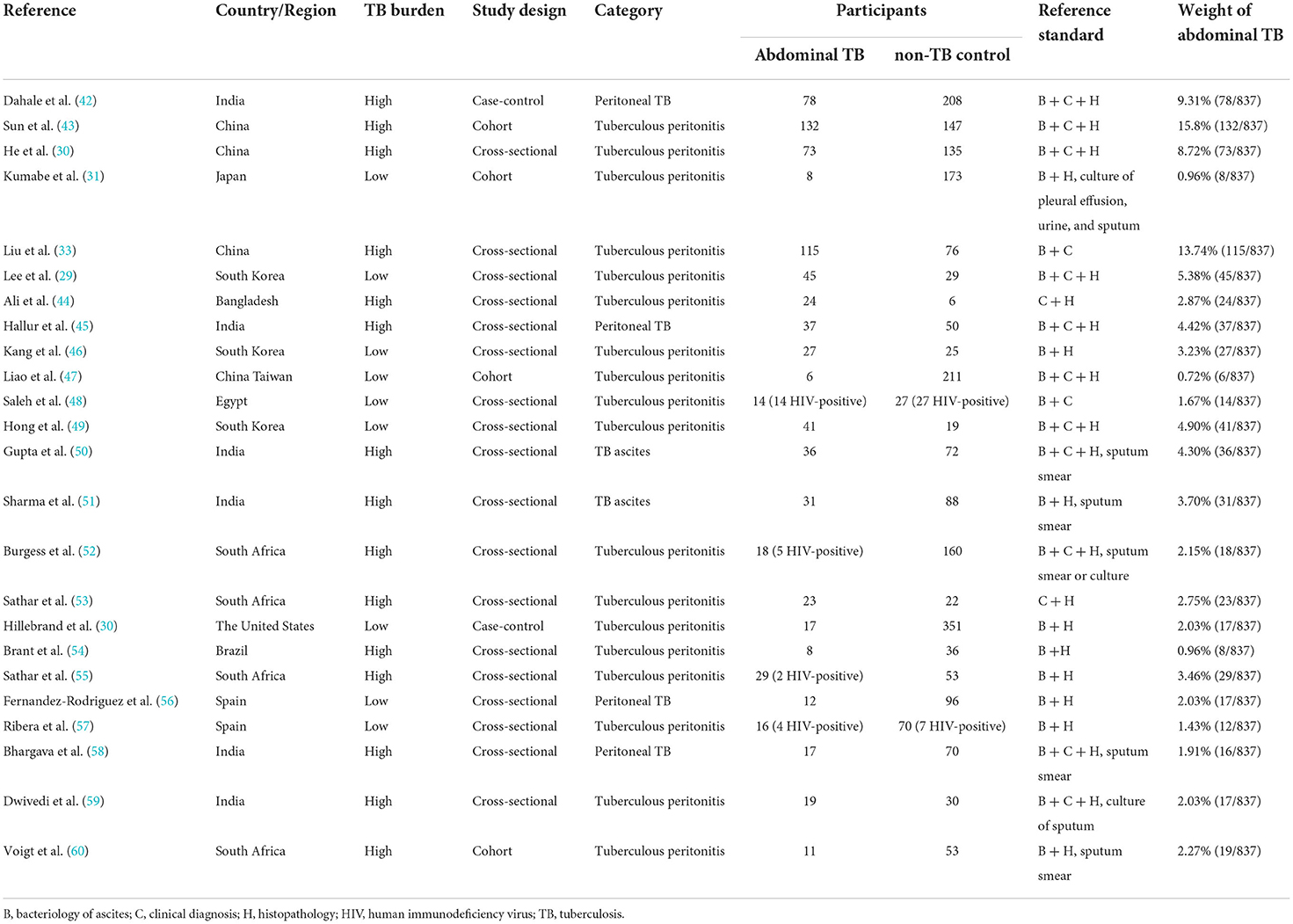
The main characteristics of 24 studies were listed in Tables 1, 2 (29–33, 42–60). Data were collected from 11 countries (five low-burden and six high-burden) between 1989 and 2021. A total of 3,044 participants' data (837 patients with abdominal TB and 2,207 participants without TB) were analyzed. Sub-categories of abdominal TB included peritoneal TB, tuberculous peritonitis, and TB ascites. The reference standards for abdominal TB met the following criteria: clinical diagnosis, bacteriology, and histopathology (61, 62). The ADA index was calculated from samples of ascitic fluid according to the methods of Slaats, Giusti et al. as required by our inclusion criteria. The ADA cut-off value to support the diagnosis of abdominal TB was 7–41.1 IU/L. ADA sensitivity, specificity, TP, FP, TN, and FN were also extracted. The weight of abdominal TB was determined by the number of participants in each literature.
Quality of the included studies
To avoid bias and ambiguity, the methodological quality of the included studies was evaluated by the four aspects through QUADAS-2 (Figures 2A,B). In patient selection bias, two high-risk articles came from a case-control study (30, 42) and one unclear article lacked an inclusion period (59). In the index test bias, three unclear articles did not report the blinding method (29, 49, 53). The same three unclear articles also did not report the reference standard blinding method (29, 49, 53). Regarding flow and timing bias, seven unclear articles showed that participants accepted different reference standards (30, 42, 43, 49, 55, 57, 60). Generally, the overall quality of the included articles was good.
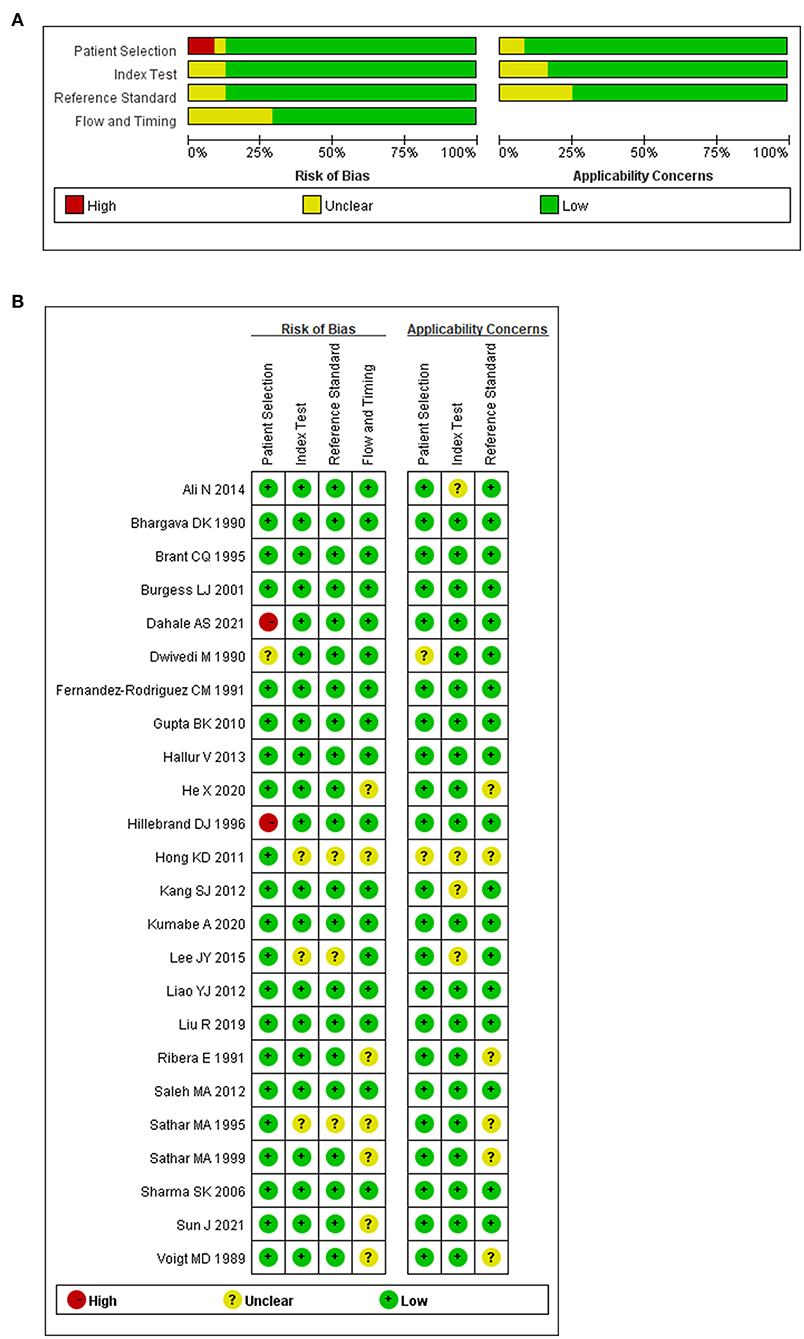
Figure 2. Methodological quality regarding ADA and abdominal TB. (A) Graph of risk of bias and applicability concerns. (B) Summary of risk bias and applicability concerns.
Summary statistics
To study the summary diagnostic value of ADA for abdominal TB, 3,044 samples of 24 studies were included. The pooled sensitivity and specificity of ADA were 93% (95% CI: 0.89–0.95) and 95% (95% CI: 0.93–0.96), respectively (Figure 3). The combined PLR was 18.6 (95% CI: 14.0–24.6) and NLR was 0.08 (95% CI: 0.05–0.12) (Figure 4). The combined DOR was 236 (95% CI: 134–415), indicating that ADA was reliable for diagnosing abdominal TB. The HSROC curve of ADA with its confidence and prediction regions is shown in Figure 5. The summary point is the optimal combination of sensitivity and specificity. The yellow dotted line around each summary point represents the 95% CI. The AUROC was 0.98 (95% CI: 0.96–0.99), suggesting that ADA had excellent diagnostic accuracy (AUROC above 0.93 was considered “excellent”) (63).
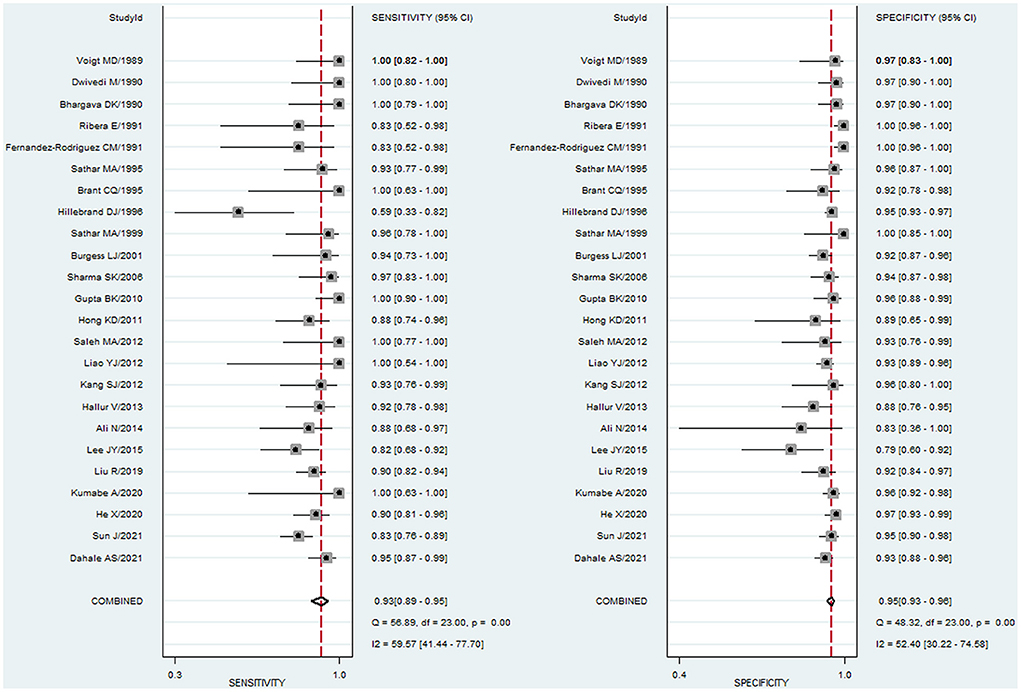
Figure 3. Paired forest plots of pooled sensitivity and specificity of ADA for the diagnosis of abdominal TB. Sensitivity and specificity in each study were represented by squares, and 95% confidence intervals were represented by horizontal bars.
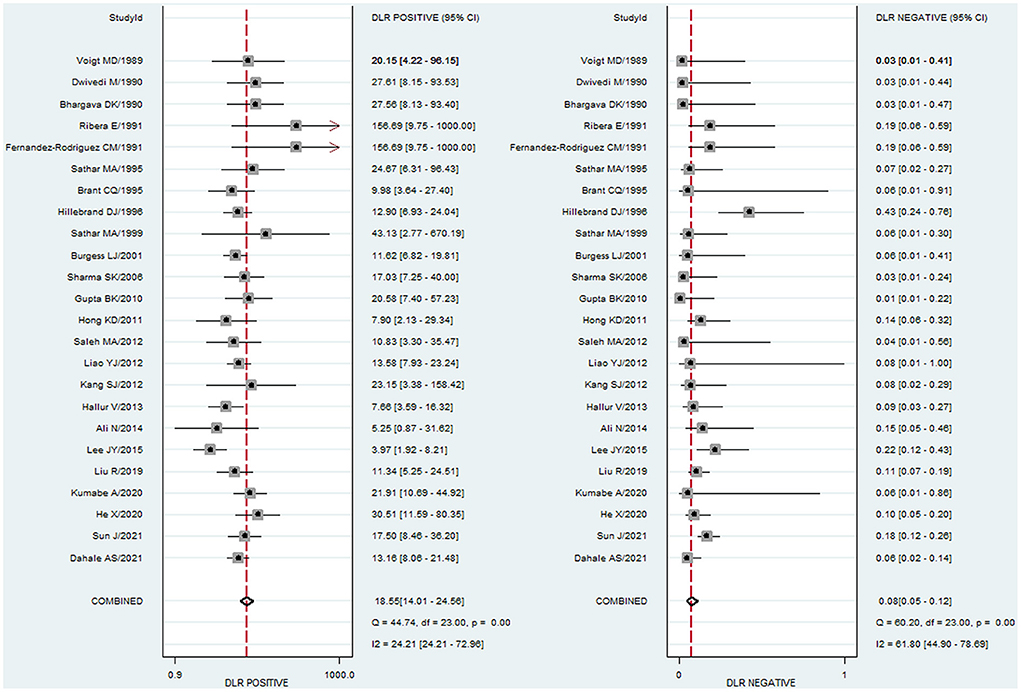
Figure 4. Paired forest plots of combined PLR and NLR of ADA for the diagnosis of abdominal TB. PLR and NLR in each study were represented by squares, and 95% confidence intervals were represented by horizontal bars.
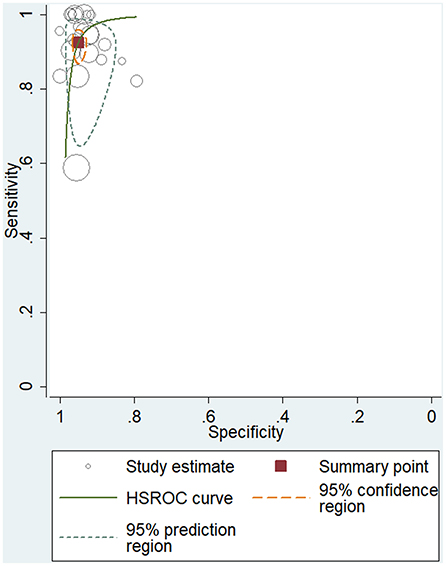
Figure 5. Hierarchical summary receiver operating characteristic (HSROC) curve for evaluating the overall diagnostic performance of ADA for the diagnosis of abdominal TB.
Heterogeneity
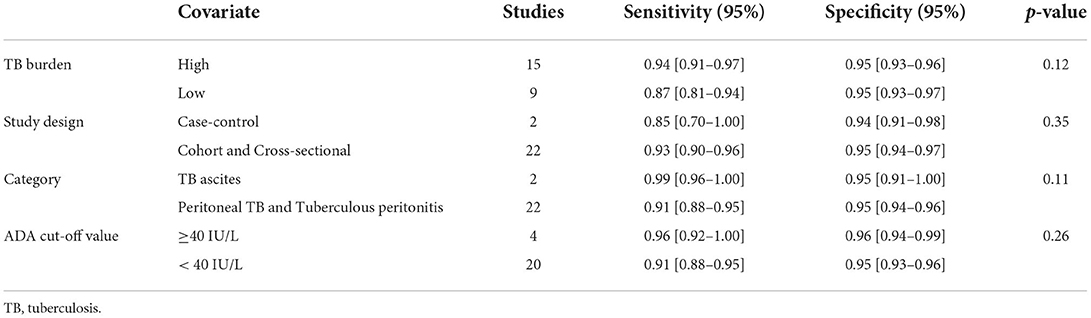
We also explored whether there was heterogeneity among potential covariates or not (Table 3). There was no heterogeneity found between the abdominal TB and control groups in the four subgroups (all p > 0.05): high-burden vs. low-burden TB countries, p = 0.12; case-control vs. cohort and cross-sectional studies, p = 0.35; TB ascites vs. peritoneal TB and tuberculous peritonitis, p = 0.11; and a cut-off value of ADA ≥40 IU/L vs. <40 IU/L, p = 0.26.
Publication bias
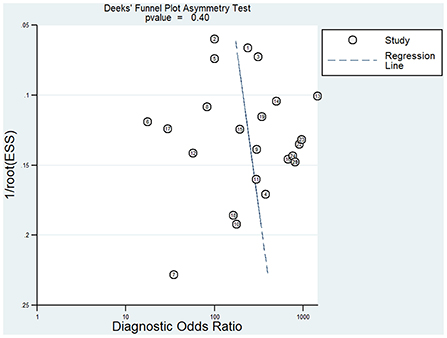
According to Deeks' funnel plot asymmetry test (Figure 6), significant publication bias (p = 0.40) was not observed in any of the included articles. Therefore, the stability of this study was confirmed.
Discussion
Abdominal TB is a disease of an insidious nature with non-specific clinical features (64). Early differentiation from other diseases and diagnosis of abdominal TB is key to successful treatment thereof (65–67). Traditional diagnostic methods can result in significant delays in the diagnosis of abdominal TB. Subsequently, severe sequelae may occur due to delayed initiation of treatment. Therefore, it is important to develop a simple, fast, and economical method to diagnose abdominal TB. Studies have found that the level of immunomodulatory enzyme ADA increases rapidly and may therefore be useful for the detection of pulmonary and/or extrapulmonary TB. However, there are no systematic studies on the diagnostic performance of ADA for abdominal TB (23, 31, 68). Hence, we performed a systematic review and meta-analysis consisting of 24 studies to assess the overall performance of ADA in abdominal TB diagnosis.
First, we evaluated the diagnostic efficacy of ADA for abdominal TB, and found the pooled sensitivity and specificity were 93 and 95%, respectively, which demonstrates that the missed diagnosis and misdiagnosis rates of abdominal TB using ADA have been as low as 7 and 5%, respectively. These findings were similar to those of ADA sensitivity and specificity for the detection of TB ascites or tuberculous peritonitis, both of which have high diagnostic efficacy (69–71). As the sensitivity and specificity were higher than 90%, the diagnostic accuracy of ADA for abdominal TB was quite high. In addition, higher than 10 of PLR values and lower than 0.1 of NLR values are considered strong diagnostic significance (72). In our meta-analysis, the PLR was 18.6, indicating that the probability of an ADA-positive diagnosis of abdominal TB was 18.6-fold higher than that of non-TB controls. Furthermore, the NLR was 0.08, suggesting that 8% of ADA-negative diagnoses were abdominal TB. DOR is a measure of diagnostic test efficiency that combines sensitivity and specificity; a higher DOR value indicates better performance of the discriminatory test (73). In this study, the DOR was 236, indicating that ADA is a good marker to distinguish abdominal TB from non-TB groups. The HSROC curve also suggested that ADA had an excellent performance in diagnosing abdominal TB; the AUROC reached 0.98, which represents a high overall accuracy with high values of sensitivity and specificity. Therefore, ADA be an accurate marker to support the diagnosis of abdominal TB, however, it cannot be used as the only diagnostic marker of abdominal TB as it has a misdiagnosis rate of 5%. In addition, ADA sensitivity was found to be as low as 58.8–83.3% in low-burden countries such as the USA, South Korea, and Spain (29, 30, 56). As a screening marker for abdominal TB, the cut-off value of ADA should be reduced in these low-burden countries to increase its sensitivity.
After evaluating the comprehensive diagnostic efficacy of ADA, bivariate analysis was carried out on TB burden, study design, category, and ADA cut-off value. No significant differences were found between these four categories (p > 0.05). As for TB burden, ADA did not bias the diagnosis of abdominal TB in high- and low-burden countries, although in the included studies, sensitivity was lower in low-burden countries than in high-burden countries. We also found no bias relating to study design, indicating that the original case-control studies we included did not reduce the quality of our meta-analysis. Different categories of abdominal TB did not lead to bias in the diagnostic performance of ADA, which was consistent with previous findings that ADA has superior diagnostic performance for TB ascites and tuberculous peritonitis (69, 70). Although different ADA cut-off values were reported in different original studies (7–41.1 IU/L), this wide range of values did not lead to bias in diagnosing abdominal TB. Recently, 40 IU/L was identified as the clinical diagnostic point in some studies (26, 40). As there was no bias in TB burden, study design, category, and ADA cut-off value, the results of this study are highly accurate.
Although we found that ADA had excellent diagnostic efficacy for abdominal TB without significant heterogeneity or publication bias, its limitations cannot be ignored. First, the combined sensitivity and specificity of ADA were very high (>90%). However, these two values were directly related to the prevalence of abdominal TB. As the positive predictive value of ADA increases with high prevalence, it is more important to diagnose abdominal TB using ADA in countries with high burdens of TB (74). Second, although the good values of PLR and NLR proved the diagnostic accuracy of ADA, missed diagnosis and misdiagnosis rates existed (<10%). Therefore, ADA cannot be used as the golden standard for the detection of abdominal TB. Third, ADA levels could be affected by other factors. For example, Delacour et al. (75) found that bilirubin > 50 μmol/L or hemoglobin > 177 μmol/L interfered with ADA values. In one of the studies included, Dahale et al. investigated the diagnostic value of ADA for peritoneal TB in cirrhosis. The ADA cut-off value of peritoneal TB in the cirrhosis subgroup (64.0 IU/L) was slightly lower than that of the peritoneal TB group (72.2 IU/L), which might be related to the interference of bilirubin changes in cirrhosis on ADA value (42). Fourth, HIV-induced immunodeficiency increases the likelihood of Mtb infection, and patients living with HIV have lower ADA levels than their seronegative counterparts (76). However, the articles included in this meta-analysis could not provide data for studying the impact of HIV infection on ADA diagnosis of abdominal TB. Finally, in the present meta-analysis, we only included English-written articles, and it is unclear whether the non-English articles would affect the results.
Conclusions
In conclusion, this study showed that ADA has excellent diagnostic value for abdominal TB, with high sensitivity and specificity, particularly in regions with a high burden of TB. ADA detection is a simple, fast, and economical auxiliary method for the clinical diagnosis of abdominal TB.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
RZ, XQ, JY, and DM conceived the study, which was refined by YY, TR, LY, QL, XS, SW, YQ, and XL. RZ and XQ conducted the literature search. RZ, XQ, JY, YY, and TR screened the full-text papers and extracted the data. RZ, XQ, TR, and SW run the analysis. RZ, XQ, JY, QL, XS, YQ, XL, and DM drafted the manuscript. All authors provided input into revisions and approved the final draft for submission.
Funding
This work was supported by the National Natural Science Foundation of China (81801629, 81971433, 81971428, and 82071353); the National Key R&D Program of China (2021YFC2701700 and 2017YFA0104200); the grants from the Science and Technology Bureau of Sichuan Province (2021YJ0017 and 2020YFS0041); the Fundamental Research Funds for the Central University (SCU2021D009).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ADA, adenosine deaminase; AUROC, area under the receiver operating characteristic curve; CI, confidence interval; DOR, diagnostic odds ratio; FN, false negative; FP, false positive; HSROC, hierarchical summary receiver operating characteristic; HIV, human immunodeficiency virus; M. tb, Mycobacterium tuberculosis; NLR, negative likelihood ratio; PLR, positive likelihood ratio; QUADAS-2, Quality Assessment of Diagnostic Accuracy Studies tool-2; TB, tuberculosis; TN, true negative; TP, true positive.
References
1. Furin J, Cox H, Pai M. Tuberculosis. Lancet. (2019) 393:1642–56. doi: 10.1016/S0140-6736(19)30308-3
2. Lonnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med. (2009) 68:2240–6. doi: 10.1016/j.socscimed.2009.03.041
3. Zurcher K, Reichmuth ML, Ballif M, Loiseau C, Borrell S, Reinhard M, et al. Mortality from drug-resistant tuberculosis in high-burden countries comparing routine drug susceptibility testing with whole-genome sequencing: a multicentre cohort study. Lancet Microbe. (2021) 2:e320–30. doi: 10.1016/S2666-5247(21)00044-6
4. Ionescu S, Nicolescu AC, Madge OL, Marincas M, Radu M, Simion L. Differential diagnosis of abdominal tuberculosis in the adult-literature review. Diagnostics. (2021) 11:2362. doi: 10.3390/diagnostics11122362
5. Vaid U, Kane GC. Tuberculous peritonitis. Microbiol Spectr. (2017) 5:1–20. doi: 10.1128/microbiolspec.TNMI7-0006-2016
6. Sartoris G, Seddon JA, Rabie H, Nel ED, Schaaf HS. Abdominal tuberculosis in children: challenges, uncertainty, and confusion. J Pediatric Infect Dis Soc. (2020) 9:218–27. doi: 10.1093/jpids/piz093
7. Lan Z, Bastos M, Menzies D. Treatment of human disease due to mycobacterium bovis: a systematic review. Eur Respir J. (2016) 48:1500–3. doi: 10.1183/13993003.00629-2016
8. Lin YS, Huang YC, Lin TY. Abdominal tuberculosis in children: a diagnostic challenge. J Microbiol Immunol Infect. (2010) 43:188–93. doi: 10.1016/S1684-1182(10)60030-8
9. Chung JJ, Choi JM, Cho ES, Kim JH, Yu JS. Multidetector CT findings of histopathologically proven peritoneal tuberculous cold abscesses. Radiol Med. (2017) 122:248–56. doi: 10.1007/s11547-017-0726-x
10. Choi EH, Coyle WJ. Gastrointestinal tuberculosis. Microbiol Spectr. (2016) 4:1–20. doi: 10.1128/microbiolspec.TNMI7-0014-2016
11. Okamoto K, Hatakeyama S. Tuberculous peritonitis. N Engl J Med. (2018) 379:e20. doi: 10.1056/NEJMicm1713168
12. Malikowski T, Mahmood M, Smyrk T, Raffals L, Nehra V. Tuberculosis of the gastrointestinal tract and associated viscera. J Clin Tuberc Other Mycobact Dis. (2018) 12:1–8. doi: 10.1016/j.jctube.2018.04.003
13. Debi U, Ravisankar V, Prasad KK, Sinha SK, Sharma AK. Abdominal tuberculosis of the gastrointestinal tract: revisited. World J Gastroenterol. (2014) 20:14831–40. doi: 10.3748/wjg.v20.i40.14831
14. Samant H, Desai D, Abraham P, Joshi A, Gupta T, Rodrigues C, et al. Acid-fast bacilli culture positivity and drug resistance in abdominal tuberculosis in Mumbai, India. Indian J Gastroenterol. (2014) 33:414–9. doi: 10.1007/s12664-014-0467-x
15. Chow KM, Chow VC, Szeto CC. Indication for peritoneal biopsy in tuberculous peritonitis. Am J Surg. (2003) 185:567–73. doi: 10.1016/S0002-9610(03)00079-5
16. Kohli M, Schiller I, Dendukuri N, Dheda K, Denkinger CM, Schumacher SG, et al. Xpert((R)) MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst Rev. (2018) 8:CD012768. doi: 10.1002/14651858.CD012768.pub2
17. Opota O, Mazza-Stalder J, Greub G, Jaton K. The rapid molecular test Xpert MTB/RIF ultra: towards improved tuberculosis diagnosis and rifampicin resistance detection. Clin Microbiol Infect. (2019) 25:1370–6. doi: 10.1016/j.cmi.2019.03.021
18. Ndege R, Ngome O, Bani F, Temba Y, Wilson H, Vanobberghen F, et al. Ultrasound in managing extrapulmonary tuberculosis: a randomized controlled two-center study. BMC Infect Dis. (2020) 20:349. doi: 10.1186/s12879-020-05073-9
19. Skoura E, Zumla A, Bomanji J. Imaging in tuberculosis. Int J Infect Dis. (2015) 32:87–93. doi: 10.1016/j.ijid.2014.12.007
20. Gao ZW, Wang X, Zhang HZ, Lin F, Liu C, Dong K. The roles of adenosine deaminase in autoimmune diseases. Autoimmun Rev. (2021) 20:102709. doi: 10.1016/j.autrev.2020.102709
21. Satti I, McShane H. Current approaches toward identifying a correlate of immune protection from tuberculosis. Expert Rev Vaccines. (2019) 18:43–59. doi: 10.1080/14760584.2019.1552140
22. Feng M, Sun F, Wang F, Cao G. The diagnostic effect of sequential detection of ADA screening and T-SPOT assay in pleural effusion patients. Artif Cells Nanomed Biotechnol. (2019) 47:3272–7. doi: 10.1080/21691401.2019.1647221
23. Pormohammad A, Riahi SM, Nasiri MJ, Fallah F, Aghazadeh M, Doustdar F, et al. Diagnostic test accuracy of adenosine deaminase for tuberculous meningitis: a systematic review and meta-analysis. J Infect. (2017) 74:545–54. doi: 10.1016/j.jinf.2017.02.012
24. Ntwari J, Dusabejambo V, Page C. Use of adenosine deaminase (ADA) to diagnose suspected peritoneal tuberculosis in Rwanda: a cross-sectional study. BMC Infect Dis. (2020) 20:239. doi: 10.1186/s12879-020-04965-0
25. Reuter H, Burgess L, van Vuuren W, Doubell A. Diagnosing tuberculous pericarditis. QJM. (2006) 99:827–39. doi: 10.1093/qjmed/hcl123
26. Yang X, Zhang J, Liang Q, Pan L, Duan H, Yang Y, et al. Use of T-SPOTTB for the diagnosis of unconventional pleural tuberculosis is superior to ADA in high prevalence areas: a prospective analysis of 601 cases. BMC Infect Dis. (2021) 21:4. doi: 10.1186/s12879-020-05676-2
27. Tarhan G, Gumuslu F, Yilmaz N, Saka D, Ceyhan I, Cesur S. Serum adenosine deaminase enzyme and plasma platelet factor 4 activities in active pulmonary tuberculosis, HIV-seropositive subjects and cancer patients. J Infect. (2006) 52:264–8. doi: 10.1016/j.jinf.2005.06.009
28. Kim SB, Shin B, Lee JH, Lee SJ, Lee MK, Lee WY, et al. Pleural fluid ADA activity in tuberculous pleurisy can be low in elderly, critically ill patients with multi-organ failure. BMC Pulm Med. (2020) 20:13. doi: 10.1186/s12890-020-1049-6
29. Lee JY, Kim SM, Park SJ, Lee SO, Choi SH, Kim YS, et al. A rapid and non-invasive 2-step algorithm for diagnosing tuberculous peritonitis using a T cell-based assay on peripheral blood and peritoneal fluid mononuclear cells together with peritoneal fluid adenosine deaminase. J Infect. (2015) 70:356–66. doi: 10.1016/j.jinf.2014.09.012
30. Hillebrand DJ, Runyon BA, Yasmineh WG, Rynders GP. Ascitic fluid adenosine deaminase insensitivity in detecting tuberculous peritonitis in the United States. Hepatology. (1996) 24:1408–12. doi: 10.1002/hep.510240617
31. Kumabe A, Hatakeyama S, Kanda N, Yamamoto Y, Matsumura M. Utility of ascitic fluid adenosine deaminase levels in the diagnosis of tuberculous peritonitis in general medical practice. Can J Infect Dis Med Microbiol. (2020) 2020:5792937. doi: 10.1155/2020/5792937
32. He X, Gao Y, Liu Q, Zhao Z, Deng W, Yang H. Diagnostic value of interferon-gamma release assays combined with multiple indicators for tuberculous peritonitis. Gastroenterol Res Pract. (2020) 2020:2056168. doi: 10.1155/2020/2056168
33. Liu R, Li J, Tan Y, Shang Y, Li Y, Su B, et al. Multicenter evaluation of the acid-fast bacillus smear, mycobacterial culture, Xpert MTB/RIF assay, and adenosine deaminase for the diagnosis of tuberculous peritonitis in China. Int J Infect Dis. (2020) 90:119–24. doi: 10.1016/j.ijid.2019.10.036
34. McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, the P-DTAG. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. (2018) 319:388–96. doi: 10.1001/jama.2017.19163
36. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
37. Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. (2001) 20:2865–84. doi: 10.1002/sim.942
38. Chappell FM, Raab GM, Wardlaw JM. When are summary ROC curves appropriate for diagnostic meta-analyses? Stat Med. (2009) 28:2653–68. doi: 10.1002/sim.3631
39. Sharma V, Soni H, Kumar-M P, Dawra S, Mishra S, Mandavdhare HS, et al. Diagnostic accuracy of the Xpert MTB/RIF assay for abdominal tuberculosis: a systematic review and meta-analysis. Expert Rev Anti Infect Ther. (2021) 19:253–65. doi: 10.1080/14787210.2020.1816169
40. Antonangelo L, Faria CS, Sales RK. Tuberculous pleural effusion: diagnosis & management. Expert Rev Respir Med. (2019) 13:747–59. doi: 10.1080/17476348.2019.1637737
41. Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. (2005) 58:882–93. doi: 10.1016/j.jclinepi.2005.01.016
42. Dahale AS, Puri AS, Sachdeva S, Agarwal AK, Kumar A, Dalal A, et al. Reappraisal of the role of ascitic fluid adenosine deaminase for the diagnosis of peritoneal tuberculosis in cirrhosis. Korean J Gastroenterol. (2021) 78:168–76. doi: 10.4166/kjg.2021.068
43. Sun J, Zhang H, Song Z, Jin L, Yang J, Gu J, et al. The negative impact of increasing age and underlying cirrhosis on the sensitivity of adenosine deaminase in the diagnosis of tuberculous peritonitis: a cross-sectional study in eastern China. Int J Infect Dis. (2021) 110:204–12. doi: 10.1016/j.ijid.2021.07.061
44. Ali N, Nath NC, Parvin R, Rahman A, Bhuiyan TM, Rahman M, et al. Role of ascitic fluid adenosine deaminase (ADA) and serum CA-125 in the diagnosis of tuberculous peritonitis. Bangladesh Med Res Counc Bull. (2014) 40:89–91. doi: 10.3329/bmrcb.v40i3.25228
45. Hallur V, Sharma M, Sethi S, Sharma K, Mewara A, Dhatwalia S, et al. Development and evaluation of multiplex PCR in rapid diagnosis of abdominal tuberculosis. Diagn Microbiol Infect Dis. (2013) 76:51–5. doi: 10.1016/j.diagmicrobio.2013.02.022
46. Kang SJ, Kim JW, Baek JH, Kim SH, Kim BG, Lee KL, et al. Role of ascites adenosine deaminase in differentiating between tuberculous peritonitis and peritoneal carcinomatosis. World J Gastroenterol. (2012) 18:2837–43. doi: 10.3748/wjg.v18.i22.2837
47. Liao YJ, Wu CY, Lee SW, Lee CL, Yang SS, Chang CS, et al. Adenosine deaminase activity in tuberculous peritonitis among patients with underlying liver cirrhosis. World J Gastroenterol. (2012) 18:5260–5. doi: 10.3748/wjg.v18.i37.5260
48. Saleh MA, Hammad E, Ramadan MM, Abd El-Rahman A, Enein AF. Use of adenosine deaminase measurements and QuantiFERON in the rapid diagnosis of tuberculous peritonitis. J Med Microbiol. (2012) 61:514–9. doi: 10.1099/jmm.0.035121-0
49. Hong KD, Lee SI, Moon HY. Comparison between laparoscopy and noninvasive tests for the diagnosis of tuberculous peritonitis. World J Surg. (2011) 35:2369–75. doi: 10.1007/s00268-011-1224-2
50. Gupta BK, Bharat V, Bandyopadhyay D. Sensitivity, specificity, negative and positive predictive values of adenosine deaminase in patients of tubercular and non-tubercular serosal effusion in India. J Clin Med Res. (2010) 2:121–6. doi: 10.4021/jocmr2010.05.289w
51. Sharma SK, Tahir M, Mohan A, Smith-Rohrberg D, Mishra HK, Pandey RM. Diagnostic accuracy of ascitic fluid IFN-gamma and adenosine deaminase assays in the diagnosis of tuberculous ascites. J Interferon Cytokine Res. (2006) 26:484–8. doi: 10.1089/jir.2006.26.484
52. Burgess LJ, Swanepoel CG, Taljaard JJ. The use of adenosine deaminase as a diagnostic tool for peritoneal tuberculosis. Tuberculosis. (2001) 81:243–8. doi: 10.1054/tube.2001.0289
53. Sathar MA, Ungerer JP, Lockhat F, Simjee AE, Gouws E. Elevated adenosine deaminase activity in patients with HIV and tuberculous peritonitis. Eur J Gastroenterol Hepatol. (1999) 11:337–41. doi: 10.1097/00042737-199903000-00020
54. Brant CQ, Silva MRJr, Macedo E P, Vasconcelos C, Tamaki N, Ferraz ML. The value of adenosine deaminase (ADA) determination in the diagnosis of tuberculous ascites. Rev Inst Med Trop São Paulo. (1995) 37:449–53. doi: 10.1590/S0036-46651995000500011
55. Sathar MA, Simjee AE, Coovadia YM, Soni PN, Moola SA, Insam B, et al. Ascitic fluid gamma interferon concentrations and adenosine deaminase activity in tuberculous peritonitis. Gut. (1995) 36:419–21. doi: 10.1136/gut.36.3.419
56. Fernandez-Rodriguez CM, Perez-Arguelles BS, Ledo L, Garcia-Vila LM, Pereira S, Rodriguez-Martinez D. Ascites adenosine deaminase activity is decreased in tuberculous ascites with low protein content. Am J Gastroenterol. (1991) 86:1500–3.
57. Ribera E, Martinez Vasquez JM, Ocana I, Ruiz I, Jiminez JG, Encabo G, et al. Diagnostic value of ascites gamma interferon levels in tuberculous peritonitis. comparison with adenosine deaminase activity. Tubercle. (1991) 72:193–7. doi: 10.1016/0041-3879(91)90007-F
58. Bhargava DK, Gupta M, Nijhawan S, Dasarathy S, Kushwaha AK. Adenosine deaminase (ADA) in peritoneal tuberculosis: diagnostic value in ascitic fluid and serum. Tubercle. (1990) 71:121–6. doi: 10.1016/0041-3879(90)90007-U
59. Dwivedi M, Misra SP, Misra V, Kumar R. Value of adenosine deaminase estimation in the diagnosis of tuberculous ascites. Am J Gastroenterol. (1990) 85:1123–5.
60. Voigt MD, Kalvaria I, Trey C, Berman P, Lombard C, Kirsch RE. Diagnostic value of ascites adenosine deaminase in tuberculous peritonitis. Lancet. (1989) 1:751–4. doi: 10.1016/S0140-6736(89)92574-9
61. Makharia GK, Ghoshal UC, Ramakrishna BS, Agnihotri A, Ahuja V, Chowdhury SD, et al. Intermittent directly observed therapy for abdominal tuberculosis: a multicenter randomized controlled trial comparing 6 months versus 9 months of therapy. Clin Infect Dis. (2015) 61:750–7. doi: 10.1093/cid/civ376
62. Cho OH, Park KH, Park SJ, Kim SM, Park SY, Moon SM, et al. Rapid diagnosis of tuberculous peritonitis by T cell-based assays on peripheral blood and peritoneal fluid mononuclear cells. J Infect. (2011) 62:462–71. doi: 10.1016/j.jinf.2011.04.001
63. Eguchi H, Horita N, Ushio R, Kato I, Nakajima Y, Ota E, et al. Diagnostic test accuracy of antigenaemia assay for PCR-proven cytomegalovirus infection-systematic review and meta-analysis. Clin Microbiol Infect. (2017) 23:907–15. doi: 10.1016/j.cmi.2017.05.009
64. Eraksoy H. Gastrointestinal and abdominal tuberculosis. Gastroenterol Clin North Am. (2021) 50:341–60. doi: 10.1016/j.gtc.2021.02.004
65. Meregildo-Rodriguez ED, Tafur-Ramirez RC, Espino-Saavedra WG, Angulo-Prentice SF. Abdominal tuberculosis misdiagnosed as acute surgical abdomen and carcinomatosis. F1000Res. (2021) 10:355. doi: 10.12688/f1000research.53036.2
66. Kim NJ, Choo EJ, Kwak YG, Lee SO, Choi SH, Woo JH, et al. Tuberculous peritonitis in cirrhotic patients: comparison of spontaneous bacterial peritonitis caused by Escherichia coli with tuberculous peritonitis. Scand J Infect Dis. (2009) 41:852–6. doi: 10.3109/00365540903214264
67. Sharma V. Differentiating intestinal tuberculosis and crohn disease: quo vadis. Expert Rev Gastroenterol Hepatol. (2020) 14:647–50. doi: 10.1080/17474124.2020.1785870
68. Han XF, Han C, Jin F, Wang JL, Wang MS. Factors associated with negative pleural adenosine deaminase results in the diagnosis of childhood pleural tuberculosis. BMC Infect Dis. (2021) 21:473. doi: 10.1186/s12879-021-06209-1
69. Tao L, Ning HJ, Nie HM, Guo XY, Qin SY, Jiang HX. Diagnostic value of adenosine deaminase in ascites for tuberculosis ascites: a meta-analysis. Diagn Microbiol Infect Dis. (2014) 79:102–7. doi: 10.1016/j.diagmicrobio.2013.12.010
70. Shen YC, Wang T, Chen L, Yang T, Wan C, Hu QJ, et al. Diagnostic accuracy of adenosine deaminase for tuberculous peritonitis: a meta-analysis. Arch Med Sci. (2013) 9:601–7. doi: 10.5114/aoms.2013.36904
71. Riquelme A, Calvo M, Salech F, Valderrama S, Pattillo A, Arellano M, et al. Value of adenosine deaminase (ADA) in ascitic fluid for the diagnosis of tuberculous peritonitis: a meta-analysis. J Clin Gastroenterol. (2006) 40:705–10. doi: 10.1097/00004836-200609000-00009
72. Deeks JJ. Systematic reviews in health care: systematic reviews of evaluations of diagnostic and screening tests. BMJ. (2001) 323:157–62. doi: 10.1136/bmj.323.7305.157
73. Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. (2003) 56:1129–35. doi: 10.1016/S0895-4356(03)00177-X
74. Laniado-Laborin R. Adenosine deaminase in the diagnosis of tuberculous pleural effusion: is it really an ideal test? a word of caution. Chest. (2005) 127:417–8. doi: 10.1378/chest.127.2.417
75. Delacour H, Sauvanet C, Ceppa F, Burnat P. Analytical performances of the diazyme ADA assay on the Cobas(R) 6000 system. Clin Biochem. (2010) 43:1468–71. doi: 10.1016/j.clinbiochem.2010.09.005
Keywords: adenosine deaminase, abdominal tuberculosis, ascites, meta-analysis, diagnostic value
Citation: Zhou R, Qiu X, Ying J, Yue Y, Ruan T, Yu L, Liu Q, Sun X, Wang S, Qu Y, Li X and Mu D (2022) Diagnostic performance of adenosine deaminase for abdominal tuberculosis: A systematic review and meta-analysis. Front. Public Health 10:938544. doi: 10.3389/fpubh.2022.938544
Received: 07 May 2022; Accepted: 29 August 2022;
Published: 21 September 2022.
Edited by:
Sergio Oscar Angel, CONICET Instituto Tecnológico de Chascomús (INTECH), ArgentinaCopyright © 2022 Zhou, Qiu, Ying, Yue, Ruan, Yu, Liu, Sun, Wang, Qu, Li and Mu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junjie Ying, eWluZ2p1bmppZTE3N0AxNjMuY29t; Dezhi Mu, bXVkekBzY3UuZWR1LmNu
†These authors have contributed equally to this work
 Ruixi Zhou
Ruixi Zhou Xia Qiu1,2†
Xia Qiu1,2† Yan Yue
Yan Yue Luting Yu
Luting Yu Qian Liu
Qian Liu Shaopu Wang
Shaopu Wang Yi Qu
Yi Qu Xihong Li
Xihong Li Dezhi Mu
Dezhi Mu