
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 25 August 2022
Sec. Life-Course Epidemiology and Social Inequalities in Health
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.937877
 Yangyang Sun1,2,3†
Yangyang Sun1,2,3† Lingling Zhou4†
Lingling Zhou4† Tao Shan5†
Tao Shan5† Qiong Ouyang2,3,6
Qiong Ouyang2,3,6 Xu Li2,3
Xu Li2,3 Yuanming Fan2,3
Yuanming Fan2,3 Ying Li2,3
Ying Li2,3 Hang Gong2,3
Hang Gong2,3 Raphael N. Alolga2,3
Raphael N. Alolga2,3 Gaoxiang Ma2,3
Gaoxiang Ma2,3 Yuqiu Ge7*
Yuqiu Ge7* Heng Zhang8*
Heng Zhang8*Objective: We investigated the association between cancer incidence and body mass index (BMI) variability calculated from the recall of weight at decades of age by participants in the USA Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial.
Methods: A total of 89,822 individuals' BMI were recorded as recalled the participant's aged 30, 40, 50, 60, 70 years, and baseline. BMI variability was assessed using four indices: SD, coefficient of variation (CV), variability independent of the mean (VIM), and average real variability (ARV). The multivariate Cox regression analysis was performed to calculate hazard ratios (HRs) of these measures for incident cancers and corresponding 95% CIs.
Results: During the median follow-up of 11.8 years, there were newly diagnosed 5,012 cases of prostate cancer, 792 cases of lung cancer, 994 cases of colon cancer, and 132 cases of ovarian cancer. Compared with the lowest quartile (Q1) group, the highest quartile (Q4) group of BMI variability indices was associated with increased lung cancer risk, including BMI_SD (HR, 1.58; 95% CI, 1.17–2.12), BMI_CV (HR, 1.46; 95% CI, 1.10–1.94), BMI_VIM (HR, 1.73; 95% CI, 1.33–2.25), and BMI_ARV (HR, 2.17; 95% CI, 1.62–2.91). Associations between BMI variability and prostate, colon, and ovarian cancer incidences were of limited significance.
Conclusion: The findings imply that maintaining a stable weight across adulthood is associated with a decreased incidence of lung cancer.
Cancer Statistics reported 19.3 million new cancer cases and almost 10.0 million cancer deaths globally in 2020 (1), including ~1.8 million new cancer cases and 0.6 million cancer deaths in the USA (2). Thereby, cancer remained one of the leading causes of death. There is an urgent need to identify and avoid exposure to potential risk factors by instituting appropriate interventions (3).
Obesity is an independent risk factor for cancer (4). Increasing evidence has revealed that body mass index (BMI) [weight (kg)/height (m2)], the most common indicator of obesity, was associated with the incidence of multiple cancer (5–7). The performance of BMI measurement at a specific time is not able to represent long-term weight changes. The variability of BMI could depict weight changes; thus, it is meaningful to assess the association between the variability of BMI and cancer risks.
Existing studies indicated that the variability of BMI may affect the incidence of adverse outcomes (8, 9). Body weight variability increased the risk of dementia and cardiovascular diseases and mortality of patients with type 2 diabetes based on a nationwide population-based cohort from Korea (10, 11). In addition, BMI variability was reported to increase the risk of atrial fibrillation, myocardial infarction, and all-cause mortality (12, 13). But no associations between BMI variability and risk of breast, endometrial, colon, or lung cancers were found in the Iowa Women's Health Study (14). To this end, the association between BMI variability and cancer risk remains largely obscure.
To address the gaps in current knowledge, this study evaluated the relationship of BMI variability from adulthood to elderhood and the incidence of prostate, lung, colon, and ovarian cancers using the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial.
The Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial was designed to intervene the prostate, lung, colon, and ovarian cancers mortality by screening methods (https://cdas.cancer.gov/plco/). The overall design of the PLCO has been described elsewhere (15–17). From November 1993 to July 2001, ~1,550,000 participants aged 55–74 years were randomly assigned to the control or intervention groups at 10 screening centers in the United States. We followed the reporting guidelines of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement for observational studies.
All the participants were asked, “Please estimate your weight and height when you were in your 30, 40, 50, 60, 70s, and current,” respectively. BMI was calculated as an individual's weight in kilograms divided by the square of their height in meters. To comprehensively investigate the association between BMI variability and the risk of cancers, BMI variability was assessed using four indices: (1) SD; (2) coefficient of variation (CV); (3) variability independent of the mean (VIM); and (4) average real variability (ARV). VIM was calculated as 100 × SD/meanβ, where β is the regression coefficient based on the ln of the SD over the ln of the mean (18). ARV is the average of the absolute differences between consecutive values and was calculated using the following formula: ARV = , where n denotes the number of anthropometric measurements (19). The earliest recorded BMI greater than the latest recorded BMI was regarded as BMI loss. On the contrary, a lower earliest recorded BMI than the latest value was defined as BMI gain.
Newly diagnosed prostate, lung, colon, and ovarian cancers were treated as the endpoints of this study. The Annual Study Update (ASU) was used to ascertain cancer diagnosis. Participants were asked if they were diagnosed with cancer, the type of cancer, date of diagnosis, hospital or clinic of diagnosis, and physician contact information. Prostate cancer (C61.9), lung cancer (C34.1 to C34.9), colon cancer (C18.0 to C18.9), and ovarian cancer (C56.9) sites had the International Classification of Disease for Oncology, third edition (ICD-O-3) codes based on initial medical records.
Self-reporting questionnaires were used to obtain demographic and lifestyle data. The baseline questionnaire (BQ) and supplemental questionnaire (SQ) included information about demographics, history of health, smoking, drugs used, and gender-specific details. The Diet History Questionnaire (DHQ) is a food frequency questionnaire. Educational level was defined as <8, 8–11, 12 years, or completed high school, posthigh school training other than college, some college, college graduate, and postgraduate. Smoking status was categorized as non-smoker, former smoker, and current smoker. Drinking status was dichotomized into never, former, and current drinker. The race was classified as white (non-Hispanic), black (non-Hispanic), Hispanic, Asian, Pacific Islander, and American-Indian. Physical activity was defined as whether participants exercised 1+ time/month. Dietary covariates (drinking statuses, vegetable and fruit consumption, and vitamin D intake) contained in the Diet History Questionnaire, which was a food frequency questionnaire that was added in 1998, not baseline data. Other variables recorded baseline questionnaire was baseline data.
Pertinent exclusion criteria for the current analysis were as follows: (1) diagnosis with any cancer prior to trial entry (n = 11,814); (2) more than three missing values of the BMI variables (n = 53,262); (3) participants withdrawal or lost contact (n = 6); and (4) BMI > 100 kg/m2 (n = 4). Finally, 89,822 individuals with three or more available body weight and height measurements were enrolled in this study. The study participants were followed-up until 31 December 2009. The mean follow-up duration was 11.0 ± 2.7 years. The flowchart of the study population is shown in Figure 1.
The baseline characteristics of the study participants are presented as mean ± SD for continuous variables and number (percentage) for categorical variables. The incidence rates of outcomes (prostate, lung, colon, and ovarian cancers) were calculated by dividing the number of events by 1,000 person-years. The multivariate Cox regression models were performed to examine the associations between quartiles of BMI variability and the risk of outcomes. Hazard ratios (HRs) and corresponding 95% CI were calculated compared with the lowest quartile group. The repeated measures Cox regression was used to investigate the association between repeated BMI measurements and cancers.
We also conducted three sensitivity analyses to determine the robustness of our findings. First, we calculated four indices of BMI variability for roundly representing variability. Second, the participants diagnosed with other cancers (cancer was not the outcome event of concern) were censored during follow-up before the onset of outcomes in our study. It is necessary to examine the possible influence of competing events on the association between BMI variability and the risk of lung cancer by applying Fine and Gray's subdistribution hazards regression model (20). Finally, considering possible reverse causality, we reanalyzed the association between quartiles of BMI variability and the risk of study outcomes by excluding participants whose outcomes event occurred in the first 2 years of follow-up.
We performed subgroup analyses of age, sex, smoking status, BMI trajectory, and baseline BMI. We evaluated the associations between BMI variability and the risk of cancer in these subgroups. To quantify dose-response relationships, we used restricted cubic spline models with four knots at the 5, 35, 65, and 95th centiles to examine the associations between BMI variability indices (SD, ARV, CV, and VIM as continuous variables) and the risk of cancer after full adjustment. All the statistical tests were two-sided, and P <0.05 was considered statistically significant. Analyses were performed using R software (version 3.6.1).
All the methods and experiments were approved by the China Pharmaceutical University Ethics Committee. All the participants provided written informed consent. This study was carried out in accordance with the Declaration of Helsinki.
The average age at baseline of the eligible PLCO study population of the 89,822 participants was 62.0 ± 5.1 years. As shown in Table 1, there were 43,510 (48.4%) males, 82,356 (91.7%) white, no Hispanic, and 7,652 (8.5%) current smokers. The outcomes of current study included prostate cancer cases (n = 5,012), lung cancer cases (n = 792), colon cancer cases (n = 994), and ovarian cancer cases (n = 132).
Table 2 presents the relationship between BMI variability indices (BMI_SD and BMI_ ARV) and prostate, lung, colon, and ovarian cancer risks. After adjusting for age, sex, and baseline BMI (model 1), individuals with the highest quartile (Q4) of BMI_SD and BMI_ARV were at significantly higher risk of lung cancer (HR, 95% CI = 1.76, 1.38–2.25, Ptrend <0.001 for BMI_SD; 2.18, 1.71–2.77, Ptrend <0.001 for BMI_ARV) compared with those with the lowest quartile (Q1). After adjusting for all the potential confounding variables (model 2), individuals with the highest quartile of BMI_SD and BMI_ARV had a higher risk of lung cancer (HR, 95% CI = 1.58, 1.17–2.12, Ptrend = 0.001 for BMI_SD; 2.17, 1.62–2.91, Ptrend <0.001 for BMI_ARV) compared with those with the lowest quartile (Table 2). These associations were also observed for BMI_CV and BMI_VIM (Supplementary Table 1).
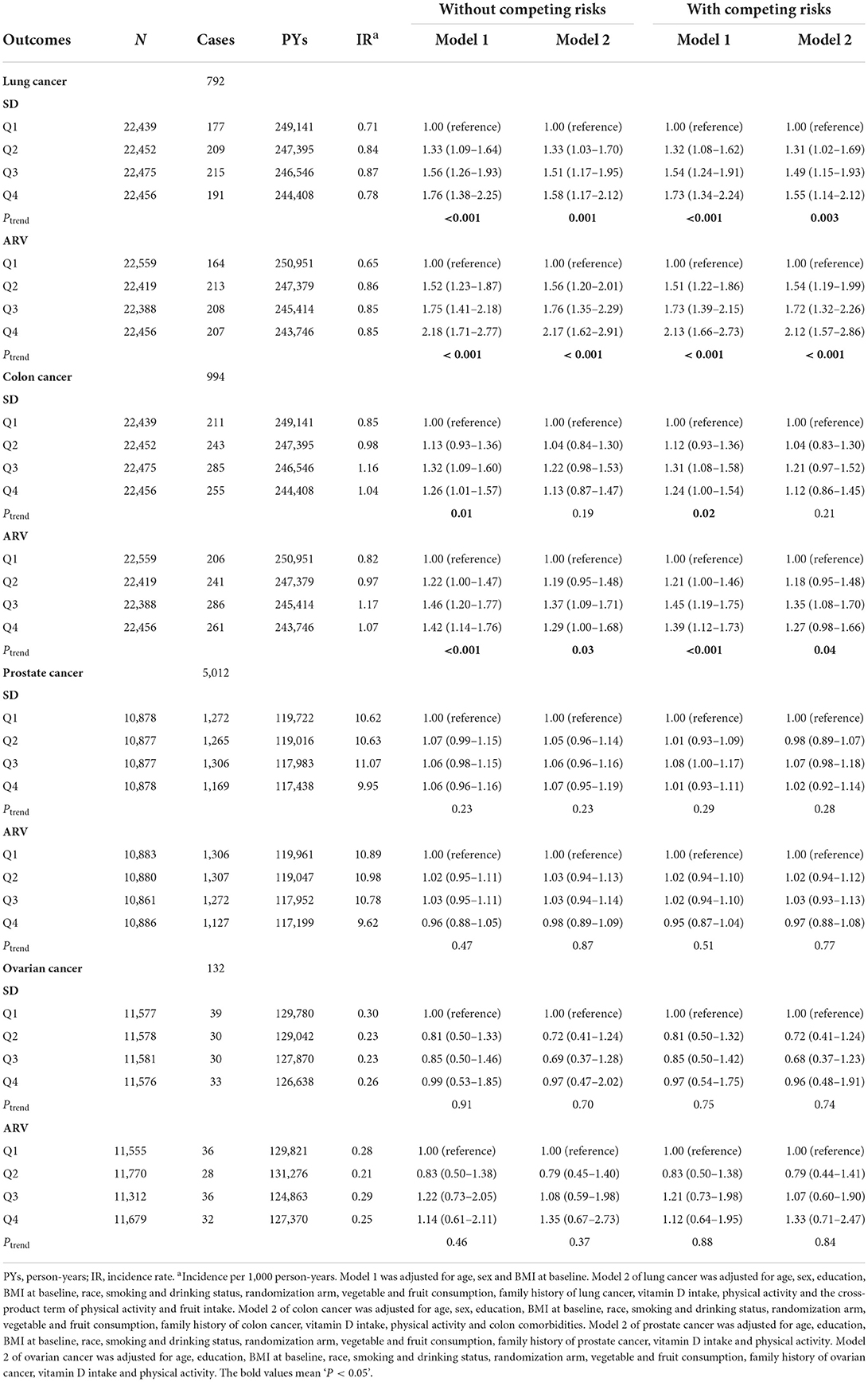
Table 2. SD and ARV for BMI variability in relation to the risks of prostate, lung, colon, and ovarian cancers.
In addition, the association between BMI variability and the risk of colon cancer was significantly increased in the higher quartile groups of SD and ARV in model 1 (HR, 1.29; 95% CI, 1.00–1.68, Ptrend = 0.005 for BMI_SD; HR, 1.29; 95% CI, 1.00–1.68, Ptrend = 0.005 for BMI_ARV, respectively) compared with the lowest quartile (Q1) group (Table 2). The association between ARV and the risk of colon cancer was significantly increased in the fully adjusted models (HR, 1.29; 95% CI, 1.00–1.68, Ptrend = 0.026) compared with the lowest quartile (Q1) group (Table 2). We did not observe any association between BMI_SD, BMI_CV, and BMI_VIM and the risk of colon cancer (Table 2; Supplementary Table 1). Similarly, no significant relationship between BMI variability and prostate and ovarian cancer risks was observed in model 1 or model 2 (data not shown). The association between repeated BMI measurements and risk of cancers was not observed based on the repeat measurement Cox model (Supplementary Table 3).
We used restricted cubic splines to flexibly model and visualize the relationship of BMI variability with the risk of lung cancer. The curves for the associations between BMI_SD (Figure 2A) and BMI_ARV (Figure 2B) and the risk of lung cancer were non-linear (P for non-linear = 0.016, P for non-linear = 0.002, respectively). For BMI_ARV, the risk curve displayed an inverse U-shape (Figure 2B). As shown in Supplementary Figure 2, the BMI_CV and BMI_VIM were linearly associated with incident lung cancer (P for non-linear = 0.098, P for non-linear = 0.404, respectively).
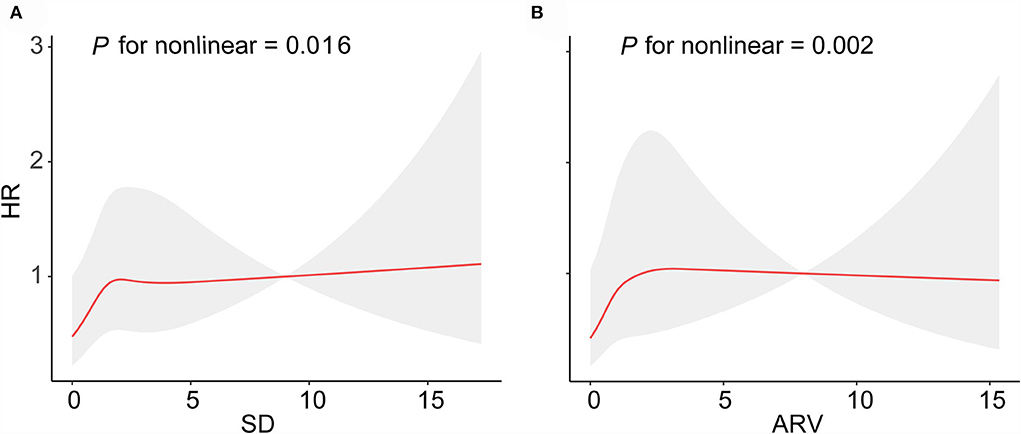
Figure 2. Curve association between SD and ARV for BMI variability and the risk of lung cancer. Shading indicates 95% CIs. (A) SD for BMI, (B) CV for BMI.
The subgroup analysis was performed for the risk of lung cancer based on the Q1–Q4 groups of BMI_SD, BMI_CV, BMI_VIM, and BMI_ARV. BMI variability (SD, ARV, CV, and VIM) was associated with higher risks of lung cancer at age <65 years (Figure 3A; Supplementary Figure 1A). For sex, higher BMI variability was consistently associated with incident lung cancer, except for BMI_CV in males (Figure 3B; Supplementary Figure 1B), and the positive association between BMI variability and lung cancer was limited to former and current smokers (Figure 3C; Supplementary Figure 1C). BMI variability was associated with higher risks of lung cancer in BMI loss, except for BMI_VIM (Figure 3D; Supplementary Figure 1D). BMI variability was associated with higher risks of lung cancer with a baseline BMI of 18.5–24.9 kg/m2, except for BMI_VIM (Figure 3E; Supplementary Figure 1E).
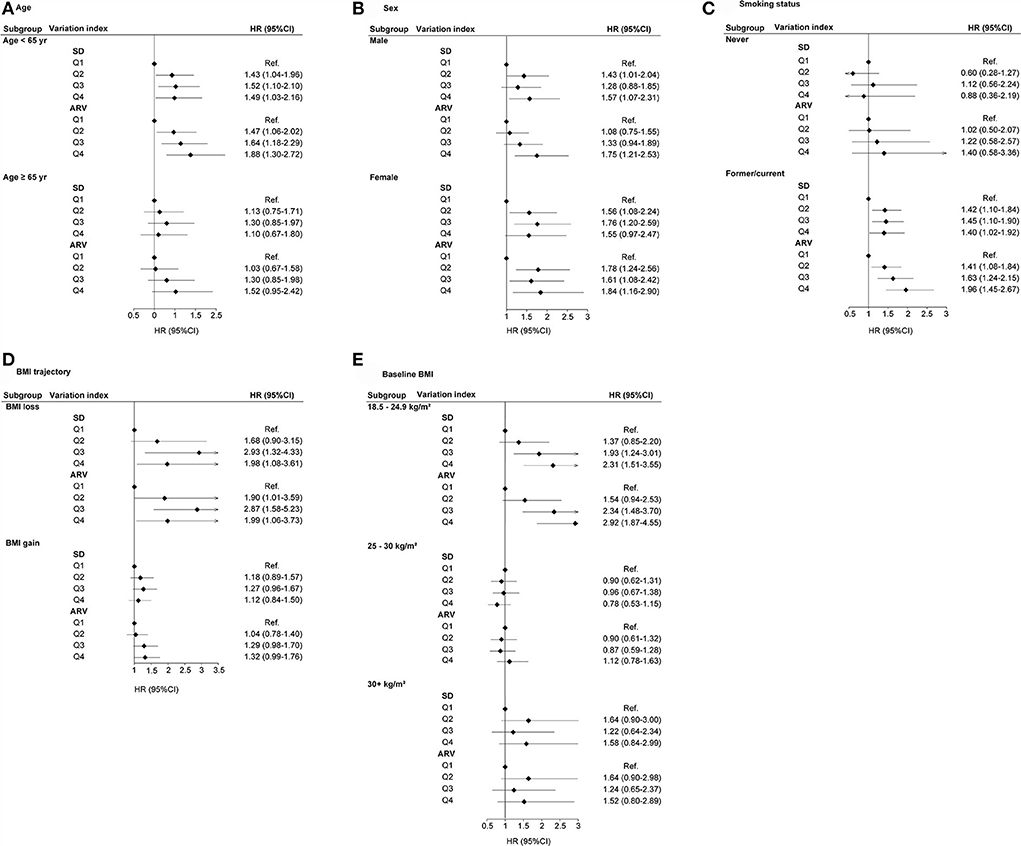
Figure 3. HR (95% CI) of lung cancer with respect to SD and ARV for BMI variability in subgroups. (A) Age, (B) Sex, (C) Smoking status, (D) BMI trajectory, and (E) Baseline BMI.
The four indices of BMI variability (SD, ARV, CV, and VIM) also demonstrated a consistent association with risks of the PLCO cancer (Table 2; Supplementary Table 1). Then, we considered the incident non-PLCO cancer as competing events, the results of which revealed similar association patterns between BMI variability and risk of the PLCO cancers (Table 2; Supplementary Table 1). Last, when we excluded the PLCO cancer cases that had been ascertained in the first 2 years of follow-up, an association between BMI variability (SD, ARV, CV, and VIM) and risks of the PLCO cancers did not change substantially (Supplementary Table 2). Yet after adjusting for potential confounding variables, SD and CV of BMI were also positively related to the incidence of colon cancer (Q3: HR, 1.33; 95% CI, 1.02–1.72, Ptrend = 0.032 for BMI_SD, Q3: HR, 1.38; 95% CI, 1.07–1.79, Ptrend = 0.041 for BMI_CV, respectively) compared to the lowest quartile (Q1) group (Supplementary Table 2). Individuals in the second quartile (Q2) and highest quartile (Q4) groups of BMI_VIM had a higher risk of colon cancer (Q2: HR, 1.35; 95% CI, 1.05–1.72, Q4: HR, 1.37; 95% CI, 1.05–1.79, Ptrend = 0.089) compared to those in the lowest quartile (Q1) group (Supplementary Table 2).
Instability in BMI after 30 years of age was found to be associated with an increased risk of lung cancer after adjusting for potential risk factors in this study. Our findings suggested that higher BMI variability may have an effect on the incidence of lung cancer. Maintaining proper weight may be beneficial for lowering the risk of lung cancer.
To the best of our knowledge, only the Iowa Women's Health Study has examined the association between body weight (not BMI) variability and incident lung and colon cancers. They found that the root mean square error around the slope of weight on age defined as body weight variability was associated with an increased risk of lung cancer in model 1, but the association was attenuated after full adjustment (14). They found no association between body weight variability and the risk of colon cancer, which is similar to our study. Our study has some strengths compared to the previous study. The Iowa Women's Health study only reported the association between the risk of lung cancer and BMI variability in the women population. We found that the association between BMI variability and lung cancer was not limited to women. The follow-up duration was 2–6 years in the Iowa Women's Health study, while the participants' median follow-up duration was 11 years in our study. Besides, we had a bigger sample size of 89,822 subjects compared to 33,834 subjects for that study (14).
The underlying reasons for the association between increased BMI variability and the risk of lung cancer remain unclear. Previous observational studies demonstrated that weight loss was associated with loss of lean muscle mass, and weight regain was related to increased adiposity (21–23). This may lead to an increase in fat mass after body weight variabilities, resulting in an increase in adipose tissues. Adipose tissue secretes numerous adipocytokines that potentially played a role in the pathogenesis of lung cancer (22). For instance, stimulation of SQ-5 human clonal squamous lung cancer-derived cell proliferation was found after the addition of recombinant human leptin (24). Leptin seemed to mediate and amplify a complex interplay between tumor and immunoinflammatory cells, resulting in the development and progression of lung cancer (25). Moreover, the expression of adiponectin receptors exclusively in lung cancer tissues suggested that adiponectin functional signaling mediated lung cancer development (26). Further studies were warranted to explore the exact mechanism underlying the association between increased BMI variability and the risk of lung cancer.
We also found interesting results. The associations between BMI_SD, BMI_CV, and BMI_VIM and incident colon cancer were not observed after full adjustment, except for BMI_ARV. However, individuals in the third quartile (Q3) of BMI_SD and BMI_CV and in the second quartile (Q2) and highest quartile (Q4) of BMI_VIM had an increased risk of incident colon cancer when excluding colon cancer cases ascertained in the first 2 years of follow-up. This result of the sensitivity analysis contradicted the above results. It showed that the association between BMI variability and incident colon cancer needed to be further analyzed using another large sample size.
In subgroup analysis, the positive association of BMI variability with incident lung cancer existed in former or current smokers. This suggested that BMI variability was also a key risk factor for lung cancer in smokers. Furthermore, BMI variability was positively associated with incident lung cancer limited to individuals aged <65 years. This shows that it may be more important for maintaining weight before 65 years. These associations needed to be examined through further studies.
Our study had several notable strengths. We used a prospective study design, a large sample size of 89,822 individuals, and a follow-up period of >10 years. BMI of subjects in their 30, 40, 50, 60, 70s, and current age was provided for measuring long-term variability. Various subgroup analyses provided interesting conclusions. The stability of the results was confirmed by sensitivity analyses. Of note, this was the first study to investigate the association between BMI variability since adulthood and incident lung cancer during elderhood.
Several limitations deserve mention. First, a potential recall bias exists since the data on weight and height were self-reported. The authenticity of the data needs verification. Second, we could not determine whether the weight loss was intentional or unintentional due to a lack of information on the same. Third, we could not examine the association between BMI variability and the risk of non-PLCO cancers, since non-PLCO cancers had a low incidence in the PLCO cohort. Fourth, considering that the PLCO cohort was a controlled trial to determine whether certain screening examinations reduce mortality from prostate, lung, colorectal, and ovarian cancer. Participants for the intervention arm undergo a chest X-ray at baseline and annually for 2 years. In particular, participants classified as “smokers” undergo an additional chest X-ray at 3 years for lung screening. Lung screening and lifestyle interventions reduced the incidence of lung cancer and influenced participants' smoking behavior. Thereby, there is a low percentage of active smoking in the cohort, leading to a lower incidence of lung cancer in the cohort. Last, there is no denying that there are residual confounding factors in the association between BMI and cancers. Dietary carbohydrates and fiber from fruits, vegetables, and whole grains are associated with lower lung cancer risk. Refined carbohydrates, such as soft drinks, appear to increase risk based on the PLCO trial (27). In addition to dietary intake, a history of metabolic diseases associated with obesity, such as diabetes, has a significantly higher risk of lung cancer (28).
This population-based study revealed that BMI variability is independently associated with an increased risk of lung cancer. Our findings suggested that maintaining a stable weight through appropriate interventions may be beneficial to preventing incident lung cancer, especially for smokers.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://cdas.cancer.gov/plco/. This work has been conducted using the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial under project ID PLCO-411. Further information is available from the corresponding author upon request.
The studies involving human participants were reviewed and approved by China Pharmaceutical University Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
HZ and YG conceived and designed the study. YS, TS, and LZ conducted data analyses and prepared the first draft. All authors provided statistical expertise, aided in interpreting the results, contributed to the critical revision of the manuscript for important intellectual content, and approved the final version of the manuscript.
This study was supported by the National Natural Science Foundation of China (81903390), the Jiangsu Provincial Natural Science Foundation (BK20190555 and BK20190600), the Science and Technology Development Foundation of Nanjing Medical University (NMUB20210063), and the Young Talent Support Project of Children's Hospital of Nanjing Medical University (TJGC2021014).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.937877/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
3. Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. (2018) 68:31–54. doi: 10.3322/caac.21440
4. Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev. (2015) 95:727–48. doi: 10.1152/physrev.00030.2014
5. Recalde M, Davila-Batista V, Diaz Y, Leitzmann M, Romieu I, Freisling H, et al. Body mass index and waist circumference in relation to the risk of 26 types of cancer: a prospective cohort study of 3.5 million adults in Spain. BMC Med. (2021) 19:10. doi: 10.1186/s12916-020-01877-3
6. Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. (2014) 384:755–65. doi: 10.1016/S0140-6736(14)60892-8
7. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body fatness and cancer–viewpoint of the IARC working group. N Engl J Med. (2016) 375:794–8. doi: 10.1056/NEJMsr1606602
8. Wannamethee SG, Shaper AG, Walker M. Weight change, weight fluctuation, and mortality. Arch Intern Med. (2002) 162:2575–80. doi: 10.1001/archinte.162.22.2575
9. Mehta T, Smith DL Jr, Muhammad J, Casazza K. Impact of weight cycling on risk of morbidity and mortality. Obes Rev. (2014) 15:870–81. doi: 10.1111/obr.12222
10. Roh E, Hwang SY, Kim JA, Lee YB, Hong SH, Kim NH, et al. Body weight variability increases dementia risk among older adults: a nationwide population-based cohort study. Front Endocrinol. (2020) 11:291. doi: 10.3389/fendo.2020.00291
11. Nam GE, Kim W, Han K, Lee CW, Kwon Y, Han B, et al. Body weight variability and the risk of cardiovascular outcomes and mortality in patients with type 2 diabetes: a nationwide cohort study. Diabetes Care. (2020) 43:2234–41. doi: 10.2337/dc19-2552
12. Lim YM, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS, et al. Body mass index variability and long-term risk of new-onset atrial fibrillation in the general population: a Korean nationwide cohort study. Mayo Clin Proc. (2019) 94:225–35. doi: 10.1016/j.mayocp.2018.10.019
13. Nam GE, Cho KH, Han K, Han B, Cho SJ, Roh YK, et al. Impact of body mass index and body weight variabilities on mortality: a nationwide cohort study. Int J Obes. (2019) 43:412–23. doi: 10.1038/s41366-018-0079-0
14. French SA, Folsom AR, Jeffery RW, Zheng W, Mink PJ, Baxter JE. Weight variability and incident disease in older women: the Iowa Women's Health Study. Int J Obes Relat Metab Disord. (1997) 21:217–23. doi: 10.1038/sj.ijo.0800390
15. Andriole GL, Crawford ED, Grubb RL III, Buys SS, Chia D, Church TR, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. (2009) 360:1310–19. doi: 10.1056/NEJMoa0810696
16. Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial. Control Clin Trials. (2000) 21:273–309S. doi: 10.1016/S0197-2456(00)00098-2
17. Sun Y, Li X, Jiang W, Fan Y, Ouyang Q, Shao W, et al. Advanced paternal age and risk of cancer in offspring. Aging. (2020) 13:3712–25. doi: 10.18632/aging.202333
18. Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlöf B, et al. Effects of β blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. (2010) 9:469–80. doi: 10.1016/S1474-4422(10)70066-1
19. Mena L, Pintos S, Queipo NV, Aizpurua JA, Maestre G, Sulbaran T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens. (2005) 23:505–11. doi: 10.1097/01.hjh.0000160205.81652.5a
20. Hsu JY, Roy JA, Xie D, Yang W, Shou H, Anderson AH, et al. Statistical methods for cohort studies of CKD: survival analysis in the setting of competing risks. Clin J Am Soc Nephrol. (2017) 12:1181–9. doi: 10.2215/CJN.10301016
21. Lee JS, Visser M, Tylavsky FA, Kritchevsky SB, Schwartz AV, Sahyoun N, et al. Weight loss and regain and effects on body composition: the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. (2010) 65:78–83. doi: 10.1093/gerona/glp042
22. Yoo HJ, Kim BT, Park YW, Park KH, Kim CW, Joo NS. Difference of body compositional changes according to the presence of weight cycling in a community-based weight control program. J Korean Med Sci. (2010) 25:49–53. doi: 10.3346/jkms.2010.25.1.49
23. Rodin J, Radke-Sharpe N, Rebuffe-Scrive M, Greenwood MR. Weight cycling and fat distribution. Int J Obes. (1990) 14:303–10.
24. Tsuchiya T, Shimizu H, Horie T, Mori M. Expression of leptin receptor in lung: leptin as a growth factor. Eur J Pharmacol. (1999) 365:273–9. doi: 10.1016/S0014-2999(98)00884-X
25. Ribeiro R, Araujo A, Lopes C, Medeiros R. Immunoinflammatory mechanisms in lung cancer development: is leptin a mediator? J Thorac Oncol. (2007) 2:105–8. doi: 10.1016/S1556-0864(15)30035-6
26. Petridou ET, Mitsiades N, Gialamas S, Angelopoulos M, Skalkidou A, Dessypris N, et al. Circulating adiponectin levels and expression of adiponectin receptors in relation to lung cancer: two case-control studies. Oncology. (2007) 73:261–9. doi: 10.1159/000127424
27. Tao J, Jatoi A, Crawford J, Lam WWT, Ho JC, Wang X, et al. Role of dietary carbohydrates on risk of lung cancer. Lung Cancer. (2021) 155:87–93. doi: 10.1016/j.lungcan.2021.03.009
Keywords: BMI, variability, lung cancer, PLCO cohort study, risk factor
Citation: Sun Y, Zhou L, Shan T, Ouyang Q, Li X, Fan Y, Li Y, Gong H, Alolga RN, Ma G, Ge Y and Zhang H (2022) Variability of body mass index and risks of prostate, lung, colon, and ovarian cancers. Front. Public Health 10:937877. doi: 10.3389/fpubh.2022.937877
Received: 06 May 2022; Accepted: 25 July 2022;
Published: 25 August 2022.
Edited by:
Joao Sollari Lopes, National Statistical Institute of Portugal, PortugalReviewed by:
Jun Tao, The University of Hong Kong, Hong Kong SAR, ChinaCopyright © 2022 Sun, Zhou, Shan, Ouyang, Li, Fan, Li, Gong, Alolga, Ma, Ge and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heng Zhang, emhhbmdoZW5nMDUxM0AxMjYuY29t; Yuqiu Ge, eXVxaXVnZTA4MTZAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.