- 1Department of Clinical Biological Resource Bank, Guangzhou Women and Children's Medical Center, Guangzhou Medical University, Guangzhou, China
- 2Department of Blood Transfusion, Guangzhou Women and Children's Medical Center, Guangzhou Medical University, Guangzhou, China
- 3Pediatric Intensive Care Unit, Guangzhou Women and Children's Medical Center, Guangzhou Medical University, Guangzhou, China
Background: Sepsis is a highly life-threatening heterogeneous syndrome and a global health burden. Studies have shown that many genetic variants could influence the risk of sepsis. Long non-coding RNA lincRNA-NR_024015 may participate in functional alteration of endothelial cell via vascular endothelial growth factor (VEGF) signaling, whereas its relevance between the lincRNA-NR_024015 polymorphism and sepsis susceptibility is still unclear.
Methods: 474 sepsis patients and 678 healthy controls were enrolled from a southern Chinese child population in the present study. The polymorphism of rs8506 in lincRNA-NR_024015 was determined using Taqman methodology.
Results: Overall, a significant association was found between rs8506 polymorphism and the risk of sepsis disease (TT vs. CC/CT: adjusted OR = 1.751, 95%CI = 1.024–2.993, P = 0.0406). In the stratified analysis, the results suggested that the carriers of TT genotypes had a significantly increased sepsis risk among the children aged 12–60 months, females, early-stage sepsis and survivors (TT vs. CC/CT: ORage = 2.413; ORfemale = 2.868; ORsepsis = 2.533; ORsurvivor = 1.822; adjusted for age and gender, P < 0.05, respectively).
Conclusion: Our study indicated that lincRNA-NR_024015 rs8506 TT genotype might contribute to the risk of sepsis in a southern Chinese child population. Future research is required to elucidate the possible immunoregulatory mechanisms of this association and advance the development of novel biomarkers in sepsis.
Introduction
Sepsis, a syndrome caused by a dysregulated immune response to infection, is a life-threatening worldwide health issue (1). Globally, there were an estimated 48.9 million cases of sepsis and 11.0 million potential sepsis-related deaths in 2017 (2). According to the age, sepsis incidence peaked in early childhood who were younger than 5 years, representing 41.5% (estimated 20.3 million) of overall cases of sepsis in 2017 (2). At present, despite advances in the diagnosis and treatment of sepsis, it remains a clinical challenge for clinicians and researchers due to the fact that sepsis is still the main cause of mortality worldwide (3). Hence, it is critical to investigate the potential biomarkers to evaluate the susceptibility and disease progression of sepsis.
Long non-coding RNA (lncRNA) is a type of RNA molecules with more than 200 nucleotides in length (4, 5). Although lacking of protein-coding capacity, lncRNA has been found to be related to so many pathological processes of human diseases, such as cancer and inflammatory response (4, 6). Recently, emerging studies have revealed that some lncRNAs, such as NEAT1, MALAT1, and ANRIL, could participate in the progression of sepsis and serve as a potential biomarker for sepsis (7–9). LincRNA-NR_024015, also known as Testis development related gene 1 (TDRG1), is a newly identified tumor-associated lncRNA. Studies have shown that lncRNA TDRG1 could serve as a proto-oncogene in multiple tumor types (10–13). Chen and colleagues revealed that lncRNA TDRG1 might promote endometrial carcinoma cell proliferation and invasion by targeting vascular endothelial growth factor (VEGF) (12). Severe vascular dysfunction is considered central to the progression of organ failure during the development of sepsis (14, 15). Furthermore, the elevated circulating level of VEGF was observed in patients with sepsis (16–20). According to these findings, we considered that LincRNA-NR_024015 might have critical effects on the pathogenesis of sepsis by interacting with VEGF. In recent years, some studies have suggested that polymorphisms of the genetic components including inflammatory mediators and lncRNAs were markedly associated with the susceptibility of human sepsis (21–27). Nevertheless, whether the polymorphism of lincRNA-NR_024015 can affect sepsis susceptibility is still unknown.
The present study aimed to investigate the association between the rs8506 polymorphism of lincRNA-NR_024015 and sepsis susceptibility in the current hospital-based case–control study with 474 cases and 678 healthy controls in a southern Chinese child population.
Materials and Methods
Study Population
The study included 474 children with sepsis, who were randomly selected from January 2016 to December 2018 at Guangzhou Women and Children's Medical Center. The diagnostic criterial for sepsis, severe sepsis and septic shock were based on the Surviving Sepsis Campaign Guidelines for Management of Severe Sepsis and Septic Shock (28). The 678 subjects in the control group were recruited from the same hospital according to the age and gender of the children in an ~1:1 ratio. Sepsis patients and healthy controls who were older than 18 years and the patients with malignancy, immunodeficiencies, autoimmune, blood diseases or other organic diseases were excluded from the present study. The characteristics of all subjects in the present study were shown in Table 1. This study had received the ethics approval of the Institutional Review Board of Guangzhou Women and Children's Medical Center (2015042202). Written informed consent was obtained from each participant guardian.
DNA Extraction and SNP Genotyping
The SNP with potential function and associated information were adopted according to the NCBI dbSNP database (http://www.ncbi.nlm.nih.gov/, http://snpinfo.niehs.nih.gov/) based on the following criteria: (1) the minor allele frequency (MAF) ≥5% in Southern Han Chinese offspring in HapMap; (2) affect the microRNA/Protein binding site activity; (3) located in the gene regulatory region. 2 ml of venous whole blood were collected in an EDTA tube from each sepsis child and control. Genomic DNA were extracted from the whole blood samples (200 μl) by the phenol–chloroform method using the TIANamp Blood DNA Kit (TianGen Biotech, Beijing, China) as described previously (29). The concentration and purity of the extracted DNA was determined by a NanoDrop 2000 (Thermo Fisher Scientific, USA). High-quality DNA samples with OD260/OD280 values between 1.6 and 1.8 were used for genotyping experiments. The allele-specific fluorescent probes (lincRNA-NR_024015 rs8506) were purchased from Applied Biosystems (Thermo Fisher Scientific, USA). Genotyping for the rs8506 C>T was performed in the 384-well plate using Taqman PCR method (30). Briefly, DNA amplification was conducted in a volume of 5 μl containing 2.5 ng template DNA, primer pool, and 2 × Multiplex PCR Mix, followed by the conditions [95°C for 3 min, 40 cycles of (95°C for 20 s, 58°C for 90 s, 72°C for 30 s) and 72°C for 1 min] in an Applied Biosystems Q6 instrument (Thermo Fisher Scientific, USA). For quality control, three replicates for each sample were performed in a 384-well plate and each plate contained four distilled water samples instead of DNA templates. Moreover, 10% of the DNA samples was randomly selected for a repeated genotyping analysis, and the results were 100% consistent. Genotyping was performed blindly to the status of the case or control.
Statistical Analysis
The differences in the genotype frequencies of lincRNA-NR_024015 rs8506 and the demographic variables between sepsis cases and healthy controls were compared applying the χ2 test. Hardy–Weinberg equilibrium (HWE) of the healthy controls for rs8506 was tested using the goodness-of-fit χ2 test. Multivariate logistic regression analyses were carried out to calculate the odd ratios (ORs) and their 95% confidence intervals (CIs) for risk of sepsis, which were also stratified by the age, gender, prognosis and the number of organs with dysfunction. All of the statistical analyses were conducted using SAS software (Version 9.3; SAS Institute, Cary, NC, USA) and a P-value < 0.05 was considered to be statistically significant.
Results
Population Characteristics
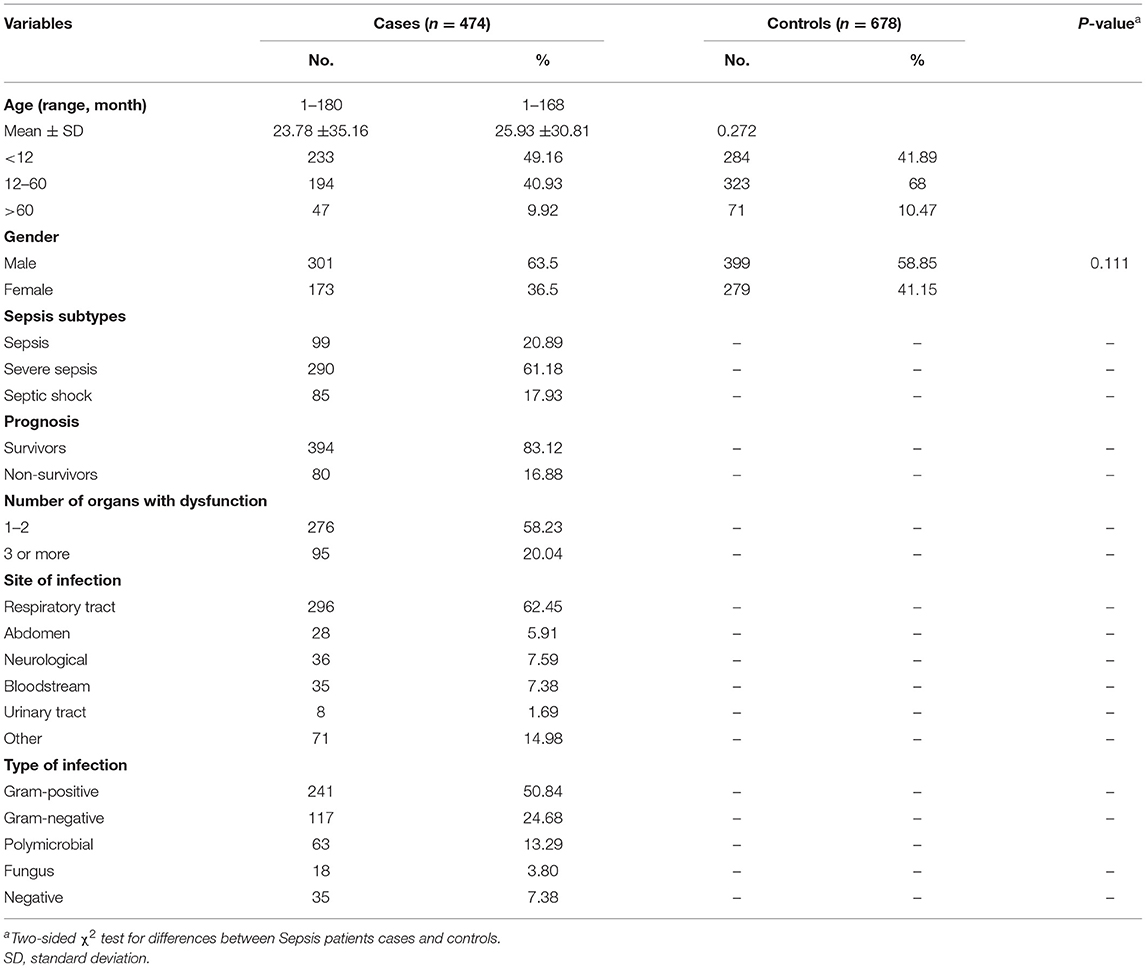
In order to describe whether SNP in the lincRNA-NR_024015 rs8506 was associated with susceptibility to sepsis in a southern Chinese child population, we carried out a case–control study with a cohort of 474 Chinese patients with sepsis and 678 healthy controls. The general and clinical characteristics of the study population were summarized in Table 1. On average, the patients were 24 months old (range: 1–180; standard deviation, SD: ±35) and the healthy controls were 26 months old (range: 1–168; SD: ±31). Of the 474 patients, 63% were male individuals and 37% were female; while 59% of the healthy controls were male and 41% were female. No statistically significant differences were found between cases and control groups with respect to age (P = 0.272) and gender (P = 0.111). During the observation period, 61.2% of the patients were in severe sepsis and 17.9% were in septic shock. Considering the prognosis of the patients, the patient group was subdivided into survivors (394; 83.1%) and non-survivors (80; 16.9%). Moreover, 58.2% of all the enrolled patients developed 1–2 organs with dysfunction; 3 or more organs with dysfunction occurred in about 20% of all the patients. Respiratory tract (62.45%), abdomen (5.91%), neurological (7.59%), bloodstream (7.38%) and urinary tract (1.69%) represented the predominant sites of infection in this study. Additionally, across all types of infection, the most frequent type was gram-positive infection (50.84%), followed by gram-negative infection (24.68%).
Association Analysis
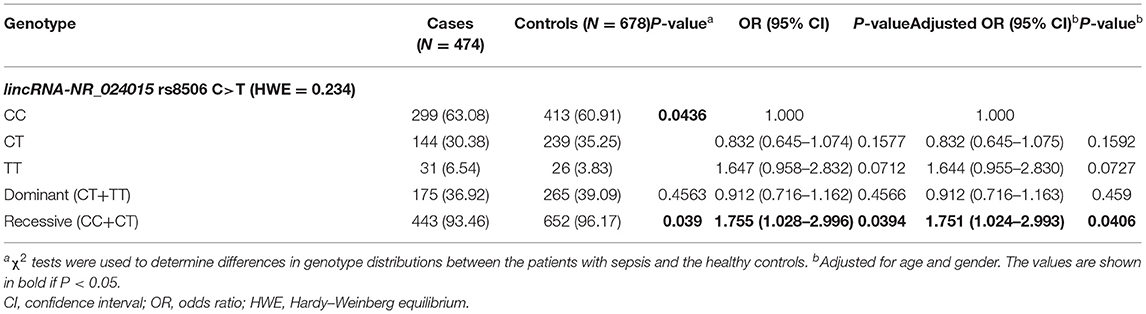
The genotypes of lincRNA-NR_024015 rs8506 were successfully evaluated in the present study. As shown in Table 2, the distribution of the genotypes agreed with the Hardy–Weinberg equilibrium in the healthy controls (P = 0.234). We observed that the population who carried rs8506 TT genotype had a 1.755-fold higher risk of sepsis than those did not carry (TT vs. CC/CT: OR = 1.755, 95%CI = 1.028–2.996, P = 0.0394). Furthermore, after adjustments for age and gender, significantly elevated risk of sepsis was also found in the rs8506 TT genotype, as compared with rs8506 CC/CT genotype (TT vs. CC/CT: adjusted OR = 1.751, 95%CI = 1.024–2.993, P = 0.0406).
Stratified Analysis
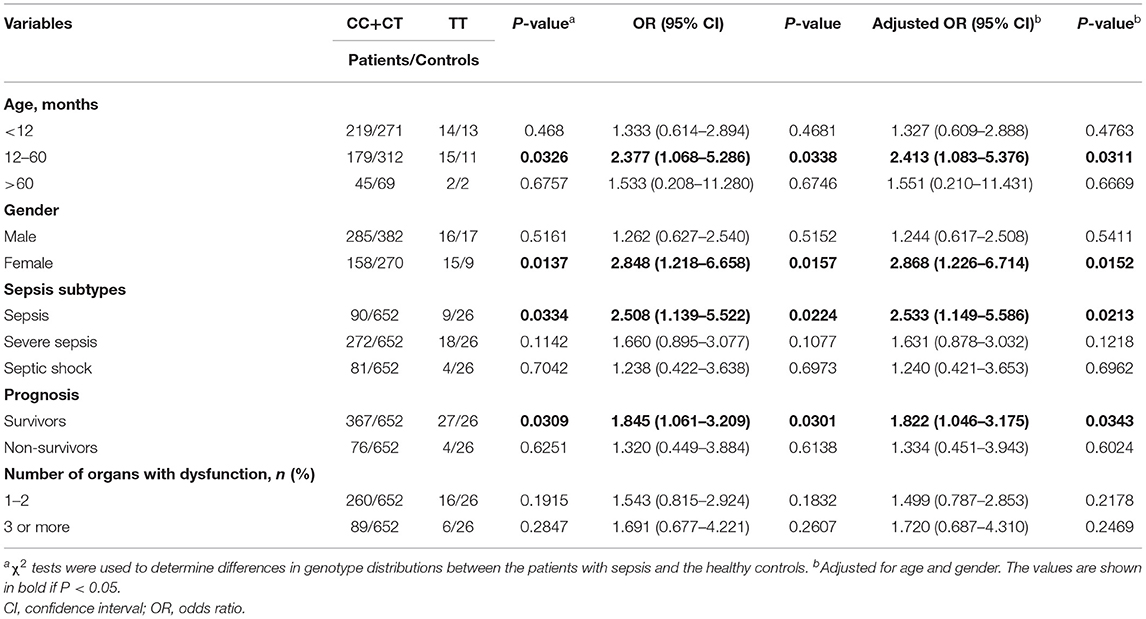
We further performed a stratified analysis of the relationship between lincRNA-NR_024015 rs8506 polymorphism and sepsis susceptibility by clinical features (Table 3). The rs8506 TT genotype was found to be markedly associated with an increased sepsis risk among the children aged 12–60 months (TT vs. CC/CT: OR = 2.377, 95%CI = 1.068–5.286, P = 0.0338; adjusted OR = 2.413, 95%CI = 1.083–5.376, P = 0.0311), females (TT vs. CC/CT: OR = 2.848, 95%CI = 1.218–6.658, P = 0.0157; adjusted OR = 2.868, 95%CI = 1.226–6.714, P = 0.0152), sepsis (TT vs. CC/CT: OR = 2.508, 95%CI = 1.139–5.522, P = 0.0224; adjusted OR = 2.533, 95%CI = 1.149–5.586, P = 0.0213), and survivors (TT vs. CC/CT: OR = 1.845, 95%CI = 1.061–3.209, P = 0.0301; adjusted OR = 1.822, 95%CI = 1.046–3.175, P = 0.0343). However, no significant associations were found in other stratified analyses.
Discussion
In the present study, we enrolled a cohort of 474 cases with sepsis and 678 controls to evaluate the association between lincRNA-NR_024015 rs8506 polymorphism and the sepsis susceptibility among southern Chinese children. The result showed that the carriers of rs8506 TT genotype had a significantly increased risk of sepsis when compared with that carrying CC/CT genotypes. Interestingly, the stratified analysis revealed that the increased risk level of rs8506 TT variant appeared more obvious in the children of 12–60 months old and female. Moreover, we also found that the rs8506 TT variant showed significantly elevated risk of sepsis in the subgroup of the patients who were in the early stage of sepsis or alive. Therefore, our findings provided evidences that lincRNA-NR_024015 rs8506 TT genotype might be associated with the susceptibility of sepsis in a southern Chinese child population.
Long non-coding RNAs (lncRNAs) are a type of non-protein-coding RNAs which exceed 200 nucleotides in length (4, 5). It has been suggested that lncRNAs play important roles in the pathogenesis of various diseases through chromatin rearrangement, transcriptional control as well as post-transcriptional processing (4, 5). However, little is known about the relationship between lncRNAs and the sepsis susceptibility (7, 31). In the present study, we found that lincRNA-NR_024015 rs8506 TT genotype was notably associated with an increased risk of sepsis. To our knowledge, this is the first study to evaluate the relationship of the rs8506 polymorphism with sepsis risk in a southern Chinese child population.
lincRNA-NR_024015 (gene ID: 732253), namely TDRG1, was initially identified as a novel human testis-specific gene which served as a regulator in sperm motility and the development of testicular germ cell tumors (32, 33). Recent studies have suggested that lincRNA-NR_024015 might play important roles in tumor progression in several cancer types including cervical (10), esophageal (34), ovarian (13) and endometrial carcinoma (12). Chen et al. (12) provided evidences that lincRNA-NR_024015 might directly bound to VEGF-A protein and upregulated its expression, thus promoting the progression of endometrial carcinoma. Moreover, lincRNA-NR_024015 and VEGF were found to be co-expressed and remarkably upregulated in fibrovascular membranes from diabetic retinopathy patients than those from epiretinal membrane (35). These reported data reveal that lincRNA-NR_024015 might be beneficial to stimulate the VEGF pathway (35). VEGF is a potent mediator that not only increases the vascular permeability, but also promotes leukocytes adhesion by eliciting the expression of adhesive molecules (36–38). Actually, emerging data have suggested that circulating VEGF levels were elevated during the development of sepsis (16–20) and the levels of this factor were associated with sepsis severity and mortality (16–18). Furthermore, blockade of VEGF signaling in a mouse model might have beneficial effects on the survival of sepsis by decreasing inflammatory responses and endothelial permeability (39). Importantly, the expression level of lincRNA-NR_024015 in esophageal tumor tissues with rs8506 CT and TT genotype was significantly higher than those with rs8506 CC genotype (34). Therefore, in combination with the findings in our study, we speculated that rs8506 TT genotype might increase the risk of sepsis via upregulating the levels of lincRNA-NR_024015 and VEGF. Further study is needed to confirmed this possibility in the future.
Epidemiological studies have showed that global sepsis apparently occurred in females and young children below 5 years old (2). Similar to the present study, the increased risk of the rs8506 TT variant genotype was more evident in the children of 12–60 months old and in females, as compared with the CC/CT genotypes. Furthermore, it is surprising in our study, the rs8506 TT genotype was markedly associated with an increased sepsis risk among the early stage of sepsis and survivor subgroup of the patient cohort, but not severe sepsis, septic shock or non-survivor. Owing to the robustly low frequency of rs8506 TT genotype in the enrolled cohort, we considered that the sample size was not enough to test the power of analysis. The current study is only an investigation that focus on the relationship between gene polymorphism and disease susceptibility. Therefore, more mechanistic studies are needed to confirm the roles of lincRNA-NR_024015 rs8506 TT in the progression of sepsis in children.
Although this is the first study to evaluate the association between lincRNA-NR_024015 rs8506 polymorphism and sepsis risk in southern Chinese children, several possible limitations should be addressed in present study. First, there are only 474 sepsis patients and 678 controls included. Therefore, the sample size in the current study might have impact on the test power of statistical analysis. Second, only rs8506 T allele was under investigation in the present study, other lincRNA-NR_024015 gene polymorphisms with potential function remain to be took into consideration. Third, we did not apply other valuable factors that are also important for sepsis severity and prognosis in the present (i.e., scores of sepsis-related organ failure assessment, length of hospital stay) (1). Forth, it has been shown that many factors (i.e., living environment, social-economic factor, population education) have impact on the incidence of sepsis (2, 40); however, we could only collect frequency-matched cases and controls by age and gender due to lack of these information.
Conclusion
In summary, we verified a significant association between lincRNA-NR_024015 rs8506 TT genotype and increased sepsis susceptibility in southern Chinese children, especially for children aged 12–60 months, females, and those with early stage of sepsis. Future studies with larger sample size and mechanistic experiments should be conducted to strengthen our findings.
Data Availability Statement
The raw analyzed datasets used in the study will be available from the corresponding authors on reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by this study had received the Ethics approval of the Institutional Review Board of Guangzhou Women and Children's Medical Center (2015042202). Written informed consent was obtained from each participant guardian. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
JL and HZ designed the experiments. JL, HZ, and BW performed the experiments. DC and YX analyzed the data. LP and LF collected the samples and clinical data. JL wrote the manuscript. JH and XG revised and finalized the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Guangdong Basic and Applied Basic Research Foundation (grant number 2021B1515230003), the Guangdong Natural Science Fund, China (grant numbers 2019A1515012061, 202102020829, and 2022A1515012558), the Guangzhou Science and Technology Program Project, China (grant numbers 201904010486, 202102010197, 202102021144, and 202102020829), the Subject Construction Project of Guangzhou Medical University (grant number 02-410-2206062), Doctoral Research Initiation Fund from Guangzhou Institute of Pediatrics, Guangzhou Women and Children's Medical Center (grant number 1600104), and Post-doctoral Research Initiation Fund from Guangzhou Institute of Pediatrics, Guangzhou Women and Children's Medical Center (grant numbers 3001162 and 3001178-04).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank the Clinical Biological Resource Bank of Guangzhou Women and Children's Medical Center for providing all the clinical samples.
References
1. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. (2020) 395:200–11. doi: 10.1016/S0140-6736(19)32989-7
3. Kissoon N, Reinhart K, Daniels R, Machado MFR, Schachter RD, Finfer S. Sepsis in children: global implications of the world health assembly resolution on sepsis. Pediatr Crit Care Med. (2017) 18:e625–7. doi: 10.1097/PCC.0000000000001340
4. Boon RA, Jae N, Holdt L, Dimmeler S. Long non-coding RNAs: from clinical genetics to therapeutic targets? J Am Coll Cardiol. (2016) 67:1214–26. doi: 10.1016/j.jacc.2015.12.051
5. Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. (2009) 10:155–9. doi: 10.1038/nrg2521
6. Winkle M, El-Daly SM, Fabbri M, Calin GA. Non-coding RNA therapeutics-challenges and potential solutions. Nat Rev Drug Discov. (2021) 20:629–51. doi: 10.1038/s41573-021-00219-z
7. Zhang TN, Li D, Xia J, Wu QJ, Wen R, Yang N, et al. Non-coding RNA: a potential biomarker and therapeutic target for sepsis. Oncotarget. (2017) 8:91765–78. doi: 10.18632/oncotarget.21766
8. Zhang CC, Niu F. LncRNA NEAT1 promotes inflammatory response in sepsis-induced liver injury via the Let-7a/TLR4 axis. Int Immunopharmacol. (2019) 75:105731. doi: 10.1016/j.intimp.2019.105731
9. Wang W, Yang N, Wen R, Liu CF, Zhang TN. Long Non-coding RNA: regulatory mechanisms and therapeutic potential in sepsis. Front Cell Infect Microbiol. (2021) 11:563126. doi: 10.3389/fcimb.2021.563126
10. Jiang H, Liang M, Jiang Y, Zhang T, Mo K, Su S, et al. The lncRNA TDRG1 promotes cell proliferation, migration and invasion by targeting miR-326 to regulate MAPK1 expression in cervical cancer. Cancer Cell Int. (2019) 19:152. doi: 10.1186/s12935-019-0872-4
11. Hu X, Mu Y, Wang J, Zhao Y. LncRNA TDRG1 promotes the metastasis of NSCLC cell through regulating miR-873-5p/ZEB1 axis. J Cell Biochem. (2019) 122:969–982. doi: 10.1002/jcb.29559
12. Chen S, Wang LL, Sun KX, Liu Y, Guan X, Zong ZH, et al. LncRNA TDRG1 enhances tumorigenicity in endometrial carcinoma by binding and targeting VEGF-A protein. Biochim Biophys Acta, Mol Basis Dis. (2018) 1864(9 Pt B):3013–21. doi: 10.1016/j.bbadis.2018.06.013
13. Chen S, Wang LL, Sun KX, Xiu YL, Zong ZH, Chen X, et al. The role of the long non-coding RNA TDRG1 in epithelial ovarian carcinoma tumorigenesis and progression through miR-93/RhoC pathway. Mol Carcinog. (2018) 57:225–34. doi: 10.1002/mc.22749
14. Joffre J, Hellman J, Ince C, Ait-Oufella H. Endothelial responses in sepsis. Am J Respir Crit Care Med. (2020) 202:361–70. doi: 10.1164/rccm.201910-1911TR
15. Peters K, Unger RE, Brunner J, Kirkpatrick CJ. Molecular basis of endothelial dysfunction in sepsis. Cardiovasc Res. (2003) 60:49–57. doi: 10.1016/S0008-6363(03)00397-3
16. Pickkers P, Sprong T, Eijk L, Hoeven H, Smits P, Deuren M. Vascular endothelial growth factor is increased during the first 48 hours of human septic shock and correlates with vascular permeability. Shock. (2005) 24:508–12. doi: 10.1097/01.shk.0000190827.36406.6e
17. van der Flier M, van Leeuwen HJ, van Kessel KP, Kimpen JL, Hoepelman AI, Geelen SP. Plasma vascular endothelial growth factor in severe sepsis. Shock. (2005) 23:35–8. doi: 10.1097/01.shk.0000150728.91155.41
18. Yano K, Liaw PC, Mullington JM, Shih SC, Okada H, Bodyak N, et al. Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality. J Exp Med. (2006) 203:1447–58. doi: 10.1084/jem.20060375
19. Karlsson S, Pettilä V, Tenhunen J, Lund V, Hovilehto S, Ruokonen E. Vascular endothelial growth factor in severe sepsis and septic shock. Anesth Analg. (2008) 106:1820–6. doi: 10.1213/ane.0b013e31816a643f
20. Whitney JE, Silverman M, Norton JS, Bachur RG, Melendez E. Vascular endothelial growth factor and soluble vascular endothelial growth factor receptor as novel biomarkers for poor outcomes in children with severe sepsis and septic shock. Pediatr Emerg Care. (2020) 36:e715–e719. doi: 10.1097/PEC.0000000000001638
21. Arnalich F, López-Maderuelo D, Codoceo R, Lopez J, Solis-Garrido LM, Capiscol C, et al. Interleukin-1 receptor antagonist gene polymorphism and mortality in patients with severe sepsis. Clin Exp Immunol. (2002) 127:331–6. doi: 10.1046/j.1365-2249.2002.01743.x
22. Montoya-Ruiz C, Jaimes FA, Rugeles MT, Lopez JA, Bedoya G, Velilla PA. Variants in LTA, TNF, IL1B and IL10 genes associated with the clinical course of sepsis. Immunol Res. (2016) 64:1168–78. doi: 10.1007/s12026-016-8860-4
23. Nakada TA, Wacharasint P, Russell JA, Boyd JH, Nakada E, Thair SA, et al. The IL20 genetic polymorphism is associated with altered clinical outcome in septic shock. J Innate Immun. (2018) 10:181–8. doi: 10.1159/000486104
24. Le KTT, Matzaraki V, Netea MG, Wijmenga C, Moser J, Kumar V. Functional annotation of genetic loci associated with sepsis prioritizes immune and endothelial cell pathways. Front Immunol. (2019) 10:1949. doi: 10.3389/fimmu.2019.01949
25. Kumar V. Toll-like receptors in sepsis-associated cytokine storm and their endogenous negative regulators as future immunomodulatory targets. Int Immunopharmacol. (2020) 89(Pt B):107087. doi: 10.1016/j.intimp.2020.107087
26. Wu Z, Yu Y, Fu L, Mai H, Huang L, Che D, et al. LncRNA SOX2OT rs9839776 polymorphism reduces sepsis susceptibility in southern Chinese children. J Inflamm Res. (2020) 13:1095–101. doi: 10.2147/JIR.S281760
27. Wu Z, Liang Y, Zuo Y, Xu Y, Mai H, Pi L, et al. The lncRNA CCAT2 Rs6983267 G variant contributes to increased sepsis susceptibility in a southern Chinese population. Infect Drug Resist. (2021) 14:2969–76. doi: 10.2147/IDR.S311717
28. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. (2013) 39:165–228. doi: 10.1007/s00134-012-2769-8
29. Fu W, Zhuo ZJ, Jia W, Zhu J, Zhu SB, Lin ZF, et al. Association between TP53 gene Arg72Pro polymorphism and Wilms' tumor risk in a Chinese population. Onco Targets Ther. (2017) 10:1149–54. doi: 10.2147/OTT.S131014
30. Che D, Pi L, Xu Y, Fu L, Zhou H, Wang Z, et al. TBXA2R rs4523 G allele is associated with decreased susceptibility to Kawasaki disease. Cytokine. (2018) 111:216–21. doi: 10.1016/j.cyto.2018.08.029
31. Ho J, Chan H, Wong SH, Wang MH, Yu J, Xiao Z, et al. The involvement of regulatory non-coding RNAs in sepsis: a systematic review. Crit Care. (2016) 20:383. doi: 10.1186/s13054-016-1555-3
32. Chen H, Sun J, He Y, Zou Q, Wu Q, Tang Y. Expression and localization of testis developmental related gene 1 (TDRG1) in human spermatozoa. Tohoku J Exp Med. (2015) 235:103–9. doi: 10.1620/tjem.235.103
33. Gan YU, Yang J, Wang Y, Tan Z, Jiang X, Tang Y. In vitro study on shRNA-mediated reduction of testis developmental related gene 1 expression and its effects on the proliferation, invasion and apoptosis of NTERA-2 cells. Oncol Lett. (2015) 10:61–6. doi: 10.3892/ol.2015.3219
34. Han L, Liu S, Liang J, Guo Y, Shen S, Guo X, et al. A genetic polymorphism at miR-526b binding-site in the lincRNA-NR_024015 exon confers risk of esophageal squamous cell carcinoma in a population of North China. Mol Carcinog. (2017) 56:960–71. doi: 10.1002/mc.22549
35. Gong Q, Dong W, Fan Y, Chen F, Bian X, Xu X, et al. LncRNA TDRG1-mediated overexpression of VEGF aggravated retinal microvascular endothelial cell dysfunction in diabetic retinopathy. Front Pharmacol. (2019) 10:1703. doi: 10.3389/fphar.2019.01703
36. Bates DO. Vascular endothelial growth factors and vascular permeability. Cardiovasc Res. (2010) 87:262–71. doi: 10.1093/cvr/cvq105
37. Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. (2019) 176:1248–64. doi: 10.1016/j.cell.2019.01.021
38. Reinders ME, Sho M, Izawa A, Wang P, Mukhopadhyay D, Koss KE, et al. Pro-inflammatory functions of vascular endothelial growth factor in alloimmunity. J Clin Invest. (2003) 112:1655–65. doi: 10.1172/JCI17712
39. Jeong SJ, Han SH, Kim CO, Choi JY, Kim JM. Anti-vascular endothelial growth factor antibody attenuates inflammation and decreases mortality in an experimental model of severe sepsis. Crit Care. (2013) 17:R97. doi: 10.1186/cc12742
Keywords: lincRNA-NR_024015, genetic polymorphisms, risk, children, sepsis
Citation: Li J, Zhou H, Wei B, Che D, Xu Y, Pi L, Fu L, Hong J and Gu X (2022) The rs8506 TT Genotype in lincRNA-NR_024015 Contributes to the Risk of Sepsis in a Southern Chinese Child Population. Front. Public Health 10:927527. doi: 10.3389/fpubh.2022.927527
Received: 24 April 2022; Accepted: 16 June 2022;
Published: 13 July 2022.
Edited by:
Arzu Didem Yalcin, Academia Sinica, TaiwanCopyright © 2022 Li, Zhou, Wei, Che, Xu, Pi, Fu, Hong and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoqiong Gu, Z3V4aWFvcWlvbmdAZ3djbWMub3Jn; Jie Hong, Z3otaG9uZ2pAdG9tLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Jinqing Li
Jinqing Li Huazhong Zhou1†
Huazhong Zhou1† Bing Wei
Bing Wei Di Che
Di Che Yufen Xu
Yufen Xu Lei Pi
Lei Pi Lanyan Fu
Lanyan Fu Xiaoqiong Gu
Xiaoqiong Gu