
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
METHODS article
Front. Public Health, 19 August 2022
Sec. Family Medicine and Primary Care
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.925232
This article is part of the Research TopicManagement of Hemodialysis PatientsView all 8 articles
Background: Dialysis-related myofascial pain in hemodialysis (HD) patients is an important issue that is associated with many other psychosomatic problems. Effective interventions are required to alleviate pain in this group. Chinese herbal medicine (CHM) may be a potential therapeutic treatment for reducing pain. The aim of this study is to evaluate the effects of a classic CHM formula intervention on pain intensity, daily function, quality of life (QOL), and safety in patients receiving HD in a dialysis center within the context of southern Taiwan.
Methods: This will be a randomized, open label, cross-over trial with two parallel groups in a pre- and post-test study. Forty patients reporting myofascial pain related to the arteriovenous (AV) fistula in the arm during regular HD sessions will be recruited. Participants will receive 4 weeks of treatment with Juan Bi Tang (JBT) and 4 weeks of no treatment in a random order, separated by a washout period of 2 weeks. Treatment doses (3 g JBT) will be consumed thrice daily. The primary outcome measure will be the Kidney Disease Quality of Life 36-Item Short-Form Survey. Secondary outcomes will include the Fugl-Meyer Assessment-arm, Visual Analogue Scale (VAS) of pain, and grip strength. Outcomes will be collected before and after each intervention, for a total of four times per participant. The safety evaluation will focus on adverse events (AEs).
Discussion: This study will be the first to use CHM to treat patients receiving HD with dialysis-related myofascial pain in their fistula arm and to perform a complete assessment of the treatment, including records of QOL, arm function and muscle power, severity of pain, and safety. The results of the study will provide convincing evidence on the use of JBT as an adjuvant treatment for dialysis-related myofascial pain.
Trial registration: Clinicaltrials.gov registry (NCT04417101) registered 30 May 2020.
Myofascial pain syndrome (MPS) is characterized by localized pain (1), paresthesia, exquisite tenderness, restricted range of motion, and hypersensitivity at specific anatomic sites, which are termed taut bands with active myofascial trigger points (MTrPs) (2–4). According to the International Association for the Study of Pain and the American Academy of Pain Medicine, the essential criteria for the diagnosis of MPS are hypersensitive spots that cause local pain and symptoms that can be recreated by palpation (5). In the United States, about 9 million people have MPS (6), and its prevalence in the general population is between 9 and 85% (5, 7).
Myofascial pain syndrome is often more serious in females than in males, and its prevalence apparently increases with age (8). Currently, the etiology of MPS is poorly understood, but various aspects of its pathogenesis are being investigated (8, 9). The negative effects of MPS on quality of life (QOL), such as loss of work tolerance, fatigue, and weakness, are well-documented (10). However, few studies have focused on MPS in specific populations.
One major complication of chronic kidney disease (CKD) or end stage kidney disease (ESRD) is rheumatic disorders, and over half (60%) of patients receiving hemodialysis (HD) develop musculoskeletal disorders (11). Therefore, it is important to consider a differential diagnosis of MPS in these patients. Another form of MPS which sometimes develops in patients receiving HD is related to the fistula arm. In such cases, the symptoms are induced by factors implicated in or related to maintenance HD.
Factors such as comorbidities, dialysis type, metabolic disorders, nutritional factors, biomechanical imbalance, and/or physio-psychological deconditioning may simultaneously contribute to the development of dialysis-related myofascial pain (12, 13). However, the pathological mechanisms involved are not well-known. Currently, the underlying pathologies are considered to be local injury from gross venipuncture or constant micro-trauma from venous pressure during each HD session in the affected arm muscle (8, 14). This stress can lead to inappropriate acetylcholine (Ach) activity at the endplate. Such activity can cause an energy crisis that favors the release of nociceptive neurotransmitters. The altered ACh in turn triggers an active contractile phenomenon (taut bands), and the nociceptive neurotransmitters, which compensate for tissue hypoxemia, cause pain neurotransmission or sensations in the form of local pain and referred pain (15–17).
There is no definitive treatment for dialysis-related myofascial pain, and the most common treatment modality is based on condition-specific and stepwise pain management for ESRD (18, 19). Aspects of dialysis-related myofascial pain can be easily mitigated with non-steroidal anti-inflammatory drugs (NSAIDs), Cyclooxygenase-2 (COX-2) inhibitors or topical analgesics (18). One problem with this approach to pain management is that the use of oral analgesics may have side effects such as bleeding, fluid retention, and cardiovascular events; thus, they are not appropriate for all patients (20). In addition, some sedative-hypnotic agents or muscle relaxants for alleviation of symptoms of anxiety and/or depression carry risks of hypotension and physical dependence (21, 22).
The aforementioned limitations of conventional treatment have led to the present study, which advocates the use of Chinese herbal medicine (CHM) therapy for pain management. CHM therapy is safe and inexpensive, and it can alleviate the pain and anxiety associated with several diseases (23, 24). Moreover, CHM use by patients with CKD has positive results on reducing the risk of ESRD, as determined by thorough analysis of the classification of CHM prescriptions in the Taiwan National Health Insurance Research Database (25). Juan Bi Tang (JBT) has a long history of use as a classic herbal prescription for treating Bi syndrome and was first documented in a classical Chinese medical book, Prescriptions for Ji Sheng Fang (CE 1253). As joint pain is one of the most common symptoms of Bi syndrome, some scholars often translate it as “Arthralgia syndrome.” Clinically, Bi syndrome covers many musculoskeletal diseases, such as rheumatoid arthritis, osteoarthritis, rheumatism, fibromyalgia, or myofascial syndrome (26). The basic pathology of Bi syndrome is the obstruction of qi (also called vital energy) and blood in the meridians due to the invasion of pathogenic wind, cold, and dampness. According to traditional Chinese medicine (TCM), open meridians and normal circulation of the qi and blood are associated with an absence of pain, while obstruction of the meridians and the flow of qi and blood are associated with the presence of pain. Juan Bi Tang is used for treating pain in the upper limbs by warming up meridians so as to dissipate cold, eliminate dampness, activate the qi and blood, and also resolve stasis (27). Although JBT is widely used in the treatment of musculoskeletal disorders, no studies have been conducted to examine its efficacy and safety for the treatment of dialysis-related myofascial pain in patients receiving HD. This randomized, open label, parallel-group, cross-over, single center clinical trial will aim to determine the possible benefits of JBT in patients with dialysis-related myofascial pain.
This single-center, two-phase study will last 12 weeks. It will be a randomized, prospective, crossover, open-label study comprising two groups and two treatment sequences, each consisting of 4 weeks of daily treatments separated by a 2-week washout period (Figure 1). In total, 40 patients will be recruited (20 patients per group) from a dialysis center in Chang Gung Memorial Hospital (CGMH), Kaohsiung, Taiwan. The subjects will be randomly and equally assigned with a central registration method into group A (JBT course in period I but not in period II) or group B (JBT course not in period I but in period II). The research protocol has been approved by the Ethics Committee of the CGMH (202000477A3) and registered at ClinicalTrials.gov (NCT04417101). The Standard Protocol Items: Recommendation for Interventional Trials (SPIRIT) 2013 checklist is provided in Table 1.
Participants will be recruited from Kaohsiung CGMH via posters and advertisements on the official hospital website. The recruitment period is planned to last 18 months. All participants will provide informed consent before randomization. The schedule of patient enrollment, intervention, and assessment is illustrated in Table 1.
Participants meeting the following criteria are eligible:
1. Age of 20 years or older;
2. Treatment with conventional HD three times a week via an arteriovenous (AV) fistula for at least 3 months;
3. Myofascial trigger points in one or more muscles around the AV fistula, namely, the brachioradialis, the flexor carpi radialis, the palmaris longus, and/or the pronator teres (Figure 2), accompanied by dialysis-related myofascial pain presenting during HD and diagnosed by a nephrologist (28);
4. Onset of symptoms within 1 month before enrollment;
5. Exquisite tenderness on palpation in the taut band, with moderate intensity of pain at baseline (i.e., a pain score >3 on a numeric rating scale);
6. No change of painkillers, muscle relaxants, or anti-inflammatory medications and no use of topical anesthetics in the past week.
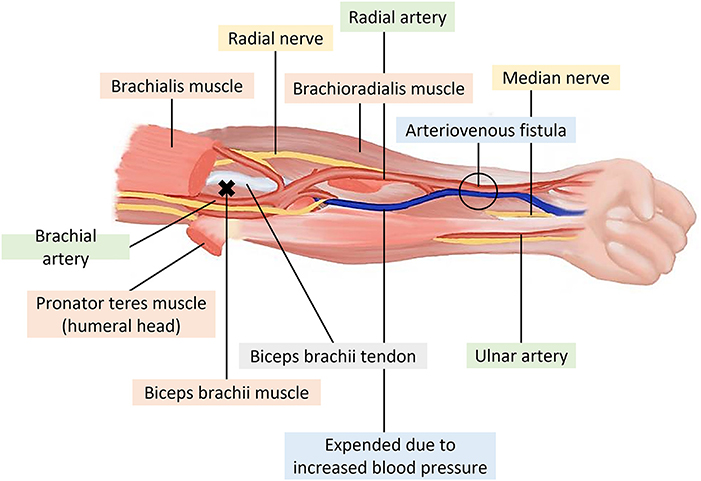
Figure 2. Muscles of the anterior forearm around the arteriovenous fistula (x: trigger points of the biceps brachii).
The exclusion criteria are as follows:
1. Severe systemic disease (i.e., sepsis, cancer, coagulopathy) interfering with therapy attendance;
2. Comorbid conditions such as rheumatoid arthritis, stroke, chronic liver disease, radiculopathies of the upper limb, recent history of cervical/shoulder/arm surgery, or trauma;
3. Depression and/or presence of a psychiatric disorder;
4. Treatment with CHM for dialysis-related myofascial pain in the past month;
5. Allergic reactions (i.e., skin rash, purpura, and vasculitis) to CHM;
6. Inability to comprehend or sign an informed consent form.
Study participation will be terminated under the following conditions:
1. Occurrence of severe complications and/or general health deterioration (i.e., sepsis, bleeding, AV fistula occlusion, and progress of electrolyte disorders such as ECG change of hyperkalemia, or uncontrolled calcium × phosphorus (Ca × P) level);
2. Occurrence of a severe adverse event (AE) of any type during the intervention;
3. Voluntary withdrawal from the trial, absence from two consecutive interviews, or loss to follow-up.
The CHM product investigated in this study will be prescription-grade JBT powder (Product code; NO.0321131, Ko Da Pharmaceutical Co., Ltd., Taoyuan, Taiwan) manufactured in accordance with Good Manufacturing Practices (GMP) and tested before factory release. Juan Bi Tang consists of 4 g Rx. Angelica Sinensis當歸, 4 g Rx. Paeoniae Rubra赤芍, 4 g Rx. Astragali黃耆, 4 g Rz. Curcuma Longae薑黃, 1 g Rx. et Rz. Notopterygii羌活, 4 g Rx. Saposhnikoviae防風, 3 g Rz. Zingiberis Recens生薑, 2 g Fr. Zizyphi Jujube大棗, and 1.5 g Rx. Glycyrrhizae Preparata炙甘草. These raw herb materials, together with starch excipient, are extracted and concentrated according to a standardized Chinese formula (see: http://www.koda.com.tw/tqm04_e.aspx). The participants will be instructed to take JBT orally in doses of 3 g (per bag) each time, thrice daily, for 4 weeks. Patients will return each week to the outpatient clinic for evaluation, medication, and counting of any unconsumed bags. Weekly compliance below 75% will be considered a deviation from the protocol and will lead to exclusion from further participation in the study. Participants in the non-treatment period will receive simple self-care measures for MPS based on the non-pharmacologic intervention in patients with CKD by Pham et al. (18). These will mainly include physical activities such as stretching, aerobic and strength exercises, and some types of combined exercises (18). The duration of the intervention will be 8 weeks, and the follow-up will be at 2 weeks.
Routine medication for maintenance HD will be continued. The medication regimen will be recorded at every visit, and participants will need to inform the study assistant of any changes to their medication, supplements, and even exercise. Additional acupuncture, herbal prescriptions, or other interventions by other TCM practitioners will not be permitted during the treatment period.
The participants will be allocated into group A (n = 20) or group B (n = 20) according to a randomized list generated in Microsoft Excel 2016. Participants will be coded with identification numbers to guarantee confidentiality during data analysis. The physicians will have no access to the sequence, and the investigators will distribute the drugs. All members of the research team will be explicitly instructed not to inform the participants of their allocations. The clinical outcomes assessor will be blinded to the allocations to decrease the risk of observer bias. Participants' files will be kept in a box in a locked cabinet only accessible by an assistant until the end of the study.
Participants will be coded with identification numbers to guarantee confidentiality during data analysis. Participants' files will be stored in a secure room in the TCM Education Research Office, Rehabilitation Building of Kaohsiung CGMH.
The subjects will be assessed five times: prior to the study, at 4 weeks (at the end of period I), at 6 weeks (at the beginning of period II), at 10 weeks (at the end of period II), and at follow-up (2 weeks after the trial period ends). A research assistant will be trained to ensure the accuracy of outcome assessments and data collection.
The primary outcome used in this study will be the Kidney Disease Quality of Life 36-Item Short-Form Survey (KDQOL-36), a self-report measure developed for health-related concerns of individuals who visit dialysis facilities for treatment (29). The KDQOL-36 is available in English and was translated into Mandarin Chinese by the RAND Corporation (see: http://www.rand.org/health/surveys_tools/kdqol.html). The instrument is composed of 12 general health items and 24 kidney-specific items. The items on general health are summarized into a Physical Component Summary (PCS) score and a Mental Component Summary (MCS) score. The 24 kidney-specific items comprise three scales: symptoms and problems, burden of kidney disease, and effects of kidney disease (12, 4, and 8 items, respectively). The raw scores are transformed into a linear range of 0–100, where higher scores indicate better QOL (30).
Secondary outcome measures will include arm motor function evaluated with the Fugl-Meyer Assessment for upper extremity (FMA-UE); muscle power threshold, measured with a grip algometer; and self-reported degree of pain [Visual Analogue Scale (VAS) score].
Upper limb function will be assessed with the FMA-UE, a stroke-specific test for measuring motor impairment and recovery (31). The FMA-UE, which is widely used to evaluate upper limb motor function in patients with stroke, is often applied in studies of pain disorders in the upper extremities (32). The maximum score of the upper extremity scale is 66 points, divided among three components: shoulder–arm (36 points), wrist–hand (24 points), and coordination (6 points). The reliability and validity of the FMA-UE are excellent, and it is sufficiently sensitive for both clinical and research applications (33). The assessment will be performed in a standardized manner to differentiate the function levels of the fistula and non-fistula arms.
Handgrip strength will be measured with a digital handgrip dynamometer (TTM-YD, Tokyo, Japan). Two consecutive measurements of the fistula-side hand will be performed at an interval of 30 s, and the maximal isometric reading will be used for data analysis (34). Hand strength is used as a simple metric of general muscle strength for identifying functional deficits (35). It can also provide good resolution for localized pathologies and provides outcomes that allow better understanding of the therapeutic response of the impaired hand (36).
Pain perception will be recorded immediately after cannulation on a VAS, a non-graduated horizontal line of 100 mm where 0 = no pain at all and 100 = as painful as possible. The VAS and grip strength measures will be assessed immediately before the first treatment; at 4, 6, and 10 weeks after the first treatment; and at follow-up.
Juan Bi Tang is described in the ancient literature of TCM as relatively safe, and previous clinical studies have reported no major toxicity in therapeutic doses (37–39). However, we will still perform a series of measures to assess the safety of JBT throughout the entire trial due to possible herb-induced toxicity or herb-dialysis interaction (40). All safety-related variables, including vital signs, physical examination, hematological test, biochemical test, and AEs, will be recorded in the case report at every visit. Laboratory testing of the participants' blood, electrolytes (i.e., potassium, phosphorus, and calcium), albumin, parathyroid hormone, and kidney and liver function will be monitored before the intervention, at 2-week intervals during the intervention, and again at 2 weeks after trial completion. In addition, all details of AEs and other ailments will also be documented accurately at every study visit, including the occurrence time, severity, duration, effective measures, and transfer. Each AE associated with the intervention drugs will be classified as mild, moderate, or severe. If severe AEs such as hyperkalemia, thrombocytopenia, liver injury, bleeding, or cardiovascular events related to JBT occur during the study, the principal investigator will provide immediate diagnosis and treatment and report the AE to the Institutional Review Board within 24 h of the time of recognition. All AEs will be monitored and recorded by an independent researcher unassociated with the study until recovery of the participant.
A statistical power analysis by G*Power version 3.1.9.2 for the differences in the outcome measure between both groups was conducted to determine the investigated sample size to achieve adequate power (80%, α = 0.05). For repeated measures ANOVA analysis, to achieve 80% power and a 25% effect size (40), at least 34 participants will be needed. Considering a 15% loss to follow-up, we plan to recruit 40 participants (20 participants for each group) to compensate for possible dropouts.
Both intention-to-treat (ITT) and per-protocol (PP) analyses will be applied in the present trial. Continuous data will be presented as mean ± SD, and categorical data will be presented as frequencies and proportions. The Student's t-test for continuous variables and chi-squared test for categorical variables will be used to compare the characteristics and clinical data of patients with and without JBT treatment. Repeated measures ANOVA will be conducted to compare the subjects' conditions over time. The Mann–Whitney U test and generalized estimating equation will be applied to compare differences when the distribution of data does not meet the assumption of normality. All analyses will be performed in IBM SPSS Statistics 22 (IBM Co., Armonk, NY, USA). A p-value <0.05 will be considered statistically significant.
Repeated myofascial pain in the fistula arm during HD sessions is a distressing symptom reported by patients receiving HD. Due to a lack of reporting in the past, this type of pain related to the fistula arm is rarely treated sufficiently to meet the needs of these patients. Uncontrolled pain in the affected muscles can degrade QOL and cause non-adherence to the recommended dialysis regimen, which can in turn increase the mortality rate due to consequent cardiovascular and pulmonary events (41, 42). Juan Bi Tang therapy uses an ancient herbal medicine to address these painful experiences, especially the inadequate flow of qi and blood. In a previous study, compared to Western medicine, JBT was found to be safe and particularly beneficial to arthritis patients with the cold and deficiency pattern (37). Most patients who receive HD have low physical performance and low immunity (43), and researchers have found that a feature of the TCM pattern in patients receiving HD is mainly deficiency syndrome (44). The etiology of TCM pays attention to the circulation of qi and blood, and insufficient or blocked circulation will cause pain. Juan Bi Tang is used clinically to treat painful obstruction of qi and blood deficiency. Thus, we propose the first pilot crossover trial to examine the effectiveness of JBT therapy in alleviating dialysis-related myofascial pain around the fistula arm in patients receiving HD.
At present, the findings of most animal studies have confirmed the benefits of JBT in improving arthritis and synovitis (45–47). Significant reductions in the serum levels of MMP-2 and MMP-9 indicate that JBT has a role to play in preventing osteoarthritis (45). Similar effects of JBT on synovial inflammation and bone destruction through inhibiting the pro-inflammatory cytokines have recently been reported by Wang et al. (46). In addition, a study by Zhao et al. has demonstrated that JBT ameliorates bone destruction and reduces bone loss induced by rheumatoid arthritis (47). Therefore, it is possible that JBT may alleviate pain by not only improving blood flow but also down-regulating inflammatory markers. This study is designed to determine whether JBT is preferable to non-pharmacologic methods for the treatment of dialysis-related myofascial pain. If this trial produces the expected results, it will provide both patients and physicians an additional option for pain control. Moreover, this trial will provide preliminary data on the effects of JBT on arm power and motor function, QOL, and safety.
HD patients may receive 10–12 medications daily, and many of these medications require multiple doses each day. Drug–drug interactions are very common due to poly-pharmacy in HD patients (48), so the involvement of JBT therapy in drug interactions is an important issue for the therapeutic efficacy and safety of medical treatment. Although several studies have reported the possibility of herb–drug interactions in CKD patients leading to nephrotoxicity, electrolyte abnormalities, or changes in kidney hemodynamics (49, 50), very few studies have tested CHM with targeted safety assessments. It is worth noting that the Rx. Angelica Sinensis component of JBT may increase the risk of bleeding when heparin is used to prevent clotting in the dialytic extracorporeal circuit (51). In addition, excessive intake of Rx. Glycyrrhizae Preparata may lead to hypertension, hypokalemia, and metabolic alkalosis (52), which may cause myopathy and arrhythmia in HD patients. Thus, the results will contribute to herb repurposing for managing myofascial pain in patients receiving HD and will provide information useful for designing a large-scale randomized controlled trial in the future.
The strengths of this study include: (1) This study is the first pilot randomized cross-over trial to evaluate the feasibility of JBT for the treatment of dialysis-related myofascial pain. (2) The design of the trial is based on TCM syndromes because dialysis-relayed myofascial pain can be inferred to inadequate qi and blood around the fistula arm in patients receiving hemodialysis. (3) The study of different safe and lower cost can be considered for scientific use of techniques related to TCM in dialytic patients. Nevertheless, a few study limitations should be noted. First, this study will be an open-label study because of the difficulty of preparing a suitable placebo; the pharmaceutical technology of CHM powder does not yet allow such a process because of the distinctive colors, tastes and smells of the preparations (51). Moreover, due to the use of patient-reported outcomes, the reliability of the conclusions of the study may be reduced due to the absence of participant blinding. Second, it will be a crossover trial and thus may entail possible carryover effects. The component of JBT with the longest half-life is Rx. et Rz. Notopterygii (T1/2 = 190–768 min); however, its washout period is only 64 h (52). To minimize this possibility, we have designed a 2-week washout period between the two groups. However, no data on the length of the washout period of JBT are currently available. The washout period involves the biological half-lives of multiple herbs and changes in the pharmacokinetics of the CHM formula (53). In the event of carry-over effects, only data from the first period will be used for the analysis, for it is a randomized parallel-group design. The current sample size was calculated on the basis of a single period of data alone; thus, the estimated size will be sufficient for specific analysis. Third, the non-treatment and JBT treatment periods will be only 4 weeks.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Institutional Review Board at Chang Gung Memorial Hospital (approval No. 202000477A3). The patients/participants provided their written informed consent to participate in this study.
M-YT and Y-YL devised the study question and design. M-YT developed the idea into the full protocol and wrote the article draft. Y-HC and H-YN reviewed the protocol. Y-CH calculated the sample size and specified the statistical strategy. M-YT and Y-TH will be in charge of enrolling participants and conducting all the procedures. All authors have read and approved the final manuscript.
This study was funded by Chang Gung Memorial Hospital with grant number CORPG-8K0091. The funders have had no role in study design and will not have any role in the trial design, manuscript writing, or decision making for publication.
The authors would like to acknowledge all physicians and nurses in the Dialysis Team of Kaohsiung Chang Gung Memorial Hospital, who received the 2022 National Healthcare Quality Award, for their kind help in the recruitment and assessment of participants, and to thank Yuan's General Hospital and Dr. Yi-Ju Chen for the professional guidance on dialysis-related myofascial pain.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ach, acetylcholine; AE, adverse event; AV, arteriovenous; CGMH, Chang Gung Memorial Hospital; CHM, Chinese herbal medicine; CKD, chronic kidney disease; ESRD, end stage kidney disease; FMA-UE, Fugl-Meyer Assessment for upper extremity; GMP, Good Manufacturing Practices; JBT, Juan Bi Tang; KDQOL-36, Kidney Disease Quality of Life 36-Item Short-Form Survey; MPS, myofascial pain syndrome; MTrPs, myofascial trigger points; HD, hemodialysis; QOL, quality of life; TCM, traditional Chinese medicine; VAS, visual analog scale.
1. Shi W, Li Y, Xu D, Lin C, Lan J, Zhou Y, et al. Auxiliary diagnostic method for patellofemoral pain syndrome based on one-dimensional convolutional neural network. Front Public Health. (2021) 9:615597. doi: 10.3389/fpubh.2021.615597
2. Cummings M, Baldry P. Regional myofascial pain: diagnosis and management. Best Pract Res Clin Rheumatol. (2007) 21:367–87. doi: 10.1016/j.berh.2006.12.006
3. Lavelle ED, Lavelle W, Smith HS. Myofascial trigger points. Anesthesiol Clin. (2007) 25:841–51. doi: 10.1016/j.anclin.2007.07.003
4. Fernández-de-Las-Peñas C, Dommerholt J. International consensus on diagnostic criteria and clinical considerations of myofascial trigger points: a Delphi study. Pain Med. (2018) 19:142–50. doi: 10.1093/pm/pnx207
5. Rivers WE, Garrigues D, Graciosa J, Harden RN. Signs and symptoms of myofascial pain: an international survey of pain management providers and proposed preliminary set of diagnostic criteria. Pain Med. (2015) 16:1794–805. doi: 10.1111/pme.12780
6. Chen Q, Bensamoun S, Basford JR, Thompson JM, An KN. Identification and quantification of myofascial taut bands with magnetic resonance elastography. Arch Phys Med Rehabil. (2007) 88:1658–61. doi: 10.1016/j.apmr.2007.07.020
7. Fleckenstein J, Zaps D, Rüger LJ, Lehmeyer L, Freiberg F, Lang PM, et al. Discrepancy between prevalence and perceived effectiveness of treatment methods in myofascial pain syndrome: results of a cross-sectional, nationwide survey. BMC Musculoskelet Disord. (2010) 11:32. doi: 10.1186/1471-2474-11-32
8. Bourgaize S, Newton G, Kumbhare D, Srbely J. A comparison of the clinical manifestation and pathophysiology of myofascial pain syndrome and fibromyalgia: implications for differential diagnosis and management. J Can Chiropr Assoc. (2018) 62:26–41.
9. Gerwin RD. Myofascial trigger point pain syndromes. Semin Neurol. (2016) 36:469–73. doi: 10.1055/s-0036-1586262
10. Henriksson KG, Bäckman E, Henriksson C, De Laval JH. Chronic regional muscular pain in women with precise manipulation work. Scand J Rheumatol. (1996) 25:213–23. doi: 10.3109/03009749609069990
11. Bardin T. Musculoskeletal manifestations of chronic renal failure. Curr Opin Rheumatol. (2003) 15:48–54. doi: 10.1097/00002281-200301000-00009
12. Unalan H, Majlesi J, Aydin FY, Palamar D. Comparison of high-power pain threshold ultrasound therapy with local injection in the treatment of active myofascial trigger points of the upper trapezius muscle. Arch Phys Med Rehabil. (2011) 92:657–62. doi: 10.1016/j.apmr.2010.11.030
13. Fleishman TT, Dreiher J, Shvartzman P. Pain in maintenance hemodialysis patients: a multicenter study. J Pain Symptom Manage. (2018) 56:178–84. doi: 10.1016/j.jpainsymman.2018.05.008
14. Wyllie AH, Kerr JFR, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. (1980) 68:251–306. doi: 10.1016/S0074-7696(08)62312-8
15. Pal US, Kumar L, Mehta G, Singh N, Singh G, Singh M, et al. Trends in management of myofacial pain. Natl J Maxillofac Surg. (2014) 5:109–16. doi: 10.4103/0975-5950.154810
16. Hong C-Z, Simons DG. Pathophysiologic and electrophysiologic mechanisms of myofascial trigger points. Arch Phys Med Rehabil. (1998) 79:863–72. doi: 10.1016/S0003-9993(98)90371-9
17. Jafri MS. Mechanisms of myofascial pain. Int Sch Res Notices. (2014) 2014:523924. doi: 10.1155/2014/523924
18. Pham PC, Khaing K, Sievers TM, Pham PM, Miller JM, Pham SV, et al. 2017 update on pain management in patients with chronic kidney disease. Clin Kidney J. (2017) 10:688–97. doi: 10.1093/ckj/sfx080
19. Santoro D, Satta E, Messina S, Costantino G, Savica V, Bellinghieri G. Pain in end-stage renal disease: a frequent and neglected clinical problem. Clin Nephrol. (2013) 79(Suppl 1):S2–11. doi: 10.5414/CNX77S104
20. Hörl WH. Nonsteroidal anti-inflammatory drugs and the kidney. Pharmaceuticals. (2010) 3:2291–321. doi: 10.3390/ph3072291
21. Zheng C, Xu J, Chen C, Lin F, Shao R, Lin Z, et al. Effects of sleep disorders and sedative-hypnotic medications on health-related quality of life in dialysis patients. Int Urol Nephrol. (2019) 51:163–74. doi: 10.1007/s11255-018-2018-3
22. Mina D, Johansen KL, McCulloch CE, Steinman MA, Grimes BA, Ishida JH. Muscle relaxant use among hemodialysis patients: prevalence, clinical indications, and adverse outcomes. Am J Kidney Dis. (2019) 73:525–32. doi: 10.1053/j.ajkd.2018.11.008
23. Sarris J, Panossian A, Schweitzer I, Stough C, Scholey A. Herbal medicine for depression, anxiety and insomnia: a review of psychopharmacology and clinical evidence. Eur Neuropsychopharmacol. (2011) 21:841–60. doi: 10.1016/j.euroneuro.2011.04.002
24. Mist S, Wright C, Jones KD, Carson JW, Shih J. Traditional Chinese medicine for fibromyalgia. Pract Pain Manag. (2010) 10. Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4223771/
25. Lin MY, Chiu YW, Chang JS, Lin HL, Lee CT, Chiu GF, et al. Association of prescribed Chinese herbal medicine use with risk of end-stage renal disease in patients with chronic kidney disease. Kidney Int. (2015) 88:1365–73. doi: 10.1038/ki.2015.226
26. Zhang EQ. Bi syndrome (Arthralgia syndrome). J Trad Chin Med. (2010) 30:145–52. doi: 10.1016/S0254-6272(10)60032-5
27. Zhang GG, Lee WL, Lao L, Bausell B, Berman B, Handwerger B. The variability of tCM pattern diagnosis and herbal prescription on rheumatoid arthritis patients. Altern Ther Health Med. (2004) 10:58–63.
28. Kuan TS. Current studies on myofascial pain syndrome. Curr Pain Headache Rep. (2009) 13:365–9. doi: 10.1007/s11916-009-0059-0
29. Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the kidney disease quality of life (KDQOL) instrument. Quality Life Res. (1994) 3:329–38. doi: 10.1007/BF00451725
30. Lacson Jr E, Xu J, Lin SF, Dean SG, Lazarus JM, et al. A comparison of SF-36 and SF-12 composite scores and subsequent hospitalization and mortality risks in long-term dialysis patients. Clin J Am Soc Nephrol. (2010) 5:252–60. doi: 10.2215/CJN.07231009
31. Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. (1975) 7:13–31.
32. Kowalczewski J, Ravid E, Prochazka A. Fully-automated test of upper-extremity function. Annu Int Conf IEEE Eng Med Biol Soc. (2011) 2011:7332–5. doi: 10.1109/IEMBS.2011.6091710
33. Lin JH, Hsueh IP, Sheu CF, Hsieh CL. Psychometric properties of the sensory scale of the Fugl-Meyer assessment in stroke patients. Clin Rehabil. (2004) 18:391–7. doi: 10.1191/0269215504cr737oa
34. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8
35. Fukuda DH, Smith-Ryan AE, Kendall KL, Moon JR, Stout JR. Simplified method of clinical phenotyping for older men and women using established field-based measures. Exp Gerontol. (2013) 48:1479–88. doi: 10.1016/j.exger.2013.10.005
36. Hall AM, Copsey B, Williams M, Srikesavan C, Lamb SE. Mediating effect of changes in hand impairments on hand function in patients with rheumatoid arthritis: exploring the mechanisms of an effective exercise program. Arthritis Care Res. (2017) 69:982–8. doi: 10.1002/acr.23093
37. He Y, Lu A, Zha Y, Tsang I. Differential effect on symptoms treated with traditional Chinese medicine and western combination therapy in RA patients. Complement Ther Med. (2008) 16:206–11. doi: 10.1016/j.ctim.2007.08.005
38. Ding Y, J B. Curative study of using Juanbi decoction combined with acupuncture to treat lumbago. J Sichuan Tradit Chin Med. (2014) 12:139–41.
39. Zhao J, Zha Q, Jiang M, Cao H, Lu A. Expert consensus on the treatment of rheumatoid arthritis with Chinese patent medicines. J Altern Complement Med. (2013) 19:111–8. doi: 10.1089/acm.2011.0370
40. Zhong Y, Deng Y, Chen Y, Chuang PY, Cijiang He J. Therapeutic use of traditional Chinese herbal medications for chronic kidney diseases. Kidney Int. (2013) 84:1108–18. doi: 10.1038/ki.2013.276
41. Claxton RN, Blackhall L, Weisbord SD, Holley JL. Undertreatment of symptoms in patients on maintenance hemodialysis. J Pain Symptom Manage. (2010) 39:211–8. doi: 10.1016/j.jpainsymman.2009.07.003
42. Madeiro AC, Machado PDLC, Bonfim IM, Braqueais AR, Fet L. Adherence of chronic renal insufficiency patients to hemodialysis. Acta Paul Enferm. (2010) 23:546–51. doi: 10.1590/S0103-21002010000400016
43. Tsai MY, Wu CH, Huang YC, Chen SY, Ng HY, Su YJ, et al. Treatment of intradialytic hypotension with an herbal acupoint therapy in hemodialysis patients: a randomized pilot study. Complement Ther Med. (2018) 38:67–73. doi: 10.1016/j.ctim.2018.04.007
44. Chen CS, Chen GW, Lin JK, Peng WH, MC X. Studies on the classical differential diagnosis of traditional Chinese medicine and biochemical parameters in hemodialysis patients. J Chin Med. (2007) 18:169–78.
45. Yuan PW, Yu HC, Zhou HZ, Zhu C, Qu Q, Liu DY. Preventive administration of Juanbi capsules for knee osteoarthritis: effects on serum MMP-2 and MMP-9 levels and cartilage repair. J Tradit Chin Med. (2011) 31:334–7. doi: 10.3109/9781841848433
46. Wang T, Jia Q, Chen T, Yin H, Tian X, Lin X, et al. Alleviation of synovial inflammation of Juanbi-Tang on collagen-induced arthritis and TNF-Tg mice model. Front Pharmacol. (2020) 11:45. doi: 10.3389/fphar.2020.00045
47. Zhao H, Xu H, Zuo Z, Wang G, Liu M, Guo M, et al. Yi Shen Juan Bi pill ameliorates bone loss and destruction induced by arthritis through modulating the balance of cytokines released by different subpopulations of T cells. Front Pharmacol. (2018) 9:262. doi: 10.3389/fphar.2018.00262
48. Al-Ramahi R, Raddad AR, Rashed AO, Bsharat A, Abu-Ghazaleh D, Yasin E, et al. Evaluation of potential drug-drug interactions among Palestinian hemodialysis patients. BMC Nephrol. (2016) 17:96. doi: 10.1186/s12882-016-0317-4
49. Dahl NV. Herbs and supplements in dialysis patients: panacea or poison? Semin Dial. (2001) 14:186–92. doi: 10.1046/j.1525-139X.2001.00051.x
50. Fasinu PS, Bouic PJ, Rosenkranz B. An overview of the evidence and mechanisms of herb-drug interactions. Front Pharmacol. (2012) 3:69. doi: 10.3389/fphar.2012.00069
51. Dube A, Manthata LN, Syce JA. The design and evaluation of placebo material for crude herbals: artemisia afra herb as a model. Phytother Res. (2007) 21:448–51. doi: 10.1002/ptr.2084
52. Teye Azietaku J, Yu XA, Li J, Hao J, Cao J, An M, et al. Simultaneous determination of bergapten, imperatorin, notopterol, and isoimperatorin in rat plasma by high performance liquid chromatography with fluorescence detection and its application to pharmacokinetic and excretion study after oral administration of notopterygium incisum extract. Int J Anal Chem. (2016) 2016:9507246. doi: 10.1155/2016/9507246
Keywords: hemodialysis, Chinese herbal medicine, randomized trial, myofascial pain, study protocol
Citation: Hsu Y-T, Ng H-Y, Chen Y-H, Huang Y-C, Lee Y-Y and Tsai M-Y (2022) Assessing the efficacy and safety of Juan Bi Tang for dialysis-related myofascial pain in the fistula arm: Study protocol for a randomized cross-over trial. Front. Public Health 10:925232. doi: 10.3389/fpubh.2022.925232
Received: 21 April 2022; Accepted: 04 August 2022;
Published: 19 August 2022.
Edited by:
Ke Han, Harbin University of Commerce, ChinaReviewed by:
Xue Xue, Hubei University of Chinese Medicine, ChinaCopyright © 2022 Hsu, Ng, Chen, Huang, Lee and Tsai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming-Yen Tsai, bWlzc3VyaWFlQHlhaG9vLmNvbS50dw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.