- 1Department of Health Policy, Health Management College, Harbin Medical University, Harbin, China
- 2Department of Social Medicine, School of Public Health, Harbin Medical University, Harbin, China
- 3Department of Pharmacy Administration, Humanities and Social Science College, Harbin Medical University, Harbin, China
- 4Department of Epidemiology and Public Health, University College London, London, United Kingdom
- 5Science and Technology Development Center, Chinese Pharmaceutical Association, Beijing, China
- 6Department of Medical Procurement, The Fourth Affiliated Hospital, Harbin Medical University, Harbin, China
Objective: Since 2016, the Chinese government has been regularly implementing the National Reimbursement Drug List Negotiation (NRDLN) to improve the accessibility of drugs. In the second round of NRDLN in July 2017, 18 anticancer drugs were included. This study analyzed the impact of the NRDLN on the accessibility of these 18 anticancer drugs in China.
Methods: National hospital procurement data were collected from 2015 to 2019. As measurements of drug accessibility, monthly average of drug availability or defined daily dose cost (DDDc) was calculated. Interrupted time series (ITS) analysis was employed to evaluate the impact of NRDLN on drug accessibility. Multilevel growth curve models were estimated for different drug categories, regions or levels of hospitals.
Results: The overall availability of 18 anticancer drugs increased from about 10.5% in 2015 to slightly over 30% in 2019. The average DDDc dropped from 527.93 CNY in 2015 to 401.87 CNY in 2019, with a reduction of 23.88%. The implementation of NRDLN was associated with higher availability and lower costs for all 18 anticancer drugs. We found an increasing level in monthly drug availability (β2 = 2.1126), which ascended more sharply after the implementation of NRDLN (β3 = 0.3656). There was a decreasing level in DDDc before July 2017 (β2 = −108.7213), together with a significant decline in the slope associated with the implementation of NRDLN (β3 = −4.8332). Compared to Traditional Chinese Medicines, the availability of Western Medicines was higher and increased at a higher rate (β3 = 0.4165 vs. 0.1108). Drug availability experienced a larger instant and slope increase in western China compared to other regions, and in secondary hospitals than tertiary hospitals. Nevertheless, regional and hospital-level difference in the effect of NRDLN on DDDc were less evident.
Conclusion: The implementation of NRDLN improves the availability and reduces the cost of some anticancer drugs in China. It contributes to promoting accessibility of anticancer drugs, as well as relieving regional or hospital-level disparities. However, there are still challenges to benefit more patients sufficiently and equally. It requires more policy efforts and collaborative policy combination.
Introduction
Cancer is one of the leading causes of death and disability worldwide (1, 2). In China, as reported by National Central Cancer Registry, there were ~4.06 million new cancer cases and 2.41 million cancer deaths in 2016 (3). Meanwhile, anticancer drug costs have imposed heavy burdens on patients and their families, as well as China's economy and healthcare system. In 2018, global expenditure on anticancer drugs was as high as $6.3 billion USD, accounting for around 70% of total expenditure on cancer treatment (4).
In order to promote equitable access to cancer care and ensure its affordability, the international society and governments are taking actions to ensure adequate supplies and access to safe, affordable, and effective quality anticancer drugs (1, 5). For example, the American Society of Clinical Oncology (ASCO) assessed a variety of cost-cutting proposals, such as allowing Medicare to negotiate the prices of anticancer drugs, legalizing anticancer drug importation, and bundled payment programs (6).
However, according to the 2018 Access to Medicine Index, anticancer drug accessibility was low in developing countries (7). Major challenges remain in increasing population's access to anticancer drugs, especially in resource-poor settings and low- and middle-income countries, mainly due to lack of government reimbursements, insufficient budget allocations for healthcare, the unavailability of quality-assured generic and biosimilar medicines, shortages, and so on (1, 7, 8).
In China, the accessibility of innovative anticancer drugs is dim. Only 6 of 49 innovative anticancer drugs launched across the world between 2010 and 2014 are available in China. In terms of global accessibility to innovative anticancer drugs, China ranks far lower than developed countries, and is also behind India (9). In this regard, Chinese government has committed a series of policies and practices in recent years to increase the accessibility of anticancer drugs, such as tariff exemptions on imported anticancer drugs, centralizing the government procurement of anticancer drugs, and the long-term strategy of controlling anticancer drug pricing (10).
Incorporating more anticancer drugs into the National Reimbursement Drug List Negotiation (NRDLN) is one of China's recent significant efforts to reduce drug price, and improve its accessibility and affordability. The NRDLN policy was initiated through a collaboration between the National Healthcare Security Administration (NHSA) and pharmaceutical companies in 2016. Whether one drug enters national procurement list is determined through a centralized strategic price negotiation when candidate drugs are assessed comprehensively in terms of safety, efficacy, clinical need, reference price and comparative value (11). After the negotiation, the drug procurement price is largely reduced and based on further economic assessment, some of them enter the national reimbursement drug list (12). Along with the procurement price, payment standards, reimbursement level of listed drugs and detailed reimbursement restrictions (such as indications, treatment duration, number of doses, etc.) are determined by NRDLN as well (13). Following the NRDLN, public hospitals must purchase listed drugs via provincial procurement websites based on the negotiated prices, and then the reimbursement is paid by national medical insurance fund (14). In July 2017, 36 drugs were added to the list, 18 of which were anticancer drugs for major cancers, such as lung cancer, rectum cancer and leukemia (15).
In some recent studies, as measured by availability and cost, changes in the accessibility of anticancer drugs are investigated (16–24). In regard to the NRDLN, evidence has been provided on the changes in cost, availability, affordability, and clinical uses of listed drugs in specific regions and provinces of China (25–29). However, there is still a lack of longer-term nationwide empirical evidence on the impact of the implementation of NRDLN on the accessibility of anticancer drugs, nor is there a comprehensive or stratified picture of the current national situation in China.
In this study, we used national hospital procurement data over a 5-year period to explore the accessibility of 18 anticancer drugs, which were added to the national reimbursement drug list in 2017. Therein, we conducted an interrupted time series (ITS) analysis and employed a multilevel growth curve model to quantify changes in availability and cost associated with the NRDLN. It was hypothesized that the NRDLN would increase the availability and reduce costs of anticancer drugs.
Materials and Methods
Data Source
We collected data from the Chinese Medicine Economic Information Network (CMEI), which was established and still managed by the Science and Technology Development Center of Chinese Pharmaceutical Association. The CMEI is one of the largest medical-economic and drug information service platforms, covering over 1,500 public hospitals in China (30). It provides hospital information and monthly drug procurement data for each hospital, including the drug category, generic name, code, dose, brand-name producer, indications, procurement volume, and cost (procurement price). However, we only have access to the average of national drug availability and cost in each month, which provides us with time series data.
Sample and Data Collection
We focused on 18 anticancer drugs incorporated into the national reimbursement drug list in July 2017, including 15 Western Medicines and 3 Traditional Chinese Medicines. Detailed information of these 18 anticancer drugs is listed in Table 1.
In data collection, first, we identified public hospitals with continuous monthly records of the 18 anticancer drugs from January 2015 to December 2019, which left us with 887 hospitals located in 10 eastern provinces, 6 central provinces, 12 western provinces and 3 north-eastern provinces. Of the 887 total hospitals, 241 (21.17%) were secondary, while 646 (72.83%) were tertiary. Then, we collected monthly average of drug availability and cost from January 2015 to December 2019 for the bundle of 18 drugs, for each drug, and for Western Medicines and Traditional Chinese Medicines separately. At last, we were able to collect yearly average of availability and cost of each drug from 2015 to 2019 for tertiary and secondary hospitals separately, and for different regions separately.
Measurements
In line with the WHO/HAI methodology and previous research (31, 32), we measured the accessibility of all 18 anticancer drugs in terms of availability and cost.
Availability
For each drug i, we defined availability as the proportion of hospitals in which it was available. This was calculated as the percentage of hospitals where drug could be found in our sample, as follows (33).
Cost
We used the defined daily dose cost (DDDc) to reflect the cost and financial burden of each drug i. DDDc is the average daily cost of the drug used by the patients, which is calculated based on the defined daily dose system (DDDs). DDDs is the drug consumption volume divided by the defined daily dose (DDD), as recommended by the WHO, clinical application guidelines, and drug package inserts approved by the National Medical Products Administration of China (NMPA). It is calculated as follows (34).
Statistical Analysis
Interrupted Time Series
The ITS employs a quasi-experimental design to evaluate the longitudinal effects of time-delimited interventions. In statistical terms, a segmented regression analysis of interrupted time can be used to assess the extent to which an intervention has changed an outcome of interest, either transiently or in the long-term context (35). We used the ITS to analyze the impact of NRDLN on the availability and cost of 18 anticancer drugs overall and separately, as well as Western Medicines and Traditional Chinese Medicines separately. We regarded July 2017 as the fixed intervention time point. The ITS regression model with one intervention is typically formulated as follows:
Where Yt is the independent outcome variable (monthly average availability and DDDc in month t), timet refers to a continuous variable indicating the number of months at time t from the beginning of the observation period (January 2015–June 2017), NRDLNt is a dummy variable for time t occurring either before (NRDLNt = 0) or after (NRDLNt = 1) NRDLN implementation in July 2017, time after NRDLNt is a continuous variable indicating the number of months at time t after NRDLN implementation (July 2017–December 2019), which was set at 0 before July 2017. β0estimates the level of the outcome at the beginning of the observation period, β1 estimates the linear trend during the pre-intervention period, and β3 estimates the change in trend in the outcome after NRDLN implementation compared with baseline. εt is error term at time t (36).
Two robustness checks were conducted including the Augmented Dickey-Fuller test and controlled ITS. The Augmented Dickey-Fuller test was to test the stationary of time series (35, 37). Controlled ITS was to control for potential seasonal fluctuations in series (38). Monthly dummy variables were usually included to avoid spurious associations (39, 40).
Multilevel Growth Curve Model
With yearly data stratified by region or hospital level, we employed a standard two-level growth curve model to estimate changes in the average level and growth rate of availability and cost. Despite the same linear growth assumption, different parameters of growth curves are allowed to different drugs in multilevel modeling (41). The specification is as follows:
Where Yit is the yearly average availability and DDDc of drug i in year t, yearit is centered to 2017, and both NRDLNit and year after NRDLNit for drug i are the same as above. We fit different intercepts and slopes for each drug, thus allowing for variation between and within drugs. The intercept and slope equations are as follows:
Statistical Analysis Software
We conducted the ITS analysis in SAS V 9.4 and multilevel growth curve models in StataSE V 17.0, with statistical significance determined at P < 0.05.
Results
Descriptive Statistics
Monthly Availability and Cost
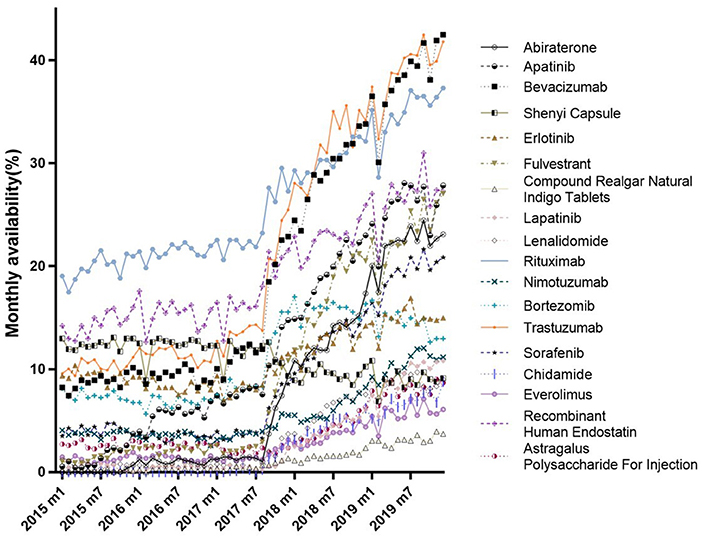
Figure 1 shows trends in the monthly availability of each 18 anticancer drug from January 2015 to December 2019. There was a fluctuating uptrend, with the exception of the Shenyi Capsule. There were obvious changes in the availabilities of most drugs between July and September 2017; for example, see Abiraterone, Trastuzumab, and Bevacizumab. While the Compound Realgar Natural Indigo Tablets, Everolimus, Astragalus Polysaccharides for Injection, and Chidamide had lower availabilities, those for Bevacizumab, Trastuzumab, and Rituximab were as high as 42.50, 41.83, and 37.32%, respectively, but none were higher than 45% until December 2019 (Supplementary Table 1).
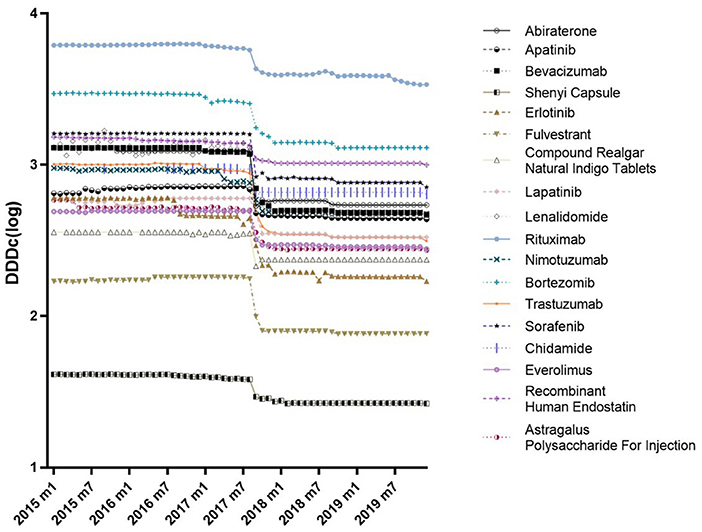
Figure 2 shows trends in the monthly DDDc for each 18 anticancer drugs from January 2015 to December 2019. The DDDc of Rituximab was the highest, while that for the Shenyi Capsule was the lowest. For each drug, there was a consistent downtrend in DDDc between July and September 2017. All the reductions in DDDc from January 2015 to December 2019 were above 32% (Supplementary Table 2). Eight drugs had a drop more than 50% in DDDc, while four drugs dropped more than 60% (i.e., Erlotinib, Trastuzumab, Bevacizumab, and Lenalidomide).
As suggested by Augmented Dickey-Fuller test results, our time series data of monthly availability and DDDc were stationary.
Yearly Availability and Cost
Based on the descriptive results in Table 2, overall availability was increasing from 2015 to 2019. In 2019, 30.47% of the surveyed hospitals could provide all 18 drugs, compared to 10.49% in 2015. The availabilities of surveyed drugs in Eastern, Central, Western, and Northeastern China had, respectively increased to 37.65, 26.96, 26.09, and 21.46% by the end of 2019. Compared with 14.59% in secondary hospitals, the availability of 18 drugs was 36.39% in tertiary hospitals. In 2019, the 15 Western Medicines (33.86%) were more widely available than the 3 Traditional Chinese Medicines (13.53%).
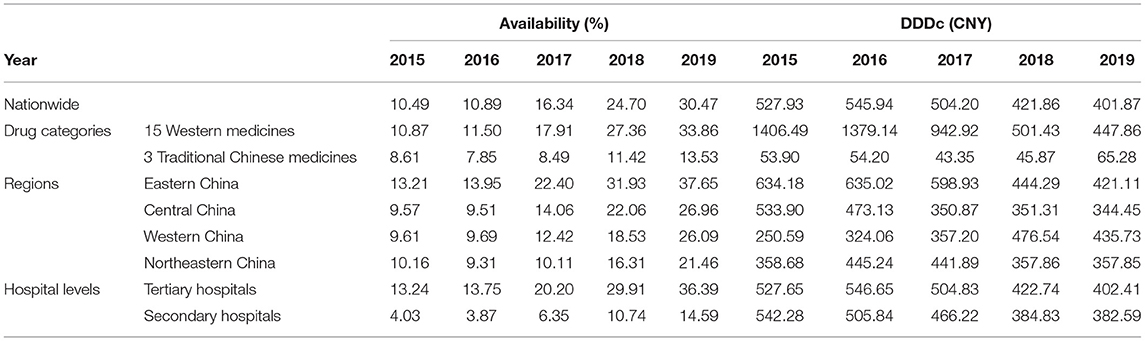
Table 2. Descriptive results of both overall yearly availability and DDDc in the nationwide, regional, hospital-level, and drug category contexts for each observed year (2015–2019).
For all 18 anticancer drugs, nationwide DDDc gradually decreased from 527.93 CNY in 2015 to 401.87 CNY in 2019, with a reduction of 23.88%. The DDDc decreased in Eastern and Central China, whilst increased in Western China. Meanwhile, DDDc was essentially flat in Northeastern China. As for tertiary and secondary hospitals, DDDc was 402.41 CNY and 382.59 CNY in 2019, respectively. Finally, the 15 Western Medicines had much higher DDDc (447.86 CNY) than the 3 Traditional Chinese Medicines (65.28 CNY).
Main Results
Changes in Availability
Table 3 shows the results of the ITS analysis for monthly availability and DDDc. There was a slight increase in the availability of all 18 anticancer drugs prior to the intervention (β1 = 0.0368, P < 0.05). After NRDLN, there was a significant increase in both the intercept (β2 = 2.1126, P < 0.001) and slope (β3 = 0.3656, P < 0.001). In other words, the increasing availability trend accelerated after NRDLN implementation, reaching nearly 10 times higher than baseline. As shown in Figure 3A, there was also an interruption point in July 2017; that is, there was an instant increase in the availability of all 18 anticancer drugs, followed by a continually increasing trend thereafter. It suggested that the NRDLN had a substantial impact on the availability of anticancer drugs.
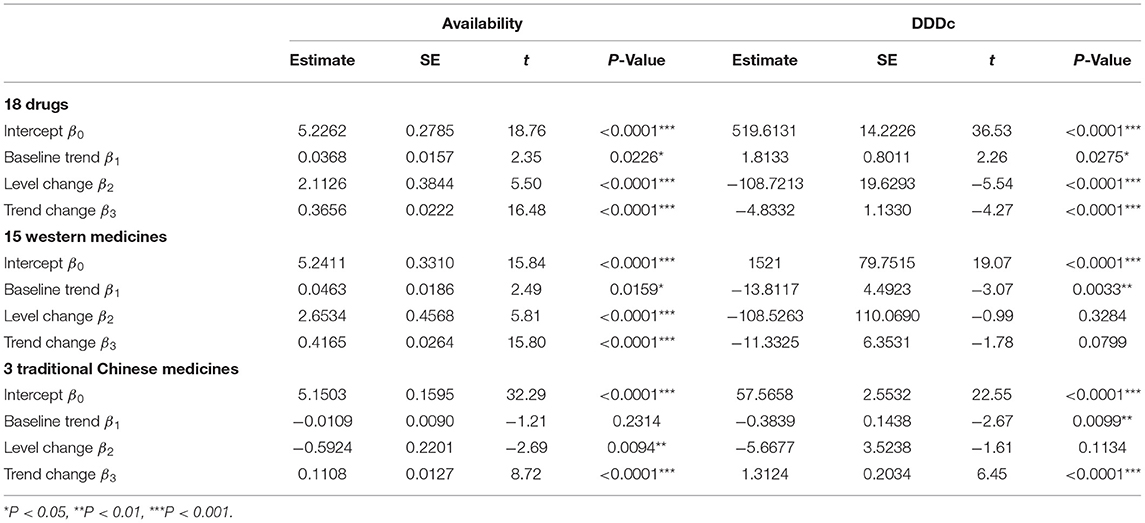
Table 3. ITS results of the impacts of NRDLN on monthly availability and DDDc for all 18 drugs, with a breakdown for the 15 Western Medicines and 3 Traditional Chinese Medicines (2015–2019).
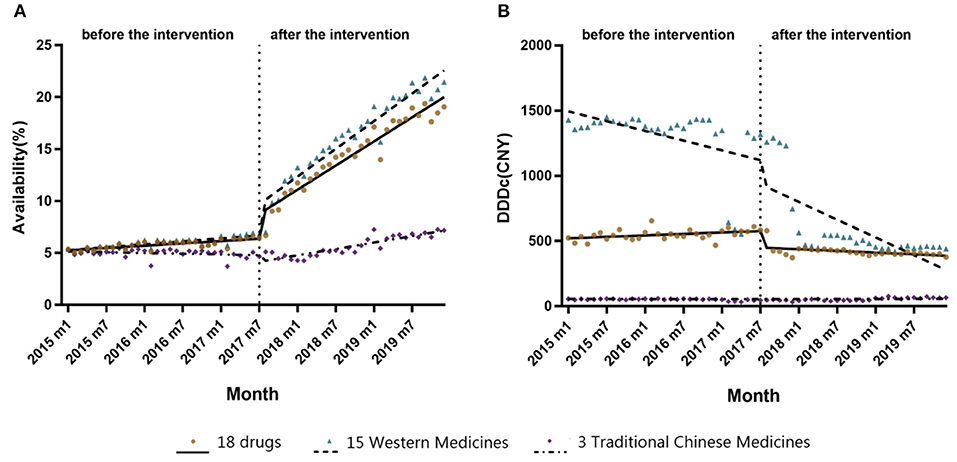
Figure 3. (A) Changes in monthly availability (%) of the 15 Western Medicines, 3 Traditional Chinese Medicines, and all 18 drugs combined both before and after NRDLN implementation in July 2017. (B) Changes in monthly DDDc (CNY) of the 15 Western Medicines, 3 Traditional Chinese Medicines, and all 18 drugs combined both before and after NRDLN implementation in July 2017.
Changes in Cost
In Table 3 and Figure 3B, we observed a statistically significant interruption point in the time series of DDDc in July 2017. There was a slow rise in DDDc for all 18 anticancer drugs before July 2017 (β1 = 1.8133, P < 0.05). At the time of policy implementation, there was a significant and substantial decrease in the DDDc (β2 = −108.7213, P < 0.001). After that, there was a significant decline in the slope (β3 = −4.8332, P < 0.001). The implementation of NRDLN catalyzed a downward trend in the cost of all 18 anticancer drugs.
Our results are robust using controlled ITS (Supplementary Table 3).
Stratification Analyses Results
Different Drug Categories
As shown in Table 3, there were significant increases in the intercept (β2 = 2.6534, P < 0.001) and regression slope (β3 = 0.4165, P < 0.001) pertaining to the availability of the 15 Western Medicines after NRDLN implementation. As shown in Figure 3A, there was a clear and immediate rise at the time of July 2017, followed by a steady and significant monthly increase. Meanwhile, there was a significant decrease in the intercept (β2 = −0.5924, P < 0.01), as well as a significant increase in the slope (β3 = 0.1108, P < 0.001) pertaining to the availability of the 3 Traditional Chinese Medicines. In addition, no significant instant decrease in DDDc was observed. A significant increase in slope was only reported in the DDDc of Traditional Chinese Medicines (β3 = 1.3124, P < 0.001).
Different Regions
As shown in Table 4, prior to 2017, there was a significant rise in yearly availability in Eastern China (λ1 = 0.228, P < 0.05). After 2017, there were significant increases in the intercepts of yearly availability for Eastern, Central, and Western China, as well as in the slopes for Central, Western, and Northeastern China. Western China experienced the highest increases in both yearly availability (λ2 = 0.723, P < 0.01) and upward trend (λ3 = 0.571, P < 0.001). Meanwhile, there was a significant decrease in yearly DDDc in terms of intercepts and slopes for all regions. The greatest decrease in yearly DDDc was found in Central China (λ2 = −0.436, P < 0.001), while the sharpest downward trend was found in Northeastern China (λ3 = −0.220, P < 0.001).
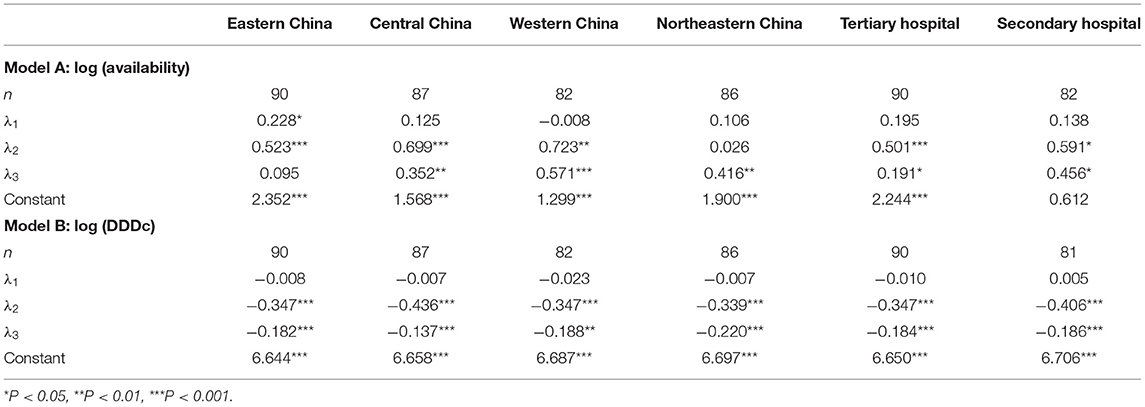
Table 4. Growth curve model estimates of yearly availability and DDDc stratified by region and hospital level (2015–2019).
Different Hospital Levels
In Table 4, we observed an insignificant time trend in both yearly availability and DDDc in the tertiary and secondary hospitals prior to 2017. However, there was a significant increase in yearly availability and significant decrease in yearly DDDc in all hospital after 2017. The tertiary hospitals had a smaller increase in the slope of yearly availability (λ3 = 0.191, P < 0.05) compared to that of secondary hospitals (λ3 = 0.456, P < 0.05). In addition, secondary hospitals had a slightly larger instant reduction in yearly DDDc (λ2 = −0.406, P < 0.001) than that of tertiary hospitals (λ2 = −0.347, P < 0.001).
Discussion
This study investigated the impact of NRDLN policy on the availability and cost of 18 anticancer drugs based on procurement data from a nationally representative sample of hospitals. As expected, the NRDLN increased the availability and decreased the cost of anticancer drugs. This finding was similar to some recent studies on specific regions of China (25–27, 42, 43). It is indicated that the implementation of NRDLN exerts a strong positive effect on the accessibility of anticancer drugs in China. This effect has not only short-term but also long-term significances.
Impact of NRDLN Policy on Availability
As for availability, our results showed an instant increase after NRDLN implementation, followed by an even sharper rise. Regardless of the drug category, hospital level, and region, a greater number of hospitals were able to provide the investigated anticancer drugs after NRDLN policy, thus entailing greater access for patients.
Despite the increased availability of most anticancer drugs, the overall availability of most anticancer drugs in China remained low (<30%). The highest availability level was less than 50% by the end of 2019, which is unsatisfactory (44). This reinforces reports from similar studies (22, 29). There are many possible reasons for this. For example, anticancer drug costs are higher than other drugs; meanwhile, there are problems with the total pre-payment of medical insurance and insufficient procurement incentives for hospitals, which can weaken policy effects (45, 46). This situation can be improved through the implementation of additional supportive measures and coordination with other policies.
We observed some variations in drug availability. First, drugs for cancers with higher incidence rates in China (NSCLC, colorectal cancer, breast cancer, gastric cancer, and NHL) were generally of higher availability. This is consistent with some previous research wherein cancer prevalence affects the availability of anticancer drugs (28). Second, drug availability was also impacted by whether and when these drugs received NMPA market approval. For example, if there was a long market approval time before market launch, drug availability tended to be lower, which was the case for Chidamide. Lastly, anticancer drugs with a larger cost decrease were more available. For instance, in our study, Bevacizumab and Trastuzumab had a decrease of more than 60% in cost, along with an increase of 416.40 and 336.50% in availability, respectively by 2019.
In addition, it was found that the availability of 15 Western Medicines increased at a higher rate than 3 Traditional Chinese Medicines. The Traditional Chinese Medicines experienced a short-term drop in availability, but a long-term increase after NRDLN implementation. Although Traditional Chinese anticancer medicines are mainly intended for anticancer and adjuvant indication applications, they are added to the national reimbursement drug list as a powerful means of cancer therapy. However, most studies have ignored the utilization data on traditional Chinese anticancer medicines, with some remaining unprovided at a number of hospitals (28).
Furthermore, we found that availability was the highest in Eastern China, followed by Central and Western China, and lowest in Northeastern China after NRDLN implementation. These regional differences in availability are consistent with previous research on essential drugs (47), which probably leads to inequity in drug accessibility. We also found varied increases in availability from 2015 to 2019 in different regions. The highest annual increase was found in Western China, which might be due to low initial availability, or different regional policy responses. Moreover, there were similar results for hospital level. Compared to tertiary hospitals, availability was lower in secondary hospitals, whereas the slope increase was higher in secondary hospitals. Overall, these findings suggest that regional and hospital-level disparities in the availability of anticancer drugs have gradually decreased by NRDLN implementation.
Impact of NRDLN Policy on Cost
As for cost, we found an instant decrease after NRDLN implementation, followed by a continuous downward trend, both of which were significant. There was a significant decline of 108.7213 CNY in DDDc in July 2017, which was equivalent to an average monthly cost reduction of 3261.60 CNY for patients. The increasing trend of drug cost was flipped over by the implementation of NRDLN. After July 2017, DDDc fell at a faster rate, equal to a monthly 145.00 CNY downward trend in anticancer drug costs, thus decreasing expenditures for patients. This is in line with some recent studies reporting that the NRDLN has helped to lower the prices of anticancer drugs, and is the direct reason for the significant reduction in average daily costs. DDDc also represented a comparable price of anticancer drugs (25, 27, 43, 48).
It should be noted that DDDc of anticancer drugs was decreasing from 2017 to 2019, same as the national trend of all drugs in China (44). The DDDc of 18 anticancer drugs in our study was over twice higher than that of all the anticancer drugs, or around 30 times higher than that of all drugs in 2019. This might be due to changes in both the variety and structure of anticancer drugs as well as the relatively high prices and expenses of innovative anticancer drugs covered by the NRDLN.
Our results showed significantly decreased DDDc associated with NRDLN in all regions, as well as in the tertiary and secondary hospitals. Contrary to the results on availability, changes in DDDc did not have significant regional or hospital-level disparities, which could be seen as a piece of evidence that the negotiated price of anticancer drugs has been well and equally adopted after NRDLN.
Suggestions
Based on our findings, the NRDLN is an effective way to promote patients' access to anticancer drugs. However, there is still a gap between current conditions and ultimate expectations for improving the accessibility to anticancer drugs. More efforts are needed to further enhance the effects of the NRDLN.
First, there should be shorter market approval duration for internationally listed innovative anticancer drugs in China (1). This requires a quicker market approval process for generic and biosimilar anticancer drugs with high effectiveness through the NRDLN. Second, unequal accessibility in different drug categories, regions, and hospitals should be addressed by implementing preferential policies, ensuring rational budget allocations, monitoring and standardizing the anticancer drug prescription structure, and objective policy evaluations in China. Lastly, there should be stronger supportive measures and enhanced coordination with other policies, such as sufficient incentives for hospitals to purchase and prescribe NRDLN drugs, coordination among multilevel medical security systems, value-based pricing and payments, and a dual channel for NRDLN drugs (49).
Limitations
This study also had some limitations. First, the CMEI database provides information on the clinical use of anticancer drugs in Chinese hospitals. Although closely related, this is not a direct substitute for information on anticancer drug prescriptions at hospitals or drug usage among individual patients. Second, we did not consider data from private hospitals or pharmacies. Third, we quantified changes in 18 anticancer drugs affected by the NRDLN, which did not represent other anticancer drugs or other NRDLN drugs. Finally, we used availability and daily costs to evaluate accessibility. Further research is needed in other aspects such as whether patients can afford anticancer drugs.
Conclusion
The NRDLN constitutes a national determination and effort to enhance population health in China. This study provides evidence that the implementation of NRDLN improves the availability and reduces the cost of some anticancer drugs in China. It contributes to promoting accessibility of anticancer drugs, as well as relieving regional or hospital-level disparities. However, there are still challenges to benefit more patients sufficiently and equally. It requires more policy efforts and collaborative policy combination.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
HZ, JZ, and QW conceptualized and undertook the analyses, and wrote the original draft of the manuscript. HZ, LS, and QW designed the study. YZ provided the data. YZ, JZ, LS, and CL contributed to the analysis method. QW and YH critically reviewed and modified the initial and subsequent drafts. All authors have read and approved the final manuscript for publication.
Funding
This study was funded by National Social Science Foundation of China (No.19AZD013), Natural Science Foundation of Heilongjiang Province, China (No.YQ2019G003), and Humanities and Social Science College Program of Harbin Medical University (No.HMURW20210101). This study was also funded by a co-operative project between Health Management College, Harbin Medical University and Science and Technology Development Center of the Chinese Pharmaceutical Association.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank CMEI for providing the data and related information on the hospital purchase data. We would like to thank Ran Wang and Yan Liu for helping with extracting the data from CMEI. We would like to thank Editage (www.editage.cn) for English language editing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.921093/full#supplementary-material
References
1. Eniu A, Cherny NI, Bertram M, Thongprasert S, Douillard JY, Bricalli G, et al. Cancer medicines in Asia and Asia-Pacific: what is available, and is it effective enough? ESMO Open. (2019) 4:e000483. doi: 10.1136/esmoopen-2018-000483
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
3. Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Center. (2022) 2:1–9. doi: 10.1016/j.jncc.2022.02.002
4. The IQVIA Institute. Global Oncology Trends 2019 (2019). Available online at: https://www.iqvia.com/insights/the-iqvia-institute/reports/global-oncology-trends-2019 (accessed March 10, 2020).
5. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. (2018) 391:1023–75. doi: 10.1016/S0140-6736(17)33326-3
6. American Society of Clinical Oncology. American society of clinical oncology position statement on addressing the affordability of cancer drugs. J Oncol Pract. (2018) 14:187–92. doi: 10.1200/JOP.2017.027359
7. The Access to Medicine Foundation. 2018 Access to Medicine Index (2018). Available online at: https://www.accesstomedicineindex.org/publications/2018-access-to-medicine-index (accessed June 21, 2019).
8. Cortes J, Perez-García JM, Llombart-Cussac A, Curigliano G, El Saghir NS, Cardoso F, et al. Enhancing global access to cancer medicines. CA Cancer J Clin. (2020) 70:105–24. doi: 10.3322/caac.21597
9. IMS Institute,. Global Oncology Trend Report: A Review of 2015 Outlook to 2020 (2016). Available online at: https://www.theimsinstitute.org/ (accessed September 22, 2018).
10. The Lancet. Cancer drugs in China: affordability and creativity. Lancet. (2018) 391:1866. doi: 10.1016/S0140-6736(18)31034-1
11. Si L, Xu L, Chen M, Jan S. Using strategic price negotiations to contain costs and expand access to medicines in China. BMJ Glob Health. (2020) 5:e002256. doi: 10.1136/bmjgh-2019-002256
12. Fang W, Xu X, Zhu Y, Dai H, Shang L, Li X. Impact of the national health insurance coverage policy on the utilisation and accessibility of innovative anti-cancer medicines in China: an interrupted time-series study. Front Public Health. (2021) 9:714127. doi: 10.3389/fpubh.2021.714127
13. Guan X, Zhang Y, Wushouer H, Shi L, Ross-Degnan D, Wagner AK. Differences in reimbursement listing of anticancer therapies in China: an observational study. BMJ Open. (2020) 10:e031203. doi: 10.1136/bmjopen-2019-031203
14. Zhang Y, Wushouer H, Han S, Fu M, Guan X, Shi L, et al. The impacts of government reimbursement negotiation on targeted anticancer medication price, volume and spending in China. BMJ Glob Health. (2021) 6:e006196. doi: 10.1136/bmjgh-2021-006196
15. Ministry of Human Resources Social Security. Releases Results of Negotiations on National Reimbursement Drug List Entry (2017). Available online at: http://www.mohrss.gov.cn/SYrlzyhshbzb/dongtaixinwen/buneiyaowen/201707/t20170719_274189.html (accessed December 31, 2021).
16. Kolasani BP, Malathi DC, Ponnaluri RR. Variation of cost among anti-cancer drugs available in Indian market. J Clin Diagn Res. (2016) 10:FC17–20. doi: 10.7860/JCDR/2016/22384.8918
17. Karikios DJ, Schofield D, Salkeld G, Mann KP, Trotman J, Stockler MR. Rising cost of anticancer drugs in Australia. Intern Med J. (2014) 44:458–63. doi: 10.1111/imj.12399
18. Cherny N, Sullivan R, Torode J, Saar M, Eniu A. ESMO European consortium study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in Europe. Ann Oncol. (2016) 27:1423–43. doi: 10.1093/annonc/mdw213
19. Goldstein DA, Clark J, Tu Y, Zhang J, Fang F, Goldstein R, et al. A global comparison of the cost of patented cancer drugs in relation to global differences in wealth. Oncotarget. (2017) 8:71548–55. doi: 10.18632/oncotarget.17742
20. Saqib A, Iftikhar S, Sarwar MR. Availability and affordability of biologic vs. non-biologic anticancer medicines: a cross-sectional study in Punjab, Pakistan. BMJ Open. (2018) 8:e019015. doi: 10.1136/bmjopen-2017-019015
21. Sarwar MR, Iftikhar S, Saqib A. Availability of anticancer medicines in public and private sectors, and their affordability by low, middle and high-income class patients in Pakistan. BMC Cancer. (2018) 18:14. doi: 10.1186/s12885-017-3980-3
22. Zhu Y, Wang Y, Sun X, Li X. Availability, price and affordability of anticancer medicines: evidence from two cross-sectional surveys in the Jiangsu province, China. Int J Environ Res Public Health. (2019) 16:3728. doi: 10.3390/ijerph16193728
23. Moye-Holz D, van Dijk JP, Reijneveld SA, Hogerzeil HV. Policy approaches to improve availability and affordability of medicines in Mexico - an example of a middle income country. Global Health. (2017) 13:53. doi: 10.1186/s12992-017-0281-1
24. World Health Organization. Technical Report: Pricing of Cancer Medicines and its Impacts: A Comprehensive Technical Report for the World Health Assembly Resolution 70.12: Operative Paragraph 2.9 on Pricing Approaches and Their Impacts on Availability and Affordability of Medicines for the Prevention and Treatment of Cancer. Geneva: World Health Organization (2018). p. 112.
25. Huang L, Wang X, Liu H. Analysis of the utilization of national-negotiation anti-tumor drugs in Nanjing area from 2016 to 2018. Chin J Pharmacoepidemiol. (2020) 29:687–90.
26. Mao L, Wen X, Yang Y, Mao Z. Macro analysis and research on the implementation of national drug negotiation: taking Hubei as an example. Chin Health Ins. (2021) 11:76–80. doi: 10.19546/j.issn.1674-3830.2021.11.009
27. Sun W, Tang Y, Zou Y, Zhang Q, Zhang B, Du X. Investigation on Clinical Use of 17 Antineoplastic Drugs Passing National Medical Insurance Negotiation in Peking Union Medical College Hospital. Beijing: Medical Journal of Peking Union Medical College Hospital (2021) vol. 12. p. 958–64. doi: 10.3969/j.issn.1674-9081.2020.00.018
28. Chen L, Xu W, Shang B, Liu C, He R, Liu H. Research on the availability of national negotiated drugs: an empirical analysis based on the purchasing data of public hospitals in Suzhou. Health Econ Res. (2020) 37:17–20. doi: 10.14055/j.cnki.33-1056/f.2020.12.005
29. Jiang H, Li X, Han F. Availability and affordability of national medical insurance negotiated innovative anticancer drugs in Changzhou. Chin J Pharmacovigilance. (2021) 18:1075–9. doi: 10.19803/j.1672-8629.2021.11.17
30. Science Technology Development Center of Chinese Pharmaceutical Association. Brief introduction to CMEI (2020). Available online at: https://en.cmei.org.cn/list/?343_1.html (accessed December 31, 2021).
31. Saeed A, Saeed H, Saleem Z, Fang Y, Babar ZU. Evaluation of prices, availability and affordability of essential medicines in Lahore Division, Pakistan: a cross-sectional survey using WHO/HAI methodology. PLoS One. (2019) 14:e0216122. doi: 10.1371/journal.pone.0216122
32. Guan X, Zhang J, Man C, Ni B, Shi L. How far have we come? Challenges to orphan drug access in China, 2011–2017. J Pharm Sci. (2019) 108:2199–205. doi: 10.1016/j.xphs.2019.01.012
33. World Health Organization Health Action International. Measuring Medicine Prices, Availability, Affordability and Price Components 2nd edition (2018). Available online at: https://www.who.int/medicines/areas/access/NPrices_prelims.pdf?ua%C2%BC1 (accessed October 15, 2020).
34. He X, Wang X, Dai H, Li X, Han F. Usage of new anti-cancer drugs in Nanjing from 2018 to 2020. Chin J Pharmacovigilance. (2021) 18:725–30. doi: 10.19803/j.1672-8629.2021.08.06
35. Yang C, Shen Q, Cai W, Zhu W, Li Z, Wu L, et al. Impact of the zero-markup drug policy on hospitalisation expenditure in western rural China: an interrupted time series analysis. Trop Med Int Health. (2017) 22:180–6. doi: 10.1111/tmi.12817
36. Guan X, Tian Y, Ross-Degnan D, Man C, Shi L. Interrupted time-series analysis of the impact of generic market entry of antineoplastic products in China. BMJ Open. (2018) 8:e022328. doi: 10.1136/bmjopen-2018-022328
37. Gillings D, Makuc D, Siegel E. Analysis of interrupted time series mortality trends: an example to evaluate regionalized perinatal care. Am J Public Health. (1981) 71:38–46. doi: 10.2105/AJPH.71.1.38
38. Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. (2002) 27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x
39. Bhaskaran K, Gasparrini A, Hajat S, Smeeth L, Armstrong B. Time series regression studies in environmental epidemiology. Int J Epidemiol. (2013) 42:1187–95. doi: 10.1093/ije/dyt092
40. He Y, Dou G, Huang Q, Zhang X, Ye Y, Qian M, et al. Does the leading pharmaceutical reform in China really solve the issue of overly expensive healthcare services? Evidence from an empirical study. PLoS One. (2018) 13:e0190320. doi: 10.1371/journal.pone.0190320
41. Hox J, Stoel RD. Multilevel and SEM approaches to growth curve modeling. In: Everitt BS, Howell DC, editors. Encyclopedia of Statistics in Behavioral Science. Chichester: John Wiley & Sons (2005). p.1296–1305. doi: 10.1002/0470013192.bsa418
42. Fang W, Xu X, Dai H, Li X. Influence of national medical insurance admittance negotiated policy on the utilization of innovative anticancer drugs. Herald Med. (2020) 39:1665–72. doi: 10.3870/j.issn.1004-0781.2020.12.014
43. Fang W, Luo C, Dai H, Meng L, Gu Z. Application analysis and medicare payments predication of new selected anti-tumor drugs of medical insurance in Nanjing city. Chin Hosp Pharm J. (2018) 38:1131–7. doi: 10.13286/j.cnki.chinhosppharmacyj.2018.11.01
44. Gong S, Cai H, Ding Y, Li W, Juan X, Peng J, et al. The availability, price and affordability of antidiabetic drugs in Hubei province, China. Health Policy Plan. (2018) 33:937–47. doi: 10.1093/heapol/czy076
45. Fang Y, Wagner AK, Yang S, Jiang M, Zhang F, Ross-Degnan D. Access to affordable medicines after health reform: evidence from two cross-sectional surveys in Shaanxi Province, western China. Lancet Glob Health. (2013) 1:e227–37. doi: 10.1016/S2214-109X(13)70072-X
46. Liu X, Zhang L, Chen W. Summarizing and analyzing the policy implementation for nationally negotiated drugs. Chin J Health Policy. (2019) 12:77–81. doi: 10.3969/j.issn.1674-2982.2019.09.014
47. Guan X, Hu H, Man C, Shi L. A survey of availability, price and affordability of essential medicines from 2011 to 2016 in Chinese secondary and tertiary hospitals. Int J Equity Health. (2018) 17:158. doi: 10.1186/s12939-018-0870-5
48. Zhou Y, Xu D, Qi Y, Kuai L. Prescription status of antitumor drugs at Chinese hospitals. Chin Hosp Pharm J. (2021) 41:1817–22. doi: 10.13286/j.1001-5213.2021.18.01
Keywords: accessibility, availability, cost, anticancer drugs, National Reimbursement Drug List Negotiation, China
Citation: Zhu H, Zhu J, Zhou Y, Shan L, Li C, Cui Y, Kang Z, Jiao M, Liu H, Gao L, Wu Q and Hao Y (2022) Impact of the National Reimbursement Drug List Negotiation Policy on Accessibility of Anticancer Drugs in China: An Interrupted Time Series Study. Front. Public Health 10:921093. doi: 10.3389/fpubh.2022.921093
Received: 15 April 2022; Accepted: 02 June 2022;
Published: 01 July 2022.
Edited by:
Chan Wang, Guangdong University of Finance and Economics, ChinaReviewed by:
You-hua Chen, South China Agricultural University, ChinaGeorgi Iskrov, Plovdiv Medical University, Bulgaria
Copyright © 2022 Zhu, Zhu, Zhou, Shan, Li, Cui, Kang, Jiao, Liu, Gao, Wu and Hao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qunhong Wu, d3VxdW5ob25nQDE2My5jb20=; Yanhua Hao, aHloeWp3QDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Hong Zhu
Hong Zhu Jingmin Zhu
Jingmin Zhu Yingyu Zhou5†
Yingyu Zhou5† Zheng Kang
Zheng Kang Mingli Jiao
Mingli Jiao Huan Liu
Huan Liu Qunhong Wu
Qunhong Wu