- 1Department of Ophthalmology, Taipei City Hospital, Taipei, Taiwan
- 2Institute of Public Health, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 3Department of Business Administration, Fu Jen Catholic University, Taipei, Taiwan
- 4General Education Center, University of Taipei, Taipei, Taiwan
- 5Li Ka Shing Faculty of Medicine, School of Public Health, The University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 6Taiwan Association of Health Industry Management and Development, Taipei, Taiwan
Purpose: This umbrella review aimed to summarize the available evidence on the association between dry eye disease and depression.
Methods: We searched the Pubmed, Embase, and Cochrane databases using the search string “(Dry eye syndrome OR Keratoconjunctivitis sicca OR KCS OR Aqueous tear deficiency OR Sjogren syndrome) AND (depression OR depressive symptoms) AND (meta-analysis OR systematic review)” from inception to 20 July 2022. We considered all meta-analyses investigating the association between dry eye disease and depression.
Results: After summarizing the included meta-analyses, it was concluded that dry eye disease is associated with depression. The symptoms of dry eye disease affect the daily lives of patients, thus affecting their mood. However, further evidence is required to confirm this association.
Conclusion: This finding highlights the importance of psychological support for patients with dry eye disease. Future clinical studies should investigate the mechanism underlying the association between dry eye disease and depression.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier: CRD42022320619.
Introduction
Dry eye disease, which affects the tear-air interface and ocular surface, is an increasing public health issue due to its influence on visual function and quality of life (1, 2). Dry eye disease is one of the most common ocular diseases worldwide, with an estimated prevalence of 11.59% (3). The usage of digital screen is associated with incident dry eye disease (4). The positive association between COVID-19 pandemic, prolonged screen times and the association between dry eye disease has already been proved (5–9). There are two subtypes of this disease, aqueous-deficient and evaporative. Keratoconjunctivitis sicca, Sjögren's syndrome and aqueous tear deficiency are all subgroups of the dry eye disease (10–12). Dry eye disease has been associated with many risk factors, including external adverse environmental factors (13). Air pollution, including higher ozone levels and particulate matter 2.5 pollution, was shown to cause ocular discomfort and induce dry eye disease (14). Seasonal changes also affect the prevalence of dry eye disease, which was found to be higher in the winter and summer (15).
Patients with dry eye disease often present with symptoms of eye discomfort, including stinging, burning, and pressure sensation, as well as sharp or throbbing pain (16). Consequently, many studies have found an association between dry eye disease and the patients' psychological status (17). A study conducted among older adults found that age-related reduction in tear production was associated with depression (18). A large population-based study identified a significant association between dry eye disease and depression in the general population (19). However, other studies have found no evidence of such significant association between Sjögren's syndrome and depression, and Sjögren's syndrome are one of the subgroups of dry eye disease (20, 21).
Given the inconsistency of prior findings and the fact that results vary according to gender, age, and other factors, the interaction between dry eye disease and depression remains unclear. Therefore, we performed an umbrella review of related meta-analyses to summarize the present knowledge on this topic and explore the possible explanations and effects.
Methods
Search strategy and eligibility criteria
In order to generate comprehensive results, we included only meta-analyses investigating the association between dry eye disease and depression. We searched the Pubmed, Cochrane, and Embase databases from inception to 20 July 2022, using the predetermined search string “(Dry eye syndrome OR Keratoconjunctivitis sicca OR KCS OR Aqueous tear deficiency OR Sjogren syndrome) AND (depression OR depressive symptoms) AND (meta-analysis OR systematic review).” There were no restrictions regarding the study language, subgroup of dry eye disease, or the age, gender, or race of participants. Only studies where depression or depressive symptoms developed subsequent to the diagnosis of dry eye disease were included. Studies that did not specify the cutoff threshold to detect depression were excluded. Studies investigating anxiety, cognitive impairment, or other psychological disorders were also excluded. This study was registered in the International Prospective Register of Systematic Reviews (PROSPERO ID: 320619).
Data extraction
Based on the PRISMA 2020 guidelines, the search process could be done either by human or by automation tools (22). In our study, two of the authors (C-YT and ZJ) independently completed the study screening process. Any discrepancies were resolved through dialogue with the senior author (T-HT.). After duplicates were removed, the retrieved studies were screened for eligibility. We excluded the following studies: (1) did not perform a quantitative synthesis; (2) did not report adequate data. Eligible studies were evaluated based on the level of comparison, random-effects summary, I2 statistic. The following data were extracted: author, year, number of included participants, patients' disease, outcome measurement tools, I2 statistic, and number of study types, statistical significance, and largest study effect size. We have also calculated 95% prediction intervals.
Assessment of methodological quality
Two of the authors (C-YT and ZJ) independently followed the AMSTAR-2 (a measurement tool to assess systematic reviews) guideline to assess the methodological quality of the included meta-analyses. The disagreements were resolved through a discussion with a senior author (T-HT). AMSTAR-2 is often used in umbrella review as it covers various aspects (23, 24). This guideline includes 16 aspects that systematically grade evidence-based medical papers (24, 25). The shortcomings in each of the aspect would result in overall quality (23). Because a high overall score may obscure some of the serious limitations of the included studies and they may be regarded as high-quality studies, a sum score for each part is not provided (26) (Table 1). The AMSTAR-2 is regarded as a reliable and valid method for evaluating the quality of systematic reviews and meta-analyses of interventional and observational research (24, 25, 32). Compared with the Risk of Bias In Non-randomized Studies-of Interventions tool, which has been commonly used in reviews, the AMSTAR-2 evaluates the determination of the study design for inclusion, reasons for exclusion of studies, sources of funding of the primary studies, and reviewers' conflict of interest (33).
Assessment of epidemiological credibility
We reanalyze all the included meta-analysis. High epidemiological credibility means highest evidence and no hints of major heterogeneity or bias (34). We further classified the included studies into the following types (35):
(1) Persuasive: statistical significance per the random-effects model of p < 0.000001, more than 1,000 cases, no high heterogeneity among the selected studies (I2 < 50%), 95% CI (excluding the null value), and no evidence of small-study effects and significant bias;
(2) Highly recommended: statistical significance of p < 0.000001, more than 1,000 cases, and most studies indicating a significant effect;
(3) Recommended: more than 1,000 cases and significant effects at p < 0.001;
(4) Weak evidence: nominally significant associations (p < 0.05); and
(5) Poor evidence: obtained from samples with <1,000 cases.
Assessment of small-study effects and excess significance bias
Small study effects describe a phenomenon that smaller studies sometimes showed larger treatment effects than larger studies (36). This is often caused by publication bias, and the p-value of Egger's test below 0.10 if small study effects exist (37). To better understand the characteristics of the included studies, we also evaluated the excess significant bias of each included study. Excess significant bias is based on the observed (O) number of studies (studies with significant result: p < 0.05) and the expected (E) number of the studies (38). Expected number (E) of the studies were calculated through the sum of the statistical power estimates for each included component study. The power of each included study was calculated using a non-central t distribution (37–39). Excess statistical significance for each meta-analyses was determined at two-sided p < 0.10 with O > E as previously proposed (37–39).
Results
Study characteristics
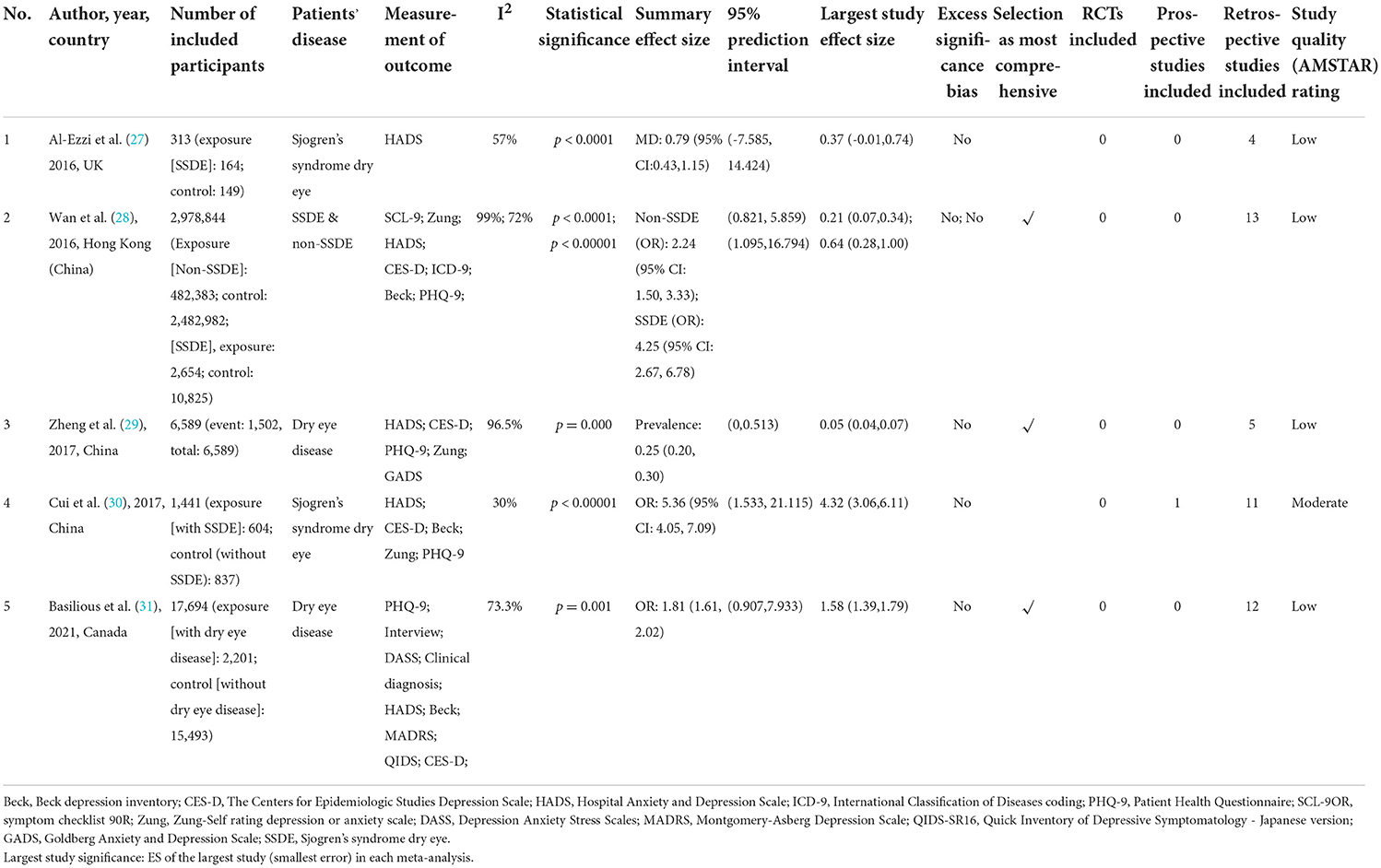
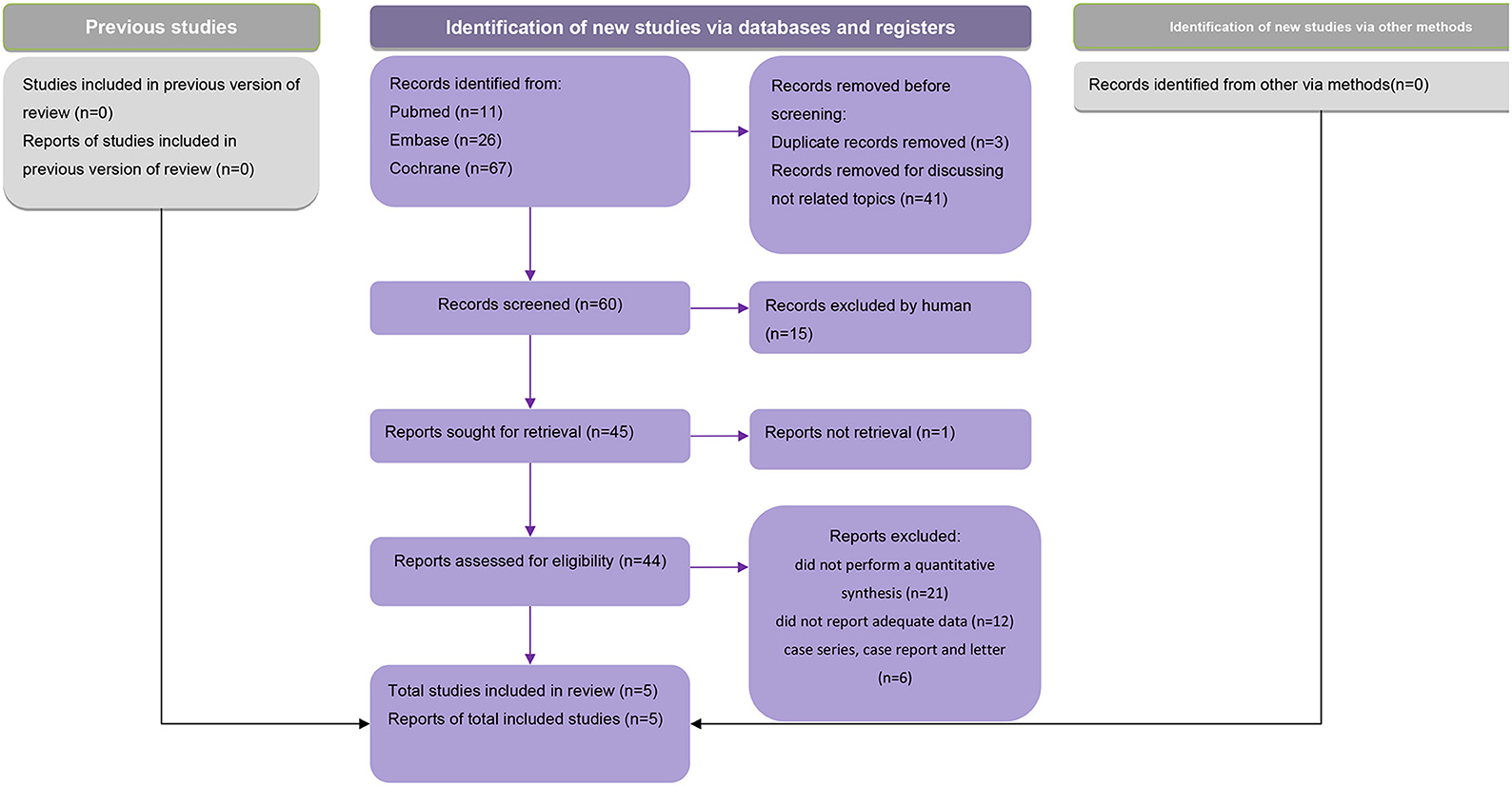
After excluding duplicates, a total of 95 meta-analyses were screened for eligibility, of which five that met the eligibility criteria were included in our analysis (Figure 1). These five studies were published between 2016 and 2021. Two of the studies defined the outcome as primary Sjogren's syndrome-related dry eye disease (27, 30), other two simply as dry eye disease (29, 31), and in one study, a subgroup analysis was performed and both Sjogren's syndrome- and non-Sjogren's syndrome-related dry eye disease were included in the outcomes (28). Based on the criteria and the characteristics of each included study, we classified our included studies into several groups. The study conducted by Cui et al. (30), Zheng et al. (29), and Wan et al. (28) could be classified as “recommended.” While the study by Al-Ezzi et al. (27) and Basilious et al. (31) were considered as “weak evidence.”
Outcome analysis
Al-Ezzi et al. (27) included five studies comprising 313 female patients and found that primary Sjogren's syndrome-related dry eye disease had an adverse effect on the psychological status of females. Cui et al. (30) found that patients with primary Sjogren's syndrome-related dry eye disease had higher prevalence and greater severity of depression compared with those in the control groups. Wan et al. (28) concluded that patients with either Sjogren's syndrome- or non-Sjogren's syndrome-related dry eye disease were more likely to develop depression. Zheng et al. (29) included 28 studies and found a significant association between dry eye disease and depression. Basilious et al. (31) concluded that depression was associated with dry eye disease symptoms but not signs.
Outcome measurement
The outcome measurement tools varied across studies and included the Hospital Anxiety and Depression Scale (HADS), Zung Self-rating depression or anxiety scale (Zung), Centers for Epidemiologic Studies Depression Scale (CES-D), and other. Although different tools were used, most of the included studies described the measurement in detail.
Publication bias
The publication bias assessment also differed among the five included studies. Al-Ezzi et al. (27) and Cui et al. (30) did not use funnel plots to assess the publication bias because the number of included studies in their meta-analyses was <10. Wan et al. (28) did not assess the publication bias in detail. Zheng et al. (29) revealed no significant publication bias based on Begg's test. Basilious et al. (31) used funnel plots to assess the publication bias, and although they found asymmetry, they did not regard it as a result of publication bias.
Small study effects and excess significance bias
In this umbrella review, none of the included studies provided the results of Egger's test. Therefore, we could not evaluate the small-study effects. The results of excess significance bias were shown in Table 1.
Residual confounding
All the included studies were observational studies; therefore, we cannot conclude the causal relationship between dry eye disease and depression. We acknowledge there may be some residual confounding or other biases when studying the association between dry eye disease and depression, and the existence of residual confounding or other biases may lead to overestimation or underestimation (40) and restricted causal interferes (41).
Discussion
All studies included in this umbrella review found a significant association between dry eye disease symptoms and depression, although the results varied across different characteristics of patients. Exploring this association may have important implications for the treatment of patients with dry eye disease.
Clinical implications
The prevalence of dry eye disease among people over 40 can be 75% (42). According to the data published by the World Health Organization, depression is estimated to affect 5.0% of adults globally (43). This review confirmed the previously reported association between dry eye disease and depression; however, the mechanism underlying this interaction remains to be explored.
The association between dry eye disease and depression tends to be bidirectional, i.e., depression can be both the cause and effect of dry eye disease (31). A prior study that demonstrated that patients with depression were likely to develop dry eye disease noted some important similarities between the pathophysiological mechanisms of the two conditions. Namely, the levels of the inflammatory cytokines interleukin-1, interleukin-6, and tumor necrosis factor (13) are increased both in depression and dry eye disease (44). These two conditions also have some risk factors in common, including female sex (45, 46), smart phone use (47, 48), gout (49, 50), and menopause (51, 52) and some other factors. Vehof et al. (45) examined the dry eye disease in a British female cohort, and discussed that hormone changes, lower pain thresholds in female may be possible reasons of the prevalence of depression among female dry eye disease patients. Zheng et al. (29) found that aged patients may easily to feel helpless and other negative feelings.
The coexistence of dry eye disease and depression was found to be associated with a series of unpleasant outcomes, including sleep disorder (53), suicide risk (54), reduced work productivity (55), and lower rates of treatment compliance (56). Furthermore, the eye discomfort affects these patients' personal feelings (57), and even their daily activities, such as reading, driving, watching television, and computer use (17, 57–59). Patients with dry eye disease often feel sad and pessimistic, and are also likely to experience cognitive disorders and pain (29). There is also a significant association between dry eye disease and anxiety, suggesting that patients with dry eye disease are more anxious and depressed compared with a healthy population (60). The biological mechanism between dry eye disease and depression is of interdisciplinary field. Some of the studies indicated that dry eye disease affects the quality of life, and then affects central nervous system sensitization (53). Another study investigated the single nucleotide polymorphisms (SNPs) in the genes in patients with dry eye disease, and they found that Val66Met, Fokl, and Apal was associated with dry eye disease. Furthermore, this association was affected by depression status (61). However, in the mechanism, which occurs first in dry eye and depression is not that clear.
The finding that depression and dry eye disease are closely related can contribute to the treatment of dry eye disease, as well as to the further investigation of the mechanism underlying the relationship between dry eye disease and psychological disorders. Based on the above, patients with dry eye disease should be provided psychological support.
Strengths and limitations
To the best of our knowledge, this is the first umbrella review to summarize the evidence on the association between dry eye disease and depression. Most of the included studies used objective measurement tools both for depression and dry eye disease, suggesting reliable results, which has high clinical value in real scenario. In view of the association between usage of digital screen and dry disease (4) and the fact of increasing screen-time around the world, we believe this study is of great interest of the public.
Nonetheless, this study also had several limitations. First, since the results and effect size varied among the included studies, we could not calculate a pooled result. Second, most of the included studies were retrospective. Hence, future prospective studies are needed to investigate the causality between dry eye disease and depression. Third, the differences among the studies in the measurement tools for depression, sample size, and other factors resulted in statistical heterogeneity; thus, the results should be interpreted with caution. Fourth, further studies should summarize the evidence on the association between the severity of dry eye disease and that of depression. Fifth, we only explored the association between dry eye and depression, however, we acknowledged that the association between other eye disease or their treatments [such as retinopathy (62, 63), cataract surgery (64), and glaucoma (65, 66)] and depression has already been proved in previously published meta-analysis. Finally, the high-quality meta-analysis investigating the association between dry eye disease and depression are lacking currently. Therefore, more high-quality meta-analysis should be conducted to include all eye disease including dry eye disease in the future.
Conclusion
This review confirmed the association between dry eye disease and patients' psychological status, emphasizing the importance of psychological support and guidance for these patients. Future clinical studies are needed to explore the mechanism underlying this relationship.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
C-YT, ZJ, and T-HT conducted the study and drafted the manuscript. C-YT and ZJ participated in the design of the study and performed statistical analyses. T-HT conceived the study and participated in its design and coordination. All authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Verjee MA, Brissette AR, Starr CE. Dry eye disease: early recognition with guidance on management and treatment for primary care family physicians. Ophthalmol Ther. (2020) 9:877–88. doi: 10.1007/s40123-020-00308-z
2. Aragona P, Giannaccare G, Mencucci R, Rubino P, Cantera E, Rolando M. Modern approach to the treatment of dry eye, a complex multifactorial disease: a P. ICASSO board review. Br J Ophthalmol. (2021) 105:446–53. doi: 10.1136/bjophthalmol-2019-315747
3. Papas EB. The global prevalence of dry eye disease: aA Bayesian view. Ophthalmic Physiol Opt. (2021) 41:1254–66. doi: 10.1111/opo.12888
4. Al-Mohtaseb Z, Schachter S, Shen Lee B, Garlich J, Trattler W. The relationship between dry eye disease and digital screen use. Clin Ophthalmol. (2021) 15:3811–20. doi: 10.2147/OPTH.S321591
5. Elhusseiny AM, Eleiwa TK, Yacoub MS, George J, ElSheikh RH, Haseeb A, et al. Relationship between screen time and dry eye symptoms in pediatric population during the COVID-19 pandemic. Ocul Surf. (2021) 22:117–9. doi: 10.1016/j.jtos.2021.08.002
6. Pandey SK, Sharma V. Mask-associated dry eye disease and dry eye due to prolonged screen time: Are we heading towards a new dry eye epidemic during the COVID-19 era? Indian J Ophthalmol. (2021) 69:448. doi: 10.4103/ijo.IJO_3250_20
7. Prescott CR. Increased screen time and dry eye: another complication of COVID-19. Eye Contact Lens. (2021) 47:433. doi: 10.1097/ICL.0000000000000820
8. Tangmonkongvoragul C, Chokesuwattanaskul S, Khankaeo C, Punyasevee R, Nakkara L, Moolsan S, et al. Prevalence of symptomatic dry eye disease with associated risk factors among medical students at Chiang Mai University due to increased screen time and stress during COVID-19 pandemic. PLoS ONE. (2022) 17:e0265733. doi: 10.1371/journal.pone.0265733
9. Neti N, Prabhasawat P, Chirapapaisan C, Ngowyutagon P. Provocation of dry eye disease symptoms during COVID-19 lockdown. Sci Rep. (2021) 11:1–9. doi: 10.1038/s41598-021-03887-4
10. Lemp MA, Foulks GN. The definition and classification of dry eye disease. Ocul Surf. (2007) 5:75–92. doi: 10.1016/S1542-0124(12)70081-2
11. Chang S-W, Wu W-L. Association between dry eye parameters depends on tear components. J Clin Med. (2022) 11:3056. doi: 10.3390/jcm11113056
12. Da Cunha E, Mariette X, Desmoulins F, Bergé E, Nocturne G, Benmalek A, et al. Associations between ocular and extra-ocular assessment in primary Sjögren's syndrome. Joint Bone Spine. (2022) 89:105426. doi: 10.1016/j.jbspin.2022.105426
13. Calonge M, Pinto-Fraga J, González-García MJ, Enríquez-de-Salamanca A, López-de la Rosa A, Fernández I, et al. Effects of the external environment on dry eye disease. Int Ophthalmol Clinics. (2017) 57:23–40. doi: 10.1097/IIO.0000000000000168
14. Kim Y, Choi Y-H, Kim MK, Paik HJ, Kim DH. Different adverse effects of air pollutants on dry eye disease: Ozone, PM2. 5, and PM10. Environ Pollut. (2020) 265:115039. doi: 10.1016/j.envpol.2020.115039
15. van Setten G, Labetoulle M, Baudouin C, Rolando M. Evidence of seasonality and effects of psychrometry in dry eye disease. Acta Ophthalmologica. (2016) 94:499–506. doi: 10.1111/aos.12985
16. Guo LW, AKPEK E. The negative effects of dry eye disease on quality of life and visual function. Turk J Med Sci. (2020) 50:1611–5. doi: 10.3906/sag-2002-143
17. Uchino M, Schaumberg DA. Dry eye disease: impact on quality of life and vision. Curr Ophthalmol Rep. (2013) 1:51–7. doi: 10.1007/s40135-013-0009-1
18. Kim KW, Han SB, Han ER, Woo SJ, Lee JJ, Yoon JC, et al. Association between depression and dry eye disease in an elderly population. Invest Ophthalmol Vis Sci. (2011) 52:7954–8. doi: 10.1167/iovs.11-8050
19. Van Der Vaart R, Weaver MA, Lefebvre C, Davis RM. The association between dry eye disease and depression and anxiety in a large population-based study. Am J Ophthalmol. (2015) 159:470–4. doi: 10.1016/j.ajo.2014.11.028
20. Bongi SM, Del Rosso A, Orlandi M, Matucci-Cerinic M. Gynaecological symptoms and sexual disability in women with primary Sjögren's syndrome and sicca syndrome. Clin Exp Rheumatol. (2013) 31:683–90.
21. Theander L, Strömbeck B, Mandl T, Theander E. Sleepiness or fatigue? Can we detect treatable causes of tiredness in primary Sjögren's syndrome? Rheumatology. (2010) 49:1177–83. doi: 10.1093/rheumatology/keq023
22. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160. doi: 10.1136/bmj.n160
23. Okoth K, Chandan JS, Marshall T, Thangaratinam S, Thomas GN, Nirantharakumar K, et al. Association between the reproductive health of young women and cardiovascular disease in later life: umbrella review. BMJ. (2020) 371:m3502. doi: 10.1136/bmj.m3502
24. Poole R, Kennedy OJ, Roderick P, Fallowfield JA, Hayes PC, Parkes J. Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. BMJ. (2017) 359:j5024. doi: 10.1136/bmj.j5024
25. Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. (2009) 62:1013–20. doi: 10.1016/j.jclinepi.2008.10.009
26. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008
27. Al-Ezzi MY, Pathak N, Tappuni AR, Khan KS. Primary Sjögren's syndrome impact on smell, taste, sexuality and quality of life in female patients: a systematic review and meta-analysis. Mod Rheumatol. (2017) 27:623–9. doi: 10.1080/14397595.2016.1249538
28. Wan KH, Chen LJ, Young AL. Depression and anxiety in dry eye disease: a systematic review and meta-analysis. Eye. (2016) 30:1558–67. doi: 10.1038/eye.2016.186
29. Zheng Y, Wu X, Lin X, Lin H. the prevalence of depression and depressive symptoms among eye disease patients: a systematic review and meta-analysis. Sci Rep. (2017) 7:46453. doi: 10.1038/srep46453
30. Cui Y, Li L, Yin R, Zhao Q, Chen S, Zhang Q, et al. Depression in primary Sjögren's syndrome: a systematic review and meta-analysis. Psychol Health Med. (2018) 23:198–209. doi: 10.1080/13548506.2017.1339895
31. Basilious A, Xu CY, Malvankar-Mehta MS. Dry eye disease and psychiatric disorders: a systematic review and meta-analysis. Eur J Ophthalmol. 2021:11206721211060963. doi: 10.1177/11206721211060963
32. Pieper D, Mathes T, Eikermann M. Can AMSTAR also be applied to systematic reviews of non-randomized studies? BMC Res Notes. (2014) 7:609. doi: 10.1186/1756-0500-7-609
33. Swierz MJ, Storman D, Zajac J, Koperny M, Weglarz P, Staskiewicz W, et al. Similarities, reliability and gaps in assessing the quality of conduct of systematic reviews using AMSTAR-2 and ROBIS: systematic survey of nutrition reviews. BMC Med Res Methodol. (2021) 21:261. doi: 10.1186/s12874-021-01457-w
34. Bellou V, Belbasis L, Tzoulaki I, Evangelou E. Risk factors for type 2 diabetes mellitus: An exposure-wide umbrella review of meta-analyses. PLoS ONE. (2018) 13:e0194127. doi: 10.1371/journal.pone.0194127
35. Gu D-T, Tung T-H, Jiesisibieke ZL, Chien C-W, Liu W-Y. Safety of cinnamon: an umbrella review of meta-analyses and systematic reviews of randomized clinical trials. Front Pharmacol. (2021) 12:790901. doi: 10.3389/fphar.2021.790901
36. Schwarzer G, Carpenter JR, Rücker G. Small-study effects in meta-analysis. In: Meta-Analysis with R. Use R. Cham: Springer (2015). doi: 10.1007/978-3-319-21416-0_5
37. Bellou V, Belbasis L, Tzoulaki I, Evangelou E, Ioannidis JPA. Environmental risk factors and Parkinson's disease: an umbrella review of meta-analyses. Parkinsonism Relat Disord. (2016) 23:1–9. doi: 10.1016/j.parkreldis.2015.12.008
38. Ioannidis JPA, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials. (2007) 4:245–53. doi: 10.1177/1740774507079441
39. Sun H, Gong T-T, Xia Y, Wen Z-Y, Zhao L-G, Zhao Y-H, et al. Diet and ovarian cancer risk: an umbrella review of systematic reviews and meta-analyses of cohort studies. Clin Nutr. (2021) 40:1682–90. doi: 10.1016/j.clnu.2020.11.032
40. Raglan O, Kalliala I, Markozannes G, Cividini S, Gunter MJ, Nautiyal J, et al. Risk factors for endometrial cancer: an umbrella review of the literature. Int J Cancer. (2019) 145:1719–30. doi: 10.1002/ijc.31961
41. Han MA, Zeraatkar D, Guyatt GH, Vernooij RWM, El Dib R, Zhang Y, et al. Reduction of red and processed meat intake and cancer mortality and incidence: a systematic review and meta-analysis of cohort studies. Ann Intern Med. (2019) 171:711–20. doi: 10.7326/M19-0699
42. Rouen PA, White ML. Dry eye disease: prevalence, assessment, and management. Home Healthcare Now. (2018) 36:74–83. doi: 10.1097/NHH.0000000000000652
43. Evaluation IoHMa. Global Health Data Exchange (GHDx). Available online at: https://vizhub.healthdata.org/gbd-results/?params=gbd-api-2019-permalink/d780dffbe8a381b25e1416884959e88b
44. Tiskaoglu NS, Yazici A, Karlidere T, Sari E, Oguz EY, Musaoglu M, et al. Dry eye disease in patients with newly diagnosed depressive disorder. Curr Eye Res. (2017) 42:672–6. doi: 10.1080/02713683.2016.1236966
45. Vehof J, Kozareva D, Hysi PG, Hammond CJ. Prevalence and risk factors of dry eye disease in a British female cohort. Br J Ophthalmol. (2014) 98:1712–7. doi: 10.1136/bjophthalmol-2014-305201
46. Albert PR. Why is depression more prevalent in women? J Psychiatry Neurosci. (2015) 40:219–21. doi: 10.1503/jpn.150205
47. Elhai JD, Dvorak RD, Levine JC, Hall BJ. Problematic smartphone use: a conceptual overview and systematic review of relations with anxiety and depression psychopathology. J Affect Disord. (2017) 207:251–9. doi: 10.1016/j.jad.2016.08.030
48. Moon JH, Kim KW, Moon NJ. Smartphone use is a risk factor for pediatric dry eye disease according to region and age: a case control study. BMC Ophthalmol. (2016) 16:1–7. doi: 10.1186/s12886-016-0364-4
49. Lin S, Zhang H, Ma A. Association of gout and depression: a systematic review and meta-analysis. Int J Geriatr Psychiatry. (2018) 33:441–8. doi: 10.1002/gps.4789
50. Lee C-Y, Chen H-C, Sun C-C, Lin H-Y, Lu K-H, Huang J-Y, et al. Gout as a risk factor for dry eye disease: a population-based cohort study. J Clin Med. (2019) 8:62. doi: 10.3390/jcm8010062
51. Freeman EW. Associations of depression with the transition to menopause. Menopause. (2010) 17:823–7. doi: 10.1097/gme.0b013e3181db9f8b
52. Versura P, Campos EC. Menopause and dry eye. A possible relationship. Gynecol Endocrinol. (2005) 20:289–98. doi: 10.1080/09513590400027257
53. JM Weatherby T, Ram Vasant Raman V, Agius M. Depression and dry eye disease: a need for an interdisciplinary approach? Psychiatr Danub. (2019) 31:619–21.
54. Um S-B, Yeom H, Kim NH, Kim HC, Lee HK, Suh I. Association between dry eye symptoms and suicidal ideation in a Korean adult population. PLoS ONE. (2018) 13:e0199131. doi: 10.1371/journal.pone.0199131
55. Uchino M, Uchino Y, Dogru M, Kawashima M, Yokoi N, Komuro A, et al. Dry eye disease and work productivity loss in visual display users: the Osaka study. Am J Ophthalmol. (2014) 157:294–300. doi: 10.1016/j.ajo.2013.10.014
56. Jamilloux Y, Sarabi M, Kérever S, Boussely N, Le Sidaner A, Valgueblasse V, et al. Adherence to online monitoring of patient-reported outcomes by patients with chronic inflammatory diseases: a feasibility study. Lupus. (2015) 24:1429–36. doi: 10.1177/0961203315585814
57. Tong L, Waduthantri S, Wong T, Saw S, Wang J, Rosman M, et al. Impact of symptomatic dry eye on vision-related daily activities: the Singapore Malay Eye Study. Eye. (2010) 24:1486–91. doi: 10.1038/eye.2010.67
58. Deschamps N, Ricaud X, Rabut G, Labbé A, Baudouin C, Denoyer A. The impact of dry eye disease on visual performance while driving. Am J Ophthalmol. (2013) 156:184–9. e3. doi: 10.1016/j.ajo.2013.02.019
59. Miljanović B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. (2007) 143:409–15. e2. doi: 10.1016/j.ajo.2006.11.060
60. Szakáts I, Sebestyén M, Németh J, Birkás E, Purebl G. The role of health anxiety and depressive symptoms in dry eye disease. Curr Eye Res. (2016) 41:1044–9. doi: 10.3109/02713683.2015.1088955
61. Hallak JA, Tibrewal S, Mohindra N, Gao X, Jain S. Single nucleotide polymorphisms in the BDNF, VDR, and DNASE 1 genes in dry eye disease patients: a case-control study. Invest Ophthalmol Vis Sci. (2015) 56:5990–6. doi: 10.1167/iovs.15-17036
62. Zou Y, You W, Wang J, Wang F, Tian Z, Lu J, et al. Depression and retinopathy in patients with type 2 diabetes mellitus: a meta-analysis. Psychosom Med. (2021) 83:239–46. doi: 10.1097/PSY.0000000000000924
63. Li S, Liu H, Zhu X. The effect of psychotherapy on anxiety, depression, and quality of life in patients with diabetic retinopathy: a protocol for systematic review and network meta-analysis. Medicine. (2021) 100:e28386. doi: 10.1097/MD.0000000000028386
64. Pellegrini M, Bernabei F, Schiavi C, Giannaccare G. Impact of cataract surgery on depression and cognitive function: systematic review and meta-analysis. Clin Exp Ophthalmol. (2020) 48:593–601. doi: 10.1111/ceo.13754
65. Liu J, Cao L, Yang G, Zhou R. Effects of non-pharmacological interventions on anxiety, depression, and sleep quality in patients with postoperative glaucoma: a protocol for systematic review and network meta-analysis. Medicine. (2021) 100:e27090. doi: 10.1097/MD.0000000000027090
Keywords: dry eye disease, depression, umbrella review, meta-analysis, psychological status
Citation: Tsai C-Y, Jiesisibieke ZL and Tung T-H (2022) Association between dry eye disease and depression: An umbrella review. Front. Public Health 10:910608. doi: 10.3389/fpubh.2022.910608
Received: 01 April 2022; Accepted: 26 October 2022;
Published: 17 November 2022.
Edited by:
Wulf Rössler, Charité Universitätsmedizin Berlin, GermanyReviewed by:
Yonathan Garfias, Universidad Nacional Autónoma de México, MexicoGyörgy Purebl, Semmelweis University, Hungary
Copyright © 2022 Tsai, Jiesisibieke and Tung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao-Hsin Tung, Y2gyODc2QGdtYWlsLmNvbQ==
 Ching-Yao Tsai1,2,3,4
Ching-Yao Tsai1,2,3,4 Zhu Liduzi Jiesisibieke
Zhu Liduzi Jiesisibieke Tao-Hsin Tung
Tao-Hsin Tung