
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Public Health, 11 July 2022
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.899846
This article is part of the Research TopicModern Molecular Era of The Mycobacterial World: Insights Into Diagnosis and Transmission of Mycobacteria and Associated DiseasesView all 10 articles
Background: Mycobacterium senegalense is a non-tuberculous mycobacterium and is found everywhere in the environment. However, M. senegalense infection in human is extremely rare, especially in immunocompetent individuals. It is difficult to detect M. senegalense infection because its symptoms are non-specific, and routine diagnostic tests are less sensitive. It is also resistant to commonly used antibiotics. Here, we report the first case of M. senegalense infection after laparoscopic cholecystectomy in China.
Case Presentation: A 55-year-old man was admitted because of repeated infections at multiple incision sites for more than 1 year. Although routine diagnostic test results were negative, metagenomic next-generation sequencing (mNGS) identified DNA sequences of M. senegalense in tissue samples from incision sites. The presence of M. senegalense was further confirmed by polymerase chain reaction and capillary electrophoresis. After 60 days of quadruple therapy with clarithromycin, moxifloxacin, rifampicin, and oxycycline, the patient's wound healed.
Conclusion: We believe the case findings contribute to the limited amount of knowledge about M. senegalense infection and raises awareness that this infection can result in poor wound healing, even in an immunocompetent host. Owing to a lack of early, precise diagnosis, it is difficult to treat M. senegalense infections. Based on our findings, mNGS is a sensitive diagnostic test for M. senegalense infections.
Mycobacterium senegalense is a non-tuberculous mycobacterium (NTM) that was first reported to cause bovine farcy in sub-Saharan Africa, exhibiting chronic granulomatous inflammation of skin lymphatics and draining lymph nodes of zebu cattle (1). However, human infections with M. senegalense are rare, and its zoonotic potential is unknown.
NTM is a complex pathogen that is usually a commensal or saprophytic organism. It is extensively found in the environment such as in soil and water sources, leading to high human–pathogen exposure (2), although only a few species infect humans. NTM is an opportunistic pathogen that usually infects immunocompromised patients with intractable conditions such as those with underlying lung diseases, human immunodeficiency virus infections, and cancers, as well as those receiving chemotherapy (3). NTM can be divided into two groups based on how long they take to grow in a culture: rapid-growing and slow-growing species. The most common pathogen among the slow-growing NTM species is M. avium-intracellulare, which primarily causes pulmonary infection (4). M. fortuitum is the most common pathogen among the rapid-growing NTM species, which usually causes colonized or transient infections in the respiratory tract, in addition to disseminated disease, lymphadenitis, and skin and soft tissue infections (5, 6). Although M. senegalense belongs to the same group as M. fortuitum, it rarely infects humans.
So far, only seven cases of humans being infected by M. senegalense have been described; however, none of them appear to have occurred in China. In this study, we report the first case of M. senegalense infection after laparoscopic cholecystectomy in China. mNGS test identified that the patient was infected with M. senegalense, even though routine diagnostic tests were negative. The patient's condition improved, and the wound healed after quadruple therapy with clarithromycin, moxifloxacin, rifampicin, and doxycycline.
A 55-year-old male patient, who present to our hospital in August 2021, because of repeated infection at multiple incisions for more than 1 year after laparoscopic cholecystectomy surgery done for gallstone complicated with cholangitis. On examination, the incisions under the xiphoid process were red and swollen, the incisions were dehiscent, local tenderness, high skin temperature and a little suppuration (Figure 1A).
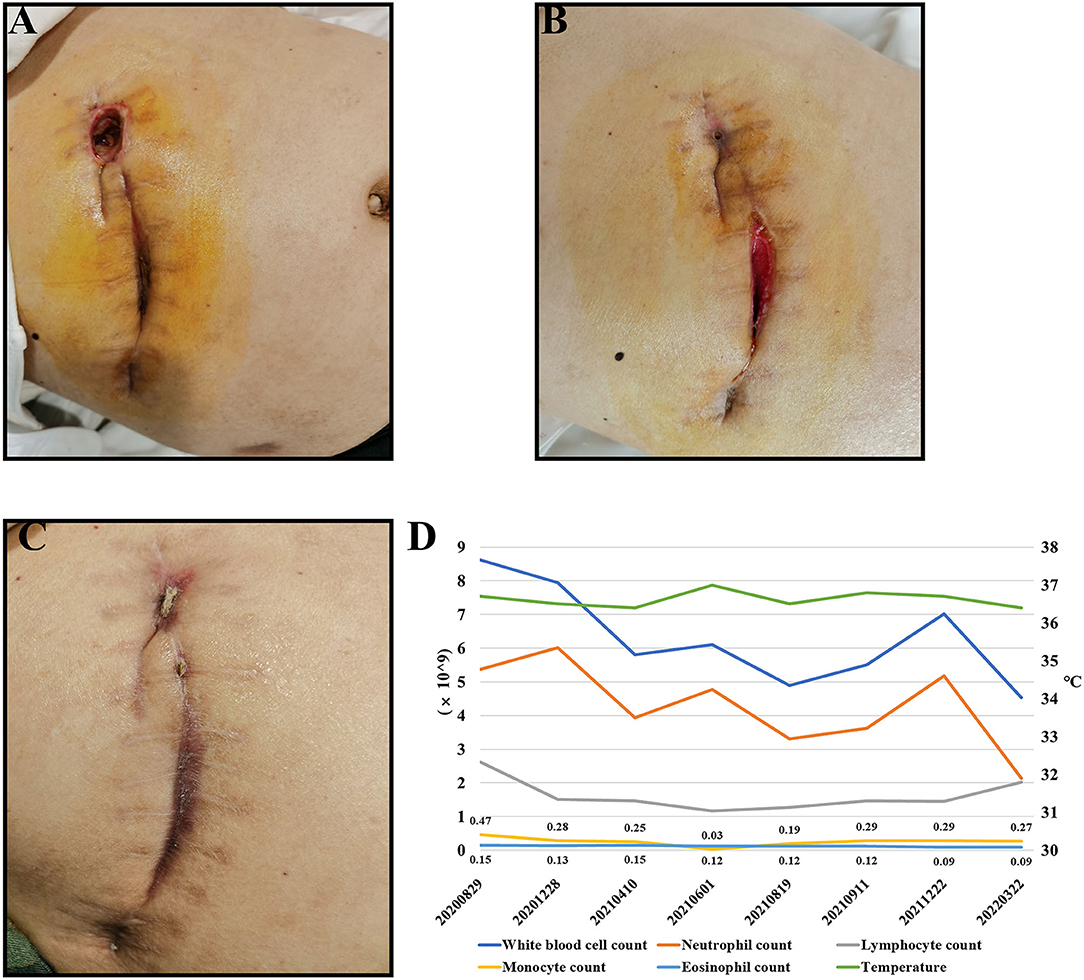
Figure 1. Condition of the patient's incisions. (A) It shows the incisions of the patient on admission. (B) It shows the incisions of the patient after second surgery. (C) It shows the patient's incisions after 60 days of quadruple therapy. (D) The patient's temperature and routine blood test results.
The patient had multiple incisions infections and repeated purulent exudates after surgery, with an average recurrence about 25 days, but the blood routine results shown no abnormality. According to the B-ultrasound results (Figure 2A), considering the formation of sinus tract under xiphoid process. In September 2021, laparoscopic re-surgery and abdominal exploration were performed, the sinus trace was cut and drainage of abscess. Then given piperacillin sodium and tazobactam sodium anti-infection treatment, but the effect was still poor after a period of time. We discovered the surgical incisions still were not healing, the skin surrounding the incisions was red, swollen and painful, and suppuration was present (Figure 1B).
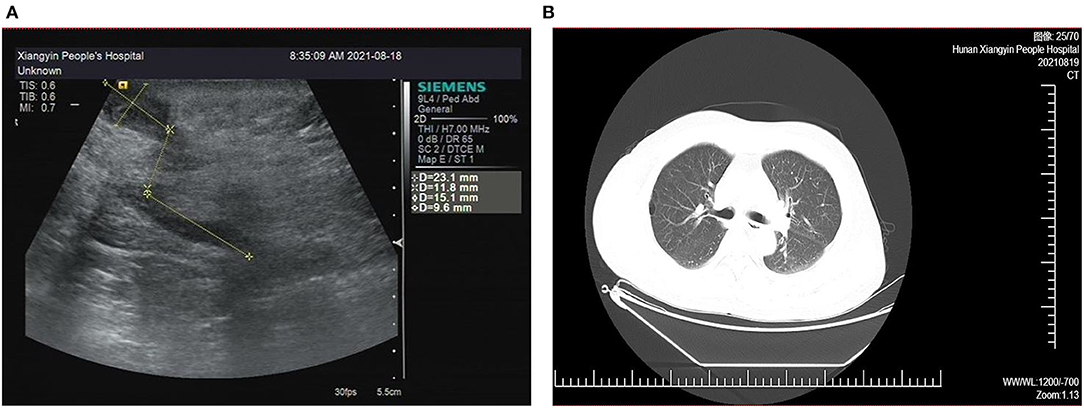
Figure 2. (A) Abdominal B-ultrasound results show the formation of sinus tract and empyema. (B) Chest computed tomography images of the lung window show no sign of tuberculosis-related diseases.
Since admission, the patient's body temperature and blood routine results were normal (Figure 1D). Additionally, regular bacterial culture and acid-fast bacillus (AFB) staining were negative, but blood samples for the tubercle bacillus antibody (TB-Ab) test were positive. Combined with the patient's chest CT (Figure 2B), tuberculosis related examination and the patient's clinical symptoms, they did not support tuberculosis-related diseases. In order to further resolve the question and determine the pathogens, the mategenomic sequencing technology covering the pathogen is used to accurately identify pathogens.
On November 29, 2021, the tissue from the incisions of the patient was sampled for DNA metagenomic next-generation sequencing (mNGS) (KingMed Diagnostics, Changsha, China). It detected 53 sequences that could be mapped to M. senegalense in a total of 113 sequences, and the coverage was 0.09%, making up 58.76% of the total microbe sequences (Table 1). Targeted PCR of M. senegalense using two pairs of primers was applied: 16S RNA forward 5′-AGCGGCGGAGCATGTGGATTA-3′, reverse 5′-GCTGATCTGCGATTACTAGCGACTC-3′ (GenBank: DQ145802.1); rpoB forward 5′-TGCGTGCCATCTTCGGTGAGA-3′, reverse 5′-GTCGATGTTCCAGCCTGCCTTG-3′ (GenBank: JF706631.1). The primers were designed and verified using Primer-BLAST based on the reference genome sequence of M. senegalense in NCBI. Subsequently, the capillary electrophoresis technique (Qsep 100TM; Bioptic) also curtained the M. senegalense infection (Figure 3).

Figure 3. Polymerase chain reaction and capillary electrophoresis technique confirmed M. senegalense infection in the patient. 16S rRNA- and rpoB-specific amplified fragments are marked with red boxes.
According to the results, the patient was initiated with oral clarithromycin (500 mg, twice daily), moxifloxacin (400 mg, once daily), rifampicin (450 mg, once daily) and doxycycline (100 mg, once every 12 hours). Then the swelling gradually subsided and there was no obvious purulent exudation. After 20 days, the patient's incisions healed well, and there was no sign of recurrence in the 60th day after quadruple therapy (Figure 1C).
We present a unique case of a patient with chronic incision site infections who underwent laparoscopic cholecystectomy and re-surgery, after which he was diagnosed as having an infection caused by an unusual NTM species.
Rapid-growing NTM species, such as M. fortuitum, have increasingly gained recognition during the last two decades because of their ability to thrive even in the harshest environments. In an immunosuppressed patient, these pathogens cause serious infections, such as bacteremia (5). M. senegalense is a rapid-growing NTM, belongs to the same group as M. fortuitum, and was first isolated from a bovine source (6). Infection with M. senegalense affects the sinus tracts and is characterized by multiple abscesses and granuloma formation. Only 7 cases about human infection with M. senegalense have been reported, the recent report found it to cause skin infections in immunocompetent patients (7). Most patients had minimal history of contact with animals and travel to areas where M. senegalense infection was more commonly endemic (8–10). And most of those cases were identified by various sequencing techniques. The first case of human infection by M. senegalense was described in 2005 (11). The patient had non-Hodgkin's lymphoma and was treated with R-CHOP. Her physical examination revealed high fever of 39.8°C, although she had no contact with any animal- or bovine-specific sources. Although it was difficult to diagnose, 16S rRNA gene, rpoB, and 16S-23S rRNA gene internal transcribed spacer sequence analyses finally revealed M. senegalense infection. Another case reported M. senegalense infection in a healthy girl with no prior history of serious illness or hospitalization (12). The patient was scratched by fish tank debris, following which the wound was sterilized and sutured. After 2 weeks, the wound failed to heal and appeared reddened and indurated. 16S gene sequencing and biochemical testing was used to diagnose the M. senegalense infection, which was the first infection reported in humans without immunodeficiency. This also indicated that immunocompetent individuals were also at a risk of infection.
It is worth mentioning that the patient had no history of contact with animals, and the patient's body temperature, routine blood test results, and other data indicated that infection was absent. These indicators were not present in previous cases, suggesting that M. senegalense may not cause changes in infection indicators. According to current literature, the prevalence and incidence of NTM infections continue to increase (11, 13). Rapid identification and subsequent drug susceptibility testing are essential for sleeting appropriate antibiotics against NTM. In addition, it is necessary to be vigilant about NTM infections, even if individuals have no contact history with animals, no travel history, and no infection-negative routine blood test results with recurrent infections at laparoscopic incision sites.
Because of the scarcity of literature on M. senegalense, we draw on treatment guidelines for NTM infections (14, 15). From the literature review, we found that the management of NTM infections is quite difficult. First, NTM infections possess several internal antimicrobial resistance mechanisms such as an impermeable cell wall and formation of granulomas, which reduces antimicrobial influx. Second, the long-term treatment of NTM infections leads to the development of drug resistance (16). According to the American Thoracic Society, Infectious Diseases Society of America, and Clinical and Laboratory Standards Institute, the latest recommended NTM drugs include macrolides, clofazimine, quinolones, sulfonamides, linezolid, aminoglycosides, bedaquiline, and tetracyclines (17). Additionally, the success rate of treatment for NTM infection is species specific. Usually, a combination of three or four drugs are administered to avoid the development of resistance to monotherapy. Based on previous experience combined with this patient's condition, we administered clarithromycin (500 mg, twice daily), moxifloxacin (400 mg, once daily), rifampicin (450 mg, once daily), and doxycycline (100 mg, once every 12 h). With this combination of therapy, the patient achieved good clinical efficacy. The case report provides a reference for the treatment of M. senegalense.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HY, FLG, and SLZ collected and analyzed patient data. HLZ, HY, YFY, and HDL wrote the manuscript. HDL and JJL provided supervision. All authors reviewed, edited, and approved the final manuscript.
This study was supported by National Natural Science Foundation of China (82073260 to HDL).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Dr. Qianhe Wei, Dr. Yan Yu, Ms. Xiaoyu Tong and their colleague from Changsha Kingmed Diagnostics for technical support of this manuscription.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.899846/full#supplementary-material
1. Hamid ME. Current Perspectives on Mycobacterium farcinogenes and Mycobacterium senegalense, the Causal Agents of Bovine Farcy. Vet Med Int. (2014) 2014:247906. doi: 10.1155/2014/247906
2. Falkinham JO 3rd. Environmental sources of nontuberculous mycobacteria. Clin Chest Med. (2015) 36:35–41. doi: 10.1016/j.ccm.2014.10.003
3. Falkinham JO 3rd. Surrounded by mycobacteria: nontuberculous mycobacteria in the human environment. J Appl Microbiol. (2009) 107:356–67. doi: 10.1111/j.1365-2672.2009.04161.x
4. Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ Jr, Andrejak C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J. (2020) 56:2000535. doi: 10.1183/13993003.00535-2020
5. Schinsky MF, McNeil MM, Whitney AM, Steigerwalt AG, Lasker BA, Floyd MM, et al. Mycobacterium septicum sp. nov., a new rapidly growing species associated with catheter-related bacteraemia. Int J Syst Evol Microbiol. (2000) 50 (Pt 2):575–81. doi: 10.1099/00207713-50-2-575
6. Mohan K. Mycobacterium senegalense from bovines in Eastern Nigeria. J Appl Bacteriol. (1985) 59:277–81. doi: 10.1111/j.1365-2672.1985.tb01789.x
7. Cheng AY, Lee CH. Skin infection by Mycobacterium farcinogenes-senegalense group in an immunocompetent patient: a case report. BMC Infect Dis. (2022) 22:445. doi: 10.1186/s12879-022-07409-z
8. Bugeja A, Hae R, Rajda E, Clark EG, Akbari A, Fairhead T, et al. A living donor kidney transplant recipient with mycobacterium senegalense bacteremia: a case report. Transpl Infect Dis. (2021) 23:e13596. doi: 10.1111/tid.13596
9. Santos-Perez JL, Delgado-Mainar P, Toro-Rueda C, Baquero-Artigao F. Surgical site infection by Mycobacterium senegalense in a pediatric patient. Enferm Infecc Microbiol Clin (Engl Ed). (2021) 39:259–61. doi: 10.1016/j.eimce.2021.02.004
10. Carretero O, Reyes C, San-Juan R, Chaves F, Lopez-Roa P. Mycobacterium senegalense infection after implant-based breast reconstruction, Spain. Emerg Infect Dis. (2020) 26:611–3. doi: 10.3201/eid2603.190230
11. Oh WS, Ko KS, Song JH, Lee MY Ryu SY, Taek S, et al. Catheter-associated bacteremia by Mycobacterium senegalense in Korea. BMC Infect Dis. (2005) 5:107. doi: 10.1186/1471-2334-5-107
12. Talavlikar R, Carson J, Meatherill B, Desai S, Sharma M, Shandro C, et al. Mycobacterium senegalense tissue infection in a child after fish tank exposure. Can J Infect Dis Med Microbiol. (2011) 22:101–3. doi: 10.1155/2011/206532
13. Furuuchi K, Morimoto K, Yoshiyama T, Tanaka Y, Fujiwara K, Okumura M, et al. Interrelational changes in the epidemiology and clinical features of nontuberculous mycobacterial pulmonary disease and tuberculosis in a referral hospital in Japan. Respir Med. (2019) 152:74–80. doi: 10.1016/j.rmed.2019.05.001
14. Quang NT, Jang J. Current molecular therapeutic agents and drug candidates for mycobacterium abscessus. Front Pharmacol. (2021) 12:724725. doi: 10.3389/fphar.2021.724725
15. Kumar K, Daley CL, Griffith DE, Loebinger MR. Management of Mycobacterium avium complex and Mycobacterium abscessus pulmonary disease: therapeutic advances and emerging treatments. Eur Respir Rev. (2022) 31:210212. doi: 10.1183/16000617.0212-2021
16. Tarashi S, Siadat SD, Fateh A. Nontuberculous mycobacterial resistance to antibiotics and disinfectants: challenges still ahead. Biomed Res Int. (2022) 2022:8168750. doi: 10.1155/2022/8168750
Keywords: Mycobacterium senegalense, infection, non-tuberculous mycobacterium, metagenomic next-generation sequencing, case report
Citation: Zhou H, Yang H, Gong F, Zhou S, Yang Y, Liu H and Liu J (2022) Case Report: Mycobacterium senegalense Infection After Cholecystectomy. Front. Public Health 10:899846. doi: 10.3389/fpubh.2022.899846
Received: 19 March 2022; Accepted: 06 June 2022;
Published: 11 July 2022.
Edited by:
Ravindra Purushottam Turankar, The Leprosy Mission Trust India, IndiaReviewed by:
Djaltou Aboubaker Osman, Center of Study and Research of Djibouti (CERD), EthiopiaCopyright © 2022 Zhou, Yang, Gong, Zhou, Yang, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haidan Liu, aGFpZGFubGl1QGNzdS5lZHUuY24=; Jijia Liu, bWNsaXVqaWppYUBjc3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.