- Department of Gastroenterology, The First Affiliated Hospital, Jinan University, Guangzhou, Guangdong, China
Background: Multifarious factors have a causal relationship with gastric cancer (GC) development. We conducted a comprehensive analysis to evaluate the strength of the evidence examining non-genetic risk factors for gastric cancer.
Methods: PubMed, Web of Science, and the Cochrane Library were searched from inception to November 10, 2021 to identify meta-analyses of observational studies examining the association between environmental factors and GC risk. For each meta-analysis, the random effect size, 95% confidence interval, heterogeneity among studies, and evidence of publication bias were assessed; moreover, the evidence was graded using predefined criteria, and the methodological quality was evaluated using AMSTAR 2.
Results: A total of 137 associations were examined in 76 articles. Among these meta-analyses, 93 associations yielded significant estimates (p < 0.05). Only 10 associations had strong epidemiologic evidence, including 2 risk factors (waist circumference and bacon), and 8 protective factors (dietary total antioxidant capacity, vegetable fat, cruciferous vegetable, cabbage, total vitamin, vitamin A, vitamin C, and years of fertility); 26 associations had moderate quality of evidence; and the remaining 57 associations were rated as weak. Ninety-four (68.61%) associations showed significant heterogeneity. Twenty-five (18.25%) associations demonstrated publication bias.
Conclusions: In this comprehensive analysis, multiple associations were found between environmental factors and GC with varying levels of evidence. Healthy dietary habits and lifestyle patterns could reduce the risk for GC. However, further high-quality prospective studies are still necessary to draw more definitive conclusions.
Background
The incidence of gastric cancer (GC) has gradually decreased in recent decades, mainly due to improved socioeconomic status, hygienic practices, and consequentially reduced Helicobacter pylori (HP) infection rates (1–3). However, GC remains the fifth most common cancer and the third major cause of oncological mortality worldwide (4, 5), and is responsible for over 1,000,000 new cases and approximately 80,000 deaths per year (4), which has posed a serious global public health burden. The etiology of GC is complicated and multifactorial; both genetic and environmental risk factors together with their interaction significantly contribute to its development (6, 7). A better understanding of these risk factors may improve the prediction and prevention of this condition.
Although many systematic reviews and meta-analyses have examined risk factors for GC (8), to our knowledge, there have been no systematic efforts to summarize and critically evaluate the evidence. Therefore, the aim of this comprehensive analysis is to provide a comprehensive overview and assess the strength, credibility, and classification of the existing epidemiological evidence examining the association between non-genetic factors and GC risk (9).
Methods
This study was registered in the International Prospective Register of Systematic Reviews (registration number: CRD42021290515). This study did not require ethical approval.
Literature search and eligible criteria
Two observers (HYQ and TCY) independently and systematically searched PubMed, Web of Science, Cochrane Library from inception to November 10, 2021, to identify observational studies of systematic reviews and meta-analyses assessing the association between non-genetic risk factors and GC using the following search algorithm: (“Stomach Neoplasms” OR “Gastric Cancer” OR “Cancer, Gastric” OR “Cancers, Gastric” OR “Gastric Cancers” OR “Gastric Neoplasm” OR “Gastric Neoplasms” OR “Stomach Neoplasm” OR “Neoplasm, Stomach” OR “Neoplasms, Stomach” OR “Neoplasms, Gastric” OR “Neoplasm, Gastric” OR “Stomach Cancers” OR “Cancers, Stomach” OR “Cancer, Stomach” OR “Stomach Cancer” OR “Cancer of the Stomach”) AND (“systematic review” OR “meta-analysis”). The reference lists of retrieved eligible papers were further hand-searched to avoid missing other potentially related studies. Only articles published in English were included.
Articles were deemed qualified if they satisfied all of the following inclusion criteria: (1) the articles were systematic reviews and meta-analyses of observational studies (i.e., cohort and case-control and cross-sectional studies); (2) the study evaluated the association of environmental (non-genetic) factors and GC risk, but not for screening, diagnostic, prognostic purposes; and (3) the study provided enough data to perform the analyses. The title and abstract of all eligible papers were initially screened, and then the full text of possible qualified articles was retrieved for further perusal based on the pre-established inclusion and exclusion criteria. Disagreements between two investigators were settled through a discussion. If multiple meta-analysis examined the identical scientific issue, we chose the largest number of studies to avoid repeated evaluation of the same primary studies (10, 11).
Data extraction
From each included meta-analysis, two researchers (HYQ and TCY) independently extracted the following data: the examined risk factors, the first author's name, year of publication, the epidemiological design and number of included studies, and the number of participants and cases. The study-specific relative risk estimates [i.e., relative risks (RRs), odds ratios (ORs), and hazard ratios (HRs) together with their corresponding 95% confidence intervals (CI)], heterogeneity, and publication bias for every risk factor were also collected in each study. Divergence during data extraction was clarified by discussion until a consensus was reached.
Assessment of methodological quality
Two researchers (HYQ and TCY) independently appraised the methodological quality of all included systematic reviews using the updated 16-item AMSTAR 2 instrument (a measurement tool for assessing the methodological quality of systematic reviews) (12). This tool is used to classify the methodological quality into four grades: high, moderate, low, and critically low. No or only one non-critical flaw is considered high quality, more than one non-critical defect is considered moderate quality, only one critical defect with or without non-critical flaws is low quality, and more than one critical defect with or without non-critical flaws is considered critically low quality. Any differences between the AMSTARS 2 scores were resolved through a discussion or arbitration by the third investigator (LDL).
Evaluation of the evidence quality
Two authors (HYQ and TCY) independently assessed the strength of the epidemiologic evidence using the following criteria (11, 13, 14):
(1) precision of the estimate (p-value <0.001 (15, 16), a threshold related to significantly fewer false-positive results, and >1,000 cases), (2) the heterogeneity between studies was not relatively large (I2 <50% and p-value of Cochran Q test>0.10), and (3) no evidence of small-study effects (p-value of Egger's test>0.10). The strength of the epidemiologic evidence was classified as high (when all of these criteria were met), moderate (when a maximum of 1 criterion was not met and p-value < 0. 001 was satisfied), or weak (in other cases, p-value < 0.05). When the p-value was not directly reported, it was recalculated from the 95% confidence interval of the pooled effect estimate using an established method (17). In case of doubt during the evaluation of evidence quality, discrepancies were settled through arbitration with a third investigator (LDL).
Data synthesis and analysis
Based on the extracted raw data from every included study, we reanalyzed and presented the random-effects estimate whenever the fixed-effects model was initially used (14). And in the case of missing measures, we calculated them when enough data were available. A p-value of the pooled estimate of effect size of < 0.05 was deemed significant. I2 and Q test was used to determine the heterogeneity (18) among studies, while Egger's test was utilized to evaluate the small-study effects (19); a p-value of < 0.10 (Q test) indicated a significant heterogeneity and a publication bias. Values (I2 test) exceeding 50% were generally considered to indicate high heterogeneity. All p-values were two tailed, and all statistical analyses were performed using Stata version 16.0.
Results
Characteristics of the included studies
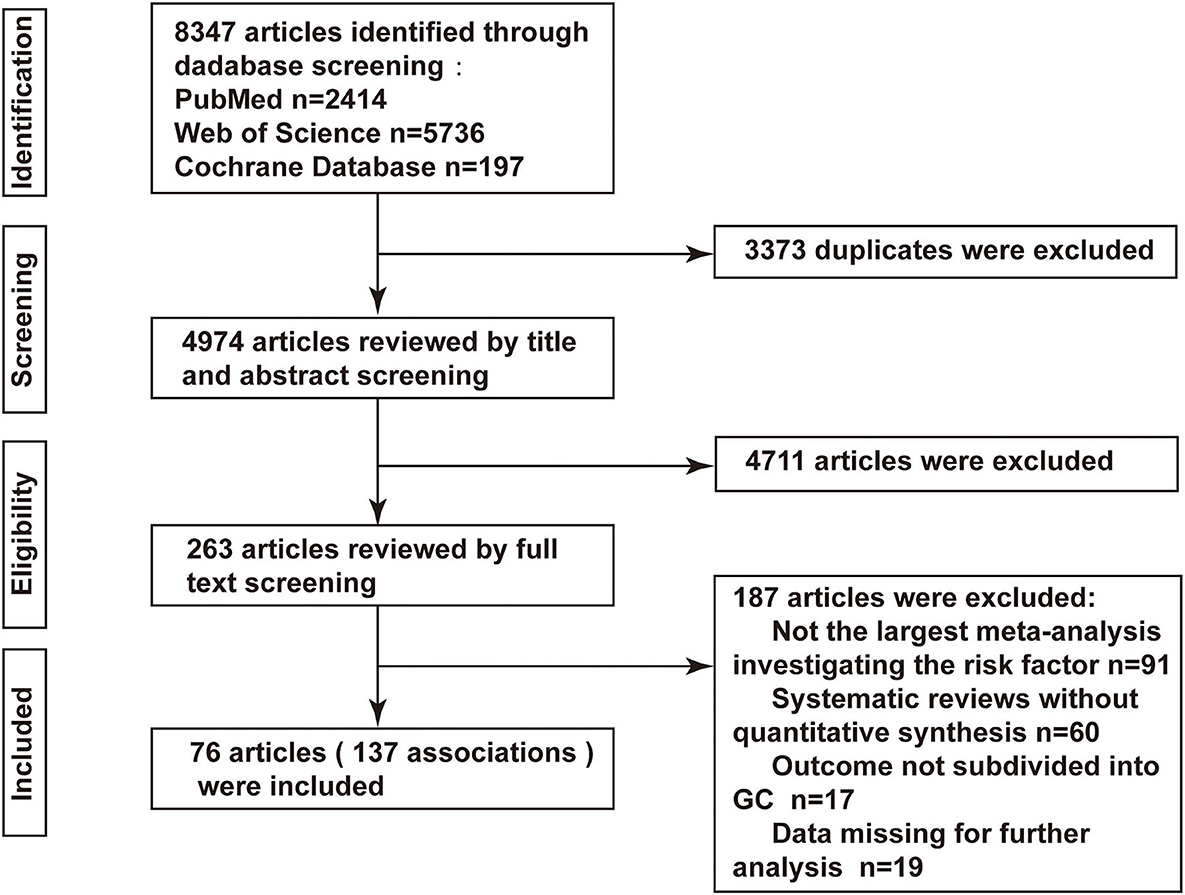
Overall, the initial search identified a total of 8,347 articles (2,414 from PubMed, 5,736 from Web of Science, and 197 from the Cochrane Library), of which 76 were finally deemed eligible (20–95). The process of selecting contained meta-analyses is displayed in Figure 1, while the general characteristics of the included eligible articles are summarized in Supplementary Table S1. The publication dates of contained studies ranged from 2008 to 2021. Among the meta-analyses reported in our study, the median number of original articles included in each meta-analysis was 12 (range: 2–73), the median number of cases was 4,745 (range: 51–137,451), and the number of cases was >1,000 in 113 (82.48%) meta-analyses. All 76 articles examined 112 unique risk factors and 137 associations, among these associations, 93 (67.88%) reported significant summary effects with a p-value of <0.05, while 47 (34.3%) reported a p-value of <0.001 (Supplementary Table S1).
Anthropometric indices
Obesity is a well-recognized risk factor for multiple adverse health outcomes. Waist circumference and waist–to–hip ratio were associated with an increased risk of GC in the highest vs. lowest comparisons (RR 1.48; 1.24–1.78 and RR 1.33; 1.04–1.70, respectively) (20). Similarly, a higher body mass index (≥30 vs. 18.5-25) was also associated with GC (OR 1.13; 1.03–1.24) (22) (Figure 2).
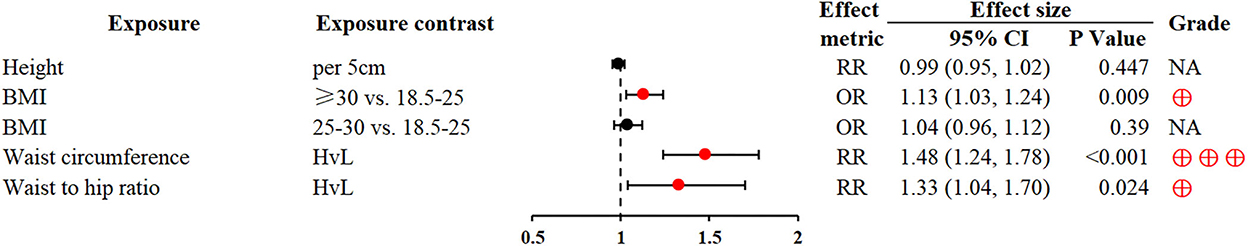
Figure 2. Forest plot: summary effect estimates of meta-analyses reporting associations between GC and factors pertaining to anthropometric indices. BMI, body mass index; HvL, highest vs. lowest; NA, not applicable; red dots represent risk factors; blue dots represent protective factors; The strength of the epidemiologic evidence was rated as high (⊕⊕⊕), moderate (⊕⊕), weak (⊕).
Dietary intake
The Mediterranean diet (MedDiet) and dietary total antioxidant capacity (D-TAC) were associated with significant reductions in GC risk for the highest vs. lowest comparisons (RR 0.7; 0.61–0.8 and RR 0.63; 0.53–0.73; respectively) (23, 26). Fiber intake was inversely associated with GC (28), whereas, refined grain consumption of the highest dosage was related to a significantly increased risk of GC (OR 1.36; 1.21–1.54) (30). Intake of meat, particularly red and processed meat was associated with GC (34, 94). High salt consumption, especially salt-preserved foods, could increase the GC risk (36, 37). Fruit and vegetable intake were widely reported as protective factors for GC (40, 41, 43); of note, vegetable fat also had beneficial effects on GC (RR 0.55; 0.41–0.74) (32). Heavy alcohol drinking and chili intake might increase the risk of GC (48, 49), but no association between tea and GC was found (50) (Figure 3).
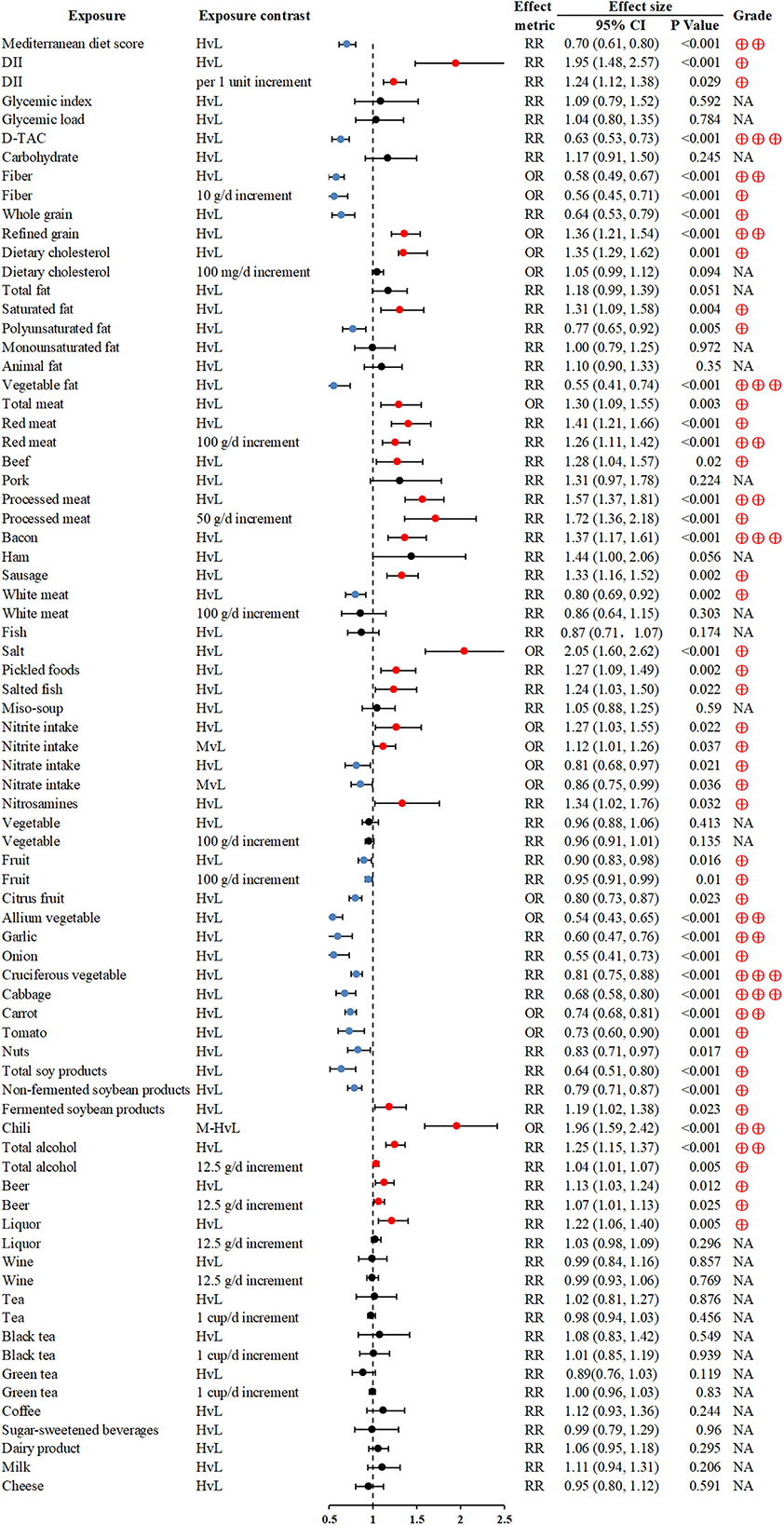
Figure 3. Forest plot: summary effect estimates of meta-analyses reporting associations between GC and factors pertaining to dietary intake. DII, dietary inflammatory index; D-TAC, dietary total antioxidant capacity.
Micronutrients
Higher vitamin consumption was associated with reduced GC risk (RR 0.73; 0.68–0.78) (55), especially antioxidant vitamins (vitamin A, vitamin C, vitamin E, β-carotene, and α-carotene). An inverse association between total polyphenol intake and GC was also found (OR 0.67; 0.54–0.81, for the highest vs. lowest intake comparisons) (62) (Figure 4).
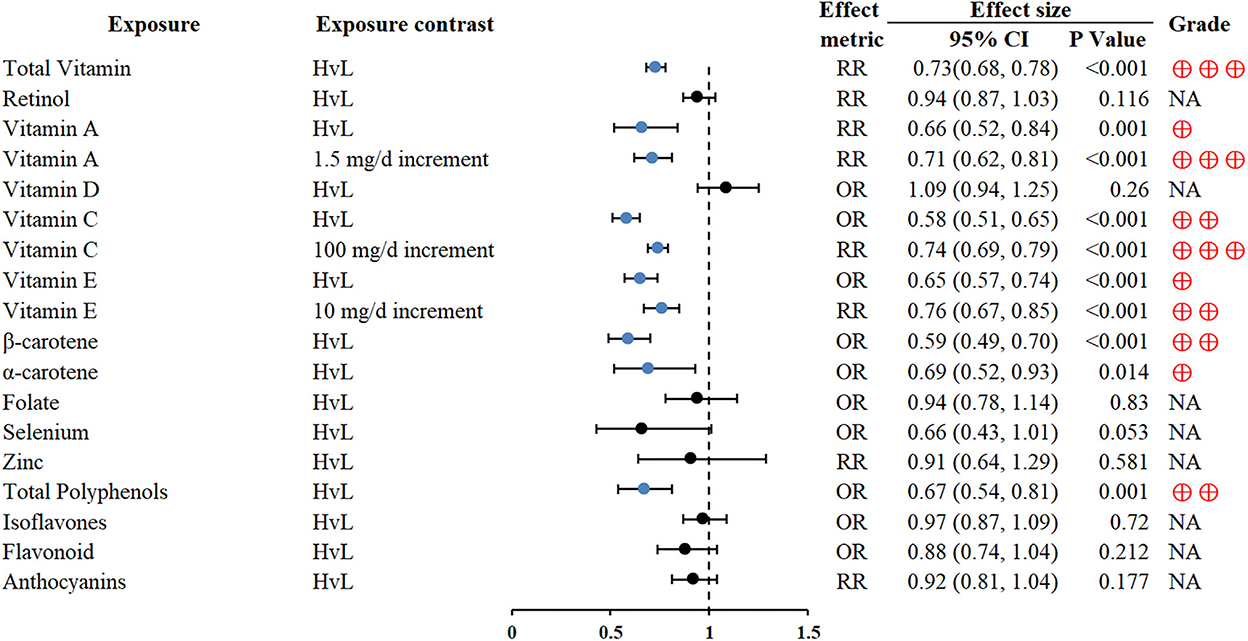
Figure 4. Forest plot: summary effect estimates of meta-analyses reporting associations between GC and factors pertaining to micronutrients.
Use of medication therapy
Regular proton pump inhibitors (PPI) use was associated with GC (RR 1.78; 1.38–2.31) (66). Conversely, regular aspirin use could reduce the risk of GC (RR 0.6; 0.51–0.82) (67). In addition, statin use and menopausal hormone therapy might also be related to a decreased risk of GC (OR 0.65; 0.45–0.93 and RR 0.77; 0.64–0.92, respectively) (71, 72) (Figure 5).
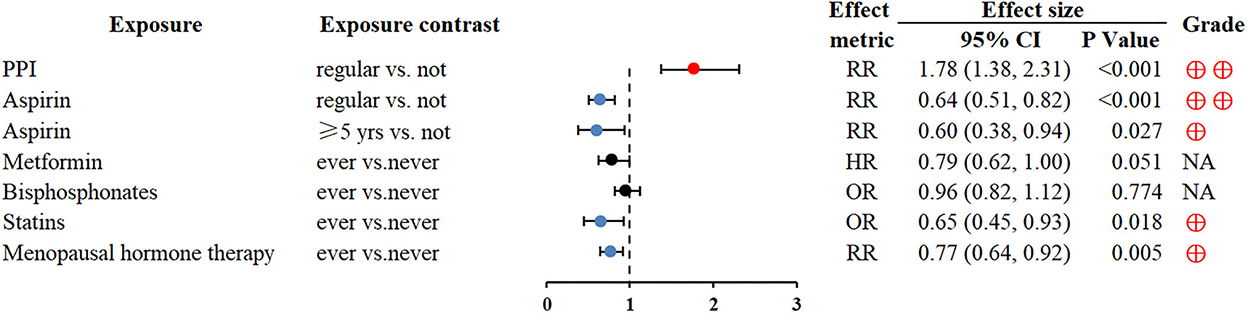
Figure 5. Forest plot: summary effect estimates of meta-analyses reporting associations between GC and factors pertaining to use of medication. PPI, proton pump inhibitors.
Lifestyle
Smoking can be linked to the development of many cancers, including GC (73, 74). Physically active people were protected from subsequent GC (RR 0.81; 0.73–0.89) (76). Higher toothbrushing frequency and refrigerator use also reduced the risk of GC (OR 0.84; 0.77–0.92 and OR 0.7; 0.56–0.88, respectively) (77, 78) (Figure 6).
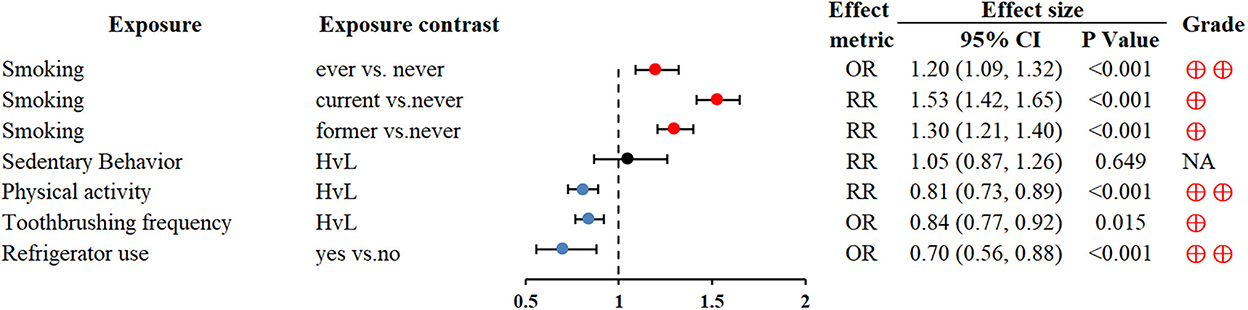
Figure 6. Forest plot: summary effect estimates of meta-analyses reporting associations between GC and factors pertaining to use of lifestyle.
Pre-existing medical history
Depression was associated with an increased GC risk (OR 1.84; 1.61–2.09) (79). Non-alcoholic fatty liver disease (NAFLD) could significantly increase the development risk of various extrahepatic cancers, including GC (OR 1.74; 1.03–2.95) (80). Based on the contribution of autoimmunity to gastric carcinogenesis, autoimmune diseases (e.g., systemic lupus erythematosus, pernicious anemia and diabetes mellitus, type 1) were closely associated with an increased risk of GC (RR 1.34; 1.05–1.72; RR 2.84; 2.30–3.50; RR 1.41; 1.2–1.67, respectively) (81, 83) (Figure 7).
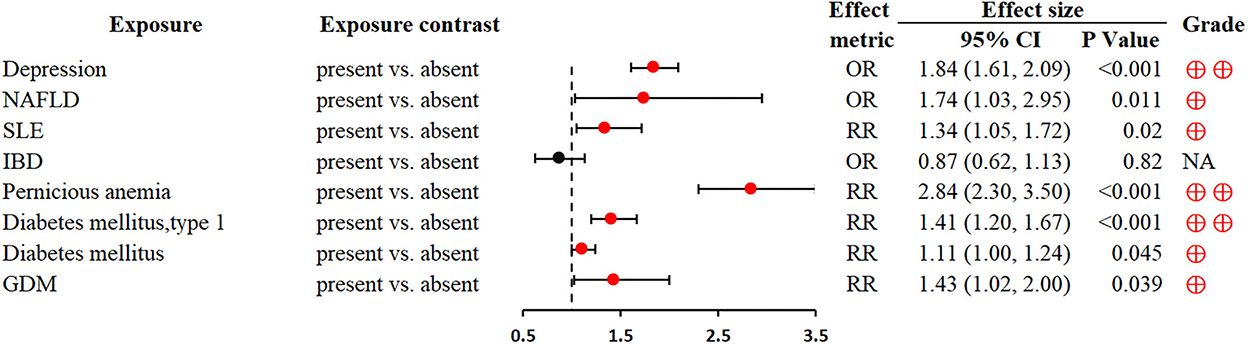
Figure 7. Forest plot: summary effect estimates of meta-analyses reporting associations between GC and factors pertaining to pre-existing medical history. NAFLD, non-alcoholic fatty liver disease; SLE, systemic lupus erythematosus; IBD, inflammatory bowel disease; GDM, gestational diabetes mellitus.
Viral or bacterial infection
In addition to well-recognized HP and Epstein-Barr virus, other viruses (e.g., hepatitis B virus, hepatitis C virus, human cytomegalovirus, human papillomavirus and John Cunningham virus) were also associated with a higher incidence of GC (86–89) (Figure 8).
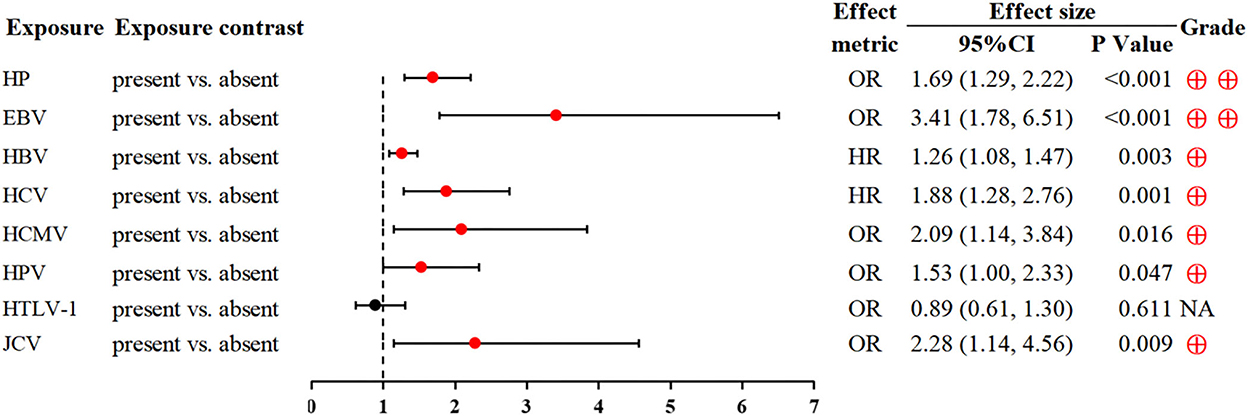
Figure 8. Forest plot: summary effect estimates of meta-analyses reporting associations between GC and factors pertaining to viral or bacterial infection. HP, Helicobacter pylori; EBV, Epstein-Barr virus; HBV, hepatitis B virus; HCV, hepatitis C virus; HCMV, human cytomegalovirus; HPV, human papillomavirus; HTLV-1, human T-cell lymphotropic virus type 1; JCV, John Cunningham virus.
Other factors
A strong inverse association was found between socioeconomic position indicators (educational level and household income) and GC risk (OR 0.60; 0.44–0.84 and OR 0.65; 0.48–0.89, respectively) (90). Of note, we found that blood group O and longer duration of fertility were protective factors of GC, conversely, blood group A was associated with a higher GC risk (72, 91) (Figure 9).
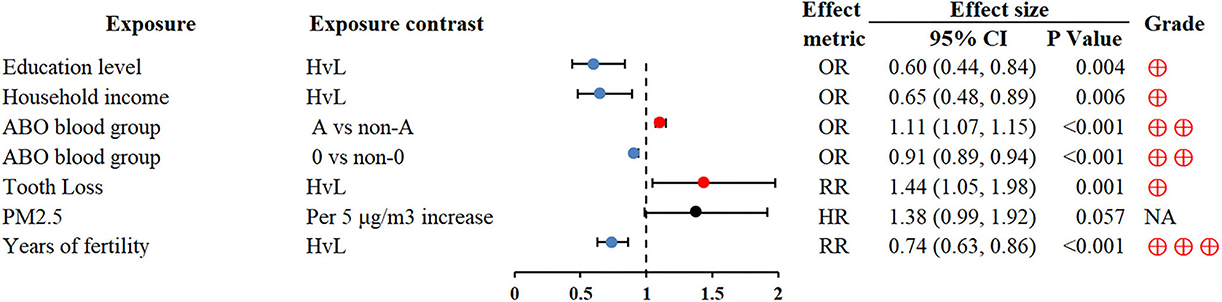
Figure 9. Forest plot: summary effect estimates of meta-analyses reporting associations between GC and factors pertaining to other factors. PM2.5, particulate matter with a diameter of 2.5 μm or less.
Heterogeneity and small study effects
With regard to heterogeneity, in the 137 unique meta-analyses, only 43 (31.39%) associations showed absence of heterogeneity (I2 < 50% and p-value of Cochran Q test ≥ 0.10), while the remaining 94 (68.61%) associations indicated significant heterogeneity (I2 ≥ 50% or p-value of Cochran Q test < 0.10). In terms of publication bias, 110 (80.29%) meta-analyses showed no evidence of significant small-study effects (p-value ≥ 0.10 of Egger's test), whereas 25 (18.25%) meta-analyses demonstrated publication bias (p-value < 0.10 of Egger's test). With regard to the associations of GC with fiber intake (10 g/d increment) (28) and HTLV-1 infection (89), small-study effects were not applicable as only 2 observational studies were included in each meta-analysis.
Quality assessment of meta-analyses
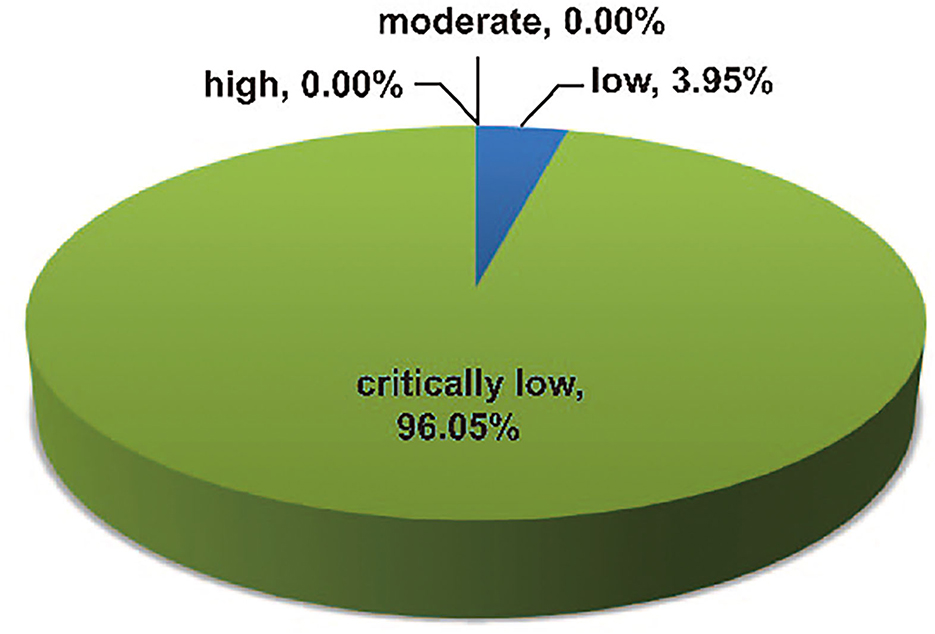
The methodological qualities of the 76 included articles were assessed and graded using the 16-item AMSTAR2 tool (Supplementary Table S2); three (3.95%) studies were determined to have low methodological quality, while the other 73 (96.05%) studies were determined to have critically low methodological quality (Figure 10). Based on the AMSTAR 2 scores, none of the eligible articles had high or moderate methodological quality. The most common critical flaws were as follows: lack of registered protocol (n = 64, 84.21%), incomplete literature search (n = 75, 98.68%), and the absence of list of excluded studies (n = 64, 84.21%).
Strength of epidemiologic evidence
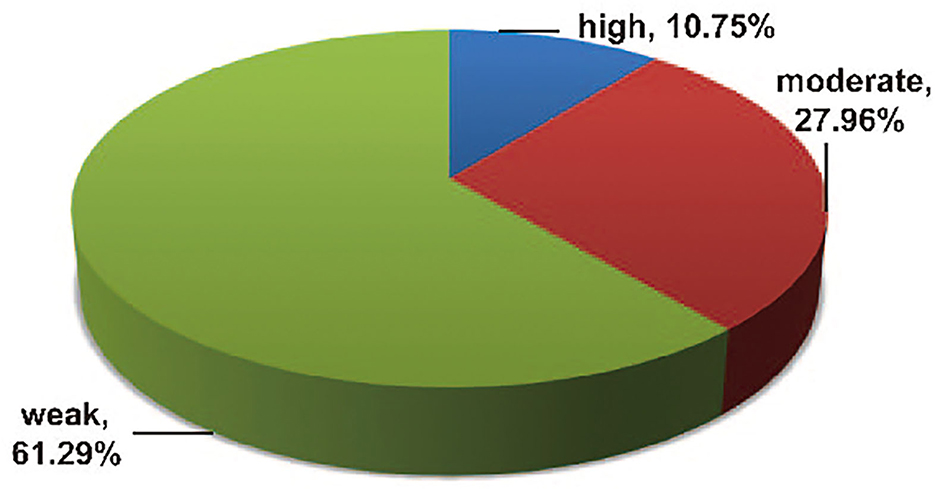
The outcomes of the epidemiologic evidence measurement are presented in Supplementary Table S3. Among the 93 statistically significant associations, only 10 (10.75%) showed high epidemiologic evidence for an association with GC according to the abovementioned prespecified credibility criteria (with >1,000 cases, p-value of < 0.001, and absence of large heterogeneity and small-study effects), including two risk factors (waist circumference and bacon) and eight protective factors (D-TAC, vegetable fat, cruciferous vegetable, cabbage, total vitamin, vitamin A [HvL], vitamin C [100 mg/d increment], and years of fertility) (20, 26, 32, 43, 55, 56, 72, 94). A total of 26 (27.96%) associations demonstrated moderate epidemiologic evidence, the remaining 57 (61.29%) associations presented weak epidemiological evidence (Figure 11).
Discussion
In this comprehensive analysis, 76 meta-analyses of observational studies were identified and appraisal of current evidence that examined the association of GC with various environmental risk factors was performed. All 137 environmental associations, covering anthropometric indices, dietary intake, micronutrients, use of medication, lifestyle, pre-existing medical history, viral or bacterial infection, and others, were assessed. Among these, two risk factors (waist circumference and bacon) and eight protective factors (D-TAC, vegetable fat, cruciferous vegetable, cabbage, total vitamin, vitamin A, vitamin C, and years of fertility) yielded high epidemiologic evidence without any evidence of bias (20, 26, 32, 43, 55, 56, 72, 94). However, we cannot confirm if other connections are not meaningful, and some uncertainties still need to be evaluated further.
The association between waist circumference and increased risk of GC was supported by high epidemiologic evidence (20), indicating that abdominal obesity plays a major role in the development of GC, and this is consistent with previous studies (96–98). The potential molecular mechanism of carcinogenesis is as follows: the metabolically active visceral adipose tissues promote the production of inflammatory mediators and cytokines (e.g., TNF-α and leptin), inhibit the secretion of adiponectin, and facilitate the development of insulin resistance (99, 100) and subsequent hyperinsulinemia to partially promote carcinogenesis by stimulating the increase in the expression of insulin-like growth factor (IGF-1) (101).
Of note, more than half of the associations examined a broad variety of dietary factors, which revealed the current direction of this line of research. The MedDiet is a recognized healthy dietary pattern, characterized by relatively high consumption of fruits, vegetables, legumes, and olive oil, moderate intake of dairy products and fish, and very limited intake of red meat and processed meat products (23, 102). Our results showed that the MedDiet is inversely associated with GC risk by 30% (23). Fruits and vegetables are rich sources of dietary fiber and antioxidant vitamins. Higher consumption of dietary fiber increases stool bulk, thereby diluting and slowing the absorption of potential carcinogens (103). Fiber can also be fermented into short-chain fatty acids by gut microbiota to exert antitumor effects (28). That is why a higher intake of refined grains increases the risk of GC (30). Antioxidant vitamins can scavenge free radicals, enhance antioxidative capacity and reduce cell oxidative damage (104). Potential carcinogens (e.g., N-nitroso compounds, polycyclic aromatic hydrocarbons and heterocyclic aromatic amines) and lipid peroxidation may explain the positive association between excessive red and processed meat intake and GC (34). Pickled foods, as a potential source of nitrosamines, are associated with a higher GC risk (37). Heavy alcohol drinking is closely related to GC, mainly due to the oxidative stress and DNA damage induced by its metabolite, acetaldehyde, and the direct damaging effect of ethanol on gastric mucosa (49).
With regard to medication therapy, long-term use of PPIs could increase GC risk, as supported by moderate epidemiologic evidence, mainly due to the accelerating progression of HP-related atrophic gastritis- and hypergastrinemia-promoting enterochromaffin-like cell hyperplasia (105). However, based on our findings, aspirin showed moderate epidemiologic evidence of GC- preventive effects, likely owing to the inhibition of cyclooxygenase-2 (COX-2) (67). In terms of pre-existing medical conditions, depression, pernicious anemia, and type 1 diabetes mellitus presented an increased risk of GC with moderate credibility. Depression can degrade the immune and endocrine systems, thus reducing resistance to cancer (106). Depression can also promote the secretion of glucocorticoids due to the influence of the hypothalamus–pituitary–adrenal axis, causing gastric mucosal erosion and ulcers (107). The positive association between pernicious anemia and GC is mainly due to the destruction of acid-producing parietal cells (108).
The estimated 60 well-established carcinogens found in cigarette smoke could explain the positive association between smoking and GC risk (109). Moderate credibility indicated that the risk of GC was increased in individuals with blood group A, but was significantly reduced in individuals with blood group O. Individuals with blood group A is more susceptible to GC partly due to the reduced immune system's response to tumors and the increased risk of pernicious anemia (110) and HP infection (111). The longer duration of fertility showed highly credible evidence of an inverse association with GC, mainly owing to the effect of estrogen (72).
Most of the assessed associations could not show high epidemiological evidence due to the significant heterogeneity and/or small-study effects. Heterogeneity usually indicates the presence of bias in some primary studies, but might also be due to the real differences among studies. Genuine heterogeneity might play a role in the field of GC, including the difference in exposure assessment, the mixture of cohort and case-control studies in some meta-analyses, differential association of risk factors due to geographical heterogeneity, and so on. As positive results are more likely to be published compared with null results, and the study participants may be a small portion of the actual population with the disease, the probability of small-study effects should be taken into consideration. The reported associations with GC need to be explained with caution, particularly in meta-analyses with a relatively small number of included studies; the heterogeneity and publication bias among researches are evident.
Strengths and limitations
Our study has several strengths. This comprehensive analysis was the first to provide a comprehensive overview of the evidence to evaluate the association of non-genetic factors with GC risk, although several studies reported the risk factors of GC. Then, the systematic literature search, article selection, and data extraction were conducted by two independent researchers. Additionally, the AMSTAR 2 tool was utilized to appraise the methodological quality of the eligible systematic reviews. Furthermore, the epidemiologic evidence was graded in accordance with the predefined criteria including the evaluation of the estimate precision, heterogeneity, and publication bias.
Nevertheless, our study has several limitations. First, only published meta-analyses of observational researches were included; thus, other risk factors with enough evidence that have not yet been evaluated by meta-analytic quantitative synthesis were possibly overlooked. Second, the quality of component meta-analyses was not evaluated as it exceeded the range reported in our study, and meta-analyses should be conducted by the researchers of the primary studies. Third, the majority of included meta-analyses showed heterogeneity, and the observational investigations were prone to selection and recall biases, especially case-control studies. Fourth, the systematic reviews and meta-analyses contained in our study were only published in English; thus, the possible missing information that were published in other languages might affect the evaluation results.
Conclusions
In conclusion, developing a healthier dietary (e.g., MedDiet) and lifestyle pattern along with promoting physical activity to prevent obesity could hopefully reduce the incidence of GC in the near future. However, further high-quality prospective studies excluding potential residual confounders are needed; the application of reporting guidelines (e.g., STROBE) (112) and registration of hypothesis-testing observational studies may be necessary to improve the credibility of evidence. Updated methodologically robust meta-analyses are also needed to better understand the association of GC with these factors and draw firmer conclusions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YH and XG contributed to the concept and design of the comprehensive analysis. YH, CT, and DL collected and analyzed the data. YH and HZ drafted the manuscript. XG revised the manuscript. All authors read and approved the final manuscript to be published.
Acknowledgments
We would like to appreciate all authors of the original meta-analysis that were included in this comprehensive analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.892468/full#supplementary-material
References
1. Luo G, Zhang Y, Guo P, Wang L, Huang Y, Li K. Global patterns and trends in stomach cancer incidence: age, period and birth cohort analysis. Int J Cancer. (2017) 141:1333–44. doi: 10.1002/ijc.30835
2. Mashta O. Stomach cancer incidence has halved over past 30 years in Britain. BMJ. (2009) 339:b3281. doi: 10.1136/bmj.b3281
3. Qin Y, Tong X, Fan J, Liu Z, Zhao R, Zhang T, et al. Global burden and trends in incidence, mortality, and disability of stomach cancer from 1990 to 2017. Clin Transl Gastroenterol. (2021) 12:e00406. doi: 10.14309/ctg.0000000000000406
4. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
5. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. (2019) 144:1941–53. doi: 10.1002/ijc.31937
6. Jin G, Lv J, Yang M, Wang M, Zhu M, Wang T, et al. Genetic risk, incident gastric cancer, and healthy lifestyle: a meta-analysis of genome-wide association studies and prospective cohort study. Lancet Oncol. (2020) 21:1378–86. doi: 10.1016/S1470-2045(20)30460-5
7. Fang X, Wei J, He X, An P, Wang H, Jiang L, et al. Landscape of dietary factors associated with risk of gastric cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Eur J Cancer (Oxford, England: 1990). (2015) 51:2820–32. doi: 10.1016/j.ejca.2015.09.010
8. Poorolajal J, Moradi L, Mohammadi Y, Cheraghi Z, Gohari-Ensaf F. Risk factors for stomach cancer: a systematic review and meta-analysis. Epidemiol Health. (2020) 42:e2020004. doi: 10.4178/epih.e2020004
9. Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. (2015) 13:132–40. doi: 10.1097/XEB.0000000000000055
10. Veronese N, Solmi M, Caruso MG, Giannelli G, Osella AR, Evangelou E, et al. Dietary fiber and health outcomes: an umbrella review of systematic reviews and meta-analyses. Am J Clin Nutr. (2018) 107:436–44. doi: 10.1093/ajcn/nqx082
11. Belbasis L, Bellou V, Evangelou E, Ioannidis JP, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. (2015) 14:263–73. doi: 10.1016/S1474-4422(14)70267-4
12. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Clinical research ed). (2017) 358:j4008. doi: 10.1136/bmj.j4008
13. Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ (Clinical research ed). (2014) 348:g2035. doi: 10.1136/bmj.g2035
14. Piovani D, Danese S, Peyrin-Biroulet L, Nikolopoulos GK, Lytras T, Bonovas S. Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta-analyses. Gastroenterology. (2019) 157:647–59.e4. doi: 10.1053/j.gastro.2019.04.016
15. Gelman A, Robert CP. Revised evidence for statistical standards. Proc Natl Acad Sci U S A. (2014) 111:E1933. doi: 10.1073/pnas.1322995111
16. Ioannidis JP, Tarone R, McLaughlin JK. The false-positive to false-negative ratio in epidemiologic studies. Epidemiology. (2011) 22:450–6. doi: 10.1097/EDE.0b013e31821b506e
17. Altman DG, Bland JM. How to obtain the P value from a confidence interval. BMJ (Clin Res). (2011) 343:d2304. doi: 10.1136/bmj.d2304
18. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clin Res)). (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
19. Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. (2018) 74:785–94. doi: 10.1111/biom.12817
20. Du X, Hidayat K, Shi BM. Abdominal obesity and gastroesophageal cancer risk: systematic review and meta-analysis of prospective studies. Bioscience Rep. (2017) 37:474. doi: 10.1042/BSR20160474
21. Seo MS, Park DK, Hwang IC, Shim JY, Ahn HY. Adult height is not associated with the risk of stomach cancer in a meta-analysis. J Gastrointest Oncol. (2020) 11:708–14. doi: 10.21037/jgo-20-199
22. Lin X-J, Wang C-P, Liu X-D, Yan K-K, Li S, Bao H-H, et al. Body mass index and risk of gastric cancer: a meta-analysis. Jpn J Clin Oncol. (2014) 44:783–91. doi: 10.1093/jjco/hyu082
23. Morze J, Danielewicz A, Przybylowicz K, Zeng H, Hoffmann G, Schwingshackl L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur J Nutr. (2021) 60:1561–86. doi: 10.1007/s00394-020-02346-6
24. Liang Y, Jiao H, Qu L, Liu H. Positive association between dietary inflammatory index and gastric cancer risk: a systematic review and meta-analysis. Nutr Cancer. (2020) 72:1290–6. doi: 10.1080/01635581.2019.1679197
25. Turati F, Galeone C, Augustin LSA, La Vecchia C. Glycemic index, glycemic load and cancer risk: an updated meta-analysis. Nutrients. (2019) 11:2342. doi: 10.3390/nu11102342
26. Parohan M, Sadeghi A, Khatibi SR, Nasiri M, Milajerdi A, Khodadost M, et al. Dietary total antioxidant capacity and risk of cancer: a systematic review and meta-analysis on observational studies. Critical Rev Oncol Hematol. (2019) 138:70–86. doi: 10.1016/j.critrevonc.2019.04.003
27. Ye Y, Wu Y, Xu J, Ding K, Shan X, Xia D. Association between dietary carbohydrate intake, glycemic index and glycemic load, and risk of gastric cancer. Eur J Nutr. (2017) 56:1169–77. doi: 10.1007/s00394-016-1166-4
28. Zhang Z, Xu G, Ma M, Yang J, Liu X. Dietary fiber intake reduces risk for gastric cancer: a meta-analysis. Gastroenterology. (2013) 145:113–20.e3. doi: 10.1053/j.gastro.2013.04.001
29. Zhang X-F, Wang X-K, Tang Y-J, Guan X-X, Guo Y, Fan J-M, et al. Association of whole grains intake and the risk of digestive tract cancer: a systematic review and meta-analysis. Nutr J. (2020) 19:6. doi: 10.1186/s12937-020-00556-6
30. Wang T, Zhan R, Lu J, Zhong L, Peng X, Wang M, et al. Grain consumption and risk of gastric cancer: a meta-analysis. Int J Food Sci Nutr. (2020) 71:164–75. doi: 10.1080/09637486.2019.1631264
31. Miao P, Guan L. Association of dietary cholesterol intake with risk of gastric cancer: a systematic review and meta-analysis of observational studies. Front Nutr. (2021) 8:722450. doi: 10.3389/fnut.2021.722450
32. Han J, Jiang Y, Liu X, Meng Q, Xi Q, Zhuang Q, et al. Dietary fat intake and risk of gastric cancer: a meta-analysis of observational studies. PLoS ONE. (2015) 10:e0138580. doi: 10.1371/journal.pone.0138580
33. Ferro A, Rosato V, Rota M, Costa AR, Morais S, Pelucchi C, et al. Meat intake and risk of gastric cancer in the Stomach cancer Pooling (StoP) project. Int J Cancer. (2020) 147:45–55. doi: 10.1002/ijc.32707
34. Kim SR, Kim K, Lee SA, Kwon SO, Lee J-K, Keum N, et al. Effect of red, processed, and white meat consumption on the risk of gastric cancer: an overall and dose-response meta-analysis. Nutrients. (2019) 11:826. doi: 10.3390/nu11040826
35. Wu S, Liang J, Zhang L, Zhu X, Liu X, Miao D. Fish consumption and the risk of gastric cancer: systematic review and meta-analysis. BMC Cancer. (2011) 11:26. doi: 10.1186/1471-2407-11-26
36. Ge S, Feng X, Shen L, Wei Z, Zhu Q, Sun J. Association between habitual dietary salt intake and risk of gastric cancer: a systematic review of observational studies. Gastroenterol Res Pract. (2012) 2012:808120. doi: 10.1155/2012/808120
37. D'Elia L, Rossi G, Ippolito R, Cappuccio FP, Strazzullo P. Habitual salt intake and risk of gastric cancer: a meta-analysis of prospective studies. Clinical Nutr (Edinburgh, Scotland). (2012) 31:489–98. doi: 10.1016/j.clnu.2012.01.003
38. Zhang FX, Miao Y, Ruan JG, Meng SP, Dong JD, Yin H, et al. Association between nitrite and nitrate intake and risk of gastric cancer: a systematic review and meta-analysis. Med Sci Monit Int Med J Exp Clin Res. (2019) 25:1788–99. doi: 10.12659/MSM.914621
39. Song P, Wu L, Guan W. Dietary nitrates, nitrites, and nitrosamines intake and the risk of gastric cancer: a meta-analysis. Nutrients. (2015) 7:9872–95. doi: 10.3390/nu7125505
40. Wang Q, Chen Y, Wang X, Gong G, Li G, Li C. Consumption of fruit, but not vegetables, may reduce risk of gastric cancer: results from a meta-analysis of cohort studies. Eur J Cancer (Oxford, England: 1990). (2014) 50:1498–509. doi: 10.1016/j.ejca.2014.02.009
41. Zhou Y, Zhuang W, Hu W, Liu G-J, Wu T-X, Wu X-T. Consumption of large amounts of allium vegetables reduces risk for gastric cancer in a meta-analysis. Gastroenterology. (2011) 141:80–9. doi: 10.1053/j.gastro.2011.03.057
42. Turati F, Pelucchi C, Guercio V, La Vecchia C, Galeone C. Allium vegetable intake and gastric cancer: a case-control study and meta-analysis. Mol Nutr Food Res. (2015) 59:171–9. doi: 10.1002/mnfr.201400496
43. Wu QJ, Yang Y, Wang J, Han LH, Xiang YB. Cruciferous vegetable consumption and gastric cancer risk: a meta-analysis of epidemiological studies. Cancer Sci. (2013) 104:1067–73. doi: 10.1111/cas.12195
44. Fallahzadeh H, Jalali A, Momayyezi M, Bazm S. Effect of carrot intake in the prevention of gastric cancer: a meta-analysis. J Gastric Cancer. (2015) 15:256–61. doi: 10.5230/jgc.2015.15.4.256
45. Yang T, Yang X, Wang X, Wang Y, Song Z. The role of tomato products and lycopene in the prevention of gastric cancer: a meta-analysis of epidemiologic studies. Med Hypotheses. (2013) 80:383–8. doi: 10.1016/j.mehy.2013.01.005
46. Zhang D, Dai C, Zhou L, Li Y, Liu K, Deng YJ, et al. Meta-analysis of the association between nut consumption and the risks of cancer incidence and cancer-specific mortality. Aging. (2020) 12:10772–94. doi: 10.18632/aging.103292
47. Wang Y, Guo J, Yu F, Tian Y, Wu Y, Cui L, et al. The association between soy-based food and soy isoflavone intake and the risk of gastric cancer: a systematic review and meta-analysis. J Sci Food Agric. (2021) 101:5314–24. doi: 10.1002/jsfa.11334
48. Du Y, Lv Y, Zha W, Hong X, Luo Q. Chili consumption and risk of gastric cancer: a meta-analysis. Nutr Cancer. (2021) 73:45–54. doi: 10.1080/01635581.2020.1733625
49. Wang P-L, Xiao F-T, Gong B-C, Liu F-N. Alcohol drinking and gastric cancer risk: a meta-analysis of observational studies. Oncotarget. (2017) 8:99013–23. doi: 10.18632/oncotarget.20918
50. Zhao L-G, Li Z-Y, Feng G-S, Ji X-W, Tan Y-T, Li H-L, et al. Tea drinking and risk of cancer incidence: a meta-analysis of prospective cohort studies and evidence evaluation. Advan Nutr. (2021) 12:402–12. doi: 10.1093/advances/nmaa117
51. Huang Y, Chen H, Zhou L, Li G, Yi D, Zhang Y, et al. Association between green tea intake and risk of gastric cancer: a systematic review and dose-response meta-analysis of observational studies. Public Health Nutr. (2017) 20:3183–92. doi: 10.1017/S1368980017002208
52. Xie F, Wang D, Huang Z, Guo Y. Coffee consumption and risk of gastric cancer: a large updated meta-analysis of prospective studies. Nutrients. (2014) 6:3734–46. doi: 10.3390/nu6093734
53. Li Y, Guo L-l, He K, Huang C, Tang S. Consumption of sugar-sweetened beverages and fruit juice and human cancer: a systematic review and dose-response meta-analysis of observational studies. J Cancer. (2021) 12:3077–88. doi: 10.7150/jca.51322
54. Sun Y, Lin LJ, Sang LX Dai C, Jiang M, Zheng CQ. Dairy product consumption and gastric cancer risk: a meta-analysis. World J Gastroenterology. (2014) 20:15879–98. doi: 10.3748/wjg.v20.i42.15879
55. Kong P, Cai Q, Geng Q, Wang J, Lan Y, Zhan Y, et al. Vitamin intake reduce the risk of gastric cancer: meta-analysis and systematic review of randomized and observational studies. PLoS ONE. (2014) 9:e116060. doi: 10.1371/journal.pone.0116060
56. Wu Y, Ye Y, Shi Y, Li P, Xu J, Chen K, et al. Association between vitamin A, retinol intake and blood retinol level and gastric cancer risk: a meta-analysis. Clin Nutr (Edinburgh, Scotland). (2015) 34:620–6. doi: 10.1016/j.clnu.2014.06.007
57. Khayatzadeh S, Feizi A, Saneei P, Esmaillzadeh A. Vitamin D intake, serum Vitamin D levels, and risk of gastric cancer: a systematic review and meta-analysis. J Res Med Sci. (2015) 20:790–6. doi: 10.4103/1735-1995.168404
58. Li P, Zhang H, Chen J, Shi Y, Cai J, Yang J, et al. Association between dietary antioxidant vitamins intake/blood level and risk of gastric cancer. Int J Cancer. (2014) 135:1444–53. doi: 10.1002/ijc.28777
59. Tio M, Andrici J, Cox MR, Eslick GD. Folate intake and the risk of upper gastrointestinal cancers: a systematic review and meta-analysis. J Gastroenterol Hepatol. (2014) 29:250–8. doi: 10.1111/jgh.12446
60. Vinceti M, Filippini T, Del Giovane C, Dennert G, Zwahlen M, Brinkman M, et al. Selenium for preventing cancer. Cochra Data Sys Rev. (2018) 1:195. doi: 10.1002/14651858.CD005195.pub4
61. Li P, Xu J, Shi Y, Ye Y, Chen K, Yang J, et al. Association between zinc intake and risk of digestive tract cancers: a systematic review and meta-analysis. Clinical Nutr (Edinburgh, Scotland). (2014) 33:415–20. doi: 10.1016/j.clnu.2013.10.001
62. Vitelli-Storelli F, Rossi M, Pelucchi C, Rota M, Palli D, Ferraroni M, et al. Polyphenol intake and gastric cancer risk: findings from the Stomach Cancer Pooling Project (StoP). Cancers. (2020) 12:3064. doi: 10.3390/cancers12103064
63. You J, Sun Y, Bo Y, Zhu Y, Duan D, Cui H, et al. The association between dietary isoflavones intake and gastric cancer risk: a meta-analysis of epidemiological studies. BMC Public Health. (2018) 18:510. doi: 10.1186/s12889-018-5424-7
64. Bo Y, Sun J, Wang M, Ding J, Lu Q, Yuan L. Dietary flavonoid intake and the risk of digestive tract cancers: a systematic review and meta-analysis. Sci Rep. (2016) 6:24836. doi: 10.1038/srep24836
65. Yang D, Wang X, Yuan W, Chen Z. Intake of anthocyanins and gastric cancer risk: a comprehensive meta-analysis on cohort and case-control studies. J Nutr Sci Vitaminol. (2019) 65:72–81. doi: 10.3177/jnsv.65.72
66. Zeng R, Cheng Y, Luo D, Wang J, Yang J, Jiang L, et al. Comprehensive analysis of proton pump inhibitors and risk of digestive tract cancers. Eur J Cancer (Oxford, England: 1990) (2021) 156:190–201. doi: 10.1016/j.ejca.2021.07.030
67. Bosetti C, Santucci C, Gallus S, Martinetti M, La Vecchia C. Aspirin and the risk of colorectal and other digestive tract cancers: an updated meta-analysis through 2019. Ann Oncol. (2020) 31:558–68. doi: 10.1016/j.annonc.2020.02.012
68. Wang L, Zhang R, Yu L, Xiao J, Zhou X, Li X, et al. Aspirin use and common cancer risk: a meta-analysis of cohort studies and randomized controlled trials. Front Oncol. (2021) 11:690219. doi: 10.3389/fonc.2021.690219
69. Shuai Y, Li C, Zhou X. The effect of metformin on gastric cancer in patients with type 2 diabetes: a systematic review and meta-analysis. Clin Transl Oncol. (2020) 22:1580–90. doi: 10.1007/s12094-020-02304-y
70. Wright E, Schofield PT, Molokhia M. Bisphosphonates and evidence for association with esophageal and gastric cancer: a systematic review and meta-analysis. BMJ Open. (2015) 5:e007133. doi: 10.1136/bmjopen-2014-007133
71. Singh PP, Singh S. Statins are associated with reduced risk of gastric cancer: a systematic review and meta-analysis. Ann Oncol. (2013) 24:1721–30. doi: 10.1093/annonc/mdt150
72. Camargo MC, Goto Y, Zabaleta J, Morgan DR, Correa P, Rabkin CS. Sex hormones, hormonal interventions, and gastric cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. (2012) 21:20–38. doi: 10.1158/1055-9965.EPI-11-0834
73. Ferro A, Morais S, Rota M, Pelucchi C, Bertuccio P, Bonzi R, et al. Tobacco smoking and gastric cancer: meta-analyses of published data versus pooled analyses of individual participant data (StoP Project). Eur J Cancer Prevent. (2018) 27:197–204. doi: 10.1097/CEJ.0000000000000401
74. Ladeiras-Lopes R, Pereira AK, Nogueira A, Pinheiro-Torres T, Pinto I, Santos-Pereira R, et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. CCC. (2008) 19:689–701. doi: 10.1007/s10552-008-9132-y
75. Schmid D, Leitzmann MF. Television viewing and time spent sedentary in relation to cancer risk: a meta-analysis. JNCI. (2014) 106:98. doi: 10.1093/jnci/dju098
76. Psaltopoulou T, Ntanasis-Stathopoulos I, Tzanninis IG, Kantzanou M, Georgiadou D, Sergentanis TN. Physical activity and gastric cancer risk: a systematic review and meta-analysis. Clin J Sport Med. (2016) 26:445–64. doi: 10.1097/JSM.0000000000000316
77. Wu H, Zhang J, Zhou B. Toothbrushing frequency and gastric and upper aerodigestive tract cancer risk: a meta-analysis. Eur J Clin Invest. (2021) 51:e13478. doi: 10.1111/eci.13478
78. Yan S, Gan Y, Song X, Chen Y, Liao N, Chen S, et al. Association between refrigerator use and the risk of gastric cancer: a systematic review and meta-analysis of observational studies. PLoS ONE. (2018) 13:e0203120. doi: 10.1371/journal.pone.0203120
79. Zhang Y, Ma N, Duan F, Yin J, He G, Wang K, et al. Depression and the occurrence of gastric cancer: a meta-analysis based on their relationship and epidemiological evaluation. J Public Health-Heidelberg. (2021) 30:1533–43. doi: 10.1007/s10389-020-01469-8
80. Liu SS, Ma XF, Zhao J, Du SX, Zhang J, Dong MZ, et al. Association between nonalcoholic fatty liver disease and extrahepatic cancers: a systematic review and meta-analysis. Lipids Health Dis. (2020) 19:118. doi: 10.1186/s12944-020-01288-6
81. Clarke AE, Pooley N, Marjenberg Z, Langham J, Nicholson L, Langham S, et al. Risk of malignancy in patients with systemic lupus erythematosus: systematic review and meta-analysis. Semin Arthritis Rheum. (2021) 51:1230–41. doi: 10.1016/j.semarthrit.2021.09.009
82. Wan Q, Zhao R, Xia L, Wu Y, Zhou Y, Wang Y, et al. Inflammatory bowel disease and risk of gastric, small bowel and colorectal cancer: a meta-analysis of 26 observational studies. J Cancer Res Clin Oncol. (2021) 147:1077–87. doi: 10.1007/s00432-020-03496-0
83. Song M, Latorre G, Ivanovic-Zuvic D, Camargo MC, Rabkin CS. Autoimmune diseases and gastric cancer risk: a systematic review and meta-analysis. Cancer Res Treatment. (2019) 51:841–50. doi: 10.4143/crt.2019.151
84. Tian T, Zhang LQ, Ma XH, Zhou JN, Shen J. Diabetes mellitus and incidence and mortality of gastric cancer: a meta-analysis. Exp Clin Endocrinol Diabetes. (2012) 120:217–23. doi: 10.1055/s-0031-1297969
85. Wang Y, Yan P, Fu T, Yuan J, Yang G, Liu Y, et al. The association between gestational diabetes mellitus and cancer in women: a systematic review and meta-analysis of observational studies. Diabetes Metab. (2020) 46:461–71. doi: 10.1016/j.diabet.2020.02.003
86. Park Y, Ki M. Population attributable fraction of helicobacter pylori infection-related gastric cancer in Korea: a meta-analysis. Cancer Res Treatment. (2021) 53:744–53. doi: 10.4143/crt.2020.610
87. Bae JM, Kim EH. Epstein-Barr virus and gastric cancer risk: a meta-analysis with meta-regression of case-control studies. J Prev Med Public Health. (2016) 49:97–107. doi: 10.3961/jpmph.15.068
88. Yang Y, Jiang Z, Wu W, Ruan L, Yu C, Xi Y, et al. Chronic hepatitis virus infection are associated with high risk of gastric cancer: a systematic review and cumulative analysis. Front Oncol. (2021) 11:703558. doi: 10.3389/fonc.2021.703558
89. Wang H, Chen X-L, Liu K, Bai D, Zhang W-H, Chen X-Z, et al. Associations between gastric cancer risk and virus infection other than epstein-barr virus: a systematic review and meta-analysis based on epidemiological studies. Clin Trans gastroenterology. (2020) 11:201. doi: 10.14309/ctg.0000000000000201
90. Rota M, Alicandro G, Pelucchi C, Bonzi R, Bertuccio P, Hu J, et al. Education and gastric cancer risk-an individual participant data meta-analysis in the StoP project consortium. Int J Cancer. (2020) 146:671–81. doi: 10.1002/ijc.32298
91. Wang Z, Liu L, Ji J, Zhang J, Yan M, Zhang J, et al. ABO blood group system and gastric cancer: a case-control study and meta-analysis. Int J Mol Sci. (2012) 13:13308–21. doi: 10.3390/ijms131013308
92. Yin X-H, Wang Y-D, Luo H, Zhao K, Huang G-L, Luo S-Y, et al. Association between tooth loss and gastric cancer: a meta-analysis of observational studies. PloS ONE. (2016) 11:653. doi: 10.1371/journal.pone.0149653
93. Nagel G, Stafoggia M, Pedersen M, Andersen ZJ, Galassi C, Munkenast J, et al. Air pollution and incidence of cancers of the stomach and the upper aerodigestive tract in the European Study of Cohorts for Air Pollution Effects (ESCAPE). Int J Cancer. (2018) 143:1632–43. doi: 10.1002/ijc.31564
94. Zhu H, Yang X, Zhang C, Zhu C, Tao G, Zhao L, et al. Red and processed meat intake is associated with higher gastric cancer risk: a meta-analysis of epidemiological observational studies. PLoS ONE. (2013) 8:e70955. doi: 10.1371/journal.pone.0070955
95. Bertuccio P, Alicandro G, Rota M, Pelucchi C, Bonzi R, Galeone C, et al. Citrus fruit intake and gastric cancer: the stomach cancer pooling (StoP) project consortium. Int J Cancer. (2019) 144:2936–44. doi: 10.1002/ijc.32046
96. Steffen A, Huerta JM, Weiderpass E. Bueno-de-Mesquita HB, May AM, Siersema PD, et al. General and abdominal obesity and risk of esophageal and gastric adenocarcinoma in the European prospective investigation into cancer and nutrition. Int J Cancer. (2015) 137:646–57. doi: 10.1002/ijc.29432
97. Liu Y, Warren Andersen S, Wen W, Gao YT, Lan Q, Rothman N, et al. Prospective cohort study of general and central obesity, weight change trajectory and risk of major cancers among Chinese women. Int J Cancer. (2016) 139:1461–70. doi: 10.1002/ijc.30187
98. Corley DA, Kubo A, Zhao W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol Biomarkers Prev. (2008) 17:352–8. doi: 10.1158/1055-9965.EPI-07-0748
99. Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. (2008) 8:915–28. doi: 10.1038/nrc2536
100. Olefson S, Moss SF. Obesity and related risk factors in gastric cardia adenocarcinoma. Gastric Cancer. (2015) 18:23–32. doi: 10.1007/s10120-014-0425-4
101. Franciosi CM, Piacentini MG, Conti M, Romano F, Musco F, Caprotti R, et al. IGF-1 and IGF-1BP3 in gastric adenocarcinoma. Preliminary study. Hepato-Gastroenterology. (2003) 50:297–300.
102. Obeid CA, Gubbels JS, Jaalouk D, Kremers SPJ, Oenema A. Adherence to the Mediterranean diet among adults in Mediterranean countries: a systematic literature review. Eur J Nutr. (2022) 61:3327–44. doi: 10.1007/s00394-022-02885-0
103. Prado SBR, Castro-Alves VC., Ferreira GF, Fabi JP. Ingestion of non-digestible carbohydrates from plant-source foods and decreased risk of colorectal cancer: a review on the biological effects and the mechanisms of action. Front Nutr. (2019) 6:72. doi: 10.3389/fnut.2019.00072
104. Phan MAT, Paterson J, Bucknall M, Arcot J. Interactions between phytochemicals from fruits and vegetables: effects on bioactivities and bioavailability. Crit Rev Food Sci Nutr. (2018) 58:1310–29. doi: 10.1080/10408398.2016.1254595
105. Jiang K, Jiang X, Wen Y, Liao L, Liu FB. Relationship between long-term use of proton pump inhibitors and risk of gastric cancer: a systematic analysis. J Gastroenterol Hepatol. (2019) 34:1898–905. doi: 10.1111/jgh.14759
106. Schechter LE. Major depressive disorder. Curr Pharm Des. (2005) 11:143–4. doi: 10.2174/1381612053382269
107. Doolin K, Farrell C, Tozzi L, Harkin A, Frodl T, O'Keane V. Diurnal hypothalamic-pituitary-adrenal axis measures and inflammatory marker correlates in major depressive disorder. Int J Mol Sci. (2017) 18:226. doi: 10.3390/ijms18102226
108. Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med. (1997) 337:1441–8. doi: 10.1056/NEJM199711133372007
109. Hecht SS. Cigarette smoking: cancer risks, carcinogens, and mechanisms. Langenbeck's Arch Surgery. (2006) 391:603–13. doi: 10.1007/s00423-006-0111-z
110. Roberts JA. Blood groups and susceptibility to disease: a review. Br J Prev Soc Med. (1957) 11:107–25. doi: 10.1136/jech.11.3.107
111. Nakao M, Matsuo K, Ito H, Shitara K, Hosono S, Watanabe M, et al. ABO genotype and the risk of gastric cancer, atrophic gastritis, and Helicobacter pylori infection. Cancer Epidemiol Biomarkers Prev. (2011) 20:1665–72. doi: 10.1158/1055-9965.EPI-11-0213
Keywords: gastric cancer, risk factors, protective factors, comprehensive analysis, quality
Citation: Hui Y, Tu C, Liu D, Zhang H and Gong X (2023) Risk factors for gastric cancer: A comprehensive analysis of observational studies. Front. Public Health 10:892468. doi: 10.3389/fpubh.2022.892468
Received: 09 March 2022; Accepted: 05 December 2022;
Published: 04 January 2023.
Edited by:
Chi Lin, University of Nebraska Medical Center, United StatesReviewed by:
Abdul Rahaman, South China University of Technology, ChinaRohit Singh, University of Vermont, United States
Copyright © 2023 Hui, Tu, Liu, Zhang and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaobing Gong, Z29uZ3hiMzQ1MEBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Yuqing Hui
Yuqing Hui Chunyi Tu†
Chunyi Tu†