- School of Kinesiology and Health Science, York University, Toronto, ON, Canada
Background: Sleep deprivation and poor sleep quality contribute to increases in oxidative stress, antioxidant imbalance, and a pro-inflammatory state which may predispose to a higher risk of diabetes. Our objective was to estimate the contributions of C-reactive protein (CRP), gamma glutamyl transferase (GGT), and micronutrient antioxidants (bilirubin, carotenoids, uric acid, vitamins A, C–E?) to the relationships between sleep-fasting insulin concentration and -glycosylated hemoglobin (HbA1c).
Methods: Data from the 2005/06 US National Health and Nutritional Examination Survey were used (N = 1,946; 20 y+). Sleep quality and quantity was assessed by the Sleep Disorders Questionnaire, and fasting blood was collected to quantify CRP, GGT, antioxidant micronutrients, insulin concentration, and HbA1c. The bootstrap method was used to estimate the amount of mediation or contribution of these mediators to the sleep–insulin concentration and -HbA1c relationships, which were quantified as large (≥0.25) or moderate (≥0.09).
Results: The sleep duration–fasting insulin relationship was mediated by GGT, carotenoids, uric acid, and vitamins C and D, whereas CRP and bilirubin were non-significant mediators of a moderate effect size. Similarly, the sleep quality–fasting insulin relationship was mediated by CRP, bilirubin and vitamin C, whereas GGT, carotenoids, uric acid, and vitamin D were non-significant large-to-moderate mediators. To a lesser degree, these micronutrients mediated for the relationship between sleep-HbA1c levels.
Conclusion: Several factors related to inflammation, oxidative stress, and antioxidant status were found to lie on the pathway of the sleep–insulin and –glycemic control relationships. Sleep hygiene, reduced systemic inflammation/oxidative stress, and optimal antioxidants intake are potentially beneficial targets for managing diabetes risk.
Introduction
Sleep is vital for optimal health, but most adults do not obtain the recommended 7–9 hours of sleep per night on a regular basis (1). Sleep duration also shares a complex relationship with sleep quality, which modifies its associations with health (2). Poor sleep quantity and quality have been associated with several physiological and pathological conditions, including cardiovascular, neurological, endocrine, and immunological disorders (3). Sleep deprivation, for example, is known to affect cardiovascular disorders by impairing autonomic function, altering glucose and lipid metabolism, and increasing the risks of atherosclerosis and ischemia (3). Further, subchronic sleep restriction may increase peripheral insulin resistance without affecting hepatic insulin sensitivity (4), while sleep extension may improve fasting insulin sensitivity (5). Poor sleep is also associated with poor diets and behaviors that compromise overall health (6, 7).
A growing body of work supports an association between inflammation, oxidative stress, and antioxidant micronutrients with sleep (8–13), including two of our own cross-sectional studies (8, 9). In this most recent work, we quantified the contributions of inflammation [i.e., C-reactive Protein (CRP)], oxidative stress [i.e., γ-glutamyl transferase (GGT) (16)], and antioxidant micronutrients (i.e., bilirubin, carotenoids, uric acid, vitamins A, C, D, and E) to the sleep duration–cardiometabolic health and sleep quality–cardiometabolic health relationships (14, 15). We did not, however, assess the contributions of CRP, GGT, and antioxidant micronutrients on sleep–insulin sensitivity or –glycemic control relationships. To date, only Kim et al. (17) has explored the association between sleep and markers of insulin resistance in an apparently healthy population; using a community-based sample of 374 participants, they found that the association between actigraphy-based sleep onset latency and HOMA-IR was partially mediated by CRP and the inflammatory cytokine, interleukin-6. What remains unclear, is the extent to which these factors may act collectively to augment, or offset, the detrimental effect of poor sleep hygiene on markers of metabolic disease or type 2 diabetes risk.
The purpose of our study is to quantify the contributions of CRP, GGT, and antioxidant micronutrients (i.e., bilirubin, carotenoids, uric acid, and vitamins A, C, D, and E) to the relationships between sleep and fasting insulin concentration or glycemic control, and thereby determine the extent to which they lie on the pathway. In this study, we use blood markers of inflammation, oxidative stress, and antioxidant micronutrients, rather than self-reported dietary data to minimize recall and healthy responder bias, and leverage data from a sample of nearly 2,000 free-living adults from the U.S.
Methods
Study Design, Setting, and Participants
Data for this analysis were obtained from the U.S. National Health and Nutrition Examination Survey (NHANES), a nationally representative cross-sectional study designed to assess the health and nutritional status of its non-institutionalized civilian population (18). Briefly, NHANES samples approximately 10,000 people bi-annually, collecting data from personal interviews, standardized physical examinations, and laboratory samples. While the most recent NHANES cycles (2019–2020) provide a comprehensive assessment of nutrition and many subjective and laboratory measures of health, NHANES 2005–2006 is the last cycle to include measures such as vitamins A, E, and carotenoids data that are relevant to the current analysis. Drawing from an initial sample of 10,348 NHANES 2005–2006 participants, exclusions for age (<20 y; N = 5,369), pregnancy (N = 336), and missing sleep/exposure (N = 2,571), mediators (N = 118), and outcomes (N = 8) were made in sequence, resulting in the final analytic sample of 1,946.
Exposures: Sleep Duration and Quality
The Sleep Disorders Questionnaire was administered to NHANES participants aged ≥16 y, who reported their typical sleep habits on weekdays or workdays for the past month (18), and we used data from those aged ≥20 y in the present analysis. This Sleep Disorders Questionnaire contains items from two previously validated sleep questionnaires, but it has not been validated in its entirety (19, 20). A single question was used to collect sleep duration information: “How much sleep do you usually get on weekdays or workdays?” Responses to this question were collected in whole numbers between 1 and 11 h, and truncated at ≥12 h. Consistent with previous literature, sleep duration was categorized as “very short” (≤ 4 h), “short” (5–6 h), “adequate” (7–8 h), and “long” (≥9 h) (14).
Overall sleep quality was determined from the following 6 questions: “How often did you have trouble falling asleep?;” “How often did you wake up during the night and had trouble getting back to sleep?;” “How often did you wake up too early in the morning and were unable to get back to sleep?;” “How often did you feel unrested during the day, no matter how many hours of sleep you had?;” “How often did you feel excessively or overly sleepy during the day?;” and “How often did you not get enough sleep?” (18). Responses to these questions [0 = Never; 1 = Rarely (1 time a month); 2 = Sometimes (2–4 times a month); 3 = Often (5–15 times a month); and, 4 = Almost always (16–30 times a month)] were summed to obtain an overall sleep quality score (15, 18, 21). The sleep quality score was subsequently categorized as: “good” (0 to <3); “fair” (3 to <7); “poor” (7 to <12); and “very poor” (≥12 to 24) on the basis of previous work (15, 21).
Outcomes: Fasting Insulin Concentration and Glycosylated Hemoglobin Levels
Fasting samples were obtained during the morning examination session after an overnight fast, and standard laboratory methods were used to quantify fasting insulin concentration (pM) and glycosylated hemoglobin (HbA1c, %), a marker for the last 2–3 months of glycemic control (18).
Mediators: Inflammation, Oxidative Stress, and Antioxidant Micronutrients
Additional laboratory measures included in this study were: C-reactive protein [CRP (nM), a marker of inflammation]; γ-glutamyl transferase [GGT (U/L), a marker of oxidative stress]; and, bilirubin (μM), carotenoids (μM), uric acid (μM), and vitamins A (μM), C (μM), D (μM), and E (μM), markers of antioxidant micronutrients (18). These mediators were modeled continuously to assess their contributions to the sleep–insulin concentration and sleep–glycemic control relationships.
Demographic and Behavioral Characteristics
Other variables used to describe the study population were age (20 to <40 y, 40 to <65 y, and ≥ 65 y), sex (M, F), ethnicity (White, Black, Latin, and Others groups), education, income, smoking history, and alcohol intake. Educational attainment was categorized as < high school, high school, college; and income as < $20,000, $20,000–44,999, and ≥$45,000. Smoking history was categorized as current (if smoking now), past (if smoked ≥100 cigarettes in one's life but not a current smoker), or never (if smoked <100 cigarettes in one's life) (14). Alcohol intake was categorized as none, <3, and ≥3 drinks per day.
Mediation Model
The mediation model helps explain the underlying relationship between an exposure variable and an outcome measure through a third (mediatory) variable (22). Briefly, the mediation model (Figure 1) is a series of regression analyses that contains four path analyses: (1) path a is a regression between the exposure and the mediator; (2) path b is a regression between the mediator and the outcome while adjusting for the exposure; (3) path c is a regression between the exposure and the outcome; and (4) path c' is a regression between the exposure and the outcome while adjusting for the mediator. In the mediation model, the products of ab and c-c' are mathematically equivalent, and ab is considered as the “amount” of mediation or contribution a mediator provides to the relationship between an exposure and an outcome. As described by Kenny (22), the contribution of each mediator can also be described as “large” (ab ≥ 0.25), “moderate” (ab ≥ 0.09), “modest” (ab ≥ 0.01), and “weak” or “modest” (ab < 0.01). We further used multiple comparisons correction, i.e., Bonferroni (p = 0.05/9) and a p-value generated through simulation, to interpret our indirect effect given that it is a common practice in mediation analysis in the fields of genetics and metabolomics (23, 24).
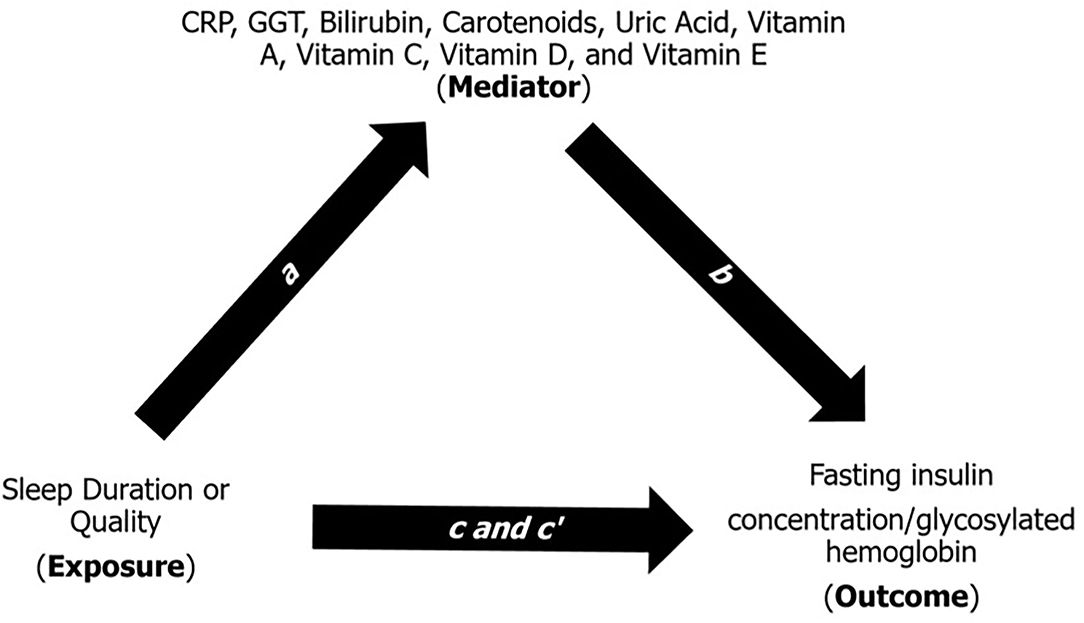
Figure 1. Multiple regression method of the indirect mediation model (22). Path a is a regression analysis between the exposure (e.g., sleep duration or quality) and the mediator (e.g., CRP, GGT, bilirubin, carotenoids, uric acid, and vitamins A, C, D, and E). Path b is a regression analysis between the mediator and the outcome (e.g., fasting insulin concentration) adjusting for the exposure. Path c is a regression analysis between the exposure and outcome. Path c' is a regression analysis between the exposure and the outcome adjusting for the mediator. The indirect effect (ab) estimate is the amount of contribution a mediator provides to the relationship between an exposure and an outcome. CRP, C-reactive protein; GGT, gamma-glutamyl transferase. Each mediator was tested one-by-one in the mediation model.
Statistical Analyses
Mean and 95% confidence interval (CI) for continuous variables, and frequency (percentage), and 95% CI for categorical variables were stratified by sleep duration. ANOVA and χ2 tests were used, as appropriate, to test for any differences in demographic and behavioral characteristics across groups. The medical exam sample weight from the demographics data file was used to weight descriptive analyses to be representative of the US adult population (18). For the mediation analysis, we used Hayes' INDIRECT SAS Macro with the bootstrap method with 5,000 iterations to estimate the amount of mediation or contribution (ab) by each mediator, and present the bias corrected ab estimates with 95% CI, and p-values (25, 26). All analyses were conducted in SAS v9.3 (Cary, NC, USA), and statistical significance was set at an α of 0.05.
Results
Demographic and Behavioral Characteristics
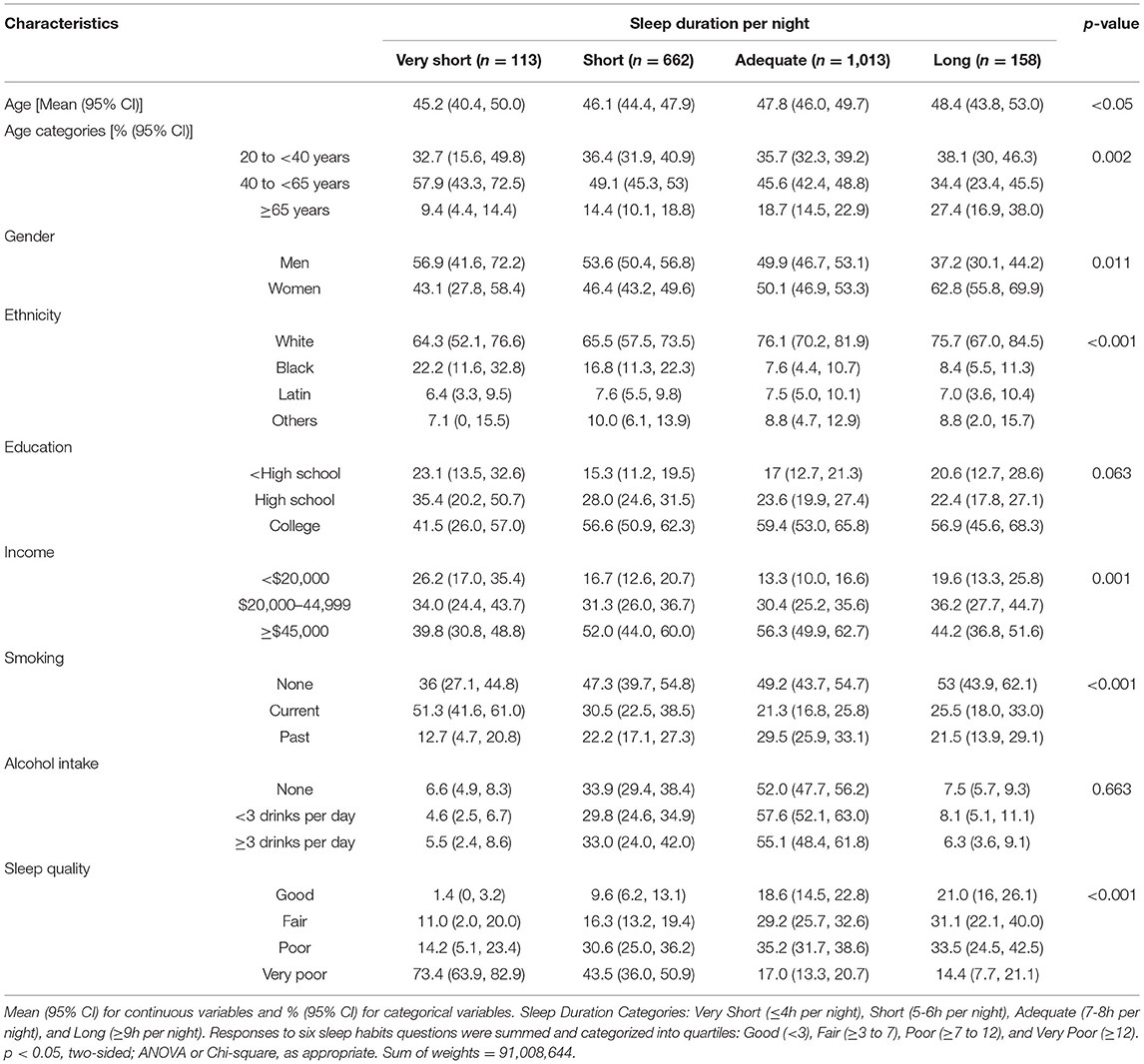
Table 1 describes the sample characteristics by sleep duration. Very short sleep was higher amongst 40–65-years-old, men, Black ethnicity, lower income, and current smokers. On the other hand, a higher proportion of adequate and long sleepers were found among White ethnicity and in those with a college education. As expected, a greater proportion of participants reporting shorter sleep duration also reported lower sleep quality, but approximately a third of the participants reporting adequate and long sleep still reported poor sleep quality.
Estimates of Mediations or Contributions
Figure 2 provides the estimates of mediation or contribution by each mediatory variable (i.e., CRP, GGT, bilirubin, carotenoids, uric acid, and vitamins A, C, D, and E) to the sleep–fasting insulin concentration relationship. GGT, carotenoids, uric acid, and vitamins C and D provided large and statistically significant contributions to the sleep duration–fasting insulin concentration relationship [ab estimate (95% CI), p-value: −0.73 (−2.02, −0.13), 0.03; −0.97 (−1.82, −0.28), 0.02; −1.04 (−2.19, −0.22), 0.02; −0.88 (−1.67, −0.33), 0.01; and −1.90 (−2.89, −1.07), <0.01, respectively]. Additionally, though non-significant, CRP and bilirubin were moderate contributors to the sleep duration–fasting insulin concentration [−0.13 (−0.83, 0.51), 0.70; and −0.12 (−0.55, 0.24), 0.54, respectively], while vitamins A and E contributed only modestly [0.01 (−0.11, 0.66), 0.79; and 0.01(−0.16, 0.27), 0.90, respectively] to the same relationship. The total effect of sleep duration–fasting insulin concentration relationship was −2.56 (SE: 2.56; p-value: 0.32).
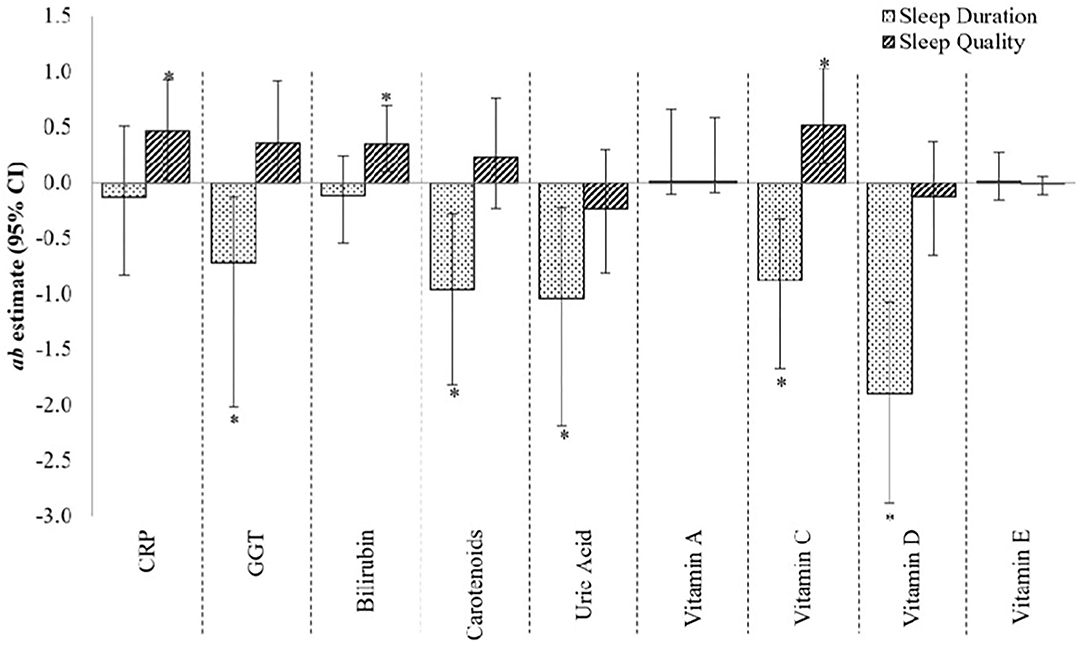
Figure 2. The contribution of CRP, GGT and micronutrient antioxidants to the sleep–fasting insulin concentration relationship. ab estimate, amount of mediation, or contribution by the mediatory variable (moderate effect when ab ≥ 0.09, and large effect when ab ≥ 0.25); CI, confidence interval. *p <0.05, 95% CI are bias-corrected, bootstrapped values. Each mediator was tested one-by-one in the mediation model.
For the relationship between sleep quality and fasting insulin, CRP, bilirubin, and vitamin C made large and statistically significant contributions [0.47 (0.02, 0.92), 0.05; 0.34 (0.09, 0.69), 0.03; and 0.51 (0.17, 1.03), 0.01, respectively] (Figure 2), whereas GGT made a large but non-significant contribution [0.35 (0.00, 0.92), 0.09]. Carotenoids, uric acid, and vitamin D, on the other hand, made moderate, non-significant contributions [0.23 (−0.23, 0.76), 0.36; −0.23 (−0.81, 0.29), 0.42; and −0.13 (−0.65, 0.36), 0.63, respectively] to the sleep quality–fasting insulin concentration relationship, while vitamin A contributed only modestly [0.01 (−0.09, 0.58), 0.82]. The total effect of sleep quality–fasting insulin concentration relationship was 2.46 (SE: 1.68; p-value: 0.14).
The mediating effect of these micronutrients was only weak-to-modest when the outcome was HbA1c (Figure 3). Vitamins C and D, for instance, were significant but modest contributions to the sleep duration–glycosylated hemoglobin relationship, while CRP, bilirubin, and vitamin C were modest, non-significant mediators of the sleep quality–glycosylated hemoglobin relationship. The total effect of sleep duration–glycosylated hemoglobin and sleep quality–glycosylated hemoglobin relationships were −0.07 (SE: 0.03; p-value: 0.04) and 0.02 (SE: 0.02; p-value: 0.41), respectively.
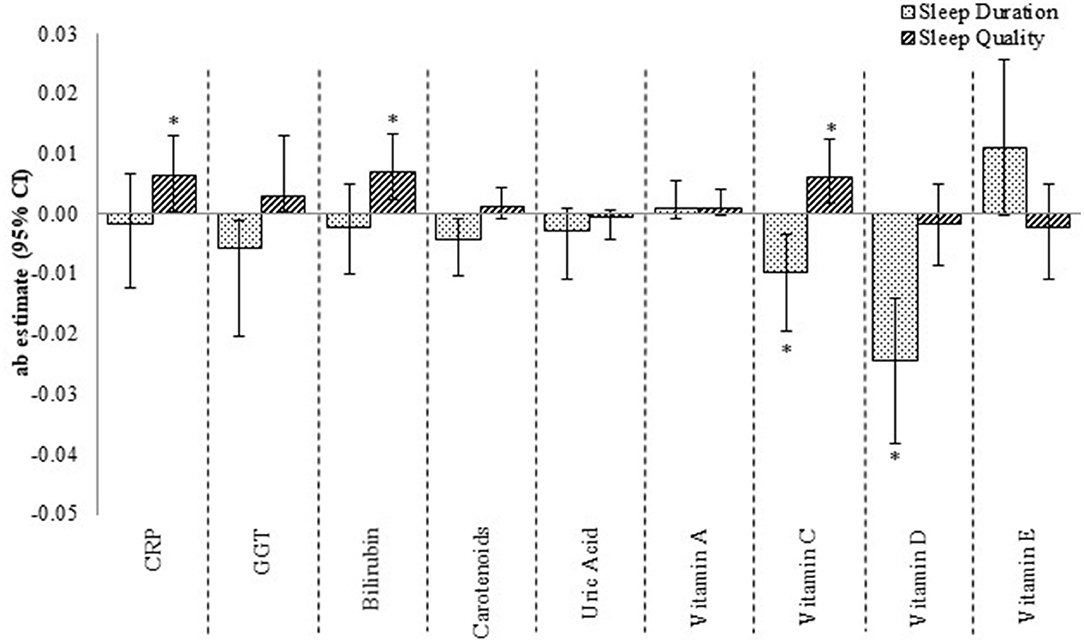
Figure 3. The contribution of CRP, GGT and micronutrient antioxidants to the sleep–glycosylated hemoglobin relationship. ab estimate, amount of mediation or contribution by the mediatory variable (moderate effect when ab ≥ 0.09, and large effect when ab ≥ 0.25) CI, confidence interval. *p < 0.05, 95% CI are bias-corrected, bootstrapped values. Each mediator was tested one-by-one in the mediation model.
When we used the Bonferroni corrected p-value of <0.0056, the only significant mediator of sleep duration–fasting insulin concentration and –glycosylated hemoglobin relationships was Vitamin D. None of our mediators contributed significantly to the relationships between sleep and insulin sensitivity or glycosylated hemoglobin if we used the simulated p-value of <0.0001 for multiple biological mediators testing.
Discussion
Our aim was to quantify the contribution of (CRP, GGT, bilirubin, carotenoids, uric acid, and vitamins A, C, D, and E) to the sleep–fasting insulin concentration and –glycemic control relationships, and thus, determine whether inflammation, oxidative stress, and antioxidant micronutrients lie on the pathway and act as mediators of these relationships. In this regard, we found that GGT, carotenoids, uric acid, and vitamins C and D contributed significantly to the relationship between sleep duration and fasting insulin concentration, while CRP, bilirubin, and vitamin C contributed significantly to the relationship between sleep quality and fasting insulin concentration. While significant, these factors were more modest mediators of the sleep–glycemic control relationship. Our findings suggest that poor sleep quality and/or duration may impact various biochemical analytes associated with oxidative stress and inflammation that may in turn promote whole body insulin resistance, particularly in middle-aged females. To our knowledge, this is the first time that the mediation effect of inflammation, oxidative stress, and antioxidant micronutrients on measures of glycemic control have been evaluated in a representative U.S. sample, and several of our findings warrant discussion.
To date, relatively few studies have examined the inter-relationship between inflammation, oxidative stress, and antioxidant micronutrients on the pathway between sleep and diabetes risk (8, 9, 14, 15, 17). Of these, our study is most comparable to Kim et al.'s (17) community-based cross-sectional study, which found partial mediation by CRP and interleukin-6 on the relationship between sleep onset latency and HOMA-IR. Main differences between this study and ours, includes Kim's use of actigraphy-based sleep measures, adjustment for diabetes and depression, and investigation of moderation by sex. Our analysis accounts for a diverse range of age, demographic, and ethnic groups while investigating diabetes-related outcomes, building upon previous analyses by our group that included sex-stratified results (14).
While it is not clear why the contributions may be stronger in women, sleep disturbance induced psychological stress and physiological changes may provide a partial explanation (27). Indeed, age, sex, smoking history, alcohol consumption, physical activity, and comorbidities are known confounders of the multiple linear relationships evaluated in the present mediation analysis (8, 28–32). For instance, moderate intensity and lifestyle-related physical activity have been found to be large mediators of the sleep–fasting insulin concentration relationship (28). It is well known that physical activity plays an important roles in glycemic control due to its acute blunting effect on insulin levels (33), and that muscular contraction stimulates insulin sensitivity by increasing AMPK activity, deactivating TCB1D1 and promoting GLUT4 translocation to the cell membrane, which increases cellular glucose uptake (34). Therefore, future attempts to quantify, and de-couple the direct and indirect paths between sleep and metabolic health would benefit from causal modeling that considers a more comprehensive set of factors (as covariates. confounders, mediators, and moderators).
Finally, in exploring mediators of the relationship between sleep, insulin sensitivity, and glycemic control, we are attempting to understand factors which underlie the pathway as possible mechanistic links. It therefore bears repeating that although we used Preacher and Hayes's (25). SAS procedure to estimate the indirect effects (path ab), the negative ab does not indicate a negative mediation, but rather that one of the paths is negative while the other is positive (22). For example, since the association between sleep duration and CRP (path a) is negative, and the relationship between CRP and fasting insulin concentration (path b) is positive (35), the resulting ab is negative. Similarly, since the association between sleep quality and CRP is positive (i.e., path a and path b is also positive), the resulting ab product is positive. Our finding of a much weaker mediation by CRP, bilirubin, and vitamins C and D to the sleep–glycosylated hemoglobin relationship (as compared to insulin sensitivity) also warrants discussion, and may be explained by the use of the bootstrap method—a non-parametric test that assumes linear relationships between paths—which likely produced conservative estimates of the indirect effect sizes (25), or a narrower homeostatic range of glycosylated hemoglobin in the non-diabetic and medicated diabetic populations (36).
Limitations
There are several limitations associated with our study. First, though mediation analysis is a causal path analysis, given the cross-sectional nature of the design, causal inference may not be made from our findings alone, and future longitudinal studies are needed to confirm the directionality and importance of these findings. Second, in applying our study exclusion criteria, our final analytic sample was only a fraction of the initial adult sample; however, all analyses were bootstrapped with replacement, which provided conservative, bias-corrected indirect effect estimates. Given that our study considered multiple mediators, the use of a lower p-value for significance testing and estimating a narrower confidence interval—a common practice in the genetics literature (23)—may be a reasonable alternative for evaluating the indirect effects. Further, mediators and outcomes were objectively measured, but sleep measures were self-reported and susceptible to recall and response bias.
Conclusions
CRP, GGT, bilirubin, carotenoids, uric acid, and vitamins C and D made large, significant contributions to the observed relationships between sleep and fasting insulin concentration, but explained less of the relationship with glycosylated hemoglobin. Since the mediators explored in this study are linked to diet, physical activity, and sleep behaviors, interventions that collectively target modifiable lifestyle behaviors including sleep hygiene, reduce systemic inflammation/oxidative stress, and optimize antioxidant intake, remain mutually beneficial targets for diabetes risk.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found at: https://www.cdc.gov/nchs/nhanes/index.html.
Author Contributions
TK designed the study, conducted the analyses, and drafted the manuscript. MR and CA consulted on the study design, analysis, interpretation of findings, and revised the drafted manuscript. All authors reviewed and approve of the final manuscript version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National sleep foundation's sleep time duration recommendations: methodology and results summary. Sleep Health. (2015) 1:40–3. doi: 10.1016/j.sleh.2014.12.010
2. Lou P, Chen P, Zhang L, Zhang P, Chang G, Zhang N, et al. Interaction of sleep quality and sleep duration on impaired fasting glucose: a population-based cross-sectional survey in China. BMJ Open. (2014) 4:e004436. doi: 10.1136/bmjopen-2013-004436
3. Liew SC, Aung T. Sleep deprivation and its association with diseases—a review. Sleep Med. (2021) 77:192–204. doi: 10.1016/j.sleep.2020.07.048
4. Rao MN, Neylan TC, Grunfeld C, Mulligan K, Schambelan M, Schwarz JM. Subchronic sleep restriction causes tissue-specific insulin resistance. J Clin Endocrinol Metab. (2015) 100:1664–71. doi: 10.1210/jc.2014-3911
5. Leproult R, Deliens G, Gilson M, Peigneux P. Beneficial impact of sleep extension on fasting insulin sensitivity in adults with habitual sleep restriction. Sleep. (2015) 38:707–15. doi: 10.5665/sleep.4660
6. St-Onge MP, Wolfe S, Sy M, Shechter A, Hirsch J. Sleep restriction increases the neuronal response to unhealthy food in normal-weight individuals. Int J Obes. (2014) 38:411–6. doi: 10.1038/ijo.2013.114
7. Mesas AE, Guallar-Castillón P, López-García E, León-Muñoz LM, Graciani A, Banegas JR, et al. Sleep quality and the metabolic syndrome: the role of sleep duration and lifestyle. Diabetes Metab Res Rev. (2014) 30:222–31. doi: 10.1002/dmrr.2480
8. Dowd JB, Goldman N, Weinstein M. Sleep duration, sleep quality, and biomarkers of inflammation in a Taiwanese population. Ann Epidemiol. (2011) 21:799–806. doi: 10.1016/j.annepidem.2011.07.004
9. Huang W, Shah S, Long Q, Crankshaw AK. Tangpricha V. Improvement of pain, sleep, and quality of life in chronic pain patients with vitamin D supplementation. Clin J Pain. (2013) 29:341–7. doi: 10.1097/AJP.0b013e318255655d
10. Wong TY, Travis RC, Tong TYN. Blood biomarker levels by total sleep duration: cross-sectional analyses in UK Biobank. Sleep Med. (2021) 88:256–61. doi: 10.1016/j.sleep.2021.10.018
11. Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. (2004) 43:678–83. doi: 10.1016/j.jacc.2003.07.050
12. Chou YT Li CH, Shen WC, Yang YC, Lu FH, Wu JS, et al. Association of sleep quality and sleep duration with serum uric acid levels in adults. PLoS ONE. (2020) 15:e0239185. doi: 10.1371/journal.pone.0239185
13. Papandreou C, Babio N, Díaz-López A, Martínez-González MÁ, Becerra-Tomas N, Corella D, et al. Sleep duration is inversely associated with serum uric acid concentrations and uric acid to creatinine ratio in an elderly Mediterranean population at high cardiovascular risk. Nutrients. (2019) 11:E761. doi: 10.3390/nu11040761
14. Kanagasabai T, Ardern CI. Contribution of inflammation, oxidative stress, and antioxidants to the relationship between sleep duration and cardiometabolic health. Sleep. (2015) 38:1905–12. doi: 10.5665/sleep.5238
15. Kanagasabai T, Ardern CI. Inflammation, oxidative stress, and antioxidants contribute to selected sleep quality and cardiometabolic health relationships: a cross-sectional study. Mediators Inflamm. (2015) 2015:824589. doi: 10.1155/2015/824589
16. Lee DH, Blomhoff R, Jacobs DR. Is Serum Gamma Glutamyltransferase a Marker of Oxidative Stress? Free Radic Res. (2004) 38:535–9. doi: 10.1080/10715760410001694026
17. Kim TH, Carroll JE, An SK, Seeman TE, Namkoong K, Lee E. Associations between actigraphy-assessed sleep, inflammatory markers, and insulin resistance in the Midlife Development in the United States (MIDUS) study. Sleep Med. (2016) 28:72–9. doi: 10.1016/j.sleep.2016.07.023
18. National Center for Health Statistics. NHANES—About the National Health and Nutrition Examination Survey. (2011). Available online at: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed December 27, 2013).
19. Plantinga L, Lee K, Inker LA, Saran R, Yee J, Gillespie B, et al. Association of sleep-related problems with CKD in the United States, 2005-2008. Am J Kidney Dis. (2011) 58:554–64. doi: 10.1053/j.ajkd.2011.05.024
20. Lee PH. Validation of the National Health and Nutritional Survey (NHANES) single-item self-reported sleep duration against wrist-worn accelerometer. Sleep Breath. (2021) doi: 10.1007/s11325-021-02542-6
21. Engeda J, Mezuk B, Ratliff S, Ning Y. Association between duration and quality of sleep and the risk of pre-diabetes: evidence from NHANES. Diabet Med. (2013) 30:676–80. doi: 10.1111/dme.12165
22. Kenny DA,. Mediation. (2013). Available online at: http://davidakenny.net/ (accessed October 3, 2013).
23. Cao-Lei L, Veru F, Elgbeili G, Szyf M, Laplante DP, King S, et al. Methylation mediates the effect of exposure to prenatal maternal stress on cytokine production in children at age 13½ years: Project Ice Storm. Clin Epigenetics. (2016) 8:54. doi: 10.1186/s13148-016-0219-0
24. Boca SM, Sinha R, Cross AJ, Moore SC, Sampson JN. Testing multiple biological mediators simultaneously. Bioinformatics. (2014) 30:214–20. doi: 10.1093/bioinformatics/btt633
25. Preacher KJ, Hayes AF. SPSS. and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. (2004) 36:717–31. doi: 10.3758/BF03206553
26. Hayes, AF,. SPSS, SAS, and R Macros and Code. Available online at: http://afhayes.com/spss-sas-and-r-macros-and-code.html (accessed May 3, 2022).
27. Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity. Brain Behav Immun. (2008) 22:960–8. doi: 10.1016/j.bbi.2008.01.011
28. Kanagasabai T, Riddell MC, Ardern CI. Physical activity contributes to several sleep–cardiometabolic health relationships. Metab Syndr Relat Disord. (2017) 15:44–51. doi: 10.1089/met.2016.0103
29. Lydon DM, Ram N, Conroy DE, Pincus AL, Geier CF, Maggs JL. The within-person association between alcohol use and sleep duration and quality in situ: an experience sampling study. Addict Behav. (2016) 61:68–73. doi: 10.1016/j.addbeh.2016.05.018
30. Ames ME, Holfeld B, Leadbeater BJ. Sex and age group differences in the associations between sleep duration and BMI from adolescence to young adulthood. Psychol Health. (2016) 31:1–17. doi: 10.1080/08870446.2016.1163360
31. Cassidy S, Chau JY, Catt M, Bauman A, Trenell MI. Cross-sectional study of diet, physical activity, television viewing and sleep duration in 233,110 adults from the UK Biobank; the behavioural phenotype of cardiovascular disease and type 2 diabetes. BMJ Open. (2016) 6:e010038. doi: 10.1136/bmjopen-2015-010038
32. Sabanayagam C, Shankar A. The association between active smoking, smokeless tobacco, secondhand smoke exposure and insufficient sleep. Sleep Med. (2011) 12:7. doi: 10.1016/j.sleep.2010.09.002
33. Henriksen EJ. Invited review: effects of acute exercise and exercise training on insulin resistance. J Appl Physiol. (2002) 93:788–96. doi: 10.1152/japplphysiol.01219.2001
34. Bird SR, Hawley JA. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc Med. (2017) 2:e000143. doi: 10.1136/bmjsem-2016-000143
35. Missel AL, Saslow LR, Griauzde DH, Marvicsin D, Sen A, Richardson CR, et al. Association between fasting insulin and C-reactive protein among adults without diabetes using a two-part model: NHANES 2005–2010. Diabetol Metab Syndr. (2021) 13:29. doi: 10.1186/s13098-021-00645-4
Keywords: mediation effect, sleep duration, sleep quality, fasting insulin, inflammation, oxidative stress, antioxidant micronutrients
Citation: Kanagasabai T, Riddell MC and Ardern CI (2022) Inflammation, Oxidative Stress, and Antioxidant Micronutrients as Mediators of the Relationship Between Sleep, Insulin Sensitivity, and Glycosylated Hemoglobin. Front. Public Health 10:888331. doi: 10.3389/fpubh.2022.888331
Received: 02 March 2022; Accepted: 13 May 2022;
Published: 09 June 2022.
Edited by:
Wilson Tam, National University of Singapore, SingaporeReviewed by:
Timothy Michael Ellmore, City College of New York (CUNY), United StatesUlf Holmbäck, Uppsala University, Sweden
Copyright © 2022 Kanagasabai, Riddell and Ardern. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chris I. Ardern, Y2FyZGVybkB5b3JrdS5jYQ==
 Thirumagal Kanagasabai
Thirumagal Kanagasabai Michael C. Riddell
Michael C. Riddell Chris I. Ardern
Chris I. Ardern