
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 27 May 2022
Sec. Clinical Diabetes
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.878845
This article is part of the Research Topic Gestational Diabetes Mellitus and Long-term Maternal Outcomes View all 19 articles
Objective: The association between gestational diabetes mellitus (GDM) and the risk of arthritis has not been reported. GDM increases the risk of long-term complications including diabetes and metabolic syndrome that are positively associated with the risk of arthritis. This study aimed to explore the association between GDM and the risk of arthritis.
Methods: Women (age ≥ 20 years) who had delivered at least one live birth were included from the 2007 to 2018 National Health and Nutrition Examination Survey cohort (N = 11,997). Patients who had a history of GDM and arthritis were identified by in-home interview. Subgroup analyses were conducted by arthritis types and status of obesity, current diabetes, metabolic syndrome, smoking, alcohol drinking, and physical activity.
Results: GDM was associated with increased odds of arthritis [multivariable-adjusted odds ratio (95% confidence interval): 1.31 (1.06–1.62)], and the result was similar in sensitivity analysis with further adjustment for metabolic syndrome [1.30 (1.05–1.60)]. In subgroup analyses, GDM was associated with increased odds of osteoarthritis [1.47 (1.05–2.06)], while no association was observed with rheumatoid arthritis [1.04 (0.69–1.57)] and other types [1.26 (0.94–1.68)]. GDM was associated with increased odds of arthritis in women without metabolic syndrome [1.34 (1.00–1.78)] and diabetes [1.35 (1.03–1.76)], in obese individuals [1.64 (1.24–2.16)], current/former smokers [1.43 (1.05–1.95)], and current drinkers [1.76 (1.00–3.14)], and in individuals engaging in higher levels of physical activity [1.53 (1.06–2.20)].
Conclusions: GDM was associated with increased odds of arthritis, and the association was independent of type 2 diabetes and metabolic syndrome.
Gestational diabetes mellitus (GDM) is diabetes that develops during pregnancy, and is currently the most common medical complication of pregnancy (1). The prevalence of GDM was estimated to be 7.6% in the US (2), and the overall weighted GDM prevalence in European countries was estimated at 10.9% (3). GDM increases the risk of long-term complications, including obesity, type 2 diabetes, metabolic syndrome, cancer, and cardiovascular disease (1, 4–7), and GDM provides unique opportunities for improving maternal health (8). Musculoskeletal conditions account for a significant proportion of non-communicable diseases contributing to disability adjusted life years, with osteoarthritis contributing most to this burden (9). The major arthritis-related disorders such as osteoarthritis and rheumatoid arthritis collectively make arthritis rank among the most common disabling health conditions (10). The global prevalence of rheumatoid arthritis was 460 per 100,000 population (11). The global age-standardized years lived with disability rate for osteoarthritis in 2017 was 118.8, an increase of 9.6% from 1990 (12).
Chronic inflammation may play a significant role in the development of arthritis-related disorders such as osteoarthritis and rheumatoid arthritis (13, 14). Inflammation and oxidative stress participate in the development of GDM and exert potentially harmful effects on the short and long-term maternal health (15). In addition, the long-term complications of GDM including obesity, type 2 diabetes, and metabolic syndrome are also positively associated with arthritis (16–20). However, the association between GDM and the risk of arthritis has not been reported in epidemiological studies. Based on the above-mentioned findings, we hypothesize that GDM is associated with increased odds of arthritis. In this study, we first explored the association between a history of GDM and the odds of arthritis in women, and then conducted stratified analyses to determine whether the association could still be observed in the absence of type 2 diabetes and metabolic syndrome. In addition, stratified analyses were also conducted by the modifiable risk factors for arthritis development, including smoking (21–23), alcohol drinking (24, 25), and low levels of physical activity (26, 27).
The National Health and Nutrition Examination Survey (NHANES) is a program of studies designed to assess the health and nutritional status of US adults and children. The survey examines a nationally representative sample of about 5,000 persons each year. We used data from six cycles of the NHANES cohort (2007/2008 to 2017/2018), as these cycles specifically provided information for a history of GDM.
The inclusion criteria are as follows: (1) women aged 20 years or older; (2) women with at least one live birth; (3) women responding to the questions regarding a history of GDM; and (4) women responding to the questions regarding arthritis. In addition, women who were diagnosed with diabetes or arthritis prior to a diagnosis of GDM were excluded. Finally, we included 11,997 women in this study.
The exposure for the analysis was the response to the question, “During your pregnancy, were you ever told by a doctor or other health professional that you had diabetes, sugar diabetes, or gestational diabetes?” and we considered women who answered yes to the above question as having a history of GDM (28, 29). The outcome for the analysis was arthritis, and patients with arthritis were identified with the questions of “doctor ever said you had arthritis?” and “which type of arthritis was it?”.
According to the previous studies (23, 30, 31), the following covariates were included: age, race/ethnicity (Mexican–American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, Other Races), body mass index (BMI, under/normal weight: <25 kg/m2, overweight: 25 to <30 kg/m2, obesity: ≥30 kg/m2), education ( ≤ high school, some college or AA degree, ≥college graduate), annual family income (< $20,000, $20,000–$44,999, $45,000–$74,999, ≥$75,000), smoking (current smoker, former smoker, never smoker), alcohol drinking (g/day), and physical activity (vigorous/moderate recreational activities for at least 10 min continuously in a typical week). In addition, current diabetes and metabolic syndrome were also considered in stratified analyses. Current diabetes was defined using a self-reported diagnosis of diabetes outside pregnancy or, if diabetes was not previously diagnosed, by a hemoglobin A1c level ≥ 6.5%, a fasting plasma glucose level ≥ 126 mg/dL, or 2-h plasma glucose ≥ 200 mg/dL (32). Type 1 diabetes was defined as an onset age of self-reported diagnosis of diabetes < 30 years and currently taking insulin (29). Any 3 of the 5 following metabolic-related disorders constitute diagnosis of metabolic syndrome (33): elevated blood pressure (≥130 mm Hg systolic blood pressure, ≥85 mm Hg diastolic blood pressure), elevated waist circumference (≥102 cm in men, ≥88 cm in women), reduced HDL-C (<40 mg/dL in men, <50 mg/dL in women), elevated triglycerides (≥150 mg/dL), and elevated fasting glucose (≥100 mg/dL).
The logistic regression was used to calculate the odds ratios (95% confidence interval) [OR (95% CI)] of arthritis for women with a GDM history compared with those without a history of GDM. We calculated three different logistic regression models. Model 1 was adjusted for demographic variables (age and race/ethnicity). Model 2 included the covariates of model 1 with additional adjustment for BMI and socioeconomic status (education and family income). Model 3 included the covariates of model 2 with additional adjustment for health behaviors (alcohol drinking, smoking, and physical activity). Stratified analyses were conducted by arthritis types (osteoarthritis, rheumatoid arthritis, and other types), current status of metabolic syndrome (yes, no), obesity (yes, no), and current diabetes (yes, no), smoking (never, current/former), alcohol drinking (yes, no), and physical activity (vigorous/ moderate recreational activities for at least 10 min continuously in a typical week: yes, no). Tests for interactions were performed by using cross-product terms of GDM with these stratified factors. In addition, because GDM was associated with lower HDL-C, and increased BMI, blood pressure, total cholesterol, triglycerides, and glucose (34), we also conducted a sensitivity analysis in which we further adjusted for metabolic syndrome to determine whether these metabolic-related disorders could account for the association between GDM and the risk of arthritis. New multi-year sample weight was computed by dividing the 2-year sample weights by 6 (six cycles of NHANES were included in this study). All analyses used sample weights, strata, and primary sampling units to account for the complex, multistage, stratified, and cluster-sampling design of NHANES. All analyses were conducted with Stata 12.0, and P ≤ 0.05 was considered statistically significant.
Among the 11,997 women included in this study, the weighted prevalence of GDM and arthritis was 8.00 and 34.72%, respectively. Women with a GDM history were more likely to be younger, engage in physical activity, and show higher prevalence of obesity, current diabetes (type 1 diabetes accounts for 2%), and metabolic syndrome. Annual family income and education levels differed significantly between women with a history of GDM and women without a history of GDM, while smoking status and alcohol drinking did not differ significantly between the two groups. Detailed characteristics of the participants are shown in Tables 1, 2. The associations between the characteristics of the participants and arthritis are shown in Table 3, and all these characteristics were associated with arthritis.
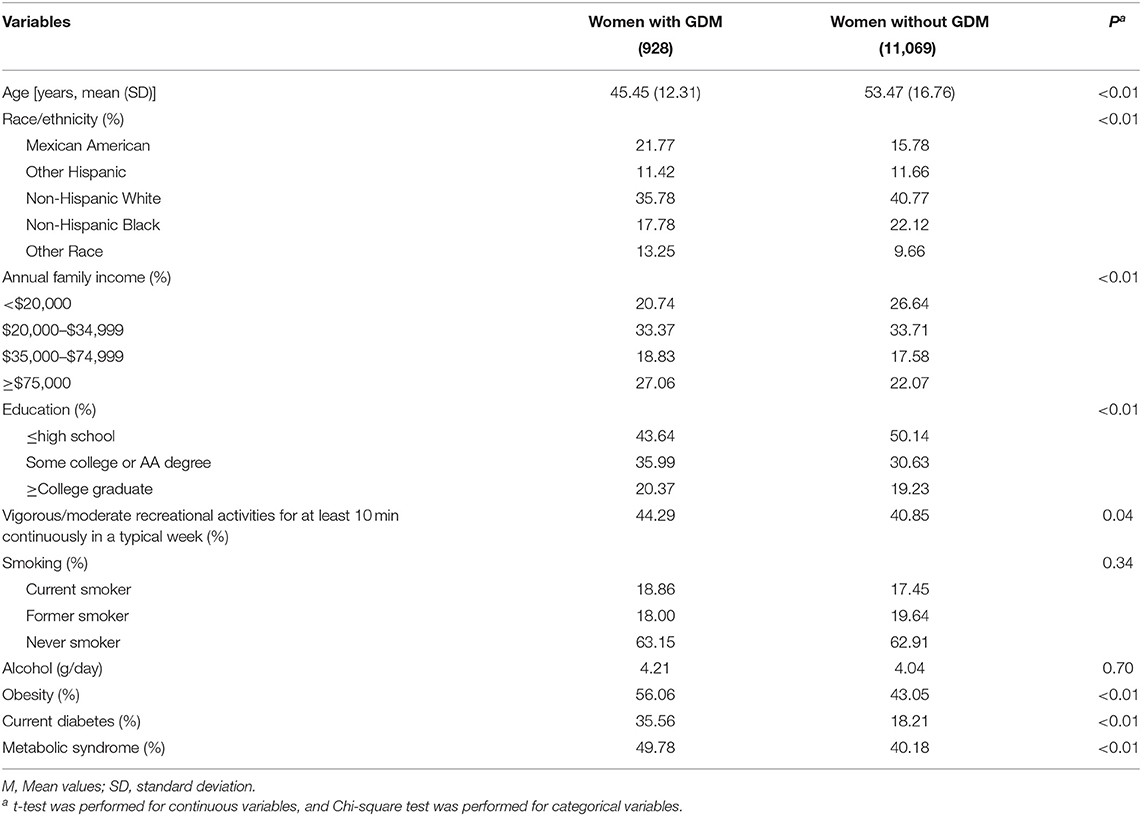
Table 1. Characteristics of the 2007–2018 NHANES adults according to the presence or absence of a history of gestational diabetes mellitus (GDM).
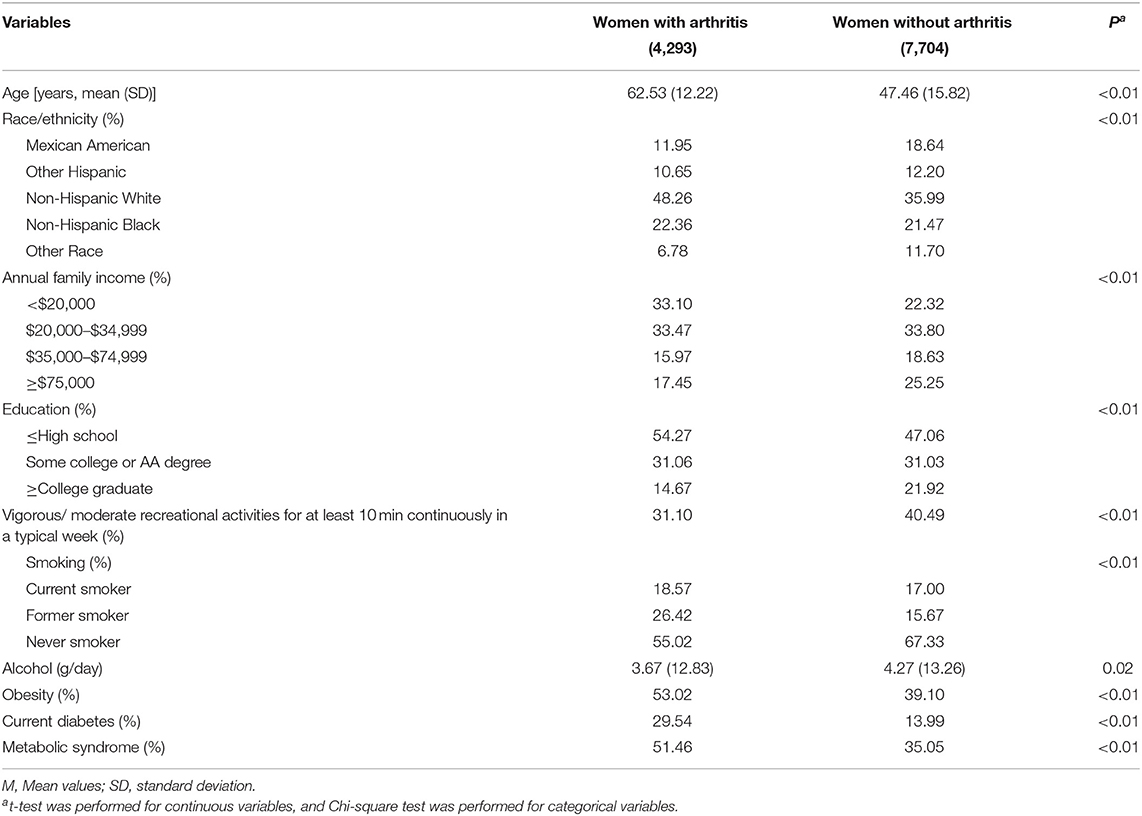
Table 2. Characteristics of the 2007–2018 NHANES adults according to the presence or absence of arthritis.
Overall, the findings on the association between a history of GDM and the odds of arthritis were similar across the three statistical models, while the observed association was attenuated slightly in model 2 and model 3. In model 3, a history of GDM was associated with increased odds of arthritis [OR (95% CI): 1.31 (1.06–1.62), P < 0.05], and the result was similar in sensitivity analysis with further adjustment for metabolic syndrome [1.30 (1.05–1.60)]. In subgroup analyses by arthritis types, a history of GDM was associated with increased odds of osteoarthritis [1.47 (1.05–2.06)], while no association was observed with rheumatoid arthritis [1.04 (0.69–1.57)] and other types [1.26 (0.94–1.68)] (Table 4, Figure 1).
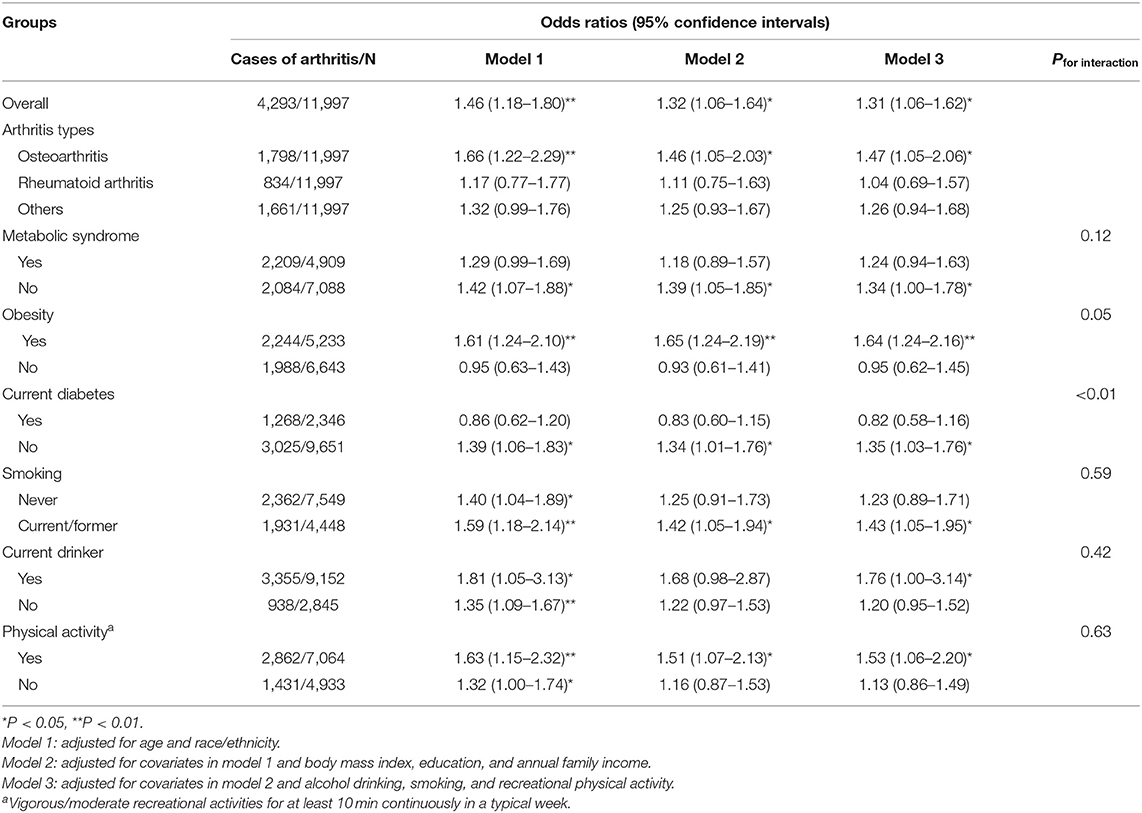
Table 4. Odds ratios of arthritis for women with a history of gestational diabetes mellitus compared with those without a history of gestational diabetes mellitus.
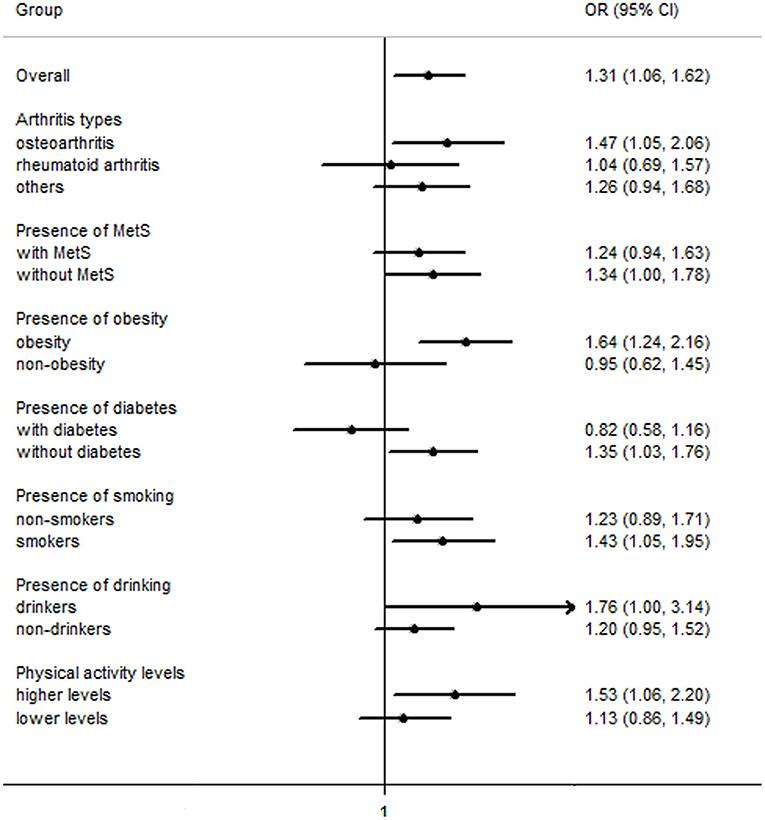
Figure 1. Odds ratios of arthritis for women with a history of gestational diabetes mellitus compared with those without a history of gestational diabetes mellitus. MetS, metabolic syndrome; OR (95% CI), Odds ratio (95% confidence interval).
In stratified analyses by current status of metabolic syndrome, diabetes, obesity and physical activity, a history of GDM was associated with increased odds of arthritis in obese women [1.64 (1.24–2.16)] and in women without metabolic syndrome [1.34 (1.00–1.78)] and current diabetes [1.35 (1.03–1.76)]. In stratified analyses by status of smoking, alcohol drinking and physical activity, a history of GDM was associated with increased odds of arthritis in current/former smokers [1.43 (1.05–1.95)], current drinkers [1.76 (1.00–3.14)], and individuals engaging in higher levels of physical activity [1.53 (1.06–2.20)]. The interactions between a history of GDM and obesity (P = 0.05) and current diabetes (P < 0.01) were significant. However, the interactions with metabolic syndrome (P = 0.12), smoking (P = 0.59), alcohol drinking (P = 0.42), and physical activity (P = 0.63) were not significant (Table 4), respectively, which may arise from the relatively wide range of 95% CIs or the possibility that these variables are independent of the history of GDM.
To our knowledge, this is the first epidemiological study to explore the association between GDM and the risk of arthritis. In this national survey cohort of 11,997 women, women with a history of GDM tended to have increased odds of arthritis, and the finding was robust even after accounting for metabolic syndrome that is potentially related to subsequent arthritis. Importantly, women with a history of GDM in whom metabolic syndrome and diabetes does not develop still have increased odds of arthritis. In stratified analyses, the association between a history of GDM and arthritis was observed in smokers, alcohol drinkers, and women engaging in higher levels of physical activity. In addition, GDM was significantly associated with increased odds of osteoarthritis, while no significant association was found with rheumatoid arthritis and other types.
Several potential reasons may explain the association between GDM and the risk of arthritis. First, women with a history of GDM were found to have a nearly 10-fold higher risk of developing type 2 diabetes than healthy controls [relative risk (95% CI): 9.51 (7.14–12.67)] (4), and a previous meta-analysis showed that type 2 diabetes was associated with increased odds of arthritis [OR (95%): 1.45 (1.18–1.78)] (20). Furthermore, patients with diabetes mellitus had 2.18 [95% (1.12–4.24)] times the odds of having osteoarthritis (35), which is the most common type of arthritis (13). Second, a recent meta-analysis found that the OR (95% CI) for metabolic syndrome was 3.45 (2.80–4.25) in women with a history of GDM compared to women without a history of GDM (5), and metabolic syndrome [OR (95% CI): 1.42 (1.16–1.73)], hypertension [1.70 (1.41–2.05), and hyperglycemia [1.23 (1.05–1.42)] were all positively associated with odds of osteoarthritis (19). Third, women with a history of GDM have significantly higher BMI (1.54 kg/m2, 95% CI: 1.32 to 2.46) (34). Compared to normal weight subjects, overweight and obese subjects had 15% (95%CI: 1.03–1.29) and 31% (1.12–1.53) higher odds of rheumatoid arthritis (36), respectively, and the associations were stronger with osteoarthritis [overweight: 2.45 (1.88–3.20), obesity: 4.55 (2.90–7.13)] (18). These findings are consistent with those from our study in which a history of GDM was associated with odds of osteoarthritis but not rheumatoid arthritis. In addition, the association between a history of GDM and odds of arthritis was only observed in subjects with obesity [1.64 (1.24–2.16)], and the association was attenuated in model 2 also adjusting for BMI [1.32 (1.06–1.64)]. These findings suggested that BMI may partially account for the observed association between GDM and the odds of arthritis. However, the association between a history of GDM and the odds of arthritis remained significant in women without metabolic syndrome [1.34 (1.00–1.78)] and current diabetes [1.35 (1.03–1.76)], and a similar result was found in sensitivity analysis with further adjustment for metabolic syndrome [1.30 (1.05–1.60)]. These findings suggested the observed association between a history of GDM and the odds of arthritis was independent of type 2 diabetes and metabolic syndrome. These findings are comparable with those from previous studies in which the long-term risk for cardiovascular disease associated with GDM was not dependent upon intercurrent type 2 diabetes (7), but maybe explained partly by BMI (28).
Results from this study showed that a history of GDM was significantly associated with increased odds of arthritis among smokers, alcohol drinkers, and women engaging in higher levels of physical activity. While smoking was inversely associated with the risk of osteoarthritis in both observational studies (22) and Mendelian randomization studies (21, 37), a positive association was found between smoking and the risk of rheumatoid arthritis in both observational studies (38) and a Mendelian randomization study (39). These results indicate that the effects of smoking on arthritis may differ by different clinical subtypes of arthritis. Low to moderate alcohol consumption was found inversely associated with the development of both osteoarthritis (24) and rheumatoid arthritis (25), while a positive association was also found between alcohol consumption and osteoarthritis prevalence in the Korean NHANES assessed by the alcohol use disorders identification test (40). Findings on the association between physical activity and arthritis are conflicting. While physical activity was found to be inversely associated with arthritis in observational studies (26, 27), physical activity may constitute an important risk factor for arthritis progression prediction with a machine learning approach (41). In this study, physical activity was also found positively associated with prevalence of arthritis [1.41 (1.27–1.58)] (Table 3). These findings indicate that the association between physical activity and arthritis may differ by clinical subtypes of arthritis, and intensity and measurement methods of physical activity (26, 27, 41), which need to be confirmed further. In summary, the findings available on the associations between smoking, alcohol drinking, and physical activity and arthritis remain contradictory, and the potential interactions between a history of GDM and these life-style factors on the risk of arthritis deserve to the confirmed further.
There are several limitations in this study. First, the causality cannot be determined because this is a cross-sectional study, and the causality should be confirmed further in prospective cohort studies. Second, a GDM history and arthritis diagnosis were based on self-report and misclassification could be of concern. However, data from the NHANES are considered to be valid to assess the prevalence of GDM and arthritis in the general population (10, 28, 29), and misclassification of patients with undiagnosed arthritis and GDM as healthy controls could have weakened the association.
In summary, a history of GDM was associated with increased odds of arthritis in this nationally representative cohort, and the association was independent of metabolic syndrome and type 2 diabetes. The association between a history of GDM and arthritis was observed in smokers, alcohol drinkers, and women engaging in higher levels of physical activity. The causality should be confirmed further in prospective cohort studies.
Analytic code will be made available from the corresponding author.
The original contributions presented in the study are included in the article/supplementary files, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by National Center for Health Statistics Research Ethics Review Board. The patients/participants provided their written informed consent to participate in this study.
YM and QL designed the study. WH conducted the statistical analysis. YM, BX, LL, and QL drafted the manuscript. QL made critical revisions. All authors contributed to the article and approved the submitted version.
The authors received support from the Maternal and Child Health Research Project of Jiangsu Province (No. F201720) and the Development Science and Technology Project of Kunshan (No. KS1646).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors are grateful to the National Center for Health Statistics of the Centers for Disease Control and Prevention for sharing the data.
1. cIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. (2019) 5:47. doi: 10.1038/s41572-019-0098-8
2. Casagrande SS, Linder B, Cowie CC. Prevalence of gestational diabetes and subsequent Type 2 diabetes among U.S. women. Diabetes Res Clin Pract. (2018) 141:200–8. doi: 10.1016/j.diabres.2018.05.010
3. Paulo MS, Abdo NM, Bettencourt-Silva R, Al-Rifai RH. Gestational diabetes mellitus in Europe: a systematic review and meta-analysis of prevalence studies. Front Endocrinol. (2021) 12:691033. doi: 10.3389/fendo.2021.691033
4. Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. (2020) 369:m1361. doi: 10.1136/bmj.m1361
5. Tranidou A, Dagklis T, Tsakiridis I, Siargkas A, Apostolopoulou A, Mamopoulos, et al. Risk of developing metabolic syndrome after gestational diabetes mellitus - a systematic review and meta-analysis. J Endocrinol Invest. (2021) 44:1139–49. doi: 10.1007/s40618-020-01464-6
6. Wang Y, Yan P, Fu T, Yuan J, Yang G, Liu, et al. The association between gestational diabetes mellitus and cancer in women: a systematic review and meta-analysis of observational studies. Diabetes Metab. (2020) 46:461–71. doi: 10.1016/j.diabet.2020.02.003
7. Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. (2019) 62:905–14. doi: 10.1007/s00125-019-4840-2
8. Saravanan P. Gestational diabetes: opportunities for improving maternal and child health. Lancet Diabetes Endocrinol. (2020) 8:793–800. doi: 10.1016/S2213-8587(20)30161-3
9. GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2017) 390:1260–344. doi: 10.1016/S0140-6736(17)32130-X
10. Dillon CF, Weisman MH. US National Health and Nutrition Examination Survey Arthritis Initiatives, Methodologies and Data. Rheum Dis Clin North Am. (2018) 44:215–65. doi: 10.1016/j.rdc.2018.01.010
11. Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C. The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int. (2021) 41:863–77. doi: 10.1007/s00296-020-04731-0
12. Safiri S, Kolahi AA, Smith E, Hill C, Bettampadi D, Mansournia, et al. A. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. (2020) 79:819–28. doi: 10.1136/annrheumdis-2019-216515
13. Grassel S, Zaucke F, Madry H. Osteoarthritis: novel molecular mechanisms increase our understanding of the disease pathology. J Clin Med. (2021) 10:1938. doi: 10.3390/jcm10091938
14. Firestein GS, McInnes IB. Immunopathogenesis of rheumatoid arthritis. Immunity. (2017) 46:183–96. doi: 10.1016/j.immuni.2017.02.006
15. de Mendonca E, Fragoso MBT, de Oliveira JM, Xavier JA, Goulart MOF, de Oliveira ACM. Gestational diabetes mellitus: the crosslink among inflammation, nitroxidative stress, intestinal microbiota and alternative therapies. Antioxidants. (2022) 11:129. doi: 10.3390/antiox11010129
16. Hart HF, Barton CJ, Khan KM, Riel H, Crossley KM. Is body mass index associated with patellofemoral pain and patellofemoral osteoarthritis? A systematic review and meta-regression and analysis. Br J Sports Med. (2017) 51:781–90. doi: 10.1136/bjsports-2016-096768
17. Jiang L, Xie X, Wang Y, Lu Y, Tian T, Chu, et al. Body mass index and hand osteoarthritis susceptibility: an updated meta-analysis. Int J Rheum Dis. (2016) 19:1244–54. doi: 10.1111/1756-185X.12895
18. Zheng H, Chen C. Body mass index and risk of knee osteoarthritis: systematic review and meta-analysis of prospective studies. BMJ Open. (2015) 5:e007568. doi: 10.1136/bmjopen-2014-007568
19. Xie Y, Zhou W, Zhong Z, Zhao Z, Yu H, Huang, et al. Metabolic syndrome, hypertension, and hyperglycemia were positively associated with knee osteoarthritis, while dyslipidemia showed no association with knee osteoarthritis. Clin Rheumatol. (2021) 40:711–724. doi: 10.1007/s10067-020-05216-y
20. Dong Q, Liu H, Yang D, Zhang Y. Diabetes mellitus and arthritis: is it a risk factor or comorbidity?: A systematic review and meta-analysis. Medicine. (2017) 96:e6627. doi: 10.1097/MD.0000000000006627
21. Johnsen MB, Vie GA, Winsvold BS, Bjorngaard JH, Asvold BO, Gabrielsen E., et al. The causal role of smoking on the risk of hip or knee replacement due to primary osteoarthritis: a Mendelian randomisation analysis of the HUNT study. Osteoarthr Cartil. (2017) 25:817–23. doi: 10.1016/j.joca.2016.12.021
22. Kong L, Wang L, Meng F, Cao J, Shen Y. Association between smoking and risk of knee osteoarthritis: a systematic review and meta-analysis. Osteoarthr Cartil. (2017) 25:809–16. doi: 10.1016/j.joca.2016.12.020
23. Sanchez-Campama J, Nagra NS, Pineda-Moncusi M, Prats-Uribe A, Prieto-Alhambra D. The association between smoking and the development of rheumatoid arthritis: a population-based case-control study. Reumatol Clin. (2021) 17:566–9. doi: 10.1016/j.reuma.2020.08.006
24. To K, Mak C, Zhang C, Zhou Y, Filbay S, Khan W. The association between alcohol consumption and osteoarthritis: a meta-analysis and meta-regression of observational studies. Rheumatol Int. (2021) 41:1577–91. doi: 10.1007/s00296-021-04844-0
25. Jin Z, Xiang C, Cai Q, Wei X, He J. Alcohol consumption as a preventive factor for developing rheumatoid arthritis: a dose-response meta-analysis of prospective studies. Ann Rheum Dis. (2014) 73:1962–7. doi: 10.1136/annrheumdis-2013-203323
26. Sun L, Zhu J, Ling Y, Mi S, Li Y, Wang T. Physical activity and the risk of rheumatoid arthritis: evidence from meta-analysis and Mendelian randomization. Int J Epidemiol. (2021) 50:1593–603. doi: 10.1093/ije/dyab052
27. Gates LS, Perry TA, Golightly YM, Nelson AE, Callahan LF, Felson, et al. Recreational Physical Activity and Risk of Incident Knee Osteoarthritis: an international meta-analysis of individual participant-level data. Arthritis Rheumatol. (2021) 74:612–22. doi: 10.1002/art.42001
28. Shostrom DCV, Sun Y, Oleson JJ, Snetselaar LG, Bao W. History of Gestational diabetes mellitus in relation to cardiovascular disease and cardiovascular risk factors in US women. Front Endocrinol. (2017) 8:144. doi: 10.3389/fendo.2017.00144
29. Ciardullo S, Bianconi E, Zerbini F, Perseghin G. Current type 2 diabetes, rather than previous gestational diabetes, is associated with liver disease in U.S. Women. Diabetes Res Clin Pract. (2021) 177:108879. doi: 10.1016/j.diabres.2021.108879
30. VanEvery H, Yang W, Olsen N, Bao L, Lu B, Wu, et al. Alcohol consumption and risk of rheumatoid arthritis among chinese adults: a prospective study. Nutrients. (2021) 13:2231. doi: 10.3390/nu13072231
31. Liu X, Tedeschi SK, Lu B, Zaccardelli A, Speyer CB, Costenbader H., et al. Long-term physical activity and subsequent risk for rheumatoid arthritis among women: a prospective cohort study. Arthritis Rheumatol. (2019) 71:1460–71. doi: 10.1002/art.40899
32. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. (2015) 314:1021–9. doi: 10.1001/jama.2015.10029
33. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin A, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. (2005) 112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404
34. Pathirana MM, Lassi Z, Ali A, Arstall M, Roberts CT, Andraweera PH. Cardiovascular risk factors in women with previous gestational diabetes mellitus: a systematic review and meta-analysis. Rev Endocr Metab Disord. (2021) 22:729–61. doi: 10.1007/s11154-020-09587-0
35. Nieves-Plaza M, Castro-Santana LE, Font YM, Mayor AM, Vila LM. Association of hand or knee osteoarthritis with diabetes mellitus in a population of Hispanics from Puerto Rico. J Clin Rheumatol. (2013) 19:1–6. doi: 10.1097/RHU.0b013e31827cd578
36. Qin B, Yang M, Fu H, Ma N, Wei T, Tang, et al. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose-response meta-analysis. Arthritis Res Ther. (2015) 17:86. doi: 10.1186/s13075-015-0601-x
37. Lee YH. Causal association between smoking behavior and the decreased risk of osteoarthritis: a Mendelian randomization. Z Rheumatol. (2019) 78:461–6. doi: 10.1007/s00393-018-0505-7
38. Di Giuseppe D, Discacciati A, Orsini N, Wolk A. Cigarette smoking and risk of rheumatoid arthritis: a dose-response meta-analysis. Arthritis Res Ther. (2014) 16:R61. doi: 10.1186/ar4498
39. Qian Y, Zhang L, Wu DJH, Xie Z, Wen C, Mao Y. Genetic predisposition to smoking is associated with risk of rheumatoid arthritis: a Mendelian randomization study. Arthritis Res Ther. (2020) 22:44. doi: 10.1186/s13075-020-2134-1
40. Kang AH, Kim MR, Shin JS, Lee J, Lee YJ, Park, et al. Association between alcohol consumption and osteoarthritis prevalence in Korea as assessed by the alcohol use disorders identification test (AUDIT): a cross-sectional study. BMC Public Health. (2020) 20:227. doi: 10.1186/s12889-020-8326-4
41. Alexos A, Moustakidis S, Christos Kokkotis, Tsaopoulos D. Physical activity as a risk factor in the progression of osteoarthritis: a machine learning perspective. In: International Conference on Learning and Intelligent Optimization. Nizhny Novgorod (2020) 12096:16–26. doi: 10.1007/978-3-030-53552-0_3
Keywords: arthritis, National Health and Nutrition Examination Survey, type 2 diabetes, metabolic syndrome, gestational diabetes mellitus
Citation: Mao Y, Hu W, Xia B, Liu L and Liu Q (2022) Association Between History of Gestational Diabetes Mellitus and the Risk of Arthritis in Women. Front. Public Health 10:878845. doi: 10.3389/fpubh.2022.878845
Received: 18 February 2022; Accepted: 28 April 2022;
Published: 27 May 2022.
Edited by:
Luis Sobrevia, Pontificia Universidad Católica de Chile, ChileReviewed by:
Paola Valero, University of Talca, ChileCopyright © 2022 Mao, Hu, Xia, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin Liu, bGl1cWluMTQzNEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.