- 1Department of Poisoning and Occupational Diseases, Emergency Medicine, Cheeloo College of Medicine, Qilu Hospital of Shandong University, Shandong University, Jinan, China
- 2Department of Digestive Internal Medicine, Cheeloo College of Medicine, Qilu Hospital of Shandong University, Shandong University, Jinan, China
- 3School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, China
- 4Department of Geriatric Medicine, School of Nursing, Cheeloo College of Medicine, Qilu Hospital of Shandong University, Shandong University, Jinan, China
Acute organophosphorus pesticide poisoning (AOPP) with cardiac arrest has an extremely high mortality rate, and corresponding therapeutic strategies have rarely been reported. Therefore, this study aimed to explore the prognostic factors and effective treatments of AOPP-related cardiac arrest. This retrospective study was conducted in our department in the years 2018–2021. We conducted a descriptive analysis of the clinical manifestations, rescue strategies, and prognosis of patients with AOPP who had experienced cardiac arrest and successful cardiopulmonary resuscitation. This study included six cases of patients with AOPP in addition to cardiac arrest; in four cases, cardiac arrest occurred <12 h after ingestion, and in two, cardiac arrest occurred more than 48 h after ingestion. Five patients had not undergone hemoperfusion therapy before cardiac arrest, and all six were treated with atropine during cardiopulmonary resuscitation and subsequent pralidoxine. Four patients recovered and were discharged from the hospital, one died in our department, and one was transferred to a local hospital and died there 2 h later. The last two patients had severe pancreatic injuries and disseminated intravascular coagulation. This, along with their death, might have been related to their prognosis. Cardiac arrest can occur in patients with severe AOPP for whom antidote administration was insufficient or not timely. Application of atropine and pralidoxine in a timely manner after cardiac arrest following AOPP is the key to successful treatment. This study provides useful guidelines for the treatment of similar cases in the future.
Introduction
Some organophosphorus pesticides (OPs) are extremely poisonous and cause rapid intoxication-induced death with minimal ingestion or exposure. Acute organophosphorus pesticide poisoning (AOPP) is a major life-threatening toxic disease in the rural areas of developing countries (1, 2). In China, AOPP cases comprise nearly 50% of all poisoning cases, with case fatality rates of 3–40%, comprising over 80% of all poisoning deaths (3, 4). OPs cause damage to multiple organs through cholinergic and non-cholinergic effects (5). Common symptoms of AOPP include central nervous system and neuromuscular complications, with cardiopulmonary arrest being the most serious complication and often having a very poor prognosis (6, 7). However, AOPP-related cardiac arrest is rarely reported. In this study, we analyzed the clinical data of patients with AOPP who experienced cardiopulmonary arrest and successful cardiopulmonary resuscitation (CPR) on-site and summarized the clinical characteristics and prognostic factors of the aforementioned disease.
Materials and Methods
Study Participants
Six patients with AOPP who suffered a cardiac and/or respiratory arrest and were successfully resuscitated on-site were selected as research participants. These patients had been admitted to the Department of Poisoning and Occupational Diseases, QILU Hospital of Shandong University (Jinan City, China) between January 1, 2018 and December 31, 2021.
Inclusion and Exclusion Criteria
The inclusion criteria included a patient age of ≥18 years, patients who ingested OPs orally, a time from ingestion to admission to our department of <48 h, and meeting the following diagnostic and exclusion criteria. The diagnostic criteria were a clear history of taking poison, a distinct garlic or petroleum odor after ingestion, a reduced acetylcholinesterase level, a cholinergic crisis, and a positive trial of atropine. Patients with previous heart disease or other diseases, such gastroenteritis, myasthenia gravis, Guillain-Barré syndrome, botulism, mushroom toxicity, and nicotine toxicity, were excluded.
Treatment Plan
When patients were transferred to our department, blood and urine routine examinations, along with coagulation function, liver function, renal function, creatine kinase-MB, amylase, lipase, blood glucose, blood lipids, electrolyte, and cholinesterase tests, were conducted. Moreover, other related examinations were obtained. The main conventional treatment drugs included penehyclidine hydrochloride injection (1 mg, twice daily), atropine (1 mg, every 6 h and adjusted as necessary), pralidoxime iodide (2.0 g, twice daily), betamethasone (8 mg, once daily), pantoprazole (40 mg, twice daily), reduced glutathione (1.8 g, once daily), alanyl glutamine (20 g, once daily), torsemide (20 g, twice daily), nalmefene (0.1 mg, twice daily), and fat emulsion, amino acid (8), and glucose (1%) injections (1,920 mL, once daily). Hemoperfusion was administered twice in the first 24 h of admission and subsequently once daily for a total of four times, which was a treatment plan that we called the “2-1-1 plan.” The treatment plan was adjusted appropriately based on disease progression. When a cholinergic crisis occurred, atropine was given in a timely manner, and when cardiac and/or respiratory arrest occurred, CPR was immediately performed, and atropine was administered simultaneously. All patients after CPR were timely treated with pralidoxime. We also administered smectite powder and injected activated carbon with mannitol into the patients' stomach through a gastric tube for gastrointestinal decontamination.
Data Collection and Analysis
The patient data described in this paper were obtained from the Department of Poisoning and Occupational Diseases, QILU Hospital of Shandong University (Jinan City, China). We conducted a descriptive analysis of the whole medical record related to this study. Data on the sex, age, type of poison, medical history, main treatment, and condition changes (e.g., prehospital treatment, treatment with CPR, and disease progression) of each patient were obtained. Continuous variables are presented as means ± standard deviations, and categorical variables are presented as counts or actual numerical values.
Registration and Ethics
This study was approved by the Ethics Committee of the Shandong University QILU Hospital (Jinan City), and written informed consent was obtained from the families of patients.
Results
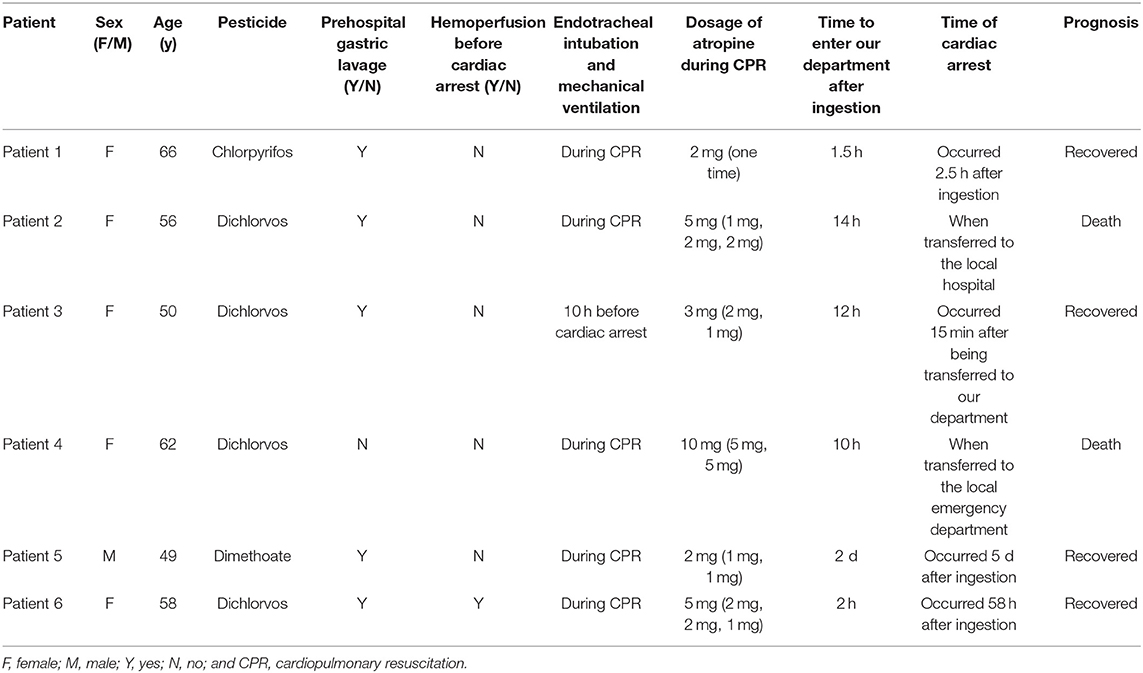
Six patients with AOPP were included in the study. There was one man and five women who were aged 49–66 years, with an average age of 56.8 ± 6.0 years. This research involved four cases of dichlorvos poisoning, one of chlorpyrifos poisoning, and one of dimethoate poisoning. All six patients developed cardiac arrest and were administered atropine by intravenous injection during CPR. Five patients received endotracheal intubation and mechanical ventilation during CPR, and one received mechanical ventilation before cardiac arrest because of respiratory failure. Five patients had not undergone hemoperfusion before the onset of cardiac and/or respiratory arrest. After treatment, four patients recovered and were discharged, and two died. The main clinical data of patients are shown in Table 1.
Four patients who had lower cholinesterase levels on admission had cardiac arrest <12 h after ingestion, that is, when they were immediately transferred to a local hospital or our hospital. Two patients who were administered reduced atropine and/or pralidoxime iodide had cardiac arrests on the fifth day and third day after ingestion, respectively. Both of the patients who died had severe pancreatic injury (amylase and/or lipase levels were three times higher than baseline values) and abnormal coagulation on admission. The patients' main laboratory tests results are shown in Table 2.
Discussion
In this study, we analyzed the clinical data of patients with AOPP who experienced cardiopulmonary arrest and successful CPR on-site. We found that cardiac arrest occurred in patients with severe AOPP for whom antidote application was insufficient or untimely. Therefore, we assumed that cardiac arrest was related to the toxic effect of OPs and administration of an insufficient amount of a specific antidote.
AOPP is a toxic disease having a main symptom of a cholinergic crisis, leading to the phosphorylation of serine residues in the active site of acetylcholine esterase (AChE) and gradual inhibition of AChE (9). The reactivation of AChE in vivo is key to the successful treatment of AOPP. The main treatment includes atropine, oxime drugs, the removal of toxins in vivo, and symptomatic supportive treatment (10–12). The determination of AChE activity can be used as an important index for the diagnosis, grading, and judgment of AOPP. OPs can inhibit acetylcholinesterase and butyrylcholinesterase, although the inhibition of butyrylcholinesterase does not produce clinical symptoms for the most part (13). In China, nearly all clinical hospitals only detect serum levels of cholinesterase, that is, butyrylcholinesterase; therefore, determining a response to AOPP therapy based on butyrylcholinesterase is not completely reliable (14, 15). The lack of a specific antidote or an impertinent rapid diagnosis and disease evaluation leads to improper treatment (16). Atropine and oxime-type antidotes should be applied in a timely manner for AOPP. If the cholinergic crisis induced by AOPP cannot be treated in a timely manner, it is highly likely to progress to cardiopulmonary arrest (17).
In patients with severe AOPP, OPs may cause central apnea or hypopnea (8), and a cholinergic crisis can lead to increased airway secretion, acute cholinergic respiratory failure, and respiratory arrest (18). OPs can also inhibit heart function and cause bradycardia and cardiac arrest. Hypoxemia, electrolyte derangements, and acidosis are major predisposing factors for cardiac arrest (19), and close monitoring and airway management are essential for prevention. The initial dose of atropine for adults is 2 to 5 mg intravenously, and if the patient does not respond to treatment, the dose must be doubled every 3 to 5 min until respiratory secretions have cleared and there is no bronchoconstriction. In severe cases, treatment may require continuous infusion over several days; at first, the toxicants in the gastrointestinal tract are not completely removed, although atropine temporarily alleviates symptoms of cholinergic crisis. However, the amount of antidote in the body remains relatively insufficient, and a cholinergic crisis can easily occur. Thus, once a patient experiences cardiac and/or respiratory arrest, atropine and oxime-type antidotes should be administered simultaneously with CPR (18, 19). In this study, patients who had successful CPR had the common characteristic of the application of atropine and establishment of advanced airway management during on-site resuscitation.
Atropine only works on muscarinic receptors, and pralidoxime works by reactivating the phosphorylated AChE by binding to OPs. The detoxification of oxime, as a specific antidote for AOPP, has saved many lives through early appropriate intervention (20–22). The standard of care includes a bolus of at least 30 mg/kg over 30 min. After the bolus, a continuous infusion of at least 8 mg/kg/h should be initiated and may be needed for several days. However, for the detoxification of oxime to work, it need to be given within 48 h of poisoning. Animal studies have shown that pralidoxime can contribute to the successful resuscitation of cardiac arrest in organophosphate-induced pig models (23). The outlook for most patients is excellent, although cardiac arrest occurred in some severe cases (3). However, owing to the limitation in the acetylcholinesterase structure-based design of oxime antidotes for AOPP, some detoxification effects are limited for some OPs (24, 25). Gastrointestinal decontamination and hemoperfusion (26) can remove OPs in the gastrointestinal tract and blood, respectively. Therefore, the timely and complete removal of toxins is another essential treatment for AOPP. This can also reduce the incidence of poisoning rebound and reduce the incidence of cardiopulmonary arrest.
The suitable dose of atropine for AOPP-related cardiac arrest has not been reported yet. In a previous edition of the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care (2020), atropine was not involved in CPR (27), and in a consensus of clinical experts on the diagnosis and treatment of AOPP (2016) in China (4), it had suggested that atropine should be actively administered for AOPP-related cardiac arrest; however, the exact dose was not discussed. In this paper, we observed that the practical dose of atropine during CPR was between 2 and 10 mg for patients undergoing an AOPP-induced cardiac arrest. Due to the limitation of only six patients in this study, the optimal dose of antidotes is not entirely clear, and further research is warranted.
Patients with severe AOPP must be timely evaluated after receiving treatment, and antidotes, such as atropine and pralidoxime, should be administered as soon as possible. Hemoperfusion, gastrointestinal decontamination, and respiratory support treatment should be administered when necessary. For patients with respiratory and cardiac arrest, atropine and pralidoxime are of great importance during CPR. We believe that our findings could potentially provide guidelines for the treatment of AOPP-related cardiopulmonary arrest. Further studies should be conducted to determine the dose and administration time of specific antidotes during CPR.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
This study was approved by the Ethics Committee of Shandong University Qilu Hospital (Jinan City). Written informed consent was obtained from the patients' families. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
GY conceived the study, drafted the manuscript, and all authors contributed substantially to its revision. GY, YL, TJ, and LZ supervised the conduct of the paper and data collection. GY, XJ, YL, LS, SC, and LZ provided statistical advice on the study design and analyzed the data. BK and XJ chaired the data oversight committee. GY and YL take responsibility for the paper as a whole. All authors contributed to the article and approved the submitted version.
Funding
This project was funded as a scientific research project of the Qilu Hospital, Shandong University (Project Number: KYLL-2019-296).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Amir A, Raza A, Qureshi T, Mahesar GB, Jafferi S, Haleem F, et al. Organophosphate poisoning: demographics, severity scores and outcomes from National Poisoning Control Centre, Karachi. Cureus. (2020) 12:e8371. doi: 10.7759/cureus.8371
2. Gheshlaghi F, Akafzadeh Savari M, Nasiri R, Wong A, Feizi A, Reza Maracy M, et al. Efficacy of fresh frozen plasma transfusion in comparison with conventional regimen in organophosphate poisoning treatment: a meta-analysis study. Crit Rev Toxicol. (2020) 50:677–84. doi: 10.1080/10408444.2020.1823313
3. Yu S, Yu S, Zhang L, Gao Y, Walline J, Lu X, et al. Efficacy and outcomes of lipid resuscitation on organophosphate poisoning patients: a systematic review and meta-analysis. Am J Emerg Med. (2019) 37:1611–17. doi: 10.1016/j.ajem.2018.11.022
4. Emergency Physicians Branch of Chinese Medical Doctors Association. Consensus of clinical experts on diagnosis and Treatment of acute organophosphorus pesticide poisoning (2016). China Emergency Med. (2016) 36:1057–65. doi: 10.3969/j.issn.1002-1949.2016.12.001
5. Mukherjee S, Gupta RD. Organophosphorus nerve agents: types, toxicity, and treatments. J Toxicol. (2020) 2020:3007984. doi: 10.1155/2020/3007984
6. Yoshida S, Okada H, Nakano S, Shirai K, Yuhara T, Kojima H, et al. Much caution does no harm! Organophosphate poisoning often causes pancreatitis. J Intensive Care. (2015) 3:21. doi: 10.1186/s40560-015-0088-1
7. Qu C, Wei M, Zhang H, Zhang J, Ke L, Li W. Acute pancreatitis caused by organophosphate poisoning complicated by spontaneous rupture of acute necrotic collection: a case report. Pancreas. (2021) 50:e10–1. doi: 10.1097/MPA.0000000000001715
8. Nomura K, Narimatsu E, Inoue H, Kyan R, Sawamoto K, Uemura S, et al. Mechanism of central hypopnoea induced by organic phosphorus poisoning. Sci Rep. (2020) 10:15834. doi: 10.1038/s41598-020-73003-5
9. Sharma R, Gupta B, Singh N, Acharya JR, Musilek K, Kuca K, et al. Development and structural modifications of cholinesterase reactivators against chemical warfare agents in last decade: a review. Mini Rev Med Chem. (2015) 15:58–72. doi: 10.2174/1389557514666141128102837
10. Sit RK, Fokin VV, Amitai G, Sharpless KB, Taylor P, Radić Z. Imidazole aldoximes effective in assisting butyrylcholinesterase catalysis of organophosphate detoxification. J Med Chem. (2014) 57:1378–89. doi: 10.1021/jm401650z
11. Eddleston M, Buckley NA, Eyer P, Dawson AH. Management of acute organophosphorus pesticide poisoning. Lancet. (2008) 371:597–607. doi: 10.1016/S0140-6736(07)61202-1
12. Rambabu L, Megson IL, Eddleston M. Does oxidative stress contribute to toxicity in acute organophosphorus poisoning? - a systematic review of the evidence. Clin Toxicol. (2020) 58:437–52. doi: 10.1080/15563650.2019.1693589
13. Eddleston M, Eyer P, Worek F, Sheriff MH, Buckley NA. Predicting outcome using butyrylcholinesterase activity in organophosphorus pesticide self-poisoning. QJM. (2008) 101:467–74. doi: 10.1093/qjmed/hcn026
14. Mahadeshwara P, Gouda HS, Hallikeri VR. Plasma cholinesterase: double-edged parameter in the diagnosis of acute organophosphorus poisoning. Med Sci Law. (2010) 50:159–60. doi: 10.1258/msl.2010.100015
15. Akutagawa K, Kunitomo K, Shimizu T, Tsuji T. Organophosphate poisoning: vital signs are not vital in diagnosis. QJM. (2020) 114:127–8. doi: 10.1093/qjmed/hcaa319
16. Dong N, Liu J, Wang Z, Gao N, Pang L, Xing J. Development of a practical prediction scoring system for severe acute organophosphate poisoning. J Appl Toxicol. (2020) 40:889–96. doi: 10.1002/jat.3950
17. Hulse EJ, Davies JO, Simpson AJ, Sciuto AM, Eddleston M. Respiratory complications of organophosphorus nerve agent and insecticide poisoning. Implications for respiratory and critical care. Am J Respir Crit Care Med. (2014) 190:1342–54. doi: 10.1164/rccm.201406-1150CI
18. Eddleston M, Mohamed F, Davies JO, Eyer P, Worek F, Sheriff MH, et al. Respiratory failure in acute organophosphorus pesticide self-poisoning. QJM. (2006) 99:513–22. doi: 10.1093/qjmed/hcl065
19. Karki P, Ansari JA, Bhandary S, Koirala S. Cardiac and electrocardiographical manifestations of acute organophosphate poisoning. Singapore Med J. (2004) 45:385–9.
20. Lorke DE, Petroianu GA. Reversible cholinesterase inhibitors as pretreatment for exposure to organophosphates. A review. J Appl Toxicol. (2019) 39:101–16. doi: 10.1002/jat.3662
21. Lorke DE, Nurulain SM, Hasan MY, Kuča K, Petroianu GA. Combined pre- and posttreatment of paraoxon exposure. Molecules. (2020) 25:1521. doi: 10.3390/molecules25071521
22. Lenina OA, Zueva IV, Zobov VV, Semenov VE, Masson P, Petrov KA. Slow-binding reversible inhibitor of acetylcholinesterase with long-lasting action for prophylaxis of organophosphate poisoning. Sci Rep. (2020) 10:16611. doi: 10.1038/s41598-020-73822-6
23. Jung YH, Lee HY, Jeung KW, Lee BK, Youn CS, Yun SW, et al. Pralidoxime administered during cardiopulmonary resuscitation facilitates successful resuscitation in a pig model of cardiac arrest. Clin Exp Pharmacol Physiol. (2020) 47:236–46. doi: 10.1111/1440-1681.13198
24. Kovalevsky A, Blumenthal DK, Cheng X, Taylor P, Radić Z. Limitations in current acetylcholinesterase structure-based design of oxime antidotes for organophosphate poisoning. Ann N Y Acad Sci. (2016) 1378:41–9. doi: 10.1111/nyas.13128
25. Gorecki L, Korabecny J, Musilek K, Nepovimova E, Malinak D, Kucera T, et al. Progress in acetylcholinesterase reactivators and in the treatment of organophosphorus intoxication: a patent review (2006-2016). Expert Opin Ther Pat. (2017) 27:971–85. doi: 10.1080/13543776.2017.1338275
26. Dong H, Weng YB, Zhen GS, Li FJ, Jin AC, Liu J. Clinical emergency treatment of 68 critical patients with severe organophosphorus poisoning and prognosis analysis after rescue. Medicine. (2017) 96:e7237. doi: 10.1097/MD.0000000000007237
27. Panchal AR, Bartos JA, Cabañas JG, Donnino MW, Drennan IR, Hirsch KG, et al. Adult Basic and Advanced Life Support Writing Group. Part 3: adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. (2020) 142:S366–68. doi: 10.1161/CIR.0000000000000918
Keywords: organophosphorus pesticide, poisoning, cardiac arrest, cardiopulmonary resuscitation, atropine
Citation: Yu G, Li Y, Jian T, Shi L, Cui S, Zhao L, Jian X and Kan B (2022) Clinical Analysis of Acute Organophosphorus Pesticide Poisoning and Successful Cardiopulmonary Resuscitation: A Case Series. Front. Public Health 10:866376. doi: 10.3389/fpubh.2022.866376
Received: 31 January 2022; Accepted: 06 May 2022;
Published: 27 May 2022.
Edited by:
Guodong Ding, Shanghai Children's Hospital, ChinaReviewed by:
Gamal Eldin Abbas Khalifa, Egyptian Resuscitation Council (EgRC), EgyptCharles Ssemugabo, Makerere University, Uganda
Copyright © 2022 Yu, Li, Jian, Shi, Cui, Zhao, Jian and Kan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangdong Jian, amlhbnhpYW5nZG9uZ3ZpcEB2aXAuMTYzLmNvbQ==; Baotian Kan, a2FuYmFvdGlhbkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Guangcai Yu
Guangcai Yu Yaqian Li1†
Yaqian Li1† Xiangdong Jian
Xiangdong Jian