
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 04 May 2022
Sec. Aging and Public Health
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.859947
 Lixian Zhong1†
Lixian Zhong1† Weiwei Chen1,2†
Weiwei Chen1,2† Tonghua Wang3†
Tonghua Wang3† Qiuting Zeng1
Qiuting Zeng1 Leizhen Lai1
Leizhen Lai1 Junlong Lai1
Junlong Lai1 Junqin Lin1
Junqin Lin1 Shaohui Tang1*
Shaohui Tang1*An umbrella review of meta-analyses was performed to summarize the evidence of associations between alcohol consumption and health outcomes and to assess its credibility. Meta-analyses of prospective cohort studies reporting the associations of alcohol consumption with health outcomes were identified. We recalculated the random-effects summary effect size and 95% confidence interval, heterogeneity, and small-study effect for each meta-analysis and graded the evidence. Fifty-nine publications reporting 224 meta-analyses of prospective cohort studies with 140 unique health outcomes were included, in which there were 49 beneficial associations and 25 harmful associations with nominally statistically significant summary results. But quality of evidence was rated high only for seven beneficial associations (renal cell carcinoma risk, dementia risk, colorectal cancer mortality, and all-cause mortality in patients with hypertension for low alcohol consumption; renal cell carcinoma risk, cardiovascular disease (CVD) risk in patients with hypertension and all-cause mortality in patients with hypertension for moderate consumption) and four harmful associations (cutaneous basal cell carcinoma risk for low alcohol consumption; cutaneous basal cell carcinoma risk and cutaneous squamous cell carcinoma risk for moderate alcohol consumption; hemorrhagic stroke risk for high alcohol consumption). In this umbrella review, only 11 health outcomes (5 in low alcohol consumption, 5 in moderate alcohol consumption and 1 in high alcohol consumption) with statistically significant showed high quality of epidemiologic evidence. More robust and larger prospective studies are needed to verify our results.
According to the data from WHO in 2018, about 2.3 billion people worldwide are current drinkers (1). Population surveys indicate that 12 to 14% of adults have current alcohol abuse and 29% had such disorder at some point in their lifetime (2, 3). More than 40 diseases and 2.8 million deaths were fully attributable to alcohol in 2016, which has aroused widespread concern and caused substantial health loss (4, 5). The American Society of Clinical Oncology (6) stated that alcohol is a cause of cancers of the oral cavity, pharynx, larynx, esophagus, liver and breast cancer. However, there has no evidence to assess the relationship between alcohol and other cancers such as endometrial cancer, ovarian cancer, renal cell carcinoma and so on. In recent decades, it is convincing that alcohol consumption had protective effects against cardiovascular disease (7–9), including total cardiovascular disease (CVD), CVD mortality, myocardial infarction (MI), coronary heart disease (CHD), ischemic stroke and heart failure. In the meanwhile, the relationship between nervous system diseases, hematological malignancy and other health outcomes are still unclear. Thus, there have been inconsistent conclusions as to the associations of alcohol consumption with human health outcomes.
Umbrella review is becoming more and more important for overviewing evidence of published systematic reviews and meta-analyses on a specific topic. Only one recent report that indicated the relationship between different types of alcohol and partial health outcomes of meta-analyses of observational studies (10). To our knowledge, there is no existing umbrella review of meta-analyses of prospective cohort studies to capture the breadth of outcomes associated with alcohol consumption and assess the quality of evidence and methodology.
Consequently, we performed this umbrella review of meta-analyses that only including prospective cohort studies to comprehensively assess the methodological quality and investigate the potential bias. More importantly, we evaluated the evidence's breadth, strength, and validity on the associations of alcohol consumption with multiple health outcomes. We believe that our work can provide a more concrete basis for formulation of alcohol consumption guidelines.
Our protocol has been registered in PROSPERO (CRD42021228480). We followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) Guidelines (11).
The systematic literature search was conducted in PubMed, Web of Science and EMBASE with no time limit for meta-analyses of prospective observational studies. Search items were “alcohol and meta-analysis,” and no other restrictions were imposed. The literature search was conducted by three authors (Zhong, Chen, and Wang). Disagreements were resolved by consensus.
Meta-analyses were included if they met the following criteria: (1) Meta-analyses included prospective cohort studies; (2) Meta-analyses reported the relationship between alcohol consumption and direct results on human outcome (incidence or mortality of diseases). The protocols, abstracts of the conference, and letters to editors were excluded. We also excluded the study involves animal research and indirect indicators on human health. When several meta-analyses simultaneously reported the same health outcome, we included the one with the largest number of studies.
One author (LZ) extracted data separately checked by the other authors (WC and TW). For each included meta-analysis, we extracted the following data: the first author's name and publication year. For each primary study in the included meta-analyses, we extracted the name of the first author, publication year, the number of cases and participants, exposure, relative risk (RR), hazard ratios (HR), odds ratios (OR), 95% confidence intervals. Since the amount of alcohol exposure varies in the primary studies, we uniformly classified alcohol consumption levels into three groups based on the primary studies: low, moderate, and high, which were defined as ethanol intake of >0 and ≤ 14.9 g/day (about >0 and <1 drink/day), 15–9.9 g/day (about 1–2.5 drinks/day), ≥30 g/day (about >2.5 drinks/day), respectively (9). The primary prospective studies in each included meta-analysis were retained if they met the following criteria: (1) The primary studies reported the number of cases and participants; (2) Reference groups were non-drinker. For the overlapping meta-analyses, we selected the one with the largest number of cases and participants combined.
We used the validated AMSTAR 2 tool (12) to evaluate the methodological quality of each included published meta-analysis of prospective studies. It has been proven to be an effective and reliable tool for assessing the quality of systematic evaluation methodologies. The AMSTAR tool includes 16 items about the conduction of a meta-analysis. No or only one non-critical defect is considered high methodologic quality, and more than one non-critical defect is considered moderate methodologic quality. Only one critical weakness with or without non-critical defects is considered low method quality, and more than one critical weakness with or without critical defects is critically low methodologic quality. Discrepancies between AMSTAR 2 scores were resolved by discussion.
We classified evidence from meta-analyses of observational studies with nominally statistically significant summary results into three categories (high, moderate, and weak). We evaluated the strength of epidemiologic evidence with the following criteria (13–17): (1) precision of the estimate [P < 0.001 (18, 19), a threshold associated with significantly fewer false-positive results, and more than 1,000 cases of the disease]; (2) consistency of results (I2 <50%; Cochran Q test, P > 0.10); (3) no evidence of small-study effects (P > 0.10). If all these criteria were satisfied, the strength of the epidemiologic evidence was rated as high. If a maximum of 1 criterion was not satisfied and P < 0.001 was found, the strength of the epidemiologic evidence was rated as moderate. If it is all other cases (P < 0.05), the strength of the epidemiologic evidence was considered weak.
According to the above information, we then took non-drinkers as the reference group and used the random-effect model to recalculate the pooled relative risks and 95% CIs of different alcohol consumption levels. Cochran's Q test and the I2 statistics are the tools to evaluate the heterogeneity between studies. I2 values equal to or exceeding 50% are usually judged to represent large heterogeneity. The Egger's test in which a p < 0.1 is taken as statistical evidence of the presence of small-study effects was used to calculate the publication bias (20). For all tests (except for the heterogeneity and small-study effects), p < 0.05 was considered statistically significant. All calculations were conducted with Stata 16.0.
The results of systematic research and selection of eligible studies are shown in Figure 1. We included 59 publications with 224 meta-analyses (9, 21–78). Eighty-four meta-analyses showed overlapping results were removed (Supplementary Table 1), and finally 39 studies with 140 unique meta-analyses were retained, with 50, 47 and 43 unique meta-analysis results in the low, moderate and high alcohol consumption groups, respectively (Supplementary Table 2). The median number of studies included in meta-analyses was five (range 2–44), the median number of participants was 170,691 (range 842–3,702,738), and the median number of cases was 2,014 (range 85–104,278).
The overall AMSTAR 2 scores of each included study are presented in Supplementary Table 3. Only 4 studies were rated as low methodological quality and the remaining 35 studies were all assessed to be critically low methodological. It is worthy to note that there has no high/moderate methodological quality based on the AMSTAR 2 strict criteria (Figure 2).
Compared with non-drinkers, low alcohol consumption decreased the risk of liver cancer (29), endometrial cancer (32) and renal cell carcinoma (72). However, low alcohol consumption increased the risk of esophageal cancer (73), breast cancer (22), cutaneous basal cell carcinoma (40) and cutaneous squamous cell carcinoma (40). Low alcohol consumption was also related to a 23% reduction in colorectal cancer mortality (43) and a 11% reduction in all cancers mortality (28) (Figure 3).
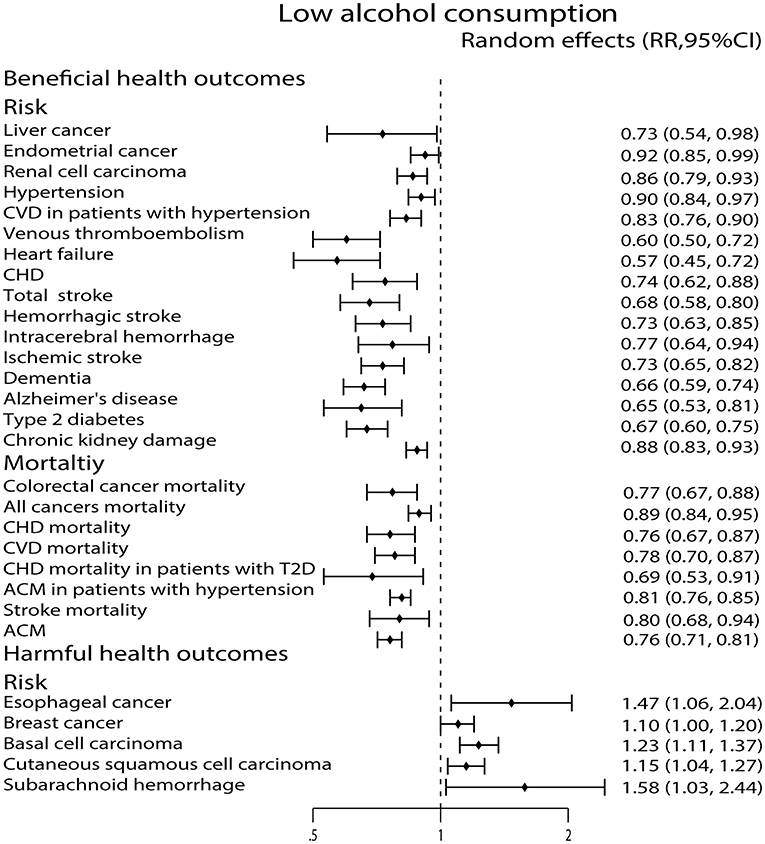
Figure 3. Forest plot: recalculated effects estimates of meta-analyses reporting significant associations of low alcohol consumption with health outcomes. RR, relative risk; CI, confidence interval; CVD, cardiovascular disease; CHD, coronary heart disease; ACM, all-cause mortality.
Moderate alcohol consumption was associated with a reduced risk of thyroid cancer (44) and renal cell carcinoma (72), while it showed increased risk of colon cancer (54), rectum cancer (54), colorectal cancer (54), esophageal cancer (73), breast cancer (22), cutaneous basal cell carcinoma (40) and cutaneous squamous cell carcinoma (40). Moreover, it reduced colorectal cancer mortality (43) and increased esophageal cancer mortality (53) (Figure 4).
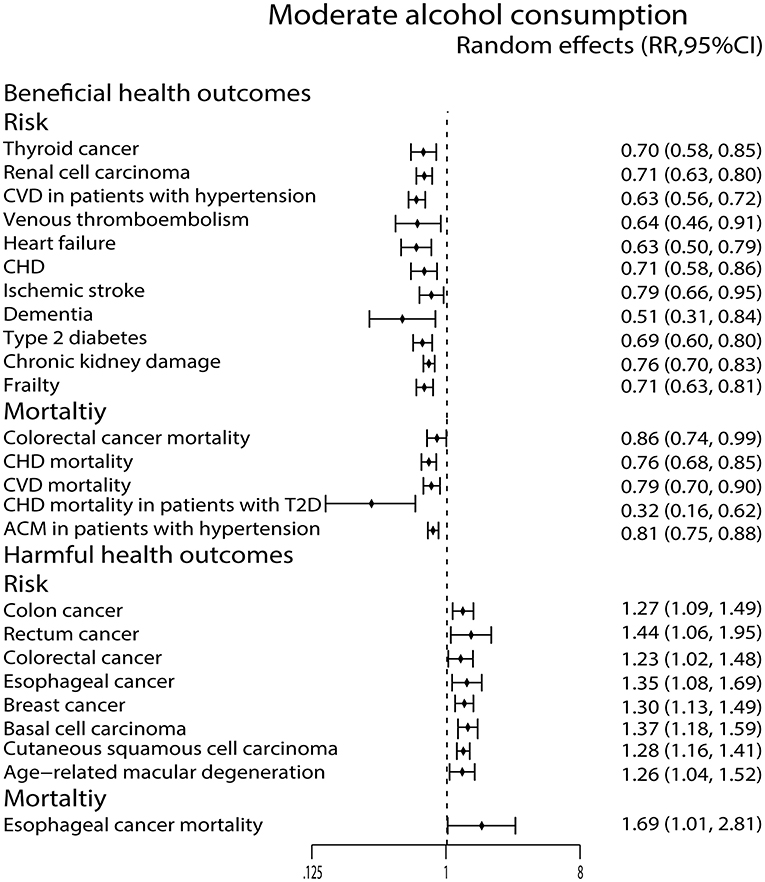
Figure 4. Forest plot: recalculated effects estimates of meta-analyses reporting significant associations of moderate alcohol consumption with health outcomes. RR, relative risk; CI, confidence interval; CHD, coronary heart disease; CVD, cardiovascular disease; ACM, all-cause mortality.
For high alcohol consumption, we found that it only decreased the incidence of thyroid cancer (44) and renal cell carcinoma (72), but it can increase the incidence of rectum cancer (54), gastric cancer (71), esophageal cancer (73), breast cancer (22), and cutaneous squamous cell carcinoma (40). In the meanwhile, high alcohol consumption was significantly related to esophageal cancer mortality (53) and all cancers mortality (28) (Figure 5).
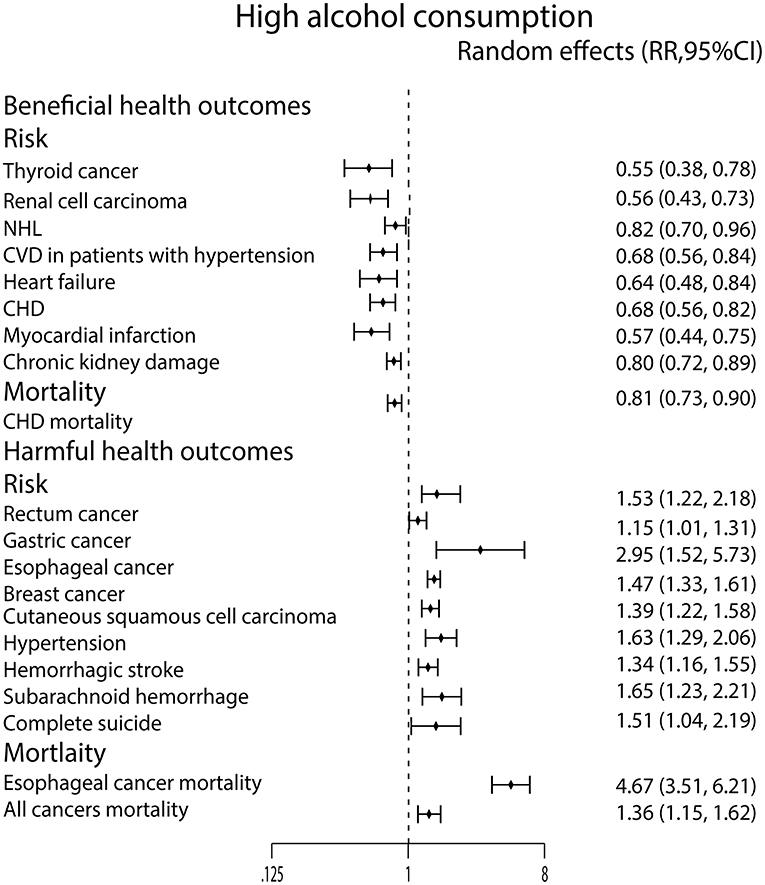
Figure 5. Forest plot: recalculated effects estimates of meta-analyses reporting significant associations of high alcohol consumption with health outcomes. RR, relative risk; CI, confidence interval; CVD, cardiovascular disease; CHD, coronary heart disease.
We found only high alcohol consumption lowered the risk of NHL (55) (Figure 5).
Low alcohol consumption lowered the risk of hypertension (59), CVD in patients with hypertension (68), venous thromboembolism (VTE) (52), heart failure (45), and CHD (9). In addition, low alcohol consumption was also related to a 24% reduction in CHD mortality (64), a 22% reduction in CVD mortality (9), a 31% reduction in CHD mortality in patients with T2D (49) and a 19% reduction in all-cause mortality (ACM) in patients with hypertension (68) (Figure 3).
We observed that the moderate alcohol consumption was related to a decreased risk of CVD in patients with hypertension (68), VTE (52), heart failure (45) and CHD (9). It also reduced the CHD mortality (64), CVD mortality (9), CHD mortality in patients with T2D (49) and ACM in patients with hypertension (68) (Figure 4).
Similarly, high alcohol consumption lowered the incidence of CVD in patients with hypertension (68), heart failure (45), CHD (9) and MI (70). What's more, it also lowered the CHD mortality (64). On the contrary, high alcohol consumption strongly increased the incidence of hypertension (59) (Figure 5).
As we see in Figure 3, low alcohol consumption strongly diminished the risk of total stroke (35), hemorrhagic stroke (35), intracerebral hemorrhage (ICH) (35), ischemic stroke (35), dementia (26) and Alzheimer's disease (AD) (26). We also found that low alcohol consumption was associated with a 20% reduction in stroke mortality (9). On the contrary, low alcohol consumption increased the risk of subarachnoid hemorrhage (SAH) (35) (Figure 3).
Moderate alcohol consumption only lowered ischemic stroke risk (35) and dementia risk (26) in this study (Figure 4). In contrast, the high alcohol consumption was associated with an increased risk of hemorrhagic stroke (35) and SAH (35) (Figure 5).
Compared with non-drinkers, low alcohol consumption was associated with decreased ACM (63) (Figure 3).
Both low and moderate alcohol consumption had protective effects against developing type 2 diabetes (T2D) (25) (Figures 3, 4).
Moderate alcohol consumption was found to increase the AMD risk (27) (Figure 4).
High alcohol consumption was associated with an increased risk of completes suicide (58) (Figure 5). For chronic kidney damage (23), the low, moderate and high alcohol consumption were all correlated to reduce the incidence (Figures 3–5). What's more, moderate alcohol consumption was found to strongly decrease the risk of frailty (21) in this study (Figure 4).
However, the low, moderate, and high drinking groups were not associated with 21, 22, and 23 health outcomes, respectively (Supplementary Figures 1–3).
Based on the criteria mentioned above, the assessment of epidemiologic evidence was not applicable for 21, 22, 23 health outcomes, respectively in low, moderate and high alcohol consumption because they show no statistically significant (P > 0.05) (Supplementary Table 4). Figure 6 showed the results of epidemiologic evidence of each group.
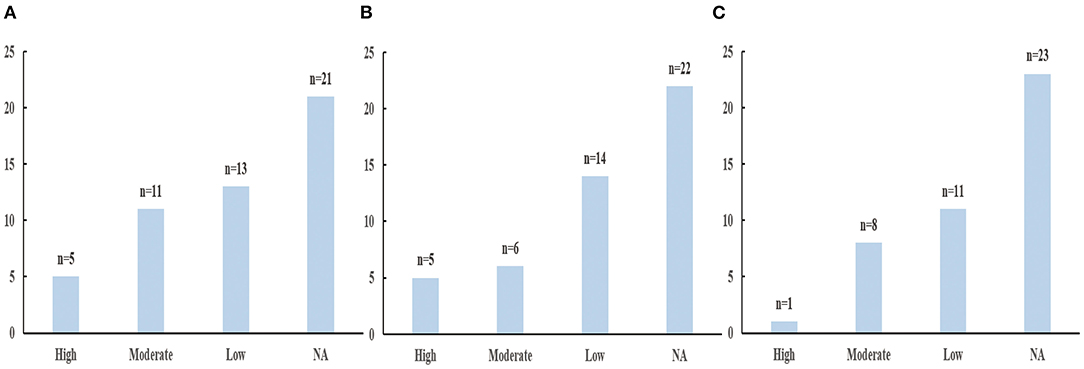
Figure 6. The results of evidecnce assessment. (A) Low alcohol consumption. (B) Moderate alcohol consumption. (C) High alcohol consumption.
In low alcohol consumption group, five health outcomes (the decreased risk of renal cell carcinoma and dementia; the decreased mortality of colorectal cancer and all-cause in patients with hypertension; the increased risk of cutaneous basal cell carcinoma) showed high epidemiologic evidence; 11 health outcomes (the decreased risk of CVD in patients with hypertension, heart failure, total stroke, HS, ischemic stroke, T2D, and AD; the decreased mortality of all cancer, CHD, all-cause; the increased risk of breast cancer) showed moderate epidemiologic evidence; and 13 health outcomes showed weak epidemiologic evidence (Supplementary Table 5).
In moderate alcohol consumption group, five health outcomes (the decreased risk of renal cell carcinoma, CVD in patients with hypertension; the decreased mortality of all-cause in patients with hypertension; the increased risk of cutaneous basal cell carcinoma and cutaneous squamous cell carcinoma) showed high epidemiologic evidence; six health outcomes (the decreased risk of heart failure, CHD, T2D and frailty; the decreased mortality of CHD; the increased risk of breast cancer) showed moderate epidemiologic evidence; 14 health outcomes showed weak epidemiologic evidence (Supplementary Table 6).
In high alcohol consumption group, only one health outcome (the increased risk of hemorrhagic stroke) showed high epidemiologic evidence; eight health outcomes (the decreased risk of renal cell carcinoma, CVD in patients with hypertension, heart failure, CHD, and CKD; the decreased mortality of CHD; the increased risk of breast cancer and cutaneous squamous cell carcinoma) showed moderate epidemiologic evidence; 11 health outcomes showed weak epidemiologic evidence (Supplementary Table 7).
We included 39 publications, which comprised 140 unique meta-analyses of prospective studies. We found the quality of evidence was graded as high for the four beneficial health outcomes (the decreased risk of renal cell carcinoma and dementia as well as the decreased mortality of colorectal cancer and all-cause in patients with hypertension) and one harmful health outcome (the increased risk of cutaneous basal cell carcinoma) in low alcohol consumption. For moderate alcohol consumption, the quality of evidence was graded as high for three beneficial health outcomes (the decreased risk of renal cell carcinoma, CVD in patients with hypertension; the decreased mortality of all-cause in patients with hypertension) and two harmful health outcomes (the increased risk of cutaneous basal cell carcinoma and cutaneous squamous cell carcinoma). In the high alcohol consumption, the quality of evidence was graded as high for only one harmful health outcome (the increased risk of hemorrhagic stroke) (Supplementary Tables 5–7).
According to ESMO Clinical Practice Guideline (79), low and moderate alcohol consumption (about 0.1–49.9 g alcohol per day) appears to have a protective effect for renal cell carcinoma. This information accords with our results that low and moderate alcohol consumption reduced the incidence of renal cell carcinoma with high epidemiologic evidence. The potential biologic mechanisms for this anti-cancer effect involve antioxidant phenolic compounds in alcohol, which can reduce oxidative stress and contribute to apoptosis by arresting the cell cycle (80, 81). Additionally, alcohol can reduce the time that carcinogenic solutes contact renal epithelial cells and control hypertension, which plays a role in protecting against renal cell carcinoma (82, 83). Furthermore, increased risk of renal cell carcinoma has been observed in individuals with diabetes or obesity and light to moderate alcohol consumption improved insulin sensitivity and lowered the risk of T2D thus lowering the incidence of renal cell carcinoma (16, 84, 85). Our umbrella review demonstrated that low alcohol consumption (includes wine, beer and liquor) reduced colorectal cancer mortality with high epidemiologic evidence. Related mechanisms may be involved in resveratrol and anthocyanin. Resveratrol found in grape skins can inhibit the occurrence, promotion and progression of tumors, and it has been found to have anti-proliferation effects in vivo and in vitro (86–89). Anthocyanin, which is presented in red wine (also in lower concentrations in white wine and beer), has been reported to have a suppressing effect on colon cancer cells in vitro, and phenolic acid and anthocyanin have been shown to inhibit colon cancer cell viability and to increase apoptosis (90–92).
However, these results should be interpreted carefully in a broader context because there was some robust evidence that alcohol increased the risk of oropharyngeal and larynx cancer, esophageal cancer, hepatocellular carcinoma, colon cancer and breast cancer by a statement of the American Society of Clinical Oncology in 2018 (6). It's worth noting that low/moderate alcohol consumption was related to the evaluated risk of cutaneous basal cell carcinoma and cutaneous squamous cell carcinoma with high epidemiologic evidence in our umbrella review, which was in accord with the above recommendation. Acetaldehyde, the terminal alcohol metabolite with carcinogenic and mutagenic effects by binding to DNA and protein, plays a key role in the pathophysiology of increasing cancer incidence (93–95). Ethanol-induced cancer also involves the induction of oncogenes or the suppression of tumor suppressor genes, which is also the main mechanism leading to cancers (95). Furthermore, the photosensitivity of alcohol metabolites can enhance cell damage and the immunosuppressive effect of alcohol (96, 97). Overall, although our results demonstrate a benefit of alcohol consumption for the risk of renal cell carcinoma and colorectal cancer mortality, caution should be taken in translating these results into guideline recommendations.
The Dietary Guidelines Advisory Committee (US) stated in 2020 (98), if alcohol is consumed, it should be consumed in moderation (≤ 1 and 2 drinks (about 12.5~25 g alcohol) /day for women and men, respectively) and only by adults of legal drinking age. A large prospective cohort of >330,000 adults emphasizes that light to moderate alcohol consumption (0.1~196 g/week for men; 0.1~98 g/week for women) reduced the all-cause mortality by up to 29% and CVD mortality by ≤ 24% in the US (99). Similarly, in our study, low/moderate alcohol consumption reduced CVD events and all-cause mortality in hypertensive patients, with high epidemiologic evidence. This is consistent with the above studies and dietary guidelines. The causal relationship between regular low alcohol consumption and prevention of CVD was supported by some biomedical evidence, which is mediated by anti-inflammatory effects, reduction of fibrinogen levels, inhibition of platelet activation and increased high-density lipoprotein level by terminal alcohol metabolite (100–102). The beneficial effects of alcohol on CVD account for 77.8% of the total beneficial effects of alcohol (103), and ACM includes deaths caused by CVD, so it is reasonable that low/moderate alcohol consumption leads to a decrease in ACM in hypertensive patients.
Our study indicated that high alcohol consumption increased the risk of hemorrhagic stroke with high epidemiologic evidence. Likewise, the ad-hoc Working Group Of The Italian Society Of Human Nutrition (104) suggests that alcohol intake should be limited to 1 drink per day for women and to 2 drinks per day for men to avoid ischemic and hemorrhagic stroke caused by heavy drinking. Alcohol has a detrimental effect on platelet function and platelet count, affecting platelet aggregation and thus damaging human hemostasis (105, 106). Overusing of alcohol may increase the hemorrhage risk linked to small-vessel vasculopathy (107). In addition, the adverse impact of alcohol consumption on blood pressure may directly increase the risk of hemorrhagic stroke. Therefore, controlling alcohol consumption is important for preventing hemorrhagic stroke.
Recently, an umbrella review (108), including 14 observational studies and RCTs, indicated that alcohol intake was a protective factor for dementia with weak evidence. Similarly, our prospective umbrella review, showing low alcohol consumption decreased the incidence of dementia with high epidemiologic evidence, seems to be more robust. Alcohol increases cerebral blood flow, reduces clotting, increases antithrombotic activity, and increases endothelium dilation, which has protective effects against atherosclerosis, vascular occlusion, and cerebral hypoperfusion (109, 110). But, WHO guideline in 2019 (111) suggests that interventions aimed at reducing or ceasing hazardous and harmful drinking should be offered to adults with normal cognition and mild cognitive impairment to reduce the risk of dementia because there is extensive evidence on excessive alcohol as a risk factor for dementia (112, 113). Therefore, while low alcohol intake is a protective factor for dementia, considering the harmful effects of heavy alcohol intake on dementia and other diseases, we are more cautious about incorporating these results into recommendations.
Our umbrella review had the following strengths. On the one hand, to our knowledge, it was the first umbrella review of prospective meta-analyses about the associations between alcohol consumption and health outcomes at present. The included primary studies were all based on prospective observational study design so that the recall bias can be reduced to a certain extent. On the other hand, we used validated tools to evaluate the methodological quality and quality of epidemiological evidence in our umbrella review. All meta-analyses included in this review had high or moderate methodological quality. What's more, we unified the alcohol grouping criteria and recalculated effect sizes, heterogeneity, and small-study effects better to explain the association between alcohol and health outcomes.
However, there were several limitations in our umbrella review. Firstly, we only included the prospective meta-analyses and therefore, we may have missed other health outcomes not yet studied through prospective meta-analysis. Secondly, we did not explore the different types of alcoholic beverages in this study. Arranz et al. (114) pointed out that significant inverse association between regular and moderate wine consumption and vascular risk, particularly red wine, and a similar relationship is reported for beer consumption, while lower protection is described for the consumption of any spirituous beverage. A cross-sectional study showed that compared to never drinkers of each type of alcoholic drink, red wine, champagne/white wine and fortified wine drinkers had a lower BMI, whereas beer and spirits drinkers had higher BMI compared to never drinkers of each type of alcoholic drink (115). Another study found that liquor consumption and binge drinking was associated with increased risk of VTE, whereas wine consumption was possibly associated with reduced risk of VTE (116). However, due to the lack of raw data, there is little literature to explore the association between various health outcomes and different types of alcoholic beverages. In addition, most of the primary studies have not reported the alcoholic type and we failed to conduct a subgroup based on the alcoholic different alcoholic drink types. Thirdly, we did not evaluated quality of the primary studies, since it was beyond the scope of the current umbrella review. Finally, we did not explore the subgroup analysis or sensitivity analysis (e.g., by sex, geography, or other factors that can influence the result).
In summary, the data presented in this study demonstrated that there were 49 beneficial associations and 25 harmful associations with nominally statistically significant summary results in 140 health outcomes for low/moderate alcohol consumption, while harmful associations mainly related to hemorrhagic stroke, hypertension, and cancers for high alcohol consumption. However, the quality of evidence was rated high only for seven beneficial associations (renal cell carcinoma risk, dementia risk, colorectal cancer mortality, and ACM in patients with hypertension for low alcohol consumption; renal cell carcinoma risk, CVD risk in patients with hypertension, and ACM in patients with hypertension for moderate consumption) and four harmful associations (cutaneous basal cell carcinoma risk for low alcohol consumption; cutaneous basal cell carcinoma risk and cutaneous squamous cell carcinoma risk for moderate alcohol consumption; hemorrhagic stroke risk for high alcohol consumption). This reminds us that we should drink in moderation and avoid binge drinking or heavy drinking. To achieve high quality of evidence for the associations of alcohol with the health outcomes and give strong recommendations, more robust and larger prospective studies are needed.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
LZ, WC, TW, and ST contributed to the conception and design of the umbrella review. LZ, WC, TW, QZ, LL, JLa, and JLi were involved in the acquisition and analysis of the data. LZ and TW interpreted the results. LZ and ST drafted the manuscript. All authors read and approved the final manuscript.
This study was supported by the Special project for high-level talents of the Affiliated Hospital of Youjiang Medical University for Nationalities, in Baise, China (No. R202011703).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to acknowledge all authors of the original studies that were included in this meta-analysis.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.859947/full#supplementary-material
CVD, Cardiovascular disease; ACM, all-cause mortality; CHD, coronary heart disease; MI, myocardial infarction; SAH, subarachnoid hemorrhage; ICH, intracerebral hemorrhage; VTE, venous thromboembolism; NHL, non-Hodgkin's lymphoma; T2D, type 2 diabetes mellitus; AD, Alzheimer's disease; RR, relative risk; OR, odd ratio; HR, hazard ratio; CI, confidence interval.
2. Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. (2007) 64:830–42. doi: 10.1001/archpsyc.64.7.830
3. Edelman EJ, Fiellin DA. Alcohol use. Ann Intern Med. (2016) 164:ITC1–16. doi: 10.7326/AITC201601050
4. Rehm J, Gmel GE Sr, Gmel G, Hasan OS, Imtiaz S, Popova S, et al. The relationship between different dimensions of alcohol use and the burden of disease—an update. Addiction. (2017) 112:968–1001. doi: 10.1111/add.13757
5. Griswold MG, Fullman N, Hawley C, Arian N, Zimsen SR, Tymeson HD, et al. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2018) 392:1015–35. doi: 10.1016/S0140-6736(18)31310-2
6. LoConte NK, Brewster AM, Kaur JS, Merrill JK, Alberg AJ. Alcohol and cancer: a statement of the American Society of Clinical Oncology. Am J Clin Oncol. (2018) 36:83–93. doi: 10.1200/JCO.2017.76.1155
7. Costanzo S, Di Castelnuovo A, Donati MB, Iacoviello L, de Gaetano G. Alcohol consumption and mortality in patients with cardiovascular disease: a meta-analysis. J Am Coll Cardiol. (2010) 55:1339–47. doi: 10.1016/j.jacc.2010.01.006
8. Imhof A, Plamper I, Maier S, Trischler G, Koenig W. Effect of drinking on adiponectin in healthy men and women: a randomized intervention study of water, ethanol, red wine, and beer with or without alcohol. Diabetes Care. (2009) 32:1101–3. doi: 10.2337/dc08-1723
9. Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. (2011) 342:d671. doi: 10.1136/bmj.d671
10. Labaca G, Segura-García L, Álvarez FJ, Bosque-Prous M. Differential health effects of alcoholic beverages: an umbrella review of observational studies. Rev Esp Salud Publica. (2020) 94: e20201114.
11. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
12. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008
13. Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. (2014) 348:g2035. doi: 10.1136/bmj.g2035
14. Belbasis L, Bellou V, Evangelou E, Ioannidis JP, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. (2015) 14:263–73. doi: 10.1016/S1474-4422(14)70267-4
15. Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. (2015) 350:g7607. doi: 10.1136/bmj.g7607
16. Neuenschwander M, Ballon A, Weber KS, Norat T, Aune D, Schwingshackl L, et al. Role of diet in type 2 diabetes incidence: umbrella review of meta-analyses of prospective observational studies. BMJ. (2019) 366:l2368. doi: 10.1136/bmj.l2368
17. Piovani D, Danese S, Peyrin-Biroulet L, Nikolopoulos GK, Lytras T, Bonovas S. Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta-analyses. Gastroenterology. (2019) 157:647–59.e4. doi: 10.1053/j.gastro.2019.04.016
18. Ioannidis JP, Tarone R, McLaughlin JK. The false-positive to false-negative ratio in epidemiologic studies. Epidemiology. (2011) 22:450–6. doi: 10.1097/EDE.0b013e31821b506e
19. Johnson VE. Revised standards for statistical evidence. Proc Nat Acad Sci. (2013) 110:19313–7. doi: 10.1073/pnas.1313476110
20. Egger M, Davey Smith G, Schneider M, Minder C. Bias in metaanalysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
21. Kojima G, Liljas A, Iliffe S, Jivraj S, Walters K. A systematic review and meta-analysis of prospective associations between alcohol consumption and incident frailty. Age Ageing. (2018) 47:26–34. doi: 10.1093/ageing/afx086
22. Sun Q, Xie W, Wang Y, Chong F, Song M, Li T, et al. Alcohol consumption by beverage type and risk of breast cancer: a dose-response meta-analysis of prospective cohort studies. Alcohol Alcohol. (2020) 55:246–53. doi: 10.1093/alcalc/agaa012
23. Li D, Xu J, Liu F, Wang X, Yang H, Li X. Alcohol drinking and the risk of chronic kidney damage: a meta-analysis of 15 prospective cohort studies. Alcoholism. (2019) 43:1360–72. doi: 10.1111/acer.14112
24. Jayasekara H, English DR, Room R, MacInnis RJ. Alcohol consumption over time and risk of death: a systematic review and meta-analysis. Am J Epidemiol. (2014) 179:1049–59. doi: 10.1093/aje/kwu028
25. Li X-H. Yu F-f, Zhou Y-H, He J. Association between alcohol consumption and the risk of incident type 2 diabetes: a systematic review and dose-response meta-analysis. Am J Clin Nutr. (2016) 103:818–29. doi: 10.3945/ajcn.115.114389
26. Anstey KJ, Mack HA, Cherbuin N. Alcohol Consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. Am J Geriatr Psychiatry. (2009) 17:542–55. doi: 10.1097/JGP.0b013e3181a2fd07
27. Chong EW-T, Kreis AJ, Wong TY, Simpson JA, Guymer RH. Alcohol consumption and the risk of age-related macular degeneration: a systematic review and meta-analysis. Am J Ophthalmol. (2008) 145:707–15.e2. doi: 10.1016/j.ajo.2007.12.005
28. Jin M, Cai S, Guo J, Zhu Y, Li M, Yu Y, et al. Alcohol drinking and all cancer mortality: a meta-analysis. Ann Oncol. (2013) 24:807–16. doi: 10.1093/annonc/mds508
29. Turati F, Galeone C, Rota M, Pelucchi C, Negri E, Bagnardi V, et al. Alcohol and liver cancer: a systematic review and meta-analysis of prospective studies. Ann Oncol. (2014) 25:1526–35. doi: 10.1093/annonc/mdu020
30. Jin Z, Xiang C, Cai Q, Wei X, He J. Alcohol consumption as a preventive factor for developing rheumatoid arthritis: a dose-response meta-analysis of prospective studies. Ann Rheum Dis. (2014) 73:1962–7. doi: 10.1136/annrheumdis-2013-203323
31. Scd SC. Alcohol dosing and total mortality in men and women. Arch Int Med. (2006) 166:2437–45. doi: 10.1001/archinte.166.22.2437
32. Zhou Q, Guo P, Li H, Chen XD. Does alcohol consumption modify the risk of endometrial cancer? A dose–response meta-analysis of prospective studies. Arch Gynecol Obstet. (2017) 295:467–79. doi: 10.1007/s00404-016-4263-y
33. Sun Q, Xu L, Zhou B, Wang Y, Jing Y, Wang B. Alcohol consumption and the risk of endometrial cancer: a meta-analysis. Asia Pac J Clin Nutr. (2011) 20:125–33.
34. Wang Y-T, Gou Y-W, Jin W-W, Xiao M, Fang H-Y. Association between alcohol intake and the risk of pancreatic cancer: a dose–response meta-analysis of cohort studies. BMC Cancer. (2016) 16:1–11. doi: 10.1186/s12885-016-2241-1
35. Larsson SC, Wallin A, Wolk A, Markus HS. Differing association of alcohol consumption with different stroke types: a systematic review and meta-analysis. BMC Med. (2016) 14:1–11. doi: 10.1186/s12916-016-0721-4
36. Yan-Hong H, Jing L, Hong L, Shan-Shan H, Yan L, Ju L. Association between alcohol consumption and the risk of ovarian cancer: a meta-analysis of prospective observational studies. BMC Public Health. (2015) 15:223. doi: 10.1186/s12889-015-1355-8
37. Yoon S-J, Jung J-G, Lee S, Kim J-S, Ahn S-k, Shin E-S, et al. The protective effect of alcohol consumption on the incidence of cardiovascular diseases: is it real? a systematic review and meta-analysis of studies conducted in community settings. BMC Public Health. (2020) 20:1–9. doi: 10.1186/s12889-019-7820-z
38. Mamluk L, Edwards HB, Savović J, Leach V, Jones T, Moore TH, et al. Low alcohol consumption and pregnancy and childhood outcomes: time to change guidelines indicating apparently ‘safe'levels of alcohol during pregnancy? A systematic review and meta-analyses. BMJ Open. (2017) 7:e015410. doi: 10.1136/bmjopen-2016-015410
39. Friberg E, Orsini N, Mantzoros CS, Wolk A. Alcohol intake and endometrial cancer risk: a meta-analysis of prospective studies. Br J Cancer. (2010) 103:127–31. doi: 10.1038/sj.bjc.6605698
40. Yen H, Dhana A, Okhovat JP, Qureshi A, Keum N, Cho E. Alcohol intake and risk of nonmelanoma skin cancer: a systematic review and dose–response meta-analysis. Br J Dermatol. (2017) 177:696–707. doi: 10.1111/bjd.15647
41. Spencer SM, Trower AJ, Jia X, Scott DJA, Greenwood DC. Meta-analysis of the association between alcohol consumption and abdominal aortic aneurysm. Br J Surg. (2017) 104:1756–64. doi: 10.1002/bjs.10674
42. Rota M, Porta L, Pelucchi C, Negri E, Bagnardi V, Bellocco R, et al. Alcohol drinking and risk of leukemia—a systematic review and meta-analysis of the dose–risk relation. Cancer Epidemiol. (2014) 38:339–45. doi: 10.1016/j.canep.2014.06.001
43. Kim Y, Je Y, Giovannucci EL. Association between alcohol consumption and survival in colorectal cancer: a meta-analysis. Cancer Epidemiol Biomark Prev. (2019) 8:1891–901. doi: 10.1158/1055-9965.EPI-19-0156
44. Hong S-H, Myung S-K, Kim HS, Group KM-AS. Alcohol intake and risk of thyroid cancer: a meta-analysis of observational studies. Cancer Res Treat. (2017) 49:534. doi: 10.4143/crt.2016.161
45. Larsson SC, Wallin A, Wolk A. Alcohol consumption and risk of heart failure: meta-analysis of 13 prospective studies. Clin Nutr. (2018) 37:1247–51. doi: 10.1016/j.clnu.2017.05.007
46. Sun K, Ren M, Liu D, Wang C, Yang C, Yan L. Alcohol consumption and risk of metabolic syndrome: a meta-analysis of prospective studies. Clin Nutr. (2014) 33:596–602. doi: 10.1016/j.clnu.2013.10.003
47. Baliunas DO, Taylor BJ, Irving H, Roerecke M, Patra J, Mohapatra S, et al. Alcohol as a risk factor for type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. (2009) 32:2123–32. doi: 10.2337/dc09-0227
48. Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ. Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes Care. (2005) 28:719–25. doi: 10.2337/diacare.28.3.719
49. Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ. Meta-analysis of the relationship between alcohol consumption and coronary heart disease and mortality in type 2 diabetic patients. Diabetologia. (2006) 49:648–52. doi: 10.1007/s00125-005-0127-x
50. Rota M, Porta L, Pelucchi C, Negri E, Bagnardi V, Bellocco R, et al. Alcohol drinking and multiple myeloma risk–a systematic review and meta-analysis of the dose–risk relationship. Eur J Cancer Prev. (2014) 23:113–21. doi: 10.1097/CEJ.0000000000000001
51. Larsson SC, Orsini N, Wolk A. Alcohol consumption and risk of heart failure: a dose–response meta-analysis of prospective studies. Eur J Heart Fail. (2015) 17:367–73. doi: 10.1002/ejhf.228
52. Chen M, Ji M, Chen T, Hong X, Jia Y. Alcohol consumption and risk for venous thromboembolism: a meta-analysis of prospective studies. Front Nutr. (2020) 7:32. doi: 10.3389/fnut.2020.00032
53. Islami F, Fedirko V, Tramacere I, Bagnardi V, Jenab M, Scotti L, et al. Alcohol drinking and esophageal squamous cell carcinoma with focus on light-drinkers and never-smokers: a systematic review and meta-analysis. Int J Cancer. (2011) 129:2473–84. doi: 10.1002/ijc.25885
54. Moskal A, Norat T, Ferrari P, Riboli E. Alcohol intake and colorectal cancer risk: a dose–response meta-analysis of published cohort studies. Int J Cancer. (2007) 120:664–71. doi: 10.1002/ijc.22299
55. Psaltopoulou T, Sergentanis TN, Ntanasis-Stathopoulos I, Tzanninis IG, Tsilimigras DI, Dimopoulos MA. Alcohol consumption and risk of hematological malignancies: a meta-analysis of prospective studies. Int J Cancer. (2018) 143:486–95. doi: 10.1002/ijc.31330
56. Gallagher C, Hendriks JM, Elliott AD, Wong CX, Rangnekar G, Middeldorp ME, et al. Alcohol and incident atrial fibrillation–a systematic review and meta-analysis. Int J Cardiol. (2017) 246:46–52. doi: 10.1016/j.ijcard.2017.05.133
57. Zhang C, Qin Y-Y, Chen Q, Jiang H, Chen X-Z, Xu C-L, et al. Alcohol intake and risk of stroke: a dose–response meta-analysis of prospective studies. Int J Cardiol. (2014) 174:669–77. doi: 10.1016/j.ijcard.2014.04.225
58. Amiri S, Behnezhad S. Alcohol use and risk of suicide: a systematic review and Meta-analysis. J Addict Dis. (2020) 38:200–13. doi: 10.1080/10550887.2020.1736757
59. Briasoulis A, Agarwal V, Messerli FH. Alcohol consumption and the risk of hypertension in men and women: a systematic review and meta-analysis. J Clin Hypertens. (2012) 14:792–8. doi: 10.1111/jch.12008
60. Huang J, Wang X, Zhang Y. Specific types of alcoholic beverage consumption and risk of type 2 diabetes: a systematic review and meta-analysis. J Diabetes Investig. (2017) 8:56–68. doi: 10.1111/jdi.12537
61. Han M. The dose-response relationship between alcohol consumption and the risk of type 2 diabetes among asian men: a systematic review and meta-analysis of prospective cohort studies. J Diabetes Res. (2020) 2020:1032049. doi: 10.1155/2020/1032049
62. Jiménez-Jiménez FJ, Alonso-Navarro H, García-Martín E, Agúndez JA. Alcohol consumption and risk for Parkinson's disease: a systematic review and meta-analysis. J Neurol. (2019) 266:1821–34. doi: 10.1007/s00415-018-9032-3
63. Stockwell T, Zhao J, Panwar S, Roemer A, Naimi T, Chikritzhs T. Do “moderate” drinkers have reduced mortality risk? A systematic review and meta-analysis of alcohol consumption and all-cause mortality. J Stud Alcohol Drugs. (2016) 77:185–98. doi: 10.15288/jsad.2016.77.185
64. Zhao J, Stockwell T, Roemer A, Naimi T, Chikritzhs T. Alcohol consumption and mortality from coronary heart disease: an updated meta-analysis of cohort studies. J Stud Alcohol Drugs. (2017) 78:375–86. doi: 10.15288/jsad.2017.78.375
65. Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol. (2014) 64:281–9. doi: 10.1016/j.jacc.2014.03.048
66. Wang C, Xue H, Wang Q, Hao Y, Li D, Gu D, et al. Effect of drinking on all-cause mortality in women compared with men: a meta-analysis. J Women's Health. (2014) 23:373–81. doi: 10.1089/jwh.2013.4414
67. Jung M-H, Shin E-S, Ihm S-H, Jung J-G, Lee H-Y, Kim C-H. The effect of alcohol dose on the development of hypertension in Asian and Western men: systematic review and meta-analysis. Korean J Intern Med. (2020) 35:906. doi: 10.3904/kjim.2019.016
68. Huang C, Zhan J, Liu Y-J, Li D-J, Wang S-Q, He Q-Q. Association between alcohol consumption and risk of cardiovascular disease and all-cause mortality in patients with hypertension: a meta-analysis of prospective cohort studies. Mayo Clin Proc. (2014) 89:1201–10. doi: 10.1016/j.mayocp.2014.05.014
69. Zhang D, Jiang H, Xie J. Alcohol intake and risk of Parkinson's disease: a meta-analysis of observational studies. Mov Disord. (2014) 29:819–22. doi: 10.1002/mds.25863
70. Yang Y, Liu D-C, Wang Q-M, Long Q-Q, Zhao S, Zhang Z, et al. Alcohol consumption and risk of coronary artery disease: a dose-response meta-analysis of prospective studies. Nutrition. (2016) 32:637–44. doi: 10.1016/j.nut.2015.11.013
71. He Z, Zhao T-T, Xu H-M, Wang Z-N, Xu Y-Y, Song Y-X, et al. Association between alcohol consumption and the risk of gastric cancer: a meta-analysis of prospective cohort studies. Oncotarget. (2017) 8:84459–72. doi: 10.18632/oncotarget.20880
72. Xu X, Zhu Y, Zheng X, Xie L. Does beer, wine or liquor consumption correlate with the risk of renal cell carcinoma? A dose-response meta-analysis of prospective cohort studies. Oncotarget. (2015) 6:13347. doi: 10.18632/oncotarget.3749
73. Li Y, Mao Y, Zhang Y, Cai S, Chen G, Ding Y, et al. Alcohol drinking and upper aerodigestive tract cancer mortality: a systematic review and meta-analysis. Oral Oncol. (2014) 50:269–75. doi: 10.1016/j.oraloncology.2013.12.015
74. Zhang X, Yu Z, Yu M, Qu X. Alcohol consumption and hip fracture risk. Osteoporos Int. (2015) 26:531–42. doi: 10.1007/s00198-014-2879-y
75. Padilla H, Gaziano JM, Djoussé L. Alcohol consumption and risk of heart failure: a meta-analysis. Phys Sportsmed. (2010) 38:84–9. doi: 10.3810/psm.2010.10.1812
76. Wang W, Zhang X. Alcohol intake and the risk of age-related cataracts: a meta-analysis of prospective cohort studies. PLoS ONE. (2014) 9:e107820. doi: 10.1371/journal.pone.0107820
77. Pereira PPdS, Mata FAFD, Figueiredo ACMG, Silva RB, Pereira MG. Maternal exposure to alcohol and low birthweight: a systematic review and meta-analysis. Rev Bras Ginecol Obstet. (2019) 41:333–47. doi: 10.1055/s-0039-1688905
78. Liu P-M, Dosieah S, Zheng H-S, Huang Z-B, Lin Y-Q, Wang J-F. Alcohol consumption and coronary heart disease in Eastern Asian men: a meta-analysis of prospective cohort studies. Zhonghua xin xue guan bing za zhi. (2010) 38:1038–44. doi: 10.3760/cma.j.issn.0253-3758.2010.016
79. Ljungberg B, Albiges L, Abu-Ghanem Y, Bensalah K, Dabestani S, Fernández-Pello S, et al. European association of urology guidelines on renal cell carcinoma: the 2019 update. Eur Urol. (2019) 75:799–810. doi: 10.1016/j.eururo.2019.02.011
80. Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet. (1994) 344:721–4. doi: 10.1016/S0140-6736(94)92211-X
81. Huang W-Y, Cai Y-Z, Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr Cancer. (2009) 62:1–20. doi: 10.1080/01635580903191585
82. Lee JE, Hunter DJ, Spiegelman D, Adami H-O, Albanes D, Bernstein L, et al. Alcohol intake and renal cell cancer in a pooled analysis of 12 prospective studies. J Natl Cancer Inst. (2007) 99:801–10. doi: 10.1093/jnci/djk181
83. Lee JE, Giovannucci E, Smith-Warner SA, Spiegelman D, Willett WC, Curhan GC. Total fluid intake and use of individual beverages and risk of renal cell cancer in two large cohorts. Cancer Epidemiol Biomarkers Prev. (2006) 15:1204–11. doi: 10.1158/1055-9965.EPI-05-0889
84. Schrieks IC, Heil AL, Hendriks HF, Mukamal KJ, Beulens JW. The effect of alcohol consumption on insulin sensitivity and glycemic status: a systematic review and meta-analysis of intervention studies. Diabetes Care. (2015) 38:723–32. doi: 10.2337/dc14-1556
85. Davies MJ, Baer DJ, Judd JT, Brown ED, Campbell WS, Taylor PR. Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: a randomized controlled trial. JAMA. (2002) 287:2559–62. doi: 10.1001/jama.287.19.2559
86. Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. (1997) 275:218–20. doi: 10.1126/science.275.5297.218
87. Schneider Y, Vincent F, Duranton Bt, Badolo L, Gossé F, Bergmann C, et al. Anti-proliferative effect of resveratrol, a natural component of grapes and wine, on human colonic cancer cells. Cancer Lett. (2000) 158:85–91. doi: 10.1016/S0304-3835(00)00511-5
88. Szende B, Tyihak E, Kiraly-Veghely Z. Dose-dependent effect of resveratrol on proliferation and apoptosis in endothelial and tumor cell cultures. Exp Mol Med. (2000) 32:88–92. doi: 10.1038/emm.2000.16
89. Schneider Y, Duranton B, Goss F, Schleiffer R, Seiler N, Raul F. Resveratrol inhibits intestinal tumorigenesis and modulates host-defense-related gene expression in an animal model of human familial adenomatous polyposis. Nutr Cancer. (2001) 39:102–7. doi: 10.1207/S15327914nc391_14
90. Kamei H, Hashimoto Y, Koide T, Kojima T, Hasegawa M. Anti-tumor effect of methanol extracts from red and white wines. Cancer Biother Radiopharm. (1998) 13:447–52. doi: 10.1089/cbr.1998.13.447
91. Yi W, Fischer J, Akoh CC. Study of anticancer activities of muscadine grape phenolics in vitro. J Agric Food Chem. (2005) 53:8804–12. doi: 10.1021/jf0515328
92. Yi W, Fischer J, Krewer G, Akoh CC. Phenolic compounds from blueberries can inhibit colon cancer cell proliferation and induce apoptosis. J Agric Food Chem. (2005) 53:7320–9. doi: 10.1021/jf051333o
93. Garro AJ, Espina N, Farinati F, Salvagnini M. The effects of chronic ethanol consumption on carcinogen metabolism and on O6-methylguanine transferase-mediated repair of alkylated DNA. Alcohol Clin Exp Res. (1986) 10:73S−7S. doi: 10.1111/j.1530-0277.1986.tb05184.x
94. Wang M, McIntee EJ, Cheng G, Shi Y, Villalta PW, Hecht SS. Identification of DNA adducts of acetaldehyde. Chem Res Toxicol. (2000) 13:1149–57. doi: 10.1021/tx000118t
95. Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. (2007) 7:599–612. doi: 10.1038/nrc2191
96. Saladi R, Nektalova T, Fox J. Induction of skin carcinogenicity by alcohol and ultraviolet light. Clin Exp Dermatol. (2010) 35:7–11. doi: 10.1111/j.1365-2230.2009.03465.x
97. Waldschmidt TJ, Cook RT, Kovacs EJ. Alcohol and inflammation and immune responses: summary of the 2006 Alcohol and Immunology Research Interest Group (AIRIG) meeting. Alcohol. (2008) 42:137–42. doi: 10.1016/j.alcohol.2007.11.003
98. –2020 Dietary Guidelines for Americans; United States. Available online at: https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf (accessed December 15, 2020).
99. Xi B, Veeranki SP, Zhao M, Ma C, Yan Y, Mi J. Relationship of alcohol consumption to all-cause, cardiovascular, and cancer-related mortality in US adults. J Am Coll Cardiol. (2017) 70:913–22. doi: 10.1016/j.jacc.2017.06.054
100. Puddey IB, Rakic V, Dimmitt S, Beilin L. Influence of pattern of drinking on cardiovascular disease and cardiovascular risk factors-a review. Addiction. (1999) 94:649–63. doi: 10.1046/j.1360-0443.1999.9456493.x
101. Rehm J, Sempos C, Trevisan M. Alcohol and cardiovascular disease–more than one paradox to consider. Average volume of alcohol consumption, patterns of drinking and risk of coronary heart disease–a review. J Cardiovasc Res. (2003) 10:15–20. doi: 10.1177/174182670301000104
102. Rosales C, Gillard BK, Gotto AM, Pownall HJ. The alcohol–high-density lipoprotein athero-protective axis. Biomolecules. (2020) 10:987. doi: 10.3390/biom10070987
103. Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. (2009) 373:2223–33. doi: 10.1016/S0140-6736(09)60746-7
104. Iacoviello L, Bonaccio M, Cairella G, Catani M, Costanzo S, D'Elia L, et al. Diet and primary prevention of stroke: Systematic review and dietary recommendations by the ad hoc Working Group of the Italian Society of Human Nutrition. Nutr Metab Cardiovasc Dis. (2018) 28:309–34. doi: 10.1016/j.numecd.2017.12.010
105. Salem RO, Laposata M. Effects of alcohol on hemostasis. Pathol Patterns Rev. (2005) 123(suppl. 1):S96–105. doi: 10.1309/113N8EUFXYUECCNA
106. Peng G-S, Yin S-J, Cheng C-A, Chiu S-W, Lee J-T, Lin W-W, et al. Increased risk of cerebral hemorrhage in Chinese male heavy drinkers with mild liver disorder. Cerebrovas Dis. (2007) 23:309–14. doi: 10.1159/000098445
107. Casolla B, Dequatre-Ponchelle N, Rossi C, Hénon H, Leys D, Cordonnier CJN. Heavy alcohol intake and intracerebral hemorrhage: characteristics and effect on outcome. Neurology. (2012) 79:1109–15. doi: 10.1212/WNL.0b013e3182698d00
108. Mentis A-FA, Dardiotis E, Efthymiou V, Chrousos GP. Non-genetic risk and protective factors and biomarkers for neurological disorders: a meta-umbrella systematic review of umbrella reviews. BMC Med. (2021) 19:1–28. doi: 10.1186/s12916-020-01873-7
109. De Gaetano G, Di Castelnuovo A, Donati MB, Iacoviello L. thrombosis. The mediterranean lecture: wine and thrombosis–from epidemiology to physiology and back. Pathophysiol Haemost Thromb. (2003) 33:466–71. doi: 10.1159/000083847
110. Volkow ND, Wang G-J, Franceschi D, Fowler JS, Thanos PPK, Maynard L, et al. Low doses of alcohol substantially decrease glucose metabolism in the human brain. Neuroimage. (2006) 29:295–301. doi: 10.1016/j.neuroimage.2005.07.004
112. Zhou S, Zhou R, Zhong T, Li R, Tan J, Zhou H. Association of smoking and alcohol drinking with dementia risk among elderly men in China. Curr Alzheimer Res. (2014) 11:899–907. doi: 10.2174/1567205011666141001123356
113. Langballe EM, Ask H, Holmen J, Stordal E, Saltvedt I, Selbæk G, et al. Alcohol consumption and risk of dementia up to 27 years later in a large, population-based sample: the HUNT study, Norway. Eur J Epidemiol. (2015) 30:1049–56. doi: 10.1007/s10654-015-0029-2
114. Arranz S, Chiva-Blanch G, Valderas-Martínez P, Medina-Remón A, Lamuela-Raventós R, Estruch R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients. (2012) 4:759–81. doi: 10.3390/nu4070759
115. Inan-Eroglu E, Powell L, Hamer M, O'Donovan G, Duncan MJ, Stamatakis E. Is there a link between different types of alcoholic drinks and obesity? An analysis of 280,183 UK biobank participants. Int J Environ Res Public Health. (2020) 17:5178. doi: 10.3390/ijerph17145178
Keywords: alcohol, health outcomes, umbrella review, meta-analysis, epidemiologic evidence
Citation: Zhong L, Chen W, Wang T, Zeng Q, Lai L, Lai J, Lin J and Tang S (2022) Alcohol and Health Outcomes: An Umbrella Review of Meta-Analyses Base on Prospective Cohort Studies. Front. Public Health 10:859947. doi: 10.3389/fpubh.2022.859947
Received: 22 January 2022; Accepted: 06 April 2022;
Published: 04 May 2022.
Edited by:
Taulant Muka, University of Bern, SwitzerlandReviewed by:
Anna Abdoshahi, Semnan University of Medical Sciences, IranCopyright © 2022 Zhong, Chen, Wang, Zeng, Lai, Lai, Lin and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaohui Tang, dGFuZ3NoYW9odWkyMDZAam51LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.