
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Public Health , 28 April 2022
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.859350
This article is part of the Research Topic Current Research on Serological Analyses of Infectious Diseases View all 11 articles
 Nur Hasnah Maamor1†
Nur Hasnah Maamor1† Nor Asiah Muhamad1*†
Nor Asiah Muhamad1*† Nor Soleha Mohd Dali2
Nor Soleha Mohd Dali2 Mohd Hatta Abdul Mutalip3
Mohd Hatta Abdul Mutalip3 Fatin Norhasny Leman1
Fatin Norhasny Leman1 Tahir Aris2
Tahir Aris2 Nai Ming Lai4
Nai Ming Lai4 Muhammad Radzi Abu Hassan5,6
Muhammad Radzi Abu Hassan5,6Introduction: The hepatitis B virus (HBV) is a blood-borne virus that can be transmitted by percutaneous and mucocutaneous contact with infected bodily fluid. Healthcare workers (HCWs) are more exposed to HBV infection. They must have a thorough understanding of HBV infection since they can contract and spread the virus. In this study, we systematically reviewed all published evidence on the seroprevalence of Hepatitis B virus (HBV) infection among HCWs. and synthesize evidence on the association between knowledge and awareness with HBV infection.
Methods: We searched PubMed, EMBASE, Cochrane Library and Scopus for studies reporting on HBV seroprevalence from January 1997 to September 2021 among healthcare workers. We used random-effects meta-analyses to estimate the pool prevalence of HBV infection.
Results: We identified 25 studies that met our inclusion criteria, with data on 10,043 adults from 11 countries and two regions: Africa and Asia. The overall seroprevalence of HBV was 5.0% (95% confidence interval [CI] 3.6%), with Africa reporting higher estimates (5.0%, 95% CI 3.7%) than Asia population (4.0%, 95% CI 1.9%). The highest pooled prevalence estimate in African countries came from studies published in the Cameroon region (8.0%, 95% CI 5–10%) while the lowest came from Ethiopia (4.0%, 95% CI 2.6%). The overall seroprevalence estimates in the African population were significantly higher than those in the Asian group. Studies in Africa found that the average knowledge and seroprevalence were 1.4% and 11.0%, respectively where, eight studies (53.3%) reported good knowledge and seven studies (46.7%) reported average knowledge. In Asia, two studies (40.0%) reported good knowledge, one study (20.0%) reporting average knowledge, and two studies (40.0%) reporting poor knowledge. African studies demonstrated good knowledge despite the fact that their HBV infection rate was higher than 6.7%.
Conclusion: Africa and Asia have the highest seroprevalence of HBV infection. Improving the comparability of epidemiological and clinical studies constitutes an important step forward. More high-quality data is needed to improve the precision of burden estimates.
Systematic Review Registration: PROSPERO CRD42021279905.
The Hepatitis B virus (HBV) is a bloodborne virus that has become a major global public health concern. HBV, which belongs to the Hepadnaviridae family, has only one known natural host: humans. The virus enters the liver through the bloodstream and replicates in the tissue of the liver (1). Acute hepatitis B infection causes inflammation and jaundice in the liver, while chronic hepatitis B infection can lead to potentially fatal diseases such as liver cirrhosis and hepatocellular carcinoma (2). Globally, HBV infected over 2 billion individuals with 250 million of them suffering from chronic HBV infection (3). According to the World Health Organization (WHO), 325 million people are infected with HBV, with the African and Western Pacific regions having the highest rates of HBV infection at 68% (4), and approximately 900,000 people dying from HBV each year (5). Hepatitis B is most common in Sub-Saharan Africa and Southeast Asia (8.0–10.0%). This is followed by Eastern and Southern Europe, the Middle East, and Japan (2.0-7.0%), and the United States and Northern Europe (0.5–2.0%) (6–8). Furthermore, it is estimated that 40% of the healthcare workers (HCWs) are infected with HBV infections in the developing countries (9).
Healthcare workers are four times more likely to be infected with HBV compared to the general population (10). This may be due to a lack of compliance with infection control recommendations from established guidelines such as the Center for Disease Control and Prevention (CDC) (11). Handwashing, glove use, and correct disposal of sharp instruments are all part of the CDC's recommended precaution, which is aimed to prevent the spread of blood-borne infections like HBV (12). In the case of HBV infection, knowledge includes information gathering, experience, skill, and disease prevention strategies (13, 14). A lack of understanding among HCWs in both low- and middle-income countries leads to low adherence to safety measures, aggravating the HBV situation (15). A better understanding of HBV infection is essential to reduce the rate of infection among HCWs in the healthcare context (16). Knowledge is usually assessed to investigate how far the community know the concepts of disease including causes and symptoms of disease. Attitude is defined as a product of a complex interaction on values, feelings and beliefs (17). Practice is defined as an action of the habitual community to prevent the disease (18). Awareness is the knowledgeable person being conscious and behavior under the receiving in taxonomy of affective domain (19).
Although HCWs are more aware of Hepatitis B, several countries lack a comprehensive grasp of the disease biology, transmission methods, risk of transmission, clinical characteristics, and vaccination availability (20). Hepatitis B virus seroprevalence among HCWs was reported in a prior study by Mahamat et al. (21), but there was no relationship study between seroprevalence and knowledge or awareness (21). Hepatitis B awareness is lower among HCWs in developing countries, which is linked to poorer preventive attitudes, including lower Hepatitis B vaccine coverage (22). On the other hand, the prevalence of HBV infection fluctuates and is influenced by a variety of factors including geographical region, host factor, and environmental or behavioral factors. The low prevalence of HBV in Europe, for example, may be attributed to the high standard of living there (10). As a result, Hepatitis B prevalence can also predict the risk factors for HBV transmission, such as injections, occupational injuries, body tattoos, and a history of not being been vaccinated, among others (23). Therefore, this study aimed to determine the pooled prevalence of hepatitis B infection among HCWs and to compare the pooled prevalence of HBV infection across different regions. We also compiled data to determine the association between seroprevalence and level of knowledge or awareness on HBV infection.
We conducted a systematic review by following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (24) and Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) statement (25). The PROSPERO registration number is CRD42021279905. Our primary outcome was the seroprevalence of HBV infection among HCWs. Our secondary outcome was the estimation of knowledge and awareness attributable to HBV infection.
A comprehensive systematic literature search was conducted in electronic databases (PubMed, EMBASE, Cochrane Library, and Scopus) to identify relevant studies from inception to September 2021. A search strategy was developed for PubMed (Supplementary Table 1) and adapted for use in the other databases. Other electronic search was performed in the WHO International Clinical Trials Registry Platform (ICTRP). In the search, key words and equivalent Medical Subject Heading (MeSH) phrases were combined when applicable, with no language or publication year restrictions. Specific search terms are as follows: “hepatitis B” OR “HBV” OR “hepatitis B virus” AND “etiology” OR “etiology” OR “prevalence” OR “epidemiology” OR “infection” AND “healthcare workers” OR “healthcare” OR “doctor” OR “doctors” OR “nurse” OR “nurses” OR “medical” OR “medical staff” OR “medical assistant” OR “health personnel” OR “health care personnel” OR “healthcare personnel” OR “health care worker” OR “health care workers” OR “healthcare worker” OR “healthcare workers” OR “health worker” OR “health workers” OR “healthcare professionals” OR “medical care personnel” AND “knowledge” AND “awareness” (Supplementary Table 1) for the MEDLINE search. We also scanned through cross-references of identified primary studies and review articles for eligible studies.
We included studies according to the following criteria; Population/Intervention/Comparator/Outcome/Study Design (PICOS) approach. For this review, we included HCWs (P) defined as individuals such as doctors, dentists, nurses, midwives, medical personnel, medical assistant or laboratory scientists who are in direct contact with the following: (i) patient bodily fluids or biological samples such as blood, saliva, sperm, sweat and stool, (ii) new-born delivery process in which mother-to-child transmission was possible via a transplacental route, and (iii) people who were exposed sexually, and (iv) sharp or needle-stick injuries. Instead of an intervention (I), we included observational studies that report the seroprevalence of HBV with either one or both of the components of knowledge or awareness in relation to HBV seroprevalence. There was no comparator (C), and lastly, the determined outcome (O) was seroprevalence of hepatitis B infection. We considered all articles published in English language. When numerous studies with the same cohort were conducted, the research with the most detailed information on the participants or the largest number of participants was chosen.
We excluded abstracts, letters to the editor, reviews, commentaries, editorials, and studies without either primary data or described study methods. We excluded systematic reviews, non-empirical studies, conference, abstracts, editorials, commentaries, book reviews, and abstracts not accompanied by a full text.
Two review authors (NHM and NAM) independently screened all titles and abstracts to look for potential studies found through the search and coded them as “retrieve” (eligible or potentially eligible/unclear) or “do not retrieve”. Two more review authors (NSMD and MHAM) independently retrieved full-text study reports/publications to identify studies for inclusion, and to identify and record reasons for studies' exclusion. When there were disagreements, a consensus was obtained through discussion with a third reviewer (MRAH).
Two review authors (FNL and TA) independently evaluated each included study ‘s methodological quality and extracted data using a piloted form; discrepancies were resolved through discussion with a third review author (NML). Data on the year of publication, country of origin, study design, sample size, sampling procedure (if available), study period, and setting (country/continent/region) were extracted using a standardized data collection form.
To assess the risk of bias for each study, a domain-based questionnaire developed from the Newcastle-Ottawa Scale (NOS) was used to assess methodological quality (26) (Supplementary Table 2). In the following domains: participant selection (selection bias), sample size justification (selection bias), outcome measurement (detection bias), and confounding adjustment, we evaluated the risk of bias as low, moderate, high, or uncertain. We assigned a score of 7 and above as good quality, and below 6 as having some concerns to determine the overall quality.
We used Stata software version 16 for all statistical analysis (27). The pooled prevalence rates, as well as their 95% confidence intervals, were calculated using a random-effects model (28). The heterogeneity of the studies was assessed using I2 statistics and Cochran's Q Test (29). The I2 statistics were used to assess the explained variance attributable to study heterogeneity, with I2 score of 25.0, 50.0 and 75.0% denoting low, moderate and high, respectively (28, 30).
We identified a total of 4,329 studies published between January 1997 and September 2021. We could not find any studies that evaluated seroprevalence of Hepatitis B among HCW and its association with knowledge, awareness and attitude of HBV in the Americas, Europe, Eurasia, Australia, or Antarctica regions. We excluded duplicates and collated multiple reports of the same study so that each study, rather than individual report, is the focus of our analysis. We meticulously documented the selection process to complete a PRISMA flow diagram (Figure 1). We shortlisted 25 studies from a pool of 28 for an in-depth full-text examination of their suitability.
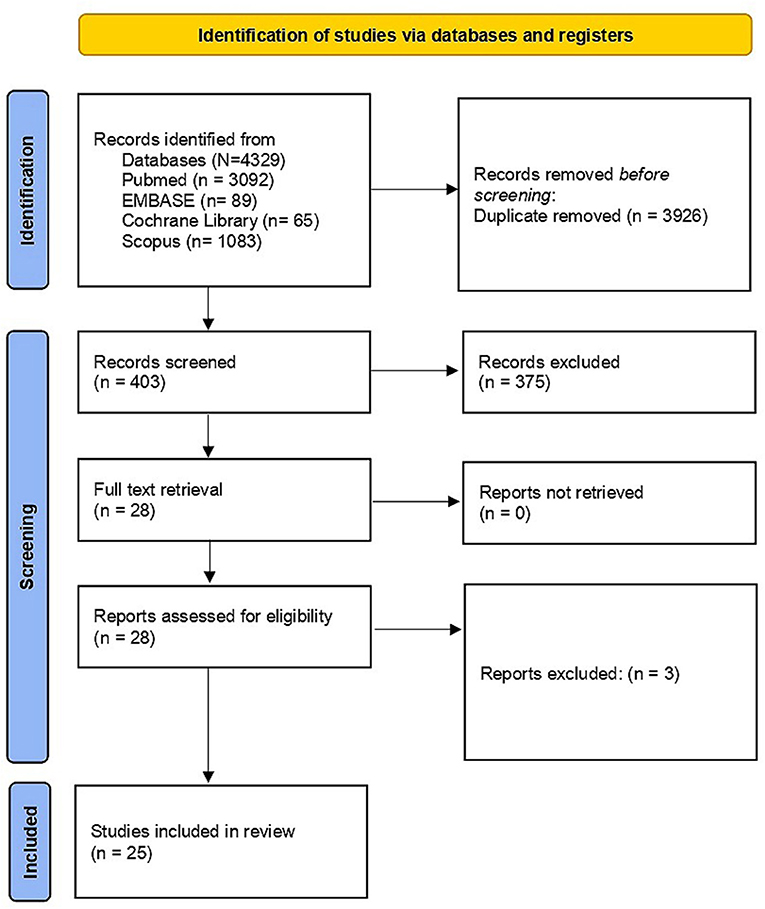
Figure 1. PRISMA flow diagram on selection process. From: Page et al. (24). For more information, visit: http://www.prisma-statement.org/.
Among the 25 included studies, nineteen were from Africa (10, 14, 20, 31–46) and six from Asia (47–52). The studies were conducted across eleven countries with a total of 10,043 participants. The characteristics of the included studies are depicted in Table 1.
Two reviewers (FNL and MHAM) independently assessed the quality of the included studies using an adapted version of the NOS for prevalence studies. The quality of evidence was rated as low to begin with, due to the non-randomized nature of the study. The quality of evidence in the outcomes were based on the NOS criteria, where a maximum score of 4 stars for selection, 2 stars for comparability, and 3 stars for exposure and outcome assessment. Studies with fewer than 2 stars were considered low quality; 2 to 6 stars, moderate quality; and 7 stars and more, high quality. Twenty-one studies were observed to be of good quality (NOS score 7 and above), and four studies were identified as fair quality (NOS score between 2 and 6 inclusive) (Supplementary Table 2).
As shown in Figure 2, the overall estimate for pooled seroprevalence of Hepatitis B was 5.0% (95% CI: 0.03–0.06), with a high-level of heterogeneity between studies (I2 = 94.6%, P = 0.001). The study was divided into two parts: Africa and Asia. In Asia, the overall pooled seroprevalence of Hepatitis B was 4.0% (95% CI: 0.01–0.07) with a high-level of heterogeneity between studies (I2 = 95.7%, P = 0.001) (Figure 3), whereas in Africa, the overall pooled seroprevalence of Hepatitis B in Africa was 5.0% (95% CI: 0.03–0.07) with a high-level of heterogeneity between studies (I2 = 92.2%, P = 0.001) (Figure 4). The subgroup analysis was performed in Africa since there were nineteen publications categorized under the African region. We pooled the seroprevalence of Hepatitis B in African countries with two or more publications for this study. This subgroup analysis was performed in Nigeria, Ethiopia, and Cameroon (Supplementary Figures 1–3). In Nigeria, the overall pooled seroprevalence of hepatitis B was 4.0% (95% CI: 0.01–0.06) with high-level heterogeneity (I2 = 94.4%, P = 0.001) (Supplementary Figure 1). Ethiopia had a pooled seroprevalence of 4.0% (95% CI: 0.02–0.06), but the level of heterogeneity was moderate (I2 = 42.6%, P = 0.19) (Supplementary Figure 2). Cameroon had the highest overall pooled seroprevalence of Hepatitis B in Africa, at 8.0% (95% CI: 0.05–0.11), with a moderate level of heterogeneity between studies (I2 = 63.7%, P = 0.03) (Supplementary Figure 3).
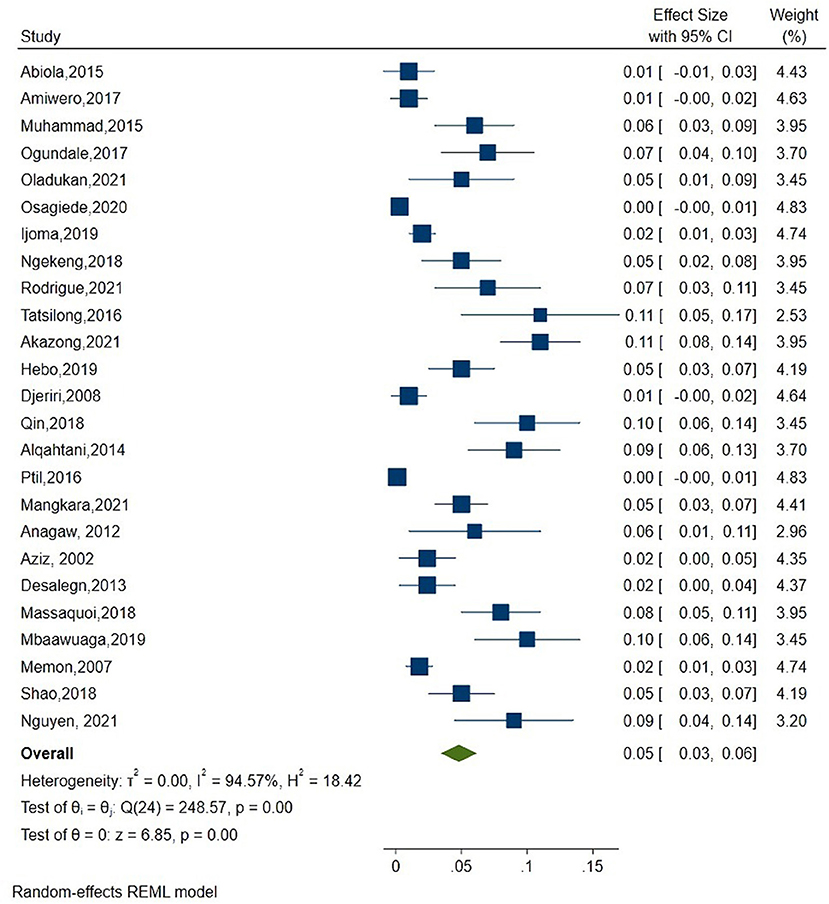
Figure 2. Forest plot of overall seroprevalence estimate of the Hepatitis B infection among healthcare workers.
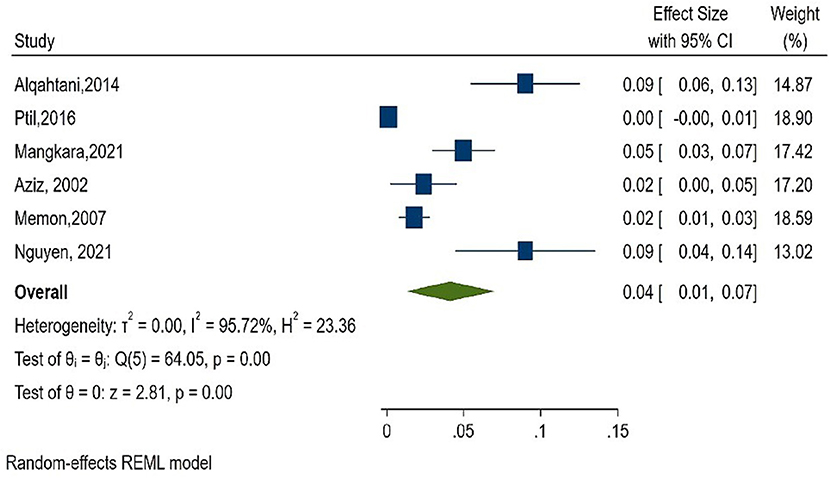
Figure 3. Forest plot of seroprevalence estimate of the Hepatitis B infection among healthcare workers in Asia.
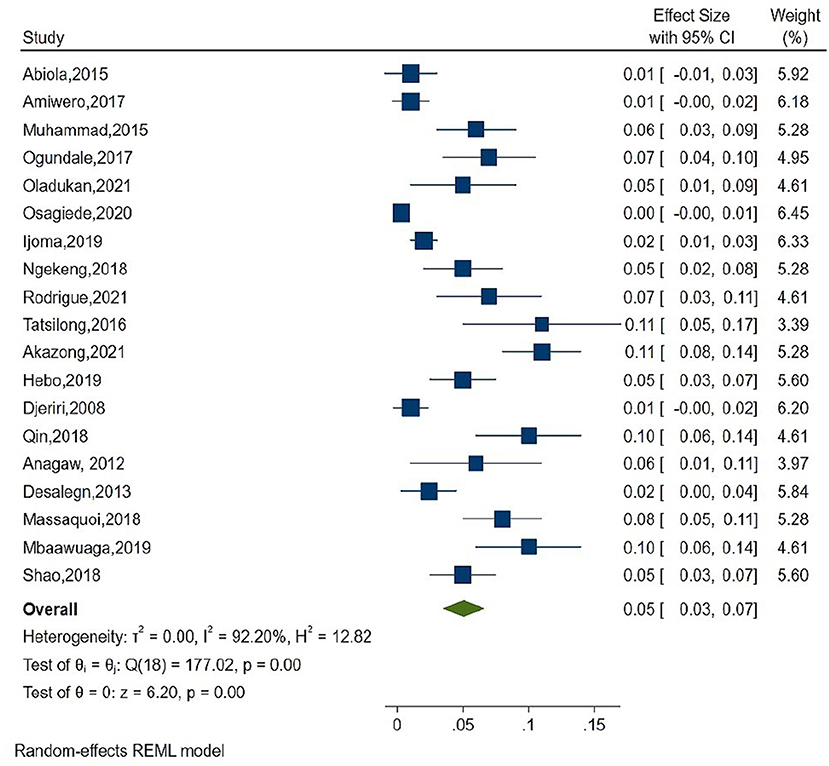
Figure 4. Forest plot of seroprevalence estimate of the Hepatitis B infection among healthcare in Africa region.
There were twenty studies that reported findings on seroprevalence and knowledge, with fifteen from Africa and five from Asia. Some of the studies mentioned the knowledge level (good, average, or poor knowledge) in the publication. In Africa, the majority of the studies found that participants have a strong knowledge of hepatitis B infection, with eight studies (53.3%) reporting good knowledge and seven studies (46.7%) reporting average knowledge. In Asia, five studies reported seroprevalence and knowledge findings, with two studies (40.0%) reporting good knowledge, one study (20.0%) reporting average knowledge, and two studies (40.0%) reporting poor knowledge. Seven studies in Africa found that the average knowledge and seroprevalence were 1.4 and 11.0%, respectively. Surprisingly, few African studies demonstrated good knowledge despite the fact that their HBV infection rate was higher than 6.7% (14, 42, 45). The average knowledge was reported in the cohort with a seroprevalence of 8.7% in the Asia region (49) Memon et al. (51) and Mangkara et al. (47) found poor knowledge in their participants with 4.7% and 5.0% HBV seroprevalence, respectively. However, Nguyen et al. (48), who found the highest seroprevalence (9.8%), claimed that participants had a good knowledge of HBV infection (48). The assessment of knowledge among HCWs were presented in the Supplementary Table 3.
There were eleven studies that found an association between seroprevalence and participants' level of awareness, including nine (81.8%) from Africa and two (18.2%) from Asia. Some of the studies mentioned the participants' level of awareness (good, average, or poor awareness). The scale for awareness level was adopted from Vaishali et al. (53) for those studies that did not mention it. Nine studies (81.8%) were reported to have good awareness, whereas two (18.2%) in the Africa region were found to have poor awareness. Seroprevalence was poor in Tanzania (32) and Sierra Leone (45), with 5.7 and 10.0%, respectively. Even though three publications had higher seroprevalence than Shao et al. (32) and Qin et al. (45), the publications showed good awareness: 6.0% Anagaw et al. (35), 6.7% Ogundele et al. (14), and 10.6% Mbaawuaga et al. (41). Supplementary Table 4 summarizes the awareness evaluation, the instrument employed, and the conclusion.
Healthcare workers have a higher risk of contracting HBV infection than the general population. Simultaneously, they play a vital role in preventing and controlling HBV infection by disseminating and transmitting HBV knowledge to the public, as well as assisting in behavior changes that may aid in infectious diseases prevention (54). After utilizing NOS to assess methodological quality, only 21 out of the 25 studies included in this review showed a good risk of bias, while another four studies exhibited a fair risk of bias. Healthcare workers must consequently have a goof level of knowledge and awareness of HBV to limit their own and the public's risk of infection. According to Rayate et al. (55), the majority of HCWs are unaware that the virus can survive outside the body for seven days (55). The same study also reported that only 27.78% of HCWs are aware that the virus can survive in dried blood type form (55). Several factors influence the likelihood of getting hepatitis B, including the prevalence of the virus in the environment or in people's behavior, the frequency of blood and body fluid exposure to HCWs, HBV infectivity (14), geographical location, and host factors (10).
In comparison to other regions, the current study found that African countries have a high seroprevalence of HBV infection (Figures 3, 4). Medical doctors, dentists, nurses, and laboratory workers made up the majority of HCWs infected with HBV. There were also cases of HBV infection among technicians, nurse assistant, cleaning operators, and housekeeping staff (17, 41, 48, 49). Accidental exposure to blood and blood products, occupational injuries such as needle-sticks and other injuries from sharp objects, lack of experience or practice with HBV infection, and not having been vaccinated were all risk factors for high seroprevalence of HBV infection in certain places (32–34, 36, 47, 51). Mahamat et al. (21) published a study on global seroprevalence among HCWs that was similar to ours. Our study, on the other hand, shows a link between HBV seroprevalence and HCWs knowledge or awareness of HBV infection, which was not addressed in the previous study (21).
Despite the high seroprevalence observed in a few studies, other publications claimed good knowledge or awareness of HBV infection (14, 35, 41, 43, 46). High HBV seroprevalence has been attributed to a lack of knowledge about HBV transmission routes in one study in Cameroon (42). Surprisingly, despite the lower seroprevalence, some studies reported average or poor knowledge or awareness of HBV infection (32, 39). This finding showed an inconsistency between the level of seroprevalence and the level of knowledge or awareness among HCWs. Notwithstanding the inconsistency, it is critical to increase HBV knowledge and awareness among HCWs (50).
A few approaches to increase an awareness of HBV infection among HCWs include strengthening immunization program, regularly screen the HBsAb and HBsAg of HCWs, media involvement, continuous medical educations, and provide trainings to the HCWs (14, 32, 33, 46, 50). This will encourage safer work practices and a higher degree of compliance with hospital policies. By assessing the level of knowledge among HCWs, not only is the general public indirectly examined, but also preventative implementation is improved. Separately, the long years of hospital service have contributed to raising increasing hepatitis B infection awareness. Furthermore, HCWs' lack of knowledge regarding Hepatitis B could have a significant impact on safety behaviors, such as vaccination. As a result, HCW awareness of Hepatitis B is vital, as knowledge plays a key role in changing prevention-related behavior (22). Lack of training or seminars for HCWs, insufficient information, or a poor awareness of HBV infection could all contribute to the lack of knowledge among HCWs. Moreover, insufficient health education programs and obtaining unreliable Hepatitis B information from friends, relatives, and co-workers may increase the likelihood of acquiring incorrect information (42). To increase HBV knowledge among HCWs, improvement in clinical practice, 53 training, and practical skills are required (56, 57).
The present study had limitations. First, we were unable to locate reports on the HBV seroprevalence and levels of knowledge or awareness in the developing countries. Thus, we were unable to compare the findings worldwide. Second, in some research, differences in score and inability to score on the level of knowledge or awareness may result in inconsistent conclusions that are either good, average, or poor. As a result, we were unable to determine some research' scores and compare them to seroprevalence.
In conclusion, hepatitis B virus was shown to be present in 4.0–5.0 % of the population tested, with an apparent higher prevalence in African countries than in Asian countries. Some HCWs were still infected with HBV despite having strong knowledge and awareness of HBV infection. Improved epidemiological data collection can help determine and identify key risk factors for a more effective public health response. Thus, if enough people are exposed to Hepatitis B virus knowledge, awareness, attitude, and practice, the goal of eliminating viral hepatitis by 2030 may be achieved.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
NHM and NAM carried out the study design, study selection, data extraction, and statistical analysis and drafted the manuscript. NSMD and MHAM participated in the study selection and data extraction and drafted the manuscript. FNL and TA evaluated the quality of included studies. MRAH and NML participated in the discussion for any discrepancies and supervised the study. All authors read and approved the final manuscript.
The open-access publication fee was supported by the National Institutes of Health, Ministry of Health, Malaysia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank the Director General of Health, Ministry of Health, Malaysia for the permission to publish this article.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.859350/full#supplementary-material
1. Yizengaw E, Getahun T, Geta M, Mulu W, Ashagrie M, Hailu D, et al. Sero-prevalence of hepatitis B virus infection and associated factors among health care workers and medical waste handlers in primary hospitals of North-West Ethiopia. BMC Res Notes. (2018) 11:437. doi: 10.1186/s13104-018-3538-8
2. Tang LSY, Covert E, Wilson E, Kottilil S. Chronic hepatitis B infection: a review. JAMA. (2018) 319:1802–13. doi: 10.1001/jama.2018.3795
3. Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. (2015) 386:1546–55. doi: 10.1016/S0140-6736(15)61412-X
5. World Health Organization. World Hepatitis Day 2020. World Health Organization (2020). Available online at: https://www.who.int/campaigns/world-hepatitis-day/2020 (accessed October 10, 2021).
6. Biset Ayalew M, Adugna B, Getachew N, Amare S, Getnet A. Knowledge and attitude of health care professionals regarding hepatitis B virus infection and its vaccination, university of gondar hospital, Ethiopia. HMER. (2016) 8:135–42. doi: 10.2147/HMER.S120477
7. Fatusi A, Esimai A, Onayade A, Ojo O. Aceptance of hepatitis B vaccine by workers in a Nigerian teaching hospital. E Af Med Jrnl. (2009) 77:608–12. doi: 10.4314/eamj.v77i11.46734
8. Hou J, Liu Z, Gu F. Epidemiology and prevention of hepatitis B virus infection. Int J Med Sci. (2005) 50–57. doi: 10.7150/ijms.2.50
9. Prüss-Üstün A, Rapiti E, Hutin YJF. Sharps Injuries: Global Burden of Disease from Sharps Injuries to Health-Care Workers. World Health Organization (2003). Available online at: https://apps.who.int/iris/handle/10665/42743
10. Abiola A-HO, Agunbiade AB, Badmos KB, Lesi AO, Lawal AO, Alli QO. Prevalence of HBsAg, knowledge, and vaccination practice against viral hepatitis B infection among doctors and nurses in a secondary health care facility in Lagos state, South-western Nigeria. Pan Afr Med J. (2016) 23:160. doi: 10.11604/pamj.2016.23.160.8710
11. Sadoh WE, Fawole AO, Sadoh AE, Oladimeji AO, Sotiloye OS. Practice of universal precautions among healthcare workers. J Natl Med Assoc. (2006) 98:722.
12. Garner JS. Hospital infection control practices advisory committee. guideline for isolation precautions in hospitals. Infect Control Hosp Epidemiol. (1996) 17:54–80. doi: 10.1017/S0195941700006123
13. Nasim A, Shah Y., ul Haq N, Tahir M, Mohammad F, Saood M, Riaz S. Knowledge, attitude and practice regarding hepatitis B in nurses working in different hospitals of Quetta city, Pakistan. Int J Nurs Crit Care. (2020) 6:24–34. Available online at: https://nursing.journalspub.info/index.php?journal=IJNCC&page=article&op=view&path%5B%5D=1427
14. Ogundele OA, Olorunsola A, Bakare B, Adegoke IA, Ogundele T, Fehintola F, et al. Seroprevalence and knowledge of hepatitis B and C among health care workers in a specialist hospital in Nigeria. EJPM. (2017) 5:7–12. doi: 10.11648/j.ejpm.s.2017050101.12
15. Elsheikh T, Balla SA, Abdalla AA, Elgasim M, Swareldahab Z, Bashir AA. Knowledge, attitude and practice of heath care workers regarding transmission and prevention of hepatitis B virus infection, White Nile state, Sudan, 2013. Am J Health Res. (2016) 4:18–22. doi: 10.11648/j.ajhr.20160402.11
16. Askarian M, McLaws M-L, Meylan M. Knowledge, attitude, and practices related to standard precautions of surgeons and physicians in university-affiliated hospitals of Shiraz, Iran. Int J Infect Dis. (2007) 11:213–9. doi: 10.1016/j.ijid.2006.01.006
17. Haq NU, Hassali MA, Shafie AA, Saleem F, Farooqui M, Aljadhey H, et al. cross sectional assessment of knowledge, attitude and practice towards Hepatiis B amng healthy population of Quetta, Pakistan. BMC Public Health. (2012) 12:692. doi: 10.1186/1471-2458-12-692
18. Kassahun CW, Mekonen AG. Knowledge, attitude, practice and their associated factors towards diabetes mellitus among non-diabetes community members of Bale zone administrative town, South East Ethiopia. a cross-sectional study. PLoS ONE. (2017) 12:e0170040. doi: 10.1371/journal.pone.0170040
19. Gafoor KA. Considerations in the Measurement of Awareness. National Seminar on Emerging trends in education (2012). Available online at: https://files.eric.ed.gov/fulltext/ED545374.pdf (accessed October 15, 2021).
20. Osagiede EF, Obekpa SA, Am IA, Tracy EO, Ehikioya JO, Johnbull J. Assessment of knowledge and seroprevalence of hepatitis B and C viral infection among health care personnel in a rural teaching hospital in South-South Nigeria. J Environ Occupational Health. (2020) 10:55–72. Available online at: https://www.jenvoh.com/abstract/assessment-of-knowledge-and-seroprevalence-of-hepatitis-b-and-c-viral-infection-among-health-care-personnel-in-a-rural-t-52567.html
21. Mahamat G, Kenmoe S, Akazong EW, Ebogo-Belobo JT, Mbaga DS, Bowo-Ngandji A, et al. Global prevalence of hepatitis B virus serological markers among healthcare workers: a systematic review and meta-analysis. WJH. (2021) 13:1190–202. doi: 10.4254/wjh.v13.i9.1190
22. Tepavčević DK, Kanazir M, Marić G, Zarić M, Lončarević G, Gazibara T. Hepatitis B-related awareness among health care workers in Belgrade, Serbia. Vojnosanitetski Pregled. (2020) 77:463–469. doi: 10.2298/VSP180227090K
23. Gyang MD, Madaki AJ, Dankyau M, Toma BO, Danjuma SA, Gyang BA. Prevalence and correlates of hepatitis B and C seropositivity among health care workers in a semi urban setting in north central Nigeria. Highland Med Res J. (2016) 16:75–9. Available online at: https://www.ajol.info/index.php/hmrj/article/view/148792
24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
25. Stevens GA, Alkema L, Black RE, Boerma JT, Collins GS, Ezzati M, et al. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. PLoS Med. (2016) 13:e1002056. doi: 10.1371/journal.pmed.1002056
26. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyse (2000)
28. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. (2011) 342:d549. doi: 10.1136/bmj.d549
29. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. (2010) 1:97–111. doi: 10.1002/jrsm.12
30. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
31. Djeriri K, Laurichesse H, Merle JL, Charof R, Abouyoub A, Fontana L, et al. Hepatitis B in moroccan health care workers. Occup Med. (2008) 58:419–24. doi: 10.1093/occmed/kqn071
32. Shao ER, Mboya IB, Gunda DW, Ruhangisa FG, Temu EM, Nkwama ML, et al. Seroprevalence of hepatitis B virus infection and associated factors among healthcare workers in northern Tanzania. BMC Infect Dis. (2018) 18:474. doi: 10.1186/s12879-018-3376-2
33. Hebo HJ, Gemeda DH, Abdusemed KA. Hepatitis B and C viral infection: prevalence, knowledge, attitude, practice, and occupational exposure among healthcare workers of Jimma university medical center, Southwest Ethiopia. Scientific World Journal. (2019) 2019:1–11. doi: 10.1155/2019/9482607
34. Desalegn Z, Selsassie SG. Prevalence of hepatitis B surface antigen (HBsAg) among health professionals in public hospitals in Addis Ababa, Ethiopia. Ethiopian J Health Dev. (2013) 27:72–79. Available online at: https://www.ejhd.org/index.php/ejhd/article/view/314
35. Anagaw B, Shiferaw Y, Anagaw B, Belyhun Y, Erku W, Biadgelegn F, et al. Seroprevalence of hepatitis B and C viruses among medical waste handlers at Gondar town health institutions, Northwest Ethiopia. BMC Res Notes. (2012) 5:55. doi: 10.1186/1756-0500-5-55
36. Ngekeng S, Chichom-Mefire A, Nde P, Nsagha D, Nkuigue A, Tiogouo K, et al. Hepatitis B prevalence, knowledge and occupational factors among health care workers in Fako division, South West region Cameroon. MRJI. (2018) 23:1–9. doi: 10.9734/MRJI/2018/40445
37. Ijoma U, Meka I, Omotowo B, Nwagha T, Obienu O, Onodugo O, et al. Sero-prevalence of hepatitis B virus infection: a cross-sectional study of a large population of health care workers in Nigeria. Niger J Clin Pract. (2021) 24:38. Available online at: link.gale.com/apps/doc/A649545545/HRCA?u=anon~118ca5b4&sid=googleScholar&xid=2b45065f
38. Oladokun AO, Agidigbi EF, Oke MA, Otebolaku-Olajide TM, Adigun GA, Alao MA. Seroprevalence and knowledge of hepatitis B among healthcare workers in Saki, Southwest, Nigeria. IOSR J Nurs Health Sci. (2021) 10:9–13. doi: 10.9790/1959-1002050913
39. Muhammad AA, Ibrahim BC, Ramadan AM. Knowledge, attitude and practice regarding hepatitis B infection among nurses in public hospitals of Niger state, Nigeria. J Obstet Gynaecol. (2016) 12:1–9. doi: 10.9734/IJTDH/2016/18663
40. Awimero CE, Nelson EA, Yusuf M, Olaosebikan OF, Adeboye MAN, Adamu UG, et al. Knowledge, awareness and prevalence of viral hepatitis among health care workers (HCWs) of the federal medical centre Bida, Nigeria (2017).
41. Mbaawuaga EM, Hembah-Hilekaan SK, Iroegbu CU, Chibuogwu Ike A. Hepatitis B virus and human immunodeficiency virus infections among health care workers in some health care centers in Benue state, Nigeria. Open J Med Micro. (2019) 9:48–62. doi: 10.4236/ojmm.2019.92007
42. Akazong WE, Tume C, Njouom R, Ayong L, Fondoh V, Kuiate J-R. Knowledge, attitude and prevalence of hepatitis B virus among healthcare workers: a cross-sectional, hospital-based study in bamenda health district, NWR, Cameroon. BMJ Open. (2020) 10:e031075. doi: 10.1136/bmjopen-2019-031075
43. Rodrigue G, Ernest D, Marcel NN, Jeanne N. Determination of the prevalence of the HBs antigen and evaluation of the vaccination status against Hepatitis B among the staff of the Dschang district hospital in west Cameroon. GSC Biol and Pharm Sci. (2021) 15:128–139. doi: 10.30574/gscbps.2021.15.2.0127
44. Tatsilong HOP, Noubiap JJN, Nansseu JRN, Aminde LN, Bigna JJR, Ndze VN, et al. Hepatitis B infection awareness, vaccine perceptions and uptake, and serological profile of a group of health care workers in Yaoundé, Cameroon. BMC Public Health. (2016) 16:706. doi: 10.1186/s12889-016-3388-z
45. Qin Y-L, Li B, Zhou Y-S, Zhang X, Li L, Song B, et al. Prevalence and associated knowledge of hepatitis B infection among healthcare workers in Freetown, Sierra Leone. BMC Infect Dis. (2018) 18:315. doi: 10.1186/s12879-018-3235-1
46. Massaquoi TA, Burke RM, Yang G, Lakoh S, Sevalie S, Li B, et al. Cross sectional study of chronic hepatitis B prevalence among healthcare workers in an urban setting, Sierra Leone. PLoS ONE. (2018) 13:e0201820. doi: 10.1371/journal.pone.0201820
47. Mangkara B, Xaydalasouk K, Chanthavilay P, Kounnavong S, Sayasone S, Muller CP, et al. Hepatitis B virus in lao dentists: a cross-sectional serological study. Ann Hepatol. (2021) 22:100282. doi: 10.1016/j.aohep.2020.10.010
48. Nguyen T, Pham T, Tang HK, Phan L, Mize G, Lee WM, et al. Unmet needs in occupational health: prevention and management of viral hepatitis in healthcare workers in Ho Chi Minh city, Vietnam: a mixed-methods study. BMJ Open. (2021) 11:e052668. doi: 10.1136/bmjopen-2021-052668
49. Ptil S, Rao RS, Agarwal A. Awareness and risk perception of hepatitis B infection among auxiliary healthcare workers. J Int Soc Prevent Communit Dent. (2013) 3:67. doi: 10.4103/2231-0762.122434
50. Alqahtani JM, Abu-Eshy SA, Mahfouz AA, El-Mekki AA, Asaad AM. Seroprevalence of hepatitis B and C virus infections among health students and health care workers in the Najran region, southwestern Saudi Arabia: the need for national guidelines for health students. BMC Public Health. (2014) 14:577. doi: 10.1186/1471-2458-14-577
51. Memon MS, Ansari S, Rashid N, Khatri NK, Mirza MA, Jafri W. Hepatitis B vaccination status in health care workers of two universiry hospitals. JLUMHS. (2007) 48–51. doi: 10.22442/jlumhs.07610115
52. Aziz S, Tily HI, Rasheed K, Memon A, Jehangir K, Quraishy MS. Prevalence of H1V, hepatitis B and C amongst health workers of civil hospital karachi. JPMA. (2022) 52. Available online at: https://www.jpma.org.pk/PdfDownload/2142.pdf
53. Vaishali K, Zulfeequer C, Aanad R, Thakrar R, Alaparthi G, Kumar SK. Awareness in patients with COPD about the disease and pulmonary rehabilitation: a survey. Lung India. (2014) 31:134. doi: 10.4103/0970-2113.129837
54. Roien R, Mehterkhail S, Faizi MA, Haidari MH, Haidari E, Alimi B, et al. Knowledge, attitude and vaccination status of health care workers against hepatitis B virus infection in kabul. Kateb Quarterly (Sci - Res). (2019) 6:119–32. Available online at: https://research.kateb.edu.af/dari/wp-content/uploads/sites/2/2019/12/9.pdf
55. Rayate AS, Barhate NR, Bhalge UU, Gavkare AM, Nagoba BS. Awareness about hepatitis B virus infection and vaccination among health care personnel at higher risk - a cross-sectional study. The European Journal of Innovative, Integrative and Translational Medicine. Eur J Integr Trans Med. (2021) 5. Available online at: https://www.resclinmed.eu/public/data_files/articles/136/article_136.pdf
56. Heinrich J. Occupational safety: selected cost and benefit implications of needlestick prevention devices for hospitals. United States General Accounting Office (2000).
Keywords: hepatitis B virus, seroprevalence, prevalence, infection, healthcare workers, knowledge, awareness, epidemiology
Citation: Maamor NH, Muhamad NA, Mohd Dali NS, Abdul Mutalip MH, Leman FN, Aris T, Lai NM and Abu Hassan MR (2022) Seroprevalence of Hepatitis B Among Healthcare Workers in Asia and Africa and Its Association With Their Knowledge and Awareness: A Systematic Review and Meta-Analysis. Front. Public Health 10:859350. doi: 10.3389/fpubh.2022.859350
Received: 21 January 2022; Accepted: 04 April 2022;
Published: 28 April 2022.
Edited by:
Kevin K. A. Tetteh, University of London, United KingdomReviewed by:
Bahador Sarkari, Shiraz University of Medical Sciences, IranCopyright © 2022 Maamor, Muhamad, Mohd Dali, Abdul Mutalip, Leman, Aris, Lai and Abu Hassan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nor Asiah Muhamad, bm9yYXNpYWhkckBnbWFpbC5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.