- 1Department of Pediatrics, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 2Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan
- 3College of Public Health, China Medical University, Taichung, Taiwan
- 4Graduate Institute of Clinical Medicine, Taipei Medical University, Taipei, Taiwan
- 5Department of Family Medicine, Taipei Medical University Hospital, Taipei, Taiwan
- 6Department of Family Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
- 7School of Nutrition and Health Sciences, College of Nutrition, Taipei Medical University, Taipei, Taiwan
- 8Graduate Institute of Metabolism and Obesity Sciences, Taipei Medical University, Taipei, Taiwan
Background: We examined the effect of sugar-sweetened beverages (SSB) and common drink intake on pubertal development in both sexes.
Methods: Data were retrieved from Taiwan Children Health Study, which involved detailed pubertal stage assessments of 2,819 schoolchildren aged 11 years in 2011–2012. Drawings of secondary sexual characteristics and self-reported age at menarche or voice breaking were used to assess pubertal stages. Dietary intake was assessed using a detailed semi-quantitative food frequency questionnaire. Generalized estimating equation modeling was applied to obtain odds ratios (ORs) and 95% confidence intervals (CIs) to represent the effects of each drink on early pubertal development outcomes.
Results: In boys, an one cup/day increment of a SSB was associated with earlier voice breaking (β = −0.12; 95% CI = −0.20, −0.04), whereas consuming yogurt (≥2 cups/day) was a protective factor against early puberty (OR = 0.78; 95% CI = 0.73, 0.83). In girls, SSB consumption was associated with increased risk of early puberty in a dose–response manner, and a similar protective effect of yogurt consumption and fermented probiotic drink (≥2 cups/day) against early puberty was observed (OR = 0.96; 95% CI = 0.94, 0.99). Furthermore, the intake of both total sugar and added sugar within SSBs increased risk of early puberty in girls but not in boys.
Conclusions: Sugar-sweetened beverages were associated with early puberty, and probiotic drinks appeared to mitigate this link. These findings indicate that the gut–brain axis could play a crucial role in sexual maturation.
Introduction
Puberty refers to the process of physical changes under the cascade of endocrine actions. It is generally regarded as a hallmark of sexual maturation (1). The pubertal progression from the initiation to full maturation varies, which implies a complex interplay of nutrition, psychological condition, socioeconomic status, and hormonal actions in an individual (2, 3). Early pubertal timing, notably defined by early age at menarche in females, has been extensively linked to adverse health outcomes, such as metabolic syndrome (4), fatty liver disease (5), cardiovascular diseases (6), and breast cancer (7). In addition to physical impacts, adolescents who mature early are disadvantaged by potential psychosocial maladaptation and are thus prone to depression and engaging in delinquent behavior, early sexual activity, as well as substance use (8–11). As the trend toward earlier pubertal timing becomes clearer (12–14), the gap between physical and psychological maturity toward the full social status of adulthood continues to lengthen (15). Taiwan is no exception, with a constantly falling age at menarche and an almost 2-year drop over three generations (16). Researchers and clinicians have continued their pursuit of the determinants of sexual maturation to address the adverse health effects associated with early maturation.
The intake of sugar-sweetened beverages (SSBs) has been associated with pro-glycemic traits, such as higher fasting glucose and fasting insulin levels (17), and a higher health risk of obesity and diabetes among adolescents (18). In total, 88.7% of Taiwanese adolescents consume more than one SSB every week (19), at an average of 446 ml per day (20). Along with the obesogenic trends in dietary habits, the consistent finding that girls with a higher bone mass index (BMI) tend to mature early supports the vital role of nutritional factors in pubertal timing (21, 22). Therefore, prior mechanistic discussions surrounding the link between SSB intake and early puberty generally focus on higher BMI (i.e., positive energy balance) as a strong driver of menarche. In addition to the effects via weight status, SSB consumption is independently associated with earlier age at menarche in a large prospective US study (23), even after participants' BMI was adjusted for. Pathways thought to be mediating mechanisms include the intake of high-glycemic foods, such as SSBs, resulting in a rapid and immediate increase in circulating insulin concentrations, which could upregulate insulin-like growth factor-1 (IGF-1), a hormone involved in the initiation of menarche via modulating the reproductive system through widespread effects on hypothalamus, pituitary, and ovaries (24). Specifically, serum levels of IGF-1 may be inversely associated with levels of sex hormone binding globulin (SHBG) in obese children, while gender difference is well noted in the association between adiposity related declines in SHBG and pubertal age in an earlier longitudinal study (25). Despite substantial evidence on girls, little is known about whether SSB intake is related to boys' sexual maturation, independent of weight status.
The mediating mechanism via SHBG and bioavailable sex steroids on pubertal onset has also been observed for other macronutrients (26). Non-sugar contents of food intakes are reported to be differentially associated with age at menarche (27–29). For instance, higher consumption of dietary fibers and vegetable proteins are associated with later age at menarche (27, 28), while higher consumption of animal proteins and poly-unsaturated fatty acids are associated with earlier age at menarche (29). The complexity of food contents also applied to dairy products that are composed of wide-ranging nutrients, thus contributing to inconsistent results of the association with age at menarche (30, 31). As there is surge in research on the physiological effects of intestinal microbiota, yogurts that are rich in probiotics have been found to have a protective effect against early breast development and menarche in a group of Chilean girls (31). However, their finding was not replicated in Iranian female samples (30). Evidence of the association between nutrition and pubertal timing in boys is even relatively scant. If nutritional factors have a direct mechanistic effect on pubertal timing, this may provide an actionable opportunity to prevent off-time puberty by manipulating pre-pubertal dietary practices.
To obtain epidemiological evidence on the relationship between SSB intake and pubertal timing in both sexes, it is imperative to prospectively survey the relationship between detailed SSB intake among growing adolescents and the risk of early puberty. We hypothesize that SSB intake is associated with pubertal timing in a gender dependent manner. Therefore, in this study, we examined the association between SSBs and several other types of drinks, with a particular focus on probiotics drinks, and longitudinal pubertal outcomes in a representative nationwide sample. Furthermore, we explored the effects of total sugar or added sugar in SSBs and other drinks on the risk of early puberty.
Materials and methods
Study design and participants
For this study, data were obtained from the Taiwan Children Health Study (TCHS), a prospective, nationwide cohort study focusing on obesity, pubertal development, and atopic diseases in adolescents. The TCHS had an open-cohort study design and comprised 3,229 children at age 11. The cross-sectional analysis herein mainly involved 2,819 schoolchildren aged 11–12 years in 2011–2012 with FFQ assessment and pubertal outcomes available (Figure 1). The detailed data collection in the TCHS was previously documented (32). Data regarding pubertal development were collected at age 11, 12, and 18. In addition, a food frequency questionnaire (FFQ) was administered at age 11. All participants received inform consent to participate in this study. The study protocol was approved by the Institutional Review Board of National Taiwan University Hospital and complied with the principles of the Declaration of Helsinki.
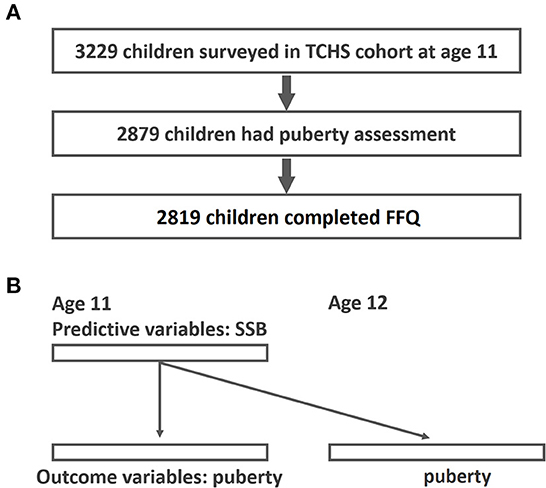
Figure 1. Study flow diagram of participant recruitment and GEE analysis for the associations of SSB and early puberty. (A) Study flow; (B) GEE analysis-time lag model of the associations between SSB and early puberty (predictive variables were SSB at age 11 and outcome variables were puberty outcomes at age 11 and age 12).
Puberty outcomes
Puberty oucomes were assessed at age 11, 12, and 18. We used two types of children self-filled questionnaire to define pubertal outcomes: the Chinese version of the puberty development scale (PDS) (33) and the Tanner derived composite stage (TDCS) (34). Early puberty outcome was determined by the TDCS, and age at menarche or voice breaking was assessed by PDS. The TDCS contains drawings of the five stages of secondary sexual characteristics (pubic hair and breast development for girls and pubic hair and genital development for boys). The two sex characteristics (pubic hair for both genders, genitalia for boys and breast development for girls) were combined and averaged to create a single Tanner score (33). Furthermore, early puberty was defined as reaching a certain pubertal stage earlier than the median age for that stage (35), referencing large-scale population-based Chinese studies (36, 37). We validated the Chinese version of the TDCS, and the consistency between Tanner stages from children self-reports and physical inspection by pediatric endocrinologist was revealed to be high in our pilot study (weighted kappa = 0.85) (38). Age at menarche and age at voice breaking were defined according to questions in the PDS at age 11–12. Most of the children had completed pubertal growth at age 18, therefore, age at menarche and age at voice breaking were confirmed again using the PDS through participants' recall.
Dietary assessment
We assessed diet using a self-administered, semi-quantitative 71-food-item FFQ in 2011 (39). It was a modified and shorter version of the Nutrition and Health Survey of Taiwan (40) and had been used in adolescents in one previous study (41) to investigate SSB intake. The original Chinese FFQ used in nationwide survey of Nutrition intake had been proved reproducible and of high validity (39). Participating children were instructed by a research dietitian for 20 min regarding how the portion size of each food item was measured in the FFQ. Participants were asked how frequently (on average in a week), they consumed a typical portion size of a certain food during the preceding month. A typical serving size was specified as 240 ml (equal to one cup) for SSBs, fresh juice, fresh milk, flavored milk, soy milk, yogurt, and fermented probiotic drinks. SSBs include all carbonated soft drinks, sweetened milk tea, and all other sugar sweetened beverages, such as flavored milk and concentrated fruit juice. Fresh juice, fresh milk, soy milk, etc… which contained no added sugar, were not included in the SSB category. Fermented probiotic drinks included Yakult, fermented milk, and other probiotic drinks. The probiotics contents in those fermented probiotic drinks mainly included Lactobacillus spp. The common probiotics contents within the yogurt include Streptococcus thermophiles, Bifidobacterium lactis, Lactobacillus bulgaricus, and Lactobacillus acidophilus. Detailed contents of fermented probiotic drinks and yogurt are listed in Supplementary Table S3. The simple sugar content in each type of drink was calculated by using a Taiwanese food composition table as the nutrient database (Taipei, Taiwan) (42). Total sugars were classified as natural sugar and added sugar. We classified natural sugars as lactose and non-lactose. Non-lactose sugars included glucose, fructose, galactose, sucrose, and maltose. Added sugar was defined as any sugar added to the drink during its production. After summing up the sugar intake by frequency and portion size to produce grams per day, we then categorized distinct sugar intake from drinks by using percentiles (≤25, 25−75%, and ≥75%).
Covariate assessment
Confounders in the statistical models were a priori confounders based on previous research (23) with data relevant to both puberty outcomes and SSBs. Body height was measured to the nearest 0.1 cm using a wall-mounted stadiometer (MAGATA, BW-120) at each school. BMI was calculated as weight divided by (height)2 [kg/m2]. Body fat was measured using a tetrapolar multi-frequency bioelectrical impedance analysis machine (IOI 353, Jawon Medical, Korea). Participants with missing covariate information were included in the model by using missing indicators (43). Moreover, all models were adjusted for BMI, parental educational level, family income, and household smoking.
Data analysis
Continuous variables were expressed as mean ± standard deviation and categorical variables were expressed as frequencies and percentages. The correlations between basic demographics and different SSB and drink consumption groups were examined by chi-square test (frequency with percentage) and analysis of variance (mean with standard deviation). To evaluate the repeated measurements of pubertal outcomes at age 11 and 12, we used generalized estimating equations (GEEs) (44)-Time lag model, to examine the interrelations among SSB intake and pubertal outcomes (Figure 1B). The primary advantage of a GEE is that it accounts for within-individual variations. The correlation structure assumed for repeated measurements was a working exchangeable correlation. In the Time-lag Model, predictors (SSB at age 11) were modeled using data from surveys that preceded the outcome variables (puberty outcome at age 11 and 12). Additionally, the time-lag model accounts for the temporal sequence in a possible cause-and-effect relationship (45). In addition to selected covariates, we adjusted all enlisted beverages and total energy with the use of variance inflation factor to test the collinearity among variables. Odds ratios (OR) and 95% confidence intervals (CIs) represented the effect of each drink on early pubertal development outcomes. Trend testing was performed to present the dose responsiveness. Missing covariate information of the participants was also included in the model by using missing indicators (43). All tests were two-sided with a 5% significance level. All analyses were performed using R version 3.3.2 (R Foundation for Statistical Computing).
Results
Participants' characteristics
Among 2,819 children analyzed in this study (Table 1), 472 (16.7%) children consumed 0 cup/day of SSB, while 628 (22.3%) children consumed ≥3 cup/day. The majority (1,719 children, 61.0%) of the children consumed 1–2 cups of SSB per day. One hundred sixty-one males (11.5%) and 139 females (9.8%) experienced early puberty. Compared with female participants, males tended to consume more SSBs with nearly 25.9% drinking ≥3 cup/day. Those consuming high quantities of SSB (≥3 cup/day) were more likely to hail from households with higher exposure to cigarette smoking, less parental education, and lower family income. However, no difference in BMI, body fat, and physical activity levels was observed across different SSB consumption groups. Moreover, intake of total energy, carbohydrates, additional sugar, and additional fat increased significantly with higher consumption frequency of SSBs, indicating that unhealthy eating habits might accompany higher levels of SSB consumption. Furthermore, intake of protein decreased as SSB consumption increased, suggesting that appropriate protein intake might be replaced by SSBs. However, parental educational level, family income, and household smoking revealed significantly differences in distribution between three SSB consumption groups, while they were adjusted in all models.
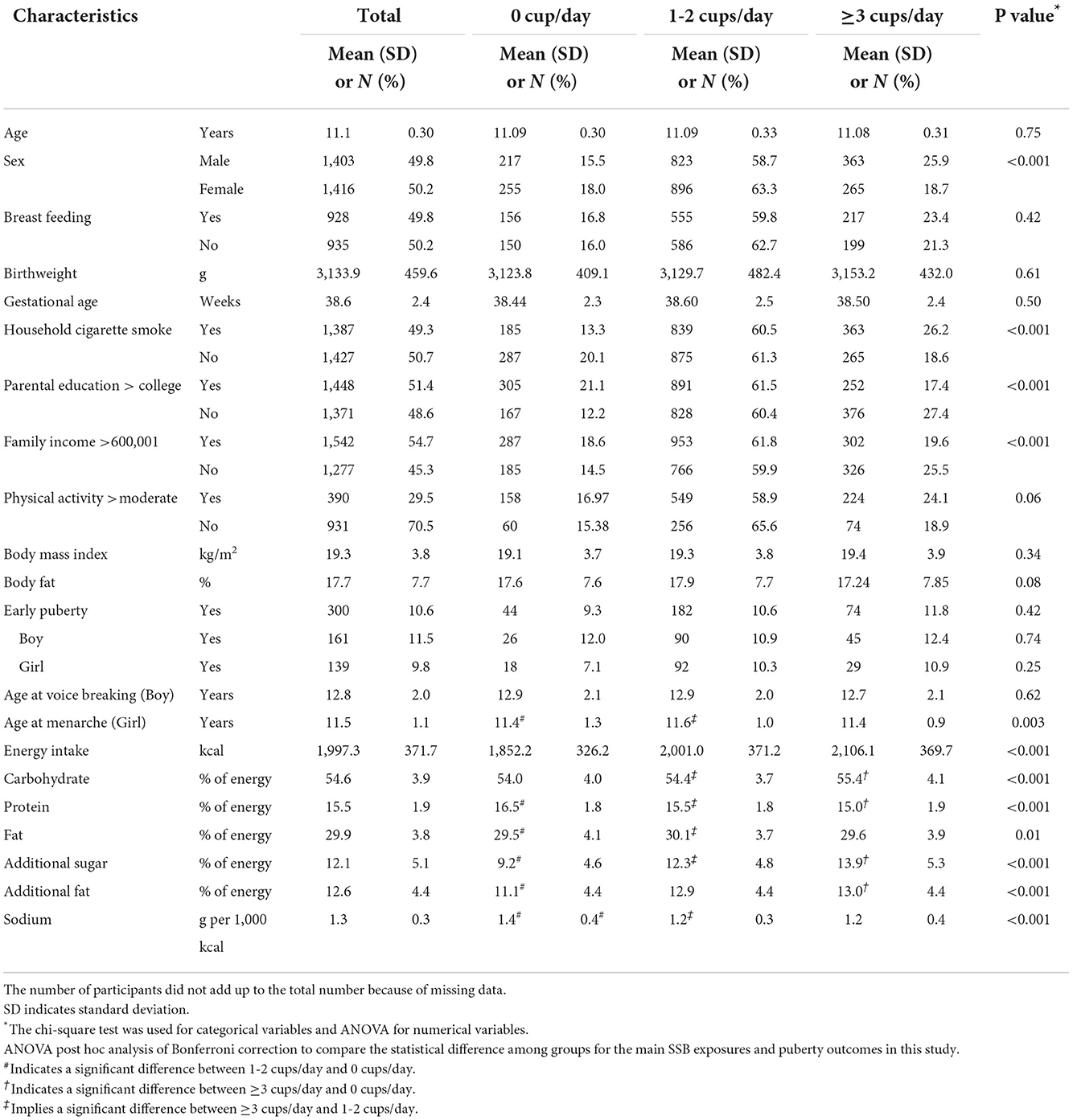
Table 1. Baseline characteristics according to categories of sugar-sweetened beverages in Taiwanese children at 11 years (N = 2,819).
The associations in boys
Using GEE modeling, we observed that a 1 cup/day increment of SSB intake was associated with voice breaking at an earlier age (β = −0.18; 95% CI =-0.29, −0.06) in male participants (Table 2). However, consuming yogurt (≥2 cups/day) was a protective factor against early puberty (OR = 0.78; 95% CI = 0.73, 0.83). By contrast, consuming fermented probiotic drinks was associated with voice breaking at an earlier age (β= −1.23; 95% CI = −3.19, −0.72). Although fresh milk seemingly did not influence the timing of puberty among boys, consuming 1 cup/day of flavored milk increased the risk of early puberty (OR = 1.05; 95% CI 1.00, 1.02) and was associated with voice breaking at an earlier age (β = −0.41; 95% CI = −0.81, −0.01).
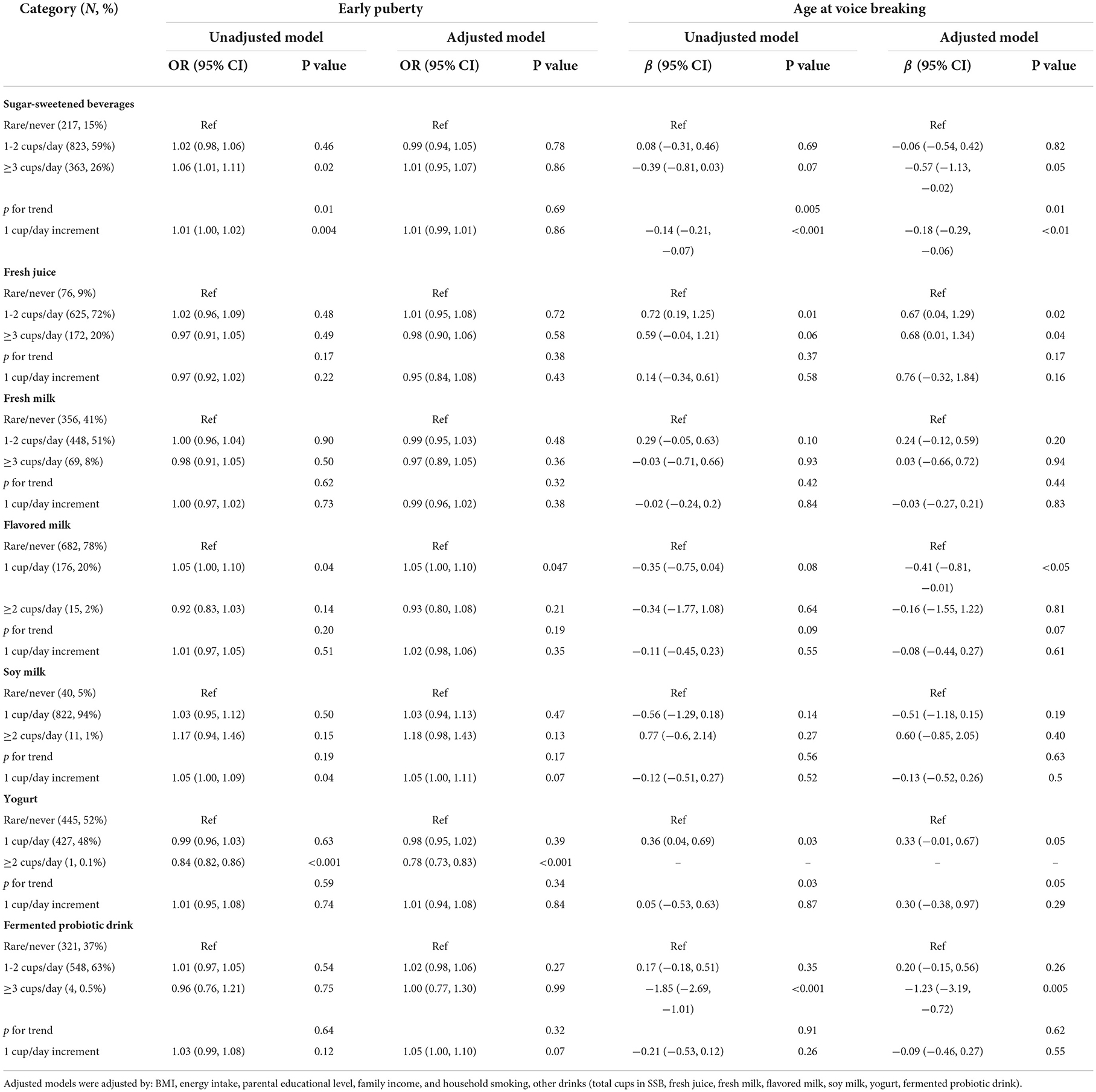
Table 2. OR and regression coefficients for the associations between consumption of selected drinks and pubertal outcomes (boys).
The associations in girls
In girls, SSB consumption was associated with an increased risk of early puberty in a dose–response manner (Table 3). Girls consuming 1 cup/day of yogurt or fermented probiotic drink exhibited an increased risk of early puberty compared with those who rarely consumed those drinks [OR = 1.03 (95% CI = 1.00, 1.05) for yogurt and OR = 1.16 (95% CI = 1.11, 1.33) for fermented probiotic drinks]. By contrast, yogurt consumption (≥2 cups/day) was a protective factor against early puberty (OR = 0.92; 95% CI = 0.89, 0.95). Similarly, consuming fermented probiotic drinks (≥2 cups/day) was associated with a reduced risk of early puberty (OR = 0.96; 95% CI = 0.94, 0.99) as well as a delayed age at menarche (β = 0.41; 95% CI = 0.24, 1.42).
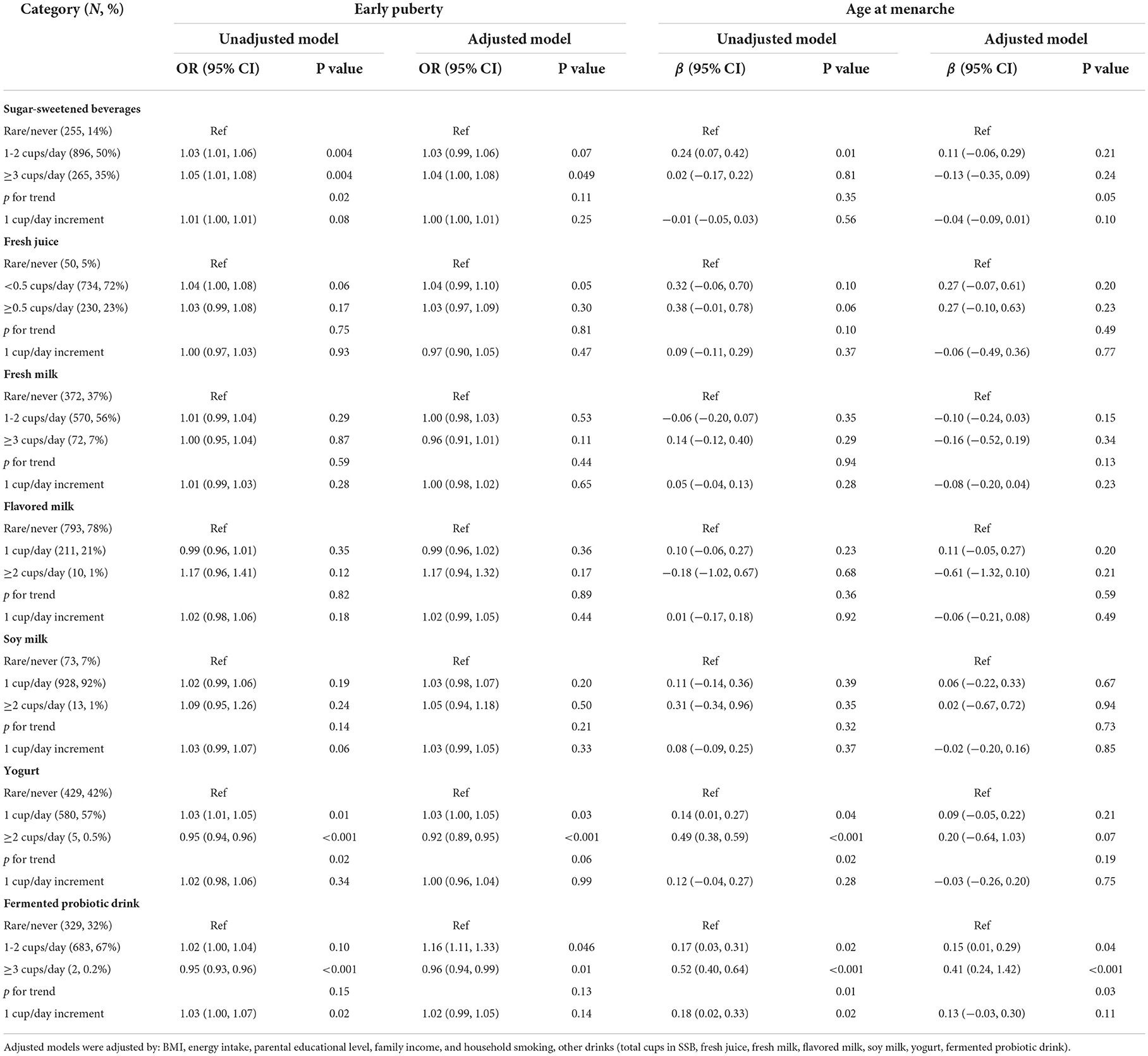
Table 3. OR and regression coefficients for the associations between consumption of selected drinks and pubertal outcomes (girls).
Total and added sugars
To investigate which component of the selected drinks might contribute to the aforementioned association with early puberty, we examined the effects of total sugar and added sugar from selected drinks on early puberty risk after adjustment for BMI, parental educational level, family income, and household smoking (Figures 2, 3). Notably, among girls, both the intake of total sugar and added sugar predicted an increased likelihood of early puberty in a significant dose–response manner. However, neither total sugar nor added sugar could fully explain the observed associations between more frequent consumption of SSBs and early puberty among boys. Detailed associations between consumption of several sugars and puberty outcomes are listed in Supplementary Tables S1, S2. The intake of total sugar, lactose from natural food, and added sugar each increased the risk of early puberty among girls in a dose–response manner.
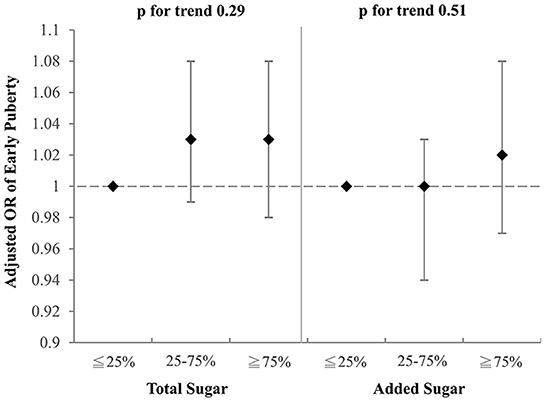
Figure 2. Adjusted OR for early puberty due to the consumption of total sugar and added sugar from selected drinks (boys). Models were adjusted for BMI, parental educational level, family income, and household smoking.
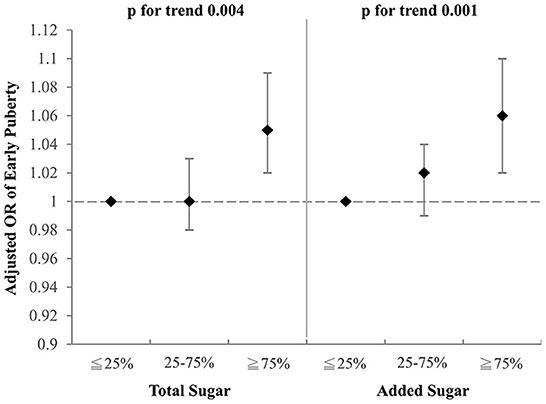
Figure 3. Adjusted OR for early puberty due to the consumption of total sugar and added sugar from selected drinks (girls). Models were adjusted for BMI, parental educational level, family income, and household smoking.
Discussion
Summary of main findings
In this study, we identified SSB-related risk and protective factors of pubertal timing among Taiwanese adolescents. To our knowledge, this is the first study to reveal that SSB consumption is significantly associated with early puberty in both sexes; probiotic drinks to a higher amount may mitigate this risk. Our findings indicate that health care providers should increase awareness of the effects of SSBs on adolescent health and pubertal development. The novel finding that fermented probiotic drinks or yogurt have a protective effect against early puberty suggests that early puberty might be a gut–brain disease. Further experimental studies are warranted to prove the aforementioned associations.
This is the first study to identify an independent association between SSB consumption and early puberty in boys. In girls, several reports have consistently indicated the association between SSB consumption and early menarche (23, 31, 46). SSB consumption might be associated with metabolic and weight status changes that potentially affect menarcheal timing. The link between obesity and early puberty in girls is well known (38). However, the relationship between obesity and early puberty among boys varies among different ethnicities (35, 47). Several large Chinese studies have reported that obesity also promotes the earlier onset of puberty in boys (47, 48). Furthermore, recent evidence has indicated that SSBs may have a direct effect on sexual maturation in addition to increasing body weight (23). Excessive sugar consumption per se is associated with an increase in the production of insulin and IGF-1, which could enhance cell proliferation and growth (49). Evidence also indicates that IGF-1 has a direct effect, through intracellular signal transduction cascades, on the proliferation and differentiation of ovaries as well as testes (50). In summary, excessive SSB consumption may cause fat accumulation and thus increase the risk of obesity, which is a known risk factor for early puberty. Moreover, increased insulin and IGF-1 as a result of excessive sugar intake contribute to dysregulation of the reproductive axis via downregulation of SHBG. Further research may be required to identify the key regulator involved in pubertal initiation and how it is linked to sugar metabolism.
We discovered that probiotic drinks appear to be a protective factor against early puberty. This finding is consistent with research (31) that revealed that yogurt delays menarche. We argue that this protection effect may be exerted through modifying dysbiosis in the gut. A paper by Cowan and Richardson (51) indicated that probiotic treatment can restore the normative timing of vaginal opening in stress-induced early-maturing rats. Researchers have also observed that probiotics reverse stress-induced learned fear behavior and stress hormone levels, thus highlighting the restorative effects of probiotics in the context of early-life stress (52). Our findings provide human evidence to further support the crucial role of the gut–brain axis in pubertal development. One prior human study measuring the urine metabolites in patients with central precocious puberty revealed significant alterations in urinary excretion levels of gut microbial-mammalian cometabolites before and after treatment with Triptorelin, suggesting that central precocious puberty may involve an alteration in symbiotic gut microbial composition (53). Given the cumulative evidence of the influence of gut microbiota on the development and functionality of the central nervous system (54), we tentatively conclude that gut microbiota may alter the function of the hypothalamus–pituitary–gonadal axis and thus the activation of puberty. From a practical perspective, changing gut microbiota through the intake of probiotics may attenuate early puberty. On the other hand, although the probiotic content within the yogurt and fermented probiotic drinks might protect children from early puberty, the high sugar contents within these drinks should still be noted. Currently, the World Health Organization (WHO) recommends that children consume foods with low sugar to <50 g/day (55). Hence, if a child consumes more than four cups of yogurt and fermented probiotic drinks, their sugar content within these drinks would exceed the WHO's recommendation. Parents and school policymakers are suggested to provide these probiotic drinks with low or no sugar content for children undergone pubertal growth.
Studies exploring the relationships between milk or diary consumptions and early puberty yielded inconsistent results. Evidence revealed that cow milk was the most commonly consumed animal product by girl, which might influence sexual maturation during adolescence (56, 57). However, a cohort study reported that milk consumption in girls aged ≥9 years failed to predict age at onset of menarche (58). No study has reported the aforementioned association in boys. Although we did not discover significant links between milk consumption and early puberty risk in female participants, consuming flavored milk increased the risk of early puberty in boys. This could be attributed to the extra sugar intake from flavored milk.
The intakes of total and added sugar were associated with an increased risk of early puberty among girls, but not boys. Carwile et al. (23) discovered that added sugar, but not total sugar, was associated with early menarche. Added sugar, such as artificial sweeteners, was characterized to influence metabolic changes and reproductive health by activation of sweet taste receptors (59). Sweet taste receptors locate not only in the mouth cavity but also in ovaries. This was proposed as a possible mechanism why sugar intake may influence the reproductive system. Moreover, routine exposure to added sugars may alter thresholds for sweet taste perception, imparting a stronger preference for sweet food, which may yield adiposity accumulation (60). The detailed mechanism of added sugars on early puberty has not been further explored. The only relevant animal studies reported that long-term in-utero exposure of added sugar to 18-day-old mice resulted in a thicker epidermal and dermal layer in the mammary glands of the mice embryos. By the age of 4 weeks after birth, these exposed mice experienced an early production of the mammary gland. This phenomenon indirectly confirmed that long-term exposure to added sugar may prompt early puberty (61).
Strengths and limitations
The TCHS cohort was nationally representative, systemically sampled, and longitudinally followed; therefore, the derived findings could provide nutritional guidance for our local population. Although same-source bias may be present in a questionnaire-based research design, a detailed FFQ, used to assess food intake, allowed us to calculate nutrient consumption and examine its effect on sexual maturation. However, we did not specify the species and doses of probiotics in our population-based epidemiological study. We were also unable to conclude the direction of the causal relationship between pubertal timing and probiotics use. Given the short time interval in which the exposures and outcomes were assessed (i.e., age 11 years for SSB or probiotic consumption and age 11–12 years for puberty), it is difficult to ascertain whether the exposure truly precedes the pubertal outcome. There could be instances where children in this study had attained puberty before 11 years; this could affect the interpretation of the study findings, which may be biased by reverse causality. Moreover, we tested several regression models in Tables 2, 3 for all exposures and outcomes. We did not specifically adjust for multiple testing, because preferences for food choices were not our primary variables of interest. Despite this, the associations found in the tables remained significant after using stringent corrections for multiple testing. Lastly, the study findings may also be biased by residual confounding from unmeasured covariates, such as water consumption that was not available in the dataset. Further investigation may be required to determine a mechanistic explanation of the association revealed in our study.
Conclusion
Our study identified an independent association between SSBs and early pubertal timing after adjustments were made for BMI. Probiotic use to a certain amount appeared to mitigate the risk of early puberty. These findings not only highlight the importance of nutritional screening when consulting adolescents going through puberty but also elucidate the gut–brain connection. From the perspective of public health nutrition, a potential way to reduce adverse effects brought by early sexual maturation is to restrict sugar consumption in growing children. More research may be required to identify the specific probiotic species and molecular pathways linking food consumption and pubertal timing.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by National Taiwan University Ethics Committees. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
M-CT contributed to data interpretation and writing the manuscript. YCC and YLL contributed to data analysis, cohort data collection, hypothesis generation, data interpretation, and writing the manuscript. All authors approved the final manuscript as submitted and published and agreed to be accountable for all aspects of the work.
Funding
This study was supported by grants 103-2314-B-002-043-MY3 and 106-2314-B-001-007-MY3 from YLL. The study was also supported by grants 104-2314-B-532-002-MY3, 107-2314-B-038-113-MY3, 109-2314-B-038-057, MOST 110-2314-B-038-156, and MOST 111-2314-B-038-004 (PI. YCC) from the Ministry of Science and Technology of Taiwan.
Acknowledgments
The authors thank the school teacher, children, and parents who supported the data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer YCC a shared affiliation with one of the authors' YCC to the handling editor at the time of review.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.854477/full#supplementary-material
Abbreviations
BMI, body mass index; CI, confidence interval; FFQ, food frequency questionnaire; GEE, generalized estimating equation; IGF-1, insulin-like growth factor-1; OR, odds ratio; PDS, Puberty Development Scale; SE, standard error; SSB, sugar-sweetened beverage; TCHS, Taiwan Children's Health Study; TDCS, tanner-derived composite stage.
References
1. Fortenberry JD. Puberty and adolescent sexuality. Horm Behav. (2013) 642:280–7. doi: 10.1016/j.yhbeh.2013.03.007
2. Lomniczi A, Wright H, Ojeda SR. Epigenetic regulation of female puberty. Front Neuroendocrinol. (2015) 36:90–107. doi: 10.1016/j.yfrne.2014.08.003
3. Ojeda SR, Lomniczi A, Sandau U, Matagne V. New concepts on the control of the onset of puberty. Endocr Dev. (2010) 17:44–51. doi: 10.1159/000262527
4. Chang C-J, Lai M-M, Lin C-C, Liu C-S, Li T-C, Li C-I, et al. Age at menarche and its association with the metabolic syndrome in Taiwan. Obes Res Clin Pract. (2016) 10:S26–34. doi: 10.1016/j.orcp.2015.10.003
5. Yi KH, Hwang JS, Lim SW, Lee JA, Kim DH, Lim JS. Early menarche is associated with non-alcoholic fatty liver disease in adulthood. Pediatr Int. (2017) 5912:1270–5. doi: 10.1111/ped.13422
6. Lee JJ, Cook-Wiens G, Johnson BD, Braunstein GD, Berga SL, Stanczyk FZ, et al. Age at menarche and risk of cardiovascular disease outcomes: findings from the national heart lung and blood institute-sponsored women's ischemia syndrome evaluation. J Am Heart Assoc. (2019) 812:e012406. doi: 10.1161/JAHA.119.012406
7. Hsieh CC, Trichopoulos D, Katsouyanni K, Yuasa S. Age at menarche, age at menopause, height and obesity as risk factors for breast cancer: associations and interactions in an international case-control study. Int J Cancer. (1990) 465:796–800. doi: 10.1002/ijc.2910460508
8. Mrug S, Elliott M, Gilliland MJ, Grunbaum JA, Tortolero SR, Cuccaro P, et al. Positive parenting and early puberty in girls: protective effects against aggressive behavior. Arch Pediatr Adolesc Med. (2008) 1628:781–6. doi: 10.1001/archpedi.162.8.781
9. Williams JM, Dunlop LC. Pubertal timing and self-reported delinquency among male adolescents. J Adolesc. (1999) 221:157–71. doi: 10.1006/jado.1998.0208
10. Tsai MC, Hsieh YP, Strong C, Lin CY. Effects of pubertal timing on alcohol and tobacco use in the early adulthood: a longitudinal cohort study in Taiwan. Res Dev Disabil. (2015) 36c:376–83. doi: 10.1016/j.ridd.2014.10.026
11. Tsai MC, Strong C, Lin CY. Effects of pubertal timing on deviant behaviors in Taiwan: a longitudinal analysis of 7th- to 12th-grade adolescents. J Adolesc. (2015) 42:87–97. doi: 10.1016/j.adolescence.2015.03.016
12. Delemarre-van de Waal HA. Secular trend of timing of puberty. Endocr Dev. (2005) 8:1–14. doi: 10.1159/000084082
13. Euling SY, Herman-Giddens ME, Lee PA, Selevan SG, Juul A, Sorensen TI, et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. (2008) 121(Suppl 3):S172–91. doi: 10.1542/peds.2007-1813D
14. Toppari J, Juul A. Trends in puberty timing in humans and environmental modifiers. Mol Cell Endocrinol. (2010) 324:39–44. doi: 10.1016/j.mce.2010.03.011
15. Patton GC, Viner R. Pubertal transitions in health. Lancet. (2007) 3699567:1130–9. doi: 10.1016/S0140-6736(07)60366-3
16. Chang SR, Chen KH. Age at menarche of three-generation families in Taiwan. Ann Hum Biol. (2008) 354:394–405. doi: 10.1080/03014460802154777
17. McKeown NM, Dashti HS, Ma J, Haslam DE, Kiefte-de Jong JC, Smith CE, et al. Sugar-sweetened beverage intake associations with fasting glucose and insulin concentrations are not modified by selected genetic variants in a ChREBP-FGF21 pathway: a meta-analysis. Diabetologia. (2018) 612:317–30. doi: 10.1007/s00125-017-4475-0
18. Yoshida Y. Simoes EJ. Sugar-sweetened beverage, obesity, and type 2 diabetes in children and adolescents: policies, taxation, and programs. Curr Diab Rep. (2018) 186:31. doi: 10.1007/s11892-018-1004-6
19. Lin WT, Lee CY, Tsai S, Huang HL, Wu PW, Chin YT, et al. Clustering of Metabolic risk components and associated lifestyle factors: a Nationwide Adolescent Study in Taiwan. Nutrients. (2019) 113:09. doi: 10.3390/nu11030584
20. Lee CY, Lin WT, Tsai S, Hung YC, Wu PW, Yang YC, et al. Association of parental overweight and cardiometabolic diseases and pediatric adiposity and lifestyle factors with cardiovascular risk factor clustering in adolescents. Nutrients. (2016) 89:13. doi: 10.3390/nu8090567
21. Juul F, Chang VW, Brar P, Parekh N. Birth weight, early life weight gain and age at menarche: a systematic review of longitudinal studies. Obes Rev. (2017) 1811:1272–88. doi: 10.1111/obr.12587
22. Fan HY, Lee YL, Hsieh RH, Yang C, Chen YC. Body mass index growth trajectories, early pubertal maturation, and short stature. Pediatr Res. (2020) 88:117–24. doi: 10.1038/s41390-019-0690-3
23. Carwile JL, Willett WC, Spiegelman D, Hertzmark E, Rich-Edwards J, Frazier AL, et al. Sugar-sweetened beverage consumption and age at menarche in a prospective study of US girls. Hum Reprod. (2015) 303:675–83. doi: 10.1093/humrep/deu349
24. Daftary SS, Gore AC. IGF-1 in the brain as a regulator of reproductive neuroendocrine function. Exp Biol Med. (2005) 2305:292–306. doi: 10.1177/153537020523000503
25. Pinkney J, Streeter A, Hosking J, Mostazir M, Jeffery A, Wilkin T. Adiposity, chronic inflammation, and the prepubertal decline of sex hormone binding globulin in children: evidence for associations with the timing of puberty (Earlybird 58). J Clin Endocrinol Metab. (2014) 999:3224–32. doi: 10.1210/jc.2013-3902
26. Biro FM, Summer SS, Huang B, Chen C, Benoit J, Pinney SM. The impact of macronutrient intake on sex steroids during onset of puberty. J Adolesc Health. (2022) 703:483–7. doi: 10.1016/j.jadohealth.2021.10.011
27. Koo MM, Rohan TE, Jain M, McLaughlin JR, Corey PN. A cohort study of dietary fibre intake and menarche. Public Health Nutr. (2002) 52:353–60. doi: 10.1079/PHN2002261
28. Gunther AL, Karaolis-Danckert N, Kroke A, Remer T, Buyken AE. Dietary protein intake throughout childhood is associated with the timing of puberty. J Nutr. (2010) 1403:565–71. doi: 10.3945/jn.109.114934
29. Rogers IS, Northstone K, Dunger DB, Cooper AR, Ness AR, Emmett PM. Diet throughout childhood and age at menarche in a contemporary cohort of British girls. Public Health Nutr. (2010) 1312:2052–63. doi: 10.1017/S1368980010001461
30. Ramezani Tehrani F, Moslehi N, Asghari G, Gholami R, Mirmiran P, Azizi F. Intake of dairy products, calcium, magnesium, and phosphorus in childhood and age at menarche in the Tehran Lipid and Glucose Study. PLoS ONE. (2013) 82:e57696. doi: 10.1371/journal.pone.0057696
31. Gaskins AJ, Pereira A, Quintiliano D, Shepherd JA, Uauy R, Corvalan C, et al. Dairy intake in relation to breast and pubertal development in Chilean girls. Am J Clin Nutr. (2017) 1055:1166–75. doi: 10.3945/ajcn.116.150359
32. Chen YC, Tu YK, Huang KC, Chen PC, Chu DC, Lee YL. Pathway from central obesity to childhood asthma. Physical fitness and sedentary time are leading factors. Am J Respir Crit Care Med. (2014) 18910:1194–203. doi: 10.1164/rccm.201401-0097OC
33. Chan NPT, Sung RYT, Nelson EAS, So HK, Tse YK, Kong APS. Measurement of pubertal status with a Chinese self-report Pubertal Development Scale. Matern Child Health J. (2010) 143:466–73. doi: 10.1007/s10995-009-0481-2
34. Taylor SJ, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Cook DG. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr Perinat Epidemiol. (2001) 151:88–94. doi: 10.1046/j.1365-3016.2001.00317.x
35. Wang Y. Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics. (2002) 1105:903–10. doi: 10.1542/peds.110.5.903
36. Sun Y, Tao FB, Su PY, Mai JC, Shi HJ, Han YT, et al. National estimates of the pubertal milestones among urban and rural Chinese girls. J Adolesc Health. (2012) 513:279–84. doi: 10.1016/j.jadohealth.2011.12.019
37. Sun Y, Tao F, Su PY. National estimates of pubertal milestones among urban and rural Chinese boys. Ann Hum Biol. (2012) 396:461–7. doi: 10.3109/03014460.2012.712156
38. Chen YC, Fan HY, Yang C, Hsieh RH, Pan WH, Lee YL. Assessing causality between childhood adiposity and early puberty: a bidirectional Mendelian randomization and longitudinal study. Metabolism. (2019) 100:153961. doi: 10.1016/j.metabol.2019.153961
39. Lee M-S, Pan W-H, Liu K-L, Yu M-S. Reproducibility and validity of a Chinese food frequency questionnaire used in Taiwan. Asia Pac J Clin Nutr. (2006) 152:161–9. doi: 10.6133/apjcn.2006.15.2.161
40. Huang YC, Lee MS, Pan WH, Wahlqvist ML. Validation of a simplified food frequency questionnaire as used in the Nutrition and Health Survey in Taiwan (NAHSIT) for the elderly. Asia Pac J Clin Nutr. (2011) 201:134–40. doi: 10.6133/apjcn.2011.20.1.134
41. Shih YH, Chang HY, Wu HC, Stanaway FF, Pan WH. High sugar-sweetened beverage intake frequency is associated with smoking, irregular meal intake and higher serum uric acid in Taiwanese adolescents. J Nutr Sci. (2020) 9:e7. doi: 10.1017/jns.2020.2
42. Administration. TFaD. Taiwanese food composition and nutrient database. (2017). Available online at: https://consumer.fda.gov.tw/Food/TFND.aspx?nodeID=178 (accessed November, 2022).
43. Huberman M, Langholz B. Application of the missing-indicator method in matched case-control studies with incomplete data. Am J Epidemiol. (1999) 15012:1340–5. doi: 10.1093/oxfordjournals.aje.a009966
44. Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. (1986) 421:121–30. doi: 10.2307/2531248
45. Lin KC, Chen PC, Twisk JWR, Lee HL, Chi LY. Time-varying nature of risk factors for the longitudinal development of disability in older adults with arthritis. J Epidemiol. (2010) 206:460–7. doi: 10.2188/jea.JE20090154
46. Mueller NT, Jacobs DR Jr, MacLehose RF, Demerath EW, Kelly SP, Dreyfus JG, et al. Consumption of caffeinated and artificially sweetened soft drinks is associated with risk of early menarche. Am J Clin Nutr. (2015) 1023:648–54. doi: 10.3945/ajcn.114.100958
47. Ma HM, Chen SK, Chen RM, Zhu C, Xiong F, Li T, et al. Pubertal development timing in urban Chinese boys. Int J Androl. (2011) 345(Pt 2):e435–45. doi: 10.1111/j.1365-2605.2011.01173.x
48. Fu J-F, Dong G-P, Liang L, Jiang Y-J, Chen L-Q, Dayan C. Early activation of the inhibin B/FSH axis in obese Tanner stage G1PH1 boys. Clin Endocrinol. (2006) 653:327–32. doi: 10.1111/j.1365-2265.2006.02597.x
49. Smajis S, Gajdosik M, Pfleger L, Traussnigg S, Kienbacher C, Halilbasic E, et al. Metabolic effects of a prolonged, very-high-dose dietary fructose challenge in healthy subjects. Am J Clin Nutr. (2020) 111:369–77. doi: 10.1093/ajcn/nqz271
50. Ipsa E, Cruzat VF, Kagize JN, Yovich JL, Keane KN. Growth hormone and insulin-like growth factor action in reproductive tissues. Front Endocrinol. (2019) 10:777. doi: 10.3389/fendo.2019.00777
51. Cowan CSM, Richardson R. Early-life stress leads to sex-dependent changes in pubertal timing in rats that are reversed by a probiotic formulation. Dev Psychobiol. (2019) 615:679–87. doi: 10.1002/dev.21765
52. Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. (2013) 339:1084-88. doi: 10.1126/science.1233521
53. Qi Y, Li P, Zhang Y, Cui L, Guo Z, Xie G, et al. Urinary metabolite markers of precocious puberty. MolCell Proteomics. (2011) 11:M111.011072. doi: 10.1074/mcp.M111.011072
54. Dinan TG, Cryan JF. Brain-gut-microbiota axis and mental health. Psychosom Med. (2017) 798:920–6. doi: 10.1097/PSY.0000000000000519
56. Wiley AS. Milk intake and total dairy consumption: associations with early menarche in NHANES 1999-2004. PLoS ONE. (2011) 62:e14685. doi: 10.1371/journal.pone.0014685
57. Wiley AS. Cow milk consumption, insulin-like growth factor-I, and human biology: a life history approach. Am J Hum Biol. (2012) 242:130–8. doi: 10.1002/ajhb.22201
58. Carwile JL, Willett WC, Wang M, Rich-Edwards J, Frazier AL, Michels KB. Milk consumption after age 9 years does not predict age at menarche. J Nutr. (2015) 1458:1900–8. doi: 10.3945/jn.115.214270
59. Rother KI, Conway EM, Sylvetsky AC. How non-nutritive sweeteners influence hormones and health. Trends Endocrinol Metab. (2018) 297:455–67. doi: 10.1016/j.tem.2018.04.010
60. Archibald AJ, Dolinsky VW, Azad MB. Early-life exposure to non-nutritive sweeteners and the developmental origins of childhood obesity: global evidence from human and rodent studies. Nutrients. (2018) 10:194. doi: 10.3390/nu10020194
Keywords: early puberty, sugar-sweetened beverages, probiotics, added sugar, menarche, voice breaking, category of study: a population study
Citation: Tsai M-C, Lee YL and Chen YC (2022) Association of the consumption of common drinks with early puberty in both sexes. Front. Public Health 10:854477. doi: 10.3389/fpubh.2022.854477
Received: 14 January 2022; Accepted: 15 November 2022;
Published: 02 December 2022.
Edited by:
Qihong Deng, Zhengzhou University, ChinaReviewed by:
Tung-Sung Tseng, Louisiana State University, United StatesYi Chun Chen, Taipei Medical University, Taiwan
Luana Lara Rocha, Federal University of Minas Gerais, Brazil
Copyright © 2022 Tsai, Lee and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Ching Chen, bWVsaXNhMjZAdG11LmVkdS50dw==; Yungling Leo Lee, bGVvbGVlQGlibXMuc2luaWNhLmVkdS50dw==
†These authors have contributed equally to this work
 Meng-Che Tsai
Meng-Che Tsai Yungling Leo Lee2,3,4*†
Yungling Leo Lee2,3,4*† Yang Ching Chen
Yang Ching Chen