- 1School of Nursing and Midwifery, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
- 2School of Public Health, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
Background: Maternal Group B Streptococcus (GBS) recto-vaginal colonization is the most common route for early onset neonatal GBS diseases. A good understanding of the rate of maternal GBS colonization, vertical transmission rate, and antibiotic susceptibility profiles is needed to formulate a broad protection mechanism, like vaccine preparation. For that reason, this meta-analysis aimed at determining the pooled prevalence of GBS recto-vaginal colonization, vertical transmission rate, and antibiotic susceptibility profiles in Ethiopia.
Methods: Both published and unpublished studies were searched from MEDLINE/PubMed, CINAHL (EBSCO), Embase, Cochrane Library, SCOPUS, Web of Sciences databases, and Google Scholar. Independent selection was then carried out by the authors based on the eligibility criteria and data extraction using Microsoft excel. The authors then used STATA version 14.1 software for further cleaning and analysis. The review was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses) PRISMA guidelines. Using the random-effect model, the prevalence with a 95% confidence interval (CI) and forest plot were used to present the findings. Besides, the studies' heterogeneity was assessed using Cochrane chi-square (I2) statistics, while Egger intercept was used to assess publication bias.
Results: This review included nineteen studies. The pooled prevalence of recto-vaginal colonization was 15% (95% CI: 11, 19), while the prevalence of vertical transmission was 51% (95% CI: 45, 58) and highest-level susceptibility to vancomycin was 99% (95% CI: 98, 100). However, the GBS susceptibility to tetracycline was 23% (95% CI: 9, 36).
Conclusions: Nearly one out of seven pregnant women in Ethiopia had recto-vaginal colonization of GBS. As a result, half of the pregnancies end with vertical transmission of GBS. Hence, the review emphasizes that policy and programs should consider planning and implementing prophylactic programs.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021287540.
Introduction
Group B streptococcus (GBS) is a member of gram-positive streptococci that causes an invasive fetal infection in newborn (1). It can be early-onset disease (EOD) and late-onset disease (LOD) if it occurs in the first week of delivery and from week 1–3 months after delivery, respectively (2–4). About 15–40% of healthy women's colon and vagina have GBS. Evidence pointed out that urinary tract infection (UTI), endometritis, chorioamnionitis, sepsis, and meningitis are associated with maternal colonization of GBS (2, 4–9). Moreover, pregnant women may encounter premature rupture of membranes (PROM), stillbirths, and low birth weight (LBW) babies due to GBS vertical transmission (10–12).
Vertical transmission of GBS occurs during pregnancy or the birth process from the genitourinary or gastrointestinal tract of colonized pregnant women (13). Mortality risk among newborns with GBS colonization is 6.6-fold higher (14). There were 48 infants with early-onset group B streptococcus (EOGBS), which is 1.4 per 1,000 neonates in the general population and 7.8 per 1,000 in women with GBS (15). Neonates with EOGBS disease are more likely to have respiratory distress disease and convulsions and require longer hospital stays (16). Case fatality is highest in Early onset of Neonatal Death when it occurs within 24 h of birth (12). Moreover, survivors suffer from uncontrolled seizures, impaired psychomotor development, profound intellectual disability, blindness, and deafness (17).
Globally, the prevalence of vaginal colonization by GBS among pregnant women varies across countries (18). For instance, its magnitudes ranged from 9.1 to 26.7% in Iran (8), and 8 to 15% across Europe (9). The prevalence of GBS in African countries, such as Egypt, Malawi, Nigeria, and Tanzania, ranges from 11.3 to 23% (19–22). Several studies across regions in Ethiopia revealed a varied proportion of recto-vaginal colonization of GBS among pregnant women (i.e., 7.2 to 20.9%) (18, 23–25). Worldwide, vertical transmission of GBS shows considerable variation within and between geographic regions, ranging from 11.2 to 57.7% (26, 27).
A cross-sectional study conducted by Woubshet et al. pointed out that the majority of the isolates were susceptible to ampicillin (95.5%) and penicillin G (90.9%). However, half of the isolates were resistant to erythromycin and clindamycin. Despite the fact that some GBS isolates are resistant to gentamicin, it is used in conjunction with penicillin to treat severe GBS-associated infections (28). Similarly, studies conducted in Nigeria (35.3%) (29) and Palestine (43%) agree with these findings (30). Countries that introduced prevention strategies, including universal routine antenatal GBS screening, identification of risk factors, and offering antibiotic prophylaxis at delivery, were able to significantly reduce the disease (22, 31). However, most of the sub-Saharan African countries do not have clear guidelines for prevention, despite the high rate of vertical transmission (27, 32, 33).
Despite few studies having been conducted on recto-vaginal colonization of GBS, its vertical transmission, and antimicrobial susceptibility among pregnant women in Ethiopia, there is, however, no national-level data or cumulative evidence to show the exact extent of the wider impact. The prevalence of maternal colonization with GBS and antibiotic susceptibility profiles in the Ethiopian context has been investigated on a small scale and in a fragmented fashion. Hence, it is imperative to conduct a comprehensive study that can aggregate the previous studies and make the findings available to policy- and decision-makers. Therefore, this systematic review and meta-analysis are conducted to generate national and reliably pooled-evidence on recto-vaginal colonization of GBS, its vertical transmission, and antimicrobial susceptibility among pregnant women in Ethiopia.
Methods
Protocol and Registration
This review was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (34). The review was registered by the International prospective register of systematic reviews with identification CRD42021287540.
Information Sources and Search Strategy
Both published and unpublished studies were searched from MEDLINE/PubMed, CINAHL (EBSCO), Embase, Cochrane Library, SCOPUS, Web of Sciences databases, and Google Scholar by using a combination of Boolean logic operators (AND, OR, NOT), Medical Subject Headings (MeSH) as well as keywords. An additional search was also conducted using Google scholar and Mednar. The search strategy includes: “Recto-vaginal colonization” [Title/Abstract] or “Vertical transmissions'“[Title/Abstract] or “Antimicrobial susceptibility””[Title/Abstract] ‘AND “Ethiopia” [MeSH Terms]), (“Recto-vaginal colonization, vertical transmission and antimicrobial susceptibility” [exactly on title] AND “Ethiopia” [any field]), (“Recto-vaginal colonization, vertical transmission and antimicrobial susceptibility ”[all field] AND “Ethiopia” [all filed]) (Supplementary File 1). In addition, the investigators searched manually for gray literature and other relevant data sources such as unpublished thesis and articles with planned dates of coverage.
Eligibility Criteria
All observational studies conducted in Ethiopia, which reported the prevalence of recto-vaginal colonization, and/or vertical transmission, and/or antimicrobial susceptibility of GBS among pregnant women, were eligible. The eligibility criteria also included English language articles and facility-based studies conducted from 12 December 2012 to October 2021. Articles, theses, and articles fulfilling the aforementioned inclusion criteria were considered for the systematic review and meta-analysis.
Study Selection
All the searched and found articles were exported to the EndNote X8 citation manager, and duplicates were deleted. These were then screened carefully by reading the titles and abstracts. The review authors (AE, TG, AD, and MA) independently screened the titles and abstracts of the identified articles using the eligibility criteria. Full-text articles were further evaluated for eligibility, as needed. Any disagreement among the authors was resolved via discussion and critical reflection. The overall study selection process is presented using the PRISMA statement flow diagram (35) (Figure 1).
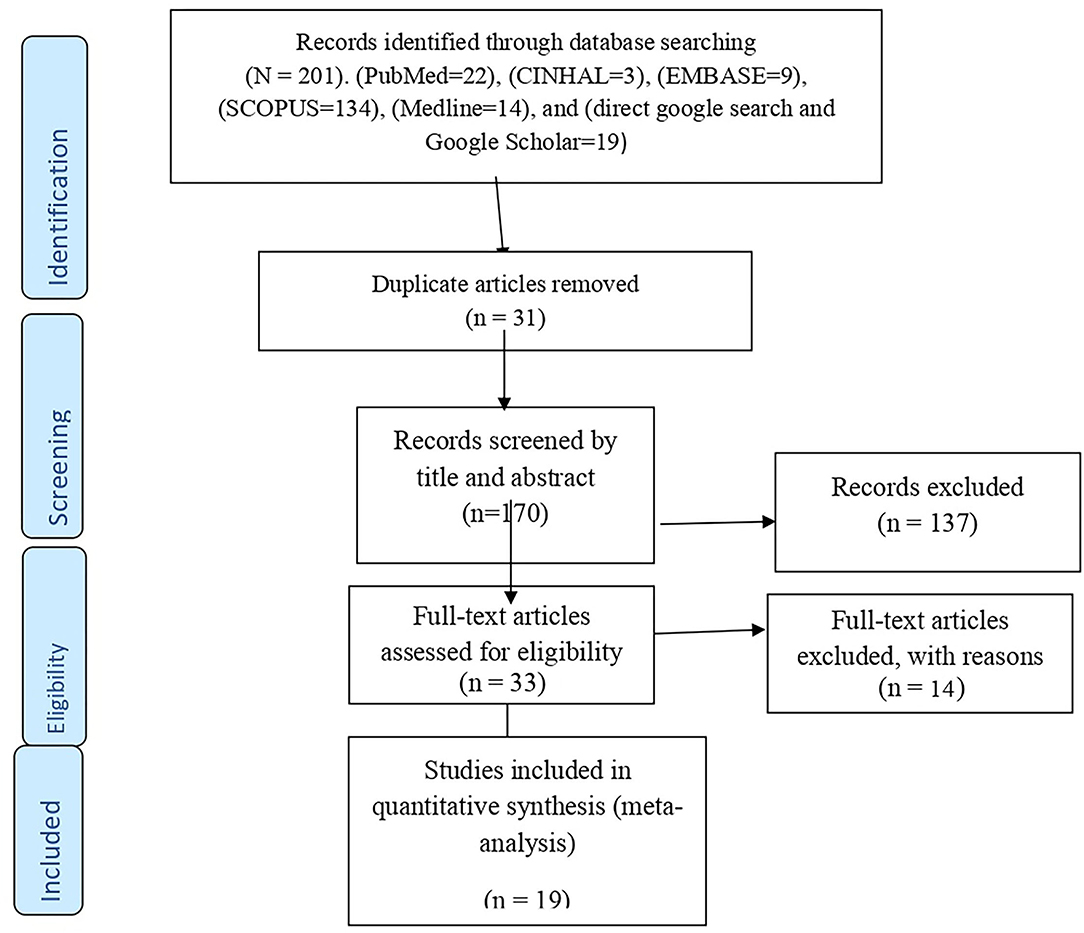
Figure 1. PRISMA flow diagram showing the selection process of eligible articles for systematic review and meta-analysis.
Data Extraction
The review authors (AE, TG, AD, and MA) independently extracted the data. A pre-defined Microsoft excel 2016 format was used to extract the data from the included articles using author(s), publication year, region of study, study setting, study design, sample size, data collection technique, and the primary outcome of interest (Table 1). The accuracy of the data extraction was verified by comparing the results of the independently extracted data.
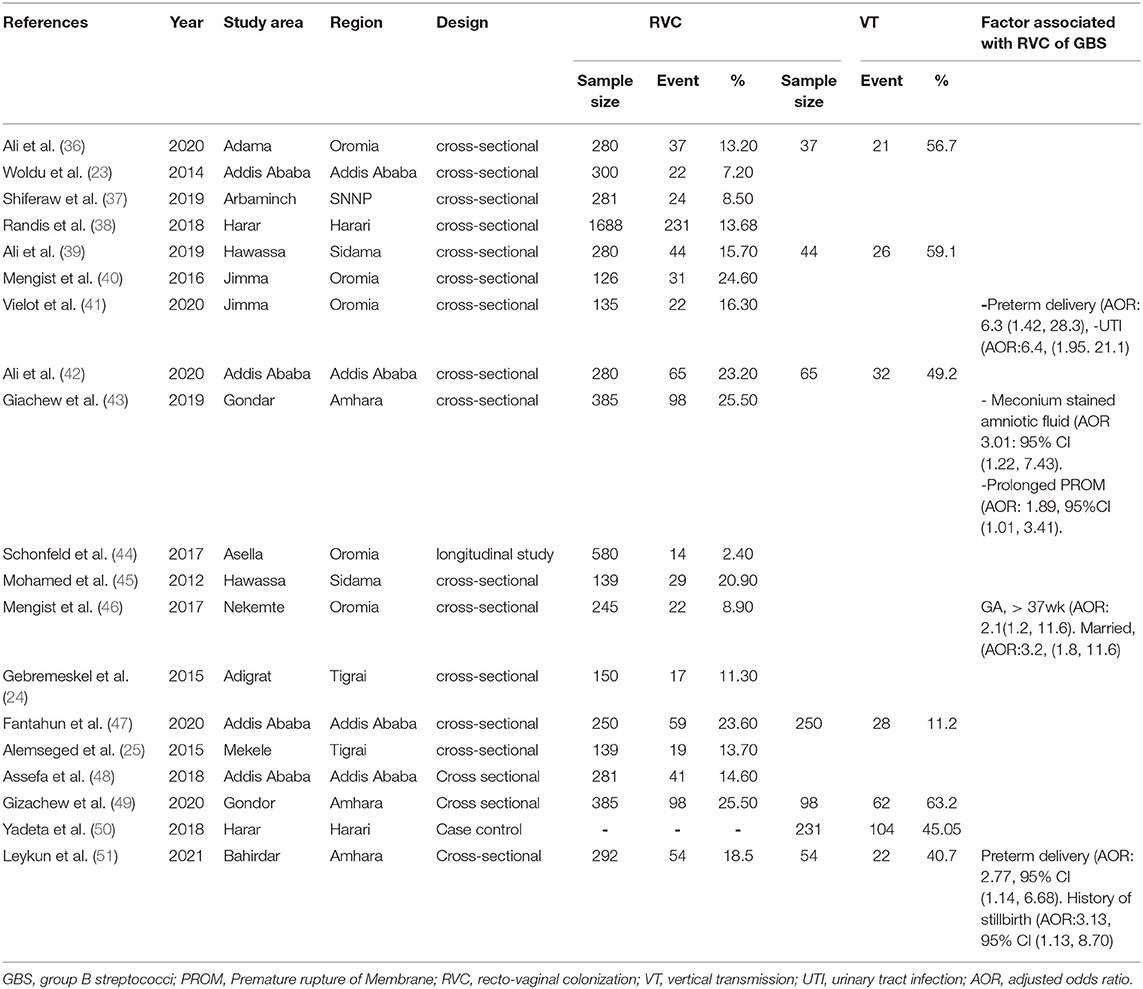
Table 1. Characteristics of studies revealing recto-vaginal colonization of Group B streptococcus Ethiopia, 2021.
Data Item
The major outcome of interest of this review was the pooled proportion of GBS colonization of pregnant women, vertical transmission of GBS, and antibiotic resistance profiles reported from different studies across Ethiopia. Sub-group analysis was done based on Ethiopia's current political-geographic regions. Measurement for recto-vaginal colonization was made using swabs collected and inoculated into 1 ml Todd Hewitt Broth supplemented with gentamicin and nalidixic acid to avoid the growth of contaminants. After 18–24 h of incubation, all broths were sub-cultured on 5% sheep blood agar for isolation of GBS. The proportion of susceptibility of GBS to the 9 different antibiotics was calculated by dividing the numbers of susceptible isolates by the total number of GBS isolated from pregnant women.
Risk of Bias
The authors critically evaluated the risk of bias from individual studies using the Joanna Briggs Institute Quality Assessment Tool for observational studies. To minimize the risk of bias, a comprehensive search (electronic/database search and manual search) for published, unpublished, institutional, or community-based studies was carried. Cooperative work among the authors was also considered seriously to reduce bias. All worked together in setting a schedule for the selection of articles based on the clear objectives and eligibility criteria, in deciding the quality of the articles, in regularly evaluating the review process, and also extracting and compiling the data.
Critical Appraisal of the Studies
The methodological reputability and quality of the findings of the included studies were critically evaluated using the quality assessment tool for observational studies (cross-sectional, case-control, and cohort studies) developed by the Joanna Briggs Institute (JBI) (52) (Supplementary File 3). The two groups of the authors, Group 1 (AD and MA) and Group 2 (AE, KS, and TG), independently evaluated the quality of the studies. The mean score of the two groups was taken for a final decision. The differences in the inclusion of the studies were resolved by consensus. The included studies were evaluated against each indicator of the tool and categorized as high-, moderate-, and low-quality. A high-quality score is above 80%, moderate-quality is 60–80%, and low-quality is below 60%. Studies with a score greater than or equal to 60% were included. This critical appraisal was conducted in order to assess the internal validity (systematic error) and external validity (generalizability) of the studies and to reduce the risk of biases.
Statistical Analysis
Data synthesis and statistical analysis were conducted using STATA version 14.1 software. The random-effect model of analysis was adopted as a method of meta-analysis because it reduced the heterogeneity of included studies. A meta-analysis of observational studies was carried out based on the recommendations of the I2 statistic described by Higgins et al. (I2 >75/100% suggesting considerable heterogeneity). The p-value of < 0.05 for I2 statistics was used to determine the presence of heterogeneity. Similarly, low, moderate, and high heterogeneity were assigned to I2 test statistics results of 25, 50, and 75%, respectively. The results were reported using the pooled prevalence with a 95% confidence interval (CI) and forest plot.
Also, sub-group analyses were conducted using publication years and regions in Ethiopia to identify the sources of heterogeneity among the studies. The investigators checked for potential publication bias using visual inspection of a funnel plot (53) and Egger's regression test. Publication bias was assumed for p > 0.10. The results of the review were reported according to the PRISMA guidelines. The findings of the included studies were first presented using a narrative synthesis and secondly by a meta-analysis chart.
Results
Search Results
A total of 201 article were identified through the electronic databases and other relevant sources. From all identified studies, 31 articles were removed due to duplication while 170 studies were reserved for further screening. Of these, 137 were excluded after being screened according to titles and abstracts. Of the 33 remaining articles, 14 studies were excluded due to studies did not present the outcome of interest and other relevant issues related to outcome variables. Finally, 19 studies that fulfilled the eligibility criteria were included for the systematic review and meta-analysis.
Study Characteristics
The review included studies from regions in Ethiopia, though the majority of the studies were from Addis Ababa and Oromia. Eighteen cross-sectional and one case-control studies (i.e., reported magnitude and total at-risk population) were included in the meta-analysis (23–25, 36, 37, 39, 40, 42–51, 54, 55). The sample size of the included studies ranged from 126 to 1,688. A total of 5,924 study samples were included, among which 927 pregnant women were with recto-vaginal colonization of GBS (Table 1).
The Pooled Effect Size of Recto-Vaginal Colonization of GBS
The pooled effect size for recto-vaginal colonization of GBS was 15% (95% CI: 11, 19) (Figure 2). Sub-group analysis showed that the highest pooled prevalence was from Addis Ababa city, i.e., 17% (95% CI: 9, 25) (Figure 3).
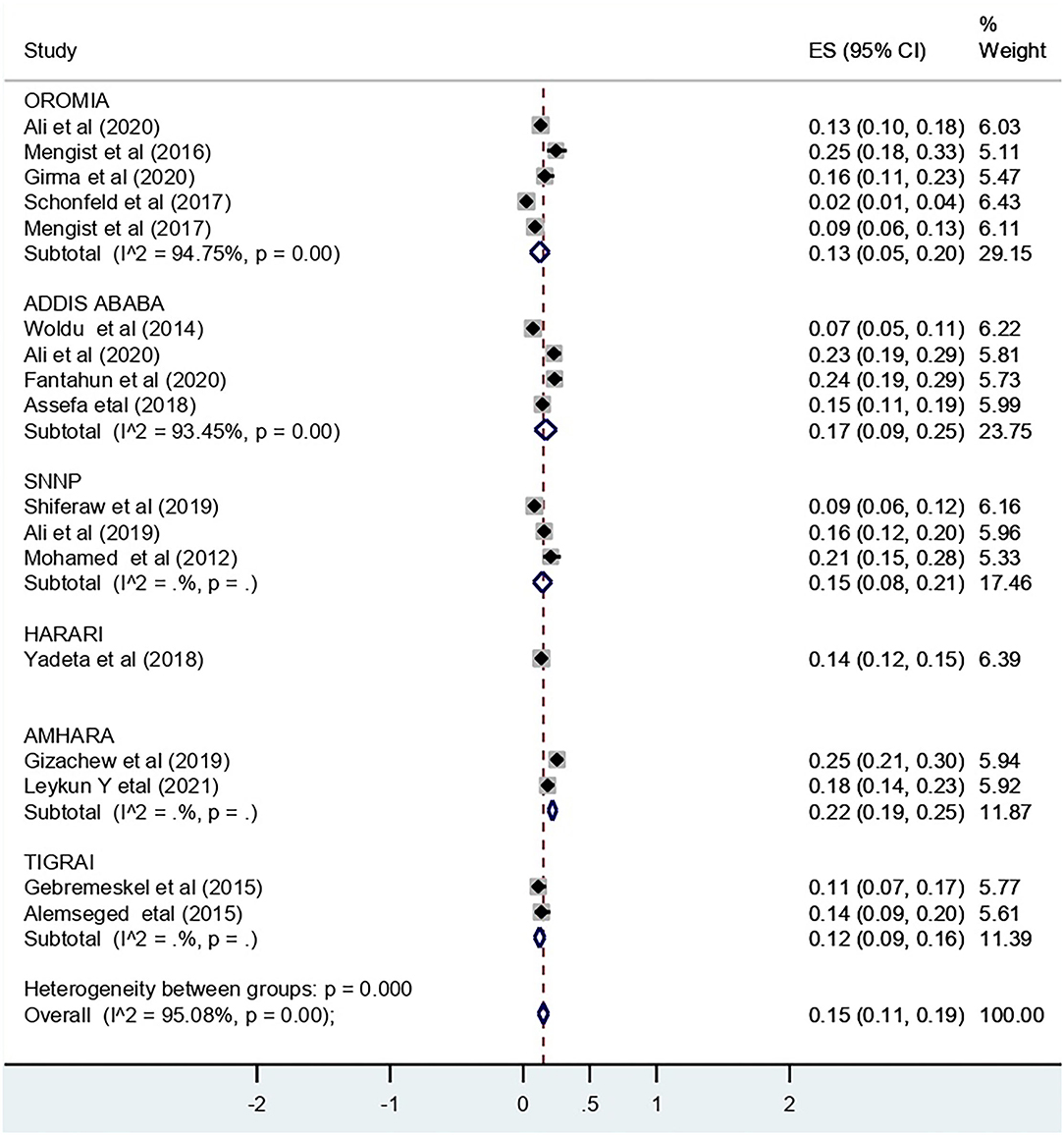
Figure 3. Subgroup analysis by region for the pooled prevalence of RVC of GBS among pregnant women in Ethiopia, 2022.
Heterogeneity of the Studies
The overall substantial heterogeneity across the studies on recto-vaginal colonization of GBS was I2 = 95.08%, p < 0.001. Sensitivity analysis was performed for the outcome variable to observe a significant change in risk ratio and CI. The meta-analysis result showed that there was no substantial difference in the overall risk ratio during the sequential removal of each study from the analysis.
Publication Bias
The Egger's regression test revealed evidence of publication bias among the included studies (p-value = 0.001). Nevertheless, a visual inspection of the funnel plot revealed symmetry (Figure 4).
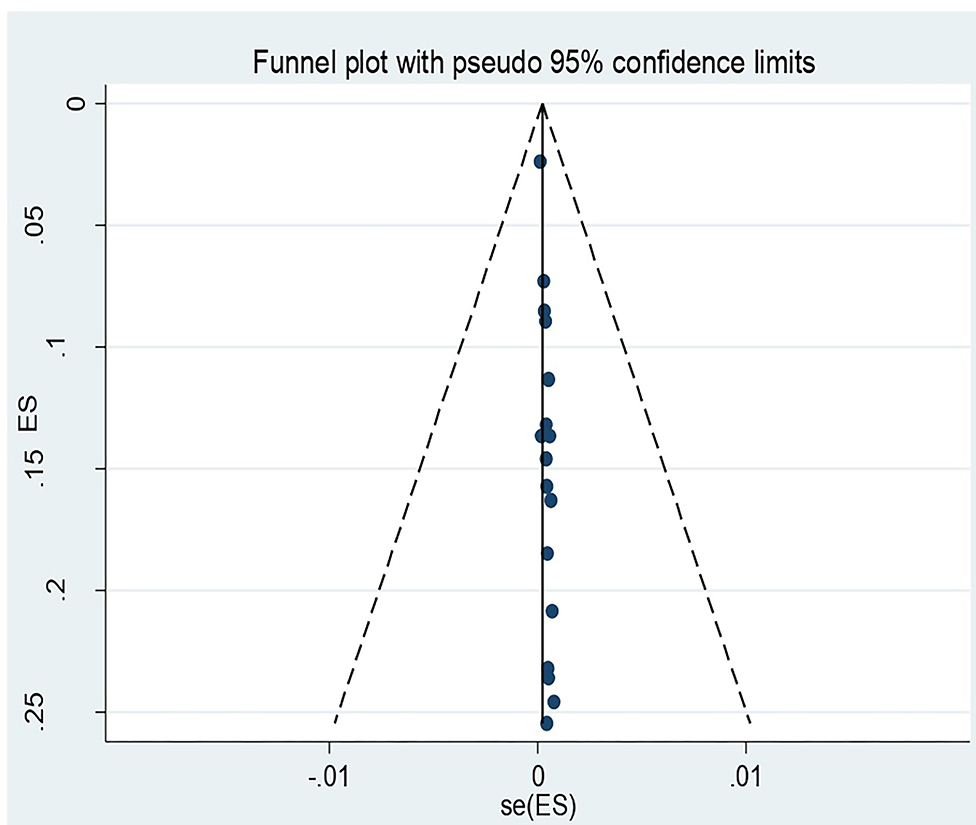
Figure 4. Funnel plot for recto vaginal colonization of Group B streptococcus among pregnant women 2022.
Meta-Regression to Check the Heterogeneity
A meta-regression analysis was conducted with assumptions of statistically significant heterogeneity of I-square test statistics <0.05. However, the meta-regression analysis found no significant variable to indicate there was any heterogeneity. What this means is that there was no statistically significant level covariation among the sample size, as well as across the publication years of the included studies. Therefore, if significantly present, heterogeneity might be explained by other factors not included in this review (Table 2).
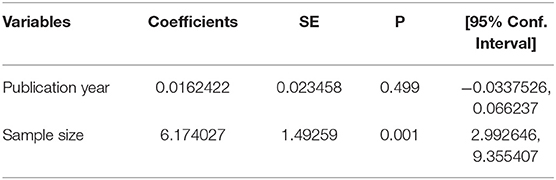
Table 2. Results of meta-regression analysis to check heterogeneity on rectovaginal colonization of GBS among pregnant women in Ethiopia, 2021.
The Pooled Effect Size of Vertical Transmission of GBS
The pooled effect size for vertical transmission of GBS was 51% (95% CI: 45, 58). The overall heterogeneity across the studies on vertical transmission of GBS from mother to child was moderate (I2 = 57.11%, p < 0.001).
Publication Bias for Vertical Transmission of GBS
The Egger's regression test revealed that there was no evidence of publication bias among the included studies (p = 0.491). In addition, a visual inspection of the funnel plot also confirmed the absence of asymmetry (Figure 5).
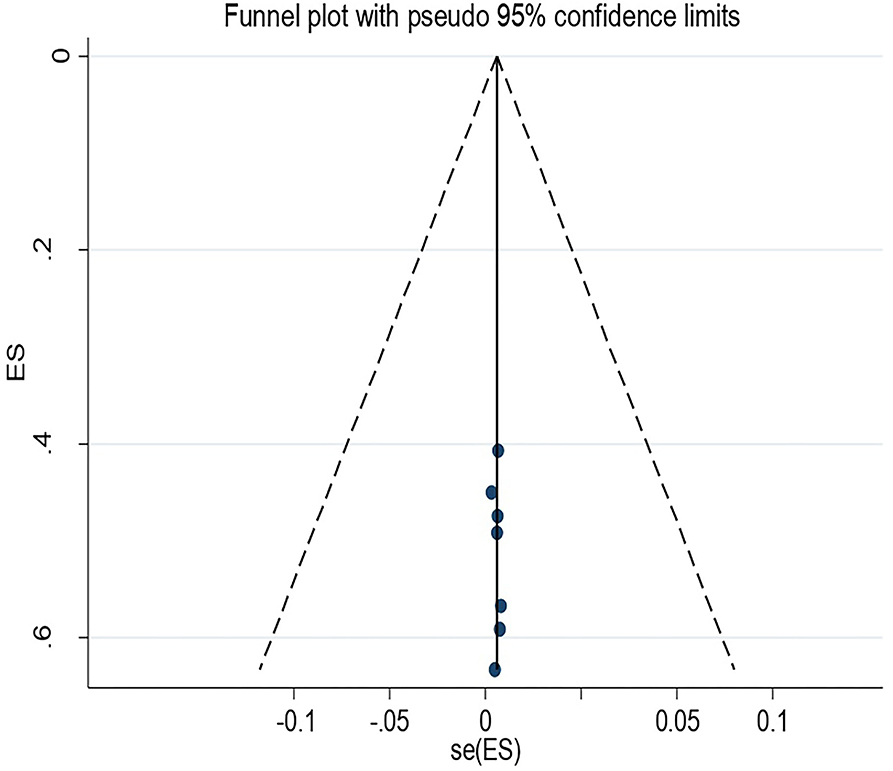
Figure 5. Funnel plot for vertical transmission of Group B streptococcus among pregnant women in Ethiopia 2022.
The Pooled Effect Size of Antibiotic Susceptibility of GBS
Among the 19 studies included in the analysis, 11 studies reported susceptibility of GBS to penicillin and erythromycin and 10 studies reported susceptibility of GBS to ampicillin and clindamycin. The highest pooled proportion of antibiotic susceptibility to GBS was observed in vancomycin, i.e., 99% (95% CI: 98, 100), followed by ampicillin, i.e., 94% (95% CI: 92, 97) (Table 2). Nonetheless, tetracycline is the lowest susceptible antibiotics, i.e., 23% (95% CI: 9, 36) (Table 3) (38).
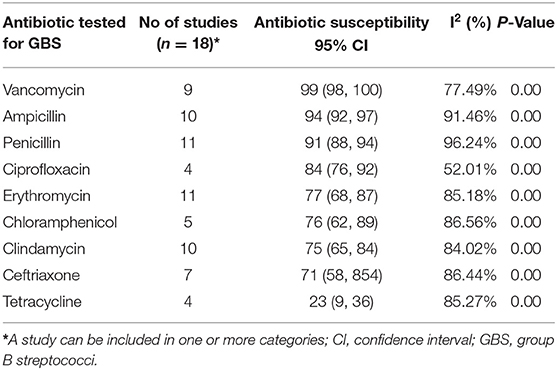
Table 3. Pooled proportion of Antibiotic susceptibility of GBS isolated from pregnant women in Ethiopia since December 2012 to November 2021.
Discussion
This systematic review and meta-analysis aimed at examining the recto-vaginal colonization of GBS, vertical transmission, and antibiotic susceptibility among pregnant women in Ethiopia. The pooled effect size for both recto-vaginal colonization of GBS and its vertical transmission to a newborn is alarming. The pooled estimate of the recto-vaginal colonization proportion of GBS was 15% (95% CI: 11, 19). Moreover, this study revealed that the pooled effect size for vertical transmission of GBS was 51% (95% CI: 45, 58).
The finding of the current meta-analysis is comparable with the meta-analysis conducted in Nicaragua, which had 14% overall estimates of maternal recto-vaginal GBS colonization proportion from 11 studies with 2,205 pregnant women (41). However, the finding from the current analysis is found to be low compared to the analysis of recto-vaginal colonization of GBS in Gabon, which was 29.8% (56). This discrepancy might be due to the variability in the number of studies involved in the meta-analysis, the variation in the number of pregnant women who participated in the two reviews, variations in the detection techniques (laboratory facilities) employed, and/or due to the biological factors among the study participants.
On the other hand, this review indicates that the pooled effect size for vertical transmission of GBS to neonates is 51%. This means that one out of two neonates, who is born from a mother with recto-vaginal colonization (RVC) of GBS, is at risk of being infected with GBS. This means that one in two pregnant women who were carriers of GBS had a chance of vertical transmission to their newborn. One of the possible explanations could be pregnant women who are infected and become carriers of GBS can transmit it to their babies easily. This might further be linked up with a delay in administering intrapartum antibiotics prophylaxis due to factors such as late presentation of the mother to the labor ward, precipitous delivery, or misidentification of risk factors, all of which can result in inadequate IAP cover (3, 33).
In a similar manner, the pooled finding of the current analysis indicated the presence of apprehension regarding the antibiotic susceptibility of GBS isolates from pregnant women. The isolates revealed susceptibility to vancomycin, ampicillin, as well as penicillin. This finding showed similarity with a meta-analysis conducted in Nicaragua, which confirmed GBS's high susceptibility to ampicillin and vancomycin (41, 57). However, in this study, the susceptibility is low for ciprofloxacillin, erythromycin, clindamycin, ceftriaxone, and tetracycline. The occurrence of the low susceptibility of GBS isolates might be due to the extensive use of antibiotics empirically for the treatment of different infectious diseases. It might also be due to the accessibility of these drugs without restriction in various areas with low cost and tradition of self-prescription.
Here, it should be noted that the expanded utilization of the beta-lactam antibiotics in the treatment of numerous infective clinical syndromes and the ease of accessibility of medication over the others also contributes to the emergence of a GBS with low susceptibility. Thus, utilization of clindamycin, erythromycin, and ceftriaxone should be directed by antimicrobial susceptibility testing. However, the absence of national guidelines on how to manage GBS colonization worsens its complication, both for pregnant women and newborns in Ethiopia. Furthermore, consistent with other studies, the non-susceptibility of GBS to tetracycline serves as a wake-up call to the need to discontinue the use of tetracycline for either treatment or prophylaxis.
Finally, in this review, a prolonged premature rupture of membrane showed association with recto-vaginal colonization of GBS. This is because GBS infection of the choriodecidua causes dysfunction of the cytokeratin network in the amniotic epithelium. Subsequently, it results in membrane weakening which leads to early rupture of the membrane (58). Thus, intrapartum antibiotic prophylaxis is essential in tackling such obstetric complications caused by GBS.
Strengths and Limitations
The investigators used extensive and comprehensive search strategies from multiple databases. Published as well as unpublished studies and gray literature were included. Studies were evaluated for methodological quality using a standardized tool. Although the literature search was systematic and assessed all related studies within the desired scope, some relevant publications, e.g., publications reported in non-English language and local languages, may have been missed. Studies with abstracts were the only ones included. This could affect the finding's inclusiveness.
Conclusion
In conclusion, nearly one out of seven pregnant women in Ethiopia had recto-vaginal colonization of GBS. As a result, half of the general pregnancy in Ethiopia is complicated by the vertical transmission of GBS. Vancomycin is a drug of choice for GBS in Ethiopia. Hence, the review highlights that the policies and programs should find mechanisms to address the reduction of GBS and vertical transmission. It is also recommended that revising first-line antibiotic treatment of GBS is vital to tackle its complications. In addition, it is better to consider the screening of pregnant women for GBS colonization.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
HB, TG, AD, KS, and BB conceived and designed the review. HB, TG, MK, DT, and GT carried out the draft of the manuscript. HB is the PI of the review. HB, TG, AD, and KS developed the search strings. AE, TG, KS, DT, and BB screened and selected studies. HB, AE, MK, GT, SM, BE, and AD extracted the data and evaluated the quality of the studies. AE, KS, SH, AD, LR, DT, MK, YD, and AA carried out the analysis and interpretation. HB, TG, AE, DT, BB, AD, GT, MK, YD, AA, and KS rigorously reviewed the manuscript. All authors read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the College of Health and Medical Sciences, Haramaya University for the support to perform these meta-analyses.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.851434/full#supplementary-material
Abbreviations
MeSH, Medical Subject Headings; OR, Odds Ratio; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analysis; GBS, Group B streptococcus; WHO, World Health Organization.
References
1. Bekker V, Bijlsma MW, van de Beek D, Kuijpers TW, van der Ende A. Incidence of invasive group B streptococcal disease and pathogen genotype distribution in newborn babies in the Netherlands over 25 years: a nationwide surveillance study. Lancet Infect Dis. (2014) 14:1083–9. doi: 10.1016/S1473-3099(14)70919-3
2. Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. (2002) 51:1–22.
3. Nandyal RR. Update on group B streptococcal infections: perinatal and neonatal periods. J Perinat Neonatal Nurs. (2008) 22:230–7. doi: 10.1097/01.JPN.0000333925.30328.fd
4. Schuchat A, Whitney CG, Zangwill K. Prevention of Perinatal group B streptococcal disease: a public health perspective. MMWR Recomm Rep. (1996) 45:1–24.
5. Joachim A, Matee MI, Massawe FA, Lyamuya EF. Maternal and neonatal colonisation of group B streptococcus at Muhimbili National Hospital in Dar es Salaam, Tanzania: prevalence, risk factors and antimicrobial resistance. BMC Public Health. (2009) 9:437. doi: 10.1186/1471-2458-9-437
6. Phares CR, Lynfield R, Farley MM, Mohle-Boetani J, Harrison LH, Petit S, et al. Epidemiology of invasive group B streptococcal disease in the United States, 1999-2005. JAMA. (2008) 299:2056–65. doi: 10.1001/jama.299.17.2056
7. Fatemi F, Chamani L, Pakzad P, Zeraati H, Rabbani H, Asgari S. Colonization Rate of Group B Streptococcus (GBS) in Pregnant Women Using GBS Agar Medium. Acta Medica Iranica (2009). p. 25–30. Available online at: https://acta.tums.ac.ir/index.php/acta/article/view/3538 (accessed December 21, 2021).
8. Berardi A, Rossi C, Guidotti I, Vellani G, Lugli L, Bacchi Reggiani ML, et al. Factors associated with intrapartum transmission of group B Streptococcus. Pediatr Infect Dis J. (2014) 33:1211–5. doi: 10.1097/INF.0000000000000439
9. Shah D, Saxena S, Randhawa VS, Nangia S, Dutta R. Prospective analysis of risk factors associated with group B streptococcal colonisation in neonates born at a tertiary care centre in India. Paediatr Int Child Health. (2014) 34:184–8. doi: 10.1179/2046905513Y.0000000112
10. Regan JA, Klebanoff MA, Nugent RP, Eschenbach DA, Blackwelder WC, Lou Y, et al. Colonization with group B streptococci in pregnancy and adverse outcome. VIP Study Group Am J Obstet Gynecol. (1996) 174:1354–60. doi: 10.1016/S0002-9378(96)70684-1
11. Regan JA, Klebanoff MA, Nugent RP. The epidemiology of group B streptococcal colonization in pregnancy. Vaginal Infections and Prematurity Study Group. Obstet Gynecol. (1991) 77:604–10.
12. Seale AC, Koech AC, Sheppard AE, Barsosio HC, Langat J, Anyango E, et al. Maternal colonization with Streptococcus agalactiae and associated stillbirth and neonatal disease in coastal Kenya. Nat Microbiol. (2016) 1:16067. doi: 10.1038/nmicrobiol.2016.67
13. Scasso S, Laufer J, Rodriguez G, Alonso JG, Sosa CG. Vaginal group B streptococcus status during intrapartum antibiotic prophylaxis. Int J Gynaecol Obstet. (2015) 129:9–12. doi: 10.1016/j.ijgo.2014.10.018
14. Islam MS, Saha SK, Islam M, Modak JK, Shah R, Talukder RR, et al. Prevalence, Serotype Distribution and Mortality Risk Associated With Group B Streptococcus Colonization of Newborns in Rural Bangladesh. Pediatr Infect Dis J. (2016) 35:1309–12. doi: 10.1097/INF.0000000000001306
15. Petersen KB, Johansen HK, Rosthøj S, Krebs L, Pinborg A, Hedegaard M. Increasing prevalence of group B streptococcal infection among pregnant women. Dan Med J. (2014) 61:A4908.
16. Al-Kadri HM, Bamuhair SS, Johani SM, Al-Buriki NA, Tamim HM. Maternal and neonatal risk factors for early-onset group B streptococcal disease: a case control study. Int J Womens Health. (2013) 5:729–35. doi: 10.2147/IJWH.S52206
17. Dangor Z, Lala SG, Cutland CL, Koen A, Jose L, Nakwa F, et al. Burden of invasive group B Streptococcus disease and early neurological sequelae in South African infants. PLoS ONE. (2015) 10:e0123014. doi: 10.1371/journal.pone.0123014
18. Schmidt J, Halle E, Halle H, Mohammed T, Gunther E. Colonization of pregnant women and their newborn infants with group B streptococci in the Gondar College of Medical Sciences. Ethiop Med J. (1989) 27:115–9.
19. Berardi A, Rossi C, Creti R, China M, Gherardi G, Venturelli C, et al. Group B streptococcal colonization in 160 mother-baby pairs: a prospective cohort study. J Pediatr. (2013) 163:1099–104.e1. doi: 10.1016/j.jpeds.2013.05.064
20. Fairlie T, Zell ER, Schrag S. Effectiveness of intrapartum antibiotic prophylaxis for prevention of early-onset group B streptococcal disease. Obstet Gynecol. (2013) 121:570–7. doi: 10.1097/AOG.0b013e318280d4f6
21. Kojima K, Tanaka R, Nakajima K, Kurihara N, Oba MS, Yamashita Y, et al. Predicting outcomes of neonates born to GBS-positive women who received inadequate intrapartum antimicrobial prophylaxis. Turk J Pediatr. (2014) 56:238–42.
22. Homer CS, Scarf V, Catling C, Davis D. Culture-based versus risk-based screening for the prevention of group B streptococcal disease in newborns: a review of national guidelines. Women Birth. (2014) 27:46–51. doi: 10.1016/j.wombi.2013.09.006
23. Woldu ZL, Teklehaimanot TG, Waji ST, Gebremariam MY. The prevalence of Group B Streptococus recto-vaginal colonization and antimicrobial susceptibility pattern in pregnant mothers at two hospitals of Addis Ababa, Ethiopia. Reprod Health. (2014) 11:80. doi: 10.1186/1742-4755-11-80
24. Gebremeskel TK, Zeleke TA, Mihret A, Tikue MD. Prevalence and antibiotic susceptibility pattern of Streptococcus agalactiae among pregnant women at Adigrat Zonal Hospital and Adigrat Health Center, Tigray, Ethiopia. J Gynecol Obstet. (2015) 3:29–35. doi: 10.11648/j.jgo.20150302.13
25. Alemseged G, Niguse S, Hailekiros H, Abdulkadir M, Saravanan M, Asmelash T. Isolation and anti-microbial susceptibility pattern of group B Streptococcus among pregnant women attending antenatal clinics in Ayder Referral Hospital and Mekelle Health Center, Mekelle, Northern Ethiopia. BMC Res Notes. (2015) 8:518. doi: 10.1186/s13104-015-1475-3
26. Kunze M, Ziegler A, Fluegge K, Hentschel R, Proempeler H, Berner R. Colonization, serotypes and transmission rates of group B streptococci in pregnant women and their infants born at a single University Center in Germany. J Perinat Med. (2011) 39:417–22. doi: 10.1515/jpm.2011.037
27. Le Doare K, Jarju S, Darboe S, Warburton F, Gorringe A, Heath PT, et al. Risk factors for Group B Streptococcus colonisation and disease in Gambian women and their infants. J Infect. (2016) 72:283–94. doi: 10.1016/j.jinf.2015.12.014
28. Nabil A, Esleem SE, Elmanama AA. Prevalence of group B streptococcus colonization among pregnant women in Gaza strip, Palestine. IUG J Nat Stud. (2017) 25. Avaiable online at: https://journals.iugaza.edu.ps/index.php/IUGNS/article/view/2473 (accessed December 02, 2021).
29. Onipede A, Adefusi O, Adeyemi A, Adejuyigbe E, Oyelese A, Ogunniyi T. Group B Streptococcus carriage during late pregnancy in Ile-Ife, Nigeria. Afr J Clin Exp Microbiol. (2012) 13:135–43. doi: 10.4314/ajcem.v13i3.2
30. Pearlman M. Prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. (2003) 102:414–5; author reply 5. doi: 10.1016/S0029-7844(03)00583-0
31. Darlow B, Campbell N, Austin N, Chin A, Grigg C, Skidmore C, et al. The prevention of early-onset neonatal group B streptococcus infection: New Zealand Consensus Guidelines 2014. N Z Med J. (2015) 128:69–76. doi: 10.1111/ajo.12378
32. Madzivhandila M, Adrian PV, Cutland CL, Kuwanda L, Schrag SJ, Madhi SA. Serotype distribution and invasive potential of group B streptococcus isolates causing disease in infants and colonizing maternal-newborn dyads. PLoS ONE. (2011) 6:e17861. doi: 10.1371/journal.pone.0017861
33. Nishihara Y, Dangor Z, French N, Madhi S, Heyderman R. Challenges in reducing group B Streptococcus disease in African settings. Arch Dis Child. (2017) 102:72–7. doi: 10.1136/archdischild-2016-311419
34. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
35. Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, et al. Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD Statement. JAMA. (2015) 313:1657–65. doi: 10.1001/jama.2015.3656
36. Ali MM, Asrat D, Fenta DA, Chaka TE, Woldeamanuel Y. Group B Streptococcus colonization rate and serotype distribution among pregnant women and their newborns at Adama Hospital Medical College, Ethiopia. Sci Rep. (2020) 10:9301. doi: 10.1038/s41598-020-66474-z
37. Shiferawu S, Mekonen M, Baza D, Lera T. Prevalence of Group B Streptococcus, Its associated factors and antimicrobial susceptibility pattern among pregnant women attending antenatal care at Arbaminch Hospital, South Ethiopia. Am J Health Res. (2019) 7:104–15. doi: 10.11648/j.ajhr.20190706.12
38. Randis TM, Gelber SE, Hooven TA, Abellar RG, Akabas LH, Lewis EL, et al. Group B Streptococcus β-hemolysin/cytolysin breaches maternal-fetal barriers to cause preterm birth and intrauterine fetal demise in vivo. J Infect Dis. (2014) 210:265–73. doi: 10.1093/infdis/jiu067
39. Ali MM, Woldeamanuel Y, Woldetsadik DA, Chaka TE, Fenta DA, Dinberu MT, et al. Prevalence of group B streptococcus among pregnant women and newborns at Hawassa University comprehensive specialized hospital, Hawassa, Ethiopia. BMC Infect Dis. (2019) 19:325. doi: 10.1186/s12879-019-3859-9
40. Mengist A, Kannan H, Abdissa A. Prevalence and antimicrobial susceptibility pattern of anorectal and vaginal group B Streptococci isolates among pregnant women in Jimma, Ethiopia. BMC Res Notes. (2016) 9:351. doi: 10.1186/s13104-016-2158-4
41. Vielot NA, Toval-Ruíz CE, Weber RP, Becker-Dreps S, Alemán Rivera TJ. Rectovaginal group B streptococcus colonization among pregnant women in Nicaragua: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. (2021) 34:2418–26. doi: 10.1080/14767058.2019.1667324
42. Ali MM, Mulate YW, Woldetsadik DA, Fenta DA, Chaka TE, Denberu MT, et al. Group B streptococci carriage rate and serotype distribution among mother newborn dyads attending Tikur Anbesa Specialized Hospital, Ethiopia. Ethiop Med J. (2020) 58. Avaiable online at https://www.semanticscholar.org/paper/Group-B-streptococci-carriage-rate-and-serotype-Ali-Mulate/aac056348b2f584e530dde2a18b8410de4e09aaf (accessed December 21, 2021).
43. Gizachew M, Tiruneh M, Moges F, Adefris M, Tigabu Z, Tessema B. Streptococcus agalactiae from Ethiopian pregnant women; prevalence, associated factors and antimicrobial resistance: alarming for prophylaxis. Ann Clin Microbiol Antimicrob. (2019) 18:3. doi: 10.1186/s12941-019-0303-3
44. Schönfeld A, Feldt T, Tufa TB, Orth HM, Fuchs A, Mesfun MG, et al. Prevalence and impact of sexually transmitted infections in pregnant women in central Ethiopia. Int J STD AIDS. (2018) 29:251–8. doi: 10.1177/0956462417723545
45. Mohammed M, Asrat D, Woldeamanuel Y, Demissie A. Prevalence of group B Streptococcus colonization among pregnant women attending antenatal clinic of Hawassa Health Center, Hawassa, Ethiopia. Ethiop J Health Dev. (2012) 26:36–42. Available online at: https://www.ajol.info/index.php/ejhd/article/view/83826(accessed December 12, 2021).
46. Mengist HM, Zewdie O, Belew A, Dabsu R. Prevalence and drug susceptibility pattern of group B Streptococci (GBS) among pregnant women attending antenatal care (ANC) in Nekemte Referral Hospital (NRH), Nekemte, Ethiopia. BMC Res Notes. (2017) 10:388. doi: 10.1186/s13104-017-2725-3
47. Fantahun Y, Sebre S, Seman A, Kumbi S. Magnitude of maternal vaginal colonization of Group B Streptococcus and neonatal transmission in pregnant women during labor and delivery at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. Ethiop Med J. (2020) 58. Available online at: https://www.emjema.org/index.php/EMJ/article/view/1705 (accessed December 28, 2021).
48. Assefa S, Desta K, Lema T. Group B streptococci vaginal colonization and drug susceptibility pattern among pregnant women attending in selected public antenatal care centers in Addis Ababa, Ethiopia. BMC Pregnancy Childbirth. (2018) 18:135. doi: 10.1186/s12884-018-1791-4
49. Gizachew M, Tiruneh M, Moges F, Adefris M, Tigabu Z, Tessema B. Proportion of Streptococcus agalactiae vertical transmission and associated risk factors among Ethiopian mother-newborn dyads, Northwest Ethiopia. Sci Rep. (2020) 10:3477. doi: 10.1038/s41598-020-60447-y
50. Yadeta TA, Worku A, Egata G, Seyoum B, Marami D, Berhane Y. Vertical transmission of group B Streptococcus and associated factors among pregnant women: a cross-sectional study, Eastern Ethiopia. Infect Drug Resist. (2018) 11:397–404. doi: 10.2147/IDR.S150029
51. Leykun Y, Genet C, Mulu W. Group B Streptococci Vaginal-Recto Colonization, Vertical Transmission to Newborns, Antimicrobial Susceptibility Profile and Associated Factors in Selected Health Facilities of Bahir Dar City: A Cross-Sectional Study. Infect Drug Resist. (2021) 14:5457–72. doi: 10.2147/IDR.S343429
52. Porritt K, Gomersall J, Lockwood C. JBI's Systematic Reviews: Study selection and critical appraisal. Am J Nurs. (2014) 114:47–52. doi: 10.1097/01.NAJ.0000450430.97383.64
53. Shi X, Nie C, Shi S, Wang T, Yang H, Zhou Y, et al. Effect comparison between Egger's test and Begg's test in publication bias diagnosis in meta-analyses: evidence from a pilot survey. Int J Res Stud Biosci. (2017) 5:14–20. doi: 10.20431/2349-0365.0505003
54. Yadeta TA, Worku A, Egata G, Seyoum B, Marami D, Berhane Y. Maternal group B Streptococcus recto vaginal colonization increases the odds of stillbirth: evidence from Eastern Ethiopia. BMC Pregnancy Childbirth. (2018) 18:410. doi: 10.1186/s12884-018-2044-2
55. Girma W, Yimer N, Kassa T, Yesuf E. Group B Streptococcus Recto-Vaginal Colonization in Near-Term Pregnant Women, Southwest Ethiopia. Ethiop J Health Sci. (2020) 30:687–96. doi: 10.4314/ejhs.v30i5.7
56. Gizachew M, Tiruneh M, Moges F, Tessema B. Streptococcus agalactiae maternal colonization, antibiotic resistance and serotype profiles in Africa: a meta-analysis. Ann Clin Microbiol Antimicrob. (2019) 18:14. doi: 10.1186/s12941-019-0313-1
57. Panda B, Iruretagoyena I, Stiller R, Panda A. Antibiotic resistance and penicillin tolerance in ano-vaginal group B streptococci. J Matern Fetal Neonatal Med. (2009) 22:111–4. doi: 10.1080/14767050802488212
58. Vanderhoeven JP, Bierle CJ, Kapur RP, McAdams RM, Beyer RP, Bammler TK, et al. Group B streptococcal infection of the choriodecidua induces dysfunction of the cytokeratin network in amniotic epithelium: a pathway to membrane weakening. PLoS Pathog. (2014) 10:e1003920. doi: 10.1371/journal.ppat.1003920
Keywords: prevalence, recto-vaginal, pregnant women, GBS, colonization, antibiotic susceptibility
Citation: Bekele H, Debella A, Getachew T, Balis B, Tamiru D, Eyeberu A, Tiruye G, Kure MA, Habte S, Eshetu B, Regassa LD, Mesfin S, Alemu A, Dessie Y and Shiferaw K (2022) Prevalence of Group B Streptococcus Recto-Vaginal Colonization, Vertical Transmission, and Antibiotic Susceptibility Among Pregnant Women in Ethiopia: A Systematic Review and Meta-Analysis. Front. Public Health 10:851434. doi: 10.3389/fpubh.2022.851434
Received: 09 January 2022; Accepted: 21 March 2022;
Published: 16 May 2022.
Edited by:
Thandavarayan Ramamurthy, National Institute of Cholera and Esnteric Diseases (ICMR), IndiaCopyright © 2022 Bekele, Debella, Getachew, Balis, Tamiru, Eyeberu, Tiruye, Kure, Habte, Eshetu, Regassa, Mesfin, Alemu, Dessie and Shiferaw. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adera Debella, YWtzYW5hZGVyYTYyJiN4MDAwNDA7Z21haWwuY29t; orcid.org/0000-0002-8060-0027
 Habtamu Bekele
Habtamu Bekele Adera Debella
Adera Debella Tamirat Getachew
Tamirat Getachew Bikila Balis
Bikila Balis Dawit Tamiru
Dawit Tamiru Addis Eyeberu
Addis Eyeberu Getahun Tiruye1
Getahun Tiruye1 Mohammed Abdurke Kure
Mohammed Abdurke Kure Bajrond Eshetu
Bajrond Eshetu Lemma Demissie Regassa
Lemma Demissie Regassa Sinetibeb Mesfin
Sinetibeb Mesfin Yadeta Dessie
Yadeta Dessie Kasiye Shiferaw
Kasiye Shiferaw