- 1College of Medical Informatics, Chongqing Medical University, Chongqing, China
- 2Medical Data Science Academy, Chongqing Medical University, Chongqing, China
- 3Collection Development Department of Library, Chongqing Medical University, Chongqing, China
- 4Department of Cardiology, University-Town Hospital of Chongqing Medical University, Chongqing, China
- 5Operation Management Office, Affiliated Banan Hospital of Chongqing Medical University, Chongqing, China
- 6Department of Cardiology, Ministry of Education Key Laboratory of Child Development and Disorders, National Clinical Research Center for Child Health and Disorders, China International Science and Technology Cooperation Base of Child Development and Critical Disorders, Children's Hospital of Chongqing Medical University, Chongqing, China
- 7Chongqing Key Laboratory of Pediatrics, Chongqing, China
Background: The objective of this study was to use machine learning algorithms to construct predictive models for atrial fibrillation (AF) in elderly patients with coronary heart disease (CHD) and type 2 diabetes mellitus (T2DM).
Methods: The diagnosis and treatment data of elderly patients with CHD and T2DM, who were treated in four tertiary hospitals in Chongqing, China from 2015 to 2021, were collected. Five machine learning algorithms: logistic regression, logistic regression+least absolute shrinkage and selection operator, classified regression tree (CART), random forest (RF) and extreme gradient lifting (XGBoost) were used to construct the prediction models. The area under the receiver operating characteristic curve (AUC), sensitivity, specificity, and accuracy were used as the comparison measures between different models.
Results: A total of 3,858 elderly patients with CHD and T2DM were included. In the internal validation cohort, XGBoost had the highest AUC (0.743) and sensitivity (0.833), and RF had the highest specificity (0.753) and accuracy (0.735). In the external verification, RF had the highest AUC (0.726) and sensitivity (0.686), and CART had the highest specificity (0.925) and accuracy (0.841). Total bilirubin, triglycerides and uric acid were the three most important predictors of AF.
Conclusion: The risk prediction models of AF in elderly patients with CHD and T2DM based on machine learning algorithms had high diagnostic value. The prediction models constructed by RF and XGBoost were more effective. The results of this study can provide reference for the clinical prevention and treatment of AF.
Introduction
Coronary heart disease (CHD) is the most common cardiovascular disease among the elderly (1). According to the data from the World Health Organization (2014), CHD is the main cause of death globally, accounting for more than 7 million deaths every year (2). As the most common type of diabetes mellitus (DM), type 2 diabetes mellitus (T2DM) has become the leading cause of morbidity and mortality worldwide. In 2019, there were 463 million individuals affected by diabetes globally (3). By 2040, the number of patients is expected to increase to 629 million, accounting for ~90% of all cases (4). T2DM is one of the most important complications of CHD. It is an important risk factor for the development of CHD and associated with the death of patients (5).
Atrial fibrillation (AF) is the most common arrhythmia, which is characterized by atrial activation disorder, that results in the deterioration of atrial function (6, 7). The prevalence of AF increased with age in recent studies. The overall prevalence was estimated to be 5.5%, rising from 0.7% in the 55–59 age group to 17.8% in the 85 years-and-above age group (8). Owing to the aging population, the prevalence of AF is expected to double by 2050 (9). AF in hospitalized elderly patients with CHD and T2DM is a very serious cardiac adverse event. Once patients develop AF, further thromboembolism will occur, leading to a high risk of disability and death (10, 11). Therefore, early identification and the timely management of AF in elderly patients with CHD and T2DM is very important. However, early detection of AF based on traditional indicators is difficult.
Therefore, this study is aimed at developing AF prediction models for the elderly patients with CHD and T2DM by using different machine learning algorithms to provide reference for the clinical prevention and treatment of AF. In order to the interpretability of results, the machine learning algorithms included in this study are mainly composed of logistic regression (LR), logistic regression+least absolute shrinkage and selection operator (LR+LASSO), classification and regression trees (CART), random forests (RF), and extreme gradient boosting (XGBoost).
Methods
Data Source
The study data were obtained from four tertiary hospitals in Chongqing, China, namely the Second Affiliated Hospital of Chongqing Medical University, the Third Affiliated Hospital of Chongqing Medical University, the University-Town Hospital of the Chongqing Medical University, and the Yong Chuan Hospital of the Chongqing Medical University. The transparent reporting of a multivariable predictive model for individual prognosis or diagnosis guidelines were followed for model development and validation. The clinical data were collected using electronic medical record systems. Data on patients with CHD and T2DM obtained for the period of 2015 to 2021 were used in the study.
The Ethics Committee of Chongqing Medical University approved the study. Written informed consent for participation was not required for this study owing to its retrospective design, and the study was undertaken in accordance with the national legislation and institutional requirements.
Definition
The diagnosis of AF (ICD-10, I48) was based on AHA/ACC/ESC 2006 atrial fibrillation guidelines (12). Two physicians with rich clinical experience were convened to diagnose together with echocardiography, electrocardiogram, and laboratory examinations.
Inclusion and Exclusion Criteria
Inclusion criteria comprised the following: (i) data obtained from 2015 to 2021, (ii) patients aged ≥ 65 years, and (iii) hospitalization (s) for CHD and T2DM.
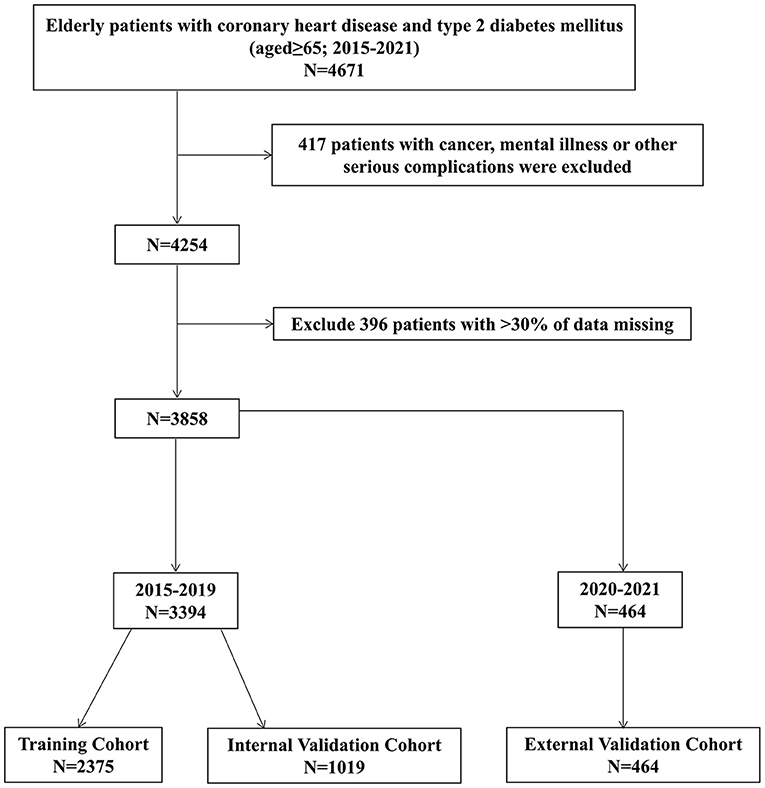
Exclusion criteria comprised the following: (i) patients with cancer, mental illness, or other serious complications and (ii) patients with >30% of missing data. The study selection process is depicted in the following flow chart (Figure 1).
Data Collection
Based on previous studies, 28 possible risk factors for predicting AF were selected, namely age, gender, smoking status, drinking status, hypertension, diastolic blood pressure (DBP), γ-glutamyltransferase (GGT), white blood cell (WBC), neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), platelet-lymphocyte (PLR), blood creatinine, total bilirubin (TB), uric acid (UA), urea Nitrogen (UN), low-density lipoprotein (LDL), hemoglobin, alanine aminotransferase (ALT), albumin (ALB), triglycerides (TGs), total cholesterol (TC), high-density lipoprotein (HDL), glycated hemoglobin (HbA1c), alkaline phosphatase (ALP), aspartate aminotransferase (AST), blood potassium, blood calcium, and blood phosphorus.
Statistical Analyses
The multiple imputation method (predictive mean matching) was used to fill in the missing continuous variables. Since the visit time period of the patients included in this study spanned from 2015 to 2021, this study can further subjected to temporal validation (13, 14). Therefore, the data included in this study were divided into two parts: 2015 to 2019 and 2020 to 2021 according to date. The data from 2015 to 2019 were divided into the training cohort and internal validation cohort based on the ratio of 7:3. The data from 2020 to 2021 were used as an external validation cohort. The training cohort was used to develop predictive models using machine learning (CART, RF, and XGBoost) and logistic regression algorithms, and then the final performance of each model was verified and compared in the internal validation cohort and external validation cohort. The area under the receiver operating characteristic curve (AUC), sensitivity, specificity, and accuracy were used as the comparison measures between different models.
Continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range), according to the distribution of normality. Categorical variables were reported as counts with percentages. Continuous variables were tested by t-test or Mann-Whitney U-test whereas categorical variables were analyzed by Chi-square test (χ2-test). All statistical analyses used two-sided tests, and P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS 22.0 and R (version 4.0.2, Vienna, Austria).
Results
Patient Characteristics
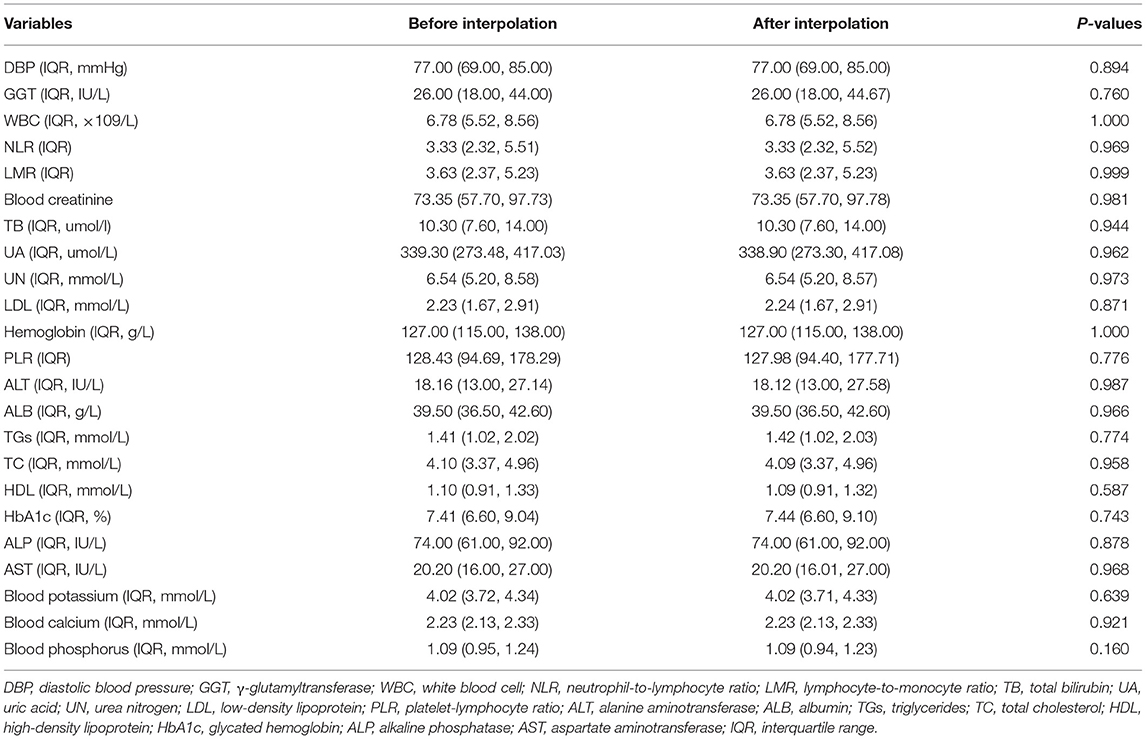
A total of 3,858 patients with CHD and T2DM were included in this study. They were divided into a training cohort (N = 2,375), an internal validation cohort (N = 1,019), and an external validation cohort (N = 464). The Mann-Whitney U-test showed that there was no significant difference before and after interpolation (Table 1).
In the training cohort, the results showed that in comparison with patients in the non-AF group, patients who suffered from AF had a higher age (P < 0.001), higher DBP (P = 0.005), GGT (P < 0.001), NLR (P < 0.001), blood creatinine (P = 0.001), higher TB (P < 0.001), UA (P < 0.001), UN (P = 0.001), and AST (P < 0.001). The levels of LMR (P < 0.001), LDL (P < 0.001), ALB (P < 0.001), TGs (P < 0.001), TC (P < 0.001), HDL (P = 0.007), and blood calcium (P < 0.001) in the AF group are lower compared with those in the non-AF group (Table 2).
Predictive Effects of Different Models
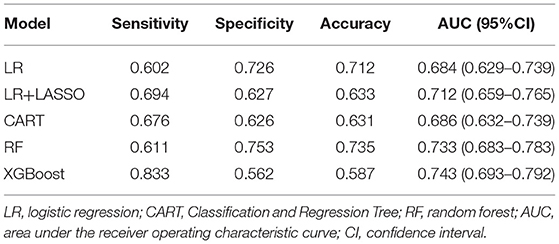
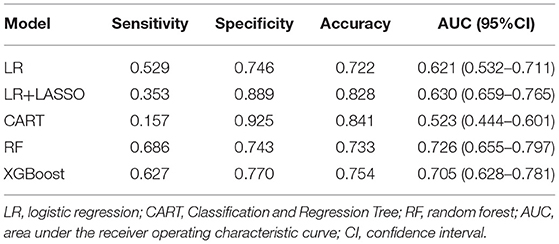
Five models were generated, including LR, LR+LASSO, CART, RF, and XGBoost, to predict the development of AF in elderly patients with CHD and T2DM after admission. Figure 2 shows the performance of the five different models in predicting AF in the internal validation cohort and external validation cohort in terms of ROC curves. In the internal validation cohort, AUC shows that the XGBoost model has the best prediction effectiveness on AF, and AUC was 0.743 compared with other models. In the external validation cohort, the highest AUC model was RF.

Figure 2. Receiver operating characteristic (ROC) curves of five different models in internal validation cohort (A) and external validation cohort (B).
Tables 3, 4, respectively, show the detailed performance indicators of the five models in the internal verification cohort and external verification cohort. In the internal validation cohort, XGBoost had the highest AUC (0.743) and sensitivity (0.833), and RF had the highest specificity (0.753) and accuracy (0.735). In the external verification, RF had the highest AUC (0.726) and sensitivity (0.686), and CART had the highest specificity (0.925) and accuracy (0.841).
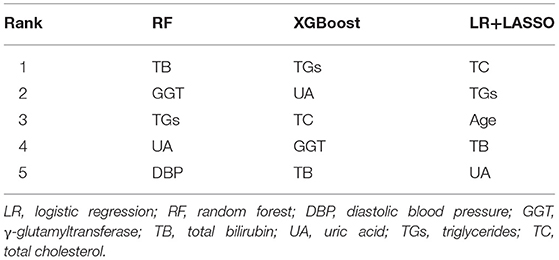
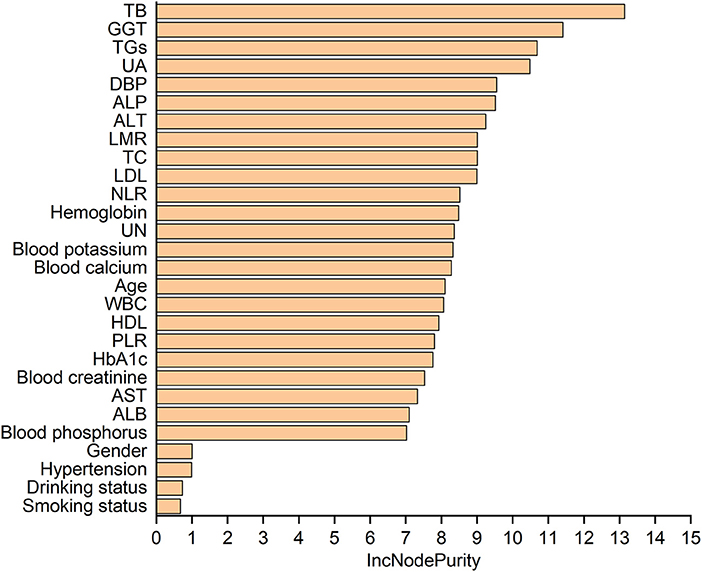
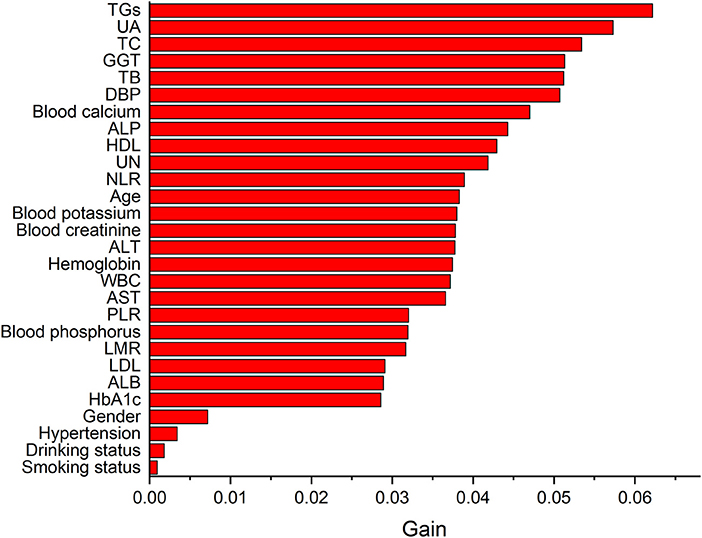
Table 5 lists the importance levels of the top five features in the top three AUC models in both the internal validation cohort and the external validation cohort. As shown in Table 5, TB, TGs and UA were the top three features that promoted the development of the prediction models for AF in coronary heart disease and type 2 diabetes mellitus patients. Figure 3 shows the feature screening process of LR+LASSO model, Figures 4, 5 shows the feature importance ranking of the RF model and the XGBoost model, respectively.
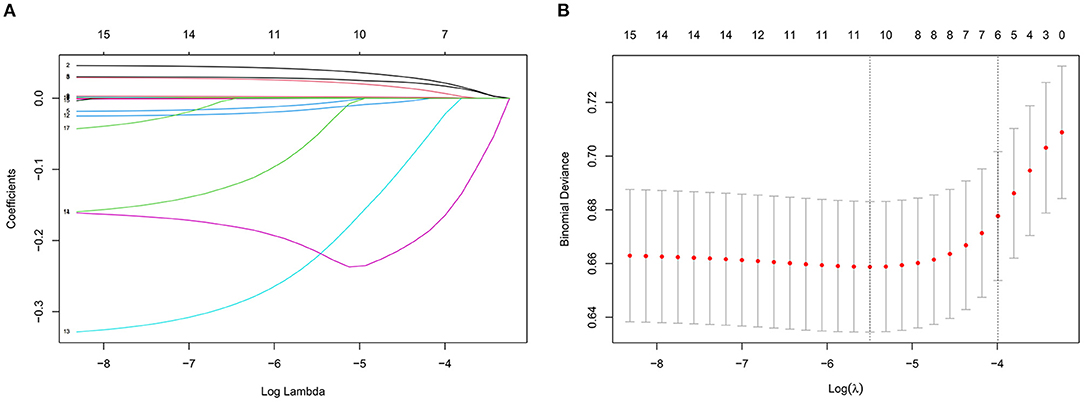
Figure 3. Features selection by LASSO. (A) LASSO coefficients profiles (y-axis) of the 16 features. The upper x-axis is the average numbers of predictors and the lower x-axis is the log (λ). (B) Five-fold cross-validation for tuning parameter selection in the LASSO model.
Discussion
In this study, A total of 3,858 patients with CHD and T2DM were included, and then 28 possible risk factors were selected for predicting AF. Five machine learning algorithms were used to construct the prediction models, and then the final performance of each model was verified and compared in the internal validation cohort and the external validation cohort. The results showed that the prediction models constructed by RF and XGBoost were more effective (delong test showed that there was no significant difference between the two models, P = 0.654). TB, TGs and UA were the three most important predictors of AF.
Machine learning algorithms could deeply mine and analyze big data, and it had been widely used in disease predictions and prognostication (15–17). RF is one of the widely used algorithms in machine learning. It uses a set model of various decision trees (18). RF rely on computers to learn all of the complex non-linear interactions between variables by minimizing the error between observation results and prediction results (19), and it uses bootstrap aggregation and randomization of predictors to obtain high disease prediction accuracy (20, 21). In addition, XGBoost is also an efficient and widely used machine learning method (22). It is an integrated algorithm belonging to a class of boosting algorithms. The core of the boosting algorithm is to integrate many weak classifiers to form a strong classifier, so as to improve the accuracy of classification. XGBoost helps to reduce overfitting compared to gradient tree boosting by only a random subset of descriptors in building a tree and is known as the “regularized boosting” technique (23).
Our study shows that high TB was a risk factor for AF in elderly patients with CHD and T2DM. Recently, studies found that TB levels are related to CVD and AF (24, 25). Bilirubin is a product of hemoglobin catabolism and has long been used as a diagnostic indicator for hepatobiliary diseases and hemolytic diseases (26). AF promotes the occurrence of inflammation and the increase of inflammatory markers, while TB, as an antioxidant stress factor, increases compensatory in order to balance oxidation and antioxidation in the body (27). Therefore, for elderly patients with CHD and T2DM, when TB level is significantly increased, there is risk of AF.
TGs are the most abundant lipids in the human body. Most tissues can use TG decomposition products to supply energy. At the same time, liver, fat, and other tissues can also synthesize TGs and store them in adipose tissue (28, 29). TGs have been proven to be a risk factor for cardiovascular disease (CVD), but its role in the development of AF in the elderly is unclear (30). The study found that low TGs were a risk factor for AF in elderly patients with CHD and T2DM. A cross-sectional survey showed that Low TGs may be a marker of CVD risk in Chinese patients with long-term T2DM (31), which is consistent with the results of our study. Of note, clinicians usually pay attention to the risk caused by elevated TGs and ignore the impact of low TGs in patients, especially in elderly patients with CHD and T2DM. The results of this study suggest that clinicians should pay attention to the TG level of elderly patients with CHD and T2DM in order to prevent atrial fibrillation in advance.
This study also showed that high UA was a risk factor for AF in elderly patients with CHD and T2DM. In recent years, several cross-sectional studies and prospective studies had shown that a high UA level is a potential risk factor for AF (32–34). Another study showed that high UA is associated with an increased prevalence of AF in hospitalized patients with T2DM, independent of multiple risk factors and potential confounders (35). Yutong Ji et al. also found that there is an association between AF and high UA in elderly patients, high UA is associated with persistent or permanent AF (36). Therefore, reducing the level of UA can reduce the risk of AF in elderly patients to a certain extent.
This study is the first to investigate the predictive model for AF in hospitalized elderly patients with coronary heart disease and type 2 diabetes mellitus. However, this study also has several limitations. Firstly, selection bias may exist due to the retrospective nature of the investigation and the imbalanced dataset. However, a multicenter and relatively large training cohort were used to build models, which was further subjected to temporal validation. Secondly, data on social support, socioeconomic status, and some other important factors were not available. Further research is warranted to explore the impact of these important indicators.
In conclusion, compared with classic logistic regression models, machine learning models (XGBoost and RF) have better performance in predicting AF in elderly patients with CHD and T2DM using features that were easily available at admission. Predictive models using machine learning algorithms can help clinicians predict AF early and implement targeted treatment measures.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The Ethics Committee of Chongqing Medical University approved the study. Written informed consent for participation was not required for this study due to its retrospective design and the study was undertaken in accordance with national legislation and institutional requirements.
Author Contributions
QX, YP, JTa, and MY: conception, methodology, and draft of the manuscript. WZ: suggestions for amendment. JTi: review and supervision. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by Chongqing Federation of Social Sciences (No. 2021NDQN65) and the Intelligent Medical Foundation of Chongqing Medical University (No. ZHYX202029).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tang M, Wang SH, Li HL, Chen H, Ma SJ. Mental health status and quality of life in elderly patients with coronary heart disease. PeerJ. (2021) 9:e10903. doi: 10.7717/peerj.10903
2. Nowbar AN, Howard JP, Finegold JA, Asaria P, Francis DP. 2014 Global geographic analysis of mortality from ischaemic heart disease by country, age and income: Statistics from World Health Organisation and United Nations. Int J Cardiol. (2014) 174:293–8. doi: 10.1016/j.ijcard.2014.04.096
3. Rong S, Birong W, Zheyun N, Hui S, Fan H. Nomogram based on risk factors for type 2 diabetes mellitus patients with coronary heart disease. Diabetes Metab Syndr Obes. (2020) 13:5025–36. doi: 10.2147/DMSO.S273880
4. Găman M-A, Cozma M-A, Dobrică E-C, Bacalba?a N, Bratu OG, Diaconu CC. Dyslipidemia: a trigger for coronary heart disease in romanian patients with diabetes. Metabolites. (2020) 10:195. doi: 10.3390/metabo10050195
5. Valgeirsson KB, Hreinsson JP, Björnsson ES. Increased incidence of autoimmune hepatitis is associated with wider use of biological drugs. Liver Int. (2019) 39:2341–9. doi: 10.1111/liv.14224
6. Matthew K, Tom B, Mark D, Derek C, John E, Brendan M, et al. A cohort study examination of established and emerging risk factors for atrial fibrillation: the Busselton Health Study. Eur J Epidemiol. (2014) 29:181–90. doi: 10.1007/s10654-013-9875-y
7. Kashou AH, Adedinsewo DA, Noseworthy PA. Subclinical atrial fibrillation: a silent threat with uncertain implications. Ann Rev Med. (2022) 73:355–62. doi: 10.1146/annurev-med-042420-105906
8. Humbert X, Roule V, Chequel M, Fedrizzi S, Brionne M, Lelong-Boulouard V, et al. Non-vitamin K oral anticoagulant treatment in elderly patients with atrial fibrillation and coronary heart disease. Int J Cardiol. (2016) 222:1079–83. doi: 10.1016/j.ijcard.2016.07.212
9. Yoko M, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000 and implications on the projections for future prevalence. Circulation. (2006) 114:119–25. doi: 10.1161/CIRCULATIONAHA.105.595140
10. Feng L, Yi W. Coronary heart disease and atrial fibrillation: a vicious cycle. Am J Physiol Heart Circ Physiol. (2020) 320:H1–12. doi: 10.1152/ajpheart.00702.2020
11. Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. (2013) 310:2050–60. doi: 10.1001/jama.2013.280521
12. Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC (2006). Guidelines for the management of patients with atrial fibrillation—executive summary. J Am Coll Cardiol. (2006) 48:854–906. doi: 10.1016/j.jacc.2006.07.009
13. Lucia C, Massimo C, Rosie C, Deborah L. External validation of the improving partial risk adjustment in surgery (PRAIS-2) model for 30-day mortality after paediatric cardiac surgery. BMJ Open. (2020) 10:e039236. doi: 10.1136/bmjopen-2020-039236
14. Tao P, JianMing X, Lin L, BingJie P, XiangKe N, XiaoHui Z, et al. Can machine learning-based analysis of multiparameter MRI and clinical parameters improve the performance of clinically significant prostate cancer diagnosis? Int J Comput Assist Radiol Surg. (2021) 16:2235–49. doi: 10.1007/s11548-021-02507-w
15. Ke W, Jing T, Chu Z, Hong Y, Jia R, Yanling L, et al. Interpretable prediction of 3-year all-cause mortality in patients with heart failure caused by coronary heart disease based on machine learning and SHAP. Comput Biol Med. (2021) 137:104813. doi: 10.1016/j.compbiomed.2021.104813
16. Chen W, Yue Z, Bingyu J, Xuedong G, Bin L, Yang X, et al. Development and validation of a predictive model for coronary artery disease using machine learning
. Front Cardiovasc Med. (2021) 8:614204. doi: 10.3389/fcvm.2021.614204
17. Hung M, Lauren E, Hon E, Xu J, Negrón BR, Rosales M, et al. Using machine learning to predict 30-day hospital readmissions in patients with atrial fibrillation undergoing catheter ablation. J Pers Med. (2020) 10:82. doi: 10.3390/jpm10030082
19. Dreiseitl S, Ohno-Machado L. Logistic regression and artificial neural network classification models: a methodology review. J Biomed Inform. (2002) 35:352–9. doi: 10.1016/S1532-0464 (03)00034-0
20. Li-Hong X, Pei-Ran C, Zhong-Ping G, Yong-Zhong L, Mei L, Liang-Cheng X, et al. Prostate cancer prediction using the random forest algorithm that takes into account transrectal ultrasound findings, age, and serum levels of prostate-specific antigen. Asian J Androl. (2017) 19:586–90. doi: 10.4103/1008-682X.186884
21. Alessia S, Antonio C, Aldo Q. Random forest algorithm for the classification of neuroimaging data in Alzheimer's disease: a systematic review. Front Aging Neurosci. (2017) 9:329. doi: 10.3389/fnagi.2017.00329
22. Zheng H, Yuan J, Chen L. Short-term load forecasting using EMD-LSTM neural networks with a xgboost algorithm for feature importance evaluation. Energies. (2017) 10:1168. doi: 10.3390/en10081168
23. Omer S, Lior R. Approximating XGBoost with an interpretable decision tree. Inform Sci. (2021) 572:522–42. doi: 10.1016/j.ins.2021.05.055
24. Zheng-Yun Z, Lu-Qin B, Sung-Joo K, Cai-Cun Z, Yoon-Ho C. Inverse relation of total serum bilirubin to coronary artery calcification score detected by multidetector computed tomography in males. Clin Cardiol. (2012) 35:301–6. doi: 10.1002/clc.21964
25. Shao-Peng L, Pei-Yi L, Hui-Lin J, You-Ming L, Xiao-Hui C. Is serum total bilirubin useful to differentiate cardioembolic stroke from other stroke subtypes? Neurol Res. (2015) 37:727–31. doi: 10.1179/1743132815Y.0000000038
26. Karl-Heinz W, Marlies W, Christine M, Silvia G, Cameron BA, Claudio T, et al. Looking to the horizon: the role of bilirubin in the development and prevention of age-related chronic diseases. Clin Sci. (2015) 129:1–25. doi: 10.1042/CS20140566
27. Troughton JA, Woodside JV, Young IS, Arveiler D, Amouyel P, Ferrières J, et al. Bilirubin and coronary heart disease risk in the prospective epidemiological study of myocardial infarction (PRIME). Eur J Cardiovasc Prevent Rehabil. (2006) 14:79–84. doi: 10.1097/01.hjr.0000230097.81202.9f
28. Wiesner P, Watson KE. Triglycerides: a reappraisal. Trends Cardiovasc Med. (2017) 27:428–32. doi: 10.1016/j.tcm.2017.03.004
29. Sergio R, Xavier R, Belén O, Leticia F, Mendiguren JM, Vicente A, et al. Triglycerides and residual atherosclerotic risk. J Am Coll Cardiol. (2021) 77:3031–41. doi: 10.1016/j.jacc.2021.04.059
30. Waters DD, Bangalore S, Fayyad R, DeMicco DA, Laskey R, Melamed S, et al. Visit-to-visit variability of lipid measurements as predictors of cardiovascular events. J Clin Lipidol. (2018) 12:356–66. doi: 10.1016/j.jacl.2017.12.003
31. Yanfeng R, Qian R, Juming L, Xiaohui G, Xiaoxu H, Linong J, et al. Low triglyceride as a marker for increased risk of cardiovascular diseases in patients with long-term type 2 diabetes: a cross-sectional survey in China. Diabetes Metab Res Rev. (2018) 34:e2960. doi: 10.1002/dmrr.2960
32. Kuwabara M, Niwa K, Nishihara S, Nishi Y, Takahashi O, Kario K, et al. Hyperuricemia is an independent competing risk factor for atrial fibrillation. Int J Cardiol. (2016) 231:137–42. doi: 10.1016/j.ijcard.2016.11.268
33. Kwon CH, Lee SH, Lee J-Y, Ryu S, Sung K-C. Uric acid and risk of atrial fibrillation in the korean general population. Circ J. (2018) 82:2728–35. doi: 10.1253/circj.CJ-18-0748
34. Liu T, Zhang X, Korantzopoulos P, Wang S, Li G. Uric acid levels and atrial fibrillation in hypertensive patients. Internal Medicine. (2011) 50:799–803. doi: 10.2169/internalmedicine.50.4587
35. Bajaj JS, Reddy KR, Tandon P, Wong F, Kamath PS, Garcia-Tsao G, et al. The 3-month readmission rate remains unacceptably high in a large North American cohort of patients with cirrhosis. Hepatology. (2016) 64:200–8. doi: 10.1002/hep.28414
Keywords: coronary heart disease, type 2 diabetes mellitus, atrial fibrillation, machine learning, prediction models
Citation: Xu Q, Peng Y, Tan J, Zhao W, Yang M and Tian J (2022) Prediction of Atrial Fibrillation in Hospitalized Elderly Patients With Coronary Heart Disease and Type 2 Diabetes Mellitus Using Machine Learning: A Multicenter Retrospective Study. Front. Public Health 10:842104. doi: 10.3389/fpubh.2022.842104
Received: 23 December 2021; Accepted: 09 February 2022;
Published: 04 March 2022.
Edited by:
Kevin Lu, University of South Carolina, United StatesReviewed by:
Kurt Neumann, Independent Researcher, Kerékteleki, HungaryPrimoz Kocbek, University of Maribor, Slovenia
Copyright © 2022 Xu, Peng, Tan, Zhao, Yang and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Tian, jietian@cqmu.edu.cn
 Qian Xu
Qian Xu Yan Peng
Yan Peng Juntao Tan
Juntao Tan Wenlong Zhao
Wenlong Zhao Meijie Yang
Meijie Yang Jie Tian
Jie Tian