- 1Department of Pharmacy, Tongji Medical College, Tongji Hospital, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Pharmacy, Tongji Medical College, Union Hospital, Huazhong University of Science and Technology, Wuhan, China
Objective: This study aims to systematically review recent economic evaluations of elbasvir/grazoprevir (EBR/GZR) for chronic hepatitis C (CHC), to critically appraise the reporting quality and to summarize the results.
Methods: A literature search was undertaken using Medline, Embase, the Cochrane Library, EconLit, China National Knowledge Infrastructure, Wanfang Data, and Chongqing VIP to identify original articles containing economic evaluations of EBR/GZR for CHC published between 1 January 2000 and 31 December 2020. The Consolidated Health Economic Evaluation Reporting Standards statement was used to assess the quality of reporting of the articles.
Results: Of 93 articles identified, 13 studies fulfilled the inclusion criteria. These studies were conducted in 4 countries, and 8 active interventions were assessed. The target population was patients infected with CHC genotype 1 infection in all studies. Eight out of 13 studies that compared EBR/GZR vs. other direct antiviral agents suggested that EBR/GZR was generally more cost-effective or dominant than daclatasvir/asunaprevir (DCV/ASV), sofosbuvir/velpatasvir (SOF/VEL), ledipasvir/sofosbuvir (LDV/SOF), ombitasvir/paritaprevir/ritonavir + dasabuvir (3D) but not more cost-effective than glecaprevir/pibrentasvir (GLE/PIB). Two studies from China and one study from the USA that compared EBR/GZR vs. pegylated interferon and ribavirin (PegIFN/RBV) consistently indicated that EBR/GZR was generally more cost-effective than PegIFN/RBV. One study from Italy compared EBR/GZR with SOF + PegIFN/RBV and suggested that EBR/GZR had a lower cost and higher effectiveness. One study from France and one study from the USA confirmed that compared with non-therapy for patients with chronic kidney disease, EBR/GZR was cost-effective at commonly accepted current standards. All included studies were of good quality of reporting, with an average score of 21.9 (range 19–23).
Conclusion: EBR/GZR for CHC genotype 1 might be cost-effective or dominant compared with PegIFN/RBV and other direct antiviral agents (SOF/VEL, 3D, DCV/ASV, LDF/SOF) or non-therapy. However, under certain assumptions, EBR/GZR was not a cost-effective alternative for CHC patients vs. GLE/PIB.
Introduction
Chronic hepatitis C (CHC) is caused by hepatitis C virus (HCV), characterized by liver damage and extreme risks of progressing liver cirrhosis or hepatocellular carcinoma (HCC) in the end stage of infection (1). According to World Health Organization (WHO) reports, ~71 million people are chronically infected with HCV, and ~399,000 people die of cirrhosis or HCC caused by HCV infection each year (2). From 2004 to 2016, a total of 2.021 million cases of CHC were reported in China, with the overall incidence increasing by 14.4% annually (3). The influencing factors of CHC disease include sex, age, lifestyle, and genotype. For instance, the most widespread genotype (GT) is HCV GT1b (62.78%) in the Chinese population, while GT1 dominates (75.3%), with GT1a being the most prevalent subtype in the USA (4–6). CHC patients suffer tremendously both physically and mentally and increase their economic burden through higher healthcare expenses. The average annual direct economic burden of CHC patients is ¥ 69,280, and the average annual direct economic burden of patients with liver cirrhosis or liver cancer is ¥ 44,750 and ¥ 101,389, respectively (7, 8). It is apparent that chronic HCV infection can lead to enormous clinical and economic burdens.
Achievement of sustained virologic response (SVR) is the endpoint criterion of treating HCV infection, which can significantly decrease the risk of liver disease progression and avoid transmutation into end-stage liver diseases (9). The treatment of HCV infection and achievement of SVR are of critical importance in decreasing the medical and economic burdens among CHC patients.
Historically, the standard of care for HCV infection is the combination of pegylated interferon (PegIFN) and ribavirin (RBV), which is usually related to low efficacy and many adverse event rates, especially in cirrhotic patients. Recently, direct-acting antivirals (DAAs) have developed rapidly with improved SVR rates and fewer adverse events in the global market (10, 11). These all-oral DAA regimens for HCV infection included elbasvir/grazoprevir (EBR/GZR), daclatasvir/asunaprevir (DCV/ASV), sofosbuvir/velpatasvir (SOF/VEL), ombitasvir/paritaprevir/ritonavir + dasabuvir (3D), glecaprevir/pibrentasvir (GLE/PIB), and ledipasvir/sofosbuvir (LDV/SOF). As more novel DAAs for HCV infection have been approved to enter the market, it is essential to evaluate the effects on both health outcomes and economics (12).
EBR/GZR is a fixed-dose DAA compound tablet with improved efficacy, safety, and higher cost that is suitable for the treatment of CHC patients with GT1 and GT4 (13). It was officially approved to enter the Chinese market in November 2019 and introduced as a new treatment regimen for chronic HCV infection in China. Since EBR/GZR entered the market, more and more studies focused on its efficacy, safety, and cost-effectiveness, which have provided massive evidence for a deeper understanding. Currently, there are few systematic reviews on EBR/GZR, and it is necessary to estimate the economic effects of these drugs in chronic HCV infection. In summary, this paper reviews and appraises the economic evidence of treatments with EBR/GZR in CHC patients. The results would provide valuable information to researchers in designing and conducting high-quality economic evaluations and to administrators as well as health workers in making best decisions.
Methods
A systematic literature search was undertaken to identify the cost-effectiveness analyses of drugs for EBR/GZR according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines followed for review and reporting procedures.
Eligibility Criteria
Articles included in the systematic review met the following criteria: (1) the full economic evaluation, examined costs with their consequences, and reported incremental cost-effectiveness ratios (ICERs) or incremental cost-utility ratios (ICURs) were identified; (2) regardless of monotherapy or combination treatment, elbasvir/grazoprevir intervention was included; (3) complete full-text formats were available.Systematic reviews, methodological articles, expert opinions, comments (commentary), conference abstracts, and proceedings were excluded.
Literature Search
We restricted the analysis to papers published between 1 January 2000 and 31 December 2020 about relevant studies estimating the cost-effectiveness of CHC treatments. The following databases were searched: Medline, Embase, the Cochrane Library, and EconLit databases for English-language studies and China National Knowledge Infrastructure (CNKI), Wanfang Data and Chongqing VIP (CQVIP) for Chinese-language studies. Literature search algorithm was shown in Supplementary Table 1.
Study Selection
Two independent reviewers screened the titles and abstracts of all articles for eligibility. Full-text formats of all potentially relevant publications were obtained and reviewed to decide whether they met the pre-specified inclusion criteria. To resolve discrepancies, another discussion could be conducted.
Data Collection
A standardized data extraction form was used to collect relevant information, such as basic information for articles (e.g., authors and publication year), characteristics of studies (e.g., design and sample size), types of economic evaluation, study objective, descriptions of the intervention and comparators, measure of benefit, cost data and respective sources, approaches for dealing with uncertainty as well as cost, and outcome results.
Quality Assessment
All included studies were appraised for quality of reporting using the 24-item Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement (14). Each item in the CHEERS checklist was scored as having met the criteria in full (“1”), not at all (“0”), or not applicable (NA). Two reviewers independently appraised the studies, and the other authors solved the disagreements through discussion and consensus. Studies with scores higher than 75% were categorized as good, studies with scores in the range of 50–74% were categorized as moderate, and studies with scores lower than 50% were categorized as low.
Data Synthesis
To summarize and evaluate the aims, methods, settings, and results of the studies reviewed, a narrative synthesis was used. If possible, information was compared across studies about the modeling technique, the cost perspective, the measures of benefit used, and incremental cost-effectiveness ratios. Cost/charge data are presented in US$ for the common price year 2020 using the “CCEMG-EPPI-Center Cost Converter” Version 1.6 (15), a web-based tool that can be used to adjust an estimate of cost expressed in one currency and price year to a target currency and/or price year.
Result
Studies Identified
Figure 1 shows the flow chart for the identification of studies. The initial database search identified 93 potentially relevant publications, of which 79 were in English language and 14 were in Chinese. Among them, 28 were excluded for repetitive publications. After the papers' initial screening, 39 publications were excluded based on title and abstract. The remaining 26 full text papers were retrieved for detailed assessment, and 13 publications were excluded for reasons such as meeting abstracts (n = 8), not written in English or Chinese (n = 1), and review articles (n = 4). A total of 13 publications were retrieved and analyzed for further data extraction and quality assessment.
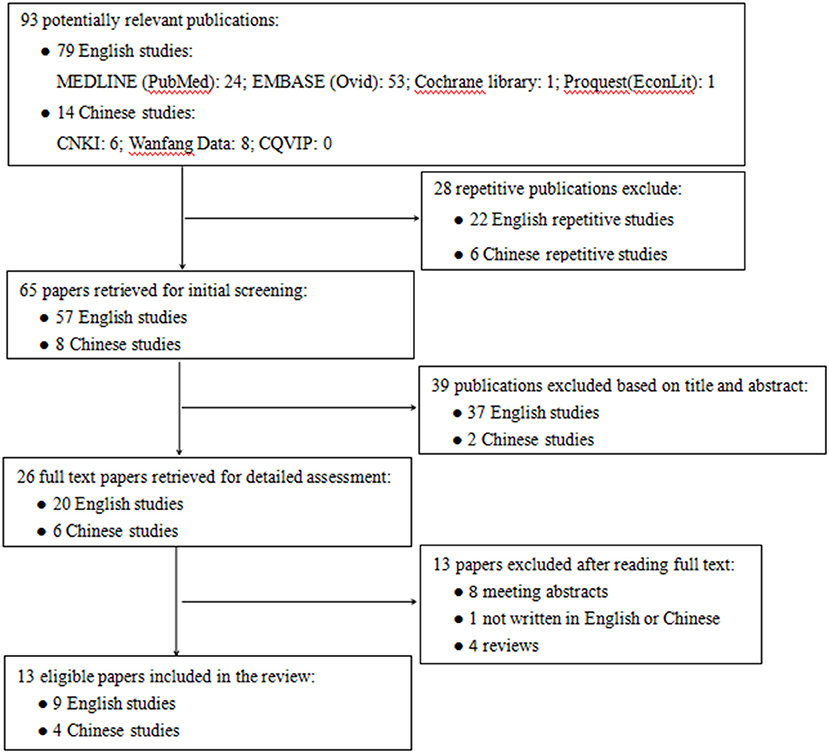
Figure 1. Flowchart of literature search. CNKI, China National Knowledge Infrastructure database; CQVIP, Chongqing VIP database.
Description of Identified Studies
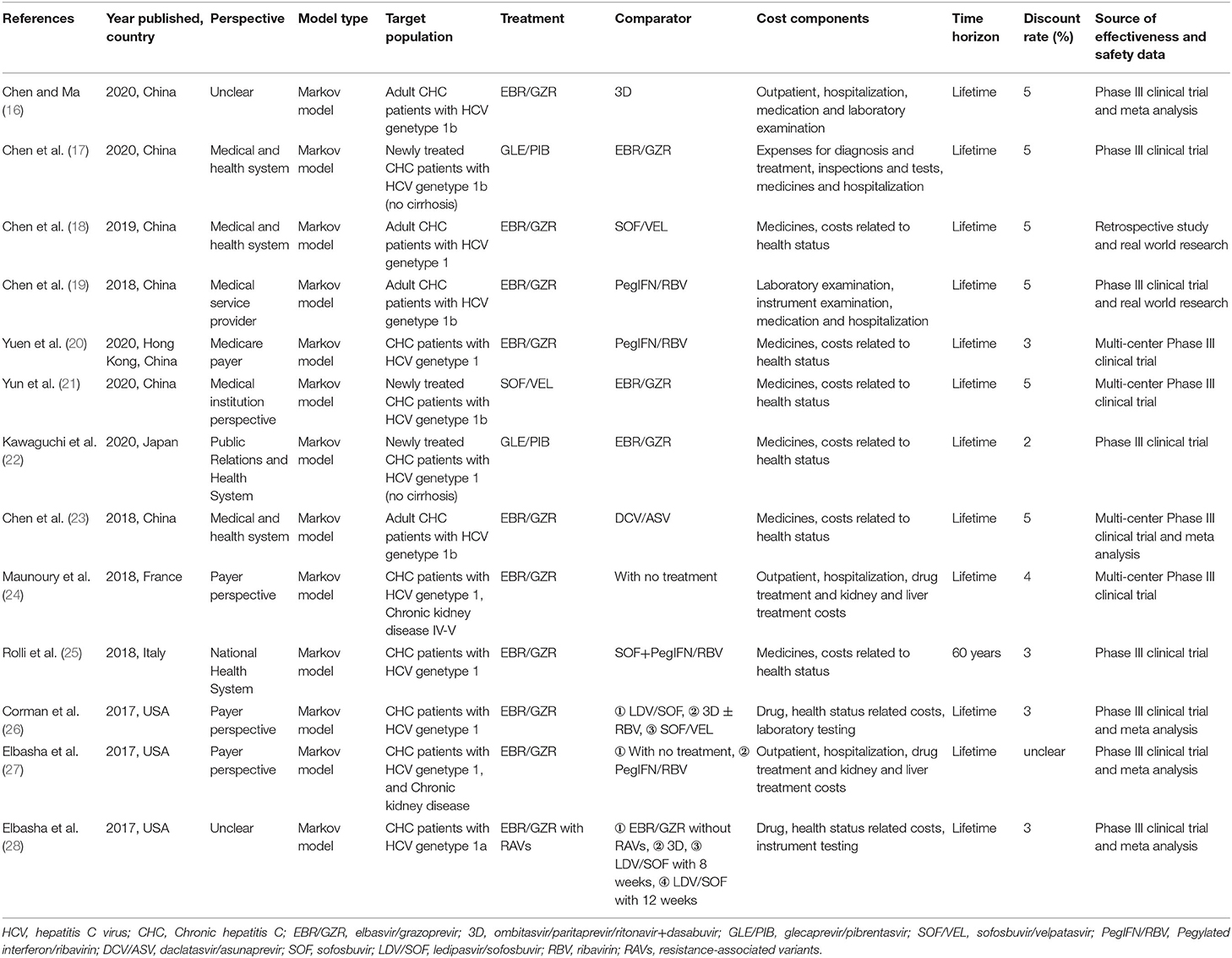
The general characteristics of the included studies are reported in Table 1. China accounted for the largest number (n = 7), and the remaining studies were conducted in the USA (n = 3), Italy (n = 1), France (n = 1), and Japan (n = 1). The Markov model were used in all of the studies. Seven studies were conducted from the healthcare perspective, and three were conducted from the payer perspective, while the perspective was not stated in two studies. Used in Markov models, most time horizons were applied for a lifetime, except one applied 60-year time horizon.
The direct antiviral agents (DAAS) for CHC in the included studies were EBR/GZR, GLE/PIB, SOF/VEL, and DCV/ASV, and the most frequent applications for comparators were PegIFN/RBV and non-therapy. Three studies were funded by Merck & Co., Inc., one by Merck Sharp & Dohme Corp., Inc., one by Gilead Sciences Shanghai Pharmaceutical Technology Co., Ltd., one by AbbVie Inc. and AbbVie GK, one by MSD Italia S.r.l., and one by National Natural Science Foundation of China. The remaining studies did not indicate the source of funding or were without funding.
Quality Assessment
The results of reporting quality per study as assessed by the CHEERS statement are summarized in Supplementary Table 2. In the CHEERS checklist, each item reported sufficiently, partially, or not at all in the review was declared. All of the included studies were evaluated for high quality. Two studies did not state the perspective (16, 28), and only one study did not report the discount rate. Meanwhile, the currency, price date, and conversion were not stated in four studies (16–19). Although some of the study findings and limitations were reported, none of the studies stated the generalizability of the results. The source of funding was not reported in four studies (16, 18, 19, 24), and four studies failed to report conflicts of interest.
Cost-Effectiveness Results of the Studies
The results of the overview of the economic evaluation outcomes of the included studies are summarized in Supplementary Table 3.
Elbasvir/Grazoprevir vs. Other Direct Anti-virus Agents
Eight studies provided economic evaluation for EBR/GZR vs. other DAAs. Five of them were conducted in China, and four studies suggested that EBR/GZR was more cost-effective than 3D (16), SOF/VEL (18, 21), and DCV/ASV (23). However, the remaining study (17) suggested that GLE/PIB has cost-effectiveness advantages over EBR/GZR in CHC GT1b treatment-naive patients without cirrhosis, which is the same as the result of a study (22) conducted in Japan. Two studies were conducted in the USA; one study (26) showed that compared with 3D, SOF/VEL, or LDV/SOF, EBR/GZR was the economically dominant regimen for treating patients with GT1a or GT1b CHC, and the other study (28) showed that resistance-associated variant (RAV) testing before treatment with EBR/GZR was more cost-effective than EBR/GZR without testing, LDV/SOF, or 3D for treating patients with GT1a CHC.
Elbasvir/Grazoprevir vs. PegIFN/RBV
Three studies considered PegIFN/RBV as comparators (19, 20, 27). Chen et al. (19) assumed that EBR/GZR provided more QALYs and lower costs than PegIFN/RBV for patients in China with cirrhosis or not. Yuen et al. (20) demonstrated that patients with GT1a or GT1b CHC in Hong Kong could gain more QALYs (1.3840 QALYs in GT1a, 0.8227 QALYs in GT1b) treated with EBR/GZR than PegIFN/RBV, while the costs were much higher ($ 6942 in GT1a, $ 7123 in GT1b). In total, EBR/GZR was cost-effective compared to PegIFN/RBV in GT1. The same result was as Elbasha et al. (27).
Elbasvir/Grazoprevir vs. PegIFN/RBV + SOF
One study (25) conducted in Italy compared EBR/GZR with SOF + PegIFN/RBV and suggested that the EBR/GZR group has a lower cost and higher effectiveness, which has an absolute advantage.
Elbasvir/Grazoprevir vs. Non-therapy
Two studies (24, 27) conducted in France and the USA compared EBR/GZR with non-therapy. Maunoury et al. (24) indicated that EBR/GZR compared to no treatment was considered cost-effective for patients with renal insufficiency at a willingness to pay of €20,000/QALY. Additionally, Elbasha et al. (27) showed that EBR/GZR resulted in higher average remaining QALYs and higher costs compared with non-therapy for patients with chronic kidney disease, and the ICER was $13201.34/QALY, which was less than the thresholds of $100,000/QALY. EBR/GZR is considered cost-effective at the commonly accepted current U.S. standards.
Discussion
Summary of Evidence
Thirteen economic evaluations of drugs for chronic hepatitis were identified in our systematic review from 2000 to 2020. Nine of them were written in English, and the other was written in Chinese. Eight active interventions were assessed in this research, including EBR/GZR, GLE/PIB, SOF/VEL, PegIFN/RBV, 3D, DCV/ASV, LDF/SOF, and PegIFN/RBV + SOF. This study aimed to evaluate the costs and cost-effectiveness of EBR/GZR for chronic hepatitis. If applicable, the thresholds were stated in the included studies. Meanwhile, we found the proper and accepted thresholds used in corresponding countries to evaluate whether the ICERs of EBR/GZR were below these thresholds. According to the results, decisions were made to determine whether they were valuable or cost-effective for CHC.
When compared to PegIFN/RBV, EBR/GZR was cost-effective or dominant for CHC patients with GT1a or GT1b or not in the included studies (19, 20, 27). Three studies assessed the cost-effectiveness analysis of EBR/GZR vs. SOF/VEL, suggesting that EBR/GZR was cost-effective in general but not for CHC patients with GT1b TN non-cirrhosis (18, 21, 26). According to the results of two studies, GLE/PIB might be a more cost-effective front-line therapy than EBR/GZR due to its economic advantages (17, 22). Two other studies investigated whether EBR/GZR was more cost-effective or dominant than 3D (26, 28).
Quality of Evidence
To appraise the quality of the included studies in our reviews, the availability of the CHEERS statement can be used to improve and hence the quality of economic evaluations of CHC. However, we find that some studies are of insufficient quality.
In terms of characterizing the study findings, limitations, generalizability, and current knowledge, it is necessary for readers to quickly obtain information on limitations and current research status. It also helps improve the reporting of economic evaluations in the future. However, in these included studies, none of them reported information that might not be objective.
In addition, some of the studies included in our review are funded by pharmaceutical companies, which might lead to potential bias of the economical evaluations. Although it is common sense for evaluations influenced by funded companies, an appropriate approach to assess this bias remains uncertain.
Key Elements of Cost-Effectiveness
Consistent with previous research, some key elements of cost-effectiveness were also found in our studies. On the one hand, the election of the comparator is crucial, and it could lead to different cost-effectiveness of the therapy regimen. For instance, EBR/GZR was evaluated to be dominant compared to SOF/VEL or DCV/ASV, while the cost-effectiveness was at a disadvantage compared to GLE/PIB. Therefore, the selection of the comparator is one of the most critical structures of cost-effectiveness analysis.
On the other hand, the included analyses were mainly for specific countries, so the results and final conclusions of economic assessments may be significantly affected by the healthcare systems and medical insurance policies of different countries. To enhance the universality and transferability of research across settings, future evaluations need to use improved methods, such as constructing multi-level models to analyze cost-effectiveness data and identifying a series of appropriate covariates to address assumptions and uncertainties in the results of economic evaluations.
Strengths and Limitations
So far as we know, this article is the first systematic review of published literature to assess the cost-effectiveness properties of EBR/GZR for chronic HCV infection. Unlike previous narrative and systematic studies that were antiquated or limited to a single comparator, this article is the most extensive review, including literature retrieval and selection, economic assessment over a longer period of time, and the use of validated tools to assess quality. Additionally, this review includes all published cost-effectiveness studies on EBR/GZR, and all cost-related parameters of different regional backgrounds are adjusted to 2020 dollars for more convenient comparison.
Although this review uses scientific and systematic methods to minimize deviations, several limitations that influence the conclusions should be considered when explicating the results.
First, in view of the possible divergences in the selected literature backgrounds and economic evaluation methods, it is exceedingly difficult to integrate these studies into a coherent whole. Although the included literature adopts the Markov model when analyzing cost-effectiveness, there are still diversified differences between them, such as research perspectives, time horizons, payers, target populations, and healthcare systems. Thus, we summarized the evidence qualitatively and then cautiously interpreted the results and conclusions.
Second, most of the literature included in this study is from the perspectives of the payer or the healthcare system and only considers direct medical costs while overlooking indirect costs. If chronic HCV infection is not treated in time, it will cause tremendous productivity and economic losses to society. Therefore, further research on pharmaceutical economics from the perspective of the whole society needs to be carried out.
Third, since some relevant studies with negative results or unfavorable findings may not be published, the literature search results are biased to a certain extent in our review. Meanwhile, due to the limitation of the author's language, some related potential documents may be ignored, especially in languages other than Chinese and English.
Conclusions
In conclusion, 13 studies were included on the cost-effectiveness of drugs for chronic hepatitis in this review. According to the available evidence, EBR/GZR for CHC might be cost-effective or dominant compared with PegIFN/RBV and other DAAs (SOF/VEL, 3D, DCV/ASV, LDF/SOF) or non-therapy. However, under certain assumptions, EBR/GZR was not a cost-effective alternative for CHC patients with cirrhosis or not or vs. GLE/PIB. More attention should be taken to improve the quality of reporting of economic evaluations.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Author Contributions
RY and JL: study design and study conduct. JL and MG: data collection. JL and LK: data analysis and drafting manuscript. RY, LK, MG, and JL: data interpretation. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.836986/full#supplementary-material
References
1. Razavi H, Elkhoury AC, Elbasha E, Estes C, Pasini K, Poynard T, et al. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. (2013) 57:2164–70. doi: 10.1002/hep.26218
2. World Health Organization. Hepatitis C. WHO (2021). Available online at: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed July 27, 2021).
3. Chinese Chinese Society of Hepatology Chinese Chinese Society of Infectious Diseases Chinese Medical Association. Guidelines for the prevention and treatment of hepatitis C(2019 version). Chin J Hepatol. (2019) 27:962–79. doi: 10.3760/cma.j.issn.1007-3418.2019.12.008
4. Rao H, Wei L, Lopez-Talavera JC, Shang J, Chen H, Li J, et al. Distribution and clinical correlates of viral and host genotypes in Chinese patients with chronic hepatitis C virus infection. J Gastroenterol Hepatol. (2014) 29:545–53. doi: 10.1111/jgh.12398
5. Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. (2017) 2:161–76. doi: 10.1016/S2468-1253(16)30181-9
6. Zhang Y, Chen LM, He M. Hepatitis C Virus in mainland China with an emphasis on genotype and subtype distribution. Virol J. (2017) 14:41. doi: 10.1186/s12985-017-0710-z
7. Yin R, Xiao D, Zhang L, Xuan J. Value assessment of elbasvir/grazoprevir in the treatment of chronic virus hepatitis C. China J Pharm Econ. (2020) 15:5–9.
8. Zhang L, Ai Y, Liu J, Yue N, Xuan J, Bal V, et al. Economic burden of needlestick injuries among healthcare workers in China. J Med Econ. (2020) 23:683–9. doi: 10.1080/13696998.2020.1737534
9. Simmons B, Saleem J, Heath K, Cooke GS, Hill A. Long-Term treatment outcomes of patients infected with hepatitis C virus: a systematic review and meta-analysis of the survival benefit of achieving a sustained virological response. Clin Infect Dis. (2015) 61:730–40. doi: 10.1093/cid/civ396
10. Sulkowski MS, Flamm S, Kayali Z, Lawitz EJ, Kwo P, McPhee F, et al. Short-duration treatment for chronic hepatitis C virus with daclatasvir, asunaprevir, beclabuvir and sofosbuvir (FOURward study). Liver Int. (2017) 37:836–42. doi: 10.1111/liv.13335
11. Bourlière M, Gordon SC, Flamm SL, Cooper CL, Ramji A, Tong M, et al. Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection. N Engl J Med. (2017) 376:2134–46. doi: 10.1056/NEJMoa1613512
12. You R, Qian X, Tang W, Xie T, Zeng F, Chen J, et al. Cost effectiveness of bosentan for pulmonary arterial hypertension: a systematic review. Can Respir J. (2018) 2018:1015239. doi: 10.1155/2018/1015239
13. EBR/GZR Information. Highlights of Prescribing Information. (2019). Available online at: https://www.merck.com/product/usa/pi_circulars/z/zepatier/zepatier_pi.pdf (accessed July 20, 2021).
14. Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Pharmacoeconomics. (2013) 31:361–7. doi: 10.1007/s40273-013-0032-y
15. Campbell Collaboration. CCEMG-EPPI-Centre Cost Converter. (2019). Available online at: http://eppi.ioe.ac.uk/costconversion/default.aspx (accessed October 18, 2021).
16. Chen P, Ma A. Cost-effectiveness analysis of elbasvir/grazoprevir for the treatment of chronic hepatitis C. Chin J New Drugs. (2020) 29:470.
17. Chen C, Ai D, Lu Y. Cost-effectiveness analysis of glecaprevir/pibrentasvir versus elbasvir/grazoprevir in chronic hepatitis C genotype 1b treatment-naive patients without cirrhosis. China Pharm. (2020) 31:1113.
18. Chen P, Xie Q. Cost-Effectiveness analysis of elbasvir/grazoprevir and sofosbuvir/velpatasvir in the treatment of hepatitis C. China J Pharm Econ. (2019) 14:14.
19. Chen P, Li H, Ma A. Elbasvir/grazoprevir for patients with chronic hepatitis C virus genotype 1b infection in China: an economic evaluation. Chin J Evid Based Med. (2018) 18:1352.
20. Yuen MF, Liu SH, Seto WK, Mak LY, Corman SL, Hsu DC, et al. Cost-Utility of all-oral direct-acting antiviral regimens for the treatment of genotype 1 chronic hepatitis c virus-infected patients in Hong Kong. Dig Dis Sci. (2021) 66:131–26. doi: 10.1007/s10620-020-06281-8
21. Yun H, Zhao G, Sun X, Shi L. Cost-utility of sofosbuvir/velpatasvir versus other direct-acting antivirals for chronic hepatitis C genotype 1b infection in China. BMJ Open. (2020) 10:e035224. doi: 10.1136/bmjopen-2019-035224
22. Kawaguchi I, Chayama K, Gonzalez YS, Virabhak S, Mitchell D, Yuen C, et al. A cost-effectiveness analysis of glecaprevir/pibrentasvir versus existing direct-acting antivirals to treat chronic hepatitis C in Japan. Adv Ther. (2020) 37:457–76. doi: 10.1007/s12325-019-01166-3
23. Chen P, Ma A, Liu Q. Cost-Effectiveness of elbasvir/grazoprevir versus daclatasvir plus asunaprevir in patients with chronic hepatitis C virus genotype 1b infection in China. Clin Drug Invest. (2018) 38:1031–9. doi: 10.1007/s40261-018-0702-9
24. Maunoury F, Clément A, Nwankwo C, Levy-Bachelot L, Abergel A, Di Martino V, et al. Cost-effectiveness analysis of elbasvir-grazoprevir regimen for treating hepatitis C virus genotype 1 infection in stage 4-5 chronic kidney disease patients in France. PLoS ONE. (2018) 13:e0194329. doi: 10.1371/journal.pone.0194329
25. Rolli FR, Ruggeri M, Kheiraoui F, Drago C, Basile M, Favaretti C, et al. Economic evaluation of zepatier for the management of HCV in the Italian scenario. Eur J Health Econ. (2018) 19:1365–74. doi: 10.1007/s10198-018-0980-4
26. Corman S, Elbasha EH, Michalopoulos SN, Nwankwo C. Cost-Utility of elbasvir/grazoprevir in patients with chronic hepatitis c genotype 1 infection. Value Health. (2017) 20:1110–20. doi: 10.1016/j.jval.2017.05.003
27. Elbasha E, Greaves W, Roth D, Nwankwo C. Cost-effectiveness of elbasvir/grazoprevir use in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and chronic kidney disease in the United States. J Viral Hepat. (2017) 24:268–79. doi: 10.1111/jvh.12639
28. Elbasha EH, Robertson MN, Nwankwo C. The cost-effectiveness of testing for NS5a resistance-associated polymorphisms at baseline in genotype 1a-infected (treatment-naïve and treatment-experienced) subjects treated with all-oral elbasvir/grazoprevir regimens in the United States. Aliment Pharmacol Ther. (2017) 45:455–67. doi: 10.1111/apt.13882
Keywords: cost-effectiveness, elbasvir/grazoprevir, direct-acting antivirals, pegylated interferon, ribavirin, hepatitis C virus
Citation: Liu J, Guo M, Ke L and You R (2022) Cost-Effectiveness of Elbasvir/Grazoprevir for the Treatment of Chronic Hepatitis C: A Systematic Review. Front. Public Health 10:836986. doi: 10.3389/fpubh.2022.836986
Received: 16 December 2021; Accepted: 04 April 2022;
Published: 13 May 2022.
Edited by:
Calvin Pan, NYU Grossman School of Medicine, United StatesCopyright © 2022 Liu, Guo, Ke and You. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruxu You, eW91cnV4dTIwMDhAMTYzLmNvbQ==; Lei Ke, MTM1NDUzNDU4MTVAMTYzLmNvbQ==
 Jinyu Liu
Jinyu Liu Min Guo1
Min Guo1 Ruxu You
Ruxu You