- 1Breast Cancer Center, West District of The First Affiliated Hospital of the University of Science and Technology of China, Hefei, China
- 2Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China
Introduction: The racial disparities of opportunity to receive the appropriate intervention and lower insurance coverage may result in survival disparities in different races. This study aims to provide a perspective on racial disparities in the survival of breast cancer patients after surgery.
Methods: Through data from the Surveillance, Epidemiology, and End Results (SEER) program, this study estimated the survival of breast cancer patients of different races from 1998 to 2017. Inverse probability weighting (IPW) was utilized to adjust the imbalanced clinicopathological features of patients of different races.
Results: This study analyzed 214,965 breast cancer patients after surgery. Among them, 130,746 patients received BCS, and the remaining 84,219 breast cancer patients underwent mastectomy. Although Asian or Pacific Islander (API) patients after surgery showed higher survival benefit than that of white patients in the primary data, after adjusting for age at diagnosis, luminal subtype, grade, T stage, and N stage in different races, white individuals had the longest period of survival was higher than that of the minority groups in BCS group [breast cancer-specific survival (BCSS): HRWhitevs.API = 0.402, HRWhitevs.Black = 0.132; P < 0.001; overall survival (OS): HRWhitevs.API = 0.689, HRWhitevs.Black = 0.254; all P < 0.001] and mastectomy group (BCSS: HRWhitevs.API = 0.325, HRWhitevs.Black = 0.128; P < 0.001; OS: HRWhitevs.API = 0.481, HRWhitevs.Black = 0.206; all P < 0.001)
Conclusions: We first identified that the survival benefit of the minority group after surgery was lower than that of white individuals, regardless of tumor chrematistics and surgery types.
Introduction
In 2020, breast cancer was the highest incidence and the second leading cause of cancer death. It was estimated that in 2020, about 276,480 women or 30% of female cancer patients were diagnosed with breast cancer, and 42,170 cancer-related deaths were caused by breast cancer (1, 2). Racial disparities in tumor incidence and survival rates have been increasingly observed recently (3). For example, compared with black breast cancer patients, white breast cancer patients were more likely diagnosed at earlier stage (4). Nowadays, several studies have identified that not only racial differences in tumor pathological characteristics, but also the receipt of systemic therapy can lead to the survival disparities of breast cancer patients (4, 5). Breast cancer is a multifactorial disease, the survival disparities of breast cancer patients are also affected by other factors beyond the tumor stage, including system therapy after surgery and socioeconomic status (6).
There have been remarkable advances in the field of breast cancer surgery in the past 50 years (7). Currently, BCS has become a standard treatment for patients with early-stage breast cancer (8). Compared with mastectomy, patients receiving BCS rely on precise imaging, detailed pathology, specialist surgery during surgery, and systemic therapy after surgery to enhance survival (9). However, compared with white breast cancer patients, minority patients have less access to effective care and lower insurance coverage (10). These racial disparities may lead to survival disparities in survival in breast cancer patients.
Ongoing nationally funded research on racial disparities has led to new concerns about human health (11). In fact, understanding racial disparities is beneficial for doctors to select an effective treatment for patients from different races. In the current study, we will provide a clear perspective on racial disparities in the survival of breast cancer patients after surgery by analysis of a multirace dataset, which contributes to the formulation of targeted public health interventions and policies.
Materials and Methods
Survival Cohort
This study utilized the population-based SEER program to collect survival data for further analysis. Before analysis, we selected female patients with M0 (no distant organ metastasis) from 1998 to 2017 because information on the molecular subtypes of breast cancer patients were provided after 1998. All patients undergoing surgery (RX sum-Surg Prim Site code: 20–39 were regarded as BCS) were included and analyzed. As American Indian (AI) and Alaska Native patients have a low incidence of breast cancer, the sample of AI breast cancer patients undergoing surgery was too small for accurate survival analysis. Thus, this study excluded the survival of AI breast cancer patients to minimize heterogeneity.
Statistical Analysis
Overall survival and BCSS were the main outcomes of this study. OS was defined as the algorithmic analysis of death, while BCSS referred to death caused by breast cancer. Other-cause mortality (OCM) was recorded as death caused by other diseases. OS and BCSS were calculated by the Kaplan–Meier method. Hazard ratios (HRs) were used for pairwise comparison.
Before pairwise comparison, this study was conducted stepwise IPW by a propensity model to adjust the baseline imbalance of patients from different races (12). IPW models were performed as follows: First, the age-adjusted IPW was only adjusted for the age imbalance at diagnosis. Second, age at diagnosis and lumen subtypes were adjusted, weighting the imbalance in age at diagnosis and luminal subtype. Third, IPW was fully adjusted, weighting the potential risk factors of the SEER program completely, including age at diagnosis, luminal subtype, grade, T stage, and N stage. Among these propensity IPW models, age at diagnosis was regarded as a continuous variable, and other factors were treated as categorical variables.
A P-value lower than 0.05 on both sides was considered statistically significant. Data were analyzed by version 3.4.3 of R software (R Foundation for Statistical Computing) and version 22 of Statistical Product and Service Solutions (SPSS).
Results
Patient Characteristics
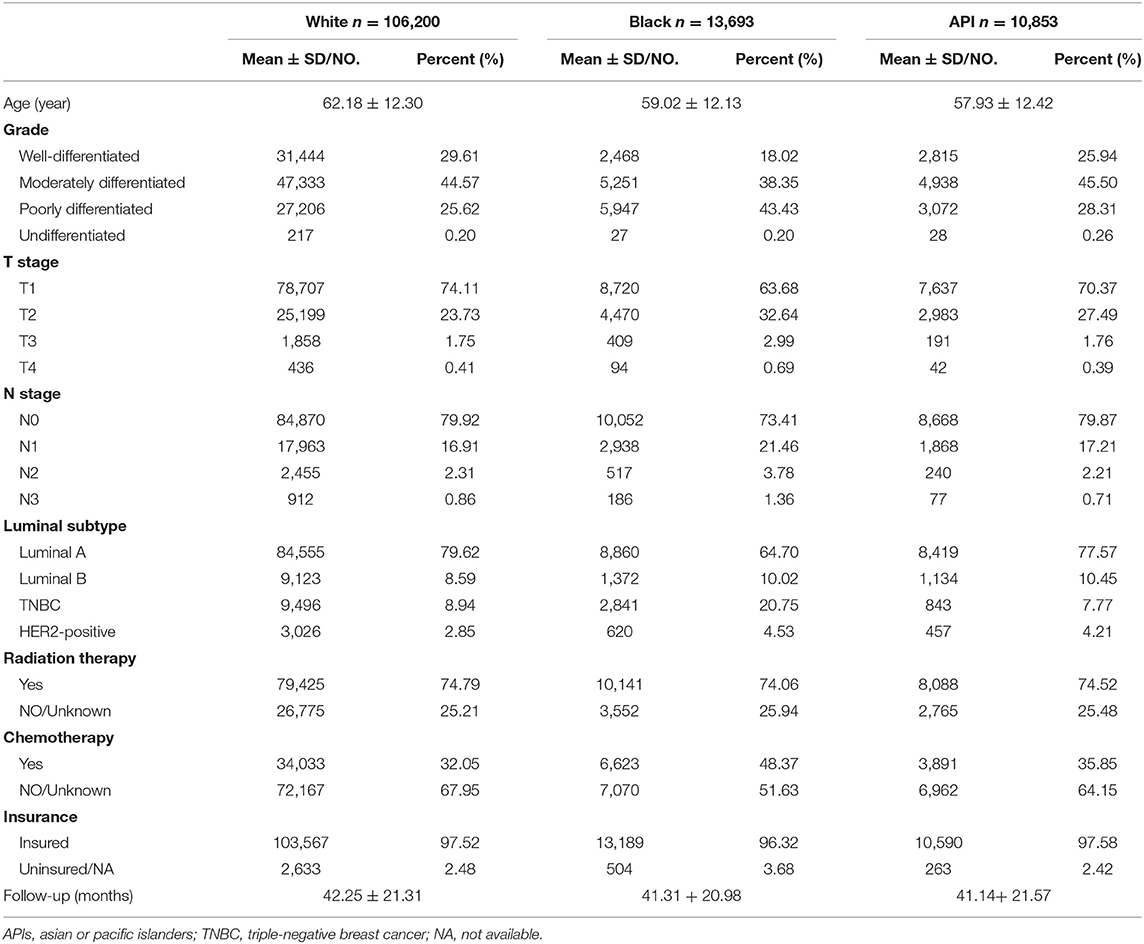
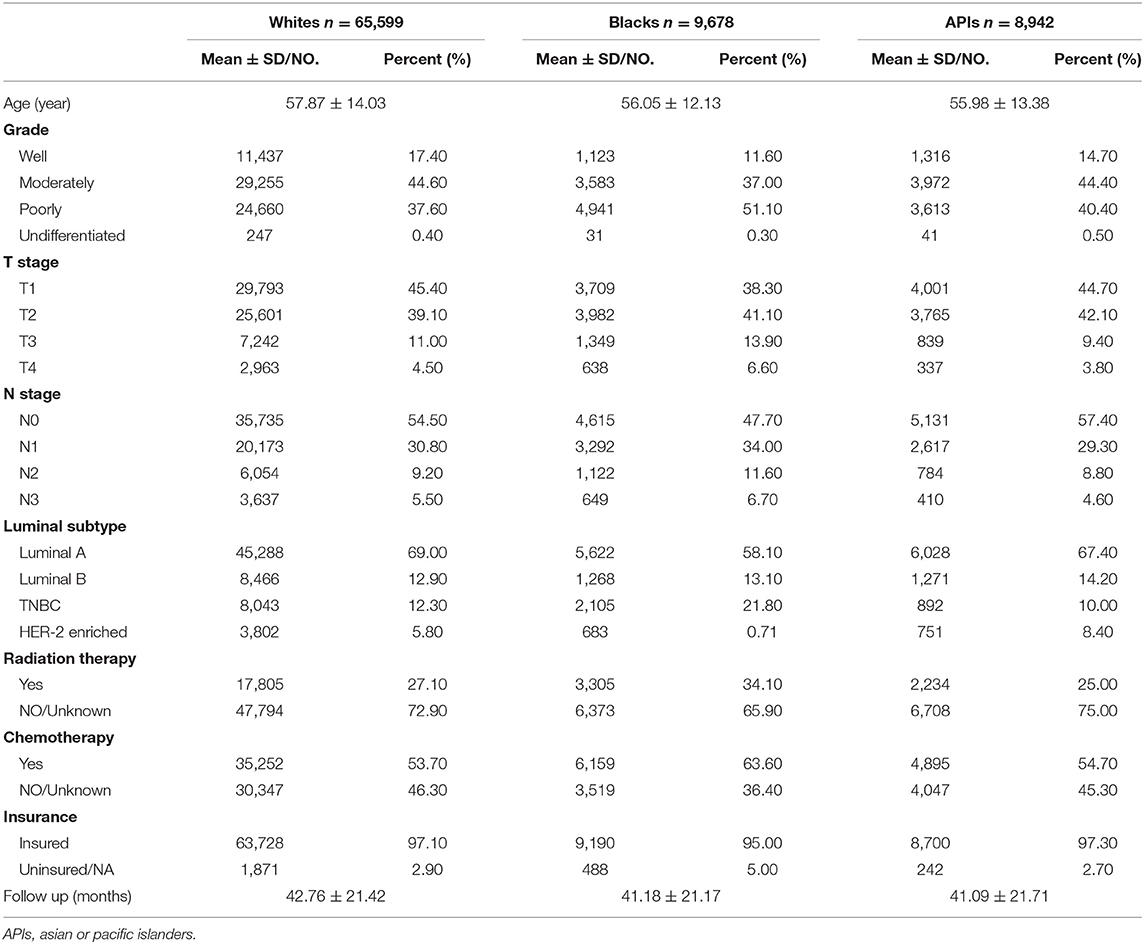
As shown in Tables 1, 2, this study contained 214,965 breast cancer patients after surgery based on all criteria. Among them, a total of 130,746 breast cancer patients underwent BCS. 84,219 breast cancer patients underwent mastectomy. There were 171,799 white patients, 23,371 black patients, and 19,795 API patients. In the BCS group, black patients with breast cancer after BCS were likely to suffer from poorly differentiated or undifferentiated tumors, with a percentage of 43.63%. White patients tended to be infected with well-differentiated tumors, with a percentage of 29.61%. In addition, black patients with breast cancer had higher rates of TNBC or HER2-positive tumors, with a percentage of 25.28%. Compared with white and black patients, it was less likely for API patients to be diagnosed at T4 and N3 stages. As shown in the Figure 1, in the main dataset, the 5-year BCSS in white, black, and API patients was 96.9, 93.1, and 97.4%, respectively, and the 5-year OS in these three groups showed similar disparities at 95.5, 88.9, and 91.9%, respectively. In the mastectomy group, the 5-year BCSS in white, black, and API patients was 90.6, 83.9, and 84.1%, respectively, and the 5-year OS in these three groups showed similar disparities at 85.4%, 79.2, and 91.0%, respectively.
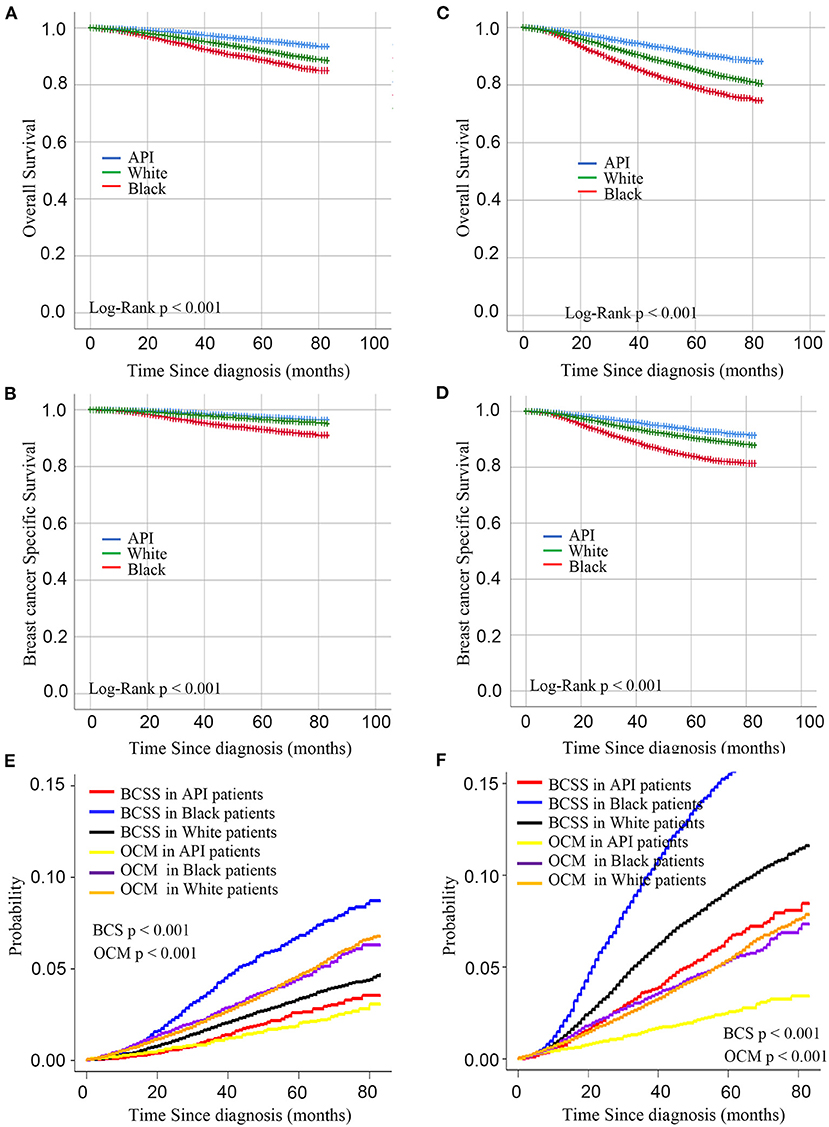
Figure 1. Kaplan– curves for overall survival and breast cancer-specific survival by race in the BCS group (A,B) and mastectomy group (C,D). (C) Cumulative incidence function curves for racial disparities of breast cancer-specific death and other-cause mortality in BCS group (E) and mastectomy (F).
Survival Analysis in BCS Group
A stepwise IPW method for sensitivity analysis in the primary dataset was performed in this study to understand the association between baseline characteristics and patient survival. First, based on the weighted imbalance of age during diagnosis, the subdistribution hazard ratio (SHR) of BCSS between white and API patients declined by 6.7% (from 1.440 to 1.373). In addition, white patients had a 74.8% reduction in the subdistribution OS hazard in the OS IPW model (from 1.892 to 1.144). In IPW models adjusted by age and luminal subtypes, survival differences between white and API patients did not disappear in either the BCSS or OS model (BCSS: HR = 0.999, CIs: 0.851–1.721, P = 0.987; OS: HR = 0.952, CIs: 0.843–1.077, P = 0.436). Compared with other races, white patients had the best BCSS and OS outcomes (as shown in Figure 2) after adjusting for an imbalanced baseline, but the survival advantages of API patients disappeared. There was no significant survival difference in black and API patients (BCSS: HR = 0.916, 0.749–1.121, P = 0.396) according to the subdistribution hazard model. Detailed SHR results are given in Table 3.
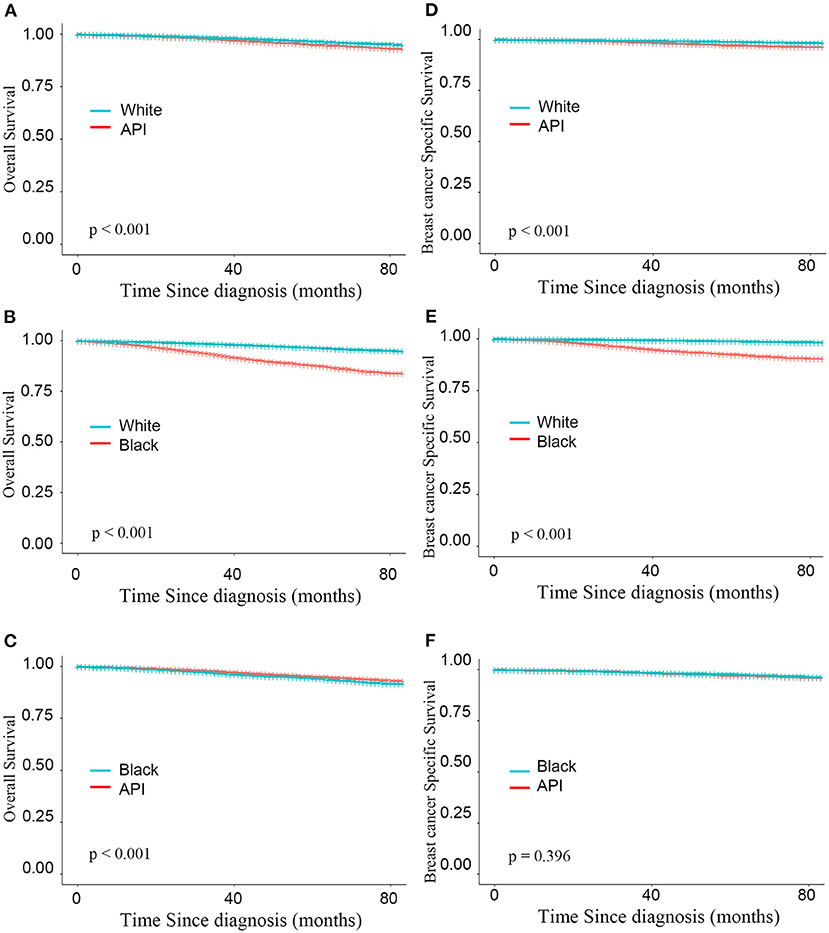
Figure 2. Kaplan–Meier curves for overall survival by race (A–C) and breast cancer-specific survival (D–F) based on the inverse probability weighting of all factors in BCS group.
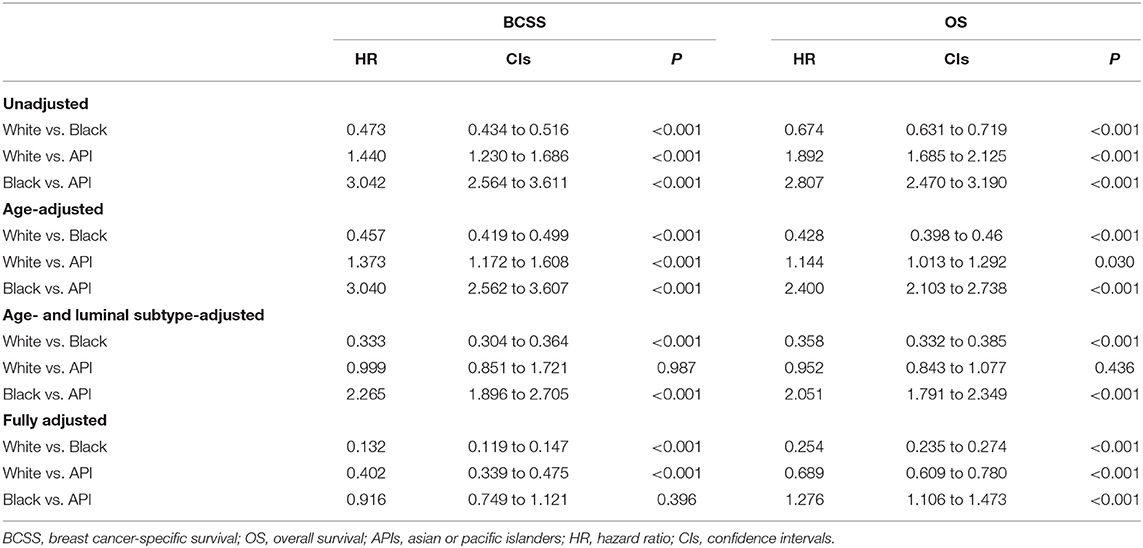
Table 3. Inverse probability weighted (IPW) estimates of BCSS and OS in patients underwent mastectomy.
Survival Analysis in Mastectomy Group
As shown in Figure 3, after adjusting the imbalanced clinicopathological characteristics in different races, the white individuals after mastectomy also had the longest survival was higher than that of the minority groups (BCSS: HRWhitevs.API = 0.325, HRWhitevs.Black = 0.128; P < 0.001; OS: HRWhitevs.API = 0.481, HRWhitevs.Black = 0.206; all P < 0.001). Interestingly, compared with BCS group, the survival advantages of whites were declined in mastectomy group (BCSS:ΔHRWhitevs.API = 19.15%, ΔHRWhitevs.Black = 3.03%; OS:ΔHRWhitevs.API = 30.19%, ΔHRWhitevs.Black = 18.90%). Detailed SHR results are given in Table 4.
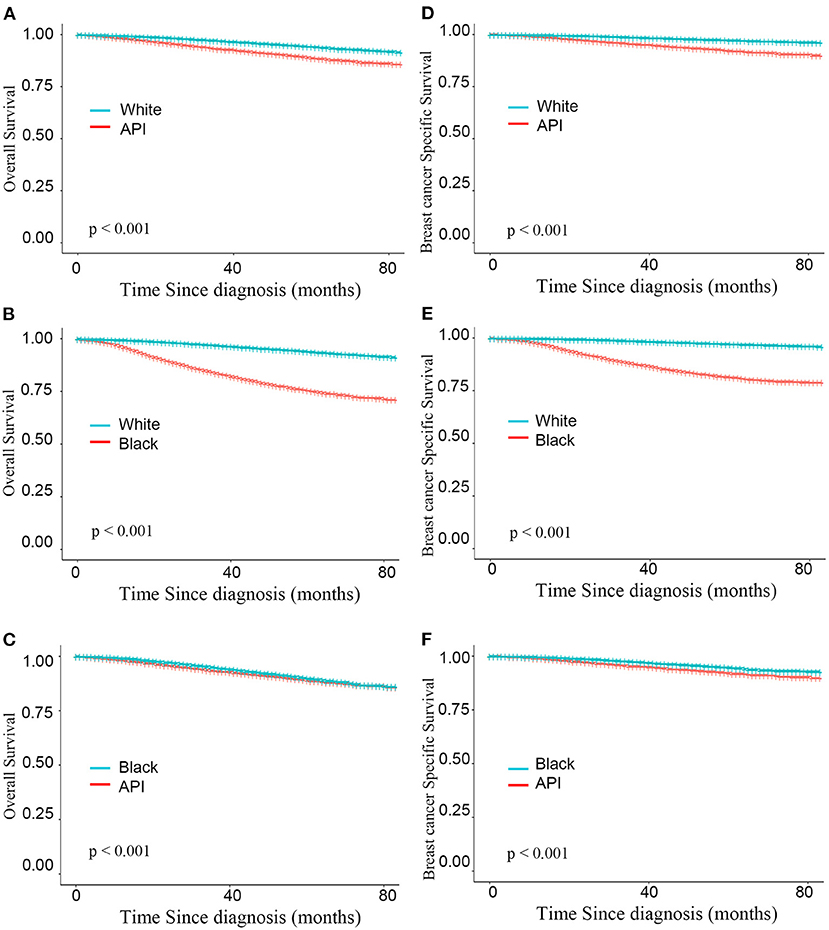
Figure 3. Kaplan–Meier curves for overall survival by race (A–C) and breast cancer-specific survival (D–F) based on the inverse probability weighting of all factors in mastectomy group.
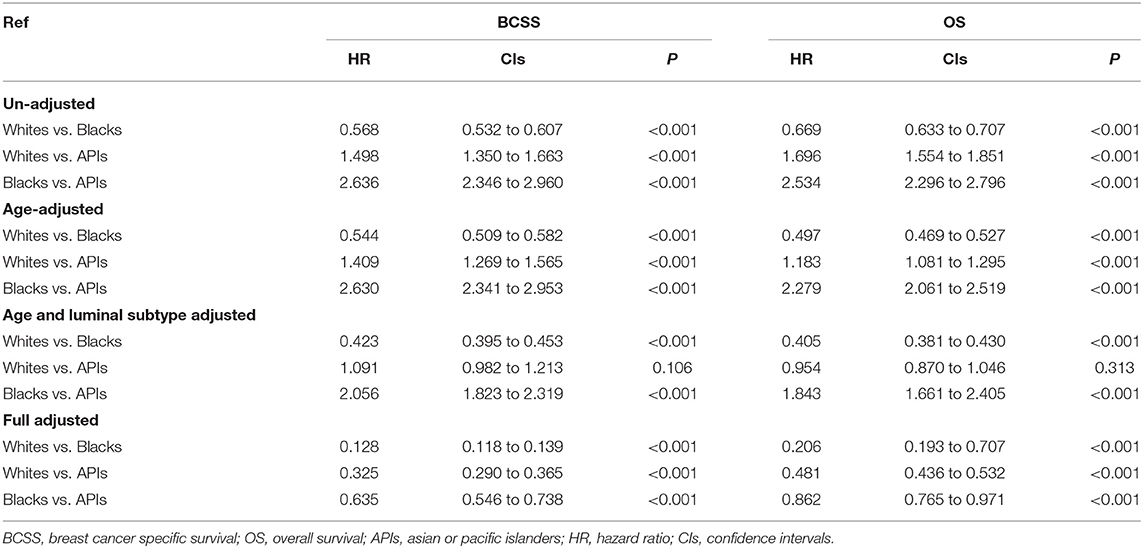
Table 4. Inverse probability weighted (IPW) estimates of BCSS and OS in patients underwent mastectomy.
Discussion
Based on a large multiracial dataset, this study found that there were distinct racial disparities in the survival of breast cancer patients after surgery. In the clinicopathological characteristics unadjusted dataset, API patients had the best BCSS and higher OS than patients of other races. However, after adjusting for the imbalanced clinicopathological features in different races by IPW, white patients had a better prognosis than minority patients regardless of surgery types. Noteworthy, we found the survival differences between whites and minorities were increased in mastectomy group.
In the past century, the total mastectomy proposed by Halsted was regarded as the standard surgery for breast cancer patients with localized cancer (13). However, this surgery was too radical to improve the quality of life of patients (14). It was gradually phased out in several large randomized clinical trials, and medical development brought less harm and higher quality of life (8, 15, 16). At 5, 8, 12, and 20 years of follow-up, initial reports of the NSABP B-06 trial consistently indicated that breast cancer patients who underwent segmental mastectomy with or without breast irradiation had no worse BCSS and OS outcomes than patients with total mastectomy (15, 17–19). Unexpectedly, BCS patients after radiotherapy even had better survival than those with total mastectomy at the 20-year follow-up (15). In fact, clinical trials and long-term follow-up analyses demonstrated that there was no worse survival between BCS and total mastectomy patients (20–23), indicating that BCS was a standard surgery for breast cancer patients in the early stage rather than total mastectomy. However, compared with mastectomy, BCS relies on precise imaging, detailed pathology, specialist surgery, and systemic therapy to prolong survival (9). The sociodemographic disparities in different races may lead to survival disparities in patients receiving different surgery types.
Although previously studies deliver evidence encouraging the use of BCS, it remains unclear why such survival disparities would exist in breast cancer patients with the same stage. In fact, BCS is a complex treatment, which relies on precise imaging and pathology, specialist surgery, and systemic therapy to enhance survival (9). Factors of ethnic heterogeneity, resulting in a worse prognosis in minorities, include younger age at diagnosis, later stage of breast cancer at diagnosis, biologic and genetic factors (24). However, in the current study, we found that adjusted for imbalanced clinicopathological features, such as age at diagnosis, grade, stage, and luminal subtype, minorities breast cancer patients still had a worse prognosis than white breast cancer patients. Thus, in addition to the adjusted clinicopathological features, several racial traits, including biologic and genetic factors, health care, and socioeconomic status, tended to affect the survival of breast cancer patients. For example, compared with white women, black women were less likely to receive appropriate treatment. Budhwani et al. (24) found that black women were less likely to receive surgical treatment in tertiary hospitals and did not receive adjuvant therapy in a timely manner. Interestingly, we found that API patients had a better prognosis than those from other races in the unadjusted data because they were less likely to be diagnosed at the T4 and N3 stages before BCS, suggesting that BCS should only be performed in APIs at early stages for a better prognosis.
In this study, we also found that in the mastectomy group, white breast cancer patients still had a better prognosis than minorities breast cancer patients. These results suggest that factors beyond clinicopathological features also affect the survival of breast cancer patients after mastectomy. Interestingly, we found that comparing the survival differences between white and minority breast cancer patients after BCS, the survival differences were increased in patients after mastectomy. These phenomena may be attributed to several unmeasurable factors, including socioeconomic status, multimorbidity, and effective therapy after surgery. For example, mastectomy is more common in women with a lower socioeconomic status, higher multimorbidity, and lower rates of adjuvant chemotherapy (25–27).
There were inherent limitations in these cancer registry datasets. First, owing to data availability, other information from SEER could not be collected, such as surgical distance, income, premenopausal endogenous hormone levels, breastfeeding, and genetic susceptibility, so it is hard to adjust for all potential confounders in this study (28–33). Second, white patients had higher survival rates, which may be influenced by adjuvant chemotherapy and radiotherapy after surgery. However, cancer registries did not collect treatment regimens systematically after surgery. In this study, the rates of radiotherapy were similar among individuals of these three races. Moreover, black patients with the worst survival rates even had the highest rate of adjuvant chemotherapy compared with women from other races. Despite limitations, this study offered a perspective on the racial disparities of breast cancer patients based on a large-scale multiracial dataset. Additionally, it adjusted for the imbalanced baseline measured by the IPW method before the analysis of cumulative racial disparities to reduce the probability of confounding.
In conclusion, we first identified that minority breast cancer patients had worse survival outcomes than white patients regardless of patients clinicopathological features and surgery types. The results suggested that survival disparities in breast cancer patients may not only be explained by the disparities of clinicopathological characteristics, but also affected by other ethnic differences. Confirming these risk factors through further research could improve the prognosis of breast cancer patients.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding authors.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conceptual construction and project administration by JL and SH. Data collection and compilation by SW and WT. Data analysis and interpretation by JL. Writing by SW, WT, and JL. Visualization by SH. Supervision by SW. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by National Natural Science Foundation of China (81802641).
Author Disclaimer
The authors are responsible for this study, which does not represent the views of the National Institutes of Health in the United States.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Zheng Xucai and Fang Jing, who work in The First Affiliated Hospital of the University of Science and Technology of China, for their help with the literature collection and data analyses during this study.
References
1. Pfeiffer RM, Webb-Vargas Y, Wheeler W. and Gail MH. Proportion of US trends in breast cancer incidence attributable to long-term changes in risk factor distributions. Cancer Epidemiol Biomarkers Prev. (2018) 27:1214–22. doi: 10.1158/1055-9965.EPI-18-0098
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
3. Zavala VA, Bracci PM, Carethers JM, Carvajal-Carmona L, Coggins NB, Cruz-Correa MR, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer. (2021) 124:315–32. doi: 10.1038/s41416-020-01038-6
4. Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA. (2015) 313:165–73. doi: 10.1001/jama.2014.17322
5. Chen L and Li CI. Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer Epidemiol Biomarkers Prev. (2015) 24:1666–72. doi: 10.1158/1055-9965.EPI-15-0293
6. Gu J, Groot G, Boden C, Busch A, Holtslander L, Lim H. Review of factors influencing women's choice of mastectomy versus breast conserving therapy in early stage breast cancer: a systematic review. Clin Breast Cancer. (2018) 18:e539–54. doi: 10.1016/j.clbc.2017.12.013
7. Matsen CB. and Neumayer LA. Breast cancer: a review for the general surgeon. JAMA Surg. (2013) 148:971–9. doi: 10.1001/jamasurg.2013.3393
8. Christian MC, McCabe MS, Korn EL, Abrams JS, Kaplan RS, Friedman MA. The National Cancer Institute audit of the National Surgical Adjuvant Breast and Bowel Project Protocol B-06. N Engl J Med. (1995) 333:1469–74. doi: 10.1056/NEJM199511303332206
9. Thompson AM. Breast conservation therapy versus mastectomy for breast cancer. Lancet Oncol. (2020) 21:493–4. doi: 10.1016/S1470-2045(20)30174-1
10. Newman LA and Martin IK. Disparities in breast cancer. Curr Probl Cancer. (2007) 31:134–56. doi: 10.1016/j.currproblcancer.2007.01.003
11. L. P. Wallner and J. J. Griggs: Advancing the Science of Cancer Health Disparities Research (1527–7755 (Electronic)).
12. Austin PC. and Schuster T. The performance of different propensity score methods for estimating absolute effects of treatments on survival outcomes: a simulation study. Stat Methods Med Res. (2016) 25:2214–37. doi: 10.1177/0962280213519716
13. Halsted WSI. A clinical and histological study of certain adenocarcinomata of the breast: and a brief consideration of the supraclavicular operation and of the results of operations for cancer of the breast from 1889 to 1898 at the Johns Hopkins Hospital. Ann Surg. (1898) 28:557–76.
14. McWhirter R. The value of simple mastectomy and radiotherapy in the treatment of cancer of the breast. Br J Radiol. (1948) 21: 599–610. doi: 10.1259/0007-1285-21-252-599
15. Fisher B, Jeong J, Anderson S, Bryant J, Fisher E, Wolmark N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. (2002) 347:567–75. doi: 10.1056/NEJMoa020128
16. McGuire K, Santillan A, Kaur P, Meade T, Parbhoo J, Mathias M et al. Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol. (2009) 16:2682–90. doi: 10.1245/s10434-009-0635-x
17. Fisher B, Bauer M, Margolese R, Poisson R, Pilch Y, Redmond C et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. (1985) 312:665–73. doi: 10.1056/NEJM198503143121101
18. Fisher B, Redmond C, Poisson R, Margolese R, Wolmark N, Wickerham L et al. Eight-year results of a randomized clinical trial comparing total mastectomy and lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. (1989) 320:822–8. doi: 10.1056/NEJM198903303201302
19. Fisher B, Anderson S, Redmond CK, Wolmark N, Wickerham DL, Cronin WM. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. (1995) 333:1456–61. doi: 10.1056/NEJM199511303332203
20. Maddox WA, Carpenter JT Jr., Laws HL, Soong SJ, Cloud G, et al. A randomized prospective trial of radical (Halsted) mastectomy versus modified radical mastectomy in 311 breast cancer patients. Ann Surg. (1983) 198:207–12. doi: 10.1097/00000658-198308000-00016
21. Blichert-Toft M, Nielsen M, Düring M, Møller S, Rank F, Overgaard M et al. Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol. (2008) 47:672–81. doi: 10.1080/02841860801971439
22. van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. (2000) 92:1143–50. doi: 10.1093/jnci/92.14.1143
23. Poggi MM, Danforth DN, Sciuto LC, Smith SL, Steinberg SM, Liewehr DJ et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer. (2003)98:697–702. doi: 10.1002/cncr.11580
24. Akinyemiju TF, Vin-Raviv N, Chavez-Yenter D, Zhao X, Budhwani H. Race/ethnicity and socio-economic differences in breast cancer surgery outcomes. Cancer Epidemiol. (2015) 39:745–51. doi: 10.1016/j.canep.2015.07.010
25. Frisell AA-O, Lagergren J, Halle M and de Boniface J. Socioeconomic status differs between breast cancer patients treated with mastectomy and breast conservation, and affects patient-reported preoperative information. Breast Cancer Res Treat. (2020) 184:977–84. doi: 10.1007/s10549-020-05911-z
26. Pathirana TI and Jackson CA. Socioeconomic status and multimorbidity: a systematic review and meta-analysis. Aust N Z J Public Health. (2018) 42:186-194. doi: 10.1111/1753-6405.12762
27. Dreyer MA-O, Nattinger AB, McGinley EL and Pezzin LE. Socioeconomic status and breast cancer treatment. Breast Cancer Res Treat. (2018) 167:1–8. doi: 10.1007/s10549-017-4490-3
28. Houssami N, Macaskill P, Marinovich ML, Dixon JM, Irwig L, Brennan ME et al. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer. (2010) 46:3219–32. doi: 10.1016/j.ejca.2010.07.043
29. Pinheiro SP, Holmes MD, Pollak MN, Barbieri RL, Hankinson SE. Racial differences in premenopausal endogenous hormones. Cancer Epidemiol Biomarkers Prev. (2005) 14:2147–53. doi: 10.1158/1055-9965.EPI-04-0944
30. Chlebowski RT, Chen Z, Anderson GL, Rohan T, Aragaki A, Lane D et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. (2005) 97:439–48. doi: 10.1093/jnci/dji064
31. Haiman CA, Chen GK, Vachon CM, Canzian F, Dunning A, Millikan RC. A common variant at the TERT-CLPTM1L locus is associated with estrogen receptor-negative breast cancer. Nat Genet. (2011) 43:1210–4. doi: 10.1038/ng.985
32. Kato I, Cichon M, Yee CL, Land S and Korczak JF. African American-preponderant single nucleotide polymorphisms (SNPs) and risk of breast cancer. Cancer Epidemiol. (2009) 33:24–30. doi: 10.1016/j.canep.2009.04.009
Keywords: racial disparities, breast conserving surgery, breast cancer, incidence, survival
Citation: Wang S, Tang W, Wang S, Hong S and Liu J (2022) Racial Disparities in Survival of Breast Cancer Patients After Surgery. Front. Public Health 10:831906. doi: 10.3389/fpubh.2022.831906
Received: 09 December 2021; Accepted: 12 April 2022;
Published: 13 May 2022.
Edited by:
Dora Il'yasova, Duke University, United StatesReviewed by:
Ambarina Faiz, The State University of New Jersey, United StatesMark Fiala, Washington University in St. Louis, United States
Copyright © 2022 Wang, Tang, Wang, Hong and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianjun Liu, bGppYW5qdW5AdXN0Yy5lZHUuY24=; Shikai Hong, c2hpa2hvbmdAMTYzLmNvbQ==
 Shuhan Wang1,2
Shuhan Wang1,2 Shengying Wang
Shengying Wang Jianjun Liu
Jianjun Liu