- 1Indian Institute of Public Health Gandhinagar (IIPHG), Gandhinagar, India
- 2HTAIn Secretariat-Department of Health Research, New Delhi, India
- 3Jhpiego, New Delhi, India
- 4Faculty of Medicine, Parul Institute of Public Health, Parul University, Vadodara, India
Background and objectives: Although a relatively recent concept for developing countries, the developed world has been using League Tables as a policy guiding tool for a comprehensive assessment of health expenditures; country-specific “League tables” can be a very useful tool for national healthcare planning and budgeting. Presented herewith is a comprehensive league table of cost per Quality Adjusted Life Years (QALY) or Disability Adjusted Life Years (DALY) ratios derived from Health Technology Assessment (HTA) or economic evaluation studies reported from India through a systematic review.
Methods: Economic evaluations and HTAs published from January 2003 to October 2019 were searched from various databases. We only included the studies reporting common outcomes (QALY/DALY) and methodology to increase the generalizability of league table findings. To opt for a uniform criterion, a reference case approach developed by Health Technology Assessment in India (HTAIn) was used for the reporting of the incremental cost-effectiveness ratio. However, as, most of the articles expressed the outcome as DALY, both (QALY and DALY) were used as outcome indicators for this review.
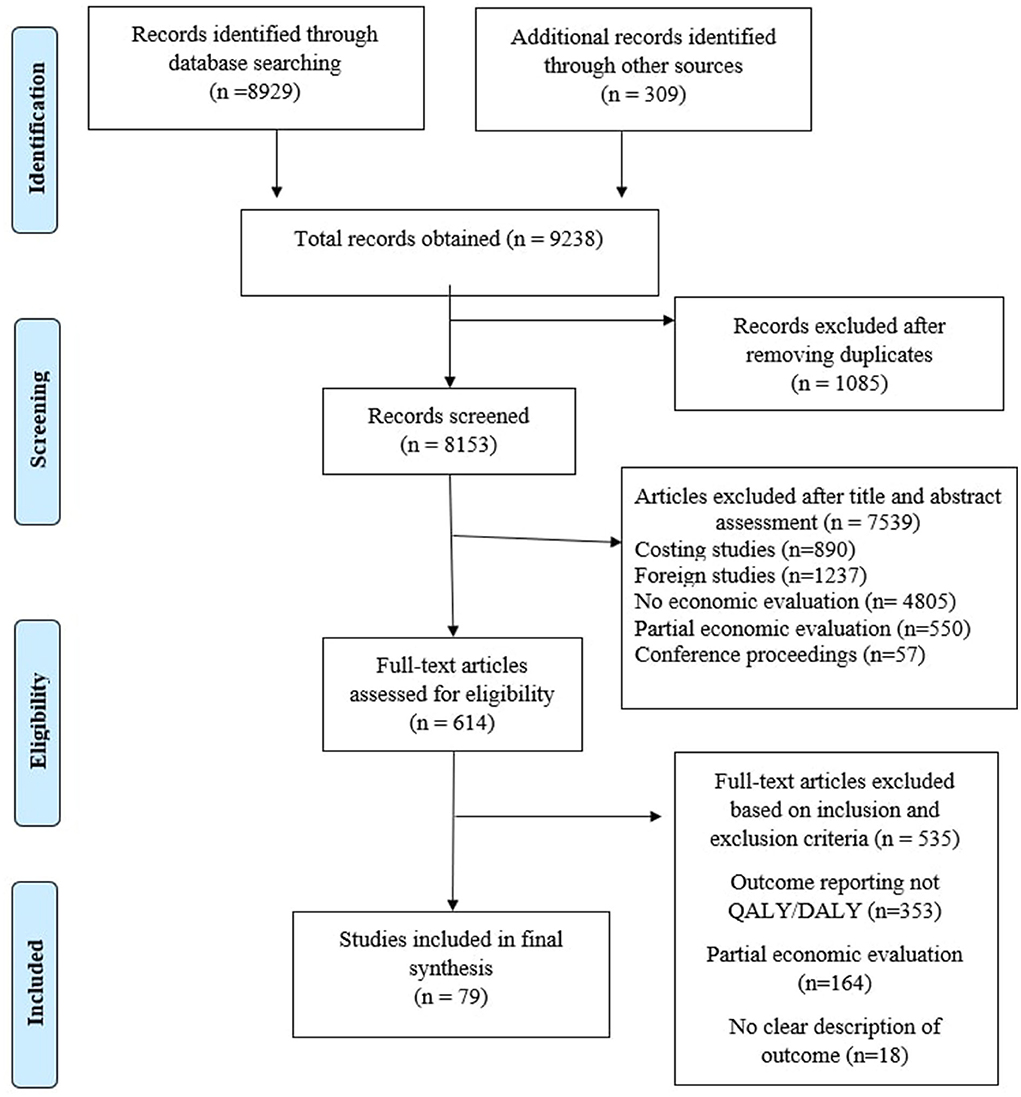
Results: After the initial screening of 9,823 articles, 79 articles meeting the inclusion criteria were selected for the League table preparation. The spectrum of intervention was dominated by innovations for infectious diseases (33%), closely followed by maternal and child health (29%), and non-communicable diseases (20%). The remaining 18% of the interventions were on other groups of health issues, such as injuries, snake bites, and epilepsy. Most of the interventions (70%) reported DALY as an outcome indicator, and the rest (30%) reported QALY. Outcome and cost were discounted at the rate of 3 by 73% of the studies, at 5 by 4% of the studies, whereas 23% of the studies did not discount it. Budget impact and sensitivity analysis were reported by 18 and 73% of the studies, respectively.
Interpretation and conclusions: The present review offers a reasonably coherent league table that reflects ICER values of a range of health conditions in India. It presents an update for decision-makers for making decisions about resource allocation.
Introduction
The Department of Health Research of India has introduced Health Technology Assessment (HTA) processes for better allocation of the resources within the health sector and a governing body, such as HTA in India (HTAIn), is established at the Department of Health Research (DHR). Its mandate is to undertake a critical appraisal of the available technologies and identify the most cost-effective interventions. This initiative recognizes the important role of economic evidence in setting health-sector priorities (1). HTAIn aims to encourage investment in cost-effective interventions that will reduce the cost of patient care, expenditure on medical equipment, the overall cost of medical treatment, out-of-pocket expenditure, and streamline medical reimbursement procedures (2).
The contribution of economic evaluation in guiding decisions on resource allocation in health care has been widely accepted (3). In this context, the “cost per quality-adjusted life-year (QALY) gained” league table, is a well-known tool for both health economists and health care decision-makers alike (2, 3).
League-table is a great tool for stakeholders, such as policymakers, decision-makers at state and central government levels, insurance companies, and pharmaceutical companies who are working on the cost-effectiveness of their new products (drug, vaccine, or medical devices) to determine threshold values to help them assess and interpret the cost-effectiveness of the health technology under study. League tables rank health technology interventions or products or programs in terms of cost-effectiveness for numerous diseases (4, 5).
League tables rank healthcare interventions based on their incremental cost-effectiveness ratios (ICERs) and inform decision-makers about the prioritization of effective healthcare programs and allocation of scarce healthcare resources.
League tables are valuable tools for prioritizing health expenses, especially for national health resources, and have been used as a policy tool by high-, midde-, and low-income countries (6–8). League tables have been used in several prioritization exercises, such as World Bank Health Sector Priorities Review; (9, 10) the WHO Choosing Interventions that are Cost-Effective (WHO-CHOICE) initiative, (11, 12) and the World Health Report since 2000 by the WHO (13).
A few regional league tables are available for some diseases. For example, there are league tables in Africa for 60 different interventions (6). The league tables are available in other countries as well (14, 15). They have been used as policy tools for high-income, as well as low- and middle-income countries (5). Country-specific “league tables” are often a useful tool for national healthcare planning and budgeting (6, 7).
Gerald and Morrey argued that the important goal of QALY league tables is the maximization of the utility of health gains within a health service budget (16). They have stressed that league tables can be a potential means to transfer the results of the original studies to the local context. Though cost-effectiveness is not the only important criterion for policy choice, it provides a useful and comprehensible reference point.
Considering India's meager public investment in the health sector, it is critical that resources are used astutely on cost-effective interventions. Evidence-based policy decisions require robust technical evaluations and, hence, we aim to construct an all-inclusive document of cost-utility ratio findings based on peer-reviewed published research from India, obtained from standard databases. Considering the country's transition to Universal Health Coverage (UHC), priorities are set for achieving the Sustainable Development Goals (SDGs), and with the inception of HTAIn, it is timely to assess that the evidence published around the cost-effectiveness of healthcare interventions, including programs in India to avoid duplication of efforts, identify and prioritize HTA study areas, and, importantly, develop a league table for the country.
In 2015, Prinja et al. (17) conducted a systematic review of economic evaluations of healthcare interventions or program published during the period from January 1980 to mid of November 2014. Findings indicated the need for better economic evaluations with robust methodologies in India, as only one-third of the studies assessed modeling structural uncertainties (33%), or run sub-group analyses to account for heterogeneity (36.5%), or analyzed methodological uncertainty (32%). The aim of the study was to develop a comprehensive league table of cost per QALY or DALY ratios derived from HTA or economic evaluation studies conducted in India from January 2003 to October 2019.
Methods
Search strategy
Cost-effectiveness studies and health technology assessments conducted in India between January 2003 to October 2019 were searched from PubMed, Scopus, and York Center for Reviews and Dissemination. Keywords were checked for controlled vocabulary under Medical Subject Headings (MeSH) of PubMed. We selected articles published from 2003, as latter part of 2002; a revised National Health Policy in India was introduced that aimed to provide more equitable health care services across all the classes of population across country. This policy essentially focused on achieving acceptable standards of good quality health care for Indian population and, hence, a general interest to look for evidence-supported cost effective solutions started around that period. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) was used for this review, where “Cochrane Handbook for Systematic Reviews of Interventions version 6.1 2020,” particularly parts 2 and 3 of the handbook, was followed for conducting the review (18).
We only included studies reporting common outcomes (QALY or DALY) and methodology to increase the generalizability of league table findings. To opt for a uniform criterion, a reference case approach developed by Health Technology Assessment in India (HTAIn) was used (19). The Indian reference case encompasses guidance for conducting and reporting economic evaluation drawn from global best practices and a principle-based approach to strike a balance between specificity and flexibility for the Indian context. Although the reference case advocates reporting of incremental cost-effectiveness ratio with respect to QALY only, a large number of studies assessed cost-effectiveness in the form of DALY, and, hence, to provide a holistic overview of the Indian HTA studies, we included both QALY and DALY for the review. The review included only peer-reviewed articles that were reported in the English language. Abstracts, non-peer-reviewed reports, expert opinion, editorial and narrative reviews, partial economic evaluations, and cost analysis were excluded from the review.
Selection of studies
Three researchers extracted records from various databases. At the initial stage, all duplicates were removed, and relevant studies were selected for evaluation by title and abstract assessment that was followed by full-text review, which involved examination of the content for key indicators of review. In the second-stage screening, only full health economic evaluations that were comparing both costs and outcomes of two or more health-care interventions or program pertaining to India, published in English during January 2003 to October 2019, were critically reviewed. At this stage, a bibliographic search of the selected studies was carried out to identify additional relevant economic evaluations. The search continued until no new article was found. A disagreement between the two authors, having access to abstracts and full text of the paper and decided on its inclusion and discrepancies between the two investigators, was resolved in discussion with the third author. Efforts were ensured to eliminate any bias by adhering to strict criteria for inclusion of studies in the review.
Selection criteria for standardized league table and quality assessment
The cost-effectiveness reported in Indian studies varied widely in terms of quality and minimum requirements as mentioned in the reference cases recently, as developed and published by DHR. Hence, the selection criteria were restricted to few vital criteria only, adapted from previously reported guidelines (17, 19). This included information on perspective, outcome reporting (QALY/DALY), appropriate comparisons of incremental comparisons, time horizon, type of data and type of model used, and type of comparator used.
To avoid any possible criticism on heterogeneity associated with methodologies of various economic evaluations that results in the poorer utilization of league tables for policy decisions, current analysis included studies, which were critically evaluated for key indicators of quality, using the CHEERs checklist (20). Broadly, the information on sensitivity analysis, budget impact analysis, and discounting of the cost and effect were chosen as quality indicators. It is important to note that limited studies have included all these indicators. The systematic search yielded a large number of Indian studies reporting cost-effectiveness in terms of life years saved; however, to maintain uniformity and to follow reference cases, these studies were excluded from the review.
Adjustment of published cost-utility ratios
To present generalizable findings that may hold value in policy decisions at the present scenario, the reported ICERs were indexed to 2020 US$ and were mentioned accordingly. In the case of studies reporting ICERs in terms of INR (Indian currency), first, the published INR were converted to US$ (for the published year) and then inflated to 2020 US$.
ICER and ICER threshold assessment
The incremental cost-effectiveness ratio (ICER) is a measure to summarize the cost-effectiveness of a health care intervention in comparison with an alternative intervention or no alternative intervention. It is defined as a ratio of the difference in cost between two possible interventions, divided by the difference in their effect based on health outcomes (usually, quality adjusted life years or disability adjusted life years) (11, 21). It represents the average incremental cost associated with 1 additional unit of the measure of effect.
The cost per QALY/DALY assessed in the present study includes various modalities of threshold assessment, such as the following: (1) widely recommended approach of economic evaluation—per capita gross domestic product (GDP) was used to identify cost-effective studies for Indian health care set up; (21) and (2) Distribution of all the cost per QALY/DALY were plotted and categorized according to quartiles.
Decision-makers can use it as a decision rule in resource allocation based on a cost-effectiveness threshold. The concept of a cost-effectiveness threshold represents the highest value that society is willing to pay for a unit of health gain or forgo by funding the intervention (opportunity cost). There are many thresholds used for decision-making in a cost-effectiveness analysis, such as supply-side threshold (a measure of allocating resources from the provider's perspective), demand-side threshold (a measure of allocating resources from patient's perspective), or GDP based thresholds (willingness-to-pay value by individuals) (21). GDP-based threshold is recommended by several guidelines in the absence of evidence on other threshold measures (2, 19). Interventions below the threshold value are judged as cost-effective and usually accepted and funded, while those above the threshold value are considered too expensive.
Results
After initial search of the previously mentioned databases, 9,238 articles were assessed for their potential inclusion in the study. The detailed strategy opted for systematic retrieval of the articles is presented in Figure 1. One thousand eighty-five articles were found as duplicated reports and were removed from the record. The remaining 8,153 articles were evaluated based on title and abstract. After careful assessment, 6,789 articles were eliminated. Full text of the shortlisted articles was retrieved and finally included in the study. Based on various reasons mentioned in the PRISMA diagram, 79 articles (13–91) were included for final evidence synthesis and league table preparation.
Characteristics of the included studies
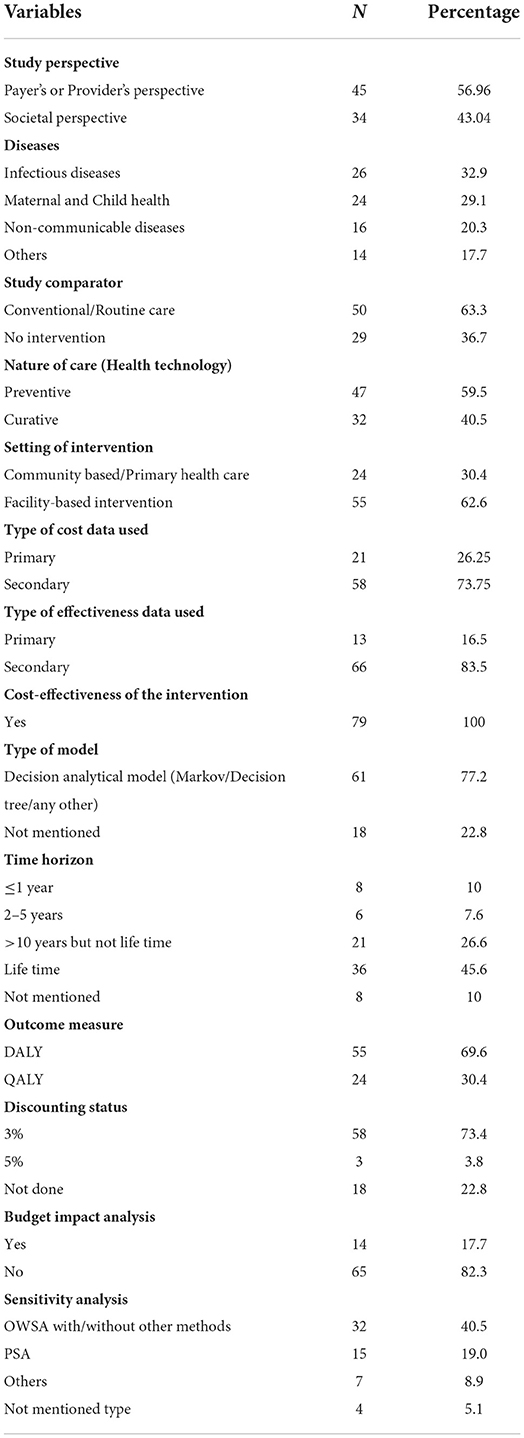
The key characteristics of the included studies are enlisted in Supplementary Table 1. The critical information needed to provide a league table, such as author, title of the study, publication year, type of disease, type of innovation and ICER (indexed for 2020), the status of budget impact analysis, and sensitivity analysis, were extracted from each study and is presented in a tabular format. General summary of other important features of the studies were analyzed and shown in Table 1. Studies assessing cost-effectiveness from the payer's or provider's perspective (56.25%) were dominated over by other studies considering societal perspective (43.75%), including consideration of out-of-pocket expenditure.
Studies were broadly classified into following categories: Infectious diseases, non-communicable diseases, maternal and child health, and others. Details of the disease type distribution are also provided in the Table 1, indicating huge share of studies addressing interventions for Infectious diseases. Popular choice of comparator was routine/conventional scenario (63.3%) over absence of any intervention (36.7%). Large number of HTA studies were for preventive intervention (59.5%), followed by the curative intervention (40.5%). Both cost and clinical efficacy studies chiefly used secondary available literature and data. Almost half (46.25%) of the studies assessed the impact of intervention over a lifetime. Outcome reporting opted for the studies was predominantly disability-adjusted life years averted (70%). The majority of the studies were of good quality as indicated by CHEER's checklist-based assessment. A considerable number of the studies undertook sensitivity analysis (73.4%). However, budget impact analysis was not explored by 18% of the studies.
ICER threshold
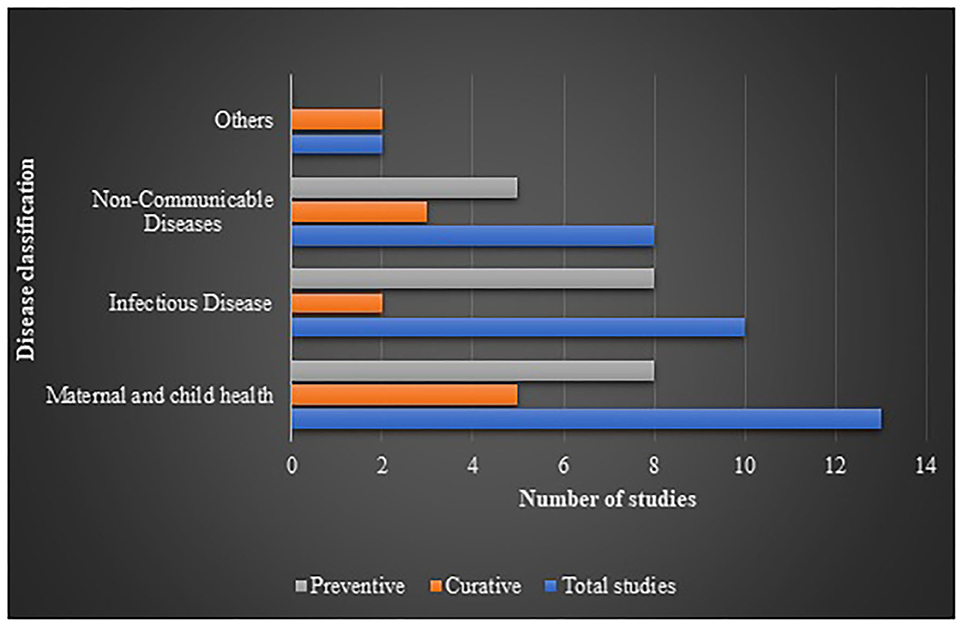
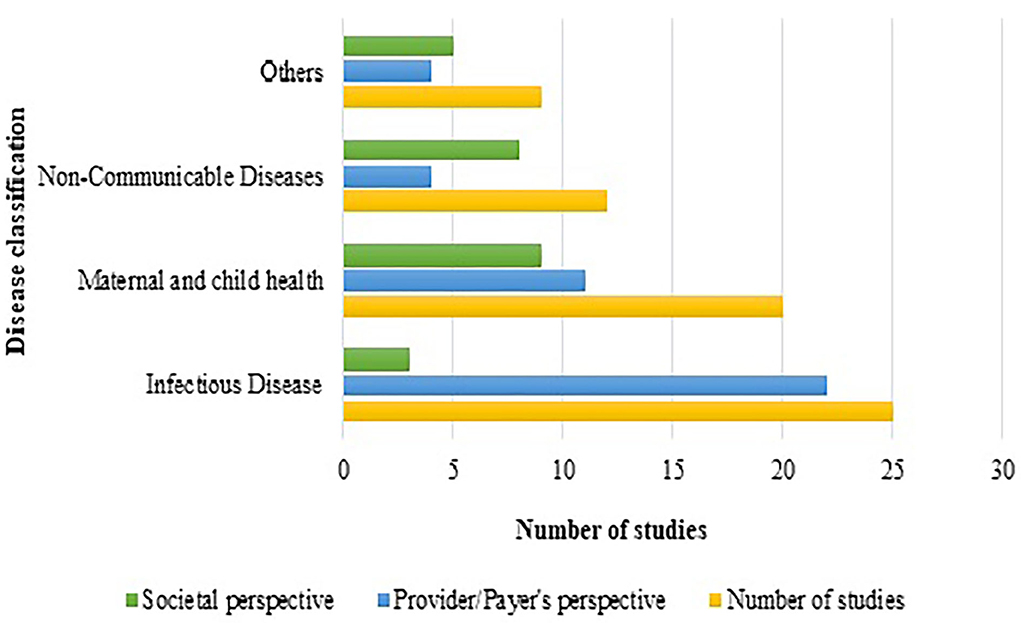
Interventions showing ICER were plotted against diseases (the diseases were broadly classified into 4 categories of interest, nature of care and shown in Figure 2. It indicated that most of the interventions addressed infectious diseases and maternal and child health, where preventive interventions preponderated the spectrum. A comprehensive assessment of ICER corresponding to per capita gross domestic product (GDP) of India (as per 2019 statistics) according to diseases and study perspective is presented as Figure 3. Sixty-six (83.5%) interventions fall below the threshold of per capita Indian GDP, where majority of the studies assessed cost-effectiveness of the innovations for maternal and child diseases and infectious diseases. Chiefly, the studies evaluated the effectiveness from provider's (healthcare services, health practitioners) and payer's perspectives (such as government, insurance, or healthcare services by the non-governmental organizations) and, hence, have not considered out-of-pocket expenditures.
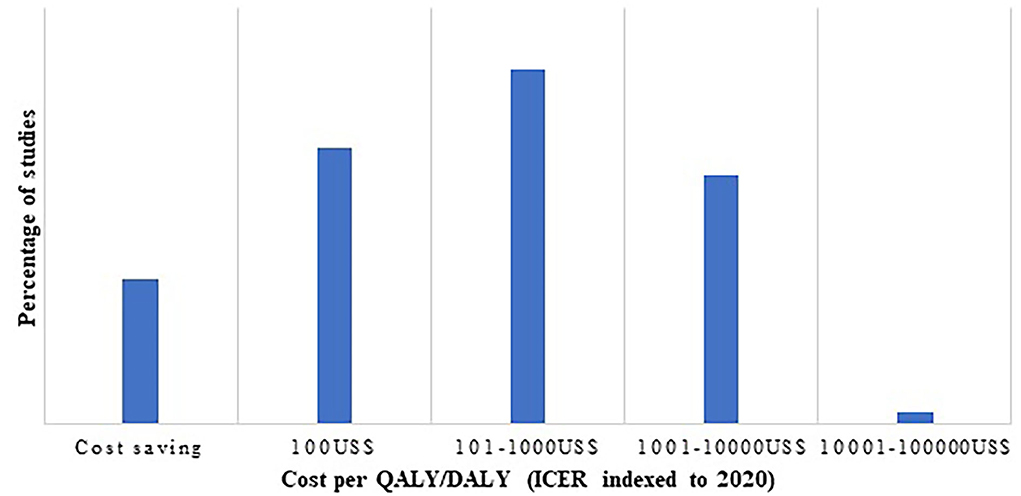
As indicated in Figure 4, cost per QALY/DALY estimates were chiefly falling in the range of cost saving to 1,000 US$ per QALY/DALY. Proportion of cost saving interventions was 13.9%, whereas 1.3% had indexed ICER (to 2020) of 100,000 US$.
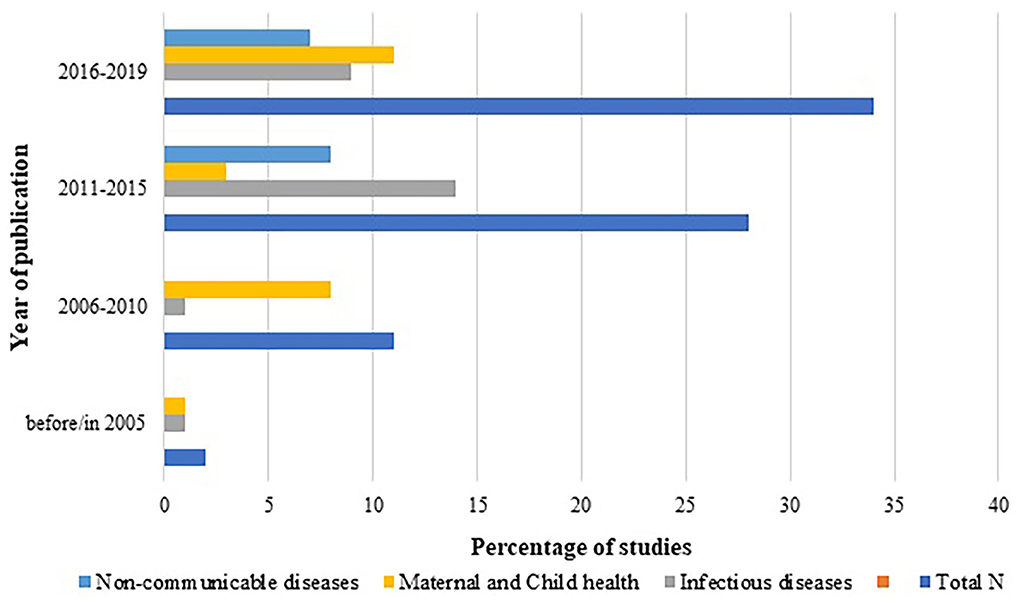
Year-wise distribution of the studies according to the disease type was also assessed and plotted as Figure 5. It showed steep increase in HTA studies with time, with maximum HTA studies reported between 2015 to 2019. Although interventions for infectious diseases have shown consistently dominant trend among all the diseases across all the years, the last decade's innovations addressing non-communicable diseases have started picking up.
Discussion
The prime challenge in development and calibration of league table is the heterogeneity among cost per QALY/DALY ratios reported by various studies. The purpose of this comprehensive review of published cost-effectiveness studies is to create a league table for India that may act as a reference document and assist in identify standardized methodologies and provide a landscape assessment of the of cost-effectiveness studies from India.
We compiled 79 studies reporting cost per QALY/DALY and observed that despite incremental cost-effectiveness ratio (ICER) varying widely, majority of the interventions were falling below the WHO recommended threshold of cost-effectiveness for India (per capita GDP 2019) (92). We retrieved and critically reviewed all the important methodological information from the 79 studies according to the reference case prepared by DHR for India (2, 19). The key exercises undertaken by the authors were the following: (1) The conversion of all the reported ICERs to corresponding value of 2020 US$. This served as an important base for “Head-to-Head Comparisons” of the interventions; (2) This league table provided entire spectrum of cost per QALY/DALY data for various disease groups that range from cost saving to 100,000 US$ per QALY/DALY and its distribution as per the perspective opted, along with the nature of care. This analysis yielded documentation of the group of interventions that are highly cost-effective; (3) The document also evaluated the quality of the finding and its potential use for informing policy decisions using surrogate markers, such as discounting, budget impact, and sensitivity analysis; (4) The league table also identified and mapped the interventions that are falling below the cost-effectiveness Indian threshold—per capita GDP (as per 2019 values) and categorized them further according to disease group and perspective of economic evaluation. The time trend analysis clearly depicted a steep increase in reporting of cost-effectiveness studies especially after the establishment of Health Technology Assessment in India (HTAIn)—an HTA body under DHR following the recommendation of 12th Plan Working Group on Health Research (93). This study may provide a detailed information and facilitate evidence-based decisions through parallel comparisons of the healthcare innovations. We aim to continuously upgrade the league tables with upcoming economic evaluations where the current report may act as a benchmark for future reference cases.
Limitations
Although reporting of cost-utility ratios from India varies widely, the review restricted article inclusion using reporting criteria of most the popular forms—QALY and DALY—to provide better generalizability of the findings. In this process, some important studies, which expressed findings as cost per Life years saved (LYS) and other critical outcomes, remained untapped. Another important limitation of the league table presented here is to completely rely on the published cost—utility ratios, which may have some inherent bias and methodological challenges. There may be underreported clinical and modeling assumption that may significantly influence the outcome and interpretation. Moreover, some of the best pieces of research in gray literature are found in India that remain unpublished in reputed databases and, hence, we may have missed some of the important observations holding contextual values. In addition to this, we could not assess the quality of the studies using any of the recommended checklist due to considerable data gaps and had to adhere to the minimum criteria of quality assessment for compilation of current league table.
Conclusion
With rapidly changing dynamics of healthcare investment, with establishment of India's own HTA body, increasing awareness about cost-effectiveness analysis of this snapshot of cost-utility studies will act as a valuable reference for healthcare planning and resource allocation. Limitations of existing health technology assessments or economic evaluation studies underscore methodological priorities for future health technology assessment studies. The disease specific league table will assist in mapping the disease burden with the investment needs and will prepare the country for combating economic burden associated with morbidity and mortality in futuristic manner.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
KS and MS conceptualized and designed the study and developed search strategy. KS prepared first draft of the manuscript. KT, PK, and AP completed search, extracted data, and synthesize evidence. SS, DS, and KR guided evidence synthesis, resolve disputes in inclusion, exclusion of studies, reviewed, revised, and provided significant inputs in manuscript draft. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Health Technology Assessment in India, Department of Health Research, Ministry of Health and Family Welfare, India.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.831254/full#supplementary-material
References
1. Rao NV, Downey L, Jain N, Baru R, Cluzeau F. Priority-setting, the Indian way. J Glob Health. (2018) 8:020311. doi: 10.7189/jogh.08.020311
2. Department of Health Research. Health Technology Assessment in India: A Manual. 2018. Ministry of Health and Family Welfare, Government of India. Available online at: https://htain.icmr.org.in/index.php/documents/publications/htain-manual.
3. Mason JM. Cost-per-QALY league tables: their role in pharmacoeconomic analysis. Pharmacoeconomics. (1994) 5:472–81. doi: 10.2165/00019053-199405060-00004
4. Schlander M, Garattini S, Holm S, Kolominsky-Rabas P, Nord E, Persson U, et al. Incremental cost per quality-adjusted life-year gained? The need for alternative methods to evaluate medical interventions for ultra-rare disorders. J Comp Effect Res. (2014) 3:399–422. doi: 10.2217/cer.14.34
5. Horton S, Gelband H, Jamison D, Levin C, Nugent R, Watkins D. Ranking 93 health interventions for low- and middle-income countries by cost-effectiveness. PLoS ONE. (2017) 12:e0182951. doi: 10.1371/journal.pone.0182951
6. Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. (2015) 93:118–24. doi: 10.2471/BLT.14.138206
7. Mauskopf J, Rutten F, Schonfeld W. Cost-effectiveness league tables. Pharmacoeconomics. (2003) 21:991–1000. doi: 10.2165/00019053-200321140-00001
8. Wilson N, Davies A, Brewer N, Nghiem N, Cobiac L, Blakely T. Can cost-effectiveness results be combined into a coherent league table? Case study from one high-income country. Popul Health Metr. (2019) 17:1–8. doi: 10.1186/s12963-019-0192-x
9. Jamison DT, Alleyne G, Breman J, Measham AR, Alleyne G, Claeson M, et al. editors. Disease Control Priorities in Developing Countries. 2nd ed. New York, NY: Oxford University Press (2006). p. 35–86.
10. Hutubessy R, Chisholm D, Edejer TT. Generalized cost-effectiveness analysis for national-level priority-setting in the health sector. Cost Eff Resour Alloc. (2003) 1:8. doi: 10.1186/1478-7547-1-8
11. World Health Organization. Cost-Effectiveness and Strategic Planning (WHO-CHOICE). Table: Threshold Values for Intervention Cost-Effectiveness by Region. Geneva: World Health Organization (2010).
12. Chisholm D. Choosing cost-effective interventions in psychiatry: results from the CHOICE programme of the World Health Organization. World Psychiatry. (2005) 4:37–44.
13. World Health Organization. The World Health Report 2002: Reducing Risks, Promoting Healthy Life. Geneva: World Health Organization (2002).
14. Hashempour R, Raei B, Safaei Lari M, Abolhasanbeigi Gallezan N, AkbariSari A. QALY league table of Iran: a practical method for better resource allocation. Cost Eff Resour Alloc. (2021) 19:1–1. doi: 10.1186/s12962-020-00256-2
15. Shillcutt SD, Walker DG, Goodman CA, Mills AJ. Cost effectiveness in low- and middle-income countries: a review of the debates surrounding decision rules. Pharmacoeconomics. (2009) 27:903–17. doi: 10.2165/10899580-000000000-00000
16. Gerard K, Mooney G. QALY league tables: handle with care. Health Econ. (1993) 2:59–64. doi: 10.1002/hec.4730020108
17. Prinja S, Chauhan AS, Angell B, Gupta I, Jan S. A systematic review of the state of economic evaluation for health care in India. Appl Health Econ Health Policy. (2015) 13:595–613. doi: 10.1007/s40258-015-0201-6
18. Higgins JP. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration (2011). Available from: www.cochrane-handbook.org (accessed July 2019).
19. Department of Health Research. Health Technology Assessment in India - HTAIn. Available online at: https://dhr.gov.in/sites/default/files/eNewsletter/img/HTAIn/HTAIn10-01-2017.pdf (accessed August 29, 2019).
20. Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated health economic evaluation reporting standards (CHEERS)—Explanation and elaboration: A report of the ISPOR health economic evaluations publication guidelines good reporting practices task force. Value Health. (2013) 16:231–50. doi: 10.1016/j.jval.2013.02.002
21. Edejer, T T-T, Baltussen R, Adam T, Hutubessy R, Acharya A, Evans DB, Murray CJL, editors. Making Choices in Health: WHO Guide to Cost Effectiveness Analysis. Geneva: World Health Organization (2003).
22. Ghoshal UC, Aggarwal R, Baba CS. Recurrent duodenal ulcer haemorrhage: a pharmacoeconomic comparison of various management strategies. Expert Opin Pharmacother. (2003) 4:1593–603. doi: 10.1517/14656566.4.9.1593
23. Fung IC, Guinness L, Vickerman P, Watts C, Vannela G, Vadhvana J, et al. Modelling the impact and cost-effectiveness of the HIV intervention programme amongst commercial sex workers in Ahmedabad, Gujarat, India. BMC Public Health. (2007) 7:195. doi: 10.1186/1471-2458-7-195
24. Dandona L, Kumar SG, Kumar GA, Dandona R. Cost-effectiveness of HIV prevention interventions in Andhra Pradesh state of India. BMC Health Serv Res. (2010) 10:117. doi: 10.1186/1472-6963-10-117
25. Dowdy DW, Steingart KR, Pai M. Serological testing versus other strategies for diagnosis of active tuberculosis in India: a cost-effectiveness analysis. PLoS Med. (2011) 8:e1001074. doi: 10.1371/journal.pmed.1001074
26. Goodchild M, Sahu S, Wares F, Dewan P, Shukla RS, Chauhan LS, et al. Cost-benefit analysis of scaling up tuberculosis control in India. Int J Tuberc Lung Dis. (2011) 15:358–62.
27. Prinja S, Bahuguna P, Rudra S, Gupta I, Kaur M, Mehendale SM, et al. Cost effectiveness of targeted HIV prevention interventions for female sex workers in India. Sex Transm Infect. (2011) 87:354–61. doi: 10.1136/sti.2010.047829
28. Reid S. Estimating the Burden of Disease from Unsafe Injections in India: a Cost-benefit assessment of the auto-disable syringe in a country with low blood-borne virus prevalence. Indian J Community Med. (2012) 37:89–94. doi: 10.4103/0970-0218.96093
29. Eaton JW, Menzies NA, Stover J, Cambiano V, Chindelevitch L, Cori A, et al. Health benefits, costs, and cost-effectiveness of earlier eligibility for adult antiretroviral therapy and expanded treatment coverage: a combined analysis of 12 mathematical models. Lancet Glob Health. (2014) 2:e23–34. doi: 10.1016/S2214-109X(13)70172-4
30. Hoog AH, Cobelens F, Vassall A, van Kampen S, Dorman SE, Alland D, et al. Optimal triage test characteristics to improve the cost-effectiveness of the Xpert MTB/RIF assay for TB diagnosis: a decision analysis. PloS ONE. (2013) 8:e82786. doi: 10.1371/journal.pone.0082786
31. Vassall A, Pickles M, Chandrashekar S, Boily MC, Shetty G, Guinness L, et al. Cost-effectiveness of HIV prevention for high-risk groups at scale: an economic evaluation of the Avahan programme in south India. Lancet Glob Health. (2014) 2:e531–e40. doi: 10.1016/S2214-109X(14)70277-3
32. Vassall A, Chandrashekar S, Pickles M, Beattie TS, Shetty G, Bhattacharjee P, et al. Community mobilisation and empowerment interventions as part of HIV prevention for female sex workers in Southern India: a cost-effectiveness analysis. PLoS ONE. (2014) 9:e110562. doi: 10.1371/journal.pone.0110562
33. Joshi S, Kulkarni V, Gangakhedkar R, Mahajan U, Sharma S, Shirole D, Chandhiok N. Cost-effectiveness of a repeat HIV test in pregnancy in India. BMJ Open. (2015) 5:e006718. doi: 10.1136/bmjopen-2014-006718
34. Kelly V, Sagili KD, Satyanarayana S, Reza LW, Chadha SS, Wilson NC. Cost-utility analysis of LED fluorescence microscopy in the diagnosis of pulmonary tuberculosis in Indian settings. Int J Tuberc Lung Dis. (2015) 19:696–701. doi: 10.5588/ijtld.14.0203
35. Little KM, Pai M, Dowdy DW. Costs and consequences of using Interferon-γ release assays for the diagnosis of active tuberculosis in India. PLoS ONE. (2015) 10:e0124525. doi: 10.1371/journal.pone.0124525
36. Maddali MV, Dowdy DW, Gupta A, Shah M. Economic and epidemiological impact of early antiretroviral therapy initiation in India. J Int AIDS Soc. (2015) 18:20217. doi: 10.7448/IAS.18.1.20217
37. Rosenthal VD, Udwadia FE, Kumar S, Poojary A, Sankar R, Orellano PW, et al. Clinical impact and cost-effectiveness of split-septum and single-use prefilled flushing device vs 3-way stopcock on central line-associated bloodstream infection rates in India: a randomized clinical trial conducted by the International Nosocomial Infection Control Consortium (INICC). Am J Infect Control. (2015) 43:1040–5. doi: 10.1016/j.ajic.2015.05.042
38. Suen SC, Bendavid E, Goldhaber-Fiebert JD. Cost-effectiveness of improvements in diagnosis and treatment accessibility for tuberculosis control in India. Int J Tuberc Lung Dis. (2015) 19:1115–xv. doi: 10.5588/ijtld.15.0158
39. Kapoor S, Gupta A, Shah M. Cost-effectiveness of isoniazid preventive therapy for HIV-infected pregnant women in India. Int J Tuberc Lung Dis. (2016) 20:85–92. doi: 10.5588/ijtld.15.0391
40. Aggarwal R, Chen Q, Goel A, Seguy N, Pendse R, Ayer T, et. al. Cost-effectiveness of hepatitis C treatment using generic direct-acting antivirals available in India. PLoS ONE. (2017) 12:e0176503. doi: 10.1371/journal.pone.0176503
41. John D, Parikh R. Cost-effectiveness and cost utility of community screening for glaucoma in urban India. Public Health. (2017) 148:37–48. doi: 10.1016/j.puhe.2017.02.016
42. Lu X, Smare C, Kambili C, El Khoury AC, Wolfson LJ. Health outcomes of bedaquiline in the treatment of multidrug-resistant tuberculosis in selected high burden countries. BMC Health Serv Res. (2017) 17:87. doi: 10.1186/s12913-016-1931-3
43. Goel A, Chen Q, Chhatwal J, Aggarwal R. Cost-effectiveness of generic pan-genotypic sofosbuvir/velpatasvir versus genotype-dependent direct-acting antivirals for hepatitis C treatment. J Gastroenterol Hepatol. (2018) 33:2029–36. doi: 10.1111/jgh.14301
44. Krishnamoorthy Y, Eliyas SK, Nair NP, Sakthivel M, Sarveswaran G, Chinnakali P. Impact and cost effectiveness of pneumococcal conjugate vaccine in India. Vaccine. (2019) 37:623–30. doi: 10.1016/j.vaccine.2018.12.004
45. Chaillon A, Mehta SR, Hoenigl M, Solomon SS, Vickerman P, Hickman M, et al. Cost-effectiveness and budgetary impact of HCV treatment with direct-acting antivirals in India including the risk of reinfection. PLoS ONE. (2019) 14:e0217964. doi: 10.1371/journal.pone.0217964
46. Chugh Y, Dhiman RK, Premkumar M, Prinja S, Singh Grover G, Bahuguna P. Real-world cost-effectiveness of pan-genotypic Sofosbuvir-Velpatasvir combination versus genotype dependent directly acting anti-viral drugs for treatment of hepatitis C patients in the universal coverage scheme of Punjab state in India. PLoS ONE. (2019) 14:e0221769. doi: 10.1371/journal.pone.0221769
47. Sohn H, Kasaie P, Kendall E, Gomez GB, Vassall A, Pai M, et al. Informing decision-making for universal access to quality tuberculosis diagnosis in India: an economic-epidemiological model. BMC Med. (2019) 17:155. doi: 10.1186/s12916-019-1384-8
48. Schulman-Marcus J, Prabhakaran D, Gaziano TA. Pre-hospital ECG for acute coronary syndrome in urban India: a cost-effectiveness analysis. BMC Cardiovasc Disord. (2010) 10:13. doi: 10.1186/1471-2261-10-13
49. Lohse N, Marseille E, Kahn JG. Development of a model to assess the cost-effectiveness of gestational diabetes mellitus screening and lifestyle change for the prevention of type 2 diabetes mellitus. Int J Gynaecol Obstet. (2011) 115:S20–5. doi: 10.1016/S0020-7292(11)60007-6
50. Buttorff C, Hock RS, Weiss HA, Naik S, Araya R, Kirkwood BR, et al. Economic evaluation of a task-shifting intervention for common mental disorders in India. Bull World Health Organ. (2012) 90:813–21. doi: 10.2471/BLT.12.104133
51. Marseille E, Lohse N, Jiwani A, Hod M, Seshiah V, Yajnik CS, et al. The cost-effectiveness of gestational diabetes screening including prevention of type 2 diabetes: application of a new model in India and Israel. J Matern Fetal Neonatal Med. (2013) 26:802–10. doi: 10.3109/14767058.2013.765845
52. Rachapelle S, Legood R, Alavi Y, Lindfield R, Sharma T, Kuper H, et al. The cost-utility of telemedicine to screen for diabetic retinopathy in India. Ophthalmology. (2013) 120:566–73. doi: 10.1016/j.ophtha.2012.09.002
53. Home P, Baik SH, Galvez GG, Malek R, Nikolajsen A. An analysis of the cost-effectiveness of starting insulin detemir in insulin-naive people with type 2 diabetes. J Med Econ. (2015) 18:230–40. doi: 10.3111/13696998.2014.985788
54. Basu S, Bendavid E, Sood N. Health and economic implications of national treatment coverage for cardiovascular disease in India: cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes. (2015) 8:541–51. doi: 10.1161/CIRCOUTCOMES.115.001994
55. Gupta V, Baabbad R, Hammerby E, Nikolajsen A, Shafie AA. An analysis of the cost-effectiveness of switching from biphasic human insulin 30, insulin glargine, or neutral protamine Hagedorn to biphasic insulin aspart 30 in people with type 2 diabetes. J Med Econ. (2015) 18:263–72. doi: 10.3111/13696998.2014.991791
56. Patel SP, Pena ME, Babcock CI. Cost-effectiveness of noninvasive ventilation for chronic obstructive pulmonary disease-related respiratory failure in Indian hospitals without ICU facilities. Lung India. (2015) 32:549–56. doi: 10.4103/0970-2113.168137
57. Basu S, Shankar V, Yudkin JS. Comparative effectiveness and cost-effectiveness of treat-to-target versus benefit-based tailored treatment of type 2 diabetes in low-income and middle-income countries: a modelling analysis. Lancet Diabetes Endocrinol. (2016) 4:922–32. doi: 10.1016/S2213-8587(16)30270-4
58. Raykar, N., Nigam, A. & Chishd, D. An extended cost-effectiveness analysis of Schizophrenia treatment in India under universal public finance. Cost Eff Resour Alloc. (2016) 14:9. doi: 10.1186/s12962-016-0058-z
59. Nadkarni A, Weiss HA, Weobong B, McDaid D, Singla DR, Park AL, et al. Sustained effectiveness and cost-effectiveness of Counselling for Alcohol Problems, a brief psychological treatment for harmful drinking in men, delivered by lay counsellors in primary care: 12-month follow-up of a randomised controlled trial. PLoS Med. (2017) 14:e1002386. doi: 10.1371/journal.pmed.1002386
60. Prinja S, Bahuguna P, Faujdar DS, Jyani G, Srinivasan R, Ghoshal S, et al. Cost-effectiveness of human papillomavirus vaccination for adolescent girls in Punjab state: Implications for India's universal immunization program. Cancer. (2017) 123:3253–60. doi: 10.1002/cncr.30734
61. Prinja S, Kaur G, Malhotra P, Jyani G, Ramachandran R, Bahuguna P, et al. Cost-effectiveness of autologous stem cell treatment as compared to conventional chemotherapy for treatment of multiple myeloma in India. Indian J Hematol Blood Transfus. (2017) 33:31–40. doi: 10.1007/s12288-017-0776-1
62. Praveen D, Peiris D, MacMahon S, Mogulluru K, Raghu A, Rodgers A, et al. Cardiovascular disease risk and comparison of different strategies for blood pressure management in rural India. BMC Public Health. (2018) 18:1264. doi: 10.1186/s12889-018-6142-x
63. Lin JK, Moran AE, Bibbins-Domingo K, Falase B, Pedroza Tobias A, Mandke CN, et al. Cost-effectiveness of a fixed-dose combination pill for secondary prevention of cardiovascular disease in China, India, Mexico, Nigeria, and South Africa: a modelling study. Lancet Glob Health. (2019) 7:e1346–e58. doi: 10.1016/S2214-109X(19)30339-0
64. Aggarwal R, Ghoshal UC, Naik SR. Assessment of cost-effectiveness of universal hepatitis B immunization in a low-income country with intermediate endemicity using a Markov model. J Hepatol. (2003) 38:215–22. doi: 10.1016/S0168-8278(02)00382-3
65. Suraratdecha C, Jacobson J, Sivalenka S, Narahari D. A cost-effectiveness analysis of strategies for controlling Japanese encephalitis in Andhra Pradesh, India. J Pharmaceut Finance Econ Pol. (2006) 15:21–40. doi: 10.1300/J371v15n01_05
66. Goldie SJ, O'Shea M, Campos NG, Diaz M, Sweet S & Kim SY. Health and economic outcomes of HPV 16,18 vaccination in 72 GAVI-eligible countries. Vaccine. (2008) 26:4080–93. doi: 10.1016/j.vaccine.2008.04.053
67. Jeuland DW, Poulos C, Clemens J, Sur D, Anh DD, Agtini M, Bhutta Z. The cost-effectiveness of typhoid Vi vaccination programs: calculations for four urban sites in four Asian countries Vaccine. (2008) 26:6305–16. doi: 10.1016/j.vaccine.2008.09.040
68. Esposito DH, Tate JE, Kang G, Parashar UD. Projected impact and cost-effectiveness of a rotavirus vaccination program in India, 2008. Clin Infect Dis. (2011) 52:171–7. doi: 10.1093/cid/ciq094
69. Dabral M. Cost-effectiveness of supplementary immunization for measles in India. Indian Pediatr. (2009) 46:957–62.
70. Jeuland M, Cook J, Poulos C, Clemens J, Whittington D. Cost-effectiveness of new-generation oral cholera vaccines: a multisite analysis. ISPOR. (2009) 12:899–908. doi: 10.1111/j.1524-4733.2009.00562.x
71. Rose J, Hawthorn RL, Watts B, Singer ME. Public health impact and cost effectiveness of mass vaccination with live attenuated human rotavirus vaccine (RIX4414) in India: model based analysis. BMJ. (2009) 339:b3653. doi: 10.1136/bmj.b3653
72. Sutherland T, Meyer C, Bishai DM, Geller S, Miller S. Community-based distribution of misoprostol for treatment or prevention of postpartum hemorrhage; cost effectiveness, mortality, and morbidity reduction analysis. Int J Gynaecol Obstet. (2010) 108:289–94. doi: 10.1016/j.ijgo.2009.11.007
73. Clark AD, Griffiths UK, Abbas SS, Rao KD, Privor-Dumm L, Hajjeh R, et al. Impact and cost-effectiveness of Haemophilus influenzae type b conjugate vaccination in India. J Pediatr. (2013) 163:S60–72. doi: 10.1016/j.jpeds.2013.03.032
74. Gupta M, Prinja S, Kumar R, Kaur M. Cost-effectiveness of Haemophilus influenzae type b (Hib) vaccine introduction in the universal immunization schedule in Haryana State, India. Health Policy Plan. (2013) 28:51–61. doi: 10.1093/heapol/czs025
75. Rheingans R, Anderson JD 4th, Anderson B, Chakraborty P, Atherly D, Pindolia D. Estimated impact and cost-effectiveness of rotavirus vaccination in India: effects of geographic and economic disparities. Vaccine. (2014) 32 (Suppl. 1):A140–50. doi: 10.1016/j.vaccine.2014.05.073
76. Plessow R, Arora NK, Brunner B, Wieser S. Cost-effectiveness of price subsidies on fortified packaged infant cereals in reducing iron deficiency anemia in 6-23-month-old-children in Urban India. PLoS ONE. (2016) 11:e0152800. doi: 10.1371/journal.pone.0152800
77. Prinja S, Bahuguna P, Mohan P, Mazumder S, Taneja S, Bhandari N, et al. Cost effectiveness of implementing integrated management of neonatal and childhood illnesses program in district Faridabad, India. PLoS ONE. (2016) 11:e0145043. doi: 10.1371/journal.pone.0145043
78. Fitzpatrick MC, Shah HA, Pandey A, Bilinski AM, Kakkar M, Clark AD, et al. One Health approach to cost-effective rabies control in India. Proc Natl Acad Sci U S A. (2016) 113:14574–81. doi: 10.1073/pnas.1604975113
79. Antillón M, Bilcke J, Paltiel AD, Pitzer VE. Cost-effectiveness analysis of typhoid conjugate vaccines in five endemic low- and middle-income settings. Vaccine. (2017) 35:3506–14. doi: 10.1016/j.vaccine.2017.05.001
80. Sinha RK, Haghparast-Bidgoli H, Tripathy PK, Nair N, Gope R, Rath S, et al. Economic evaluation of participatory learning and action with women's groups facilitated by Accredited Social Health Activists to improve birth outcomes in rural eastern India. Cost Eff Resour Alloc. (2017) 15:2. doi: 10.1186/s12962-017-0064-9
81. Zhang S, Incardona B, Qazi SA, Stenberg K, Campbell H, Nair H, et al. Working Group. Cost-effectiveness analysis of revised WHO guidelines for management of childhood pneumonia in 74 Countdown countries. J Glob Health. (2017) 7:010409. doi: 10.7189/jogh.07.010409
82. Goudet S, Jayaraman A, Chanani S, Osrin D, Devleesschauwer B, Bogin B, et al. Cost effectiveness of a community based prevention and treatment of acute malnutrition programme in Mumbai slums, India. PLoS ONE. (2018) 13:e0205688. doi: 10.1371/journal.pone.0205688
83. Powell-Jackson T, Fabbri C, Dutt V, Tougher S, Singh K. Effect and cost-effectiveness of educating mothers about childhood DPT vaccination on immunisation uptake, knowledge, and perceptions in Uttar Pradesh, India: a randomised controlled trial. PLoS Med. (2018) 15:e1002519. doi: 10.1371/journal.pmed.1002519
84. Prinja S, Bahuguna P, Gupta A, Nimesh R, Gupta M, Thakur JS. Cost effectiveness of mHealth intervention by community health workers for reducing maternal and newborn mortality in rural Uttar Pradesh, India. Cost Eff Resour Alloc. (2018) 16:25. doi: 10.1186/s12962-018-0110-2
85. Bettampadi D, Boulton ML, Power LE, Hutton DW. Are community health workers cost-effective for childhood vaccination in India? Vaccine. (2019) 37:2942–51. doi: 10.1016/j.vaccine.2019.04.038
86. Kashi B, Godin CM, Kurzawa ZA, Verney A, Busch-Hallen J, De-Regil L. Multiple micronutrient supplements are more cost-effective than iron and folic acid: modeling results from 3 high-burden Asian countries. J Nutr. (2019) 149:1222–9. doi: 10.1093/jn/nxz052
87. Frick K, Riva-Clement L, Shankar MB. Screening for refractive error and fitting with spectacles in rural and urban India: cost-effectiveness. Ophthal Epidemiol. (2009) 16:378. doi: 10.3109/09286580903312277
88. Rob B, Vinod JA, Monica P, Balraj A, Job A, Norman G, et al. Costs and health effects of screening and delivery of hearing aids in Tamil Nadu, India: an observational study. BMC Public Health. (2009) 9:135. doi: 10.1186/1471-2458-9-135
89. Cecchini MF, Sassi JA, Lauer YY, Lee V, Guajardo-Barron D. Chisholm Tackling of unhealthy diets, physical inactivity, and obesity: health effects and cost-effectiveness. Lancet. (2010) 376:1775–84. doi: 10.1016/S0140-6736(10)61514-0
90. Chow J, Klein EY, Laxminarayan R. Cost-effectiveness of “golden mustard” for treating vitamin A deficiency in India. PLoS ONE. (2010) 5:e12046. doi: 10.1371/journal.pone.0012046
91. Brown HS, Stigler M, Perry C, Dhavan P, Arora M, Reddy KS. The cost-effectiveness of a school-based smoking prevention program in India. Health Promot Int. (2013) 28:178–86. doi: 10.1093/heapro/dar095
92. Megiddo I, Colson A, Chisholm D, Dua T, Nandi A, Laxminarayan R. Health and economic benefits of public financing of epilepsy treatment in India: An agent-based simulation model. Epilepsia. (2016) 57:464–74. doi: 10.1111/epi.13294
93. Department of Health Research, Ministry of Health and Family Welfare, Government of India. Available online at: https://www.dhr.gov.in (accessed on March 15, 2020).
Keywords: country-specific “league table”, Health Technology Assessment, India, cost-effectiveness, policy decision-making process
Citation: Shah K, Singh M, Kotwani P, Tyagi K, Pandya A, Saha S, Saxena D and Rajshekar K (2022) Comprehensive league table of cost-utility ratios: A systematic review of cost-effectiveness evidence for health policy decisions in India. Front. Public Health 10:831254. doi: 10.3389/fpubh.2022.831254
Received: 13 December 2021; Accepted: 22 August 2022;
Published: 13 October 2022.
Edited by:
Piyameth Dilokthornsakul, Chiang Mai University, ThailandReviewed by:
Suresh Munuswamy, Public Health Foundation of India, IndiaJulie Abimanyi-Ochom, Deakin University, Australia
Copyright © 2022 Shah, Singh, Kotwani, Tyagi, Pandya, Saha, Saxena and Rajshekar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Komal Shah, a3NoYWhAaWlwaGcub3Jn
 Komal Shah
Komal Shah Malkeet Singh
Malkeet Singh Priya Kotwani
Priya Kotwani Kirti Tyagi2
Kirti Tyagi2 Apurvakumar Pandya
Apurvakumar Pandya Somen Saha
Somen Saha Deepak Saxena
Deepak Saxena