
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Public Health , 24 March 2022
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.809356
 Sarah E. Smith-Jeffcoat1*
Sarah E. Smith-Jeffcoat1* Sadia Sleweon1*
Sadia Sleweon1* Mitsuki Koh1
Mitsuki Koh1 George M. Khalil1
George M. Khalil1 Marcos C. Schechter2,3
Marcos C. Schechter2,3 Paulina A. Rebolledo2,4
Paulina A. Rebolledo2,4 Vyjayanti Kasinathan2,3
Vyjayanti Kasinathan2,3 Adam Hoffman3,5
Adam Hoffman3,5 Rebecca Rossetti1
Rebecca Rossetti1 Talya Shragai1
Talya Shragai1 Kevin O'Laughlin1
Kevin O'Laughlin1 Catherine C. Espinosa1
Catherine C. Espinosa1 Bettina Bankamp1
Bettina Bankamp1 Michael D. Bowen1
Michael D. Bowen1 Ashley Paulick1
Ashley Paulick1 Amy S. Gargis1
Amy S. Gargis1 Jennifer M. Folster1
Jennifer M. Folster1 Juliana da Silva1
Juliana da Silva1 Caitlin Biedron1
Caitlin Biedron1 Rebekah J. Stewart1
Rebekah J. Stewart1 Yun F. Wang3,6
Yun F. Wang3,6 Hannah L. Kirking1
Hannah L. Kirking1 Jacqueline E. Tate1 and CDC COVID-19 Emergency Response GA-10 Field1
Jacqueline E. Tate1 and CDC COVID-19 Emergency Response GA-10 Field1We aimed to describe frequency of COVID-19 exposure risk factors among patients presenting for medical care at an urban, public hospital serving mostly uninsured/Medicare/Medicaid clients and risk factors associated with SARS-CoV-2 infection. Consenting, adult patients seeking care at a public hospital from August to November 2020 were enrolled in this cross-sectional investigation. Saliva, anterior nasal and nasopharyngeal swabs were collected and tested for SARS-CoV-2 using RT-PCR. Participant demographics, close contact, and activities ≤14 days prior to enrollment were collected through interview. Logistic regression was used to identify risk factors associated with testing positive for SARS-CoV-2. Among 1,078 participants, 51.8% were male, 57.0% were aged ≥50 years, 81.3% were non-Hispanic Black, and 7.6% had positive SARS-CoV-2 tests. Only 2.7% reported COVID-19 close contact ≤14 days before enrollment; this group had 6.79 adjusted odds of testing positive (95%CI = 2.78–16.62) than those without a reported exposure. Among participants who did not report COVID-19 close contact, working in proximity to ≥10 people (adjusted OR = 2.17; 95%CI = 1.03–4.55), choir practice (adjusted OR = 11.85; 95%CI = 1.44–97.91), traveling on a plane (adjusted OR = 5.78; 95%CI = 1.70–19.68), and not participating in an essential indoor activity (i.e., grocery shopping, public transit use, or visiting a healthcare facility; adjusted OR = 2.15; 95%CI = 1.07–4.30) were associated with increased odds of testing positive. Among this population of mostly Black, non-Hispanic participants seeking care at a public hospital, we found several activities associated with testing positive for SARS-CoV-2 infection in addition to close contact with a case. Understanding high-risk activities for SARS-CoV-2 infection among different communities is important for issuing awareness and prevention strategies.
Since January 2020, and as of January 2022, over 75,000,000 confirmed cases of COVID-19 and 800,000 deaths from COVID-19 have occurred in the United States (1). Furthermore, people of color have been disproportionally affected by SARS-CoV-2 infection and Black, non-Hispanic people have been underrepresented in COVID-19 investigations (2). SARS-CoV-2, the virus that causes COVID-19, reaches peak viral load soon after symptom onset which increases the likelihood of transmission and, consequentially, is the time period of focus for prevention strategies (3). Documented pre-symptomatic and asymptomatic transmission of SARS-CoV-2 reduces the effectiveness of preventing spread through isolation strategies (4). Initially, state and local governments enacted a myriad of restrictions on different types of indoor and outdoor activities to limit community transmission. Many of these decisions emerged from laboratory-based experiments or small outbreak investigations focused on COVID-19 transmission dynamics. More recently, several studies have investigated high-risk activities associated with testing positive for SARS-CoV-2; however, Black, non-Hispanic people represented only 2–13% of the participants (5–7). A few additional reports investigated exposures and potential high-risk activities among groups that have been disproportionately affected by the COVID-19 epidemic including people experiencing homelessness or incarceration (8, 9). More recently, the mass vaccination campaign in the U.S. has helped reduce COVID-19 severe illness and death (10). However, due to the introduction of the Omicron variant in the U.S., non-pharmaceutical interventions have again become essential to halting the pandemic.
As part of an investigation to understand the diagnostic performance of self-collected saliva and anterior nasal (AN) swabs compared to healthcare worker-collected nasopharyngeal (NP) swab, participants at a large, public hospital in Fulton County, Georgia, were interviewed about COVID-19 exposures, employment, and social activities that may be potential risk factors for exposure as part of a larger epidemiologic questionnaire at the time of specimen collection (11). In Fulton County, people identifying as Black, non-Hispanic, make up 44.5% of the total population and 31–53% of reported weekly COVID-19 cases are among Black, non-Hispanic people; however, nearly 16% of reported COVID-19 cases were missing race/ethnicity (12, 13). This study examined known exposures to persons with COVID-19, and potential unknown exposures (risk-factors) and estimated the risk for SARS-CoV-2 infection associated with such exposures among patients presenting for medical care at an urban, public hospital serving mostly low-income clients.
Enrollment, specimen collection, and testing procedures have been previously described (11). Briefly, this cross-sectional investigation enrolled patients aged ≥18 years presenting for medical care at the emergency department, preoperative screening clinic, or labor and delivery department at Grady Memorial Hospital during August–November 2020. Grady Memorial Hospital, an urban hospital in Fulton County, GA, serves the Atlanta metropolitan area including five surrounding counties and about two thirds of its clients are insured by Medicaid/Medicare or uninsured (14). Patients whose treating clinician ordered NP SARS-CoV-2 testing by reverse transcription polymerase chain reaction (RT-PCR)—for any reason (e.g., diagnostic or screening)—were eligible for study enrollment. Patients were excluded if they were unable to or declined consent, <18 years of age, unable to conduct self-collection of specimens, were enrolled previously during a prior visit, or if an NP swab was contraindicated. Trained interviewers as part of this investigation administered structured questionnaires in English or Spanish to collect patient demographic characteristics, known COVID-19 exposures, and risk factors for exposure to COVID-19 within the 14 days prior to enrollment. The sample size of this secondary analysis was based on the primary objective of the parent investigation (11).
Patients provided self-collected saliva and AN swab and a healthcare worker-collected NP swab. NP swab aliquots, saliva, and AN swabs were tested at the Centers for Disease Control and Prevention (CDC) using the CDC 2019-nCoV Real-Time RT-PCR Diagnostic Panel in accordance with the Emergency Use Authorization Instructions for Use (15).
Data were entered and stored in the Research Electronic Data Capture (REDCap) software (Vanderbilt University, Nashville, TN) hosted at CDC. Our outcome of interest was any SARS-CoV-2 positive RT-PCR result from specimens collected on the same day as the interview and tested at CDC. A “known close contact” was defined as being within 6 ft of a known COVID-19 case for a total of ≥15 min over a 24-h period. “Close contact within the past 14 days” was defined as being a known close contact where the reported date of last exposure was ≤14 days before the date of the interview (16). “Essential worker” was defined as a participant who reported a job or job location that meets the U.S. Department of Homeland Security's definition of “essential critical infrastructure workforce” (17). “Essential activity” was defined in this analysis as grocery shopping, using public transit, or visiting a healthcare facility.
We described the demographics, close contacts, employment, and social activities reported by participants. Age was grouped into categories based on distribution and alignment with other reports. As we were interested in recent infections, participants reporting a prior positive COVID-19 test were excluded from the bivariate and multivariate analyses of potential risk factors and SARS-CoV-2 detection. Bivariate associations were assessed using Chi-square, Fishers exact, and Mann-Whitney U tests, where appropriate. An alpha of <0.05 was considered statistically significant. Logistic regression models were used to understand crude and adjusted odds ratios (cOR and aOR) of potential risk factors and testing positive for SARS-CoV-2. Age, gender, and race and Hispanic ethnicity were included in the multivariate models a priori. In the multivariate models' race/ethnicity variable, the non-Hispanic categories of American Indian/Alaska Native, Asian, Multiple Race, and Native Hawaiian/Pacific Islander were grouped with Unknown/Other/Refused due to small numbers. Other potential risk factors with a bivariate association p < 0.1 were considered for inclusion in the model. Collinearity of model covariates was assessed by reviewing correlation matrices, and the best fitting model was chosen based on Akaike's information criterion. Because there was some interaction of being a known close contact with the other risk factor variables and the outcome, we then repeated the analysis of potential risk factors and SARS-CoV-2 detection after removing participants who responded “yes” or “unknown” to having close contact with a COVID-19 case to better understand potential risk factors when the participant had no known close contact to a case. RStudio, R version 4.0.3 (Boston, MA) and SAS 9.4 (Cary, NC) were used for data analysis.
All participants provided written consent before enrollment. This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy (See e.g., 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. 241(d); 5 U.S.C. 552a; 44 U.S.C. 3501 et seq.). This investigation was determined to be an exempt public health activity by the Emory University Institutional Review Board and Grady Memorial Hospital Research Oversight Committee.
Among 1,078 enrolled participants, 559 (51.8%) were male, 516 (47.9%) were female, 2 (0.2%) were non-binary and 1 (0.1%) refused to answer (Table 1). Over half (57.0%) of participants were aged ≥50 years including 309 (28.7%) participants aged 50–59 years and 305 (28.3%) aged ≥60 years. Most participants identified as Black, non-Hispanic (n = 876; 81.3%) followed by White, non-Hispanic (n = 81; 7.5%) and Hispanic or Latino (n = 71; 6.6%).
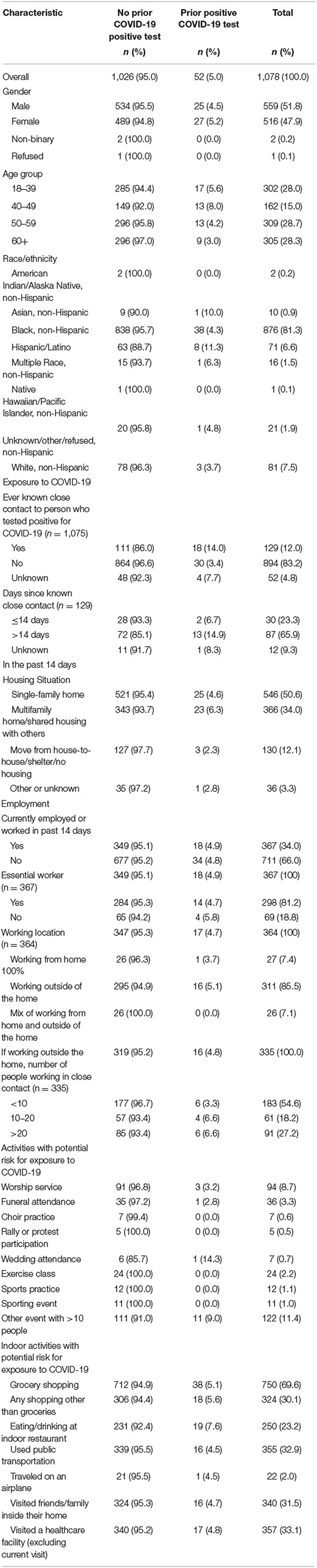
Table 1. Frequency of exposures and potential risk factors for exposure to COVID-19 among persons accessing medical care and tested for SARS-CoV-2 by previous COVID-19 test result at a large urban, public hospital in Fulton County, GA, August-November 2020.
Most participants (n = 894; 83.2%) reported no known close contact to a COVID-19 case. Of the 129 (12%) participants who reported known close contact, 30 (23.3%) reported the last known exposure ≤ 14 days before enrollment (Table 1). Among those with known close contact, the close contact was a household member (n = 31; 24.3%), non-household family member (n = 26; 20.3%), or close acquaintance (i.e., friend, boyfriend, girlfriend, significant other) (n = 30; 23.4%) (Supplementary Table 1). More than half of the participants enrolled in the investigation reported living in a single-family home (n = 546; 50.6%), one third lived in a multifamily home or shared housing (n = 366; 34.0%), and 12.1% (n = 130) reported living in a shelter or unstable housing. Most participants reported always wearing a mask when leaving home to go inside another building (non-work location) (n = 864; 81.4%) and an additional 102 (9.6%) and 67 (6.3%) participants wore a mask most of the time and sometimes, respectively. A few participants reported never wearing a mask (n = 17; 1.6%).
One third of participants were employed or worked in the past 14 days (n = 367; 34.0%). The majority of employed participants reported working outside the home (n = 311; 85.5%) and working indoors (n = 209, 62.6%). Most common places of work for employed participants include working at a restaurant or bar (n = 63; 17.3%), health care facility (n = 45; 12.3%), or construction or landscaping service (n = 38; 10.4%). Among those employed, 81.2% (n = 298) reported jobs that met the definition of an essential worker. Most employed participants reported, on average, working in close contact each hour with <10 people (e.g., coworkers, clients; n = 183; 54.6%), 61 (18.2%) participants reported working in close contact with 10–20 people, and 91 (27.2%) participants reported working in close contact with >20 people. Most participants reported that they always wore a mask (n = 250; 75.1%), while 11.1% (n = 37) and 10.2% (n = 34) of participants reported wearing a mask most of the time and sometimes, respectively, while at work.
Nearly all (n = 982; 91.1%) reported participating in at least one of 16 queried social activities in the past 14 days (median = 2; range = 0–9 activities). Of the nine indoor or outdoor social activities queried, the most frequently reported activities were attending some other event with >10 people (11.4%; n = 122), attending a worship service (8.7%; n = 94), attending a funeral (3.4%; n = 36), and attending an exercise class (2.2%; n = 24) (Table 1). Of the seven indoor only activities, the most frequently reported activities were grocery shopping (69.6%; n = 750), visiting a healthcare facility (excluding current visit; 33.1%; n = 357), using public transportation (32.9%; n = 355), and visiting friends or family inside their home (31.5%; n = 340).
Fifty-two (4.8%) of 1,078 participants reported a previous positive COVID-19 test. Among 1,026 participants who did not report a previous positive test, 78 (7.6%) had a current positive SARS-CoV-2 RT-PCR result, 111 (10.8%) had close contact with a COVID-19 case, and 51 (5.0%) did not know if they had close contact with a COVID-19 case (Table 2). Responding “yes” or “unknown” to having close contact to COVID-19 case had 2.71 higher odds of a positive test compared to no close contact to COVID-19 case [crude Odds Ratio (cOR) = 2.71; 95%CI = 1.51–4.87 and cOR = 2.39; 95%CI = 1.03–5.55, respectively). Reporting known close contact with a COVID-19 case ≤14 days before enrollment had six times the odds of a positive test compared to those not reporting known close contact within 14 days (cOR = 6.38; 95%CI = 2.78–14.63). Participants working in close contact with ≥10 people had twice the odds of testing positive than those who worked with <10 people or who were not employed (cOR = 2.01; 95%CI = 1.15–3.52). Participants attending a sports practice or sporting event within 14 days had statistically higher odds of testing positive than those not attending (cOR = 4.17; 95%CI = 1.11–15.74 and 7.27; 95%CI = 2.08–25.39, respectively). In the selected multivariable model (AUC = 0.70; AIC= 539.90) controlling for age, gender, and race/ethnicity, known close contact with a COVID-19 case ≤ 14 days before enrollment (aOR = 6.79; 95%CI = 2.78–16.62), living in a multifamily home (aOR = 2.52; 95%CI = 1.09–5.86), working in close contact with ≥10 people (aOR = 2.04; 95%CI = 1.10–3.78), attending a sporting event (aOR = 6.25; 95%CI = 1.16–33.68), and not participating in any essential indoor activity (aOR = 2.47; 95%CI = 1.35–4.51) had statistically increased odds of testing positive for SARS-CoV-2 (Figure 1A).
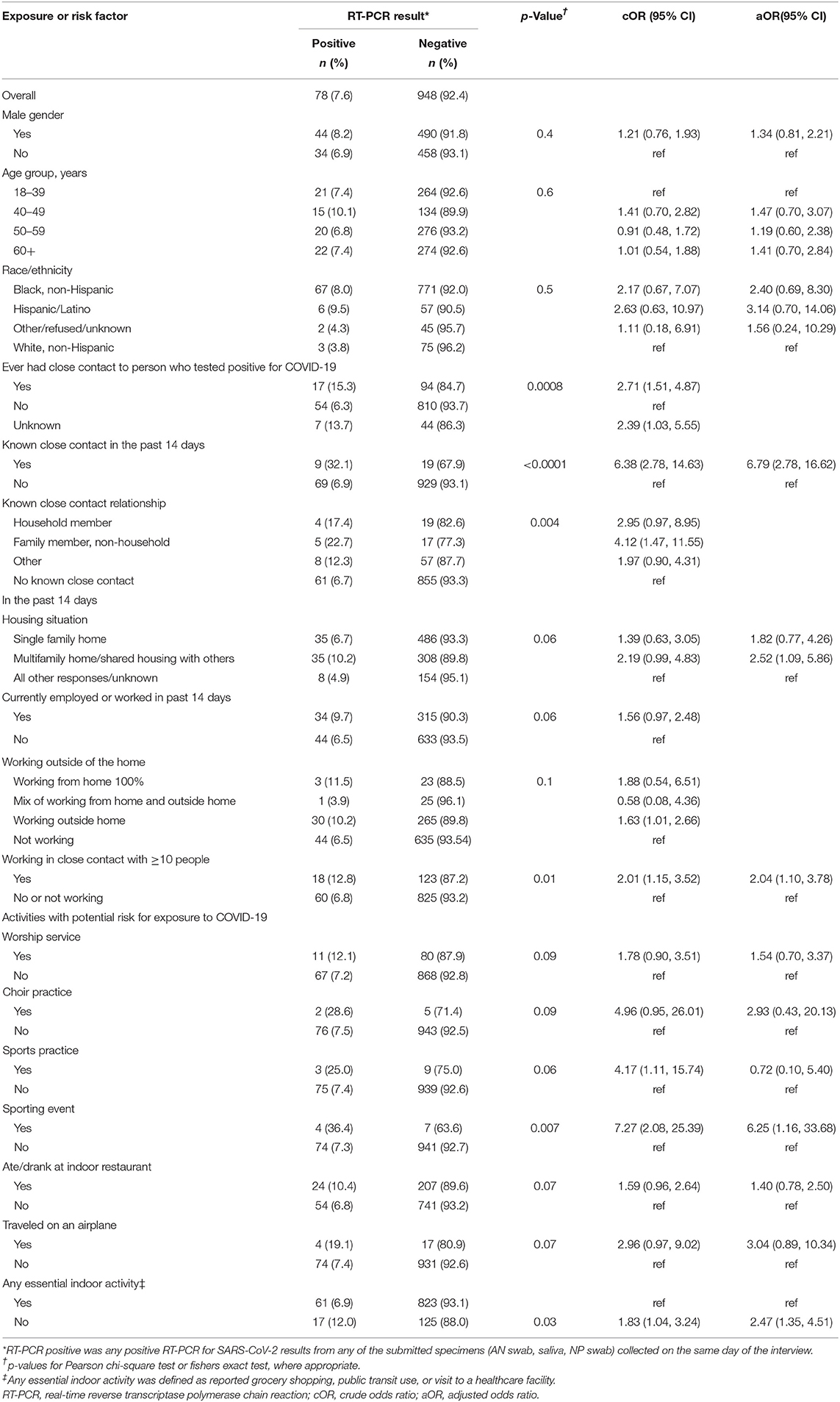
Table 2. Associations between known and potential exposures to SARS-CoV-2 infected persons and testing positive for the virus; a study at a large urban, public hospital in Fulton County, GA, August-November 2020 (n = 1,026).
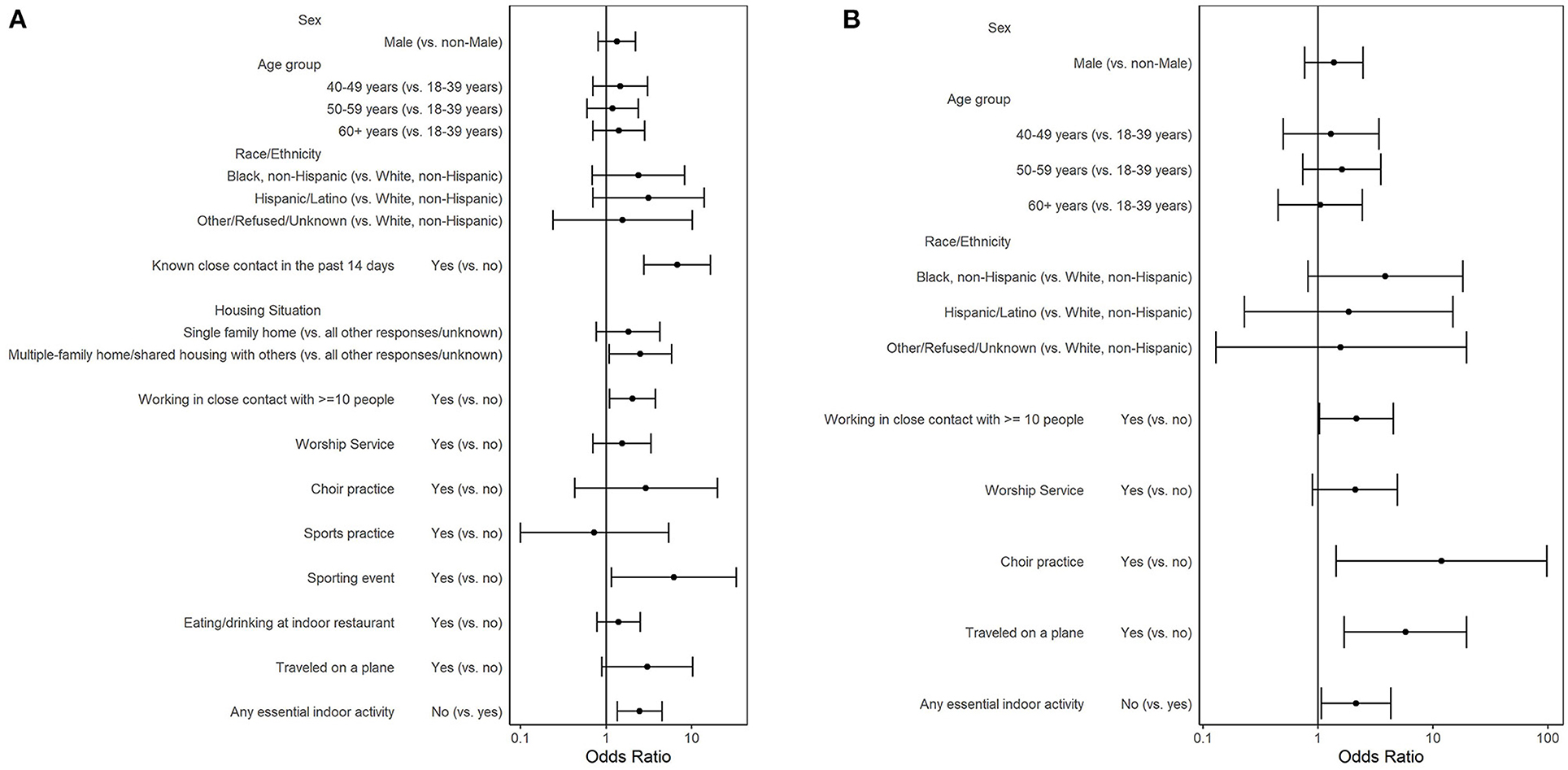
Figure 1. Adjusted odds ratios of a positive SARS-CoV-2 RT-PCR result: by COVID-19 exposure and by risk factors for COVID-19 exposure among (A) participants who did not report a prior positive COVID-19 test (n = 1,026) and (B) participants who did not report a prior positive SARS-CoV-2 test and had no known close contact to a COVID-19 case (n = 864); a study at a large urban, public hospital in Fulton County, GA, August-November 2020.
To understand risk factors when no known close contact was reported, we constructed an additional model where we excluded the 162 (15.8%) participants who responded “yes” or “unknown” to having close contact to a COVID-19 case. Among 864 participants who did not report a previous positive test nor a close contact to a COVID-19 case, 54 (6.3%) tested positive for SARS-CoV-2 (Table 3). Participants reporting attending worship service (cOR = 2.55; 95%CI = 1.19–5.46), choir practice (cOR = 15.54; 95%CI = 2.15–112.53), or traveling on an airplane (cOR = 4.55; 95%CI = 1.44–14.33) had statistically higher odds of testing positive for SARS-CoV-2 than those not reporting these activities. In the selected multivariable model (AUC = 0.67; AIC = 401.53) controlling for age, gender, and race/ethnicity, persons who worked in close contact with ≥10 people (aOR = 2.17; 95%CI = 1.03–4.55), attended choir practice (aOR = 11.85; 95%CI = 1.44–97.91), traveled on an airplane (aOR = 5.78; 95%CI = 1.70–19.68), or did not participate in an essential indoor activity (aOR = 2.15; 95%CI = 1.07–4.30) had statistically greater odds of testing positive for SARS-CoV-2 (Figure 1B).
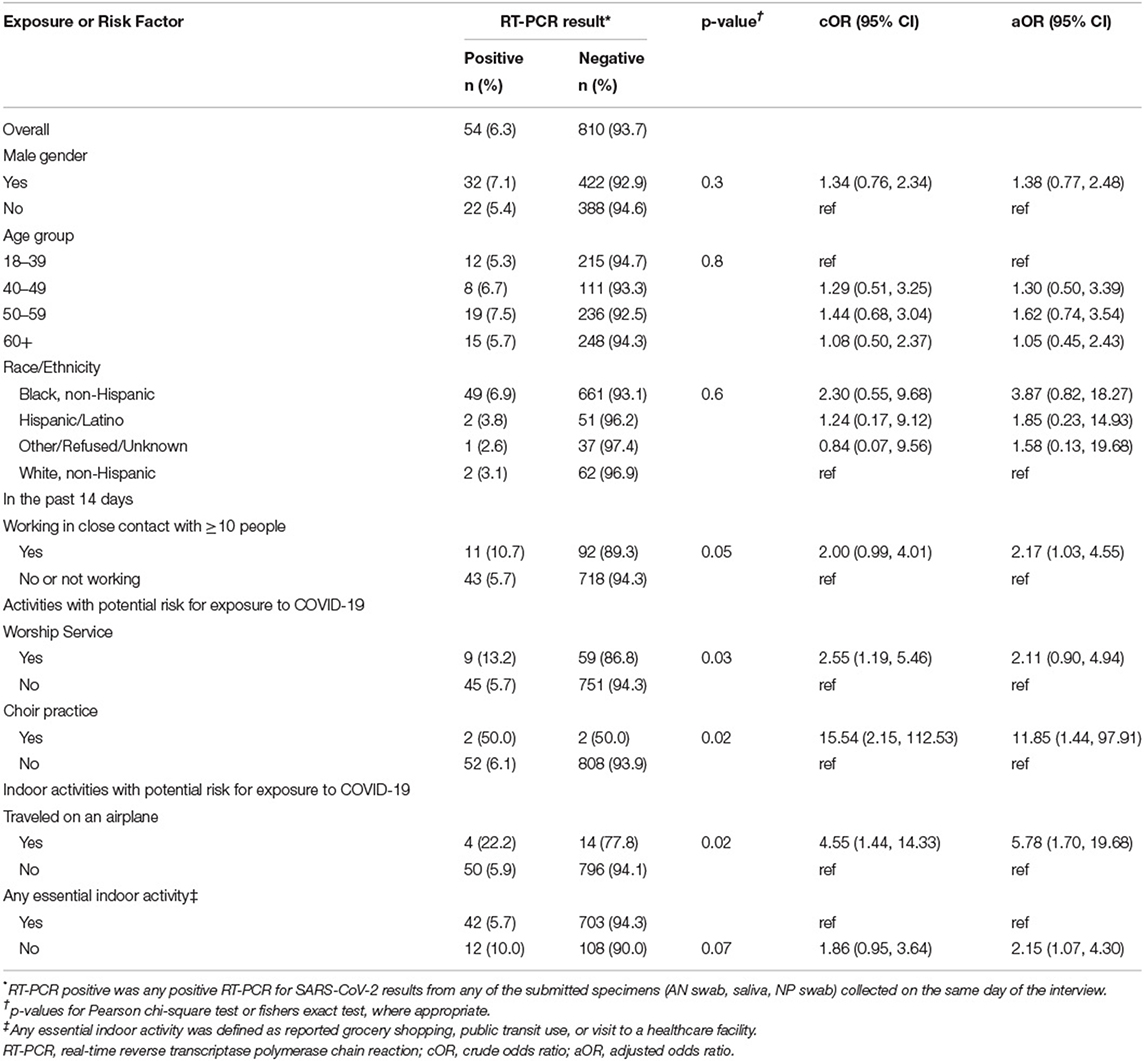
Table 3. Association of risk factors for COVID-19 exposure and SARS-CoV-2 RT-PCR result among participants without a reported prior positive SARS-CoV-2 test and who reported no known close contact to a COVID-19 case; a study at a large urban, public hospital in Fulton County, GA, August-November 2020 (n = 864).
We aimed to describe frequency of COVID-19 exposure risk factors and risk factors associated with SARS-CoV-2 infection. This investigation, which focused on a low-income, urban population seeking medical care at a public hospital, found that most social activities were infrequent in the 14 days prior to enrollment. Grocery shopping, visiting a healthcare facility, and using public transit were the most frequently reported activities, all of which represent essential indoor activities and none of which were statistically associated with testing positive for SARS-CoV-2. Enrollment started just after the summer peak and ended right before Thanksgiving, during a period when testing percent positivity ranged from 3 to 5% in Fulton County (13). Participants' frequency of social activities may have been lower than normal as a reaction to the many cases and deaths in the surrounding community over the summer. Just over one third of participants reported being employed or working in the past 14 days, mostly as essential workers, highlighting vulnerability to SARS-CoV-2 among many of the participants. Like other investigations of exposure risk factors, we found that having close contact with a COVID-19 case especially in the 2 weeks prior to testing was a very strong risk factor for testing positive for SARS-CoV-2 (2, 7, 18–24). Living in a multifamily household and having close contact with non-household family members were associated with testing positive for COVID-19, further supporting the importance of these close social networks as a driver of SARS-CoV-2 transmission.
Contact tracing relies on patients identifying known individuals with whom they have had close contact and places they have visited. Additionally, it becomes much harder to name unknown individuals in the same room or within 6 feet of someone for ≥15 min. By excluding those who responded “yes” or “unknown” to having close contact to a COVID-19 case, we identified activities or work circumstances that could increase the risk for SARS-CoV-2 transmission to persons involved in such activities or circumstances. Among those who reported no known close contact to a COVID-19 case, working in close contact with ≥10 people, attending choir practice, traveling on an airplane, and not participating in an essential activity were associated with increased odds of testing positive for SARS-CoV-2. Being in close contact most days of the week with many different people, through one's job or living situation, increases the risk of SARS-CoV-2 transmission. While mask wearing was relatively high in this population, employers could increase efforts to promote similar prevention practices by encouraging time off to get vaccinated to reduce SARS-CoV-2 transmission in workplaces that require employees to work in close contact with each other or the public. In our analysis, during the 14 days prior to testing, choir practice showed a strong association with testing positive for SARS-CoV-2. In March 2020, an outbreak of COVID-19 among a church choir highlighted the ease of SARS-CoV-2 transmission among those singing indoors, in close range without masks (25). Singing is accompanied by jets of air from the lungs passing through moving vocal cords and out past the sinus, nasal, and oral cavities, all areas where SARS-CoV-2 has been detected. Singing has previously been shown to transmit other infectious diseases like TB (26). Encouraging virtual or outdoor, distanced, and masked choir sessions could help prevent SARS-CoV-2 transmission. Our finding that traveling by airplane was associated with increased odds of testing positive for SARS-CoV-2 could be from close contact on the airplane itself or from the many casual close contacts that one may experience in the act of traveling (e.g., waiting in line for ticket or luggage, traveling by taxi or metro to and from airport, etc.). A prospective cohort seroprevalence study found traveling by air was associated with incident seroconversion (6).
Previously published investigations have found some different social activities associated with a positive SARS-CoV-2 test. Several studies have found that dining at a restaurant or going to a bar or coffee shop were associated with testing positive for SARS-CoV-2 (6, 7). We did not find that indoor eating or drinking at a restaurant was associated with testing positive. However, participants in our investigation were recruited from a low-income, urban population seeking medical care at a public hospital which was substantially different from the populations included in the other previously published investigations that noted this association.
Our investigation had several limitations. First, participants were interviewed by individuals identifying themselves as CDC employees, which could have led to social desirability bias in the responses (the participants may have felt the need to provide a response they perceived to be satisfactory to CDC). Second, the cross-sectional design of this investigation does not allow us to make any claims of causation because we cannot say if the infection or the potential exposure from an activity occurred first. Third, this was a secondary analysis of a larger investigation; therefore, we were underpowered to detect small differences in social activities not commonly reported. Some of the associations that were detected were based on a very small number reporting the exposure and should be interpreted with caution. Fourth, interviewers did not explicitly ask about mask wearing during each individual activity, but rather an overall frequency of mask wearing when leaving the home to go inside another building. Lastly, our participants were recruited from a single public hospital in Fulton County, GA that serves largely low-income individuals, and may not represent the broader community in Atlanta or beyond.
This investigation among a population of mostly Black, non-Hispanic participants seeking care at a public hospital found several exposures and risk factors for exposure to COVID-19 associated with testing positive for SARS-CoV-2. Understanding the frequency of social activities and determining which activities increase the risk of SARS-CoV-2 transmission in different types of communities is important for both communicating with and preventing transmission among vulnerable populations. COVID-19 vaccine access and uptake has been slow in some populations at high risk of COVID-19 and there was high vaccine hesitancy among some of the most disproportionately affected populations in the United States (27, 28). Since Spring of 2021, there have been great strides in vaccination uptake within the United States which helps to prevent COVID-19 hospitalization and death (10). However, the partial immune evasion seen by the Omicron variant and waning immunity from initial vaccination series means that a return to focusing on non-pharmaceutical prevention measures will be key to reducing SARS-CoV-2 transmission in the Omicron variant era and beyond.
The datasets presented in this article are not readily available because data from this investigation were approved by CDC for COVID-19 pandemic response use and will be made public as aggregated data shared with CDC leadership and the public in the form of publications, presentations, or web content. Requests to access the datasets should be directed to SS-J, dXlpN0BjZGMuZ292.
The studies involving human participants were reviewed and approved by Centers for Disease Control and Prevention, Emory University Institutional Review Board, and Grady Memorial Hospital Research Oversight Committee. The patients/participants provided their written consent to participate in this study.
Halie K. Miller, Ph.D.; AdeSubomi O. Adeyemo, PharmD; Anne C. Moorman, MPH; Brenda L. Bauman, MSPH; Kahaliah Joseph, MSc; Michelle O'Hegarty, Ph.D.; Nazia Kamal, Ph.D.; Mila Cohen, MSc; Amadea Britton, MD; Courtney T. Callahan, Ph.D.; Jamila Fonseka, MPH; Elfriede Agyemang, MD; Miriam J. Lawson, MS; Molly Deutsch-Feldman, Ph.D.; Tejpratap S. P. Tiwari, MD; Samira Sami, DrPH; Hong Tao, BS.
MS, PR, RS, YW, HK, and JT contributed to conceptualization, project design, and overall project leadership. SS-J, GK, VK, AH, RR, TS, KO'L, CE, BB, MB, AP, AG, JF, JS, CB, and the CDC COVID-19 Emergency Response GA-10 Field Team contributed to data specimen collection and specimen testing. SS-J, SS, MK, GK, and JT contributed to analysis and initial manuscript development. All authors contributed to manuscript editing and approval.
Funding for this activity was provided by the U.S. Centers for Disease Control and Prevention and CDC Foundation Project #1085.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U. S. Centers for Disease Control and Prevention (CDC).
YW, PR, VK, AH, and MS received funding for this study from the CDC Foundation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Francisco Averhoff (CDC), Nicolas Wiese (CDC), Luis Lowe (CDC), and Susan Ray (Emory University) for feedback on the initial concept development. We thank Hany Atallah (Grady Memorial Hospital) and Brooks L. Moore (Grady Memorial Hospital) for their approval and support to recruit from Grady Memorial Hospital and Gerardo Garcia-Lerma (CDC), Lauren Franco (CDC), Anupama Shankar (CDC), Adam Wharton (CDC), and Tiffiany M. Aholou (CDC) for their logistical and field support. We want to thank the CDC COVID-19 Surge Laboratory for specimen extraction and SARS-CoV-2 rRT-PCR testing (Raydel Anderson, Kay W. Radford, Gimin Kim, Dexter Thompson, Congrong Miao, Min-hsin Chen, Claire Hartloge, Magdalena Medrzycki, Patricia Shewmaker, Brent Jenkins, Nhien Tran, Srinivasan Velusamy, Lalitha Gade, Kashif Sahibzada, Renee Galloway, Phili Wong, HaoQiang Zheng, Michelle Adamczyk, Erin Breaker, Davina Campbell, Lori Spicer, Lisa Tran, William Furin, Carrie A. Sanders, K. Allison Perry-Dow, Monica Chan, Amanda Lyons, Hollis Houston, Karlos Crayton, J. Carrie Whitworth, Gillian McAllister, Alison L. Halpin, Natashia R. Reese, Kamile Rasheed, David R. Lonsway, Uzma A. Ansari, Amelia Bhatnagar, Rocio Balbuena, Maria Karlsson, M. Shannon Keckler, Baoming Jiang, Jan Vinje, Leslie Barclay, Rashi Gautam, Slavica Mijatovic-Rustempasic, Amy Hopkins, Eric Katz, Hannah Browne, Kenny Nguyen, Mathew Esona, Sung-Sil Moon, Theresa Bessey, Preeti Chhabra, Leeann Smart). We thank Katherine Butler, Caitlin D. Bohannon, D. Joseph Sexton, Laura Hughes-Baker, Wendi Kuhnert-Tallman Brandi Limbago, and Christopher Elkins for their leadership and support from the Laboratory Task Force of the CDC COVID-19 Response. Finally, we would like to thank the healthcare workers and laboratory technologists at Grady Memorial Hospital for their dedication and the participants for their participation despite the circumstances that brought them to the hospital.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.809356/full#supplementary-material
1. Centers for Disease Control and Prevention. COVID Data Tracker. (2021). Available online at: https://covid.cdc.gov/covid-data-tracker/index.html#cases_casesper100klast7days (accessed October 20, 2021).
2. Baker JM, Nelson KN, Overton E, Bejamin AL, Lash TL, Photakis M, et al. Quantification of occupational and community risk factors for SARS-CoV-2 seropositivity among health care workers in a large U.S. health care system. Ann Intern Med. (2021) 174:649–54. doi: 10.7326/M20-7145
3. Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. The Lancet Microbe. (2021) 2:e13–22. doi: 10.1016/S2666-5247(20)30172-5
4. Buitrago-Garcia D, Egli-Gany D, Counotte MJ, Hossmann S, Imeri H, Ipekci AM, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med. (2020) 17:e1003346. doi: 10.1371/journal.pmed.1003346
5. Chamie G, Marquez C, Crawford E, Peng J, Petersen M, Schwab D, et al. SARS-CoV-2 Community transmission disproportionately affects Latinx population during shelter-in-place in San Francisco. Clin Infect Dis. (2021) 73(Suppl. 2):S127–35. doi: 10.1093/cid/ciaa1234
6. Nash D, Rane M, Chang M, Kulkarni SG, Zimba R, You W, et al. Recent SARS-CoV-2 seroconversion in a national, community-based prospective cohort of U.S. adults. medRxiv. (2021). doi: 10.1101/2021.02.12.21251659
7. Fisher KA, Tenforde MW, Feldstein LR, Lindsell CJ, Shapiro NI, Files DC, et al. Community and close contact exposures associated with COVID-19 among symptomatic adults ≥18 years in 11 outpatient health care facilities - United States, July 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:1258–64. doi: 10.15585/mmwr.mm6936a5
8. Ghinai I, Davis ES, Mayer S, Toews KA, Huggett TD, Snow-Hill N, et al. Risk factors for severe acute respiratory syndrome Coronavirus 2 infection in homeless shelters in Chicago, Illinois-March-May, 2020. Open Forum Infect Dis. (2020) 7:ofaa477. doi: 10.1093/ofid/ofaa477
9. Kennedy BS, Richeson RP, Houde AJ. Risk factors for SARS-CoV-2 in a statewide correctional system. N Engl J Med. (2020) 383:2479–80. doi: 10.1056/NEJMc2029354
10. Rosenberg ES, Holtgrave DR, Dorabawila V, Conroy M, Greene D, Lutterloh E, et al. New COVID-19 cases and hospitalizations among adults, by vaccination status - New York, May 3-July 25, 2021. MMWR Morb Mortal Wkly Rep. (2021) 70:1306–11. doi: 10.15585/mmwr.mm7037a7
11. Smith-Jeffcoat SE, Koh M, Hoffman A, Rebolledo PA, Schechter MC, Miller HK, et al. Effects of patient characteristics on diagnostic performance of self-collected samples for SARS-CoV-2 testing. Emerg Infect Dis. (2021) 27:2081–9. doi: 10.3201/eid2708.210667
12. Bureau USC. QuickFacts: Fulton County, Georgia. United States Census Bureau (2019). Available online at: https://www.census.gov/quickfacts/fultoncountygeorgia (accessed October 20, 2021).
13. Fulton County Board of Health. Fulton County Board of Health Epidemiology Report. Atlanta, GA: Fulton County Government.
14. Grady Health System. Grady Fast Facts. (2018). Available online at: https://www.gradyhealth.org/wp-content/uploads/2018/07/Grady_Fast_Facts.pdf (accessed January 29, 2021).
15. Centers for Disease Control Prevention. Emergency Use Authorization.: CDC 2019-nCoV Real-Time RT-PCR Diagnostic Panel: FDA. (2020). Available online at: https://www.fda.gov/media/134919/download (accessed March 2, 2021).
16. Centers for Disease Control Prevention. COVID-19: Case Investigation & Contract Tracing Guidance Atlanta, GA. (2021). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/php/contact-tracing/contact-tracing-plan/appendix.html (accessed March 24, 2021).
17. Cybersecurity and Infrastructure Security Agency. Guidance on the Essential Critical Infrastructure Workforce: Ensuring Community and National Resilience in COVID-19 Response. Washington, DC: U.S. Department of Homeland Security (2020).
18. Bahrs C, Kimmig A, Weis S, Ankert J, Hagel S, Maschmann J, et al. Prospective surveillance study in a 1,400-bed university hospital: COVID-19 exposure at home was the main risk factor for SARS-CoV-2 point seroprevalence among hospital staff. Transbound Emerg Dis. (2021) 1–11. doi: 10.1111/tbed.14041
19. Dimcheff DE, Schildhouse RJ, Hausman MS, Vincent BM, Markovitz E, Chensue SW, et al. Seroprevalence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection among Veterans Affairs healthcare system employees suggests higher risk of infection when exposed to SARS-CoV-2 outside the work environment. Infect Control Hosp Epidemiol. (2021) 42:392–8. doi: 10.1017/ice.2020.1220
20. Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J Hosp Infect. (2021) 108:120–34. doi: 10.1016/j.jhin.2020.11.008
21. Hobbs CV, Martin LM, Kim SS, Kirmse BM, Haynie L, McGraw S, et al. Factors associated with positive SARS-CoV-2 test results in outpatient health facilities and emergency departments among children and adolescents aged <18 years - Mississippi, September-November 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:1925–9. doi: 10.15585/mmwr.mm6950e3
22. Jacob JT, Baker JM, Fridkin SK, Lopman BA, Steinberg JP, Christenson RH, et al. Risk factors associated with SARS-CoV-2 seropositivity among US health care personnel. JAMA Netw Open. (2021) 4:e211283. doi: 10.1001/jamanetworkopen.2021.1283
23. Maechler F, Gertler M, Hermes J, van Loon W, Schwab F, Piening B, et al. Epidemiological and clinical characteristics of SARS-CoV-2 infections at a testing site in Berlin, Germany, March and April 2020—a cross-sectional study. Clin Microbiol Infect. (2020) 26:1685.e7–12. doi: 10.1016/j.cmi.2020.08.017
24. Sun Y, Koh V, Marimuthu K, Ng OT, Young B, Vasoo S, et al. Epidemiological and Clinical Predictors of COVID-19. Clin Infect Dis. (2020) 71:786–92. doi: 10.1093/cid/ciaa322
25. Hamner L, Dubbel P, Capron I, Ross A, Jordan A, Lee J, et al. High SARS-CoV-2 Attack Rate Following Exposure at a Choir Practice - Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:606–10. doi: 10.15585/mmwr.mm6919e6
26. Mangara BT, Napolitano EC, Passannante MR, McDonald RJ, Reichman LB. Mycobacterium tuberculosis miniepidemic in a church gospel choir. Chest. (1998) 113:234–7. doi: 10.1378/chest.113.1.234
27. Hughes MM, Wang A, Grossman MK, Pun E, Whiteman A, Deng L, et al. County-level COVID-19 vaccination coverage and social vulnerability - United States, December 14, 2020-March 1, 2021. MMWR Morb Mortal Wkly Rep. (2021) 70:431–6. doi: 10.15585/mmwr.mm7012e1
Keywords: SARS-CoV-2, COVID-19, risk factors, exposure, underrepresented
Citation: Smith-Jeffcoat SE, Sleweon S, Koh M, Khalil GM, Schechter MC, Rebolledo PA, Kasinathan V, Hoffman A, Rossetti R, Shragai T, O'Laughlin K, Espinosa CC, Bankamp B, Bowen MD, Paulick A, Gargis AS, Folster JM, da Silva J, Biedron C, Stewart RJ, Wang YF, Kirking HL, Tate JE and CDC COVID-19 Emergency Response GA-10 Field (2022) Risk-Factors for Exposure Associated With SARS-CoV-2 Detection After Recent Known or Potential COVID-19 Exposures Among Patients Seeking Medical Care at a Large Urban, Public Hospital in Fulton County, Georgia — A Cross-Sectional Investigation. Front. Public Health 10:809356. doi: 10.3389/fpubh.2022.809356
Received: 04 November 2021; Accepted: 28 February 2022;
Published: 24 March 2022.
Edited by:
Nuno Sepulveda, Warsaw University of Technology, PolandReviewed by:
Nicola Magnavita, Università Cattolica del Sacro Cuore, Medicine and Surgery, ItalyCopyright © 2022 Smith-Jeffcoat, Sleweon, Koh, Khalil, Schechter, Rebolledo, Kasinathan, Hoffman, Rossetti, Shragai, O'Laughlin, Espinosa, Bankamp, Bowen, Paulick, Gargis, Folster, da Silva, Biedron, Stewart, Wang, Kirking, Tate and CDC COVID-19 Emergency Response GA-10 Field. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah E. Smith-Jeffcoat, dXlpN0BjZGMuZ292; Sadia Sleweon, cXBnOUBjZGMuZ292
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.