
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Public Health, 02 February 2022
Sec. Environmental Health and Exposome
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.802167
This article is part of the Research TopicEnvironmental Exposures and Cardiometabolic DiseaseView all 5 articles
Background: Established evidence suggests risks of developing cardiovascular disease are different by sex. However, it remains unclear whether associations of PM2.5 with cardiovascular risk are comparable between women and men. The meta-analysis aimed to examine sex differences in associations of ischemic heart disease (IHD) and stroke with long-term PM2.5 exposure.
Methods: PubMed, EMBASE and Cochrane Library were searched until May 2, 2021. We included cohort studies reporting sex-specific associations of long-term PM2.5 exposure (e.g., ≥1 year) with IHD and stroke. The primary analysis was to estimate relative risk (RR) of PM2.5-outcome in women and men separately, and the additional women-to-men ratio of RR (RRR) was explored to compare sex differences, using random-effect models.
Results: We identified 25 eligible studies with 3.6 million IHD and 1.3 million stroke cases among 63.7 million participants. A higher level of PM2.5 exposure was significantly associated with increased risk of IHD in both women (RR = 1.21; 95% CI, 1.15–1.27) and men (RR = 1.12; 95% CI, 1.07–1.17). The women-to-men RRR of IHD was 1.05 (95% CI, 1.02–1.08) per 10 μg/m3 increment in PM2.5 exposure, indicating significant excess risk of IHD in women. The significant risks of stroke associated with PM2.5 were obtained in both women (RR = 1.11; 95% CI, 1.08–1.13) and men (RR = 1.11; 95% CI, 1.07–1.14), but no significant women-to-men RRR was observed in stroke (RRR = 1.00; 95% CI, 0.96–1.04).
Conclusions: The study identified excess risk of IHD associated with long-term PM2.5 exposure in women. The findings would not only have repercussions on efforts to precisely evaluate the burden of IHD attributable to PM2.5, but would also provide novel clues for cardiovascular risk prevention accounting for sex-based differences.
Cardiovascular disease (CVD) is the leading cause of the global disease burden (1), and accumulating evidence highlighted that sex differences existed in the risk factors, manifestation, and treatment of the cardiovascular clinical spectrum (2, 3). Meta-analyses have illustrated that several factors (e.g., smoking and diabetes) had significantly greater cardiovascular risk in women than men (4, 5).
In addition, established evidence identified a relationship of cardiovascular morbidity linked to long-term exposure to PM2.5 (i.e., particulate matter <2.5 μm in diameter) (6). However, there is a debate on sex-based discrepancies for the PM2.5-CVD associations. Several studies observed higher risks of CVD associated with PM2.5 in women (7–9), while others reported similar effect estimations between sexes (10–12). To our knowledge, there is no quantitative synthesis of published literature, comparing sex differences in the relationship between long-term PM2.5 exposure and CVD. A comprehensive investigation of potential sex differences in PM2.5-related risk of CVD would extend our understanding of deleterious effects due to air pollution. If the different cardiovascular risks associated with long-term exposure to PM2.5 could be confirmed in women and men, it would have implications for precise assessment of disease burden attributable to PM2.5 exposure. Meanwhile, it could also provide novel clues for cardiovascular risk prevention, accounting for sex-based differences.
In this study, considering various types of CVD, ischemic heart disease (IHD) and stroke were selected as two main endpoints since they have been the top leading causes of CVD burden (1) and mostly reported by previous original studies on associations between PM2.5 and CVD (13). Herein, we conducted a meta-analysis of cohort studies to examine sex-specific risks of long-term exposure to PM2.5 with incident IHD and stroke, and further to identify whether a more detrimental association of PM2.5 exposure might exist in women, using the pooled estimations of relative risk ratio between women and men.
This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines with a checklist in Supplementary Table 1 (14). Briefly, we systematically searched the relevant articles in PubMed, EMBASE and Cochrane Library until May 2nd, 2021. Search terms included PM2.5 exposure, cardiovascular outcome, and study design, including keywords as follows: (1) particulate matter: PM2.5, fine particulate matter, (2) cardiovascular outcome: cardiovascular disease, cardiovascular event, stroke, cerebrovascular disease, myocardial ischemia, coronary artery disease, heart failure, myocardial infarction, ischemic heart disease, angina pectoris, coronary heart disease, heart attack, acute coronary syndrome, and (3) cohort study: cohort, longitudinal study, longitudinal, odds ratio, relative risk, hazard ratio. The full electronic search strategies for each database are shown in Supplementary Tables 2–4. Additionally, we manually checked the relevant reviews and references of included studies to complement articles.
Two authors (J.Z. and X.W.) independently screened the titles and abstracts, and then full texts of the potential qualified studies were further assessed. A third reviewer (M. Y.) would check the article and make a decision if there was any disagreement. Studies eligible for inclusion met the following conditions: (1) Participants: general human population with ambient PM2.5 exposure, excluding those with workplace exposure to PM2.5; (2) Exposures: the exposure of interest included long-term exposure (i.e., ≥1 year) to PM2.5; (3) Comparisons: studies provided sex-specific effect estimates of the PM2.5-outcome association with relative risk (RR) or hazard ratio (HR) as well as their 95% confidence intervals (CIs) per 10-μg/m3 increment of PM2.5 exposure; (4) Outcomes: study outcomes included at least either IHD or stroke; (5) Designs: studies were restricted to cohort design. Those excluded studies were: (1) reviews or animal experiments; (2) targeting short-term exposures or acute effects; (3) irrelevant research outcomes; (4) with other study designs (e.g., ecological studies, cross-sectional studies, or case-control studies, etc.); (5) cohorts among patients with specific diseases; (6) studies unavailable to explicit sex-subgroup results; (7) studies from the same cohort with overlapping participants. When multiple articles examined the same outcome based on the same cohort, only one study per cohort was included with the longest duration of follow-up or the most recent published article.
Two investigators (J.Z. and X.W.) independently extracted data on author name, publication year, country, study name, study period, population characteristics, sample size, methods of PM2.5 exposure measurement, International Classification of Diseases (ICD) codes of outcomes, number of cases, covariates adjusted in the statistical model, and sex-specific effect estimates (HRs or RRs with 95% CIs). When a study reported multiple results using regression models with different covariates, the result of fully adjusted model was chosen. The quality of included studies was evaluated using Newcastle-Ottawa Assessment Scale (NOS) (15). Briefly, the NOS is based on eight items from three main aspects: (1) Selection of study population; (2) Comparability of cohorts; (3) Assessment of outcomes and adequacy of follow up of cohorts. There were four, one, and three items for the categories of Selection, Comparability, and Outcome, respectively. Each study could be awarded a maximum of one point for each numbered item within the Selection and Outcome categories, but a maximum of two points could be given for Comparability (Supplementary Table 5). The total score of NOS ranged from 0 to 9, and studies with 7 or more were considered as high-quality in the meta-analysis.
In this meta-analysis, the major endpoints were incident risks of IHD and stroke. The primary estimates were the pooled sex-specific relative risk (RR) and the women-to-men ratio of RR (RRR) per 10-μg/m3 increment of PM2.5 exposure. The women-to-men RRRs with 95% CIs have been commonly used to assess the excessive risk for exposure-disease association in women compared to men (4, 5).
For each study, sex-specific RRs and 95% CIs were used for associations between cardiovascular outcomes and long-term PM2.5 exposure. Hazard Ratios (HRs) were considered equivalent to RRs. All the effect estimates with 95% CIs were converted to a comparable unit of 10 μg/m3. The pooled RR for women or men was separately obtained using the random-effect model by the method of DerSimonian and Laird, prior accounting for between-study heterogeneity (16).
Furthermore, the sex-specific RR was log-transformed, and the women-to-men difference in log-RRs was computed within each study. The differences were then pooled across studies using random-effect meta-analysis weighted by the inverse variances of the log-RRs, and finally back-transformed to the raw scale, obtaining the pooled women-to-men RRR. The standard error of the log RRR was derived from the sum of variance of the sex-specific log-RR for each study, followed by taking the square root. The details of the identical approach have been described elsewhere (4, 5).
Heterogeneity of between-study was tested by the coefficient of inconsistency (I2 statistic). Sensitivity analysis by excluding one study at a time was conducted, and publication bias was graphically examined using funnel plots along with the Begg's test. Stata version 12.0 software (StataCorp, TX) was used for all meta-analyses. All P-values were two-sided with a significant level at 0.05.
The flowchart of study inclusion and exclusion is shown in Figure 1. After screening 1,365 records, 219 articles were moved to the full-text review. Finally, there were 25 eligible publications among 67.3 million participants included in the further meta-analyses (7–12, 17–35), and the summarized characteristics of studies are shown in Table 1. Of the 25 eligible articles, 11 were based on cohorts from North America (7, 9, 10, 19–24, 26, 34), 5 from European countries (17, 18, 25, 28, 30), 6 from Asian populations (11, 12, 27, 31, 33, 35), 1 from Australia (29), and 2 from multiple countries (8, 32). Information on the quality assessment of studies is shown in Supplementary Table 5. After evaluating the design and description of the included studies using the NOS, it indicated 21 studies (84%) scored ≥7 as high-quality research. Furthermore, details of study characteristics for 17 publications on the outcome of IHD are listed in Supplementary Table 6 (7–12, 17–27), and characteristics of 18 articles on stroke are shown in Supplementary Table 7 (8, 9, 17, 19, 21–23, 25–35).
In the 17 studies on associations of long-term PM2.5 exposure with risk of IHD, 10 studies defined the outcome of IHD using the same codes of ICD (ICD-9: 410–414; ICD-10: I20-I25) (Supplementary Table 6). The outcome of 1 study focused on myocardial infarction (MI) (ICD-10: I21–I22) (8), and another 3 used a narrower definition of MI (ICD-8 and ICD-9: 410; ICD-10: I21) (10, 18, 19). Only 3 articles did not list ICD codes of their IHD descriptions (11, 22, 24). Based on scores of the NOS, 14 of the 17 included studies were high-quality.
Of the 17 studies, 14 results in women were combined to obtain a RR of 1.21 (95% CI, 1.15–1.27) for incident risk of IHD per 10 μg/m3 increment in long-term PM2.5 exposure, while a lower RR of 1.12 (95% CI, 1.07–1.17) was shown after pooling 13 results in men (Supplementary Figures 1, 2). No evidence of publication bias was found either in the funnel plots (Supplementary Figure 3) or by Begg's tests (P = 0.33 for women and P = 0.73 for men). Further analyses were limited to the 9 studies conducted in both men and women, and the women-to-men RRR for IHD was 1.05 (95% CI, 1.02–1.08) (Figure 2). There was no heterogeneity of between-study observed (I2=27.1%, P = 0.20), and limited publication bias was presented by the funnel plot (Supplementary Figure 4) with Begg's test (P = 0.69). The sensitivity analyses showed no substantial changes in the RRRs after excluding the studies one by one.
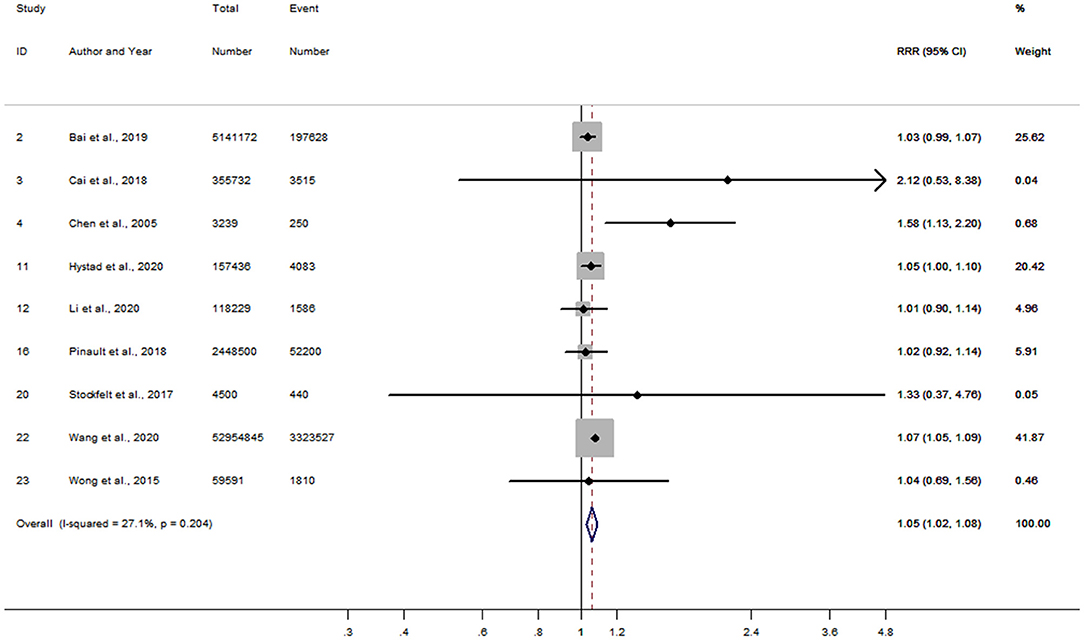
Figure 2. Forest plot for the women-to-men RRR of IHD per 10 μg/m3 increase in PM2.5 exposure. CI, confidence interval; IHD, ischemic heart disease; RRR, ratios of relative risk.
A total of 18 articles were included for the association of long-term PM2.5 exposure with risk of stroke (Supplementary Table 7). Generally, 11 of the 18 studies defined the outcome using very similar ICD codes, of which 8 studies defined the stroke with the same coding (ICD-9: 430–438; ICD-10: I60–I69) and the other 3 used slightly narrow definitions [i.e., ICD-9: 430–436 (33), ICD-9: 430–437 (19), and ICD-9: 431–438 (27)]. Six studies narrowed the definitions which excluded certain specific codes within the commonly used ICD ranges (ICD-8 or ICD-9: 430–438; ICD-10: I60–I69) (8, 21, 25, 28, 29, 34), and only 1 study did not describe the ICD code (32). Fifteen of the 18 included studies were scored as high-quality.
After combining RRs in women reported from 16 studies, a 10 μg/m3 increment in long-term PM2.5 exposure was associated with an 11% increased risk of stroke (RR = 1.11; 95% CI, 1.08–1.13) (Supplementary Figure 5). The pooled RR in men was also 1.11 (95% CI, 1.07–1.14), similar to that in women (Supplementary Figure 6). A slight publication bias was observed in the analysis of women by the funnel plots (Supplementary Figure 7) with Begg's tests (P = 0.05), while no publication bias was found in men (Begg's P = 0.22). Moreover, based on 11 articles that reported RRs in women and men within the same study, the combined women-to-men RRR was 1.00 (95% CI, 0.96–1.04) for risk of stroke per 10 μg/m3 increase in PM2.5 exposure (Figure 3). Heterogeneity of between-study for the analysis was moderate (I2 = 50.6%, P = 0.03), while no publication bias with Begg's test (P = 0.14) was observed (Supplementary Figure 8). Sensitivity analysis showed little change on those estimates of RRR after leaving out one study at a time.
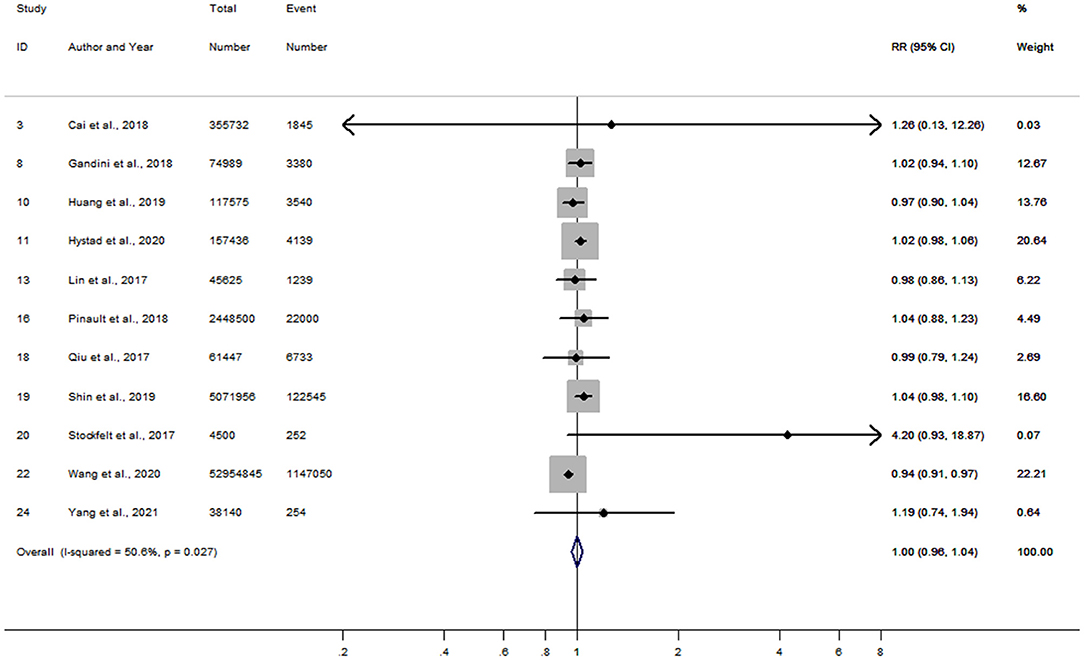
Figure 3. Forest plot for the women-to-men RRR of stroke per 10 μg/m3 increase in PM2.5 exposure. CI, confidence interval; RRR, ratios of relative risk.
The meta-analysis incorporated cohort data of 25 articles among over 36.8 million women and 30.5 million men, which systematically investigated sex-specific associations of long-term PM2.5 exposure with risks of IHD and stroke. The meta-analysis obtained a stronger RR for IHD associated with PM2.5 exposure in women than that in men. The quantitative estimation of women-to-men RRR indicated that women had a 5% greater risk of IHD per 10 μg/m3 increment of PM2.5. The associations of long-term PM2.5 exposure with stroke were significant in both women and men with similar effect magnitudes between sexes.
Ambient PM2.5 pollution has been identified as one of the risk factors contributing to acute cardiac arrest and long-term CVD burden (6, 36). However, it is controversial whether substantial differences would exist in the association of PM2.5 exposure with CVD between women and men. Several studies have observed higher risks of CVD or subtype endpoints associated with long-term PM2.5 exposure in women (7–9), while others reported similar risks between sexes (10–12). Although a recent meta-analysis has examined effect sizes of long-term exposure to air pollution on the risk of CVD (13), few studies systematically investigated potential sex differences in effect magnitudes for PM2.5-CVD association. In this meta-analysis, we extracted data on sex-specific estimations of associations between long-term PM2.5 exposure and the mostly reported outcomes of CVD (i.e., IHD and stroke). The pooled women-to-men RRR indicated that women had a 5% higher risk of IHD per 10 μg/m3 increase in PM2.5. The between-study heterogeneity and publication bias were not observed for the pooled RRR, which enhanced the robustness of the meta-analysis results.
Beyond the novel findings, the design and methods in this meta-analysis had several strengths. First, most of the included studies (21 of 25) were of high quality according to NOS evaluation (Supplementary Table 5), which improved the level of evidence. Second, the data used for the calculation of women-to-men RRR were extracted from the studies that included both men and women. The sex-specific RRs of PM2.5 on outcomes were compared in men and women from the same study, which reduced the possibility that potential sex differences were derived from disparities in the background risks of different study populations. Finally, compared to previous meta-analyses searching literature until 2019 (13), one-fourth (7 articles) of the included studies were published after 2019, providing contemporary evidence on sex differences in PM2.5-CVD association.
Accumulated studies have documented that sex differences exist in associations of IHD with classic risk factors, such as smoking (4) and diabetes (5), which were more detrimental to women. For instance, one meta-analysis showed that an excess risk of coronary heart disease associated with diabetes existed in women compared with men (5). The present study is the first meta-analysis to identify the significant excess risk of incident IHD associated with long-term exposure to PM2.5. The biological mechanisms behind the pooled results are not very clear. One of plausible reasons suggested that pulmonary deposition of inhaled particles under the controlled breathing conditions was found more pronounced in women than in men, which could lead to higher health risk in women (37). Also, studies on personal exposure and biomarkers suggested that women might be more sensitive to inflammatory and oxidative influences of particulate matter (38). Moreover, meta-analyses of epidemiological studies found that PM2.5 exposure increased the risk of diabetes (39). It is inferred that diabetes might mediate the sex difference of PM2.5-IHD association, considering the evidence on a higher risk of IHD associated with diabetes in women (5). Further epidemiological and experimental researches are needed to explain sex differences in the deleterious impacts of PM2.5, and explore specific biological mechanisms involved in PM2.5-induced heart disease.
Up to date, although the biological pathways remained unclear, the high-level evidence from meta-analysis reminds us to pay more attention to cardiovascular health in women, when we conduct health risk assessments on air pollution and intervention practices. Accurate health risk assessment is essential to deliver optimal preventive medical care, while it is no longer acceptable to use a one-size-fits-all model of cardiovascular risk stratification which ignores sex differences (40). Many tools or equations of cardiovascular risk assessment widely recommended by guidelines were developed based on sex-specific models along with different effect estimations even for the same risk factor (41, 42). In future studies on air pollution and cardiovascular health, it is encouraged to routinely report sex-specific results of exposure-risk relationship, which may help to accumulate more evidence for risk evaluation and prediction precisely. In the practice of prevention and treatment for IHD, data in US and China showed that women were less likely to be diagnosed appropriately and less frequently receive preventive care, which may be related to a lower perceived risk in women by clinicians and patients (43, 44). Although the knowledge of both health risk from air pollution and measures of CVD prevention should be delivered to everyone, more health education or intervention may be enhanced in women, especially in those middle- and low-income countries where dual challenges of low education in women and heavy air pollution exist (45, 46).
In this meta-analysis, several limitations inherent to the use of the summarized data should be addressed. First, PM2.5 is composed of numerous elements, and a recent research has also shown differences in cardiovascular health associations related to different PM2.5 components (47). It is unknown whether the sex differences in cardiovascular health are associated with various PM2.5 components. Second, PM2.5 exposure assessments in the included cohort studies were based on ambient PM2.5 levels rather than personal exposure assessment methods, which might ignore the indoor air pollution and result in potential misclassification of exposure. Measurements of personal exposure would be encouraged to obtain more accurate assessment of air pollutant exposure in future studies. Third, temperature extremes may elevate cardiovascular risk independently or jointly with air pollution (35, 48), but the sex-specific effect estimations of long-term PM2.5 exposure did not adjust for climate conditions in most of the included studies. Fourth, it is inconsistent for the adjusted covariates in regression models across the original studies. However, most of the studies have adjusted for critical traditional risk factors of CVD, such as age, body mass index (BMI), and smoking. No substantial heterogeneity of between-study in the estimations of RRRs suggested good internal reliability of the meta-analysis results. Last, most of the included articles lacked adjustment for women reproductive factors except that two studies adjusted for the use of oral contraceptives (18) and menopausal status (21). Potential residual confounding may exist due to missing adjustment for reproductive factors in women. More sex-specific quantitative analyses would be encouraged to further validate sex differences in associations of PM2.5 exposure on CVD.
In sum, the meta-analysis provided evidence on the sex-specific risk of CVD associated with long-term PM2.5 exposure, and identified a significantly stronger association between PM2.5 and risk of IHD in women, compared with men. It suggests to become a routine practice that studies on the association of CVD with air pollution report sex-specific results in the future, which would help to develop evidence-based and sex-specific health policies to reduce disease burden attributable to air pollution.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
JZ, XW, and XY designed the research. JZ, XW, and MY performed the literature search and extracted and analyzed the data. JZ and XW drafted the initial manuscript. AS, CW, XY, and NT critically reviewed and revised the article. All authors read and approved the submitted version.
The work was supported by National Natural Science Foundation of China (grant number 82103928) from the Ministry of Science and Technology of China, and the Fundamental Research Funds for Higher Education of Tianjin Municipal Education Commission (grant number 2021ZD038).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.802167/full#supplementary-material
1. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
2. Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, et al. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. (2016) 133:916–47. doi: 10.1161/CIR.0000000000000351
3. Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2014) 45:1545–88. doi: 10.1161/01.str.0000442009.06663.48
4. Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet. (2011) 378:1297–305. doi: 10.1016/S0140-6736(11)60781-2
5. Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. (2014) 57:1542–51. doi: 10.1007/s00125-014-3260-6
6. Brauer M, Casadei B, Harrington RA, Kovacs R, Sliwa K, Group WHFAPE. Taking a stand against air pollution-the impact on cardiovascular disease: a joint opinion from the World Heart Federation, American College of Cardiology, American Heart Association, and the European Society of Cardiology. Circulation. (2021) 143:e800–4. doi: 10.1161/CIRCULATIONAHA.120.052666
7. Chen LH, Knutsen SF, Shavlik D, Beeson WL, Petersen F, Ghamsary M, et al. The association between fatal coronary heart disease and ambient particulate air pollution: are females at greater risk? Environ Health Perspect. (2005) 113:1723–9. doi: 10.1289/ehp.8190
8. Hystad P, Larkin A, Rangarajan S, AlHabib KF, Avezum Á, Calik KBT, et al. Associations of outdoor fine particulate air pollution and cardiovascular disease in 157 436 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet Planetary Health. (2020) 4:e235–45. doi: 10.1016/S2542-5196(20)30103-0
9. Wang B, Eum KD, Kazemiparkouhi F, Li C, Manjourides J, Pavlu V, et al. The impact of long-term PM2.5 exposure on specific causes of death: exposure-response curves and effect modification among 53 million U.S. Medicare beneficiaries. Environ Health. (2020) 19:20. doi: 10.1186/s12940-020-00575-0
10. Bai L, Shin S, Burnett RT, Kwong JC, Hystad P, van Donkelaar A, et al. Exposure to ambient air pollution and the incidence of congestive heart failure and acute myocardial infarction: a population-based study of 5.1 million Canadian adults living in Ontario. Environ Int. (2019) 132:105004. doi: 10.1016/j.envint.2019.105004
11. Li J, Liu F, Liang F, Huang K, Yang X, Xiao Q, et al. Long-term effects of high exposure to ambient fine particulate matter on coronary heart disease incidence: a population-based Chinese cohort study. Environ Sci Technol. (2020) 54:6812–21. doi: 10.1021/acs.est.9b06663
12. Wong CM, Lai HK, Tsang H, Thach TQ, Thomas GN, Lam KB, et al. Satellite-based estimates of long-term exposure to fine particles and association with mortality in elderly Hong Kong residents. Environ Health Perspect. (2015) 123:1167–72. doi: 10.1289/ehp.1408264
13. Alexeeff SE, Liao NS, Liu X, Van Den Eeden SK, Sidney S. Long-term PM2.5 exposure and risks of ischemic heart disease and stroke events: review and meta-analysis. J Am Heart Assoc. (2021) 10:e016890. doi: 10.1161/JAHA.120.016890
14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
15. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed June 27, 2021).
16. DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. (2015) 45:139–45. doi: 10.1016/j.cct.2015.09.002
17. Cai Y, Hodgson S, Blangiardo M, Gulliver J, Morley D, Fecht D, et al. Road traffic noise, air pollution and incident cardiovascular disease: a joint analysis of the HUNT, EPIC-Oxford and UK Biobank cohorts. Environ Int. (2018) 114:191–201. doi: 10.1016/j.envint.2018.02.048
18. Cramer J, Jørgensen JT, Hoffmann B, Loft S, Bräuner EV, Prescott E, et al. Long-term exposure to air pollution and incidence of myocardial infarction: a Danish nurse cohort study. Environ Health Perspect. (2020) 128:57003. doi: 10.1289/EHP5818
19. Elliott EG, Laden F, James P, Rimm EB, Rexrode KM, Hart JE. Interaction between long-term exposure to fine particulate matter and physical activity, and risk of cardiovascular disease and overall mortality in U.S. Women. Environ Health Perspect. (2020) 128:127012. doi: 10.1289/EHP7402
20. Hart JE, Garshick E, Dockery DW, Smith TJ, Ryan L, Laden F. Long-term ambient multipollutant exposures and mortality. Am J Respir Crit Care Med. (2011) 183:73–8. doi: 10.1164/rccm.200912-1903OC
21. Lipsett MJ, Ostro BD, Reynolds P, Goldberg D, Hertz A, Jerrett M, et al. Long-term exposure to air pollution and cardiorespiratory disease in the California teachers study cohort. Am J Respir Crit Care Med. (2011) 184:828–35. doi: 10.1164/rccm.201012-2082OC
22. Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. (2007) 356:447–58. doi: 10.1056/NEJMoa054409
23. Pinault L, Brauer M, Crouse DL, Weichenthal S, Erickson A, van Donkelaar A, et al. Diabetes status and susceptibility to the effects of PM2.5 exposure on cardiovascular mortality in a National Canadian Cohort. Epidemiology. (2018) 29:784–94. doi: 10.1097/EDE.0000000000000908
24. Puett RC, Hart JE, Suh H, Mittleman M, Laden F. Particulate matter exposures, mortality, and cardiovascular disease in the health professionals follow-up study. Environ Health Perspect. (2011) 119:1130–5. doi: 10.1289/ehp.1002921
25. Stockfelt L, Andersson EM, Molnár P, Gidhagen L, Segersson D, Rosengren A, et al. Long-term effects of total and source-specific particulate air pollution on incident cardiovascular disease in Gothenburg, Sweden. Environ Res. (2017) 158:61–71. doi: 10.1016/j.envres.2017.05.036
26. Villeneuve PJ, Weichenthal SA, Crouse D, Miller AB, To T, Martin RV, et al. Long-term exposure to fine particulate matter air pollution and mortality among Canadian women. Epidemiology. (2015) 26:536–45. doi: 10.1097/EDE.0000000000000294
27. Yin P, Brauer M, Cohen A, Burnett RT, Liu J, Liu Y, et al. Long-term fine particulate matter exposure and nonaccidental and cause-specific mortality in a large National Cohort of Chinese Men. Environ Health Perspect. (2017) 125:117002. doi: 10.1289/EHP1673
28. Amini H, Dehlendorff C, Lim YH, Mehta A, Jørgensen JT, Mortensen LH, et al. Long-term exposure to air pollution and stroke incidence: a Danish Nurse cohort study. Environ Int. (2020) 142:105891. doi: 10.1016/j.envint.2020.105891
29. Dirgawati M, Hinwood A, Nedkoff L, Hankey GJ, Yeap BB, Flicker L, et al. Long-term exposure to low air pollutant concentrations and the relationship with all-cause mortality and stroke in older men. Epidemiology. (2019) 30(Suppl. 1):S82–9. doi: 10.1097/EDE.0000000000001034
30. Gandini M, Scarinzi C, Bande S, Berti G, Carnà P, Ciancarella L, et al. Long term effect of air pollution on incident hospital admissions: results from the Italian Longitudinal Study within LIFE MED HISS project. Environ Int. (2018) 121:1087–97. doi: 10.1016/j.envint.2018.10.020
31. Huang K, Liang F, Yang X, Liu F, Li J, Xiao Q, et al. Long term exposure to ambient fine particulate matter and incidence of stroke: prospective cohort study from the China-PAR project. BMJ. (2019) 367:l6720. doi: 10.1136/bmj.l6720
32. Lin H, Guo Y, Di Q, Zheng Y, Kowal P, Xiao J, et al. Ambient PM(2.5) and stroke: effect modifiers and population attributable risk in six low- and middle-income countries. Stroke. (2017) 48:1191–7. doi: 10.1161/STROKEAHA.116.015739
33. Qiu H, Sun S, Tsang H, Wong CM, Lee RSY, Schooling CM, et al. Fine particulate matter exposure and incidence of stroke: a cohort study in Hong Kong. Neurology. (2017) 88:1709–17. doi: 10.1212/WNL.0000000000003903
34. Shin S, Burnett RT, Kwong JC, Hystad P, van Donkelaar A, Brook JR, et al. Ambient air pollution and the risk of atrial fibrillation and stroke: a population-based cohort study. Environ Health Perspect. (2019) 127:87009. doi: 10.1289/EHP4883
35. Yang X, Zhang L, Chen X, Liu F, Shan A, Liang F, et al. Long-term exposure to ambient PM(2.5) and stroke mortality among urban residents in northern China. Ecotoxicol Environ Saf. (2021) 213:112063. doi: 10.1016/j.ecoenv.2021.112063
36. Kim JH, Hong J, Jung J, Im JS. Effect of meteorological factors and air pollutants on out-of-hospital cardiac arrests: a time series analysis. Heart. (2020) 106:1218–27. doi: 10.1136/heartjnl-2019-316452
37. Kim CS, Hu SC. Regional deposition of inhaled particles in human lungs: comparison between men and women. J Appl Physiol. (1998) 84:1834–44. doi: 10.1152/jappl.1998.84.6.1834
38. Sørensen M, Daneshvar B, Hansen M, Dragsted LO, Hertel O, Knudsen L, et al. Personal PM2.5 exposure and markers of oxidative stress in blood. Environ Health Perspect. (2002) 111:161–5. doi: 10.1289/ehp.5646
39. Yang B-Y, Fan S, Thiering E, Seissler J, Nowak D, Dong G-H, et al. Ambient air pollution and diabetes: a systematic review and meta-analysis. Environ Res. (2020) 180:108817. doi: 10.1016/j.envres.2019.108817
40. Agarwala A, Michos ED, Samad Z, Ballantyne CM, Virani SS. The use of sex-specific factors in the assessment of women's cardiovascular risk. Circulation. (2020) 141:592–9. doi: 10.1161/CIRCULATIONAHA.119.043429
41. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. (2016) 37:2315–81. doi: 10.1093/eurheartj/ehw106
42. Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB Sr., Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. (2014) 63:2935–59. doi: 10.1161/01.cir.0000437741.48606.98
43. Mosca L, Linfante AH, Benjamin EJ, Berra K, Hayes SN, Walsh BW, et al. National study of physician awareness and adherence to cardiovascular disease prevention guidelines. Circulation. (2005) 111:499–510. doi: 10.1161/01.CIR.0000154568.43333.82
44. Hao Y, Liu J, Liu J, Yang N, Smith SC Jr, et al. Sex differences in in-hospital management and outcomes of patients with acute coronary syndrome. Circulation. (2019) 139:1776–85. doi: 10.1161/CIRCULATIONAHA.118.037655
45. Kennedy E, Binder G, Humphries-Waa K, Tidhar T, Cini K, Comrie-Thomson L, et al. Gender inequalities in health and wellbeing across the first two decades of life: an analysis of 40 low-income and middle-income countries in the Asia-Pacific region. Lancet Global Health. (2020) 8:e1473–88. doi: 10.1016/S2214-109X(20)30354-5
46. Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. (2017) 389:1907–18. doi: 10.1016/S0140-6736(17)30505-6
47. Badaloni C, Cesaroni G, Cerza F, Davoli M, Brunekreef B, Forastiere F. Effects of long-term exposure to particulate matter and metal components on mortality in the Rome longitudinal study. Environ Int. (2017) 109:146–54. doi: 10.1016/j.envint.2017.09.005
Keywords: air pollution, meta-analysis, cohort, cardiovascular diseases, sex differences
Citation: Zhang J, Wang X, Yan M, Shan A, Wang C, Yang X and Tang N (2022) Sex Differences in Cardiovascular Risk Associated With Long-Term PM2.5 Exposure: A Systematic Review and Meta-Analysis of Cohort Studies. Front. Public Health 10:802167. doi: 10.3389/fpubh.2022.802167
Received: 26 October 2021; Accepted: 11 January 2022;
Published: 02 February 2022.
Edited by:
Guang Hao, Jinan University, ChinaReviewed by:
Yi Zhang, Chinese Center for Disease Control and Prevention, ChinaCopyright © 2022 Zhang, Wang, Yan, Shan, Wang, Yang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueli Yang, eWFuZ3h1ZWxpMjAxOUB0bXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.