- 1Section of Hygiene and Public Health, Department of Life Sciences and Public Health, Università Cattolica del Sacro Cuore, Roma, Italy
- 2Department of Public Health, University of Verona, Verona, Italy
- 3Statistics Division, The George Institute for Global Health, University of New South Wales, Newtown, NSW, Australia
Background: Digital health interventions have significant potential to improve safety, efficacy, and quality of care, reducing waste in healthcare costs. Despite these premises, the evidence regarding cost and effectiveness of digital tools in health is scarce and limited.
Objectives: The aim of this systematic review is to summarize the evidence on the cost-effectiveness of digital health interventions and to assess whether the studies meet the established quality criteria.
Methods: We queried PubMed, Scopus and Web of Science databases for articles in English published from January 1, 2016 to December 31, 2020 that performed economic evaluations of digital health technologies. The methodological rigorousness of studies was assessed with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS). The review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2009 checklist.
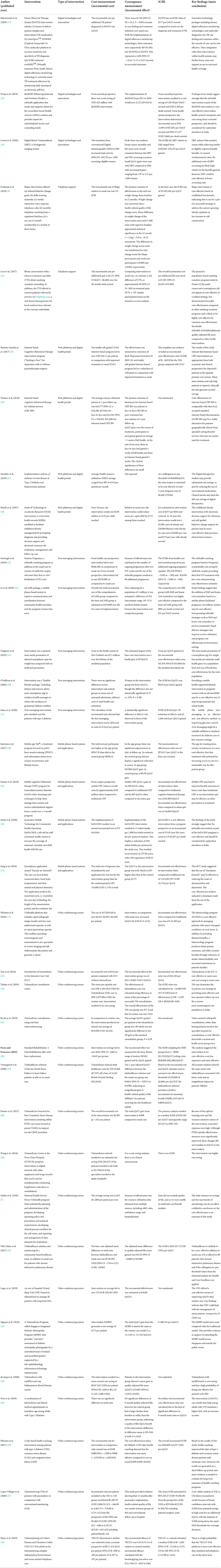
Results: Search identified 1,476 results, 552 of which were selected for abstract and 35 were included in this review. The studies were heterogeneous by country (mostly conducted in upper and upper-middle income countries), type of eHealth intervention, method of implementation, and reporting perspectives. The qualitative analysis identified the economic and effectiveness evaluation of six different types of interventions: (1) seventeen studies on new video-monitoring service systems; (2) five studies on text messaging interventions; (3) five studies on web platforms and digital health portals; (4) two studies on telephone support; (5) three studies on new mobile phone-based systems and applications; and (6) three studies on digital technologies and innovations.
Conclusion: Findings on cost-effectiveness of digital interventions showed a growing body of evidence and suggested a generally favorable effect in terms of costs and health outcomes. However, due to the heterogeneity across study methods, the comparison between interventions still remains difficult. Further research based on a standardized approach is needed in order to methodically analyze incremental cost-effectiveness ratios, costs, and health benefits.
Introduction
In a rapidly evolving society, the progress of digital technology used to improve human health and well-being needs to be constantly evaluated, both in its effectiveness and its efficiency. The WHO defines eHealth as “the cost-effective and secure use of information and communications technologies in support of health and health-related fields, including health-care services, health surveillance, health literature, and health education, knowledge and research” (1).
Digital technology encompasses many areas of eHealth, such as e-learning, telemedicine, mobile health and health information systems. eHealth also benefits from progress in related fields, such as artificial intelligence, big data analytics and genomics.
Digitized health-related data is easier to store and quickly analyze, especially when structuring a data-driven approach to build analytical models for safety improvement, managing clinical risk and increasing the quality of healthcare organizations (2).
During the COVID-19 pandemic, digital health technologies were successfully implemented to aid contact tracing, isolation management, primary care improvement and communication between citizens and decision makers (3).
South Korea is a prime example of a country with widespread digital health implementation, where remotely located supercomputers are used to secure and analyze medical big data and about 50% of digitized hospitals already use a paperless and comprehensive health care system. A rapid response and a cutting-edge government-run digital contact tracing system allowed South Korea to have early success in flattening the curve during its first wave of COVID-19 (4).
Today more than 120 countries are prioritizing health-related digital progress, with a growing need to systematically implement standards-based interoperable solutions.
Despite the institutional fervor and wide applicability of digital health strategies, healthcare facilities and services are struggling to assess the cost-effectiveness of different solutions. The absence of standards and tools for the comparative assessment of functionality and value of fast-evolving digital health solutions exacerbates the pressing need for quality evidence to navigate normative change (5).
This systematic review aims to describing the cost effectiveness of digital health interventions by assessing their impact on standardized indicators, such as Quality Adjusted Life Years (QALYs) and Disease Adjusted Life Years (DALYs), on healthcare expenditure by comparing the strategies with the Median-Based Incremental Cost-Effectiveness Ratio (ICER), and by evaluating the quality of the evidence reported.
Methods
Search strategy
A systematic review of relevant articles published on the cost-effectiveness of digital health technologies was developed in March 2021. The researchers developed the search strategies from January to February 2021 to include a wide range of digital health innovations. The academic databases and systems inquired were PubMed, Web of Science and Scopus, using the query reported in Appendix 1. A manual search of reference lists of both relevant systematic reviews and included studies was also performed. Detailed information and query strings used for the search are disclosed the manuscript.
The systematic literature review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2009 checklist.
Inclusion/exclusion criteria
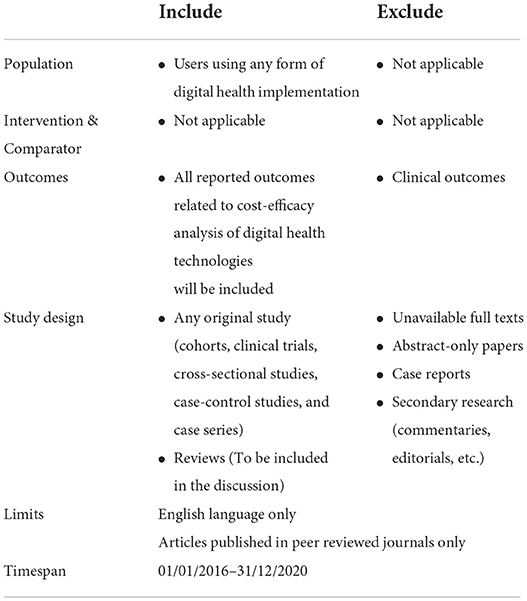
Eligible studies included any original study (cohorts, clinical trials, cross-sectional studies, case-control studies and case series) that reported an analysis of the cost effectiveness of digital health applications and innovations, with or without a comparison to standard care. Due to the rapidly changing nature of digital technologies and because a similar systematic review was released in 2015 (6), only articles published from January 1, 2016 to December 31, 2020 and written in English were included. Unavailable full texts, abstract-only papers, case reports and secondary research (commentaries, editorials, etc.) were excluded from the study. An exclusion criterion included studies where the digital health innovation was only used for recording patients' information. More detailed information is contained in the PICO criteria table (Table 1).
Selection process and data extraction
The results of the electronic search were downloaded into a reference manager library (EndNote). After duplicates were removed, titles and abstracts were reviewed by 2 experienced systematic reviewers working independently to determine whether each study met the eligibility criteria. Full-text copies of potentially relevant studies were retrieved and further assessed against inclusion/exclusion criteria by two independent reviewers. At both stages, disagreements were resolved by discussion or a third reviewer. At the end of the full-text review, the articles that met all predefined criteria were read by two researchers to confirm the inclusion of these articles.
A pilot data extraction was conducted by two of the investigators. Any discrepancy pertinent to data extraction was discussed to reach a consensus. The collected information included the following items: (1) general information (including authors, publication date, title, and country); (2) study characteristics (including discipline examined and kind of intervention); (3) methodology (including modeling method, time horizon, and perspective); (4) cost-effectiveness information (including cost measurement, consequence measurement, and ICER); and (5) key findings (and conclusion).
Assessment of methodological quality
The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist, developed by the International Society for Pharmacoeconomics and Outcomes Research, was the questionnaire used to assess the methodological quality of each study included at the end of the selection process. The CHEERS checklist included 24 items, and the recommendations were subdivided into six categories: (1) title and abstract, (2) introduction, (3) methods, (4) results, (5) discussion, and (6) other. One point was assigned to each item when the quality criteria were fulfilled (and zero points for not entirely conforming to the criteria) to generate a total score (maximum score is 24).
Two of the investigators independently assessed the quality of each study and assigned the scores based on the CHEERS checklist. Any disagreement was resolved by discussion and consensus with a third investigator.
Results
Search results
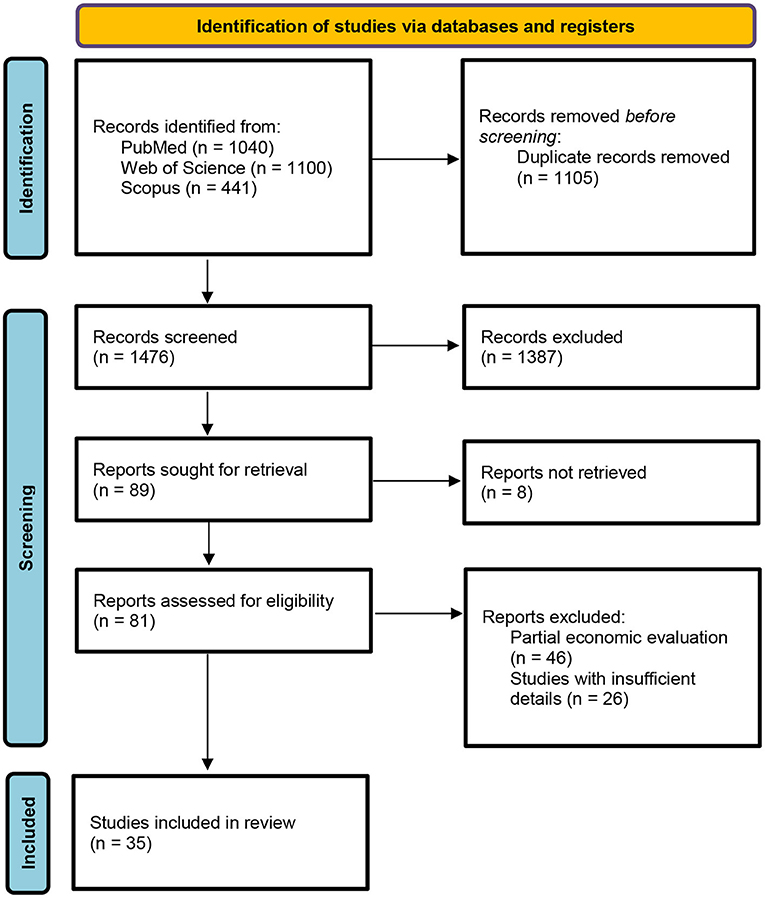
The results of the data extraction and selection process are shown in Figure 1. The database search, after duplicates were removed, returned a total of 1,476 records. In compliance with inclusion/exclusion criteria, the screening by title determined the inclusion of 552 abstracts. The abstract screening reported the inclusion of 81 full-text articles. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, a total of 35 out of the 81 articles were included in the final review.
Study characteristics
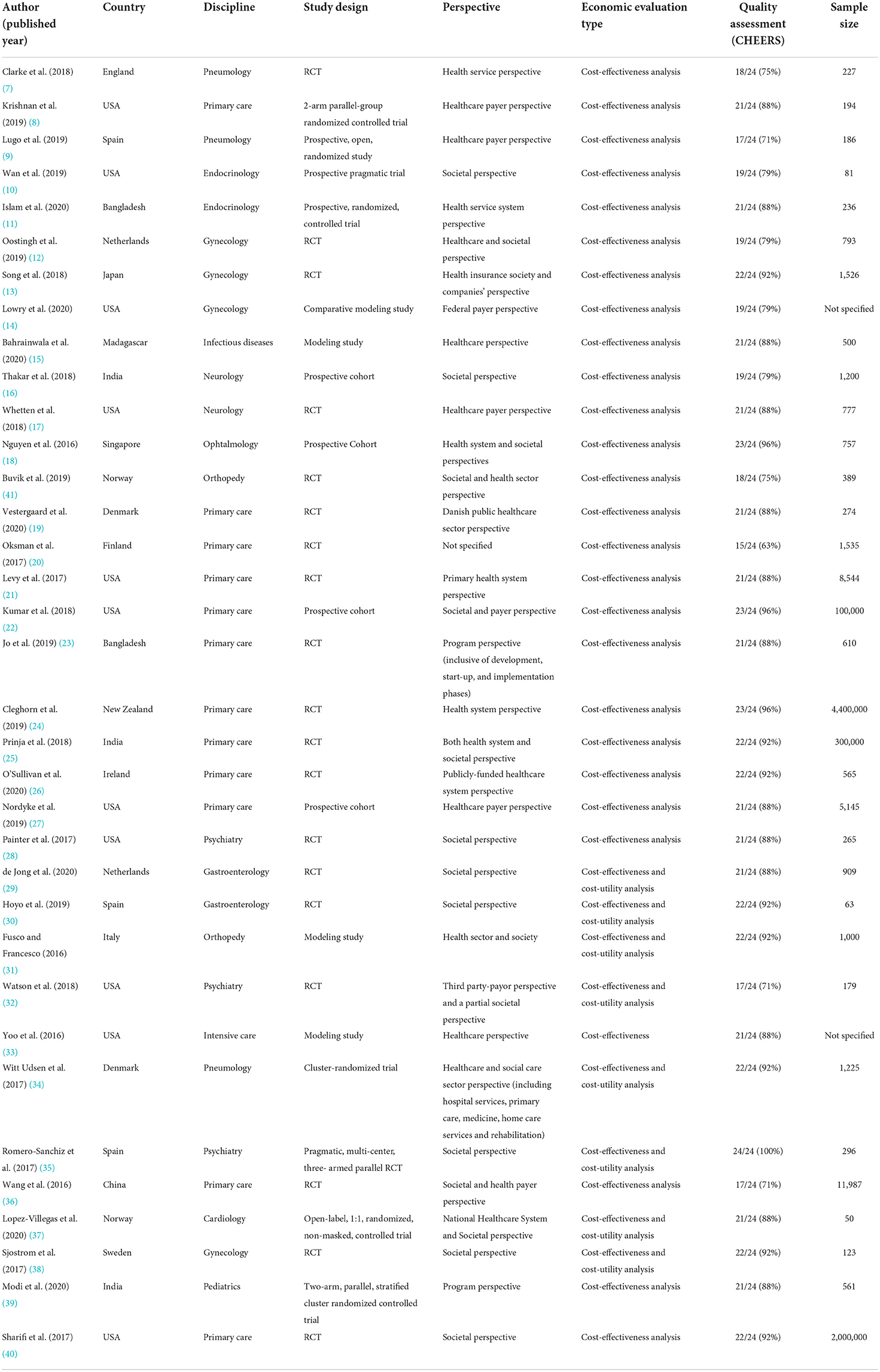
Records information and study characteristics have been subsequently extracted and summarized in Table 2.
The 35 studies spanned 17 countries, the majority of which were conducted in high income economies (28, 80%), compared to 7 in low-income economies (1, 2.9%), lower-middle income economies (5, 14.3%) and upper-middle income economies (1, 2.9%).
The most represented country was the United States of America, with 11 studies (31.4%).
The articles covered a wide range of disciplines: 12 studies discussed primary care (34.3%); three discussed gynecology (11.4%); pneumology and psychiatry had three studies each (8.6%); endocrinology, gastroenterology, neurology and orthopedy had two studies each (5.7%); cardiology, infectious disease, ophthalmology and pediatrics had one study each (2.9%).
The types of digital health intervention implemented to support citizens and patients and to reduce costs from both societal and health payer perspective, were:
- Video-conferencing system: 17/35, 48.6% (7, 9, 10, 16–20, 28–31, 33, 34, 36, 37, 41).
- Text messaging intervention: 5/35, 14.3% (11, 12, 23, 24, 26).
- Web platforms and digital health portals: 5/35, 14.3% (27, 32, 35, 38, 40).
- Telephone support: 2/35, 5.7% (8, 21).
- Mobile phone-based systems and applications: 3/35, 8.6% (13, 22, 39).
- Digital technologies and innovations: 3/35, 8.6% (14, 15, 25).
Cost-effectiveness analysis (CEA) was a criterion of selection, therefore all the studies included a CEA, whereas a cost-utility analysis (CUA) has been performed in addition to the primary CEA in eight studies (22.9%) (29–32, 34, 35, 37, 38).
The costing perspective was mostly from payer/program/health service provider perspective (24, 68.7%), whereas the costing perspective was societal in 19 studies (54.3%). Only one study did not clearly report the costing perspective (2.9%).
In order to obtain QALYs, the studies of this review referred to different instruments deriving from the trials of reference. The questionnaires and surveys used to estimate QALYs were: ShortForm-6 Dimensions (32); a multistate body mass table model (24); European Quality of Life 5-Dimensions (9–11, 19, 29, 30, 33, 34, 37, 41); Health Related Quality of Life (8, 14, 20, 31); Generalized Anxiety Disorder 7 (22); Quebec Sleep Questionnaire and Epworth Sleepiness Scale (9); Quality of Well-being Scale and Short Form Health Survey for Veterans (28); International Consultation on Continence Modular Questionnaire on Lower Urinary Tract Symptoms and Quality of Life (38). For the rest of the articles, QALYs were estimated from existing literature.
Study quality
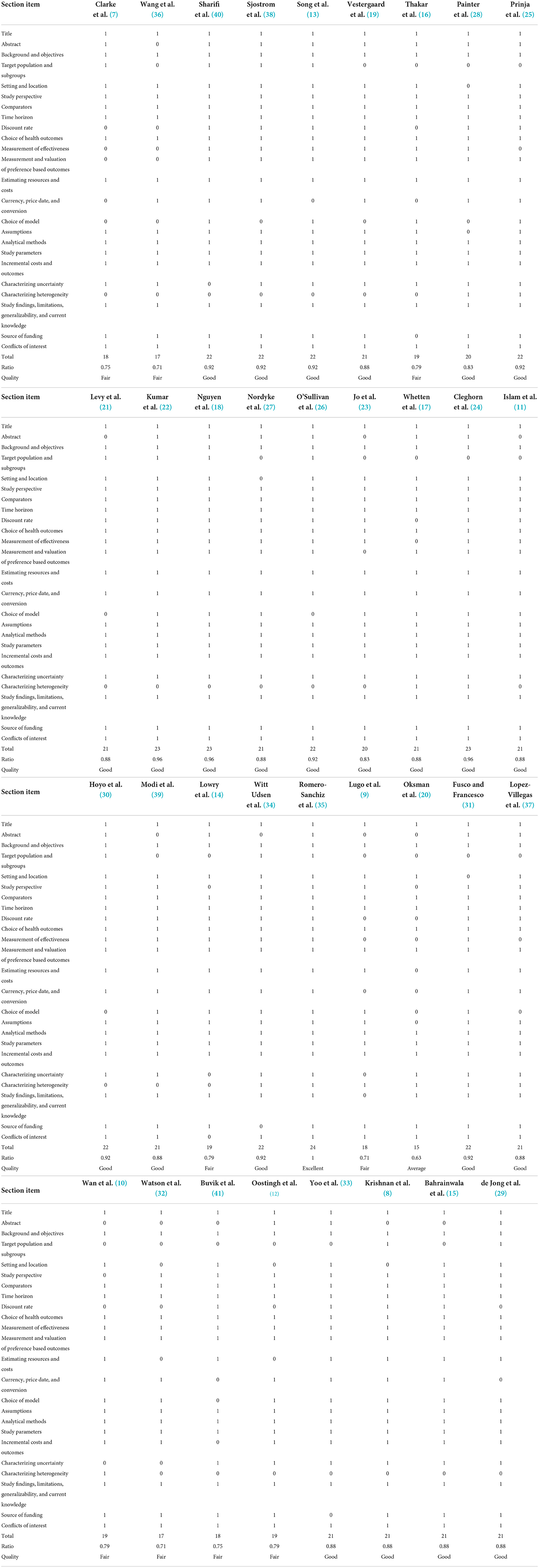
The number of items of the CHEERS Checklist satisfied by each study and the relative percentage are shown in Table 3.
The studies were graded on the bases of the number of items accomplished and classified as follows:
- Excellent, if all items were present in the study: only one study (3%) reported (35).
- Good, if at least 80% of the items were satisfied: 24 studies (69%) reported (8, 11, 12, 15, 17–19, 21–31, 33, 34, 37–40).
- Fair, if at least 70% of the items were satisfied: nine studies (26%) reported (7, 9, 10, 12, 14, 16, 32, 36, 41).
- Average, if at least 60% of the items were satisfied: one study (3%) reported (20).
The five items most likely to not be reported were: Characterizing heterogeneity (11, 31%); Target population and subgroups (13, 37%); Abstract (22, 63%); Choice of model (24, 69%); Discount rate (25, 71%).
In contrast, seven CHEERS checklist items were fulfilled by all studies (100%): Title; Background and objectives; Comparators; Time horizon; Choice of health outcomes; Analytical methods; Study parameters.
All the other 12 CHEERS items were included in almost 80% of the studies: Measurement of effectiveness (28, 80%); Currency, price date, and conversion (28, 80%); Setting and location (29, 83%); Characterizing uncertainty (30, 86%); Study perspective (32, 91%); Measurement and valuation of preference-based outcomes (32, 91%); Estimating resources and costs (32, 91%); Source of funding (32, 91%); Assumptions (33, 94%); Incremental costs and outcomes (34, 97%); Study findings, limitations, generalizability, and current knowledge (34, 97%); Conflicts of interest (34, 97%).
Type of technologies or interventions for digital health innovation
A summary of findings and evidence found is reported in Table 4.
Video-conferencing system
Video conferencing systems consist in programs that allow the delivery of specialist consultations via video for remote patients with any kind of condition or disease.
In this review, a total of 17 of the included studies evaluated the cost-effectiveness of video conferencing systems (7, 9, 10, 16–20, 28–31, 33, 34, 36, 37, 41).
The disciplines examined were the following: (1) two studies focused on a video conferencing system applied in a teletrauma/telestroke context, delivering neurology care to remote patients (16, 17); a network of audiovisual communications and data systems allowed to link hospital intensive care units to intensivists and other critical care professionals at remote locations (33); (2) two studies compared a standard rehabilitation program to a telerehabilitation program for patients that aimed to improve access and quality of care, avoid patient travel, and reduce health care costs (31, 41); (3) two videoconferencing services for general practices consisted of telehealthcare equipment for continuous monitoring of physiological measurements (19, 36); telemedicine outreach for Post-Traumatic Stress Disease (PTSD) examined the impact of telemedicine-based collaborative care for PTSD with enhanced usual care without on-site psychiatrists (28); (4) three studies focused on the effects of a videoconferencing system for patients with chronic obstructive pulmonary disease (7, 9, 34); (5) three studies implemented telemedicine interventions for patients with diabetes (10, 18, 20); (6) two video conferencing platforms were used for telemonitoring complex inflammatory bowel disease compared to standard care (29, 30); (7) one study examined patients with implanted pacemakers who received home monitoring with internet-based remote monitoring service and video-consultation service (37).
Two out of the 17 articles included in this section reported not to be cost-effective (28, 37).
The remaining 15 articles stated the digital health interventions to be cost-effective: video conferencing systems gained higher QALYs with cost-saving in 9 (7, 9, 16–18, 29–31, 41); video conferencing systems gained QALYs with higher cost at an acceptable ICER in 6 (10, 19, 20, 33, 34, 36).
Text messaging intervention
Text message-based health interventions provide patients with reminders, education or self-management assistance for a broad spectrum of health conditions.
In this review, the text messaging interventions concerned the following topics: (1) a mobile phone text messaging program for people with type 2 diabetes mellitus was implemented in Bangladesh (11); (2) Smarter Pregnancy, a text messaging coaching program in addition to the usual care for women of subfertile couples who start their first in vitro fertilization cycle (12); (3) mCARE package, a short message service and home visit reminders sent to pregnant women to promote the care-seeking of essential maternal and newborn care services (23); (4) a New Zealand national mass media promotion of selected smartphone apps with text messaging service for weight loss (24); (5) the Pregnancy, Exercise And nutrition Research study (PEARs) intervention, a ‘healthy lifestyle package,' that included dietary and exercise advice and text messages to reinforce health reminders (26).
All five of the included studies in this category found that text messaging interventions were cost-effective. Intervention groups gained higher QALYs with cost-savings in one study (12); intervention groups gained QALYs with slightly higher cost at an acceptable ICER in the other four studies (11, 23, 24, 26).
Web platforms and digital health portals
A digital health portal is a secure online web portal that gives patients convenient, 24-h access to personal health information from anywhere via an Internet connection, often “tethered” to their integrated electronic health records.
In this review, the web portals for citizens and patients retrieved during the screening process are the following: (1) “Smiling is fun”, an Internet-delivered, self-help web portal for the treatment of depression, consisting of 10 cognitive behavioral therapy modules to cope with depression (35); (2) an e-Health portal that gives internet-based cognitive-behavioral therapy designed for patients with bulimia nervosa (32); (3) a web portal that provides digital behavioral and lifestyle intervention for type 2 diabetes mellitus and hypertension patients (27); (4) an electronic health record (EHR)-based decision support program for parents with 6- to 12-year-old children with obesity, providing behavioral therapy and support (40); and (5) Tät service, a web portal support service designed to inform patients on stress urinary incontinence, that provides a pelvic floor muscle training program and prescribes pelvic floor muscle training 3 times daily during treatment (38).
The cost-effectiveness acceptability analysis indicated improved health outcomes with similar, or even lower costs for all the 5 studies of this section (27, 32, 35, 38, 40).
Telephone support
Telephone support is the use of phone calls by specialists, such as nurses, doctors and healthcare professionals in general, to deliver self-care support and/or management.
Studies selected in this category focused on the following topics: (1) a tobacco treatment telephone support program, that provides smoking cessation counseling to participants in the intervention group (21); and (2) Shape Program, an adaptive telephone-based coaching system, designed to prevent weight gain in black female primary care patients that consists of personalized obesogenic behavior change goals assigned every 2 months, a tailored skills training curriculum, patient self-monitoring delivered via a fully automated interactive voice response system and 12 counseling calls with a registered dietitian (8).
All of the studies in this section reported greater gains in quality-adjusted life years at a similar or slightly higher cost, resulting in cost-effectiveness based on established benchmarks (8, 21).
Mobile phone-based systems and applications
Mobile phone-based applications include all of the services and systems that provide support, delivery and promotion of care through the monitoring and sharing of health information via mobile technology, such as wearables and health tracking apps.
Mobile phone-based applications retrieved in this review included: (1) a mobile cognitive-based therapy program set to provide learnings and techniques to help users manage their anxiety (22); (2) Karada-no-kimochi, a mobile application that predicts the menstrual cycle based on recorded data and provides information regarding menstruation (13); and (3) Innovative Mobile Technology for Community Health Operation (ImTeCHO), a mHealth-based intervention that enhances health promotion using multimedia and short message reminders to increase coverage of maternal, neonatal, and child health care (39).
All 3 studies declared improved QALYs with lower cost, suggesting the interventions were highly cost effective (13, 22, 39).
Digital technologies and innovations
This section includes all articles that focused on the cost-effectiveness of digital health interventions that do not fall within any of the above categories, such as experimental digital diagnostic imaging or experimental technologies.
Digital health innovations in this group were represented by: (1) Digital Breast Tomosynthesis (DBT), a new breast imaging modality that reconstructs cross-sectional slices of the breast, minimizing soft-tissue overlap (14); (2) ReMiND, a program that assists healthcare professionals in the early identification, treatment, and rapid referral for appropriate care of any danger signs among pregnant women or neonates (25); and (3) Drone Observed Therapy System (DrOTS), a project to support community-based tuberculosis case finding using drones to deliver sputum samples and tuberculosis medication between rural communities, diagnostics and treatment facilities (15).
Two out of the three studies in this section found the digital health interventions to be highly cost-effective, resulting in higher QALYs with cost-savings (15, 25), while one study reported to be cost-effective but with higher costs (14).
Discussion
Standardized cost-effectiveness analysis should be used to compare different interventions in terms of their consequences and costs so it can be used as a crucial tool to help decision makers or funders understand if digital health interventions and innovations actually determine an increase of QALYs or DALYs with contained costs (42).
In our systematic review, we opted to exclude studies that utilized digital technologies only to record information without any active participation from healthcare personnel as we wanted to focus on interventions that facilitate the communication between citizen/patient and healthcare staff. However, the number of economic evaluations included in the review is in line with the previous review on cost-utility and cost-effectiveness of telehealth interventions published in 2015, where 35 studies assessed effectiveness, utility and costs (6).
Studies show a broad range of digital interventions, reference population, focus disease and discipline interested. The majority included a comparison of a digital health innovation costs and effectiveness in terms of health-related outcomes vs. standard care. However, some did not report all recommended economic and consequence outcome items. For example, Watson et al. (32), Buvik et al. (41), Wang et al. (36), Lugo et al. (9), and De Jong et al. (29), did not calculate the incremental cost effectiveness ratio, while Nordyke et al. (27), did not indicate the incremental effect of the use of intervention to treat disease in type 2 diabetes and hypertension patients.
The transferability of findings across economic evaluations is based on a rigorous data collection strategy, a coherent methodology, and an explicit description of target population, study design and sample size (43). In our review, over half of the studies were randomized controlled trials which are considered to be of high quality and have a low risk of bias in comparison with other study designs, such as expert opinion studies. Sample size varied significantly and ranged from 50 participants in a study where telemedicine was used to monitor patients with pacemakers compared to conventional monitoring (37) to 4.4 million participants where a text messaging intervention promoted weight loss in all of New Zealand's population (24). One article missed to indicate the sample size (14).
Economic evaluations conducted from a societal perspective are generally preferred since one of the principal objectives of public health is to improve the health and the quality of life of the general population (44). Moreover, economic evaluations based on a fixed budget may lead to suboptimal decisions; they are inconsistent with decisions based on willingness to pay for QALYs and are considered to be of low quality in comparison to societal perspective. Of the 35 studies of the review, 19 articles conducted their economic evaluations from a societal perspective, while only one study did not report the perspective (20). On the contrary, articles with a societal perspective took into account all the losses and expenses, direct and indirect, supported by society as a whole, irrespective of who the benefactors were (e.g. production losses, travel costs, absenteeism, presenteeism, premature death, etc.). Articles with a third party payer perspective encompassed intervention costs, outpatient (incl. general practitioners and specialists) and inpatient services, medication and societal service costs.
Modeling techniques are generally used to predict the effect and the potential cost (or savings) of a determined technology where it is not feasible to wait for lifetime data to validate the cost-effectiveness (42). In our review, several studies used modeling techniques to predict the intervention effect and cost over a long period, or even a lifetime (14, 15, 17, 18, 21, 22, 24, 25, 31, 36, 40, 41). In these cases, it became fundamental to clearly express the uncertainty of the analysis described. All studies selected in the review that used predictive models indicated uncertainty (mostly Monte Carlo simulation, but also one-way and multiway sensitivity analyses, threshold analyses and probabilistic sensitivity analyses).
According to previous reviews (6), “Videoconferencing system” was the most represented digital health intervention type but it was also the one that had the most discordant results: five studies report that the applied technology is not cost-effective or that in any case there is not enough evidence to define the cost-effectiveness (7, 19, 28, 34, 37). Vestegard et al. (19) found that telehealth care was associated with lower costs but had an insignificant impact on patients' HRQoL. Painter et al. (28) underlined effectiveness of the digital measures, but admitted that they did not improve QALYs in the main analysis. Clarke et al. (7) could not give a definitive conclusion on the cost-effectiveness as an outcome of their study due to a wide variance on savings and the uncertainty of monitoring cost. Also Lopez-Villegas et al. (37) had inconclusive results due to broad confidence intervals with ICER from potential savings to high costs for an additional QALY, with the majority of ICERs being above the usual NHS thresholds for coverage decisions.
Witt Udsen et al. (34) through their study revealed that telehealthcare is unlikely to be cost-effective in addition to usual care.
A total of three out of the 35 studies (3/35, 8.6%) were found to not be cost-effective: two studies in the videoconferencing system category (28, 37) and one study in the digital technologies and innovations (14). Of the remaining articles, 12 studies (12/35, 34.3%) found digital health interventions gained QALYs with a higher cost at an acceptable ICER when compared with a relative national benchmark (six studies in videoconferencing systems, four studies in text messaging, two studies in telephone support) (8, 10, 11, 19–21, 23, 24, 26, 33, 34, 36). Finally, a total of 20 out of the 35 studies (20/35, 57.1%) found the digital health interventions gained higher QALYs with cost-savings (nine studies in videoconferencing systems, one study in text messaging, five studies in web platforms and digital health portals, three studies in mobile phone-based systems and applications, two studies in digital health technologies and innovations) (7, 9, 12, 13, 15–18, 22, 25, 27, 29–32, 35, 38–41).
Various benefits of digital tools to rural realities were underlined in different studies (15, 17, 23, 25, 28, 32, 39). This is a very important aspect as it could improve and revolutionize the access to care and quality of treatment for a very large number of patients. Video-conferencing systems offered the greatest advantages in reaching rural areas (17, 23, 28, 36), followed by digital technologies and innovations (15, 25), web platforms and digital health portals (32), mobile phone-based systems and applications (39). The most significant achievements in reaching rural areas were found in the USA (17, 28, 32), followed by India (25, 39), China (36) and Madagascar (15).
It is known that cost-effectiveness is a subjective concept since it depends on the willingness to pay (WTP) for specific outcomes. The decision makers' WTP threshold to establish the cost-effectiveness of intervention differs in the literature. For example, in studies from the United Kingdom, a threshold range of £20,000–£30,000/QALY gained is normally used (45). While in America (46) and Australia (47) they use the amount of 50,000/QALY gained, each in their respective currencies (48).
Some studies analyzed digital health interventions that exceeded the threshold (14, 28, 34, 37) while others had costs that remained below the threshold (7). However, it is crucial to consider that interventions are preferable, even with costs over the threshold, if they improve the outcome with minor or the same costs when compared with standard care.
Limitations
Firstly, due to the heterogeneity of interventions in the included studies, we could not provide synthetic and general conclusions about costs because most costs were expressed in different values and it was not always possible to make systematic comparisons between them. Secondly, by focusing only on articles written in English, our study may be subject to publication bias and the results should be interpreted appropriately. Thirdly, another source of potential bias - typical of economic studies – is the systematic tendency of including/excluding cost items in the analysis. Therefore, results are driven toward a specific perspective, i.e., the social perspective rather than the health systems' perspective. Finally, even if extensively used to evaluate the quality of economic studies in systematic review of economic evaluations, the CHEERS checklist has structural limitations (e.g. different aspects of the study have the same importance, so the completeness of the abstract and methodological issues such as the choice of the model, the assumptions and the management of uncertainties are given the same relevance).
Conclusion
Despite a growing interest in investing in digital tools in healthcare, the evidence regarding cost-effectiveness of digital tools in the health sector remains scarce and limited.
Through this review it some evidence was found that digital health interventions can affect cost-effectiveness with a favorable effect both in terms of costs and health outcomes. In particular, the findings showed a positive impact especially for studies that implemented a new mobile application or a web portal intervention. We strongly believe that the findings of this research could be used to better inform and orient health policies. More than half of the studies included report that the use of digital health intervention led to the achievement of a better efficiency and outcomes for patients, the optimization of available human and technological resources and the consistent reduction in the costs of the healthcare services provided. Recognized international examples of digital health practices being successful could be the first step to informing and orienting decision makers to structure a new, evidence-based, digital health maturity.
However, due to the heterogeneity across study methods, cost perspectives, disciplines and diseases involved, the comparison between interventions still remains difficult. Further research based on a standardized approach is needed in order to methodically analyze incremental cost-effectiveness ratios, costs and health benefits.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AG, FC, GF, and WR: conception or design of the work and final approval of the version to be published. AG: data collection. AG, GF, GT, AM, and VP: articles screening, data analysis, interpretation, and drafting the article. FC, AG, GF, AM, and VP: critical revision of the article. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. WHO. mHealth, use of appropriate digital technologies for public health-Report by the Director-General. Exec Board, 142nd Sess provisional agenda item 44 EB142/20. (2017) 2015(November 2017):1–5. Available from: http://apps.who.int/gb/e/e_wha71.html (accessed March 1, 2021).
2. Cascini F, Santaroni F, Lanzetti R, Failla G, Gentili A, Ricciardi W. Developing a data-driven approach in order to improve the safety and quality of patient care. Front Public Health. (2021) 9:667819. doi: 10.3389/fpubh.2021.667819
3. Cascini F, Hoxhaj I, Zaçe D, Ferranti M, Di Pietro ML, Boccia S, et al. How health systems approached respiratory viral pandemics over time: a systematic review. BMJ Global Health. (2020). doi: 10.1136/bmjgh-2020-003677
5. Mendoza G, Okoko L, Konopka S, Jonas E. mHealth compendium. Afr Strateg Health Proj. (2013) 3:82. Available online at: http://www.africanstrategies4health.org/ (accessed March 1, 2021).
6. De La Torre-Diéz I, López-Coronado M, Vaca C, Aguado JS, De Castro C. Cost-utility and cost-effectiveness studies of telemedicine, electronic, and mobile health systems in the literature: a systematic review. Telemed J E Health. (2015) 21:81–5. doi: 10.1089/tmj.2014.0053
7. Clarke M, Fursse J, Brown-Connolly NE, Sharma U, Jones R. Evaluation of the National Health Service (NHS) direct pilot telehealth program: cost-effectiveness analysis. Telemed E Health. (2018) 24:67–76. doi: 10.1089/tmj.2016.0280
8. Krishnan A, Finkelstein EA, Levine E, Foley P, Askew S, Steinberg D, et al. A digital behavioral weight gain prevention intervention in primary care practice: cost and cost-effectiveness analysis. J Med Internet Res. (2019) 21:e12201. doi: 10.2196/12201
9. Lugo VM, Garmendia O, Suarez-Girón M, Torres M, Vázquez-Polo FJ, Negrín MA, et al. Comprehensive management of obstructive sleep apnea by telemedicine: clinical improvement and cost-effectiveness of a virtual sleep unit. A randomized controlled trial. PLoS ONE. (2019) 14:e0224069. doi: 10.1371/journal.pone.0224069
10. Wan W, Nathan AG, Skandari MR, Zarei P, Reid MW, Raymond JK, et al. Cost-effectiveness of shared telemedicine appointments in young adults with T1D: CoYoT1 trial. Diabetes Care. (2019) 42:1589–92. doi: 10.2337/dc19-0363
11. Islam M, Peiffer R, Chow CK, Maddison R, Lechner A, Holle R, Niessen L, Laxy M. Cost-effectiveness of a mobile-phone text messaging intervention on type 2 diabetes—a randomized-controlled trial. Health Policy Technol. (2020) 9:79–85. doi: 10.1016/j.hlpt.2019.12.003
12. Oostingh EC, Ophuis RH, Koster WPH, Polinder S, Lingsma HF, Laven JSE, et al. Mobile health coaching on nutrition and lifestyle behaviors for subfertile couples using the smarter pregnancy program: model-based cost-effectiveness analysis. JMIR Mhealth Uhealth. (2019) 7:e13935. doi: 10.2196/preprints.13935
13. Song M, Kanaoka H. Effectiveness of mobile application for menstrual management of working women in Japan: randomized controlled trial and medical economic evaluation. J Med Econ. (2018) 21:1131–8. doi: 10.1080/13696998.2018.1515082
14. Lowry KP, Trentham-Dietz A, Schechter CB, Alagoz O, Barlow WE, Burnside ES, et al. Long-term outcomes and cost-effectiveness of breast cancer screening with digital breast tomosynthesis in the United States. J Natl Cancer Inst. (2020) 112:582–9. doi: 10.1093/jnci/djz184
15. Bahrainwala L, Knoblauch AM, Andriamiadanarivo A, Diab MM, McKinney J, Small PM, et al. Drones and digital adherence monitoring for community-based tuberculosis control in remote Madagascar: a cost-effectiveness analysis. PLoS ONE. (2020) 15:e0235572. doi: 10.1371/journal.pone.0235572
16. Thakar S, Rajagopal N, Mani S, Shyam M, Aryan S, Rao AS, et al. Comparison of telemedicine with in-person care for follow-up after elective neurosurgery: results of a cost-effectiveness analysis of 1200 patients using patient-perceived utility scores. Neurosurg Focus. (2018) 44:E17. doi: 10.3171/2018.2.FOCUS17543
17. Whetten J, van der Goes DN, Tran H, Moffett M, Semper C, Yonas H. Cost-effectiveness of Access to Critical Cerebral Emergency Support Services (ACCESS): a neuro-emergent telemedicine consultation program. J Med Econ. (2018) 21:398–405. doi: 10.1080/13696998.2018.1426591
18. Nguyen H V, Tan GSW, Tapp RJ, Mital S, Ting DSW, Wong HT, et al. Cost-effectiveness of a National Telemedicine Diabetic Retinopathy Screening Program in Singapore. Ophthalmology. (2016) 123:2571–80. doi: 10.1016/j.ophtha.2016.08.021
19. Vestergaard AS, Hansen L, Sorensen SS, Jensen MB, Ehlers LH. Is telehealthcare for heart failure patients cost-effective? An economic evaluation alongside the Danish TeleCare North heart failure trial. BMJ Open. (2020) 10:e031670. doi: 10.1136/bmjopen-2019-031670
20. Oksman E, Linna M, Hörhammer I, Lammintakanen J, Talja M. Cost-effectiveness analysis for a tele-based health coaching program for chronic disease in primary care. BMC Health Serv Res. (2017) 17:138. doi: 10.1186/s12913-017-2088-4
21. Levy DE, Klinger E V, Linder JA, Fleegler EW, Rigotti NA, Park ER, et al. Cost-effectiveness of a health system-based smoking cessation program. Nicotine Tob Res. (2017) 19:1508–15. doi: 10.1093/ntr/ntw243
22. Kumar S, Bell MJ, Juusola JL. Mobile and traditional cognitive behavioral therapy programs for generalized anxiety disorder: a cost-effectiveness analysis. PLoS ONE. (2018) 13:e0190554. doi: 10.1371/journal.pone.0190554
23. Jo Y, LeFevre AE, Healy K, Singh N, Alland K, Mehra S, et al. Costs and cost-effectiveness analyses of mCARE strategies for promoting care seeking of maternal and newborn health services in rural Bangladesh. PLoS ONE. (2019) 14:e0223004. doi: 10.1371/journal.pone.0223004
24. Cleghorn C, Wilson N, Nair N, Kvizhinadze G, Nghiem N, McLeod M, et al. Health benefits and cost-effectiveness from promoting smartphone apps for weight loss: multistate life table modeling. JMIR Mhealth Uhealth. (2019) 7:e11118. doi: 10.2196/11118
25. Prinja S, Bahuguna P, Gupta A, Nimesh R, Gupta M, Thakur JS. Cost effectiveness of mHealth intervention by community health workers for reducing maternal and newborn mortality in rural Uttar Pradesh, India. Cost Eff Resour Alloc. (2018) 16:25. doi: 10.1186/s12962-018-0110-2
26. O'Sullivan EJ, Rokicki S, Kennelly M, Ainscough K, McAuliffe FM, O'Sullivan EJ, et al. Cost-effectiveness of a mobile health-supported lifestyle intervention for pregnant women with an elevated body mass index. Int J Obes. (2020) 44:999–1010. doi: 10.1038/s41366-020-0531-9
27. Nordyke RJ, Appelbaum K, Berman MA. Estimating the impact of novel digital therapeutics in type 2 diabetes and hypertension: health economic analysis. J Med Internet Res. (2019) 21:e15814. doi: 10.2196/preprints.15814
28. Painter JT, Fortney JC, Austen MA, Pyne JM. Cost-effectiveness of telemedicine-based collaborative care for posttraumatic stress disorder. Psychiatr Serv. (2017) 68:1157–63. doi: 10.1176/appi.ps.201600485
29. de Jong MJ, Boonen A, van der Meulen-de Jong AE, Romberg-Camps MJ, van Bodegraven AA, Mahmmod N, et al. Cost-effectiveness of telemedicine-directed specialized vs standard care for patients with inflammatory bowel diseases in a randomized trial. Clin Gastroenterol Hepatol. (2020) 18:1744–52. doi: 10.1016/j.cgh.2020.04.038
30. Hoyo JD, Nos P, Bastida G, Faubel R, Muñoz D, Garrido-Marín A, et al. Telemonitoring of Crohn's Disease and Ulcerative colitis (TECCU): cost-effectiveness analysis. J Med Internet Res. (2019) 21:e15505. doi: 10.2196/15505
31. Fusco F, Turchetti G. Telerehabilitation after total knee replacement in Italy: cost-effectiveness and cost-utility analysis of a mixed telerehabilitation-standard rehabilitation programme compared with usual care. BMJ Open. (2016) 6:e009964. doi: 10.1136/bmjopen-2015-009964
32. Watson HJ, McLagan N, Zerwas SC, Crosby RD, Levine MD, Runfola CD, et al. Cost-effectiveness of internet-based cognitive-behavioral treatment for bulimia nervosa: results of a randomized controlled trial. J Clin Psychiatry. (2018) 79:16m11314. doi: 10.4088/JCP.16m11314
33. Yoo B-K, Kim M, Sasaki T, Melnikow J, Marcin JP. Economic evaluation of telemedicine for patients in ICUs. Crit Care Med. (2016) 44:265–74. doi: 10.1097/CCM.0000000000001426
34. Witt Udsen F, Lilholt PH, Hejlesen O, Ehlers L. Cost-effectiveness of telehealthcare to patients with chronic obstructive pulmonary disease: results from the Danish ‘TeleCare North' cluster-randomised trial. BMJ Open. (2017) 7:e014616. doi: 10.1136/bmjopen-2016-014616
35. Romero-Sanchiz P, Nogueira-Arjona R, García-Ruiz A, Luciano J V, Campayo JG, Gili M, et al. Economic evaluation of a guided and unguided internet-based CBT intervention for major depression: results from a multicenter, three-armed randomized controlled trial conducted in primary care. PLoS ONE. (2017) 12:e0172741. doi: 10.1371/journal.pone.0172741
36. Wang T-T, Li J-M, Zhu C-R, Hong Z, An D-M, Yang H-Y, et al. Assessment of utilization and cost-effectiveness of telemedicine program in western regions of China: a 12-year study of 249 hospitals across 112 cities. Telemed E Health. (2016) 22:909–20. doi: 10.1089/tmj.2015.0213
37. Lopez-Villegas A, Catalan-Matamoros D, Peiro S, Lappegard KT, Lopez-Liria R. Cost–utility analysis of telemonitoring versus conventional hospital-based follow-up of patients with pacemakers. The NORDLAND randomized clinical trial. PLoS ONE. (2020) 15:e0226188. doi: 10.1371/journal.pone.0226188
38. Sjostrom M, Lindholm L, Samuelsson E. Mobile app for treatment of stress urinary incontinence: a cost-effectiveness analysis. J Med Internet Res. (2017) 19:e154. doi: 10.2196/jmir.7383
39. Modi D, Saha S, Vaghela P, Dave K, Anand A, Desai S, et al. Costing and cost-effectiveness of a mobile health intervention (ImTeCHO) in improving infant mortality in tribal areas of Gujarat, India: cluster randomized controlled trial. JMIR Mhealth Uhealth. (2020) 8:e17066. doi: 10.2196/17066
40. Sharifi M, Franz C, Horan CM, Giles CM, Long MW, Ward ZJ, et al. Cost-effectiveness of a clinical childhood obesity intervention. Pediatrics. (2017) 140:e20162998. doi: 10.1542/peds.2016-2998
41. Buvik A, Bergmo TS, Bugge E, Smaabrekke A, Wilsgaard T, Olsen JA. Cost-Effectiveness of telemedicine in remote orthopedic consultations: randomized controlled trial. J Med Internet Res. (2019) 21:e11330. doi: 10.2196/11330
42. Iribarren SJ, Cato K, Falzon L, Stone PW. What is the economic evidence for mHealth? A systematic review of economic evaluations of mHealth solutions. PLoS ONE. (2017) 12:e0170581. doi: 10.1371/journal.pone.0170581
43. Bergmo TS. How to measure costs and benefits of ehealth interventions: An overview of methods and frameworks. J Med Internet Res. (2015) 11:e254. doi: 10.2196/jmir.4521
44. Jönsson B. Editorial: ten arguments for a societal perspective in the economic evaluation of medical innovations. Eur J Health Econ. (2009) 10:357–9. doi: 10.1007/s10198-009-0173-2
45. McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics. (2008) doi: 10.2165/00019053-200826090-00004
46. Weinstein MC. How much are Americans willing to pay for a quality-adjusted life year? Med Care. (2008) 26:733–44. doi: 10.1097/MLR.0b013e31816a7144
47. Taylor C, Jan S. Economic evaluation of medicines. Aust Prescr. (2017) 40:76–8. doi: 10.18773/austprescr.2017.014
48. Ghani Z, Jarl J, Berglund JS, Andersson M, Anderberg P. The cost-effectiveness of mobile health (Mhealth) interventions for older adults: systematic review. Int J Environ Res Public Health. (2020) 17:1–13. doi: 10.3390/ijerph17155290
Appendix 1
Search string
(“digital health”[Mesh Terms] OR “digital health” OR “telemedicine”[Mesh Terms] OR “telemedicine” OR “e health”[Mesh Terms] OR “e health” OR “electronic health” OR “m health”[Mesh Terms] OR “m health” OR “mobile health” OR “remote consultation” OR “digital transformation” OR “home care services” OR “telenursing” OR “health innovation”[Mesh Terms] OR “telemetry”[Mesh Terms] OR “telehealth”[Mesh Terms] OR “telehealth” OR “telecare” OR “digital care”[Mesh Terms]) AND (“cost analysis”[Mesh Terms] OR “cost analysis” OR “cost benefit”[Mesh Terms] OR “cost benefit” OR “cost efficacy”[All Fields] OR “cost effectiveness”[All Fields] OR “cost consequence”[All Fields] OR “economic evaluation” OR “economic outcome” OR “economic assessment” OR “hta”). Search criteria have been adjusted to each database searched.
Keywords: digital health, telemedicine, mobile health, electronic health, telehealth, digital care, cost-effectiveness
Citation: Gentili A, Failla G, Melnyk A, Puleo V, Tanna GLD, Ricciardi W and Cascini F (2022) The cost-effectiveness of digital health interventions: A systematic review of the literature. Front. Public Health 10:787135. doi: 10.3389/fpubh.2022.787135
Received: 30 September 2021; Accepted: 21 July 2022;
Published: 11 August 2022.
Edited by:
Mohammed Ayalew, Hawassa University, EthiopiaReviewed by:
Victoria Ramos Gonzalez, Instituto de Salud Carlos III (ISCIII), SpainSanjib Saha, Lund University, Sweden
Copyright © 2022 Gentili, Failla, Melnyk, Puleo, Tanna, Ricciardi and Cascini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Gentili, YW5kcmVhLmdlbnRpbGkxOTg5QGdtYWlsLmNvbQ==
 Andrea Gentili
Andrea Gentili Giovanna Failla
Giovanna Failla Andriy Melnyk
Andriy Melnyk Valeria Puleo
Valeria Puleo Gian Luca Di Tanna3
Gian Luca Di Tanna3 Walter Ricciardi
Walter Ricciardi Fidelia Cascini
Fidelia Cascini