- 1Internal Medicine Department, Security Forces Hospital, Ministry of Interior, Riyadh, Saudi Arabia
- 2Pediatric Cardiology Department, Prince Mohammed Medical City, Ministry of Health, Aljouf, Saudi Arabia
- 3General Pediatric Department, Prince Sultan Military Medical City, Ministry of Defense, Riyadh, Saudi Arabia
- 4Pediatrics Department, Ad-Diriyah Hospital, Ministry of Health, Riyadh, Saudi Arabia
- 5Division of Infectious Diseases, Department of Internal Medicine, Ad-Diriyah Hospital, Ministry of Health, Riyadh, Saudi Arabia
Objective: Parents' hesitancy (PH) toward childhood vaccination, including the vaccine of coronavirus disease (COVID-19), is one of the top public health threats. We aim to assess the PH toward children COVID-19 vaccination as compared to PH toward children routine vaccination among the residents of Saudi Arabia.
Method: Before the official approval of children's COVID-19 vaccination in the country, a cross-sectional study using an electronically distributed survey was performed. Responses from parents of children younger than 18 years of age were accepted. The Oxford COVID-19 vaccine hesitancy scale (OC19-VHS) and the routine vaccination hesitancy scale (R-VHS) were used. Parents were classified as hesitant, non-hesitant, and unsure.
Results: Between June 18th−30th, 2021, we included 1,052 parents. More than half of the parents were positive toward the childhood COVID-19 vaccination (63%) while 10% were unsure. Higher parental hesitancy toward children COVID-19 vaccination among mothers, parents younger than 40 years, did not receive COVID-19 nor influenza vaccines, had higher educational levels, and parents who recovered from COVID-19 infection. Hesitancy was mainly driven by the novelty of the vaccines and the fear of serious adverse effects. Compared to the routine vaccination, parents were more hesitant toward COVID-19 vaccination (6 vs. 27%).
Conclusion: Generally, parents in Saudi Arabia were positive toward children's COVID-19 vaccination. Focused education to reassure hesitant parents on the safety of the vaccine is essential to achieve larger vaccination coverage.
Introduction
Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-2), as of yet, has caused hundreds of millions of infections and millions of deaths among elderly, adults, and children (1), with mortality risk among hospitalized patients ranging between 10 and 20% including studies from Saudi Arabia (2–4). The urgency to combat such pandemics has led to accelerated competition among scientists and pharmaceutical companies around the globe to create potent and safe vaccines against this disease (5). Such rapid development of these vaccines, as well as other factors, have raised concerns among the general population on safety and efficacy (6). However, these vaccines have been granted emergency use authorizations after they showed large safety and efficacy levels (7, 8).
Hesitancy to vaccination is listed among the top ten threats to global health (9). As per the WHO Strategic Advisory Group of Experts on Immunization (SAGE), vaccine hesitancy is defined as the delay in acceptance or refusal of vaccines despite the availability of vaccine services (10). At our country level, a pre-pandemic study revealed that almost 20% of parents were hesitant with childhood routine vaccination (11), highlighting the magnitude of the vaccination hesitancy in a country that mandates childhood vaccination.
During the pandemic of COVID-19, the crucial role of the COVID-19 vaccine against the pandemic was opposed by a massive infodemic of misinformation and conspiracy theories (12, 13). For many reasons, COVID-19 vaccines evaluation was delayed in the pediatric age group and therefore authorization was granted later in the pandemic. Although COVID-19 had a milder rate of morbidity and mortality among children compared to adults, there are more reasons to vaccinate children against COVID-19. Children might have similar nasopharyngeal viral loads to adults (14), therefore they potentially have a similar risk of viral transmission and rates of secondary infections (15, 16). Moreover, if infected, children are at risk to develop multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19) (17).
The assessment of parents' willingness to provide their children with a vaccine against COVID-19 was performed in different parts of the world, but not in our region. A report by Goldman et al. from six different countries (USA, Canada, Japan, Switzerland, and Spain) where they interviewed parents presenting to emergency departments with their children revealed that only 65% of the parents were willing to vaccinate their children. The likelihood of vaccination was higher if the child was older, free from any comorbid conditions, and in those who were up-to-date in their routine vaccination schedule. The main concerns of hesitant parents were the novelty of the COVID-19 vaccine (18), not being convinced by the vaccine's effectiveness (19), or being unconcerned about COVID severity in childhood (20).
In Saudi Arabia, since July 2021, the BNT162b2 vaccine (Pfizer, USA), mRNA-1273 vaccine (Moderna, USA), ChAdOx1 vaccine (AZD1222, AstraZeneca, UK), and Ad26.COV2.S vaccine (Janssen Inc., USA), remains part of the national vaccination campaign and had shown a high degree of safety (21–23). Until January 2022, 51 million doses of these vaccines have been administered in the kingdom (24). When this study was undertaken, people younger than 18 years of age were denied vaccination as per the Saudi ministry of health protocol.
Thus, we aim to assess the willingness of parents to vaccinate their children against COVID-19 compared to the parent's willingness on the childhood routine vaccination. We also aimed to explore the determinants of the parents' hesitancy on the childhood COVID-19 vaccine.
Methods
Study Design and Population
National cross-sectional questionnaire-based survey. Using a pre-designed questionnaire, a link to the survey was distributed electronically to the parents in the general population using social media platforms between June 18 to June 30, 2021. Parents of children aged 18 years or younger who were residents in Saudi Arabia and able to interact with the Arabic questionnaire were eligible to participate. Those respondents who do not complete all the responses were automatically excluded.
Sample Size Calculation
It is estimated to be 961,392 families in the country (25), for which there are 1,922,784 parents. The sample size was calculated with an estimated 50% response rate, 95% confidence interval, 5% margin of error, and the assumption of normal distribution, the minimum representative sample size is 384. We planned to stop enrollment by the end date of enrollment if more than the minimum enrollment is achieved.
Study Tool and Questionnaire Development
The questionnaire is divided into three segments: Part-1: Parents and child demographic and past medical history. Part-2: Routine vaccination hesitancy Scale (R-VHS): adopted from the Vaccine hesitancy 9-item scale (26, 27) that asks parents about their views of childhood vaccines. Each item was rated on a 1 (strongly disagree) to 5 (strongly agree) scale. Higher scores indicate greater hesitancy. Part-3: COVID-19 vaccine hesitancy: adopted from the Oxford COVID-19 vaccine hesitancy 7-item scale (OC19-VHS) (28) which has item-specific response options, coded from 1 to 5. Code 4 or 5 was considered a hesitant response, 3 was considered unsure, and code 2 or 1 was considered a positive response, as used by Freeman et al. (28). A ‘Don't know' option was also provided and excluded from the scoring analysis. The scores can range between 7 and 35, with higher scores indicating higher COVID-19 vaccine hesitancy. No modifications to these tools were made. The adopted validated questionnaires were originally written in the English language. Arabic translation and face validation were performed by senior residents fluent in both languages. Next, an English language academician validated the questionnaire with back-translation. A pilot study was carried out among randomly selected volunteers. The Arabic version was modified as per provided feedback in each stage.
Ethical Considerations
The anonymous survey data were confidentially stored with password-protected security standards. Participants were consented and asked to voluntarily participate and were informed that they, at any time, can terminate their participation and they were told that no incentive to participate. The study was approved by the institutional research board dated June 13, 2021.
Statistical Analysis
Independent-samples T-test was utilized to investigate the difference in the mean score of the COV-Oxford hesitancy scale between study variables. Then, based on the number of hesitant responses on the scale, the respondents were grouped into (1) Hesitant group, when the majority of the individual responses were regarded as hesitant responses (> 60%). The group has a subgroup of strong hesitant if >80%. (2) Non-hesitant group, when the minority of the individual responses were regarded as hesitant responses (< 40%), with a subgroup of strong non-hesitant if no single hesitant response was chosen. (3) Unsure group, when neutral responses account for> 50% of the responses, or there were equal responses between hesitant and non-hesitant responses. Multivariate analysis was performed to explore predictors for parents in the hesitant group. We used the frequency, percentage, mean, and median to present the numerical variables. For comorbid condition definition, we used the United States Center for Disease Control and Prevention definition, conditions that last 1 year or more and require ongoing medical attention or limit activities of daily living, or both. Further statistical analysis was carried out by using the SPSS software (version-23, IBM Corp., Armonk, N.Y., USA). A p-value lower than 0.05 was considered statistically significant.
Results
Respondents
During the study period, 1,240 visits to the online questionnaire were received, 1,063 individuals confirmed having a child who is under 18 years of age. After excluding 11 incomplete responses, 1,052 participants were included in the analysis. Slightly higher participation was observed from mothers (51.5%, n = 542) compared to fathers (48.5%, n = 510). The majority of the included parents had Saudi nationality (93.7%) and were from healthy medical backgrounds (82.2%). Most of the parents have reported their level of education as a University degree (62.5%) or postgraduate studies (24.2%), while the remaining participants had a secondary school degree (8.1%) or others. The top sector of job among the parents in this cohort was the education sector (27.4%), followed by the healthcare sector (20.3%), administrative jobs sectors (17.3%), housewives (15.6%), or others. Half of the included parents have reported receipt of the influenza vaccine (51.2%) over the last year. Most of the respondents themselves had already received at least one dose of the COVID-19 vaccine (n = 854/1052, 81.1%) and only the minority had a history of laboratory-confirmed COVID-19 (n = 202/1052, 19.2%). Nevertheless, among those who yet did not receive COVID-19 vaccine (18.8%, n = 198/1052), ten percent have no intention to vaccinate themselves soon (n = 105/1052). When parents were asked about the burden of COVID-19, at least one in each three had a close relative or friend who died of COVID-19, while one in each two knows a child who suffered from laboratory-confirmed COVID-19. Most of the parents (77.7%) have disclosed that their decision of vaccinating their kids will not change according to the child's age, gender, or comorbid conditions. Each parent had a median of four children, who were predominantly boys (41.4%), predominantly girls (37.4%), or have an equal distribution between the two genders (21.2%). Only 13.4% of the parents have disclosed a history of laboratory-confirmed COVID-19 among their children. The majority of parents have reported a full immunization status for their kids at least until 2 years of age (95.9%). There was a lower rate of children with comorbid disease (8.5%), history of self-reported allergy that at some point required medical attention (13.7%), or serious adverse effects following any vaccine (4.8%) (Table 1).
Parents' Hesitancy on the Childhood COVID-19 Vaccine
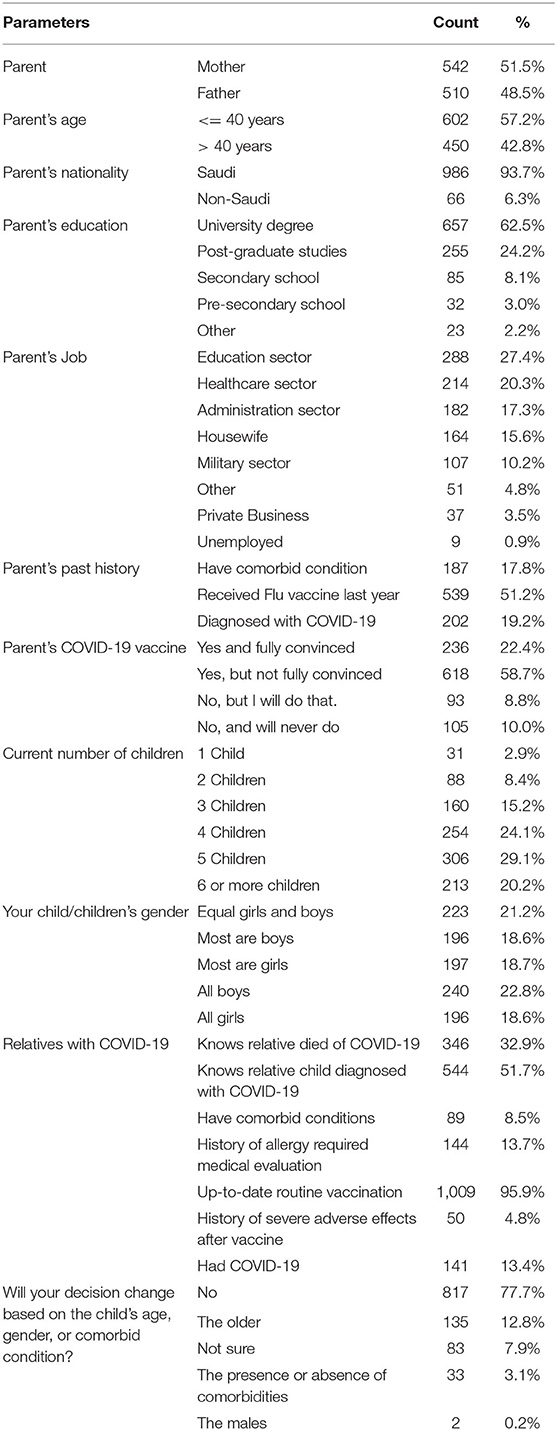
Using the Oxford COVID-19 vaccine hesitancy score (OC19-VHS), we found that the mean score of hesitancy toward COVID-19 vaccination was 18 out of 35 points. Most of the parents fell in the non-hesitant group (63%, n = 663/1052), while the remaining were either in the hesitant group (27%, n = 281/1052) or unsure about their decision (10%, n = 108/1052) (Figure 1). Furthermore, 6% were considered a strongly hesitant subgroup. On the other side, 48% of the parents were among the strongly non-hesitant group.
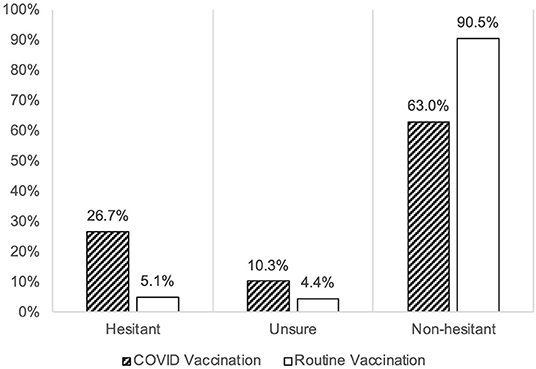
Figure 1. The parental hesitancy toward childhood COVID-19 vaccination (Oxford COVID-19 Vaccine Scale) as compared to the hesitancy toward routine childhood vaccination (VHS scale), among 1,052 parents in Saudi Arabia between June 18 to June 30, 2021.
Comparison to the Childhood Routine Vaccination
The mean hesitancy score among parents toward childhood COVID-19 vaccination was found higher (18/35 points (51%) in OC19-VHS) compared to the hesitancy toward the routine childhood vaccination [19.5/45 points (44%) in VHS]. The major drive for such difference was the higher proportion of the hesitant group against the childhood COVID-19 vaccine (27%) compared to the hesitant group on the childhood routine vaccination (6%). Additionally, the proportion of the unsure group was higher in the childhood COVID-19 vaccination (10%) compared to the unsure group in the childhood routine vaccination (4%) (Figure 1). Detailed results on the responses to both scales are presented in Figure 2.
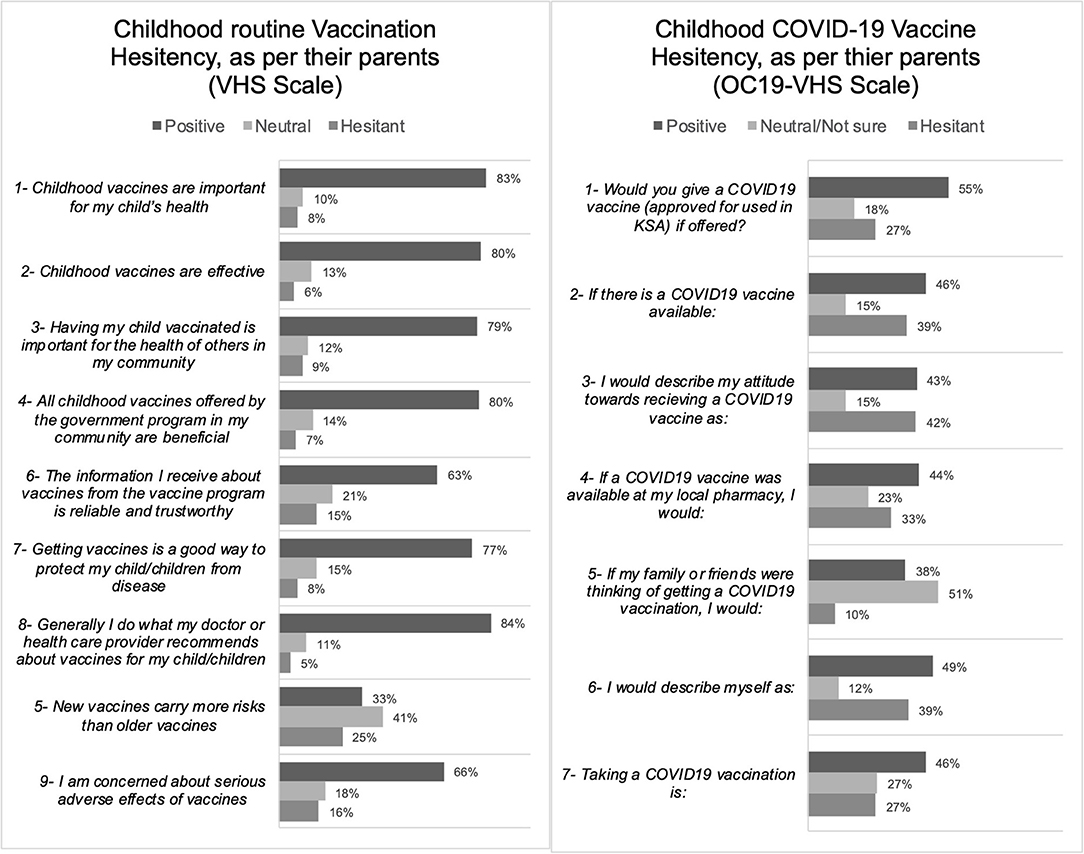
Figure 2. The frequencies of endorsement of each item are categorized into hesitant (Category 4 and 5), positive (Category 1 and 2), or neutral (n = 1,052).
Determinant of the Parent's Hesitancy on the Childhood COVID-19 Vaccination
We investigated the determinants of the parent's hesitancy on the childhood COVID-19 vaccination and also the childhood routine vaccination. In both types of vaccinations, we found significantly higher hesitancy mean scores in mothers (compared to fathers), parents who are younger than 41 years of age (compared to older than 40 years of age), parents who never received influenza or COVID-19 vaccines (compared to those who did), and those whom their children had a history of allergy after any previous vaccine (compared to those who did not). Further, the unique determinants of the hesitancy on the childhood COVID-19 vaccination alone (but not childhood routine vaccination) were those parents with an education level of a University degree or above (compared to a lower education level) and parents with a history of COVID-19 diagnosis (compared to those never diagnosed with COVID-19). On the other side, the unique determinants of the hesitancy on the routine childhood vaccinations alone (but not the childhood COVID-19 vaccination) were parents with lower than 4 children (compared to those with more than 4 children), parents with children who had a history of COVID-19 diagnosis (compared to those who did not), parents with children who did not complete the routine childhood vaccination schedule (compared to those who did) (Table 2).
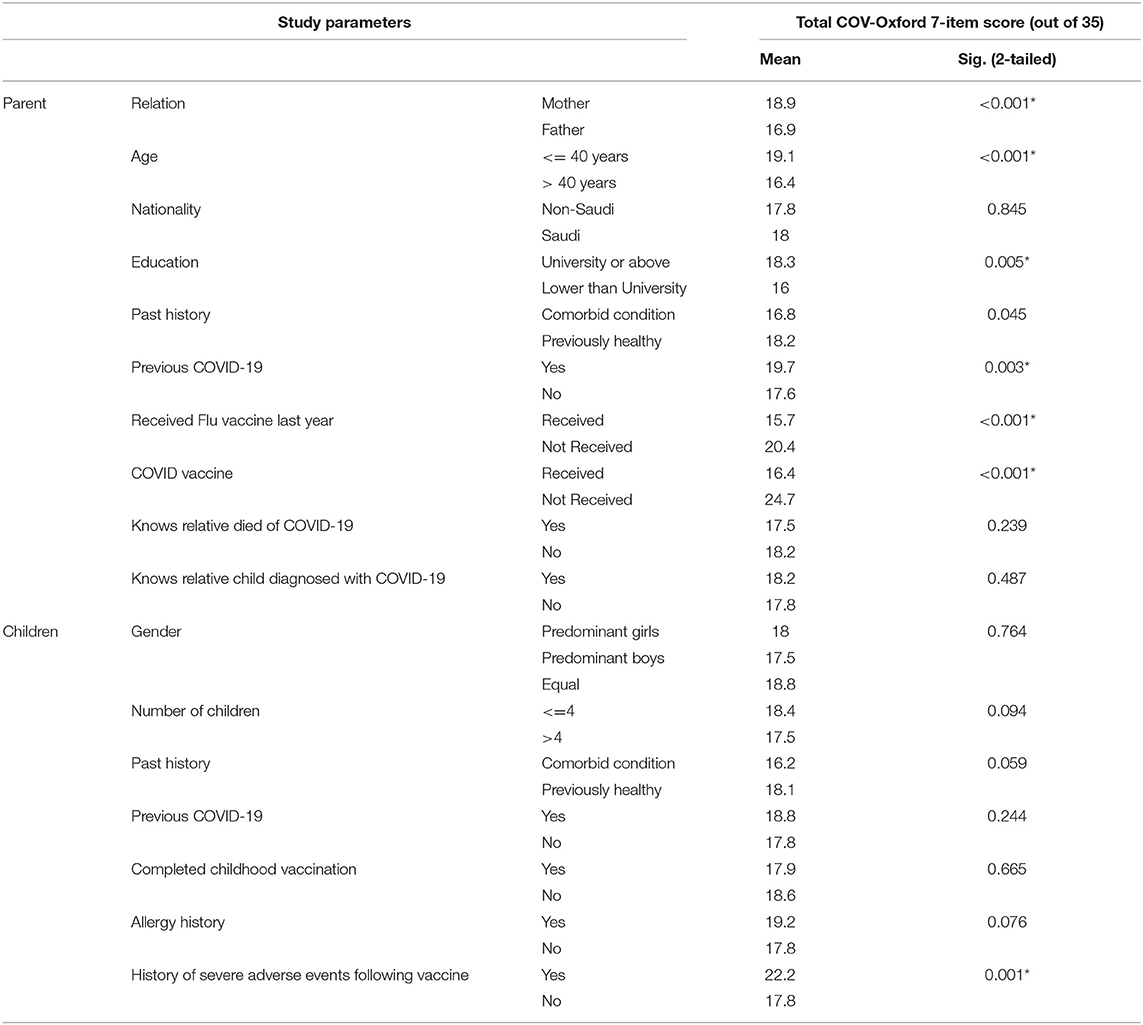
Table 2. Association and means comparisons between the study variables for COV-Oxford 7-item score (n = 1,052), higher scoring in the scales indicate higher hesitancy.
Multivariate regression analysis was performed to determine independent predictors for hesitant or strongly hesitant parents on childhood COVID vaccination, and resulted in a significant association with hesitancy toward routine childhood vaccination (adjusted odds ratio (aOR) 8.08 [95% CI 3.821–17.086)]), COVID-19 unvaccinated parents (aOR 3.398, 95% CI 2.342–4.93), flu unvaccinated parents (aOR 1.690, 95% CI 1.235–2.313), or parents with University education and higher (aOR 2.016, 95% CI 1.217–3.339) (Table 3).

Table 3. Multivariate analysis on predictors of hesitant or strongly hesitant position in parents toward childhood COVID-19 vaccination based on Oxford COVID-19 vaccine hesitancy scale (OC19-VHS) (28).
Discussion
In this study, we found a quarter of parents in Saudi Arabia were hesitant toward children's COVID-19 vaccination. In the same cohort, such hesitancy was found three times more than the hesitancy toward routine childhood vaccination, especially among mothers, parents who were younger the 40 years of age, and parents of children with allergies.
Children's vaccination has been successful in preventing many infectious diseases and was considered more effective than elderly vaccination to achieve community-wide prevention of certain infectious diseases (29). In COVID-19, children's vaccination does not only protect them but might provide an indirect advantage in protecting the older unvaccinated people (30). Globally, early in the pandemic or recent data both have revealed an elevated parental hesitancy toward the children's COVID-19 vaccination (18, 31, 32). Regionally, a recent study from Kuwait showed substantially higher parental rejection (55.8%) for children's COVID-19 vaccination (33). In our study, however, the majority of the participants were not hesitant toward the COVID-19 or routine childhood vaccination. COVID-19 vaccine-hesitant respondents in the current study were concerned that it is a new vaccine of unknown adverse events and do not trust its effectiveness, which replicates similar local studies' findings (34, 35). Some of them have expressed a lack of trust in the information provided by the official agencies. The fact that the hesitancy is more when it comes to COVID-19 compared to the routine childhood vaccination disfavor anti-vaccination ideology, but rather a lack of enough public awareness on the safety and efficacy among the pediatric age group. An additional consideration is the timing of the survey to the status of the pandemic. For example, a surge in the acceptance of enrollment in the COVID-19 trial was noted during the stages of higher disease spread (36), thus, a relatively controlled COVID-19 status in Saudi Arabia during the study period of June 2021 (37), might have affect intention of vaccination. Without a doubt, the massive spread of misinformation during the pandemic of COVID-19 has caused significant challenges to public health strategies, especially in the era of social media, and is still a hard task to counteract (12).
Sociodemographic determinants of a higher parental hesitancy toward children COVID-19 vaccination in this study were replicated from previous studies. First, parents who were mothers were found more hesitant, compared to fathers (18, 38, 39). Further exploration in our cohort revealed that the mothers had higher rejection of flu vaccination (57 vs. 41%, p < 0.001) and self-vaccination against COVID-19 (25.1 vs. 12.1%, p < 0.001) compared to the fathers. Although this needs further investigations including special concerns on pregnancy outcome, but also could alarm early antivaccination behavior among mothers in Saudi Arabia. The other consideration is that mothers in our cohort know more children with a history of COVID-19 diagnosis (61 vs. 42%, p < 0.001) than fathers, which probably has decreased their anxiety toward COVID-19 in children. Second, parents who were younger than 40 years of age or those with higher educational backgrounds were more hesitant toward children's COVID-19 vaccination, as seen in previous studies (18, 31, 35, 38, 40). Previous studies found these groups were more likely to have selected sources of information and they base their decision on a critical-thinking attitude (40). Thus, public health messaging should consider such targets of the population with the appropriate mode of messaging. It is worth mentioning that such parent gender and age differences were not found significant after the multivariate regression analysis.
Consistent with previous studies (18, 38), vaccinated parents would be more willing to vaccinate their children, while those who had COVID-19 in a mild form would be more hesitant. This alerts toward the effect of parental self-experience on children's healthcare decisions.
This study has several limitations. We did not evaluate the impact of family income and the impact of the child's age on the parent's decision of childhood COVID vaccination. Although the sample size was deemed adequate statistically, the recruited sample was at risk of selection bias to those who use social media as the recruitment tool which might underestimate vaccine hesitancy among other populations. Future studies investigating the beliefs and cultural influences of parental hesitancy are recommended.
Conclusion
Only one in every four parents in Saudi Arabia was hesitant toward children's COVID-19 vaccination, which is similar to the rate reported worldwide. The focus of public health campaigns on the hesitant population, including mothers, younger parents, highly educated parents, and parents with a history of COVID-19 infection might help to decrease parents' hesitancy toward children's COVID-19 vaccination aiming to achieve further control of COVID-19 in the community.
Data Availability Statement
The datasets presented in this article are not readily available because it could compromise the privacy of research participants. Requests to access the datasets should be directed to AAlm, YWxtb2hheWFhbSYjeDAwMDQwO2dtYWlsLmNvbQ==.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Research Board (KFMC/KACST-IRB: H-01-R-012, OHRP/NIH: IRB00010471) dated June 13, 2021. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AAlm, SFA, and BA: proposal preparation. AAlm, SFA, AAlt, and KA: manuscript writing. All authors contributed in study concept and methodology, data collection, analysis, interpretation, manuscript review, and final approval.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. COVID-19 Map - Johns Hopkins Coronavirus Resource Center (2021). Available online at: https://coronavirus.jhu.edu/map.html (accessed July 3, 2021).
2. Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. (2021) 21:1–28. doi: 10.1186/S12879-021-06536-3/FIGURES/10
3. Barry M, AlMohaya AE, AlHijji A, Akkielah L, AlRajhi A, Almajid F, et al. Clinical characteristics and outcome of hospitalized COVID-19 patients in a MERS-CoV endemic area. J Epidemiol Glob Health. (2020) 10:214–21. doi: 10.2991/JEGH.K.200806.002
4. Alguwaihes AM, Al-Sofiani ME, Megdad M, Albader SS, Alsari MH, Alelayan A, et al. Diabetes and Covid-19 among hospitalized patients in Saudi Arabia: a single-centre retrospective study. Cardiovasc Diabetol. (2020) 2020:19. doi: 10.1186/S12933-020-01184-4
5. McDonald I, Murray SM, Reynolds CJ, Altmann DM, Boyton RJ. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. Npj Vaccines. (2021) 6:1–14. doi: 10.1038/s41541-021-00336-1
6. Khubchandani J, Sharma S, Price JH, Wiblishauser MJ, Sharma M, Webb FJ. COVID-19 vaccination hesitancy in the united states: a rapid national assessment. J Community Health. (2021) 46:270–7. doi: 10.1007/s10900-020-00958-x
7. Vasireddy D, Atluri P, Malayala SV, Vanaparthy R, Mohan G. Review of COVID-19 vaccines approved in the United States of America for emergency use. J Clin Med Res. (2021) 13:204–13. doi: 10.14740/jocmr4490
8. Barry M, Bahammam AS. COVID-19 vaccine in the Kingdom of Saudi Arabia: A true operation warp speed. J Nat Sci Med. (2021) 4:92–8. doi: 10.4103/jnsm.jnsm_8_21
9. Dubé E, Gagnon D, Nickels E, Jeram S, Schuster M. Mapping vaccine hesitancy-Country-specific characteristics of a global phenomenon. Vaccine. (2014) 32:6649–54. doi: 10.1016/j.vaccine.2014.09.039
10. MacDonald NE, Eskola J, Liang X, Chaudhuri M, Dube E, Gellin B, et al. Vaccine hesitancy: Definition, scope and determinants. Vaccine. (2015) 33:4161–4. doi: 10.1016/j.vaccine.2015.04.036
11. Alsubaie SS, Gosadi IM, Alsaadi BM, Albacker NB, Bawazir MA, Bin-Daud N, et al. Vaccine hesitancy among Saudi parents and its determinants. Saudi Med J. (2019) 40:1242–50. doi: 10.15537/smj.2019.12.24653
12. Puri N, Coomes EA, Haghbayan H, Gunaratne K. Social media and vaccine hesitancy: new updates for the era of COVID-19 and globalized infectious diseases. Hum Vaccines Immunother. (2020) 2020:2586–93. doi: 10.1080/21645515.2020.1780846
13. Singh K, Lima G, Cha M, Cha C, Kulshrestha J, Ahn YY, et al. Misinformation, believability, and vaccine acceptance over 40 countries: Takeaways from the initial phase of the COVID-19 infodemic. PLoS ONE. (2022) 17:e0263381. doi: 10.1371/JOURNAL.PONE.0263381
14. Heald-Sargent T, Muller WJ, Zheng X, Rippe J, Patel AB, Kociolek LK. Age-related differences in nasopharyngeal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) levels in patients with mild to moderate coronavirus disease 2019 (COVID-19). JAMA Pediatr. (2020) 174:902–3. doi: 10.1001/jamapediatrics.2020.3651
15. Yonker LM, Neilan AM, Bartsch Y, Patel AB, Regan J, Arya P, et al. Pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): clinical presentation, infectivity, immune responses. J Pediatr. (2020) 227:45–52.e5. doi: 10.1016/j.jpeds.2020.08.037
16. Laws RL, Chancey RJ, Rabold EM, Chu VT, Lewis NM, Fajans M, et al. Symptoms and transmission of SARS-CoV-2 among children - Utah and Wisconsin, March-May 2020. Pediatrics. (2021) 147:27268. doi: 10.1542/PEDS.2020-027268
17. Eberhardt CS, Siegrist CA. Is there a role for childhood vaccination against COVID-19? Pediatr Allergy Immunol. (2021) 32:9–16. doi: 10.1111/pai.13401
18. Goldman RD, Yan TD, Seiler M, Parra Cotanda C, Brown JC, Klein EJ, et al. Caregiver willingness to vaccinate their children against COVID-19: Cross sectional survey. Vaccine. (2020) 38:7668–73. doi: 10.1016/j.vaccine.2020.09.084
19. Ali M, Ahmed S, Bonna AS, Sarkar A, Islam MA, Urmi TA, et al. Parental coronavirus disease vaccine hesitancy for children in Bangladesh: a cross-sectional study. F1000Research. (2022) 11:90. doi: 10.12688/F1000RESEARCH.76181.2
20. Ali M, Proma TS, Tasnim Z, Islam MA, Urmi TA, Ahmed S, et al. Parental COVID-19 vaccine hesitancy for children with neurodevelopmental disorders: a cross-sectional survey. Trop Med Heal. (2022) 50:1–9. doi: 10.1186/S41182-022-00415-6
21. Swilam A. Saudi Arabia Approves Moderna's COVID Vaccine -State News Agency | Reuters. Thomson Reuters (36). Available online at: https://www.reuters.com/business/healthcare-pharmaceuticals/saudi-arabia-approves-modernas-covid-vaccine-state-news-agency-2021-07-09/ (accessed March 31, 2022).
22. Almohaya AM, Alsubie H, Alqarni B, Alzayad B, Alghar A, Alshahrani K, et al. Acute unsolicited adverse events following BNT162b2 vaccine in Saudi Arabia, a real-world data. Vaccine. (2022) 40:477–82. doi: 10.1016/J.VACCINE.2021.12.001
23. Almohaya AM, Qari F, Zubaidi GA, Alnajim N, Moustafa K, Alshabi MM, et al. Early solicited adverse events following the BNT162b2 mRNA vaccination, a population survey from Saudi Arabia. Prev Med Rep. (2021) 24:10195. doi: 10.1016/J.PMEDR.2021.101595
24. Ritchie H, Mathieu E, Rodés-Guirao L, Appel C, Giattino C, Ortiz-Ospina E. Coronavirus Pandemic (COVID-19). (2020). Retrieved from: https://ourworldindata.org/coronavirus
25. Saudi General Authority for statistics. Family Registrations Report - Statistical Yearbook - 5th Edition. (2021).
26. SAGE: Strategic Advisory Group of Experts on Immunization. Report of the Sage Working Group on Vaccine Hesitancy. (2014)
27. Shapiro GK, Tatar O, Dube E, Amsel R, Knauper B, Naz A, et al. The vaccine hesitancy scale: Psychometric properties and validation. Vaccine. (2018) 36:660–7. doi: 10.1016/j.vaccine.2017.12.043
28. Freeman D, Loe BS, Chadwick A, Vaccari C, Waite F, Rosebrock L, et al. COVID-19 vaccine hesitancy in the UK: The Oxford coronavirus explanations, attitudes, and narratives survey (Oceans) II. Psychol Med. (2021) 2021:5188. doi: 10.1017/S0033291720005188
29. Kim TH, Johnstone J, Loeb M. Vaccine herd effect. Scand J Infect Dis. (2011) 43:683. doi: 10.3109/00365548.2011.582247
30. Velavan TP, Pollard AJ, Kremsner PG. Herd immunity and vaccination of children for COVID-19. Int J Infect Dis. (2020) 98:14. doi: 10.1016/J.IJID.2020.06.065
31. Lazarus JV, Ratzan SC, Palayew A, Gostin LO, Larson HJ, Rabin K, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. (2021) 27:225–8. doi: 10.1038/s41591-020-1124-9
32. Miraglia del Giudice G, Napoli A, Corea F, Folcarelli L, Angelillo IF. Evaluating COVID-19 vaccine willingness and hesitancy among parents of children aged 5-11 years with chronic conditions in Italy. Vaccines. (2022) 10:396. doi: 10.3390/VACCINES10030396
33. AlHajri B, Alenezi D, Alfouzan H, Altamimi S, Alzalzalah S, Almansouri W, et al. Willingness of parents to vaccinate their children against influenza and the novel coronavirus disease-2019. J Pediatr. (2021) 231:298–9. doi: 10.1016/j.jpeds.2020.11.059
34. Samannodi M, Alwafi H, Naser AY, Alabbasi R, Alsahaf N, Alosaimy R, et al. Assessment of caregiver willingness to vaccinate their children against COVID-19 in Saudi Arabia: a cross-sectional study. Hum Vaccin Immunother. (2021) 17:4857–64. doi: 10.1080/21645515.2021.2004054
35. Temsah MH, Alhuzaimi AN, Aljamaan F, Bahkali F, Al-Eyadhy A, Alrabiaah A, et al. Parental attitudes and hesitancy about COVID-19 vs. routine childhood vaccinations: a national survey. Front Public Heal. (2021) 9:e752323. doi: 10.3389/FPUBH.2021.752323
36. Detoc M, Bruel S, Frappe P, Tardy B, Botelho-Nevers E, Gagneux-Brunon A. Intention to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in France during the pandemic. Vaccine. (2020) 38:7002–6. doi: 10.1016/j.vaccine.2020.09.041
38. Fedele F, Aria M, Esposito V, Micillo M, Cecere G, Spano M, et al. (2021) COVID-19 vaccine hesitancy: a survey in a population highly compliant to common vaccinations. Hum Vaccin Immunother. 17:3348–54. doi: 10.1080/21645515.2021.1928460
39. Chen F, He Y, Shi Y. Parents' and guardians' willingness to vaccinate their children against COVID-19: A systematic review and meta-Analysis. Vaccines. (2022) 10:S1. doi: 10.3390/VACCINES10020179/S1
Keywords: coronavirus, vaccine, childhood vaccination, hesitancy, Saudi Arabia
Citation: Alhazza SF, Altalhi AM, Alamri KM, Alenazi SS, Alqarni BA and Almohaya AM (2022) Parents' Hesitancy to Vaccinate Their Children Against COVID-19, a Country-Wide Survey. Front. Public Health 10:755073. doi: 10.3389/fpubh.2022.755073
Received: 07 August 2021; Accepted: 04 April 2022;
Published: 28 April 2022.
Edited by:
Walid Alali, Kuwait University, KuwaitReviewed by:
Yihan Lu, Fudan University, ChinaMohammad Ali, Uttara Adhunik Medical College Hospital, Bangladesh
Copyright © 2022 Alhazza, Altalhi, Alamri, Alenazi, Alqarni and Almohaya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdulellah M. Almohaya, YWxtb2hheWFhbSYjeDAwMDQwO2dtYWlsLmNvbQ==
 Sultan F. Alhazza
Sultan F. Alhazza Ali M. Altalhi
Ali M. Altalhi Khaled M. Alamri3
Khaled M. Alamri3 Abdulellah M. Almohaya
Abdulellah M. Almohaya