
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 26 January 2023
Sec. Health Economics
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.1086393
This article is part of the Research Topic From Clinical Trials to Real-World Data Sciences for Value in Health: Access, Utilization, and Quality View all 8 articles
Objectives: Maintenance therapy with capecitabine after induction chemotherapy for patients with newly diagnosed metastatic nasopharyngeal carcinoma (mNPC) has been confirmed to be effective. This study aimed to evaluate the cost-effectiveness of capecitabine as maintenance therapy for patients with mNPC from the Chinese payers' perspective.
Methods: Markov model was conducted to simulate the disease progress and evaluated the economic and health outcomes of capecitabine maintenance plus best-supported care (CBSC) or best-supported care (BSC) alone for patients with mNPC. Survival data were derived from the NCT02460419 clinical trial. Costs and utilities were obtained from the standard fee database and published literature. Measured outcomes were total costs, quality-adjusted life-years (QALYs), life-years (LYs), incremental cost-utility ratios (ICURs), incremental cost-effectiveness ratios (ICERs), incremental net monetary benefit (INMB), and incremental net-health benefit (INHB). Sensitivity analyses were performed to assess model robustness. Additional subgroup cost-effectiveness analyses were accomplished.
Results: Throughout the course of the disease, the CBSC group provide an incremental cost of $9 734 and additional 1.16 QALYs (1.56 LYs) compared with the BSC group, resulting in an ICUR of $8 391/QALY and ICER of $6 240/LY. Moreover, the INHB was 0.89 QALYs, and the INMB was $32 034 at the willingness-to-pay threshold of $36 007/QALY. Subgroup analyses revealed that CBSC presented a positive trend of gaining an INHB in all subgroups compared with the BSC group. The results of sensitivity analyses supported the robustness of our model.
Conclusion: Compared with BSC, after induction chemotherapy, CBSC as a first-line treatment was cost-effective for newly diagnosed mNPC. These results suggest capecitabine maintenance therapy after induction chemotherapy as a new option for patients with newly diagnosed mNPC.
Nasopharyngeal carcinoma (NPC) is an epithelial carcinoma with distinct geographical distribution and is characterized by distinct geographical distribution. According to the Global cancer statistics in 2020, almost 80,000 deaths due to NPC are reported annually most frequently in southern China, Southeastern Asia, and North Africa (1, 2). NPC is an asymptomatic, intrinsically invasive disease, which results in 60–70% of patients being diagnosed with advanced stages, and approximately 10% of patients present with metastases (3, 4). Moreover, there is still a significant percentage of patients who develop distant metastases, becoming a leading cause of treatment failure and a major health concern (5, 6). Thus, developing new treatment options for cancer metastasis is urgently necessary.
Usually, as recommended by the guidelines, platinum-based chemotherapy is the first-line treatment for patients with metastatic nasopharyngeal carcinoma (mNPC) (7, 8). The median progression-free survival (PFS) was 5.0–7.0 months for patients receiving chemotherapy alone (9–11). Recent two clinical trials CAPTAIN-1st and JUPITER-02 have confirmed that combination therapy with chemotherapy and immunotherapy in the first-line treatment for mNPC improves the median PFS to over 10 months (12, 13). However, chemoimmunotherapy improves the therapeutic effect, its high price also represents a substantial financial burden to our society (14, 15). Moreover, high drug prices can lead to reduced adherence in countries where patients have to contribute to treatment costs (16).
Therefore, new therapeutic strategies are needed with stronger efficacy, less expensive, and more readily available. Accumulating evidence suggests that tolerable maintenance of low-dose chemotherapy prolongs the progression-free interval for patients without disease progression after first-line treatment (17–20). Capecitabine, an orally administered fluoropyrimidine used widely as low-dose monotherapy to prevent a recurrence (21, 22). Capecitabine is converted to fluorouracil in tumors without complications related to central venous catheterization, improving compliance and convenience. Results from recent studies have assessed and confirmed the effectiveness of capecitabine as maintenance therapy in breast cancer, metastatic colorectal cancer, as well as resected biliary tract cancer (22–24). Recently, a clinical trial also showed superiority in metronomic capecitabine as adjuvant therapy for patients with locoregionally advanced NPC (25).
In NPC, moreover, a phase 3 randomized clinical trial (NCT02460419) provided evidence supporting the efficacy of capecitabine maintenance therapy as a first-line treatment for mNPC (26). This trial randomized patients with newly diagnosed mNPC who achieved disease control after 4–6 cycles of induction chemotherapy to receive either capecitabine maintenance therapy plus best supported care (CBSC) or best supported care (BSC) alone (26). Patients in the CBSC group had a significantly higher median PFS survival compared to the BSC group (35.9 vs. 8.2 mo, HR 0.44, 95% CI 0.26–0.74, P = 0.002) (26). The CBSC group showed higher objective response rates (25 vs. 11.5%) and a longer median duration of response than BSC group (26).
These results suggest capecitabine maintenance therapy after induction chemotherapy as a new option for patients with newly diagnosed mNPC and will be suggested by the 2022 Chinese Society of Clinical Oncology (CSCO) guidelines. Chemoimmunotherapy treatments, which were currently recommended for patients with mNPC by the CSCO clinical guideline, however, its high price often causes a significant economic burden in China. In contrast, capecitabine maintenance therapy has lower drug prices with better patient compliance. Therefore, further detailed cost-effectiveness evaluation on the capecitabine maintenance therapy in mNPC is necessary for policymakers, suppliers, and patients to make a rational decision. This study aimed to compare the cost-effectiveness of CBSC and BSC as first-line treatments after induction chemotherapy for patients with mNPC from the Chinese payers' perspective.
Comprehensive mathematical Markov model was established to evaluate the economic and health outcomes of adding capecitabine maintenance therapy after induction chemotherapy for patients with newly diagnosed mNPC (Figure 1). We simulated a hypothetical cohort of populations with similar characteristics as those patients enrolled in the NCT02460419 clinical trial (26) (Supplementary Table 1). Eligible patients achieved disease control after 4–6 cycles of paclitaxel (150 mg/m2), cisplatin (60 mg/m2), and capecitabine (1 000 mg/m2 orally twice daily on days 1 to 14) every 21 days. Then they were randomly assigned to two groups in our model according to the first-line treatments: (1) BSC group: provide appropriate palliative care to reduce symptoms and improve quality of life to the greatest extent possible; (2) CBSC group: capecitabine maintenance (1 000 mg/m2 orally twice daily on days 1 to 14 of 21-day cycle) for a maximum of 2 years plus BSC treatment until disease progression or intolerable toxicity. Supplementary Table 2 details the drug dosage and schedule.
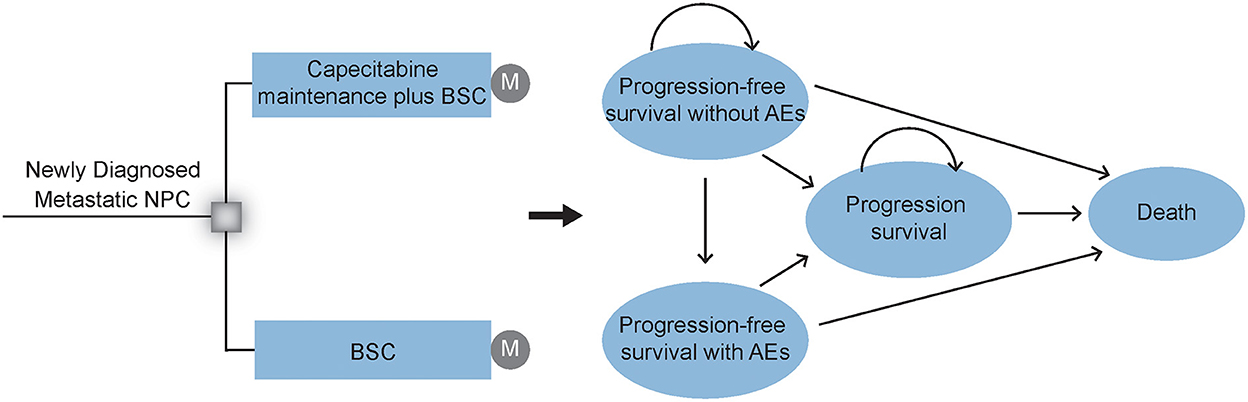
Figure 1. Markov model structure. Markov model structure and Markov states are used to evaluating the economic and health outcomes of CBSC and BSC groups as a first-line treatment after induction chemotherapy for patients with mNPC. After initial treatment, patients could experience a response with or without AEs, or experience the disease progression until death. NPC, nasopharyngeal carcinoma; CBSC, Capecitabine maintenance therapy plus best supported care group; BSC, best supported care; AEs, adverse events.
The Markov model structure used in this analysis was based on previously conducted studies and other economic models. However, the input data and group of the Markov model in this analysis were designed by ourselves. In addition, our Markov model assumed that in each Markov cycle, a patient is always in one exclusive health state. Further, the property of the Markov model was “memoryless” in a mathematical sense. In other words, transitions between the different states depend only on the current state rather than the previous state, is stochastic game.
The Markov model was conducted by using the TreeAge, version 2019 (TreeAge Software, Inc.). As shown in Figure 1, this Markov survival model was composed of 4 exclusive health states to model the disease progress: progression-free survival (PFS) without adverse events (AEs), PFS with AEs, progression disease (PD), and death. During the PFS health state, patients would achieve a response and continue to recieve different first-line therapies, either with or without adverse events (AEs) until progression or unacceptable AEs. All groups received second-line subsequent treatment after progression. The model terminates when all patients die of the disease. Consistent with the treatment cycle, each model cycle represents 3 weeks with a lifetime horizon. Moreover, 3% annual discount rate and half-cycle correlation were adopted for cost and survival estimates (27).
Patients in the model transitioned between health states due to the calculated transition probabilities from PFS and OS Kaplan-Meier curves of the NCT02460419 clinical trial (26). The GetData Graph Digitizer software was used to attain the data points to the date of the last follow-up from the PFS and OS curves using the process designated by Hoyle et al. (28). Next, the data were used to fit flexible parametric survival models, including the Exponential, Weibull, Log-logistic, Lognormal, and Gompertz models using the R software. The Log-logistic models provided a good fit for all curves in the two groups according to the visual fit, clinical rationality, and statistical goodness-of-fit (Akaike's information criterion and Bayesian information criterion) (29). The parameters of the Log-logistic distributions, detailed statistical fitting results, and fitting curves in both groups are shown in Supplementary Table 3 and Supplementary Figure 1. The disease-cause mortality rate of transitioning to death was estimated from the OS curves in the NCT02460419 trial, while the other-cause mortality rate was estimated from the recently published Chinese life table (Supplementary Table 4) (30).
The direct medical costs considered were drug costs, best supportive care, radiation therapy costs, administration, management of severe AEs, laboratory tests, and imaging. Additionally, disease-caused death costs and other-caused death costs were included. The unit costs, such as the price per drug were available from the real-world cost database of West China Hospital, 2022. The prices were recorded in Chinese yuan and then converted into US dollars at an exchange rate of 2022 (1 US dollar = 6.7467 Chinese yuan, August 1 2022) (31). Drug dosage, administration route, medication schedule, and rates of serious AEs in both groups were consistent with the NCT02460419 clinical trial (26). The median medication costs were calculated using the mean weight of 65.0 kg and body surface area of 1.79 m2 (32). Further details of the calculation of specific medication costs are available in Supplementary Table 2.
During the first-line treatment, management costs on 3 or 4 grade drug-related AEs were included by multiplying the cost derived from published literature (32, 33) by incidence rates obtained from the NCT02460419 clinical trial (26). In addition, subsequent therapy strategies and their proportions for disease progression were gained from the NCT02460419 clinical trial (26). Costs used for the model analysis are listed in Table 1.
Quality of life was modeled using health state utility weights. Each health state was assigned with a health utility preference on a scale of 0 (death) to 1 (perfect health). Due to the lack of mature quality-of-life data in the NCT02460419 trial, estimates for the utilities in PFS and PD states were derived from previous literature (34). In addition, the disutility due to the drug-related AEs was considered in the model (35). Detailed information is mentioned in Table 1.
The main measured outcomes were total costs, quality-adjusted life-years (QALYs), life-years (LYs), incremental cost-utility ratios (ICURs), and incremental cost-effectiveness ratios (ICERs). These calculations are presented in the following equations:
Where C, U, and E represent the total costs, QALYs, and LYs of CBSC (1) or BSC (0), respectively. Based on the recommendation of the China guidelines for pharmacoeconomic evaluations and the World Health Organization (WHO), we used three times the gross domestic product (GDP) per capita ($36 007, in 2022 US$) (31, 36) in China indicator for willingness-to-pay (WTP) threshold (37, 38). The ICERs—incremental costs divided by incremental QALYs gained—were calculated to be compared with a WTP threshold of $36 007/ QALY in two groups.
Moreover, we also calculated the incremental monetary benefit (INMB) and incremental net-health benefit (INHB) based on the following methods:
Where U represent the QALYs, and C represent the total costs of CBSC (1) or BSC (0), respectively.
Additionally, we considered the cost-effectiveness in subgroups using the publishing subgroup analysis data in the NCT02460419 trial (26). Patients were stratified according to age, smoking, disease stage, metastases type, liver metastases, lung metastases, bone metastases, response, and Epstein-Barr virus (EBV) DNA copy numbers. All subgroups were assumed to have the same data apart from the available PFS HRs in this model because of lacking sufficient data.
Sensitivity analyses were completed to ascertain the robustness of the model and uncertainty of the variables impact on the results. A series of one-way sensitivity analyses were carried out with all parameters varied within reasonable bounds of ±20% from their baseline values, as shown in Table 1 (39). Furthermore, probabilistic sensitivity analyses were conducted to estimate variations in inputs changed simultaneously with a specific pattern of statistical distributions as revealed in Table 1 by conducting 10 000 Monte Carlo estimations (40).
Markov transition probabilities between each state were calculated based on Log logistic model until death, which is accessible in Supplementary Figure 2. Within a lifetime horizon, the baseline results in each group are summarized in Table 2.
In the PFS state, patients in the CBSC group provided an additional 1.14 PFS QALYs with an incremental cost of $23 118 compared with the BSC group. Throughout the course of the disease, patients with newly diagnosed mNPC received CBSC providing an additional cost of $9 734 and incremental 1.16 QALYs (1.56 LYs) in the comparison with the BSC, resulting in an ICUR of $8 391/QALY, which was less than the WTP threshold suggesting that the CBSC was cost-effective from the payer's perspective. Moreover, the INHB was 0.89 QALYs, and the INMB was $32 034 at the WTP threshold of $36 007/QALY in the entire disease course, indicating that the CBSC was cost-effective.
Prespecified subgroup analyses revealed that compared with the BSC group, CBSC presented a positive trend of gaining an INHB and a high probability of cost-effectiveness at the WTP threshold of $36 007/QALY in all subgroups. For these subgroups, the INHBs concerning the health benefit ranked the subgroup from high to low as stable disease response to first-line chemotherapy [1.52, range (0.5–1.64)], oligometastatic type [1.11, range (0.27–2.53)], primary metastasis stage [1.05, range (0.54–1.71)], and absent of liver metastasis [1.05, range (0.54–1.71)]. Refer to Figure 2 for additional details.
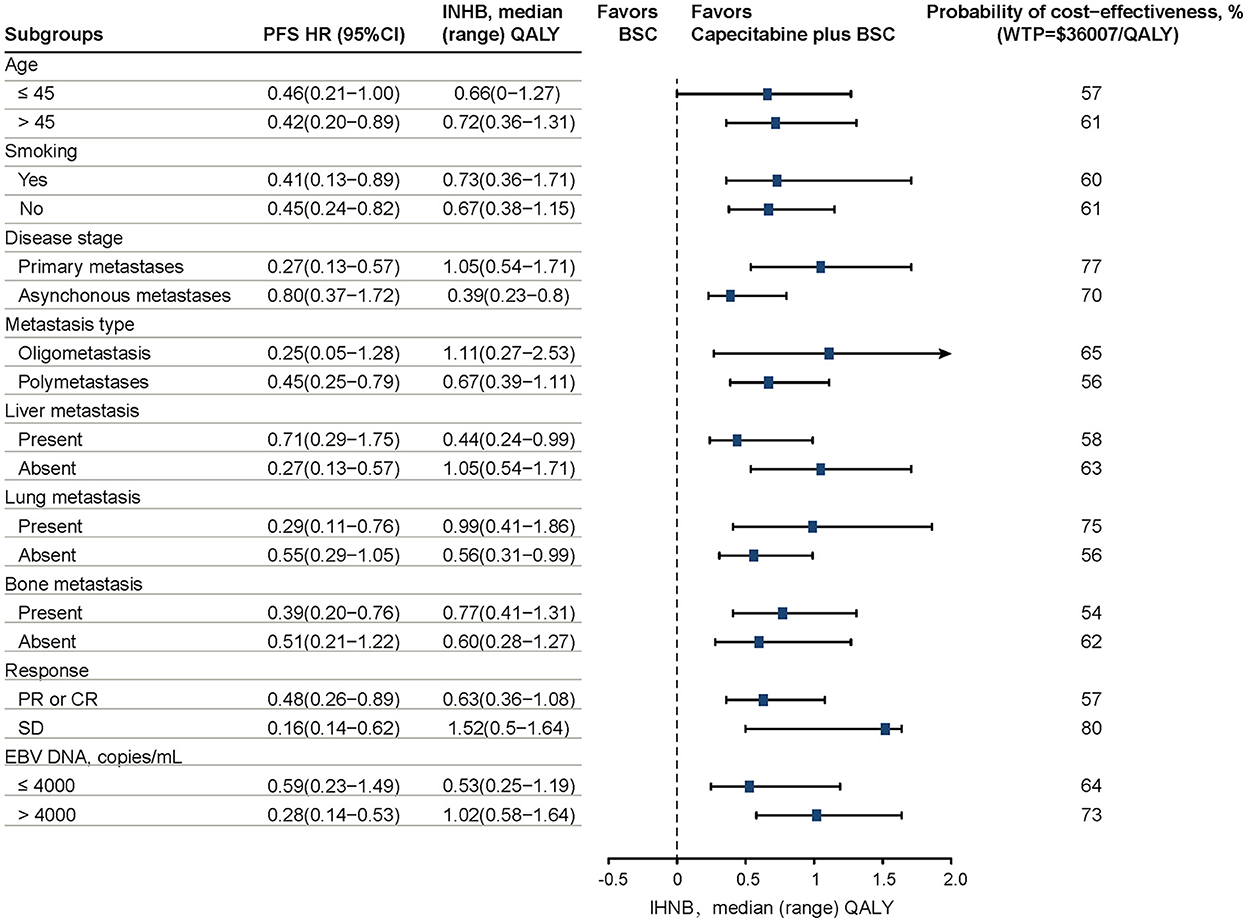
Figure 2. Results of prespecified subgroup analyses in INHBs and probabilities of cost-effectiveness by varying HRs of PFS. The vertical dashed line indicates the point of no effect (INHB = 0), the blue squares indicate the median INHBs, and the black solid bars indicate the ranges of INHB adjusted by HRs. PFS, progression-free survival; HR, hazard ratio; INHB, incremental net health benefit; BSC, best-supported care; WTP, willingness-to-pay; QALY, quality-adjusted life-year.
Results from the one-way sensitivity analysis between two treatment strategies are presented in the tornado diagram (Figure 3). The results demonstrated that the body weight of patients, the proportion receiving subsequent immunotherapy in the BSC and CBSC groups, and the proportion receiving concomitant radiotherapy in the CBSC group played a vital role in the results of ICURs. Overall, varying the input parameters did not alter the conclusion that ICURs were lower than the WTP threshold.
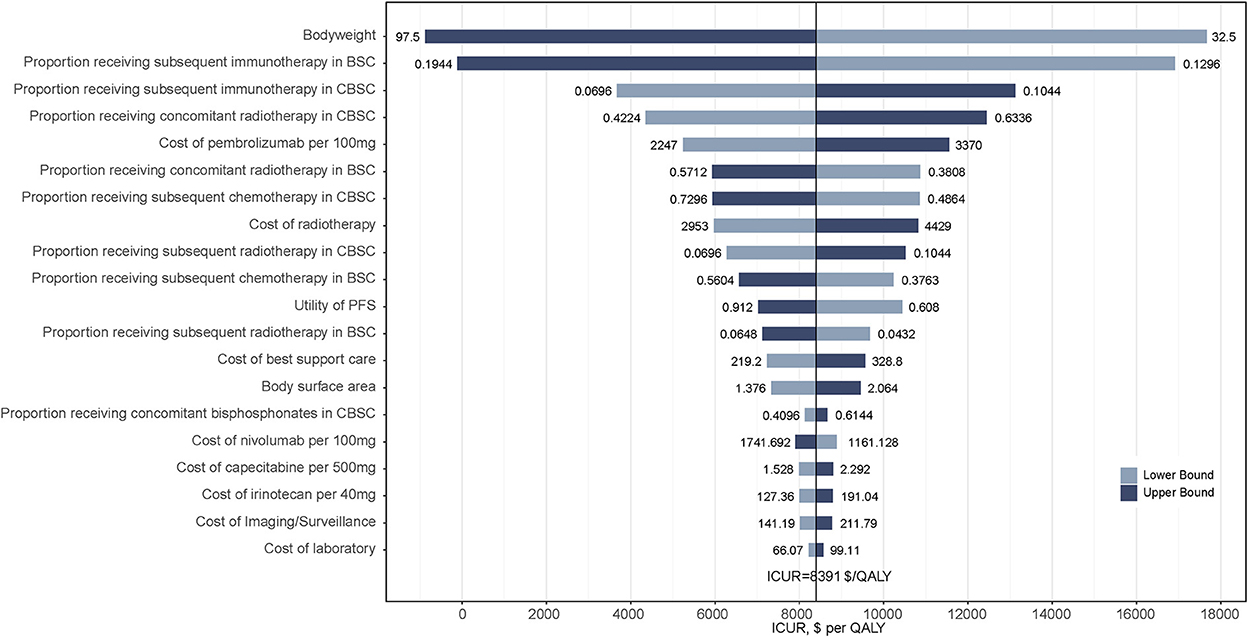
Figure 3. Tornado diagrams derived from the one-way sensitivity analysis of CBSC vs. BSC. Only the top 20 parameters that had the most influence on the results were shown. The black solid line indicates the ICURs. The dashed line indicates the WTP threshold in China ($36 007/QALY). CBSC, Capecitabine maintenance therapy plus best supported care group; BSC, best supported care; ICUR, incremental cost-utility ratios; QALY, quality-adjusted life-year.
The cost-effectiveness acceptability curve by 10 000 Monte Carlo simulations revealed that compared with the BSC group, the probability of the CBSC group being cost-effective is 65.9% at the WTP threshold was $36 007/QALY (Figure 4).
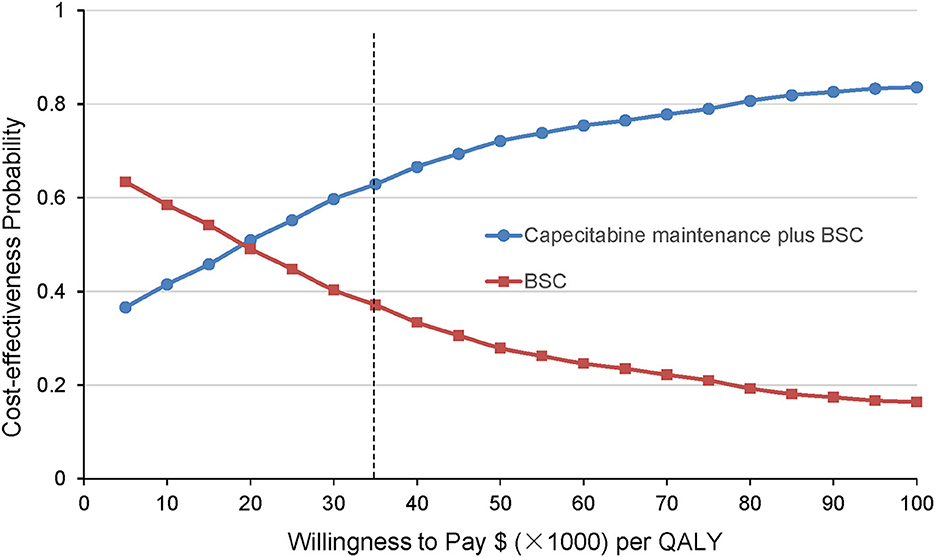
Figure 4. Cost-effectiveness acceptability curves derived from the probabilistic sensitivity analysis. The results are presented as probabilities that the treatment option is cost-effective at different willingness-to-pay thresholds. The black dashed line indicates the WTP threshold in China ($36 007/QALY). BSC, best-supported care; QALY, quality-adjusted life-year.
Most patients with early-stage NPC are responsive to the standard systemic radiotherapy and chemotherapy and are associated with a good prognosis, in addition to the mNPC (2). Furthermore, in the absence of further intensive therapy, most mNPC patients with disease recurrence will experience progression soon after first-line treatment. Although oncologists and patients are gradually interested in the promising immunotherapy maintenance option for recurrent or metastasis NPC based on the outcomes of the CAPTAIN-1st and JUPITER-02 clinical researches (12, 13), the high drug prices might be an important impediment to scale up. Low-dose maintenance chemotherapy was well tolerated with a low discontinuation rate and may be an attractive therapeutic intervention for the treatment of NPC. A recent clinical trial (NCT02460419) reported the efficacy and safety of capecitabine maintenance therapy in addition to systemic induction chemotherapy for patients with mNPC (26).
Based on the latest data released by the China's National Bureau of Statistic in 2021, the annual healthcare costs increased to ~7 trillion yuan ($1 trillion), carrying an enormous substantial economic burden on the health care system in China (36). Cost-effectiveness analysis based on randomized controlled trials (RCTs), that is, collection of patient-level cost data of treatments along with the measures of effectiveness is becoming increasingly common (26). This adds dimensions to interpret the results of RCTs and is designed to answer questions of health economic policy in addition to clinical benefits alone (15, 18). Therefore, we synthesized the latest evidence in the NCT02460419 trial and conducted the analysis to estimate the cost-effectiveness of the capecitabine maintenance therapy plus best supportive care, and best supportive care alone in the first-line treatment of newly diagnosed mNPC from the Chinese payers' perspective.
Overall, our analysis meets the CHEERS Checklist (Supplementary Table 5). According to the baseline analysis, after induction chemotherapy capecitabine maintenance treatment of mNPC was more cost-effective than induction chemotherapy alone at the WTP threshold of $36 007/QALY in China. As shown in Table 2, the ICUR was $8 391 per QALY in the baseline results, which was significantly lower than the WTP threshold. Meanwhile, the gained INHB in the CBSC group was positive at the threshold of $36 007 per QALY gained. The combined baseline results in the PFS state and across disease stages support the role of additional capecitabine in preventing disease progression was the primary driver of economic outcomes. All subgroups favored induction chemotherapy plus CBSC treatment due to the positive trend in INHB compared with the BSC treatment. After first-line induction chemotherapy, the stable disease response subgroup treated with CBSC had the highest probability to be cost-effective. According to the plasma EBV DNA status at baseline, patients with EBV DNA copy number > 4000 received CBSC treatment was more cost-effective than EBV DNA copy number ≤ 4000. Moreover, patients in the CBSC treatment group with lung or bone metastasis were more cost-effective than no present metastases, but those without liver metastasis were more cost-effective than present liver metastasis. It should be noted that the results of the subgroup analyses should be interpreted with caution owing to the lack of sufficient data and the heterogeneity of the population.
Finding on the one-way sensitivity analysis revealed that none of the key conclusions are changed by altering each parameter, which underlined the robustness of our Markov model. Further, the results of the probabilistic sensitivity analysis further demonstrate the stability of the model and the higher probability that the CBSC group was more cost-effective than the BSC group.
Most of the treatment-related AEs occurred in patients treated with capecitabine were manageable and no treatment-related deaths occurred. Therefore, capecitabine maintenance therapy does not introduce high additional possible costs for AEs treatment. In addition, a relatively appropriate price with a significant PFS benefit could be responsible for the capecitabine maintenance treatment after induction chemotherapy was more cost-effective compare to induction chemotherapy alone. Based on the results of the current study, capecitabine maintenance therapy could be an effective, safe, and cost-effective treatment, which appeared to be a promising new option for patients with mNPC.
To our knowledge, this is the first cost-effectiveness analysis to estimate the economic outcomes of the capecitabine maintenance therapy plus best supportive care in the first-line treatment of newly diagnosed mNPC from the Chinese payers' perspective. Though maintenance capecitabine has shown promising results for NPC in early trials (25, 26), further data for health and economic outcomes are needed before the drug can be accepted as a standard first-line treatment in the future. A previous cost-effectiveness analysis has shown that capecitabine and bevacizumab maintenance therapy for patients with metastatic colon cancer was not cost-effective at an ICER of $725 601/QALY from the US Medicare payer's perspective (41). Our study differs from this research that evaluated a combination regimen including capecitabine for metastatic colon cancer in the US. Another analysis evaluated the cost-effectiveness of metronomic capecitabine as adjuvant chemotherapy for locoregionally advanced NPC patients from the perspective of China (42). The results indicated that metronomic capecitabine as adjuvant chemotherapy is a cost-effective strategy, which obtained an ICER of $ 9669.99/QALY (42). Their results are consistent with ours, which revealed that capecitabine treatment was an effective and cost-effective choice for NPC in China.
Several strengths need to be emphasized. First, the NCT02460419 clinical trial was conducted in China and all enrolled patients were Chinese; thus, our study using price and clinical data in China provided an actionable and valuable evidence for policymakers, providers, and patients to make an optional decision. Second, in the present study, we used data from the life table to capture other causes of background mortality including cardiovascular diseases, allowing the model to better reflect reality. Third, multiple economic outcomes of prespecified subgroups in the NCT02460419 clinical trial were evaluated in our current analysis. Economic analysis of subgroups provides more precise information that may be helpful for clinicians and patients.
Some limitations of this study must be acknowledged. First, we did not include other standard immunotherapy-related first-line treatment options, e.g., camrelizumab or toripallimab plus chemotherapy due to the different inclusion criteria between the clinical trials. There is a lack of randomized controlled clinical trials testing maintenance immunotherapy vs. capecitabine maintenance therapy in patients with mNPC. Second, the OS data in the NCT02460419 clinical trial were not mature at the time of the analysis, which could have some impact on fitted survival data. Nevertheless, the long-term survival data of the two groups was extrapolated from the limited available survival curves from the NCT02460419 clinical trial using a specific mathematical model, thus, our results are unlikely to be strongly affected by immature OS data. Our analysis would be updated as new additional evidence becomes available. Third, because of the quality-of-life data have not been published in the NCT02460419 clinical trial, we used relevant data in the published literature. Subsequent one-way sensitivity analyses, therefore, indicate that changing the utility values would not alter our conclusions.
Based on the analysis, compared with BSC treatment, after induction chemotherapy, capecitabine maintenance treatment plus BSC as first-line treatment was a more cost-effective strategy for patients with newly diagnosed mNPC from the Chinese payers' perspective. Exploring treatment strategies tailored by the characteristics of the individual patient could be a way to improve the economic outcomes.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
JiaH and KT: conceptualization. JiaH and XL: methodology. XS and XL: formal analysis and investigation. JiaH: writing—original draft preparation. JiaH and KT: writing—review and editing. JiaH and NC: funding acquisition. XS: resources. NC: supervision. All authors contributed to the article and approved the submitted version.
This work was supported by the Natural Science Foundation of Sichuan province (grant number 2022NSFSC1520).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JZ declared a shared affiliation with the authors JiaH, XL, KT, XS, JinH, and NC to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1086393/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. (2019) 394:64–80. doi: 10.1016/S0140-6736(19)30956-0
3. Lee AWM, Ng WT, Chan YH, Sze H, Chan C, Lam TH. The battle against nasopharyngeal cancer. Radiother Oncol. (2012) 104:272–8. doi: 10.1016/j.radonc.2012.08.001
4. Tan WL, Tan EH, Lim DWT, Ng QS, Tan DSW, Jain A, et al. Advances in systemic treatment for nasopharyngeal carcinoma. Chin Clin Oncol. (2016) 5:2. doi: 10.21037/cco.2016.03.03
5. Li AC, Xiao WW, Shen GZ, Wang L, Xu AA, Cao YQ, et al. Distant metastasis risk and patterns of nasopharyngeal carcinoma in the era of imrt: long-term results and benefits of chemotherapy. Oncotarget. (2015) 6:24511–21. doi: 10.18632/oncotarget.4312
6. Wong KCW, Hui EP, Lo KW, Lam WKJ, Johnson D, Li LL, et al. Nasopharyngeal carcinoma: an evolving paradigm. Nat Rev Clin Oncol. (2021) 18:679–95. doi: 10.1038/s41571-021-00524-x
7. National Comprehensive Cancer Network. Nccn Clinical Practice Guidelines in Oncology: Head and Neck Cancers, Version 3. (2021) Available online at: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed August 1, 2022).
8. Tang LL, Chen YP, Chen CB, Chen MY, Chen NY, Chen XZ, et al. The chinese society of clinical oncology (Csco) clinical guidelines for the diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun. (2021) 41:1115–27. doi: 10.1002/cac2.12218
9. Jin Y, Cai XY, Shi YX, Xia XY, Cai YC, Cao Y, et al. Comparison of five cisplatin-based regimens frequently used as the first-line protocols in metastatic nasopharyngeal carcinoma. J Cancer Res Clin. (2012) 138:1717–25. doi: 10.1007/s00432-012-1219-x
10. Wang J, Li J, Hong X, Tang W, Hu X, Wang B, et al. Retrospective case series of gemcitabine plus cisplatin in the treatment of recurrent and metastatic nasopharyngeal carcinoma. Oral Oncol. (2008) 44:464–70. doi: 10.1016/j.oraloncology.2007.06.004
11. Zhang L, Huang Y, Hong S, Yang Y, Yu G, Jia J, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet. (2016) 388:1883–92. doi: 10.1016/S0140-6736(16)31388-5
12. Yang YP, Qu S, Li JA, Hu CS, Xu MJ Li WD, et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (captain-1st): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncology. (2021) 22:1162–74. doi: 10.1016/S1470-2045(21)00302-8
13. Mai HQ, Chen QY, Chen D, Hu C, Yang K, Wen J, et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat Med. (2021) 27:1536–43. doi: 10.1038/s41591-021-01444-0
14. Dolgin E. Bringing down the cost of cancer treatment. Nature. (2018) 555:S26–S9. doi: 10.1038/d41586-018-02483-3
15. Tian K, Han J, Wang Z, Chen J. Immune checkpoint inhibition in first-line treatment for recurrent or metastatic nasopharyngeal carcinoma: a captain-1st and jupiter-02 trial-based cost-effectiveness analysis. Oral Oncol. (2022) 128:105842. doi: 10.1016/j.oraloncology.2022.105842
16. Kluender R, Mahoney N, Wong F, Yin W. Medical debt in the US, 2009-2020. JAMA. (2021) 326:250–6. doi: 10.1001/jama.2021.8694
17. Obasaju C, Bowman L, Wang P, Shen W, Winfree KB, Smyth EN, et al. Identifying the target nsclc patient for maintenance therapy: an analysis from a placebo-controlled, phase iii trial of maintenance pemetrexed (H3e-Mc-Jmen). Ann Oncol. (2013) 24:1534–42. doi: 10.1093/annonc/mdt123
18. Han J, Tian K, Yang J, Gong Y. Durvalumab vs placebo consolidation therapy after chemoradiotherapy in stage III non-small-cell lung cancer: an updated pacific trial-based cost-effectiveness analysis. Lung Cancer. (2020) 146:42–9. doi: 10.1016/j.lungcan.2020.05.011
19. Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Three-year overall survival with durvalumab after chemoradiotherapy in stage iii nsclc-update from pacific. J Thorac Oncol. (2020) 15:288–93. doi: 10.1016/j.jtho.2019.10.002
20. Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. (2010) 7:455–65. doi: 10.1038/nrclinonc.2010.82
21. Zielinski C, Gralow J, Martin M. Optimising the dose of capecitabine in metastatic breast cancer: confused, clarified or confirmed? Ann Oncol. (2010) 21:2145–52. doi: 10.1093/annonc/mdq069
22. Wang X, Wang SS, Huang H, Cai L, Zhao L, Peng RJ, et al. Effect of capecitabine maintenance therapy using lower dosage and higher frequency vs observation on disease-free survival among patients with early-stage triple-negative breast cancer who had received standard treatment: the sysucc-001 randomized clinical trial. JAMA. (2021) 325:50–8. doi: 10.1001/jama.2020.23370
23. Luo HY Li YH, Wang W, Wang ZQ, Yuan X, Ma D, et al. Single-agent capecitabine as maintenance therapy after induction of xelox (or folfox) in first-line treatment of metastatic colorectal cancer: randomized clinical trial of efficacy and safety. Ann Oncol. (2016) 27:1074–81. doi: 10.1093/annonc/mdw101
24. Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. Capecitabine compared with observation in resected biliary tract cancer (bilcap): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. (2019) 20:663–73. doi: 10.1016/S1470-2045(18)30915-X
25. Chen YP, Liu X, Zhou Q, Yang KY, Jin F, Zhu XD, et al. Metronomic capecitabine as adjuvant therapy in locoregionally advanced nasopharyngeal carcinoma: a multicentre, open-label, parallel-group, randomised, controlled, phase 3 trial. Lancet. (2021) 398:303–13. doi: 10.1016/S0140-6736(21)01123-5
26. Liu GY Li WZ, Wang DS, Liang H, Lv X, Ye YF, et al. Effect of capecitabine maintenance therapy plus best supportive care vs best supportive care alone on progression-free survival among patients with newly diagnosed metastatic nasopharyngeal carcinoma who had received induction chemotherapy: a phase 3 randomized clinical trial. JAMA Oncol. (2022) 8:553–61. doi: 10.1001/jamaoncol.2021.7366
27. Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. (2016) 316:1093–103. doi: 10.1001/jama.2016.12195
28. Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol. (2011) 11:139. doi: 10.1186/1471-2288-11-13
29. Latimer NR. Survival analysis for economic evaluations alongside clinical trials–extrapolation with patient-level data: inconsistencies, limitations, and a practical guide. Med Decis Making. (2013) 33:743–54. doi: 10.1177/0272989X12472398
30. Yang GH, Wang Y, Zeng YX, Gao GF, Liang XF, Zhou MG, et al. Rapid health transition in China, 1990-2010: findings from the global burden of disease study 2010. Lancet. (2013) 381:1987–2015. doi: 10.1016/S0140-6736(13)61097-1
31. National Bureau of Statistics of China: National Data. (2022) Available online at: http://www.pbc.gov.cn/rmyh/108976/109428/index.html (Accessed August 1st, 2022)
32. Gu XH, Zhang Q, Chu YB, Zhao YY, Zhang YJ, Kuo D, et al. Cost-effectiveness of afatinib, gefitinib, erlotinib and pemetrexed-based chemotherapy as first-line treatments for advanced non-small cell lung cancer in China. Lung Cancer. (2019) 127:84–9. doi: 10.1016/j.lungcan.2018.11.029
33. Chen J, Hu G, Chen Z, Wan X, Tan C, Zeng X, et al. Cost-effectiveness analysis of pembrolizumab plus axitinib versus sunitinib in first-line advanced renal cell carcinoma in China. Clin Drug Investig. (2019) 39:931–8. doi: 10.1007/s40261-019-00820-6
34. Chen X, Liang W, Wan N, Zhang L, Yang Y, Jiang J, et al. Cost-effectiveness analysis of gemcitabine plus cisplatin versus fluorouracil plus cisplatin for first-line treatment of recurrent or metastatic nasopharyngeal carcinoma. Oral Oncol. (2019) 94:80–5. doi: 10.1016/j.oraloncology.2019.04.022
35. Van den Bosch L, van der Laan HP, van der Schaaf A, Oosting SF, Halmos GB, Witjes MJH, et al. Patient-reported toxicity and quality-of-life profiles in patients with head and neck cancer treated with definitive radiation therapy or chemoradiation. Int J Radiat Oncol. (2021) 111:456–67. doi: 10.1016/j.ijrobp.2021.05.114
36. China Statistical Yearbook. Compiled by National Bureau of Statistics of China. (2021) Available online at: http://www.stats.gov.cn/tjsj/ndsj/2021/indexch.htm (accessed August 1, 2022).
37. Eichler HG, Kong SX, Gerth WC, Mavros P, Jonsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value in Health. (2004) 7:518–28. doi: 10.1111/j.1524-4733.2004.75003.x
38. Murray CJL, Evans DB, Acharya A, Baltussen RMPM. Development of who guidelines on generalized cost-effectiveness analysis. Health Econ. (2000) 9:235–51.
39. Kohn CG, Zeichner SB, Chen QS, Montero AJ, Goldstein DA, Flowers CR. Cost-effectiveness of immune checkpoint inhibition in braf wild-type advanced melanoma. J Clin Oncol. (2017) 35:1194–202. doi: 10.1200/Jco.2016.69.6336
40. Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD, et al. Model parameter estimation and uncertainty analysis: a report of the ispor-smdm modeling good research practices task force working group-6. Med Decis Making. (2012) 32:722–32. doi: 10.1177/0272989X12458348
41. Sherman SK, Lange JJ, Dahdaleh FS, Rajeev R, Gamblin TC, Polite BN, et al. Cost-effectiveness of maintenance capecitabine and bevacizumab for metastatic colorectal cancer. JAMA Oncol. (2019) 5:236–42. doi: 10.1001/jamaoncol.2018.5070
Keywords: nasopharyngeal carcinoma, cost-effectiveness, capecitabine, maintenance therapy, real-world data
Citation: Han J, Lan X, Tian K, Shen X, He J and Chen N (2023) Cost-effectiveness analysis of capecitabine maintenance therapy plus best supportive care vs. best supportive care alone as first-line treatment of newly diagnosed metastatic nasopharyngeal carcinoma. Front. Public Health 10:1086393. doi: 10.3389/fpubh.2022.1086393
Received: 01 November 2022; Accepted: 29 December 2022;
Published: 26 January 2023.
Edited by:
Jing Yuan, Fudan University, ChinaReviewed by:
Longjiang She, First People's Hospital of Foshan, ChinaCopyright © 2023 Han, Lan, Tian, Shen, He and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nianyong Chen,  bl95Y2hlbkBob3RtYWlsLmNvbQ==
bl95Y2hlbkBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.