- 1China CDC Key Laboratory of Environment and Population Health, National Institute of Environmental Health, Chinese Center for Disease Control and Prevention, Beijing, China
- 2Chaoyang District Center for Disease Control and Prevention, Beijing, China
- 3National Institute for Occupational Health and Poison Control, Chinese Center for Disease Control and Prevention, Beijing, China
Background: Exposure to PM2.5 will accelerate the progression of cardiovascular diseases. Air purifier can reduce the PM2.5 exposure and theoretically alleviate the influence of PM2.5 on patients with stable coronary artery disease (SCAD). However, few studies of the protective effect showed significant results because the interferent effects of routine medication had not been taken into account. In order to explore the actual effect on patients with SCAD, we conducted a randomized single-blind crossover air purifier intervention trial.
Method: Levels of PM2.5 exposure during intervention and cardiovascular indicators (inflammation, coagulation, plaque stability, and blood lipids) after intervention were detected, meanwhile the information of drug use was obtained by questionnaire. The kinds of drug used by more than 20% of the subjects were sorted out. And the influence of these drugs on cardiovascular indicators was summarized through literature review. Based on that, the drug use was included as a variable in linear mixed effects models that used to analyze the associations between PM2.5 exposure reduction by air purifier and cardiovascular indicators.
Results: The result revealed that the interpretation contribution rate of drug use was more than that of PM2.5 exposure. The level of C-reactive protein significantly decreased by 20.93% (95%CI: 6.56%, 33.10%), 23.44% (95%CI: 2.77%, 39.39%) and 24.11% (95%CI: 4.21%, 39.69%) on lag1, lag01 and lag02 respectively, while the level of high-density lipoprotein cholesterol significantly increased by 5.10% (95%CI: 0.69%, 9.05%), 3.71% (95%CI: 0.92%, 6.60%) and 6.48% (95%CI: 2.58%, 10.24%) respectively on lag0, lag1 and lag01 associated with an interquartile range decrease of 22.51 μg/m3 in PM2.5 exposure.
Conclusion: The study shows positive effects of air purifier on SCAD, and also provides methodological reference for future related research.
1. Introduction
PM2.5 exposure has been confirmed to cause changes of cardiovascular indicators, thus promoting the occurrence and development of cardiovascular diseases (1). Air purifier with high efficiency particulate air filter (HEPA) can significantly reduce the indoor PM2.5 concentration (2, 3) then the personal exposure (4). Therefore, there is theoretical basis for using air purifier to reduce health hazards, in areas and seasons with high incidence of PM2.5 pollution.
The results of a series of intervention studies demonstrated that the use of air purifiers has different effects on the cardiovascular indicators of different groups. For healthy adults, the use of air purifiers has significant effects on the levels of monocyte chemokines, interleukin-1beta, soluble cluster of differentiation 40 ligand (sCD40L) and endothelin-1 (ET-1) in their circulatory system (5, 6), while the effects on C-reactive protein (CRP) (5, 7–9) and fibrinogen (FIB) (5, 10) are still controversial, and no significant effects on tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6) and cluster of differentiation 62 platelet (CD62P) have been found (5, 7, 8, 11). For the elderly, most studies showed no significant impacts on circulatory CRP, TNF-α, IL-6 and FIB levels by using air purifier, except on a physiological index microvascular function (2, 4, 12). But stratification analysis in one correlational study (2) found that the protective effect of air purifiers on microvascular function was not statistically significant in the elderly taking cardiovascular disease medicine. Thus, it can be seen that the conventional cardiovascular disease medicine may interfere the cardiovascular benefits of air purifiers for the elderly.
Patients with stable coronary artery disease (SCAD) usually receive comprehensive drug treatment, including mutiple drugs, to control the development of disease by regulating various functions of the circulatory system. For example, statins are mainly used to regulate the blood lipid balance; β-blockers, angiotensin-converting enzyme inhibitors (ACEI), angiotensin-receptor blockers (ARB) and calcium-channel blockers (Ca-channel blockers) are used to regulate the cardiac load and vasoconstriction; and aspirin is used to inhibit the platelet aggregation. These drugs may independently or synthetically change some cardiovascular indicators, involving inflammation, coagulation, plaque stability and blood lipids, which can also be affected by PM2.5 exposure (1, 13, 14). Therefore, it is essential to comprehensively control the interference effects of these drugs, when intervention studies on the protective effect of air purifiers on the cardiovascular system are carried out.
To explore the actual protective effect of PM2.5 exposure reduction by air purifiers, a randomized crossover intervention design was adopted to the enrolled patients with SCAD. Air purifiers were used in their residences to reduce the indoor PM2.5 concentration. At the same time of PM2.5 exposure monitoring, the drug use of the subjects during the intervention was investigated. And levels of the cardiovascular indicators of inflammation, coagulation, plaque stability and blood lipids after the intervention were detected. Interference effects of drug use were controlled in liner mixed effect models, which used to analyze the associations between PM2.5 exposure and cardiovascular indicators.
2. Materials and methods
2.1. Study design and subject recruitment
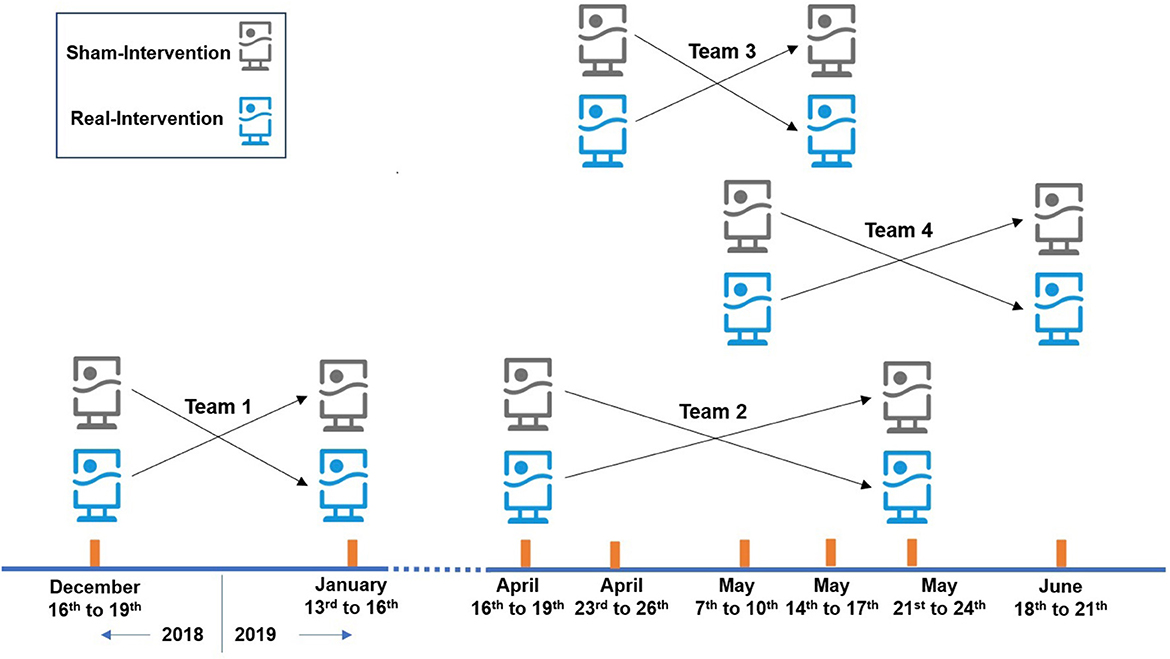
In this study, the randomized single-blind crossover intervention design was adopted. 24 SCAD patients were recruited, and the intervention trial was conducted in their residences, with air purifiers placed in their main activity space. Air purifiers equipped with HEPA filter were “real-intervention,” with a clean air delivery rate of 500 m3/h for PM2.5, while those without HEPA were “sham-intervention.” The experiment was completed in 4 batches with 6 subjects in each batch. Every intervention period was 3 days and the washout period was more than 14 days. The arrangement of the intervention is shown in Figure 1.
The subjects were recruited from 3 community-level (or above) general hospitals in Beijing, who were clinically diagnosed with SCAD according to the diagnostic requirements of 2013 European Society Cardiology Guidelines on the Management of Stable Coronary Artery Disease (15) published by the European College of Cardiology and 2010 Chinese Consensus on the Management of Chronic Stable Coronary Artery Disease (16) issued by China. In addition, the inclusion criteria include: (a) the age was between 55 and 80 years old; and (b) they lived near the hospital for a long time and the house was not decorated within 1 year. The exclusion criteria are as follows: (a) someone in their house smoked or themselves had just quitted smoking for <1 year; (b) patients were diagnosed with acute myocardial infarction within 3 months, or receive percutaneous coronary intervention and bypass transplantation within 6 months; (c) patients wore a pacemaker; and (d) patients suffered from liver injury, cancer and other serious diseases.
During the intervention, the individual exposure to PM2.5 and the concentration of PM2.5 indoor and outdoor the residence was monitored continuously. The age and body mass index (BMI) of the subjects was investigated, and their travel behaviors, window opening time and medication were recorded. The subjects stayed at home for more than 20 h/d, and the window opened for no more than 2 h/d during the intervention. Drug use records focused on conventional medicine for CAD treatment. Blood samples were collected at the end of each intervention, and the following indicators were detected: (a) inflammation indicators such as IL-6, TNF-α, CRP and FIB; (b) coagulation indicators such as CD62P, CD40L, intercellular adhesion molecule-1 (ICAM-1), nitric oxide (NO) and ET-1; (c) plaque stability indicators such as matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-2 (MMP-9); (d) blood lipid indicators such as total cholesterol (CHO), triglyceride (TG), high-density lipoprotein (HDL-C) and low-density lipoprotein (LDL-C).
2.2. PM2.5 exposure
Particulate matter sampling pumps MicroPEMTM (RTI Company, USA) based on the principle of light scattering were used to monitor PM2.5 concentration with sampling flow of 500 mL/min. It can monitor the environmental temperature and relative humidity (RH) simultaneously. Daily average concentration of PM2.5 was calculated from 8 a.m. to 8 a.m. Lag and moving average levels of PM2.5 exposure are represented by “lag.”
Sampling pumps for monitoring the indoor PM2.5 concentration were placed in the open area of the house to avoid air conditioner vent, kitchen, doors and windows, about 1.5 m above the ground, more than 1 m far from the wall, and at least 2 m far from the air purifier. Sampling pumps for monitoring the outdoor PM2.5 concentration were placed outside the residential window and avoid air conditioner unit and kitchen and its air outlet. Sampling pumps for monitoring individual exposure to PM2.5 were taken along by the subjects.
2.3. Blood collection and indicator measurements
Fasting blood samples (5 mL anticoagulant and 5 mL non-anticoagulant) were collected from 8:00 to 9: 00 on the day at the end of each intervention. IL-6, TNF-α, CRP, FIB, CD62P, CD40L, ICAM-1, NO, ET-1, MMP-2 and MMP-9 levels were tested by a professional testing institution. CHO, TG, HDL-C and LDL-C levels were tested by the laboratory of the hospital. IL-6, TNF-α, CD62P, CD40L and ICAM-1 was detected by a bead-based multiple flow cytometry on Luminex 200 system (Luminex Corporation, Austin, TX, USA) with chips (IL-6 and TNF-α: Merck Millipore, Germany; CD62P, CD40L and ICAM-1: R&D, USA). CRP, NO, MMP-2, MMP-9, FIB and ET-1 was separately detected by ELISA kit (CRP, NO, MMP-2, MMP-9: R&D, USA; FIB: NOVUS, USA; ET-1: Elabscience Biotechnology Co. Ltd, Wuhan, China).
In addition, a compound index-atherogenic index of plasma (AIP) was calculated by the results of TG and HDL-C levels and the formula: .
2.4. Statistical analyses
Descriptive statistics were conducted for general characteristics of the environmental exposure and effects. Data with normal distribution were described by mean and standard deviation (Mean±SD), and data with skewed distribution were described by median and interquartile range as M (P25, P75). The paired Student's t-test was employed to compare the PM2.5 concentrations between two intervention periods, or the indoor and outdoor environment. The Linear mixed effect models were conducted to estimate the correlation between PM2.5 exposure and cardiovascular indicators. The results were reported by the percentage change of indicators associated with an interquartile range (IQR) decreases of PM2.5 exposure. The interpretation contribution rate of independent variables in the model were calculated by the determination coefficient R2 of independent variables/ of the model.
In the linear mixed effect models, the “effective drug” variable is defined as one or more kinds of the effective drug had been taken by the subject (dichotomous variable). The kinds of drug need to be controlled were selected according to Guidelines for Rational Drug Use of Coronary Artery Disease (2nd Edition) (17) and the actual medication of the subjects (taken by more than 20% of the subjects). Whether the drug is effective was determined by searching the literatures on “Pubmed,” “Web of Science,” “China National Knowledge Infrastructure,” “Wanfang” and “VIP” (before December 30, 2021). It needs to meet the following requirements: (a) there were two or more case-control trials or pre-and post-treatment control trials of cardiovascular disease patients treated with the drug, and the results of the trials were statistically significant; or (b) there was a case-control trial or pre-and post-treatment control trial of cardiovascular disease patients treated with the drug, and the results were statistically significant, and there was one or more animal experiment of the drug, and the results were statistically significant. The Newcastle-Ottawa Scale (NOS) was used to assess the quality of literatures relevant to case control/pre-and post-control studies in patients with cardiovascular disease, and the CAMARADES Scales was used to assess the quality of literatures relevant to animal studies. Literatures with a score more than 6 points would be included in the summary.
Influencing factors of PM2.5 exposure and cardiovascular indicators need to be considered as independent variables in the linear mixed effects models. It includes the “effective drug,” age, gender and BMI, as well as temperature and relative humidity. The stepwise elimination method based on the principle of minimization of Akaike's Information Criterion (AIC) was adopted for the validity of models. We screened three additional independent variables on the basis of preserving key variables (PM2.5 exposure and the “effective drug”). The model was established for each indicator respectively, given the influencing factors differences.
The parameters of the model were tested by bilateral t-test, with statistical significance of 0.05. The “lme4” package and “r2glmm” package in the R (Version 4.0.3) was separately used for linear mixed effect model and R2 calculation.
2.5. Quality control
The monitoring instruments were cleaned and calibrated before sampling. Instrument condition review was done at the end of the first and last day of each intervention. The blood samples were collected by the nurses. Blood lipid indicators were detected on the same day, and samples were stored at −80°C before further detection. The detection process was controlled strictly in accordance with the testing procedures and the requirements of the chips/kits.
The subjects of the study were trained by professional personnel before recording the questionnaire themselves. Questionnaires were re-checked at the end of the first and last day of each intervention for ensuring recording accuracy. Questionnaire and data input were done by two personnel, and then reviewed and analyzed by special personnel.
3. Results
3.1. The general characteristics of the subjects
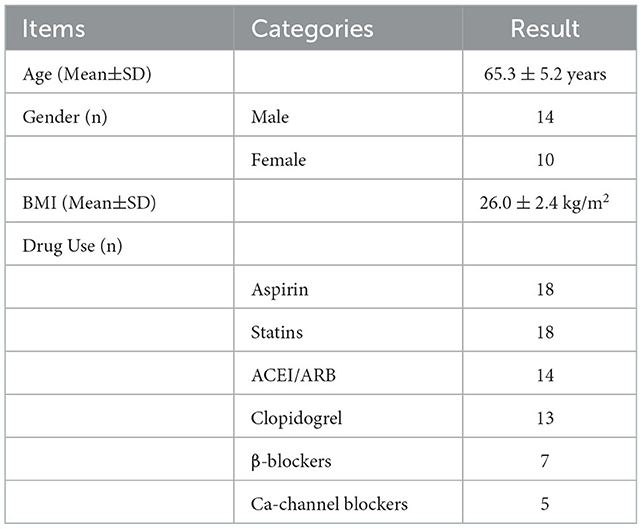
The residences of the subjects were all located within 5 km of the hospitals, in apartment buildings built from 2003 to 2015, with an area between 45.1 and 96.0 m3. The windows of the rooms were made of plastic steel or aluminum alloy to ensure the sufficient air tightness. The average age of the subjects was 65.3 ± 5.2 years old, and the ratio of male to female was 14:10. The average BMI of the subjects was 26.0 ±2.4 kg/m2. The drug use during the intervention is shown in Table 1.
3.2. Air purification effect and individual PM2.5 exposure level
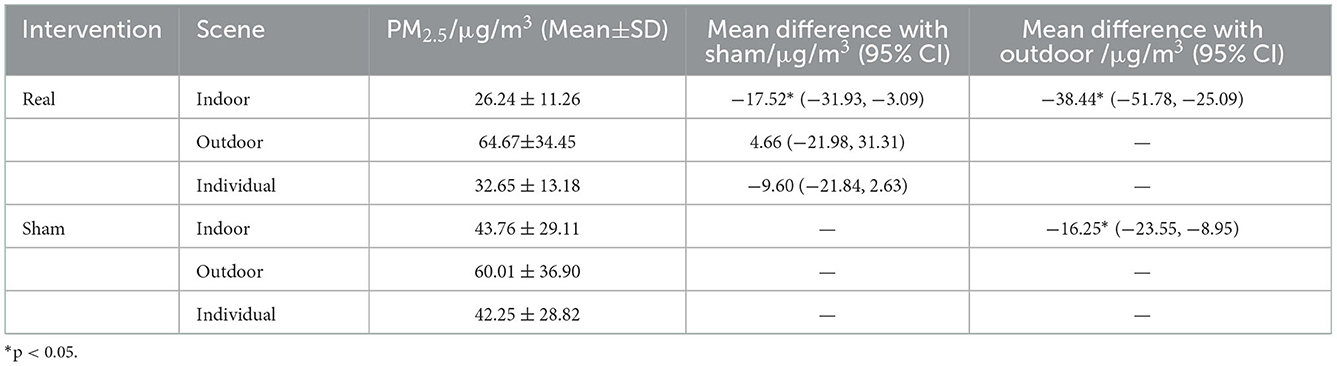
The 72 h average indoor PM2.5 concentration in the real-intervention period was 40.58% of the outdoor. It was significantly reduced by 40.04% when compared with the sham-intervention period. The change of individual PM2.5 exposure level was consistent with indoor concentration. During the real-intervention period, the individual PM2.5 exposure level was reduced by 22.72% when compared with the sham-intervention period. The use of air purifiers in residences can effectively reduce the indoor PM2.5 concentration, and then the individual PM2.5 exposure (Table 2).
3.3. Drug to be controlled and their effects on indicators
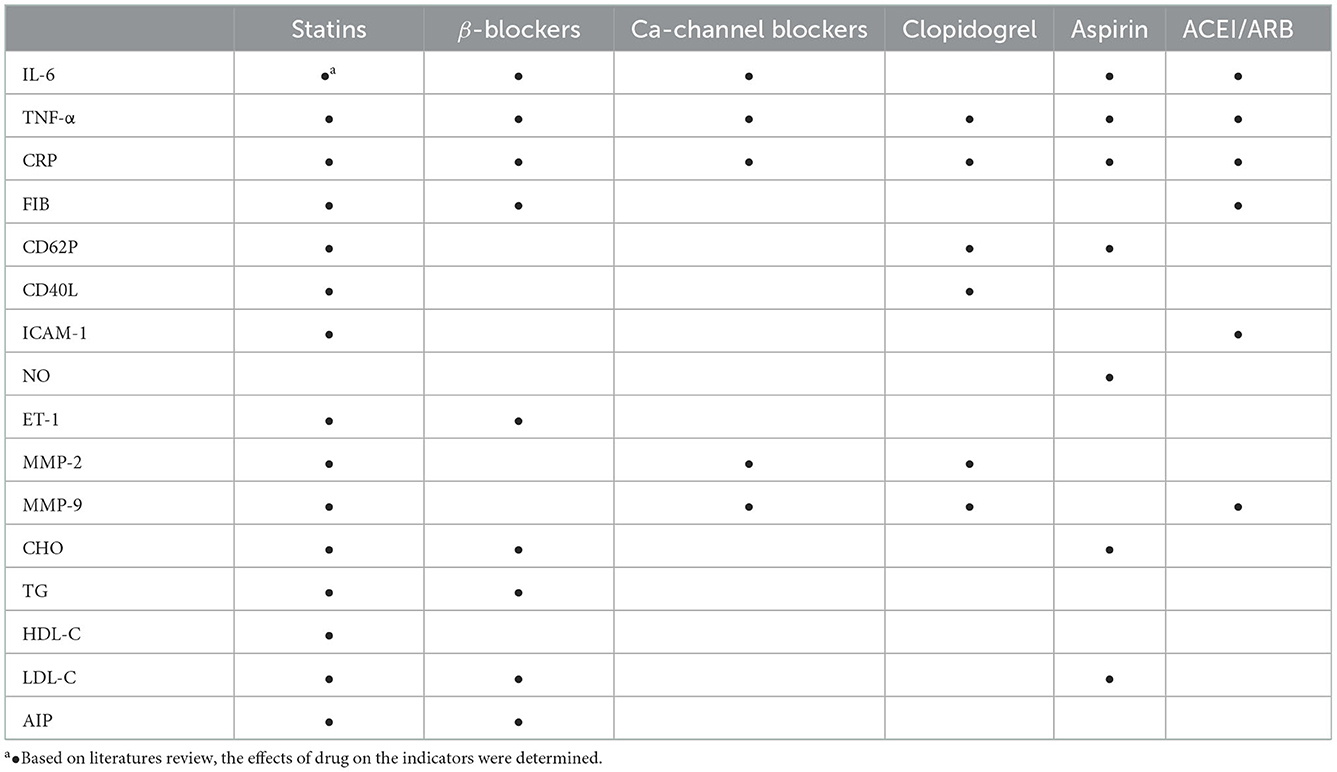
According to the questionnaire survey, 6 kinds of drug were taken by more than 20% of the subjects during the intervention period: statins, β-blockers, Ca-channel blockers, clopidogrel, aspirin and ACEI/ARB. Based on literatures review (Supplementary Tables S1–S6) (18–77), the effects of these 6 kinds of drug on the indicators of inflammation, coagulation, plaque stability and blood lipids were determined, as shown in Table 3.
3.4. Model selection and interpretation contribution rate of variables
In this study, each cardiovascular indicator was selected by minimizing AIC in the linear mixed effect analysis model, except for CHO and LDL-C. Based on the previous association research of blood lipid indicators, the influence of age, gender and BMI variables were greater than that of ambient temperature and RH. And the AIC values of CHO and LDL-C models (“log(Indicator) ~PM2.5+Age+Gender+BMI+ED+(1|ID)” and “log(Indicator) ~PM2.5+Gender+BMI+Temp+ED+ (1|ID)”) were similar, which were 12.74 vs. 12.21 and 47.03 vs. 46.54, respectively, so “log(Indicator) ~PM2.5+Age+Gender+BMI+ED+(1|ID)” was selected as the model. The final selection of linear mixed effect models for each cardiovascular indicator is shown in Supplementary Table S7.
The determination coefficient R2 of the independent variables of each model and its interpretation contribution rate in the model were calculated (Supplementary Table S7), we found that in the models corresponding to IL-6, TNF-α, FIB, CD62P, ICAM-1, ET-1, MMP-9, CHO, TG and AIP, the contribution rate of “effective drug” to the interpretation of the model was greater than that of PM2.5 exposure, and the contribution rate of “effective drug” to the interpretation of IL-6, TG and AIP models was more than 58%. In the model corresponding to the CRP, CD40L, NO, HDL-C and LDL-C, the interpretation contribution rate of PM2.5 exposure to the model was slightly greater than that of “effective drug.” In the model corresponding to MMP-2, the interpretation contribution rate of PM2.5 exposure to the model was much greater than that of drug intake (60% vs. 3%).
3.5. Effects of PM2.5 reduction on inflammation indicators
The levels of TNF-α, CRP and FIB were decreased with each IQR decrease (22.51 μg/m3) of PM2.5 exposure on lag0 when the subjects used the “real” air purifier, but the changes were not statistically significant. And CRP level of the subjects was significantly reduced by 20.93% (95%CI: 6.56%, 33.10%), 23.44% (95%CI: 2.77%, 39.39%) and 24.11% (95%CI: 4.21%, 39.69%) for each PM2.5 exposure IQR decrease, on lag1, lag01, and lag02 (Figure 2).
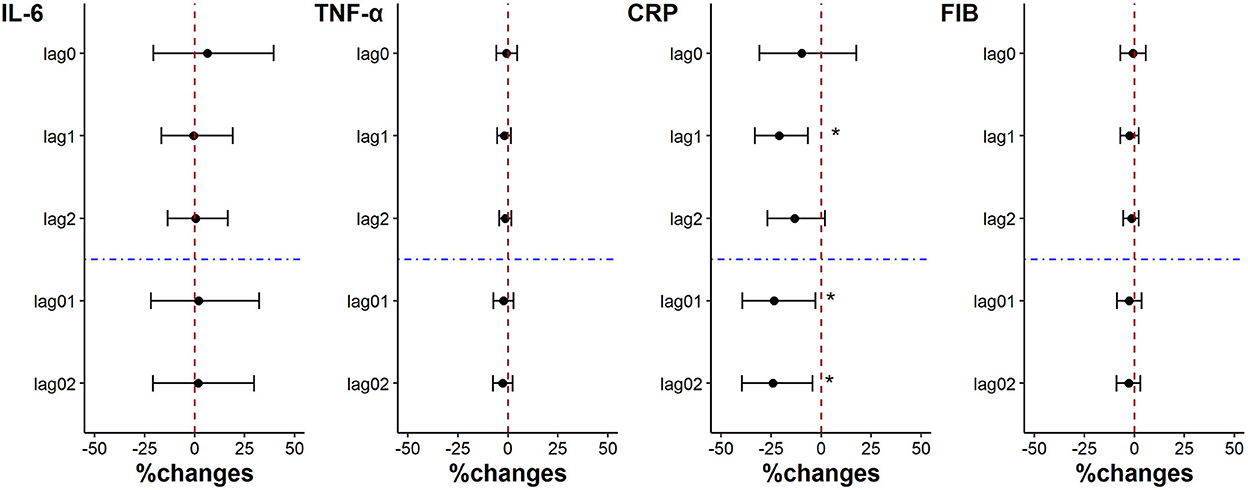
Figure 2. Percent changes in inflammation indicators associated with an IQR decrease in PM2.5 exposure. *p < 0.05.
3.6. Effects of PM2.5 reduction on coagulation indicators
There was no statistically significant change in coagulation indicators when the individual PM2.5 exposure was decreased by each IQR on lag0, lag1, lag2, lag01, and lag02. Except that ET-1 level was significantly reduced by 5.64% (95%CI: 0.83%, 10.23%) for each PM2.5 exposure IQR decrease on lag1 (Figure 3).
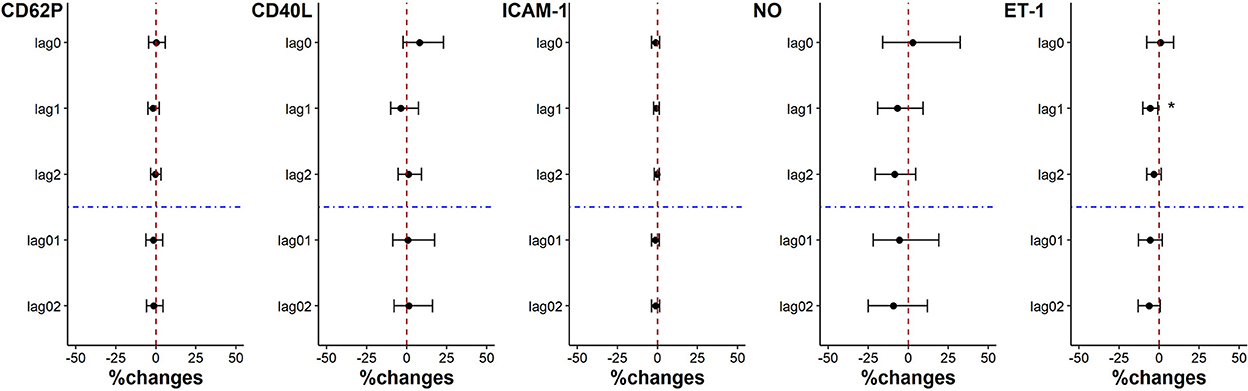
Figure 3. Percent changes in coagulation indicators associated with an IQR decrease in PM2.5 exposure. *p < 0.05.
3.7. Effects of PM2.5 reduction on plaque stability indicators
There was no significantly change in plaque stability indicators. And while the level of MMP-9 showed a downward trend for each IQR decrease of PM2.5 exposure on lag0, lag1, lag2, lag01 and lag02, the level of MMP-2 level showed an opposite trend (p > 0.05) (Figure 4).
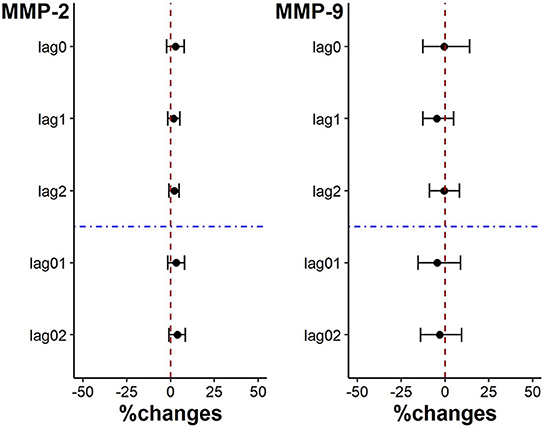
Figure 4. Percent changes in plaque stability indicators associated with an IQR decrease in PM2.5 exposure.
3.8. Effects of PM2.5 reduction on blood lipid indicators
The levels of CHO, HDL-C and LDL-C increased associated with each IQR decrease of PM2.5 exposure on lag0, lag1, lag2, lag01 and lag02. But only the increase of HDL-C was significant on lag0, lag1 and lag01, which was 5.10% (95%CI: 0.69%, 9.05%), 3.71% (95%CI: 0.92%, 6.60%) and 6.48% (95%CI: 2.58%, 10.24%) respectively. Although with no statistical significance, the level of AIP showed a decreasing trend on lag1 and lag01 (p = 0.06 and p = 0.07, respectively) with the decrease of PM2.5 exposure. There was no significant change in the level of TG (Figure 5).
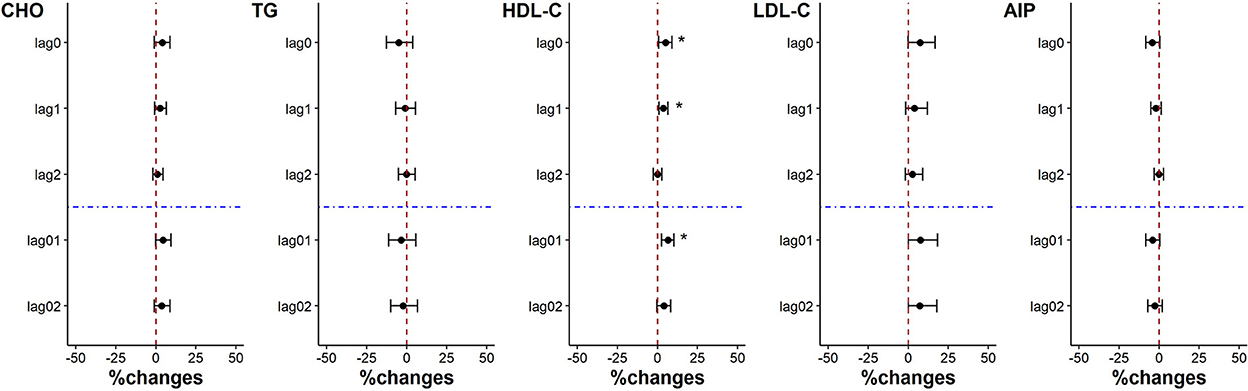
Figure 5. Percent changes in blood lipid indicators associated with an IQR decrease in PM2.5 exposure. *p < 0.05.
4. Discussion
Most of previous air purifiers intervention studies of the elderly did not consider the role of medication, leading to bias in results. In this study, the use of 6 conventional SCAD drugs (statins, β-blockers, Ca-channel blockers, ACEI/ARB, clopidogrel and aspirin) was combined and included into the linear mixed effect model in the form of one “effective drug” variable to analyze the influence of PM2.5 exposure on indicators, so as to establish a set of evaluation methods for the health protection effect of air purifier on patients with SCAD. And through a randomized crossover intervention study with small sample size, using the established evaluation method, it was found that the use of “true” air purifier to reduce the PM2.5 exposure level of patients with stable CAD had a certain protective effect on the levels of inflammatory indicator CRP and lipid indicator HDL-C under the control of the influence of drugs. But there were no significant effects on other inflammation and lipid indicators, or on coagulation and plaque stability indicators. It is suggested that CRP and HDL-C may be sensitive indicators of the acute effect of PM2.5 exposure on SCAD patients, and it is more worthy of attention in the air purifier intervention trials for stable CAD patients. Therefore, the method established in this study provided methodological reference for relevant studies, and the results of the intervention study broadened the understanding on health protection for patients with stable CAD using the air purifier, which has an important public health significance.
Inflammation is recognized as a key step in the development of CAD. Clinical studies have indicated that inflammation indicator CRP is a risk biomarker of atherosclerosis, which can mediate atherosclerosis at different stages (78). Elevated CRP level can predict the occurrence of cardiovascular events (79). In previous intervention studies of air purifiers for healthy adults, a significant association was found between the decrease of CRP and PM2.5 exposures levels (8, 9), confirming the health protection of air purifiers. However, no significant association has been found in studies for the elders, including those with cardiovascular disease (7). This study found that CRP level in patients with SCAD significantly decreased by 20.93 to 24.11% when the daily exposure to PM2.5 was reduced by 22.51 μg/m3 on day lag1, lag01, and lag02. After the interference of drug use was controlled, the air purifiers were found to have a protective effect on CRP levels of the subjects. In addition, the study revealed that the interpretation contribution rate of drug use to the analysis model corresponding to CRP was similar with that of PM2.5 exposure, which further illustrates the necessity of controlling the drug use of the subjects in the air purifier intervention studies for patients with SCAD and even patients with cardiovascular diseases who need to take drugs daily.
Blood lipid is one of the risk factors to be controlled in patients with CAD. The meta-analysis (14) showed that for each 10 μg/m3 increase in PM2.5 long-term exposure, the levels of CHO and LDL-C were increased significantly by 4.53 and 5.36%, respectively, with no significant change in HDL-C and TG levels. In a large cohort study conducted by Wu et al. (80) on middle-aged women (42–52 years old), it was found that the HDL-C level changed significantly by −0.7% when the average yearly concentration of PM2.5 was increased by 3 μg/m3, but the correlation between the average daily concentration of PM2.5 and HDL-C level was not statistically significant. At present, there are only a few population-based studies on the acute effects of PM2.5 exposure on blood lipid indicators, and there is no effective evidence to prove a significant correlation between PM2.5 exposure and changes in blood lipid indicators, which may be due to the failure to control the effects of drug use on blood lipid. In this study, it's showed that the interpretation contribution rate of drug use to the model of HDL-C was similar to that of PM2.5 exposure. And under controlling the drug effects, we analyzed the association between exposure to PM2.5 and acute effect of HDL-C in patients with SCAD. The AIP of the subjects in this study tended to improve with the decrease of PM2.5 exposure (p = 0.06 and 0.07), which proved, together with the results of HDL-C, that air purifiers used to reduce PM2.5 exposure might have a positive effect on the improvement of the quality of lipid-related indicators in patients with SCAD. These findings not only illustrate the necessity of considering drug use in relevant studies, but also provide experimental epidemiological evidence for the research of the association between PM2.5 exposure and acute effect of blood lipid indicators.
In the environmental health studies of SCAD patients, medications have a greater impact on cardiovascular indicators than environmental factors, so it's important to control their interference with the results. Stratified analyses by single kind of drug usage are usually used for this purpose (2). However, this stratified analysis is too simple considering that SCAD patients often require multiple drugs to control disease progression. The approach used in this study was to integrate multiple medications into an “effective drug” variable, which not only allows for a comprehensive consideration of single and combination drug use, but also reduces the sample size required for such studies.
The intervention study considered the influence of daily drug use on cardiovascular indicators of the patients with SCAD, and revealed the real protective effect of air purifiers on the patients with SCAD after reducing PM2.5 exposure level. However, compared with other air purifier intervention trials, the study is an experimental epidemiological study with small sample size, which may have limitation on the research results. The limitation had been noted, and individual measurements had been used to more precisely evaluated PM2.5 exposure levels of each study subject, which in turn could increase the accuracy of PM2.5 exposure-response evaluation results. In addition, the small sample size limits the stratified analysis for different drug use. If the sample size is expanded, the interference effect of various drug on the results of PM2.5 impacts can be analyzed in depth. When considering the influence of drug on cardiovascular indicators, only six kinds of conventional drug for coronary heart disease were controlled in the study. With the development and application of new drug, as well as the new discovery of the effect of drug on cardiovascular indicators, it will be necessary to conduct in-depth studies and to reveal the protective effect of air purifiers on people with cardiovascular diseases by expanding the sample size and increasing the control of the effects of other drug in the future.
5. Conclusions
In the evaluation method established in this study, the way to control the influence of drug use can provide methodological reference for future research in related fields, and the comprehensive analysis of the protective effects of air purifiers on cardiovascular system from multiple indicators of inflammation, coagulation, plaque stability and blood lipid can also provide scientific basis for screening sensitive indicators of air purification protection for patients with SCAD. In addition, through a randomized crossover intervention study with small sample size, the study found that the use of air purifier had a clear protective effect on patients with SCAD, in the case of daily routine medication management for disease management, and could significantly improve patients' inflammation indicator CRP and blood lipid indicator HDL-C. It shows that using air purifiers is one of the effective measures to protect the health of patients with SCAD in PM2.5 polluted weather.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The study was approved by the Ethics Committee of National Institute of Environmental Health, Chinese Centre for Disease Control and Prevention and all participants provided their written informed consent.
Author contributions
ZL, QW, NL, CX, YL, and DX: conceptualization and methodology. ZL and NL: project administration. ZL, NL, CX, YL, JZ, LL, HZ, YM, and FH: investigation. ZL and CX: formal analysis. ZL: visualization and writing–original draft. DX: writing–review and editing, supervision, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Program for key issues in Air Pollution Control (DQGG0405).
Acknowledgments
We thank the medical personnel and patients participating in the project. This manuscript was previously published as a preprint at: https://www.researchsquare.com/article/rs-234663/v1 (81).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1082327/full#supplementary-material
Abbreviations
SCAD, stable coronary artery disease; HEPA, high efficiency particle air filter; sCD40L, soluble CD40 ligand; ET-1, endothelin-1; CRP, C-reactive protein; FIB, fibrinogen; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; CD62P, cluster of differentiation 62 platelet; MMP-2, matrix metalloprotein-2; MMP-9, matrix metalloprotein-9; CHO, cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blockers; Ca-channel blockers, calcium-channel blockers; BMI, body mass index; ICAM-1, intercellular cell adhesion molecule-1; NO, nitric oxide; RH, relative humidity; AIP, atherogenic index of plasma; SD, standard deviation; IQR, interquartile range; NOS, Newcastle-Ottawa Scale; AIC, Akaike's Information Criterion; hsCRP, high-sensitivity C-reactive protein.
References
1. Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. (2010) 121:2331–78. doi: 10.1161/CIR.0b013e3181dbece1
2. Karottki DG, Spilak M, Frederiksen M, Gunnarsen L, Brauner EV, Kolarik B, et al. An indoor air filtration study in homes of elderly: cardiovascular and respiratory effects of exposure to particulate matter. Environ Health. (2013) 12:116–25. doi: 10.1186/1476-069X-12-116
3. Chu M, Dong W, Chi R, Pan L, Li H, Hu D, et al. Effects of high-efficiency particulate air purifiers on indoor fine particulate matter and its constituents in a district of Beijing during winter. J Peking Univ. (2018) 50:482–7. doi: 10.3969/j.issn.1671-167X.2018.03.015
4. Brauner EV, Forchhammer L, Moller P, Barregard L, Gunnarsen L, Afshari A, et al. Indoor particles affect vascular function in the aged: an air filtration-based intervention study. Am J Respir Crit Care Med. (2008) 177:419–25. doi: 10.1164/rccm.200704-632OC
5. Chen R, Zhao A, Chen H, Zhao Z, Cai J, Wang C, et al. Cardiopulmonary benefits of reducing indoor particles of outdoor origin: a randomized, double-blind crossover trial of air purifiers. J Am Coll Cardiol. (2015) 65:2279–87. doi: 10.1016/j.jacc.2015.03.553
6. Chen R, Li H, Cai J, Wang C, Lin Z, Liu C, et al. Fine particulate air pollution and the expression of microRNAs and circulating cytokines relevant to inflammation, coagulation, and vasoconstriction. Environ Health Perspect. (2018) 126:017007. doi: 10.1289/EHP1447
7. Kajbafzadeh M, Brauer M, Karlen B, Carlsten C, van Eeden S, Allen RW. The impacts of traffic-related and woodsmoke particulate matter on measures of cardiovascular health: a HEPA filter intervention study. Occup Environ Med. (2015) 72:394–400. doi: 10.1136/oemed-2014-102696
8. Allen RW, Carlsten C, Karlen B, Leckie S, van Eeden S, Vedal S, et al. An air filter intervention study of endothelial function among healthy adults in a woodsmoke-impacted community. Am J Respir Crit Care Med. (2011) 183:1222–30. doi: 10.1164/rccm.201010-1572OC
9. Chuang H, Ho K, Lin L, Chang T, Hong G, Ma C, et al. Long-term indoor air conditioner filtration and cardiovascular health: a randomized crossover intervention study. Environ Int. (2017) 106:91–6. doi: 10.1016/j.envint.2017.06.008
10. Lin L, Chuang H, Liu I, Chen H, Chuang K. Reducing indoor air pollution by air conditioning is associated with improvements in cardiovascular health among the general population. Sci Total Environ. (2013) 463–4:176–81. doi: 10.1016/j.scitotenv.2013.05.093
11. Wang Y, Zhao Y, Xue L, Wu S, Wang B, Li G, et al. Effects of air purification of indoor PM25 on the cardiorespiratory biomarkers in young healthy adults. Indoor air. (2021) 31:1125–33. doi: 10.1111/ina.12815
12. Shao D, Du Y, Liu S, Brunekreef B, Meliefste K, Zhao Q, et al. Cardiorespiratory responses of air filtration: a randomized crossover intervention trial in seniors living in Beijing: Beijing Indoor Air Purifier StudY, BIAPSY. Sci Total Environ. (2017) 603–4:541–9. doi: 10.1016/j.scitotenv.2017.06.095
13. Xu H, Wang T, Liu S, Brook RD, Feng B, Zhao Q, et al. Extreme levels of air pollution associated with changes in biomarkers of atherosclerotic plaque vulnerability and thrombogenicity in healthy adults. Circ Res. (2019) 124:e30–43. doi: 10.1161/CIRCRESAHA.118.313948
14. Liu F, Zhang X, Huang S, Gong H, Wang X, Huang K, et al. Adverse effects of air pollutant exposure on blood lipid levels in adults: a systematic review and meta-analysis. Cardiol Plus. (2020) 5:118–29. doi: 10.4103/cp.cp_18_20
15. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. (2013) 34:2949–3003. doi: 10.1093/eurheartj/eht296
16. Hu D. Guidelines and Consensus for Cardiovascular Disease Management 2010. Beijing: People's Medical Publishing House. (2010).
17. Expert Committee on Rational Drug Use of National Health and Family Planning Commission, Chinese Pharmacists Association. Guidelines for Rational Drug Use of Coronary Artery Disease (2nd Edition). Chin J Front Med Sci. (2018) 10:7–136. doi: 10.12037/YXQY.2018.06-01
18. Vukovic PM, Maravic-Stojkovic VR, Peric MS, Jovic MD, Cirkovic MV, Gradinac SD, et al. Steroids and statins: an old and a new anti-inflammatory strategy compared. Perfusion. (2010) 26:31–7. doi: 10.1177/0267659110385607
19. Ascer E, Bertolami MC, Venturinelli ML, Buccheri V, Souza J, Nicolau JC, et al. Atorvastatin reduces proinflammatory markers in hypercholesterolemic patients. Atherosclerosis. (2004) 177:161–6. doi: 10.1016/j.atherosclerosis.2004.07.003
20. Gómez-García A, Torres GM, Ortega-Pierres LE, Rodríguez-Ayala E, Alvarez-Aguilar C. Rosuvastatin and metformin decrease inflammation and oxidative stress in patients with hypertension and dyslipidemia. Rev Esp Cardiol. (2007) 60:1242–9. doi: 10.1157/13113929
21. Kinlav S, Rifai N, Libby P, Ganz P. Effect of atorvastatin on C-reactive protein in patients with acute coronary syndromes: a substudy of the MIRACL trial. J Am Coll Cardiol. (2002) 39:304. doi: 10.1016/S0735-1097(02)81367-5
22. Riesen WF, Engler H, Risch M, Korte W, Noseda G. Short-term effects of atorvastatin on C-reactive protein. Eur Heart J. (2002) 23:794–9. doi: 10.1053/euhj.2001.2967
23. Seljeflot I, Tonstad S, Hjermann I, Arnesen H. Reduced expression of endothelial cell markers after 1 year treatment with simvastatin and atorvastatin in patients with coronary heart disease. Atherosclerosis. (2002) 162:179–85. doi: 10.1016/S0021-9150(01)00696-7
24. Barale C, Frascaroli C, Senkeev R, Cavalot F, Russo I. Simvastatin effects on inflammation and platelet activation markers in hypercholesterolemia. Biomed Res Int. (2018) 2018:1–11. doi: 10.1155/2018/6508709
25. Chu C, Lee K, Lee M, Su H, Voon W, Sheu S, et al. Effects of atorvastatin and atorvastatin withdrawal on soluble CD40L and adipocytokines in patients with hypercholesterolaemia. Acta Cardiol. (2006) 61:263–9. doi: 10.2143/AC.61.3.2014826
26. Li J, Fang C, Wang C, Hui R. Effects of simvastatin on exercise-induced myocardial ischemia and plasma endothelin-1 concentrations in patients with stable angina. Clin Chim Acta. (2005) 354:205–8. doi: 10.1016/j.cccn.2004.10.012
27. Glorioso N, Troffa C, Filigheddu F, Dettori F, Soro A, Parpaglia P, et al. Effect of the HMG-CoA reductase inhibitors on blood pressure in patients with essential hypertension and primary hypercholesterolemia. Hypertension. (1999) 34:1281–6. doi: 10.1161/01.HYP.34.6.1281
28. Atalar E, Ozmen F, Haznedaroglu I, Açil T, Ozer N, Ovünç K, et al. Effects of short-term atorvastatin treatment on global fibrinolytic capacity, and sL-selectin and sFas levels in hyperlipidemic patients with coronary artery disease. Int J Cardiol. (2002) 84:227–31. doi: 10.1016/S0167-5273(02)00148-1
29. Cortellaro M, Cofrancesco E, Boschetti C, Cortellaro F, Mancini M, Mariani M, et al. Effects of fluvastatin and bezafibrate combination on plasma fibrinogen, t-plasminogen activator inhibitor and C reactive protein levels in coronary artery disease patients with mixed hyperlipidaemia (FACT study). Thromb Haemost. (2000) 83:549–53. doi: 10.1055/s-0037-1613861
30. Qu H, Xiao Y, Jiang G, Wang Z, Zhang Y, Zhang M. Effect of atorvastatin versus rosuvastatin on levels of serum lipids, inflammatory markers and adiponectin in patients with hypercholesterolemia. Pharm Res. (2009) 26:958–64. doi: 10.1007/s11095-008-9798-6
31. Koh KK, Son JW, Ahn JY, Choi YM, Jin DK, Park GS, et al. Non-lipid effects of statin on hypercholesterolemic patients established to have coronary artery disease who remained hypercholesterolemic while eating a step-II diet. Coron Artery Dis. (2001) 12:305–11. doi: 10.1097/00019501-200106000-00006
32. Koh KK, Son JW, Ahn JY, Jin DK, Kim HS, Choi YM, et al. Comparative effects of diet and statin on NO bioactivity and matrix metalloproteinases in hypercholesterolemic patients with coronary artery disease. Arterioscler Thromb Vasc Biol. (2002) 22:e19–23. doi: 10.1161/01.ATV.0000030997.02059.BB
33. Tousoulis D, Andreou I, Tentolouris C, Antoniades C, Papageorgiou N, Gounari P, et al. Comparative effects of rosuvastatin and allopurinol on circulating levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with chronic heart failure. Int J Cardiol. (2010) 145:438–43. doi: 10.1016/j.ijcard.2009.05.051
34. Fujiwara T, Saito S, Osanai T, Kameda K, Abe N, Higuma T, et al. Decreased plasma and cardiac matrix metalloproteinase activities in patients with coronary artery disease and treated with pravastatin. Eur J Pharmacol. (2008) 594:146–51. doi: 10.1016/j.ejphar.2008.07.039
35. Koh KK, Ahn JY, Jin DK, Han SH, Kim HS, Choi IS, et al. Comparative effects of statin and fibrate on nitric oxide bioactivity and matrix metalloproteinase in hyperlipidemia. Int J Cardiol. (2004) 97:239–44. doi: 10.1016/j.ijcard.2003.09.007
36. Karalis DG, Ross AM, Vacari RM, Zarren H, Scott R. Comparison of efficacy and safety of atorvastatin and simvastatin in patients with dyslipidemia with and without coronary heart disease. Am J Cardiol. (2002) 89:667–71. doi: 10.1016/S0002-9149(01)02337-2
37. Li Z, Zhu S, Huang L, Tian Y, Yu L, Zhou Y, et al. Therapeutic effects of metoprolol on ventricular remodeling and proinflammatory cytokines in patients with chronic heart failure. Chin Heart J. (2005) 17:517–20. doi: 10.3969/j.issn.1009-7236.2005.06.005
38. Ohtsuka T, Hamada M, Hiasa G, Sasaki O, Suzuki M, Hara Y, et al. Effect of beta-blockers on circulating levels of inflammatory and anti-inflammatory cytokines in patients with dilated cardiomyopathy. J Am Coll Cardiol. (2001) 37:412–7. doi: 10.1016/S0735-1097(00)01121-9
39. Doo YC, Kim DM, Oh DJ, Ryu KH, Rhim CY, Lee Y. Effect of beta blockers on expression of interleukin-6 and C-reactive protein in patients with unstable angina pectoris. Am J Cardiol. (2001) 88:422–4. doi: 10.1016/S0002-9149(01)01693-9
40. Rizos E, Bairaktari E, Kostoula A, Hasiotis G, Achimastos A, Ganotakis E, et al. The combination of nebivolol plus pravastatin is associated with a more beneficial metabolic profile compared to that of atenolol plus pravastatin in hypertensive patients with dyslipidemia: a pilot study. J Cardiovasc Pharmacol Ther. (2003) 8:127–34. doi: 10.1177/107424840300800206
41. Seljeflot I, Arnesen H, Andersen P, Aspelin T, Kierulf P. Effects of doxazosin and atenolol on circulating endothelin-1 and von Willebrand factor in hypertensive middle-aged men. J Cardiovasc Pharmacol. (1999) 34:584–8. doi: 10.1097/00005344-199910000-00016
42. Dong Y, Yang D. Efficacy of carvedilol and metoprolol for treatment of primary hypertension and their effects on vascular endothelial function. Zhejiang Med J. (2012) 34:904–6. doi: 10.3969/j.issn.1006-2785.2012.11.016
43. Makris TK, Stavroulakis GA, Krespi PG, Hatzizacharias AN, Triposkiadis FK, Tsoukala CG, et al. Fibrinolytic/hemostatic variables in arterial hypertension: response to treatment with irbesartan or atenolol. Am J Hypertens. (2000) 13:783–8. doi: 10.1016/S0895-7061(00)00262-4
44. Herrmann JM, Mayer EO. A long-term study of the effects of celiprolol on blood pressure and lipid-associated risk factors. Am Heart J. (1988) 116:1416–21. doi: 10.1016/0002-8703(88)90133-0
45. Fallois JV, Faulhaber HD. Nebivolol, a beta blocker of the 3rd generation: modern therapy of arterial hypertension. Results of a multicenter observation study. Praxis. (2001) 90:435–41.
46. Lacourcière Y, Poirier L, Lefebvre J, Provencher P, Arnott W. Comparative effects of a new cardioselective beta-blocker nebivolol and nifedipine sustained-release on 24-hour ambulatory blood pressure and plasma lipoproteins. J Clin Pharmacol. (1992) 32:660–6. doi: 10.1002/j.1552-4604.1992.tb05778.x
47. Liu W, Shimada M, Xiao J, Hu D, Matsumori A. Nifedipine inhibits the activation of inflammatory and immune reactions in viral myocarditis. Life Sci. (2009) 85:235–40. doi: 10.1016/j.lfs.2009.05.018
48. Derosa G, Mugellini A, Pesce RM, D'Angelo A, Maffioli P. Perindopril and barnidipine alone or combined with simvastatin on hepatic steatosis and inflammatory parameters in hypertensive patients. Eur J Pharmacol. (2015) 766:31–6. doi: 10.1016/j.ejphar.2015.09.030
49. Jiang S, Wang Y. Clinical efficacy of atorvastatin calcium combined with nifedipine sustained-release tablets in the treatment of hypertension and its effect on TNF-α, CRP, IL-4 and IL-10. Chin J Integ Med Cardio/Cerebrovasc Dis. (2019) 17:555–7. doi: 10.12102/j.issn.1672-1349.2019.04.020
50. Martinez MLL, Lopes LF, Coelho EB, Nobre F, Rocha JBT, Gerlach RF, et al. Lercanidipine reduces matrix metalloproteinase-9 activity in patients with hypertension. J Cardiovasc Pharmacol. (2006) 47:117–22. doi: 10.1097/01.fjc.0000196241.96759.71
51. Derosa G, Mugellini A, Pesce RM, D'Angelo A, Maffioli P. Barnidipine compared to lercanidipine in addition to losartan on endothelial damage and oxidative stress parameters in patients with hypertension and type 2 diabetes mellitus. BMC Cardiovasc Disord. (2016) 16:66–72. doi: 10.1186/s12872-016-0237-z
52. Chen Y, Xu F, Zhang Y, Ji Q, Sun Y, Lü R, et al. Effect of aspirin plus clopidogrel on inflammatory markers in patients with non-ST-segment elevation acute coronary syndrome. Chin Med J. (2006) 119:32–6. doi: 10.1097/00029330-200601010-00006
53. Solheim S, Pettersen AA, Arnesen H, Seljeflot I. No difference in the effects of clopidogrel and aspirin on inflammatory markers in patients with coronary heart disease. Thromb Haemost. (2006) 96:660–4. doi: 10.1160/TH06-06-0337
54. Heitzer T, Rudolph V, Schwedhelm E, Karstens M, Sydow K, Ortak M, et al. Clopidogrel improves systemic endothelial nitric oxide bioavailability in patients with coronary artery disease: evidence for antioxidant and antiinflammatory effects. Arterioscler Thromb Vasc Biol. (2006) 26:1648–52. doi: 10.1161/01.ATV.0000225288.74170.dc
55. Azar RR, Kassab R, Zoghbi A, Aboujaoude S, El-Osta H, Ghorra P, et al. Effects of clopidogrel on soluble CD40 ligand and on high-sensitivity C-reactive protein in patients with stable coronary artery disease. Am Heart J. (2006) 151:521.e1–e4. doi: 10.1016/j.ahj.2005.10.021
56. Klinkhardt U, Bauersachs R, Adams J, Graff J, Lindhoff-Last E, Harder S. Clopidogrel but not aspirin reduces P-selectin expression and formation of platelet-leukocyte aggregates in patients with atherosclerotic vascular disease. Clin Pharmacol Ther. (2003) 73:232–41. doi: 10.1067/mcp.2003.13
57. Xiao Z, Theroux P. Clopidogrel inhibits platelet-leukocyte interactions and thrombin receptor agonist peptide-induced platelet activation in patients with an acute coronary syndrome. J Am Coll Cardiol. (2004) 43:1982–8. doi: 10.1016/j.jacc.2003.10.071
58. Xu E, Chen X. The effect of clopidogrel on serum metalloproteinase-2, 3, 9 levels in patients with unstable angina and its clinical efficacy. J Radioimmunol. (2013) 26:801–3. doi: 10.3969/j.issn.1008-9810.2013.06.054
59. Zhang Y, Ding G. Effect and curative observation of Clopidogrel on serum matrix metalloproteinase-3, 8 and 9 of cerebral ischemic stroke patients. China Med Herald. (2013) 10:76–8. doi: 10.3969/j.issn.1673-7210.2013.24.025
60. Zhang X, Sun D, Wang W, Lin L. The effect of clopidogrel on serum matrix metalloproteinase-9 and its inhibitor-1 in patients with ischemic stroke. China Mod Doctor. (2013) 51:76–8.
61. Korish AA. Clopidogrel prophylaxis abates myocardial ischemic injury and inhibits the hyperlipidemia-inflammation loop in hypercholestrolemic mice. Arch Med Res. (2020) 51:515–23. doi: 10.1016/j.arcmed.2020.05.003
62. Ikonomidis I, Andreotti F, Economou E, Stefanadis C, Toutouzas P, Nihoyannopoulos P. Increased proinflammatory cytokines in patients with chronic stable angina and their reduction by aspirin. Circulation. (1999) 100:793–8. doi: 10.1161/01.CIR.100.8.793
63. Wang Y, Shi Y, Li S, Xu J, Li J. Effects of clopidogrel and aspirin on the serum levels of hs-CRP, TNF-α and IL-6 in patients with coronary heart disease and angina pectoris. Prog Mod Biomed. (2017) 17:327–30. doi: 10.13241/j.cnki.pmb.2017.02.032
64. Yin Y. Effect of aspirin combined with clopidogrel on serum levels of hs-CRP, TNF- alpha and IL-6 in patients with coronary heart disease and angina pectoris. Anat Res. (2018) 40:178–81.
65. Nadar S, Blann AD, Lip GY. Effects of aspirin on intra-platelet vascular endothelial growth factor, angiopoietin-1, and p-selectin levels in hypertensive patients. Am J Hypertens. (2006) 19:970–8. doi: 10.1016/j.amjhyper.2006.03.001
66. Hou L, Chi C, Yang Z, Wei L. Correlated Study of Aspirin Resistance and P-selection in Platelet in Patients with Coronary Heart Disease. Pract J Card Cereb Pneumal Vasc Dis. (2010) 18:740–1. doi: 10.3969/j.issn.1008-5971.2010.06.023
67. Zhai G, Zhu H, Qu B, Cai J. Effect of low-dosage aspirin on vascular endothelial function of angina patients. J Fourth Mil Med Univ. (2005) 26:2175–7. doi: 10.3321/j.issn:1000-2790.2005.23.020
68. Hetzel S, DeMets D, Schneider R, Borzak S, Schneider W, Serebruany V, et al. Aspirin increases nitric oxide formation in chronic stable coronary disease. J Cardiovasc Pharmacol Ther. (2013) 18:217–21. doi: 10.1177/1074248413482753
69. Su K. Effect of combination of aspirin and atorvastatin on the concentration of serum sCD40L, MMP?2 in unstable angina pectoris patients. J Trop Med. (2017) 17:226–9. doi: 10.3969/j.issn.1672-3619.2017.02.024
70. Hua Y, Xue J, Sun F, Zhu L, Xie M. Aspirin inhibits MMP-2 and MMP-9 expressions and activities through upregulation of PPARalpha/gamma and TIMP gene expressions in ox-LDL-stimulated macrophages derived from human monocytes. Pharmacology. (2009) 83:18–25. doi: 10.1159/000166183
71. Schieffer B, Bünte C, Witte J, Hoeper K, Böger RH, Schwedhelm E, et al. Comparative effects of AT1-antagonism and angiotensin-converting enzyme inhibition on markers of inflammation and platelet aggregation in patients with coronary artery disease. J Am Coll Cardiol. (2004) 44:362–8. doi: 10.1016/j.jacc.2004.03.065
72. Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, et al. Angiotensin II type 1 receptor antagonist decreases plasma levels of tumor necrosis factor alpha, interleukin-6 and soluble adhesion molecules in patients with chronic heart failure. J Am Coll Cardiol. (2000) 35:714–21. doi: 10.1016/S0735-1097(99)00594-X
73. Cottone S, Vadala A, Vella M, Nardi E, Mulé G, Contorno A, et al. Changes of plasma endothelin and growth factor levels, and of left ventricular mass, after chronic AT1-receptor blockade in human hypertension. Am J Hypertens. (1998) 11:548–53. doi: 10.1016/S0895-7061(98)00027-2
74. Lopez Santi RG, Valeff EC, Duymovich CR, Mazziotta D, Mijailovsky NE, Filippa GC, et al. Effects of an angiotensin-converting enzyme inhibitor (ramipril) on inflammatory markers in secondary prevention patients: RAICES Study. Coron Artery Dis. (2005) 16:423–9. doi: 10.1097/00019501-200510000-00002
75. Li D, Feng Y, Zhang H, Bai Z. Inhibition of early atherogenesis by ramipril and losartan in male rats with diet-induced hypercholesterolemia. J Clin Cardiol. (2003) 19:280–2. doi: 10.3969/j.issn.1001-1439.2003.05.011
76. Fogari R, Zoppi A, Lazzari P, Preti P, Mugellini A, Corradi L, et al. ACE inhibition but not angiotensin II antagonism reduces plasma fibrinogen and insulin resistance in overweight hypertensive patients. J Cardiovasc Pharmacol. (1998) 32:616–20. doi: 10.1097/00005344-199810000-00014
77. Onal IK, Altun B, Onal ED, Kirkpantur A, Oz SG, Turgan C. Serum levels of MMP-9 and TIMP-1 in primary hypertension and effect of antihypertensive treatment. Eur J Intern Med. (2009) 20:369–72. doi: 10.1016/j.ejim.2008.10.003
78. Oh J, Teoh H, Leiter LA. Should C-reactive protein be a target of therapy? Diabetes Care. (2011) 34 Suppl 2:S155–60. doi: 10.2337/dc11-s211
79. Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. (2000) 321:199–204. doi: 10.1136/bmj.321.7255.199
80. Wu X, Broadwin R, Basu R, Malig B, Ebisu K, Gold EB, et al. Associations between fine particulate matter and changes in lipids/lipoproteins among midlife women. Sci Total Environ. (2019) 654:1179–86. doi: 10.1016/j.scitotenv.2018.11.149
Keywords: air purifier, intervention trial, PM2.5, SCAD, drug use, health protection
Citation: Liu Z, Wang Q, Li N, Xu C, Li Y, Zhou J, Liu L, Zhang H, Mo Y, Han F and Xu D (2023) Cardiovascular benefits of air purifier in patients with stable coronary artery disease: A randomized single-blind crossover study. Front. Public Health 10:1082327. doi: 10.3389/fpubh.2022.1082327
Received: 28 October 2022; Accepted: 09 December 2022;
Published: 09 January 2023.
Edited by:
Ang Li, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Jie Song, Xinxiang Medical University, ChinaRenjie Chen, Fudan University, China
Wei Wei Xing, Academy of Military Medical Sciences, China
Copyright © 2023 Liu, Wang, Li, Xu, Li, Zhou, Liu, Zhang, Mo, Han and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongqun Xu,  eHVkcUBjaGluYWNkYy5jbg==
eHVkcUBjaGluYWNkYy5jbg==
 Zhe Liu
Zhe Liu Qin Wang1
Qin Wang1 Feng Han
Feng Han