
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 02 December 2022
Sec. Public Health and Nutrition
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.1079543
This article is part of the Research Topic Assessment Methods in Human Nutrition and Metabolism for the Monitoring of Non-Communicable Chronic Diseases View all 16 articles
 Xin Yin1
Xin Yin1 Gillianne Geet Yi Lai2,3
Gillianne Geet Yi Lai2,3 Adeline Seow1,4
Adeline Seow1,4 Daniel Shao Weng Tan2,3
Daniel Shao Weng Tan2,3 Darren Wan-Teck Lim2,3*†
Darren Wan-Teck Lim2,3*† Wei Jie Seow1,4*†
Wei Jie Seow1,4*†Background: Previous studies have reported differential associations of certain dietary factors such as soy consumption by epidermal growth factor receptor mutant (EGFR +) subtype of non-small cell lung cancer (NSCLC). However, whether the other dietary factors including meat, fruits, and vegetables have differential risks on different histological and molecular subtypes of lung cancer remains unclear. Therefore, we conducted a case-control study to evaluate these associations.
Methods: A total of 3,170 cases and 4,238 controls from three different studies (Genes and Environment in Lung Cancer Study, Lung Cancer Consortium Singapore Study, and Multi-ethnic Cohort Study) were included. Information on demographics, lifestyle, and dietary consumption was obtained using questionnaires. Diet was assessed by using the number of standard servings of each item consumed per week. Multivariable logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the association between meat, vegetables, and fruits consumption with lung cancer risk after adjusting for potential confounders.
Results: We identified a significant inverse association between higher consumption of fruits and the risk of lung cancer (2nd tertile: OR = 0.54, 95%CI = 0.46–0.65; 3rd tertile: OR = 0.77, 95%CI = 0.65–0.91), compared with the lower (1st tertile) consumption of fruits. Higher vegetable consumption was significantly associated with a lower risk of EGFR + lung cancer (OR = 0.69, 95% CI = 0.54–0.88), however, this association was not significant among EGFR wild-type (−) lung cancer. Conversely, higher consumption of total meat (OR = 2.10, 95%CI = 1.58–2.79) was significantly associated with higher lung cancer risk, as compared with the lower consumption group.
Conclusions: Differential associations between vegetable consumption with EGFR mutation status in NSCLC were found. Further prospective studies are warranted to assess this association and elucidate the biological mechanisms.
Lung cancer is the leading cause of cancer death and disability-adjusted life-years (DALYs) worldwide (1–3). In 2019, there were an estimated 2.26 million incident cases of lung cancer and 2.04 million deaths that occurred globally, accounting for 45.9 million DALYs (3). Non–small cell lung cancer (NSCLC), including adenocarcinoma, squamous cell carcinoma, and large cell carcinoma histologic subtypes, accounted for approximately more than 80% of lung cancers (4). Different histological types of lung cancer have different age and sex distribution, smoking status, clinical performance status, biological pathways, and overall survival rate (5, 6). Epidermal growth factor receptor (EGFR), a transmembrane protein with tyrosine kinase activity, is one of the most well-documented and investigated pathways in NSCLC (7), and has been identified as an oncogenic driver, playing an important role in regulating the proliferation, survival, and differentiation of tumor cells (7, 8). EGFR mutations are found in approximately 60% of never-smoking Asian patients with adenocarcinomas compared to 5–10% in Caucasians, and thus represent a significant proportion of NSCLC in our local context (9, 10).
The associations between dietary factors and lung cancer risk have been explored by previous studies. A healthy dietary pattern was associated with a lower risk of lung cancer (11). For example, fruits and vegetables are a rich source of vitamin C, vitamin E, carotenoids, and other micronutrients, which are previously reported to have a protective association with the risk of lung cancer and other cancers (12). A meta-analysis showed that the highest consumption group of fruits and vegetables was inversely associated with the risk of lung cancer, as compared with the lowest consumption group (13). In contrast, the literature on the association between meats and lung cancer was conflicting. Some studies suggested that red meat and processed meat were both positively associated with the risk of lung cancer (14, 15), especially among never-smokers (16, 17). However, other studies revealed either a null association or a statistically significant inverse association between meat and the risk of lung cancer (18, 19). When stratified by the types of meat, a meta-analysis demonstrated an inverse association between poultry consumption and lung cancer, based on 11 studies, but not for total white meat or fish (16). A similar trend was identified among never-smokers; higher consumption of red meat was found to be associated with an increased risk of lung cancer, and no significant associations were observed between other types of meat and lung cancer risk (20).
Associations between dietary factors and lung cancer risk have been shown to vary by histological and molecular subtypes. Some studies demonstrated that when stratified by histological subgroups of lung cancer, including adenocarcinoma, squamous cell carcinoma, and large cell carcinoma, the above-mentioned positive or inverse associations became statistically insignificant (13, 14). A previous study in Japan has reported differential associations of soy consumption by EGFR lung cancer subtypes (21); the protective effect of soybean products was found only among EGFR mutated lung cancer. Another study demonstrated that an alkaline diet prolonged overall survival among NSCLC patients with EGFR mutations (22). Furthermore, anthocyanidin extracted from fruits and vegetables was identified as an effective inhibitor of EGFR mutated cancers (23), and a low-protein diet combined with an EGFR inhibitor was reported to be a promising cancer therapy method (24). These studies demonstrated the potential differential associations between some dietary factors and EGFR lung cancer subtypes. EGFR can be abnormally activated by various mechanisms, and constitutive EGFR tyrosine kinase activation caused by mutations in the tyrosine kinase binding pocket is one of the key targets of specific small molecule inhibitors (25). EGFR tyrosine kinase inhibitors have been found to significantly improve outcomes in patients with advanced NSCLC that contain an activating EGFR mutation compared with platinum-based chemotherapy (26–29). However, whether other dietary factors are differentially associated with different histological and molecular subtypes of lung cancer remains unclear (13), particularly among the Asian population (21).
In this study, we evaluated the association between the consumption of meats, vegetables, and fruits with the risk of lung cancer by histological and molecular subtypes among Asians.
A total of three studies were included: the Genes and Environment in Lung Cancer (GEL) Study (case-control), Lung Cancer Consortium Singapore (LCCS) Study (case-only), and the Multi-ethnic Cohort Study (MEC) study (cohort). The LCCS is a case-only study of lung cancer with clinical data from three hospitals, including Singapore General Hospital (SGH), Changi General Hospital (CGH), and the National Cancer Center Singapore (NCCS). A total of 3,245 lung cancer patients, including 1,252 females and 1,993 males, with a diagnosis mean age of 63.4 years were included in the LCCS study between 2007 and 2017 (30).
The GEL study is a hospital-based case-control study of 815 controls and 399 cases recruited from 2005 to 2008, from Singapore public hospitals, including SGH, CGH, National University Hospital (NUH), and Tan Tock Seng Hospital (TTSH) (31, 32). Controls and cases were recruited from the same hospitals and frequency-matched by 10-year age groups. Controls were selected within one month after the date of diagnosis of the corresponding cases.
The MEC is a cohort study that was formed by combining two existing population-based studies, the Singapore Prospective Study Program (SP2) and the Singapore Cardiovascular Cohort Study (SCCS2), with additional recruitment of participants from 2007 to 2010 (33). The baseline of the MEC study recruited 13,777 participants. After excluding those who have been diagnosed with cancer at the baseline, a total of 13,149 cancer-free controls were included.
Lung cancer subtypes were extracted from medical records. Lung tumor tissues from the LCCS study were tested for their EGFR mutation status (mutation/+, or wildtype/-) using direct Sanger sequencing, or the real-time polymerase chain reaction (PCR) test. All EGFR tests were done at the Singapore General Hospital. Lung cancer cases in this current study were obtained from the LCCS and GEL studies. Healthy controls were obtained from the GEL and MEC baseline studies. Therefore, a total of 3,644 lung cancer cases and 13,964 controls were included in our study.
This current study of using three datasets was approved by the National University of Singapore Institutional Review Board (NUS-IRB Ref: N-20-053E). GEL study and MEC study (NUS-IRB Ref: 04-044; NUS-IRB Ref: 12–140 and CIRB Ref: 2001/001/C) were approved by the Institutional Review Board of the National University of Singapore and SingHealth Centralized Institutional Review Board (CIRB), and all participants gave informed consent prior to their participation. For the LCCS study, written informed consent was obtained from all patients and the study was approved by the SingHealth CIRB (CIRB Ref: 2018/2963).
Similar semi-quantitative Food Frequency Questionnaires (FFQ) were used in all three studies. For each study, consumption frequency and standard portion size were collected, and pictures of each item portion were used during the interview. Consumption frequency was converted into average frequency per week, and the portion size was converted into the number of standard servings. The average frequency per week and number of standard servings were multiplied to obtain the number of standard servings consumed per week (Supplementary Table S1). Fresh fruit consumption was the summed weekly consumption of fresh fruits. Vegetable consumption was defined as the sum of green, leafy, and other vegetables. Fish, chicken/poultry, pork/other meat, and preserved meat intake were summed as total meat consumption. Preserved meat was summed weekly consumption of bacon, ham, luncheon meat (canned), and sausages (Supplementary Table S2). The tertile cut-off values were chosen based on the consumption among the controls. Total energy intake per week was calculated based on the energy and nutrient composition of food by the Health Promotion Board (HPB) Singapore (34) (Supplementary Table S3). To reduce information bias, we calculated the total energy intake of participants and excluded outliers to improve the robustness of our study. Outliers were defined as those with a total energy intake < 2.5th or higher than 97.5th centiles (35).
All covariates were collected in the questionnaire and adjusted in all logistic models, including sex (male vs. female), age (years, continuous), ethnicity, educational level, family history of lung cancer, smoking status, body mass index (BMI), and total energy intake (kcal, continuous). Smoking status was divided into never and ever smokers. To avoid residual confounding by smoking, ever smokers were further categorized as smoking duration <20 years, 20–40 years, and ≥40 years. Ethnicity was categorized as Chinese, Malay, Indian and others. Body mass index (BMI, kg/m2) was categorized as underweight (< 18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (≥ 30 kg/m2). Educational level was categorized as 0 years, ≤ 6 years, and >6 years of education. Family history of lung cancer was categorized as no family history of any cancers, family history of lung cancer, and family history of other cancers.
Differences in baseline characteristics between the cases and controls were assessed using the t-test or Wilcoxon rank test based on the normality distribution for continuous variables, and Fisher's exact test for categorical variables. The multivariable logistic regression model was used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) of the association between meat, vegetables, and fruits consumption with lung cancer risk.
In order to reduce the potential selection bias from the age difference between cases and controls, a sensitivity analysis was conducted to match cases and controls using the propensity score nearest neighbor matching (1:1 matching by age, no replacement) (36). The matching caliper width was set as 0.2 as suggested in the previous studies (37, 38). The conditional logistic regression was performed in the sensitivity analysis for matched cases and controls to estimate the ORs and 95% CIs.
Stratification analyses by smoking status, different subtypes of lung cancer (non-small cell lung cancer, adenocarcinoma, squamous cell carcinoma), and EGFR status were also conducted. We also did a further subgroup analysis among non-smoking Chinese females as they are at a higher risk of EGFR-positive lung cancer (10, 39). All statistical tests were conducted as two-sided, and a P-value < 0.05 was considered as being statistically significant. All analyses were performed in Stata 16.1 (Stata Corporation, College Station, Texas, USA).
A total of 3,644 lung cancer cases and 13,964 controls were included. After excluding participants with missing information, 3,170 cases and 4,238 controls were included in the final analysis. As shown in Table 1, among cases with known EGFR status, EGFR mutation (EGFR+) was detected in 1,084 (57.29%) lung cancer cases, and EGFR wildtype (EGFR-) was detected in 808 cases (42.71%). Non-small cell lung cancer accounted for about 87.98% (2,789 cases) of the total lung cancer cases, of which the majority (2,242 cases, 80.39%) were adenocarcinoma. Compared with controls, lung cancer cases were significantly older and more likely to be males, have a family history of lung cancer, have lower educational levels, and lower BMI.
We found a significant inverse association between high fruit consumption (3rd tertile) and the risk of lung cancer (OR = 0.77, 95% CI = 0.65–0.91), as compared to low fruit consumption (1st tertile) (Table 2). Significant inverse associations were also observed among EGFR+ (OR = 077, 95% CI = 0.61–0.96) and EGFR- lung cancer (OR = 0.72, 95% CI = 0.55–0.94). For total vegetable consumption, as compared to low vegetable consumption (1st tertile), although the third tertile did not reach statistical significance (ORs = 0.85, 95% CI = 0.71–1.02), a significantly lower risk of lung cancer was observed among those with median consumption of vegetables, with an OR of 0.77 (95% CI = 0.65–0.91). A similar trend was observed in both EGFR + lung cancer and EGFR–lung cancer, however, the high consumption of total vegetables was statistically significant only among EGFR+ lung cancer (OR = 0.69, 95% CI = 0.54–0.88).
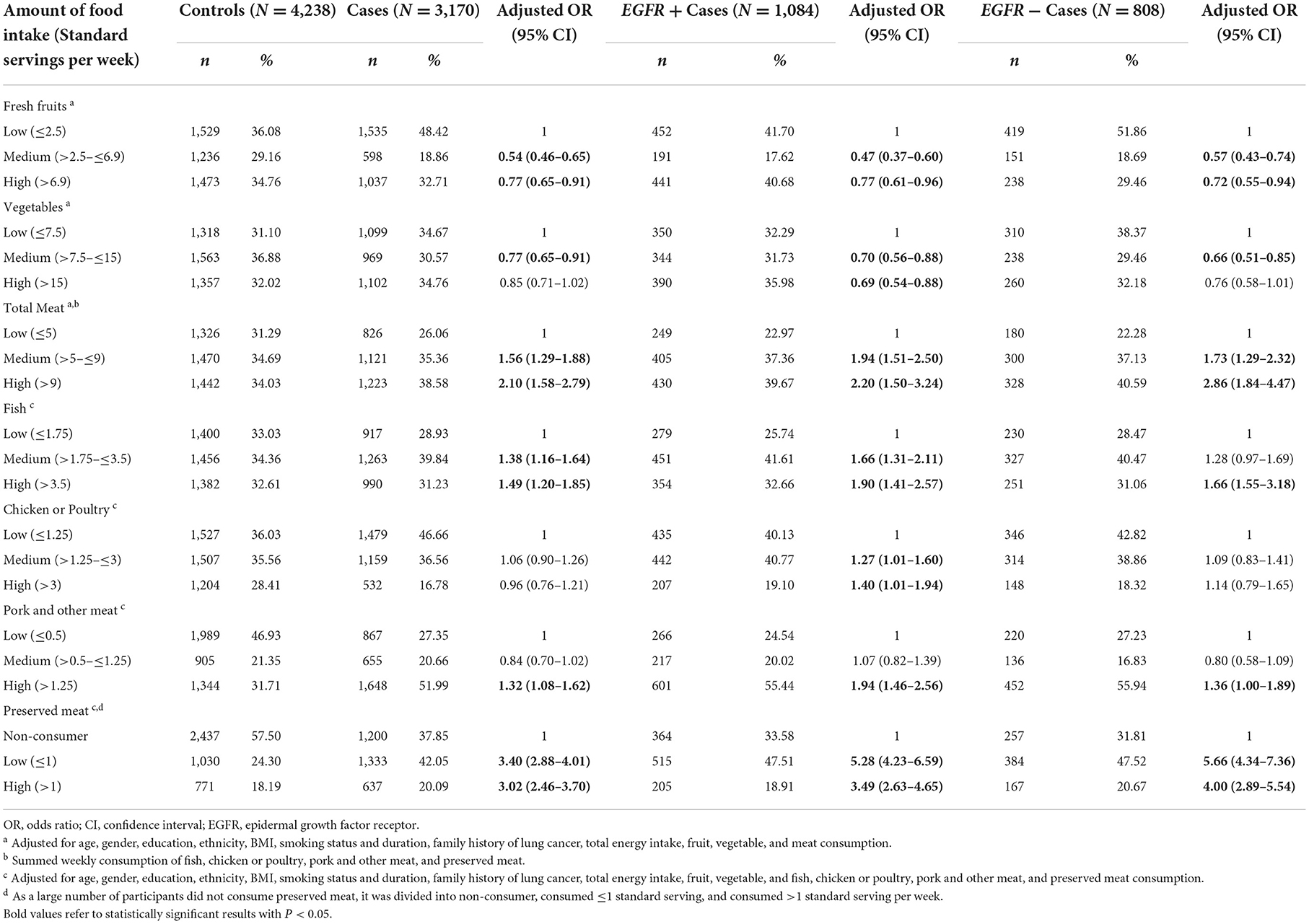
Table 2. Association between consumption of fruits, vegetables, and meat with risk of lung cancer subtypes.
Overall, positive associations between total meat intake and lung cancer were reported in our study population. Compared with low meat consumption (1st tertile), a statistically significant positive association between higher consumption (3rd tertile) of total meat and the elevated risk of lung cancer was observed (OR = 2.10, 95% CI = 1.58–2.79). When the analysis was stratified by EGFR status, statistically significant positive associations were also found for EGFR+ lung cancer (OR = 2.20, 95% CI = 1.50–3.24) and EGFR- lung cancer (OR = 2.86, 95% CI = 1.84–4.47). In addition, we observed positive associations between higher consumption of fish (OR = 1.49, 95% CI = 1.20–1.85), pork and other meats (OR = 1.32, 95% CI = 1.08–1.62), preserved meat (OR = 3.02, 95% CI = 2.46–3.70), with the risk of lung cancer.
When stratified by different subtypes of lung cancer, we observed similar associations among non-small cell lung cancer, adenocarcinoma, and squamous cell carcinoma (Table 3). Higher fruit consumption was significantly and inversely associated with the risk of all subtypes of lung cancer. A statistically significant positive association between higher consumption of total meat and the elevated risk of non-small cell lung cancer (OR = 1.99, 95% CI = 1.49–2.67), and adenocarcinoma (OR = 2.06, 95% CI = 1.52–2.79) were observed, except for squamous cell carcinoma (OR = 1.21, 95% CI = 0.59–2.51).
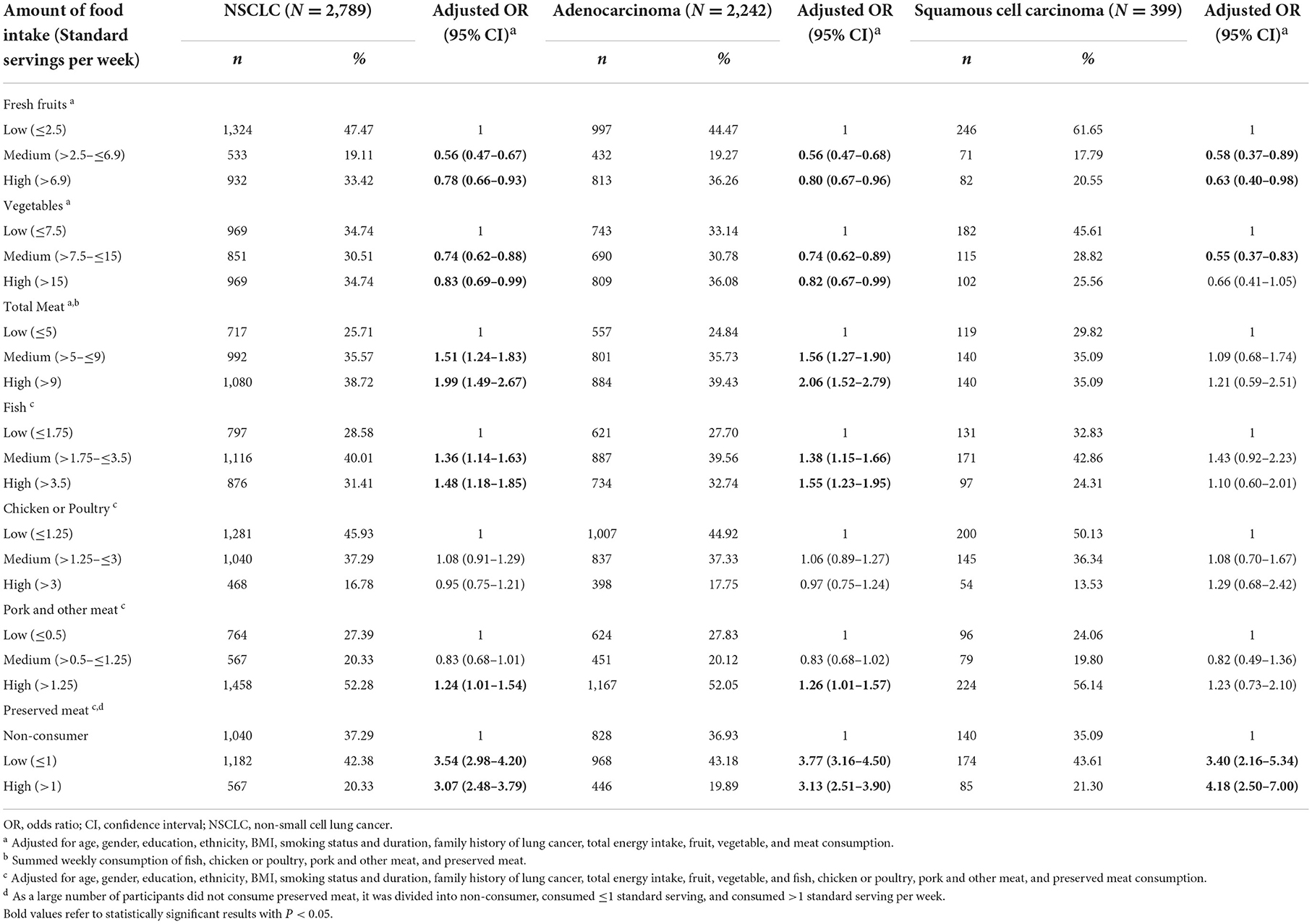
Table 3. Association between consumption of fruits, vegetables, and meat with risk of different histologic types of lung cancer.
Among never smokers, as compared to the lowest tertile, the highest total meat consumption group was associated with a higher risk of lung cancer across all strata of never smokers (never smokers, never-smoking females, and never-smoking Chinese females) (Table 4). No statistically significant associations between total vegetable consumption and risk of lung cancer were observed. For the age-matched sensitivity analyses 2,340 cases were age-matched with 2,340 controls. A total of 1,084 EGFR+ and 909 EGFR- cases were also age-matched with the same number of controls, respectively. Overall, the results were similar with the main analyses (Supplementary Tables S4–S6). Compared with low fruit consumption, a significant inverse association between high fruit consumption and the risk of lung cancer remained (OR = 0.79, 95% CI = 0.64–0.98). The statistically significant positive association between higher consumption (3rd tertile) of total meat and the elevated risk of lung cancer was also observed (OR = 1.92, 95% CI = 1.34–2.75), as compared with low meat consumption (1st tertile).
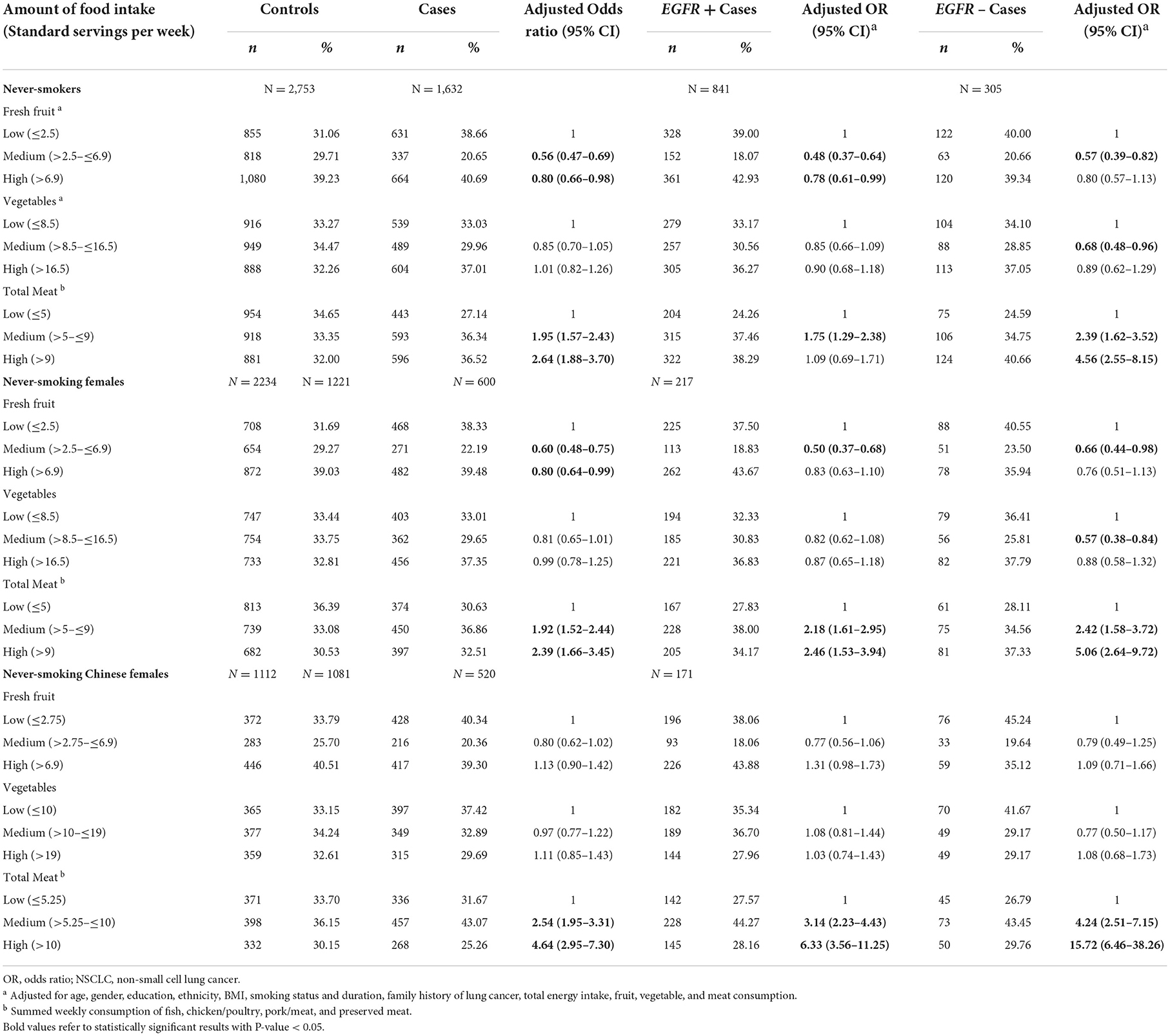
Table 4. Association between consumption of fruits, vegetables, and meat with risk of lung cancer among non-smokers.
In this study, we assessed the association between dietary factors and the risk of different histological and molecular subtypes of lung cancer. After adjusting for covariates, we identified higher consumption of total fresh fruits associated with a lower risk of lung cancer. In contrast, the higher total meat consumption, fish, pork, and preserved meat were statistically associated with elevated lung cancer risk. A significant inverse association between higher vegetable consumption and risk of EGFR+ lung cancer was identified, however, this association was not statistically significant among EGFR- lung cancer.
Consistent with previous studies, our findings showed that higher fruit consumption was correlated with a lower risk of lung cancer (40, 41). However, we did not find the monotonic decreasing ORs when comparing medium and higher fruit consumption groups. This may be attributed to the non-linear association reported in the previous study: lung cancer risk decreased for fruit consumption up to 200–300 grams per day, and no further decrease for higher consumption (42, 43). Compared with vegetables, we observed a pronounced association between fruits consumptions and lung cancer across all subtypes of lung cancer and among all subgroup populations. According to several previous studies, this pronounced protective evidence of fruits was repeatedly reported, however, the potential mechanisms still need to be investigated (41, 44, 45).
For vegetable consumption, our findings concur with previous work that an inverse association among higher consumption groups was reported (46), although we did not find a clear dose-response relationship. Similarly, a recent literature review by the World Cancer Research Fund supported the non-linear relationship between vegetable consumption and the risk of lung cancer, with decreasing risks for 300–400 grams per day and no further decrease for higher intake levels (42, 43). When stratified by smoking status, we did not find any significant associations among never-smokers, never-smoking females, or never-smoking Chinese females. Vieira et al. (13) and Smith-Warner et al. (47) also demonstrated that this protective effect was only significant among current smokers but was not statistically significant among former and never smokers. Interestingly, in the stratified analysis by EGFR status, a significantly decreased lung cancer risk was found only among EGFR+ lung cancer. Hamaguchi et al. reported that an alkaline diet (more vegetables and fruits, and less meat and dairy products) enhanced the effect of EGFR-TK inhibitor treatment in lung cancer patients with EGFR mutations (22). Our results may provide some insights into the potential mechanisms. Furthermore, the curcumin from turmeric (48, 49), Lupeol (a kind of phytosterol derived from fruits and vegetables) (50), and procyanidins-rich diets (51) have been shown to inhibit EGFR activation and have anti-cancer effects in lung cancer in multiple steps. However, we noted that the 95% CIs of the estimates in association for vegetable consumption and lung cancer by EGFR status were largely overlapping, i.e., 0.69 (0.54–0.88) and 0.76 (0.58–1.01). In the sensitivity analysis, no significant associations between vegetable consumption and EGFR +/- lung cancer were found. Therefore, this difference may be due to chance.
We found a significant positive association between total meat consumption and the risk of lung cancer after adjusting for covariates and total energy intake. Similar to our findings, a dose-response association was also found by Xue et al. with every increase of 120 g per day of red meat consumption, the risk of lung cancer increased by 35% (RR = 1.35, 95%CI = 1.25–1.46) (14). Lam et al. reported a significant positive association between higher meat intake and the risk of lung adenocarcinoma and squamous cell carcinoma (17). Similarly, our results also support the statistically significant relationship between higher meat intake and the risk of lung adenocarcinoma. As for the lack of statistically significant results for squamous cell carcinoma, this may be due to the limited number of cases in this group.
In this study, we were able to assess the effect of the consumption of total fruits, vegetables, and meat on the risk of lung cancer by specific subtypes. Based on our results, we did not observe any huge differences between different lung cancer subtypes. Higher consumption of fruits and vegetables was less pronounced among adenocarcinoma cases as compared to squamous cell carcinoma cases. Few studies have analyzed the effect of fruits and vegetables among specific lung cancer subtypes, and the results were inconsistent: four previous studies demonstrated statistically insignificant associations for small-cell carcinoma, adenocarcinoma, squamous cell carcinoma, and large cell lung carcinoma (13, 14, 40, 52); whereas Voorrips et al. revealed a weaker protective effect for adenocarcinomas than for other types of tumors, which was consistent with our results (53).
Some potential mechanisms have been proposed but the conclusions from different studies remained inconsistent. The protective effect of fruits and vegetables was attributed to biologically active compounds, including flavonoids and carotenoids (54, 55). Flavonoids found in fruit modular cytochrome P450 enzyme systems are involved in the metabolism of carcinogens (56). However, another study indicated that the intake of carotene supplementation was not associated with a decreasing risk of lung cancer (57). Besides, the protective effect may likely result from a combination of each constituent in influences several pathways involved in lung carcinogenesis (43). Red meat and processed meat are sources of saturated fats and heme iron, and several mutagens when cooked at a high temperature, including polycyclic aromatic hydrocarbons (PHAs) and heterocyclic amines (HCAs). These chemicals and mutagens may contribute to an increased risk of lung cancer (16, 58–60). However, a cohort study demonstrated a non-significant association between cooking methods, intake of specific meat mutagens or heme iron, and the risk of lung cancer (19). Therefore, further studies may be needed to characterize the mechanisms in these associations.
Although the results of this study support the hypothesis that fruit consumption is inversely associated with the risk of lung cancer and the consumption of meat is positively associated with the risk of lung cancer, there are several caveats to consider. Firstly, the cases and controls were taken from three different studies, which were carried out over different periods and used different questionnaires. Consumption of fruits, vegetables, and meat was collected in different ways; interviewers might have been trained differently, eliciting different responses from subjects. Although we have tried our best to combine those datasets appropriately and harmonize the variables, these limitations may affect the robustness of our findings, which may attenuate the results. The three studies enrolled subjects from different time periods, although all the cohorts started recruitment in 2005–2007. To control for the potential effects of the different enrollment time periods, we adjusted for the enrollment period in our model, and we found that the overall ORs and 95% CIs remained similar. In addition, in these recent 10 years, although Singaporean diet format and categories did not change much, we cannot deny that some participants may tend to eat healthily or improve their diet quality during this time (61, 62). Based on the Singapore National Nutrition Survey Report, from 2004 to 2018, the average daily intake increased a bit, from 2290 to 2470 kcal per day. The consumption of fruits and vegetables increased, but the percentage of protein remained stable at 14–15% of the total energy (63, 64). Overall, as more than half of our controls were recruited after 2008, this difference in the recruitment period may slightly overestimate our current results.
Secondly, recall bias is a major limitation for case-control studies. Although the food frequency questionnaire has been used previously and showed validity (18, 65), the participants may underreport or overreport some specific food items when asked to recall their past diet. Cases, especially among females and non-smokers, may be more likely than controls to report unhealthy diet habits and vice versa. We have made efforts to minimize this limitation by training the interviewers to limit investigator bias. Further research in a prospective cohort study is warranted to validate our findings.
Thirdly, we were unable to access the relative importance of each constituent and the effect of other food items, such as flavonoids, carotenoids rice, eggs, fast foods, soy, and dairy products due to the questionnaires. Furthermore, because the questionnaires of LCCS and MEC datasets were limited to each fruit and vegetable item portion size and frequency, we can only use the average energy intake to present each category to calculate the total energy intake (including fruits, green leafy, or other vegetables, and each kind of meat). Therefore, there are likely to be measurement errors. To avoid an under- or over-estimation of total energy intake, a total energy intake of < 2.5th or higher than 97.5th percentiles was excluded. Despite the limitations, to our knowledge, this is the first study of dietary factors and the risk of lung cancer by EGFR +/- and histologic subtypes among Southeast Asians.
In summary, we found that higher vegetable consumption was significantly associated with a decreased risk of EGFR+ lung cancer. Consistent with prior studies, an inverse association between higher fruit consumption and lung cancer, and a positive association between higher meat consumption and lung cancer were identified. Both associations remained significant when stratified by different molecular and histological types of lung cancer. Further prospective studies are warranted to assess this association and characterize the underlying biological mechanisms.
The data analyzed in this study is subject to the following licenses/restrictions: the data are not publicly available due to privacy or ethical restrictions. Requests to access these datasets should be directed to WS, ZXBoc3dqQG51cy5lZHUuc2c=.
This current study of using three datasets was approved by the National University of Singapore Institutional Review Board (NUS-IRB Ref: N-20-053E). The patients/participants provided their written informed consent to participate in this study.
XY: conceptualization, formal analysis, and writing–original draft. GL and DT: writing–review and editing. AS: resources and writing–review and editing. DL and WS: writing–review and editing and supervision. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1079543/full#supplementary-material
1. Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health. (2019) 85:2419. doi: 10.5334/aogh.2419
2. Global Burden of Disease Cancer C. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. (2017) 3:524–48. doi: 10.1001/jamaoncol.2016.5688
3. Global Burden of Disease Cancer C. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: a systematic analysis for the global burden of disease study 2019. JAMA Oncol. (2022) 8:420–44. doi: 10.1001/jamaoncol.2021.6987
4. Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. (2014) 14:535–46. doi: 10.1038/nrc3775
5. Wang BY, Huang JY, Chen HC, Lin CH, Lin SH, Hung WH, et al. The comparison between adenocarcinoma and squamous cell carcinoma in lung cancer patients. J Cancer Res Clin Oncol. (2020) 146:43–52. doi: 10.1007/s00432-019-03079-8
6. Chen JW, Dhahbi J. Lung adenocarcinoma and lung squamous cell carcinoma cancer classification, biomarker identification, and gene expression analysis using overlapping feature selection methods. Sci Rep. (2021) 11:13323. doi: 10.1038/s41598-021-92725-8
7. Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nature reviews Molecular cell biology. (2001) 2:127–37. doi: 10.1038/35052073
8. da Cunha Santos G, Shepherd FA, Tsao MS, EGFR. mutations and lung cancer. Annu Rev Pathol. (2011) 6:49–69. doi: 10.1146/annurev-pathol-011110-130206
9. Tan DS, Mok TS, Rebbeck TR. Cancer genomics: diversity and disparity across ethnicity and geography. J Clin Oncol. (2016) 34:91–101. doi: 10.1200/JCO.2015.62.0096
10. Zhang YL, Yuan JQ, Wang KF, Fu XH, Han XR, Threapleton D, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. (2016) 7:78985–93. doi: 10.18632/oncotarget.12587
11. Sun Y, Li Z, Li J, Li Z, Han J. A healthy dietary pattern reduces lung cancer risk: a systematic review and meta-analysis. Nutrients. (2016) 8:134. doi: 10.3390/nu8030134
12. Ruano-Ravina A, Figueiras A, Freire-Garabal M, Barros-Dios JM. Antioxidant vitamins and risk of lung cancer. Curr Pharm Des. (2006) 12:599–613. doi: 10.2174/138161206775474396
13. Vieira AR, Abar L, Vingeliene S, Chan DS, Aune D, Navarro-Rosenblatt D, et al. Fruits, vegetables and lung cancer risk: a systematic review and meta-analysis. Ann Oncol. (2016) 27:81–96. doi: 10.1093/annonc/mdv381
14. Xue XJ, Gao Q, Qiao JH, Zhang J, Xu CP, Liu J. Red and processed meat consumption and the risk of lung cancer: a dose-response meta-analysis of 33 published studies. Int J Clin Exp Med. (2014) 7:1542–53.
15. Lippi G, Mattiuzzi C, Cervellin G. Meat consumption and cancer risk: a critical review of published meta-analyses. Crit Rev Oncol Hematol. (2016) 97:1–14. doi: 10.1016/j.critrevonc.2015.11.008
16. Yang WS, Wong MY, Vogtmann E, Tang RQ, Xie L, Yang YS, Wu QJ, Zhang W, Xiang YB. Meat consumption and risk of lung cancer: evidence from observational studies. Ann Oncol. (2012) 23:3163–70. doi: 10.1093/annonc/mds207
17. Lam TK, Cross AJ, Consonni D, Randi G, Bagnardi V, Bertazzi PA, et al. Intakes of red meat, processed meat, and meat mutagens increase lung cancer risk. Cancer Res. (2009) 69:932–9. doi: 10.1158/0008-5472.CAN-08-3162
18. Lim WY, Chuah KL, Eng P, Leong SS, Lim E, Lim TK, et al. Meat consumption and risk of lung cancer among never-smoking women. Nutr Cancer. (2011) 63:850–9. doi: 10.1080/01635581.2011.589961
19. Tasevska N, Cross AJ, Dodd KW, Ziegler RG, Caporaso NE, Sinha R. No effect of meat, meat cooking preferences, meat mutagens or heme iron on lung cancer risk in the prostate, lung, colorectal and ovarian cancer screening trial. Int J Cancer. (2011) 128:402–11. doi: 10.1002/ijc.25327
20. Gnagnarella P, Caini S, Maisonneuve P, Gandini S. Carcinogenicity of high consumption of meat and lung cancer risk among non-smokers: a comprehensive meta-analysis. Nutr Cancer. (2018) 70:1–13. doi: 10.1080/01635581.2017.1374420
21. Matsuo K, Hiraki A, Ito H, Kosaka T, Suzuki T, Hirose K, et al. Soy consumption reduces the risk of non-small-cell lung cancers with epidermal growth factor receptor mutations among Japanese. Cancer Sci. (2008) 99:1202–8. doi: 10.1111/j.1349-7006.2008.00812.x
22. Hamaguchi R, Okamoto T, Sato M, Hasegawa M, Wada H. Effects of an alkaline diet on EGFR-TKI therapy in EGFR mutation-positive NSCLC. Anticancer Res. (2017) 37:5141–5. doi: 10.21873/anticanres.11934
23. Afaq F, Zaman N, Khan N, Syed DN, Sarfaraz S, Zaid MA, et al. Inhibition of epidermal growth factor receptor signaling pathway by delphinidin, an anthocyanidin in pigmented fruits and vegetables. Int J Cancer. (2008) 123:1508–15. doi: 10.1002/ijc.23675
24. Proske A, Bossen J, von Frieling J, Roeder T. Low-protein diet applied as part of combination therapy or stand-alone normalizes lifespan and tumor proliferation in a model of intestinal cancer. Aging (Albany NY). (2021) 13:24017–36. doi: 10.18632/aging.203692
25. Singh D, Attri BK, Gill RK, Bariwal J. Review on EGFR inhibitors: critical updates. Mini Rev Med Chem. (2016) 16:1134–66. doi: 10.2174/1389557516666160321114917
26. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. (2011) 12:735–42. doi: 10.1016/S1470-2045(11)70184-X
27. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. (2018) 378:113–25. doi: 10.1056/NEJMoa1713137
28. Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. (2014) 15:213–22. doi: 10.1016/S1470-2045(13)70604-1
29. Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. (2017) 18:1454–66. doi: 10.1016/S1470-2045(17)30608-3
30. Huat TE. Lung Cancer Consortium Singapore (LCCS). Lung Cancer Consortium Singapore. Available online at: https://lccs.com.sg/ (accessed April 5, 2022).
31. Tang L, Lim WY, Eng P, Leong SS, Lim TK, Ng AW, et al. Lung cancer in Chinese women: evidence for an interaction between tobacco smoking and exposure to inhalants in the indoor environment. Environ Health Perspect. (2010) 118:1257–60. doi: 10.1289/ehp.0901587
32. Yin X, Chan CPY, Seow A, Yau WP, Seow WJ. Association between family history and lung cancer risk among Chinese women in Singapore. Sci Rep. (2021) 11:21862. doi: 10.1038/s41598-021-00929-9
33. Multiethnic Cohort (MEC). Singapore: Saw Swee Hock School of Public Health. (2020). Available online at: https://blog.nus.edu.sg/sphs/multiethnic-cohort/ (accessed December 21, 2021).
34. Singapore HPB. Enegry & nutrient composition of food. In: Ministry of Health Singapore. Health Promotion Board, Ministry of Health of Singapore (2011). Available online at: https://focos.hpb.gov.sg/eservices/ENCF (accessed April 5, 2022).
35. Bradshaw PT, Siega-Riz AM, Campbell M, Weissler MC, Funkhouser WK, Olshan AF. Associations between dietary patterns and head and neck cancer: the Carolina head and neck cancer epidemiology study. Am J Epidemiol. (2012) 175:1225–33. doi: 10.1093/aje/kwr468
36. Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Boston College Department of Economics (2003).
37. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. (2011) 10:150–61. doi: 10.1002/pst.433
38. Wang Y, Cai H, Li C, Jiang Z, Wang L, Song J, et al. Optimal caliper width for propensity score matching of three treatment groups: a Monte Carlo study. PLoS ONE. (2013) 8:e81045. doi: 10.1371/journal.pone.0081045
39. Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. (2009) 361:958–67. doi: 10.1056/NEJMoa0904554
40. Büchner F. Bueno-de-Mesquita H, Linseisen J, Boshuizen H, Kiemeney L, Ros M, Overvad K, et al. Fruits and vegetables consumption and the risk of histological subtypes of lung cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Causes Control. (2010) 21:357–71. doi: 10.1007/s10552-009-9468-y
41. Wakai K, Matsuo K, Nagata C, Mizoue T, Tanaka K, Tsuji I, et al. Lung cancer risk and consumption of vegetables and fruit: an evaluation based on a systematic review of epidemiological evidence from Japan. JPN J Clin Oncol. (2011) 41:693–708. doi: 10.1093/jjco/hyr027
42. Continuous Update Project. The Associations between Food, Nutrition and Physical Activity and the Risk of Lung Cancer. Systmetic Literature Review. World Cancer Research Fund International (2015). Available online at: https://www.wcrf.org/diet-and-cancer/ (accessed April 15, 2022).
43. Continuous Update Project Expert Report 2018. Diet Nutrition Physical Activity and Lung Cancer. World Cancer Research Fund/American Institute for Cancer Research. Available online at: https://www.wcrf.org/diet-and-cancer/ (accessed April 15, 2022).
44. Takezaki T, Inoue M, Kataoka H, Ikeda S, Yoshida M, Ohashi Y, et al. Diet and lung cancer risk from a 14-year population-based prospective study in Japan: with special reference to fish consumption. Nutr Cancer. (2003) 45:160–7. doi: 10.1207/S15327914NC4502_04
45. Sauvaget C, Nagano J, Hayashi M, Spencer E, Shimizu Y, Allen N. Vegetables and fruit intake and cancer mortality in the Hiroshima/Nagasaki Life Span Study. Br J Cancer. (2003) 88:689–94. doi: 10.1038/sj.bjc.6600775
46. Yang T, Wang C, Li S, Guo XF Li D. Dietary intakes of fruits and vegetables and lung cancer risk in participants with different smoking status: a meta-analysis of prospective cohort studies. Asia Pac J Clin Nutr. (2019) 28:770–82.
47. Smith-Warner SA, Spiegelman D, Yaun SS, Albanes D, Beeson WL, van den Brandt PA, et al. Fruits, vegetables and lung cancer: a pooled analysis of cohort studies. Int J Cancer. (2003) 107:1001–11. doi: 10.1002/ijc.11490
48. Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. (2006) 71:1397–421. doi: 10.1016/j.bcp.2006.02.009
49. Wan Mohd Tajuddin WNB, Lajis NH, Abas F, Othman I, Naidu R. Mechanistic understanding of curcumin's therapeutic effects in lung cancer. Nutrients. (2019) 11:989. doi: 10.3390/nu11122989
50. Rauth S, Ray S, Bhattacharyya S, Mehrotra DG, Alam N, Mondal G, et al. Lupeol evokes anticancer effects in oral squamous cell carcinoma by inhibiting oncogenic EGFR pathway. Mol Cell Biochem. (2016) 417:97–110. doi: 10.1007/s11010-016-2717-y
51. Zhu W, Li MC, Wang FR, Mackenzie GG, Oteiza PI. The inhibitory effect of ECG and EGCG dimeric procyanidins on colorectal cancer cells growth is associated with their actions at lipid rafts and the inhibition of the epidermal growth factor receptor signaling. Biochem Pharmacol. (2020) 175:113923. doi: 10.1016/j.bcp.2020.113923
52. Liu Y, Sobue T, Otani T, Tsugane S. Vegetables, fruit consumption and risk of lung cancer among middle-aged Japanese men and women: JPHC study. Cancer Causes Control. (2004) 15:349–57. doi: 10.1023/B:CACO.0000027507.22124.20
53. Voorrips LE, Goldbohm RA, Verhoeven DT, van Poppel GA, Sturmans F, Hermus RJ, et al. Vegetable and fruit consumption and lung cancer risk in the Netherlands Cohort Study on diet and cancer. Cancer Causes Control. (2000) 11:101–15. doi: 10.1023/A:1008906706084
54. Woo HD, Kim J. Dietary flavonoid intake and smoking-related cancer risk: a meta-analysis. PLoS ONE. (2013) 8:e75604. doi: 10.1371/journal.pone.0075604
55. Tang N-P, Zhou B, Wang B, Yu R-B, Ma J. Flavonoids intake and risk of lung cancer: a meta-analysis. Jpn J Clin Oncol. (2009) 39:352–59. doi: 10.1093/jjco/hyp028
56. Alexandrov K, Cascorbi I, Rojas M, Bouvier G, Kriek E, Bartsch H. CYP1A1 and GSTM1 genotypes affect benzo[a]pyrene DNA adducts in smokers' lung: comparison with aromatic/hydrophobic adduct formation. Carcinogenesis. (2002) 23:1969–77. doi: 10.1093/carcin/23.12.1969
57. Gallicchio L, Boyd K, Matanoski G, Tao XG, Chen L, Lam TK, et al. Carotenoids and the risk of developing lung cancer: a systematic review. Am J Clin Nutr. (2008) 88:372–83. doi: 10.1093/ajcn/88.2.372
58. Puangsombat K, Gadgil P, Houser TA, Hunt MC, Smith JS. Heterocyclic amine content in commercial ready to eat meat products. Meat Sci. (2011) 88:227–33. doi: 10.1016/j.meatsci.2010.12.025
59. Sinha R, Knize MG, Salmon CP, Brown ED, Rhodes D, Felton JS, et al. Heterocyclic amine content of pork products cooked by different methods and to varying degrees of doneness. Food Chem Toxicol. (1998) 36:289–97. doi: 10.1016/S0278-6915(97)00159-2
60. Phillips DH. Polycyclic aromatic hydrocarbons in the diet. Mutat Res. (1999) 443:139–47. doi: 10.1016/S1383-5742(99)00016-2
61. Gan P, Er JC, Chow K, Er B, Chan JSH, Li A, Aung KT. Consumption patterns of processed foods in singapore-a cross-sectional study. Foods. (2022) 11:2782. doi: 10.3390/foods11182782
62. Reddy G, van Dam RM. Food, culture, and identity in multicultural societies: Insights from Singapore. Appetite. (2020) 149:104633. doi: 10.1016/j.appet.2020.104633
63. Health Promotion Board S. Report of the National Nutrition Survey 2010. Singapore: Research & Strategic Planning Division HPB. Health Promotion Board, Singapore (2013).
64. Health Promotion Board S. Report of the National Nutrition Survey 2018. Singapore: Health Promotion Board (2018).
Keywords: diet, lung cancer, EGFR, case-control, non-small cell lung cancer
Citation: Yin X, Lai GGY, Seow A, Tan DSW, Lim DW-T and Seow WJ (2022) Dietary factors and the risk of lung cancer by epidermal growth factor receptor mutation status and histological subtypes. Front. Public Health 10:1079543. doi: 10.3389/fpubh.2022.1079543
Received: 25 October 2022; Accepted: 15 November 2022;
Published: 02 December 2022.
Edited by:
Guiju Sun, Southeast University, ChinaReviewed by:
Xin Liu, Jiangsu Provincial Center for Disease Control and Prevention, ChinaCopyright © 2022 Yin, Lai, Seow, Tan, Lim and Seow. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Jie Seow, ZXBoc3dqQG51cy5lZHUuc2c=; Darren Wan-Teck Lim, ZGFycmVuLmxpbS53LnRAc2luZ2hlYWx0aC5jb20uc2c=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.