- 1Department of Health Service Management, Virtual School of Medical Education and Management, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 3Social Determinants of Health Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Background: Payments to physicians by the pharmaceutical industry are common, but recent evidence shows that these payments influence physician prescribing behavior in the form of increased prescription of brand-name drugs, expensive and low-cost drugs, increased prescription of payer company drugs, etc. Considering that these payments increase drug costs for patients and health systems, there is a public interest in controlling them. Therefore, this study aimed to identify and propose policy options for managing physician-pharmaceutical industry interactions in the context of Iran's health system.
Methods: In the first phase, a systematic search was conducted to identify relevant policies and interventions in Web of Science, PubMed, and ProQuest databases from 2000 to 2022. Then, the opinions of the research team and an expert group (physicians, health policy and transparency experts, and industry representatives) were used to categorize the interventions and propose policy options along with their advantages, disadvantages, and implementation considerations.
Results: In the search, 579 articles were retrieved, and 44 articles were found suitable for the final analysis. Twenty-nine interventions and strategies were identified, and based on these; Five policy options were identified: prohibition, restriction, physician self-regulation, voluntary industry disclosure, and mandatory industry disclosure.
Conclusion: The proposed policies in our study include advantages, challenges, and implementation considerations based on up-to-date evidence that can help policymakers use them to manage COI in physician-pharmaceutical industry interactions in Iran's health system. A combination of measures seems to help manage COI: firstly, using self-regulating physicians and industry to institutionalize transparency, and in the next step, implementing mandatory industry disclosure policies and establishing restrictions on some financial interactions.
Introduction
The pharmaceutical industry is a strategic partner in advancing the goals of the healthcare sector (1). Physicians attend professional meetings with pharmaceutical representatives, participate in research, and participate in development and investment for health-related industries, all of which are often important opportunities for advancing medical knowledge and patient care (2). Physicians and the industry have a common interest in advancing medical knowledge. Nevertheless, the physician's primary goal is to promote their patient's interests, while the industry's goal is to promote profitability and uses all available tools to promote its products, which are not necessarily in line with the patient benefit (1, 2).
Therefore, these interactions can create conflicts of interest (COI) that affect clinical judgments, prescribing, research, education, and treatment outcomes. There is considerable evidence that this COI often favors companies (3). Some studies show considerable concern about these interactions, especially in financial interest cases, as such links potentially lead to systematic biases in patient care (4). Physicians are the gatekeepers determining how money is spent in the health system. Hence, they are the target group of marketing activities of the pharmaceutical industry (5), and potential COI, real or perceived, is pervasive in this area (2).
Payment by the pharmaceutical industry to physicians is the most common form of physician-industry relationship, which is in the form of cash (for consulting services, lectures, travel, accommodation, etc.) or non-cash such as meals, gifts, stocks, licenses, etc. (6, 7). About 90% of pharmaceutical companies advertising costs are allocated to physicians and other prescribers (3). Industry payments to physicians and teaching hospitals in the United States, excluding scientific research funding, amounted to $3.6 billion in 2019 (8). Almost half of the physicians receive annual payments from the industry (9). The findings of a systematic review showed that financial relationships between physicians and the pharmaceutical industry are common in low- and middle-income countries (10).
There is not much documentary evidence about these payments in Iran. Tabrizi et al. claimed that in Iran, pharmaceutical companies choose their target physicians among health policymakers who play an essential role in pharmaceutical decisions to ensure the success of their marketing (11). According to a news report in 2019, there are cash incentives, free drug samples, and various rewards to doctors, such as “foreign trips” for prescribing products of a particular company in Iran's health system (12). The financial relationship of physicians with pharmaceutical companies has also been mentioned as one of the important reasons for the irrational prescription of drugs in Iran (13, 14). In Ebrahimi et al.'s study, 90% of breast cancer specialists stated that they participated in congresses sponsored by pharmaceutical companies to introduce new drugs (15).
Payments by the pharmaceutical industry to physicians have some negative consequences. These payments can affect the independent clinical decision-making of physicians and thus endanger the quality of patient care, increase healthcare costs and reduce patients' trust in physicians and the health system (1, 6, 16, 17). Almost all published studies reported a positive relationship between payments and changes in physician prescribing behavior (1, 6, 7, 18).
It has been reported as the consequences of the industry's payments to physicians, such as increased use of brand-name drugs, increased prescription of medicines produced by the paying company, prescription of low-value and expensive drugs, preference and rapid prescription of new drugs, requests to add promoted drugs to the country's official list of drugs and finally increasing drug costs (6, 7, 16, 19). A study in the United States showed that a 10% increase in pharmaceutical industry payments to physicians is associated with a 1.3% increase in medical costs and a 1.8% increase in drug costs (20).
But physician-industry interactions also have defenders. They argue that industry funding leads to the development of drugs and devices that benefit patients. Product development, production, and marketing depend on the industry and are not possible only with government funds. The industry also needs physicians to test products to use those products in clinical trials. Humanity owes a huge collection of life-saving drugs and surgical devices to the cooperation of industry and physicians (21). In general, physician-industry relationships are not inherently good or bad. There are positive interactions between physicians and the industry that help medical advances. Physicians are and should be compensated for this work, but such interactions may create the potential for bias (22). Fear of industry bias should not prevent beneficial clinical interactions or innovative research that helps patients. on the other hand, maintaining public trust in the health system is also important (23).
That is why these interactions must be properly structured and managed. In addition, some inappropriate interactions and payments need to be monitored and addressed (22). With the lack of effective monitoring and management of COI, such interactions may eventually threaten the integrity, justice, and sustainability of health systems and negatively affect patients (1). Therefore, a middle ground should be sought to control physician-industry interactions; Neither a complete prohibition can help nor a complete freedom without transparency of these interactions (22). A more balanced and transparent interaction between physicians and the pharmaceutical industry approach would recognize beneficial collaborations while eliminating inappropriate relationships such as sham payments, promotional activities, meals provided by industry representatives, and speakers' bureau activities.
Therefore, there is a public interest in controlling them (7), and policymakers worldwide are looking for effective strategies to protect physicians from the industry's undue influence and manage COI from unregulated and non-transparent interactions (5, 24). To our knowledge, a comprehensive guide on proposed interventions and policies regarding physician-industry interactions was not found. Therefore, this study aimed to identify and propose policy options for managing physician-pharmaceutical industry interactions in the context of Iran's health system.
Methods
Eligibility criteria
The following criteria were used to select studies: (1) articles that dealt with specific interventions, strategies, or policies regarding managing financial interactions between physicians and pharmaceutical companies; (2) All types of articles such as reviews, originals, etc., except Conference articles and book chapters; (3) English language; (4) Studies whose target or discussed population for intervention or strategy proposal were physicians; (5) articles whose full text was available.
Information sources
A literature search was conducted on August 30, 2022. Three databases, PubMed, ProQuest, and Web of Science, were searched. The time range was considered from January 1, 2000, to 30, August 2022.
Search
The search was conducted using the following keywords: doctor, physician, pharmaceutical, industry, interaction, relation, collaboration, payment, influence, and conflict of interest (see the complete search strategy in Appendix 1).
Selection of sources of evidence
We used the PRISMA model for screening and selecting articles (25). All retrieved records were exported to EndNote X9. After removing duplicate articles, the titles and abstracts of the remaining articles were reviewed by two team members (EZ and AG) independently. After determining the relevance, the full text of the articles was retrieved for detailed review and data extraction.
Data charting process
Each article was read by two authors independently, and relevant strategies/interventions were extracted and then discussed and agreed to be mentioned in the data form.
Data items
Only strategies/interventions proposed in studies or tested in real-world settings were extracted in this review.
Synthesis of results
Data extraction and synthesis were done in two stages. First, the authors extracted interventions and strategies from the articles. These were then categorized into proposed policy options. There are two approaches to managing COI: eliminating all situations of COI by prohibitions and restrictions and controlling COI by transparency and disclosure of interactions (26). We first categorized the proposed interventions and strategies based on these approaches: 1- prohibition and restriction and 2- disclosure. In the next step, we realized that disclosure could be done from two sides: the payer (pharmaceutical companies or the industry) and the receiver (physicians). Also, industry disclosure is now voluntary and mandatory so that these options can be separated according to their specific implementation considerations.
In the second stage, we formed a team of experts. These people were selected purposefully and related to the research subject, including two physicians, two industry representatives, and three health policy experts (two of them are researchers in the field of transparency). We shared our proposed policy options with the experts' team, who confirmed our classification. In the next step, we asked the experts to tell us their opinions on these five options: “What are your opinions about the advantages and disadvantages of this policy option? What considerations should be taken into account in implementing this policy?” The interview was conducted online, and the audio was recorded. Some of the interviewees said were in the evidence, and some new points were added, especially considering the context of Iran's health system.
Results
Selection of sources of evidence
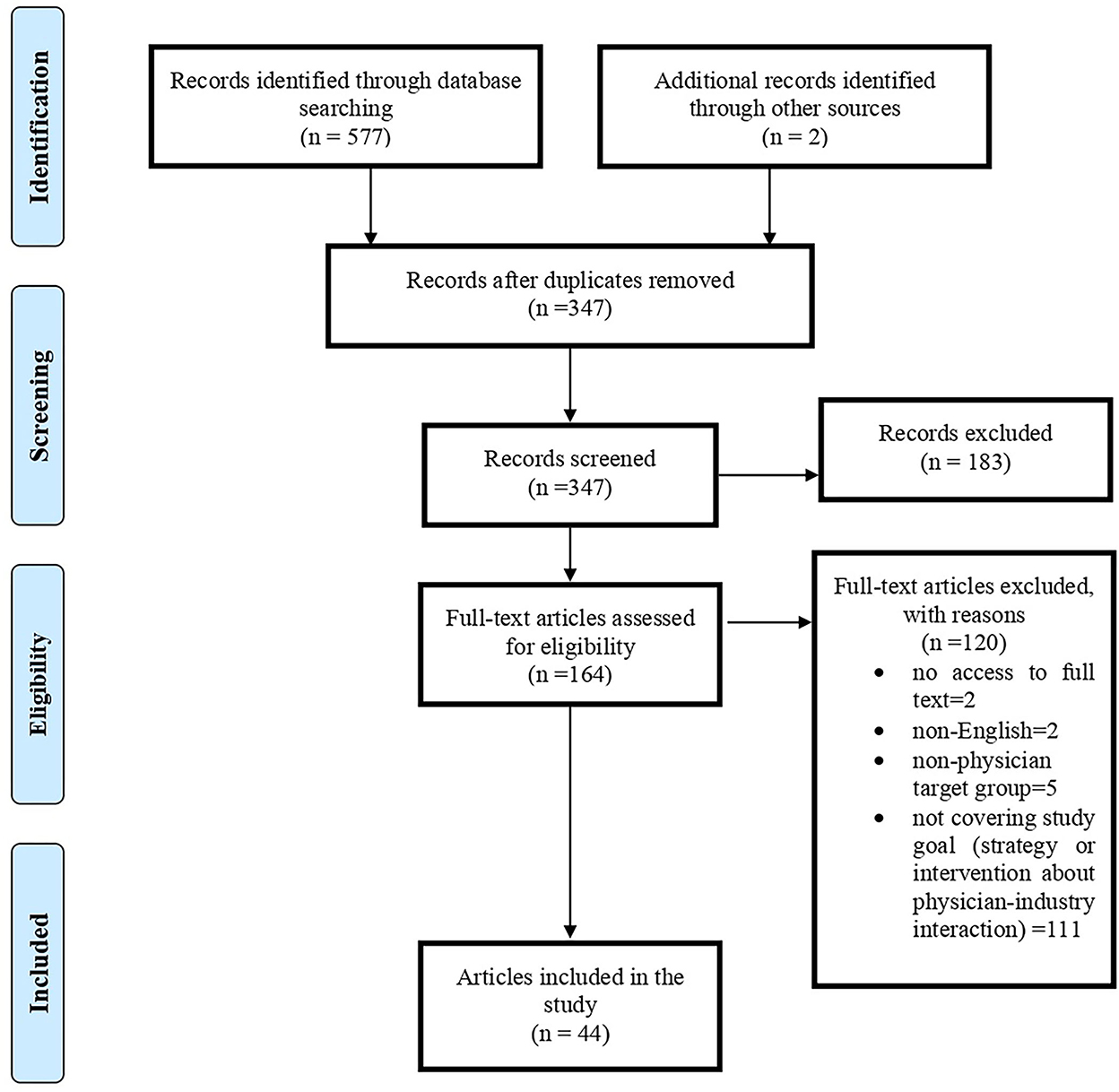
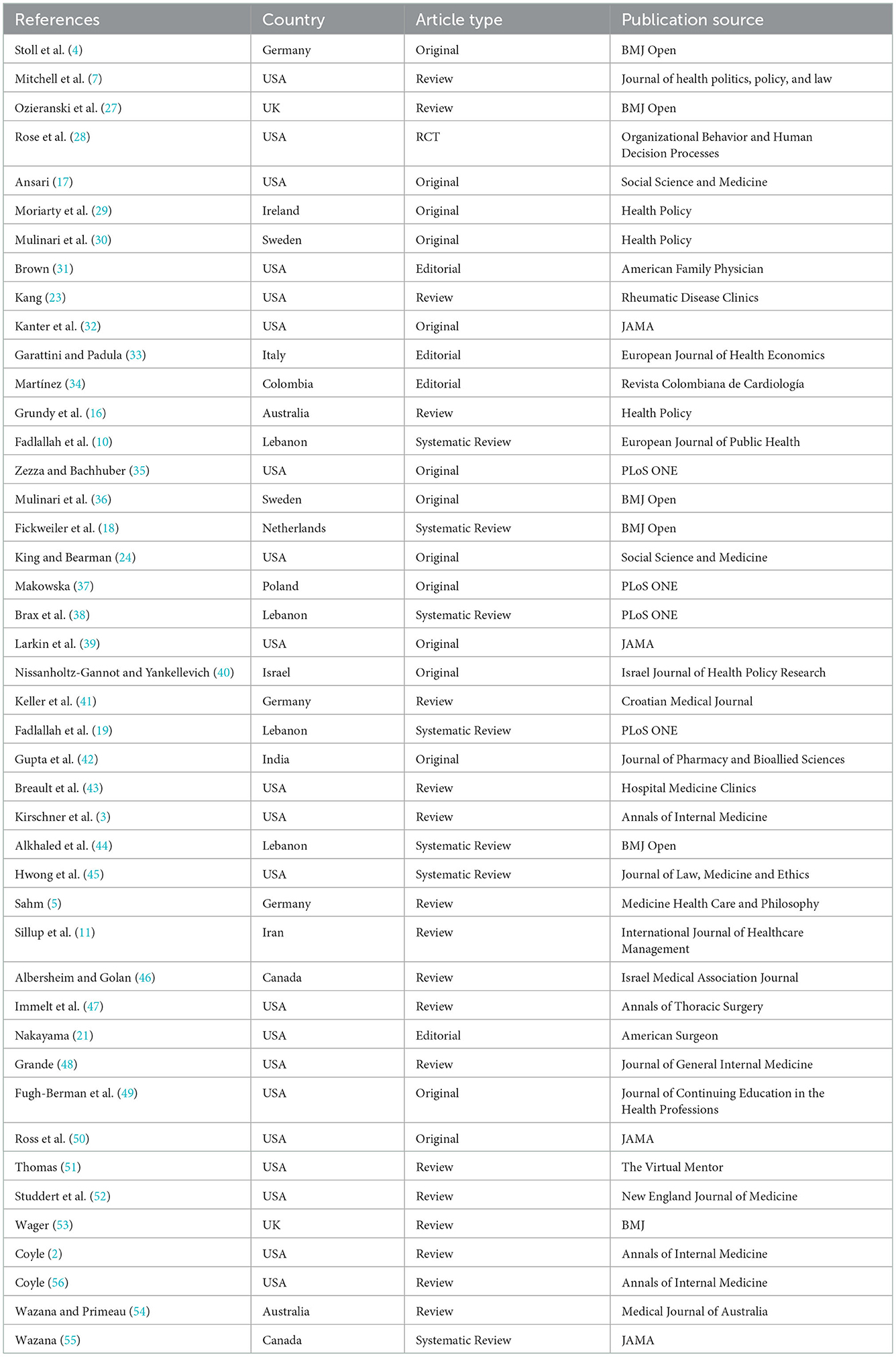
In the search, 579 articles were retrieved; after removing duplicate titles (232 articles), the titles and abstracts of the remaining articles were reviewed. From the remaining 164 articles, 44 were found suitable for the research topic (Figure 1). Of 44 papers, 32% (N = 14) was original, 57% (N = 25) was review articles (including seven systematic reviews), four editorial, and one RCT. Most articles were published in 2017 and 2021 (N = 6); generally, 70% of included articles were published in recent 10 years. Regarding affiliation of authors, 48% (N = 21) were related to the USA and then to Lebanon (N = 4) and Germany (N = 3). In addition, we had articles from 15 countries, including one from Iran. BMJ Open (N = 5), JAMA (N = 4), PLoS ONE (N = 3), Health Policy (N = 3), and Annals of Internal Medicine (N = 3) provided the most paper for our study. The characteristics of included papers are presented in Table 1.
Results of individual sources of evidence
A list of elicited strategies and interventions for managing COI arising from the physicians-pharmaceutical industry interaction is presented in Table 2.
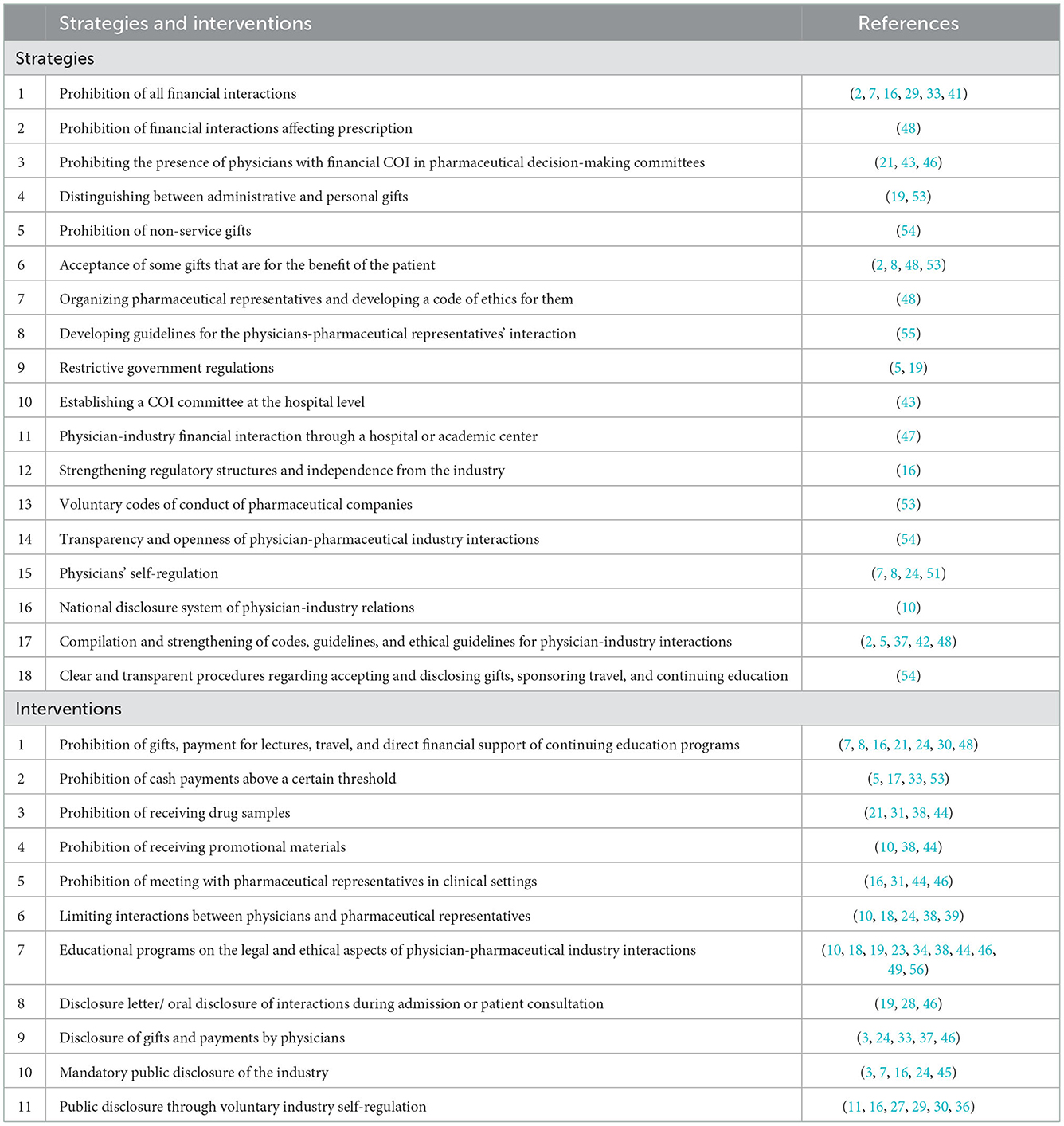
Table 2. Strategies and interventions extracted from the systematic review for managing COI of the physician-pharmaceutical industry.
Synthesis of results
In the second phase, with the collaboration of the experts' team, five policy options were proposed (Figure 2):
• Prohibition: ban on all physician-pharmaceutical industry financial interactions.
• Restriction: permission to receive certain gifts, payments, and interactions that benefit patients.
• Physician self-regulation: regulation of relationships with industry based on codes of ethics and voluntary disclosure by physicians.
• Voluntary industry disclosure: disclosure of gifts, payments, and other interactions by pharmaceutical companies on an optional and voluntary basis.
• Mandatory industry disclosure: all pharmaceutical companies must disclose payments to physicians by law.
The policy options' advantages, disadvantages, and implementation considerations are presented in Tables 3–7.
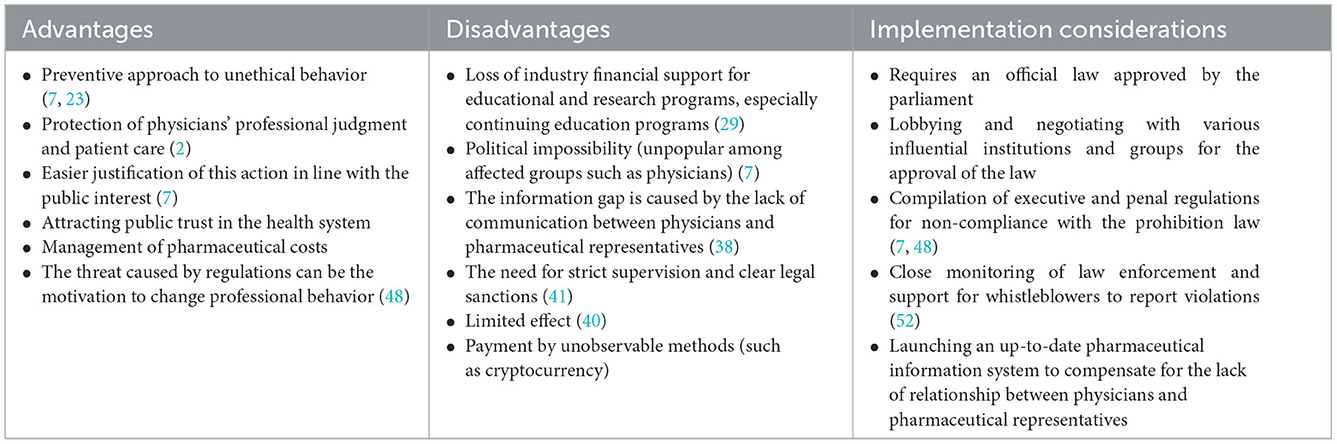
Table 3. Advantages, disadvantages, and implementation considerations of policy options: 1. Prohibition.
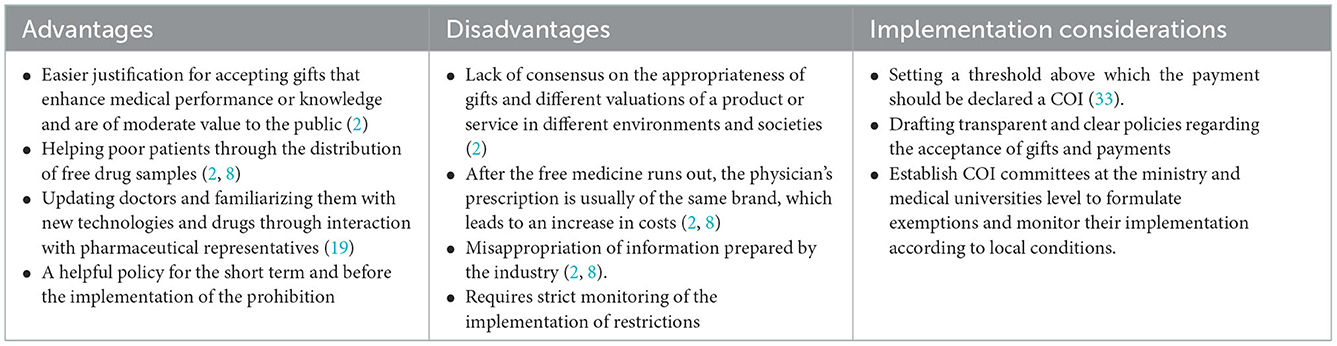
Table 4. Advantages, disadvantages, and implementation considerations of policy options: 2. Restriction.
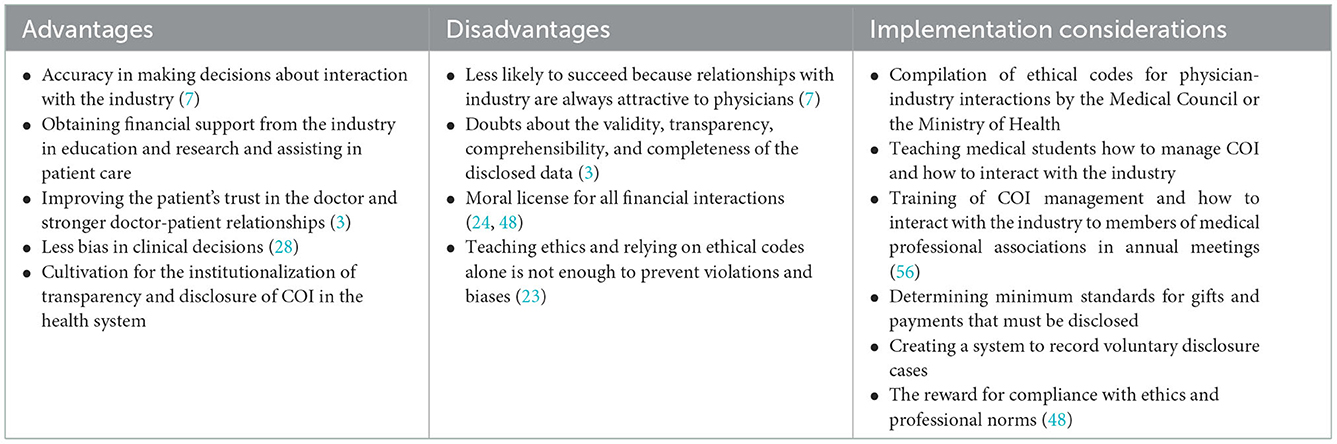
Table 5. Advantages, disadvantages, and implementation considerations of policy options: 3. Physician self-regulation.
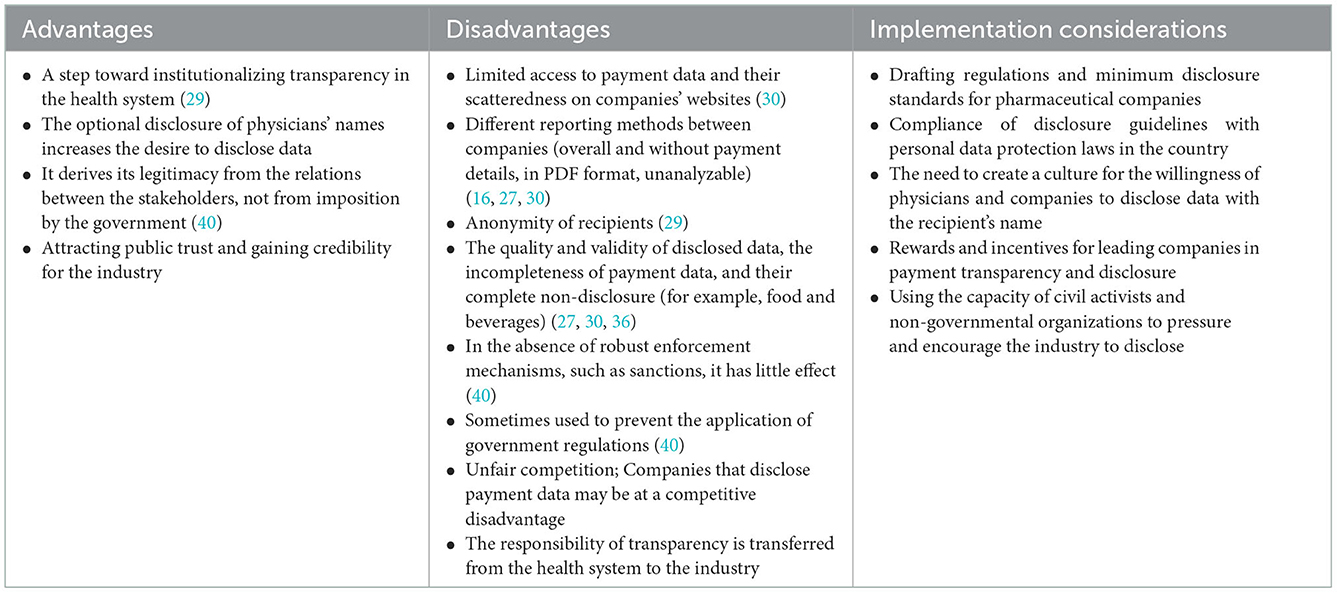
Table 6. Advantages, disadvantages, and implementation considerations of policy options: 4. Voluntary industry disclosure.
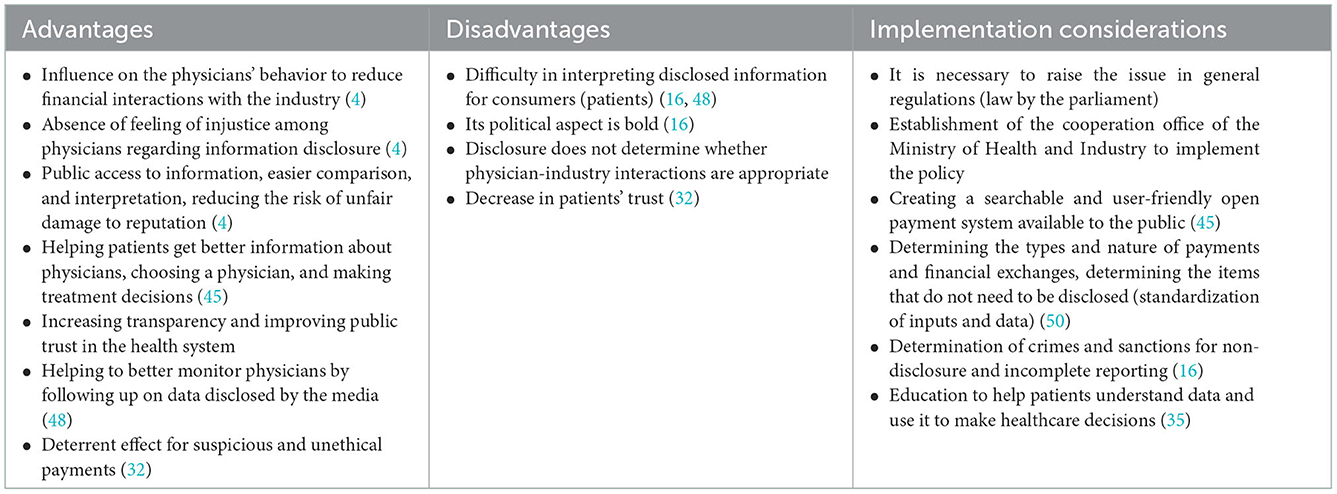
Table 7. Advantages, disadvantages, and implementation considerations of policy options: 5. Mandatory industry disclosure.
Discussion
This study was conducted to identify policy options for managing physician-industry interactions in Iran, and five policy options were proposed. In this section, we have discussed these options with an emphasis on their implementation considerations.
Prohibition
There is a strong relationship between receiving industry benefits and supporting their products (2). Since the primary goal of pharmaceutical representatives is to promote their products—not to serve the interests of patients—so the best tactic for physicians is to avoid them (33). A concern often raised in defense of these interactions is that meetings between pharmaceutical representatives and physicians accompany the presentation of information about new drug products and allow busy physicians to stay up-to-date more efficiently (7). If the prohibition policy is implemented, the relationship between physicians and pharmaceutical representatives will be cut off, which should be addressed by designing and setting up an updated drug information system. Prohibition policy has a preventive approach toward unethical behavior and possible adverse consequences of physician-industry interaction (7). Passing and implementing the law prohibiting physician-industry interaction has many implementation challenges. Prohibition of such interactions requires legislators to formulate and pass clear and precise laws to ban all types of industry payments to physicians (7), which will probably face reactions from the medical community. Monitoring the implementation of the prohibition law is challenging and may transfer these payments to unobservable ways (such as cryptocurrency). Government regulators can reduce physician-industry COI by increasing sanctions for such activities.
Restriction
An alternative policy to the prohibition is to restrict physician-industry interactions and distinguish between acceptable and unacceptable payments and gifts. Evidence shows that restrictive policies may have a positive effect on improving the prescribing behavior of physicians (38). In implementing this policy, receiving inexpensive gifts for use in the physician's office (such as notebooks or pens) and items related to patient care (drug samples and medical booklets) that do not hurt care is not prohibited, but gifts and payments such as recreational events for physicians, payment for lectures or free meals are strictly prohibited due to the increased potential for COI (2, 7). The Iranian Medical Council has also declared it acceptable gifts that benefit patients (such as drug samples for poor patients) (57). Also, according to Iran's Pharmaceutical Code of Ethics, it is allowed to accept low-value gifts, such as calendars, pens, etc., from pharmaceutical companies (58).
Implementing restrictive policies requires that medical universities and hospitals have clear and transparent procedures and guidelines for accepting gifts and payments that specify what is acceptable and prohibited. Necessary executive action determines a “threshold” above which payment should be declared a financial COI. This difficult task depends on the countries' culture, history, and wealth (33). It is suggested that COI committees be formed at the health ministry and medical sciences university level to formulate exemptions and monitor their implementation according to local conditions and the extent of the relationship with the industry.
Physicians' self-regulation
The medical profession traditionally relies on self-regulation to implement the ethical standards of medicine and protect the patient's interests (51). When the primary goals of patient care, education, and clinical research may be threatened by financial or other secondary interests, physicians have a responsibility to self-regulate; as such, COI may undermine public trust in the physicians (8). Physicians often believe that a conscious commitment to ethical behavior and professionalism protects them from undue industry influence (59). Therefore, physicians' self-regulation relies on strengthening moral norms (60). Self-regulatory tools include voluntary disclosure, ethical guidelines, and educational interventions to reduce COI. Physician self-regulation can be implemented as a transitional policy to institutionalize a transparency culture before enforcing mandatory transparency laws.
In the Pharmaceutical Code of Ethics, as well as the Professional Ethics Guide of the Iranian Medical Council, there are cases of how to interact with pharmaceutical companies. For example, it is prohibited to give cash and non-cash gifts to physicians by pharmaceutical companies and to accept any money for travel and accommodation costs for vacations, participation in conferences, seminars, workshops, and continuing education programs from companies and industries, for themselves or their families (57). To implement this policy, it is necessary to compile a complete ethical guide by the Iranian Medical Council or the Ministry of Health to determine acceptable cases and ethical behaviors in physician-industry interactions.
Disclosure of physician-industry interactions can be made voluntarily on the physician's website or a central website. Another approach for physicians is to disclose their interactions with the industry through printed materials or a disclosure form during admission, an informed consent form, or verbally during consultation and examination of patients (19, 28). Physician disclosure does not lead to a behavior becoming ethical, but it is a step toward promoting ethical behavior (3); It can also lead to the promotion of the view among physicians that whatever is disclosed is no longer a problem (a moral license) (24). To promote a transparency culture, incentives are also determined to comply with ethics and professional norms (48).
One of the most critical executive measures in the physician self-regulation policy is empowerment, informing, and training about interactions with the industry and its consequences for physicians. Training is available at two academic levels for medical students and continuing education programs for practicing physicians. Educating members of medical professional societies on issues related to physician-industry interactions can be accomplished at annual meetings (56). These programs aim to help physicians better understand the COI associated with accepting gifts and other financial incentives and their potential impact on patient care (48). Evidence shows that educational programs are effective in increasing awareness and changing the attitude of physicians regarding their sensitivity toward interaction with pharmaceutical companies (18, 49).
Voluntary industry disclosure (self-regulation)
Disclosure of payments and financial interactions with pharmaceutical companies can be voluntary (self-regulation) by the industry. Self-regulation allows the industry to design, implement, and monitor payment disclosure rules (27). In this policy, pharmaceutical companies are required to disclose their payments to physicians annually (30), and a trade body (such as the Pharmaceutical Manufacturers Association) supervises the implementation of the policy (16). The European Federation of Pharmaceutical Industries and Associations (EFPIA) requires pharmaceutical companies to report physician payments (36). In Australia, the Pharmaceutical Industry Association requires member companies to publicly report payments to physicians and medical facilities (16). The industry's voluntary disclosure policy is implemented in more than 30 European countries (27, 30).
Implementing this policy requires formulating regulations of minimum disclosure standards for pharmaceutical companies, which the health system can determine. The data can be published on a central platform (for example, in the UK on the Disclosure UK database) or on the company's website. Companies have considerable discretion over publishing and accessing data, leading to different reporting or general reports without detailing payments (30). The incompleteness of payment data and lack of full disclosure of them (for example, meals) is a significant flaw of the industry's self-regulation mode (27, 30). The findings of a study showed that in 23 European countries with a self-regulation approach, disclosures are published in PDF documents on companies' websites, preventing the public from understanding payment patterns (27).
One of the critical shortcomings of industry self-regulation is that it makes disclosure by paying companies conditional on recipients' consent, and a physician can request anonymity (27, 36). In this regard, there is a need to adapt the data disclosure criteria to the personal information protection law in the country. Efforts should be made to create a culture of the willingness of physicians and companies to disclose data with names and details. In this regard, individuals and companies can use financial and non-financial incentives. Due to the voluntary nature of disclosure and the less willingness of physicians and industry to disclose information, the capacity of non-governmental organizations and civil activists can be used to pressure and encourage disclosure.
Mandatory industry disclosure
In implementing the policy of mandatory disclosure of payments by the industry, a government institution such as the Ministry of Health is responsible for implementation and monitoring (16). The most famous policy in this field is the Sunshine Payment Act, which was passed in 2010 in the United States, and a system called “open payment” was launched to implement this law in 2013, which requires pharmaceutical companies to disclose their payments to physicians in this system (3). The French initiative is the Bertrand law and the Transparency Santé system (16). Mandatory disclosure regulations have also been implemented in countries such as Japan, Turkey, Portugal, Greece, Denmark, Romania, Latvia, and Slovenia (16).
Mandatory industry disclosure must be proposed in the public regulations, and the law must be approved in the parliament, which may be influenced by the lobby of the physician's union and groups. Also, disclosure standards and criteria, along with relevant guidelines, should be developed, and a searchable and user-friendly open payment system with public access should be designed (45). In this regard, there is a need for continuous cooperation and interaction between the Ministry of Health and industry representatives, and a joint office should be established for this purpose. One of the important measures of this office is to determine the types and nature of payments and financial exchanges, to select the items that do not need to be disclosed, and in general, to standardize inputs, data, time frame, and publishing methods (50).
Types of reportable payments include cash, gifts, and stock in the form of consulting fees, food, and beverage, payments for participation in continuing education programs and lectures, grants, and research payments made directly or indirectly (through a third party) to physicians. Also, ownership interests, such as shares in pharmaceutical companies, must be reported. Financial interactions exempt from disclosure can include drug samples and items with a value of <$10 per transaction or $100 per year (3). Physicians preview the data before it is publicly released and, if necessary, object to corrections.
Supporters of public reporting policies support it as a tool to manage industry influence and COI and believe that public reporting acts as a deterrent to inappropriate relationships between physicians and industry (16). Evidence shows that public disclosure reduces the recipients of financial benefits from companies (3).
Limitations
This systematic review had limitations. At the time of the search, we, unfortunately, did not have access to the Scopus database. Therefore, we may have missed some relevant studies. We only included English-language studies, so strategies and interventions published in non-English-language articles may have been unique that we did not consider.
Conclusion
The proposed policies in our study include advantages, challenges, and implementation considerations based on up-to-date evidence that can help policymakers to manage COI in physician-pharmaceutical industry interactions in Iran's health system. Transparency is an essential part of resolving COI. Disclosure of industry payments to physicians is necessary but insufficient for addressing COI and ensuring the independence of physicians, regulators, and health systems. Disclosure of COI does not necessarily lead to eliminating or preventing bias in clinical decision-making. Paradoxically, transparency may normalize financial COI or increase their influence through a moral license. Therefore, transparency should be accompanied by policies that seek to reduce or eliminate some of these COIs. Although the government must play an essential role through regulation and supervision, physicians must rely on self-regulation and professional ethics to rid the profession of undue commercial influence. It seems that a combination of measures can help to reduce the adverse effects of COI: firstly, using self-regulation of physicians and industry to institutionalize transparency, and in the next step, implementing mandatory industry disclosure policies and establishing restrictions on some financial interactions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
EZ and AG conceived and designed the study. EZ, AG, AN, and SB conducted the literature searches and extracted the data. HH performed the interviews. EZ and AN wrote the manuscript. HH and SB revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chimonas S, Mamoor M, Zimbalist SA, Barrow B, Bach PB, Korenstein D. Mapping conflict of interests: Scoping review. Br Med J. (2021) 375:e066576. doi: 10.1136/bmj-2021-066576
2. Coyle SL. Physician-industry relations. Part 1: Individual physicians. Ann Internal Med. (2002) 136:396–402. doi: 10.7326/0003-4819-136-5-200203050-00014
3. Kirschner NM, Sulmasy LS, Kesselheim AS. Health policy basics: The physician payment sunshine act and the open payments program. Ann Intern Med. (2014) 161:519–21. doi: 10.7326/M14-1303
4. Stoll M, Hubenschmid L, Koch C, Lieb K, Egloff B. Physicians' attitudes towards disclosure of payments from pharmaceutical companies in a nationwide voluntary transparency database: A cross-sectional survey. Br Med J Open. (2022) 12:e055963. doi: 10.1136/bmjopen-2021-055963
5. Sahm S. Of mugs, meals and more: The intricate relations between physicians and the medical industry. Med Health Care Philos. (2013) 16:265–73. doi: 10.1007/s11019-012-9391-y
6. Mitchell AP, Trivedi NU, Gennarelli RL, Chimonas S, Tabatabai SM, Goldberg J, et al. Are financial payments from the pharmaceutical industry associated with physician prescribing? A systematic review. Ann Internal Med. (2021) 174:353–61. doi: 10.7326/M20-5665
7. Mitchell A, Sarpatwari A, Bach PB. Industry payments to physicians are kickbacks. How should stakeholders respond? J Health Polit Pol Law. (2022) 2022:10041205. doi: 10.1215/03616878-10041205
8. American College of Obstetricians and Gynecologists' Committee on Ethics. Professional relationships with industry: ACOG committee statement no. 2. Obstet Gynecol. (2022) 140:904–11. doi: 10.1097/AOG.0000000000004963
9. Guo T, Sriram S, Manchanda P. The effect of information disclosure on industry payments to physicians. J Market Res. (2021) 58:115–40. doi: 10.1177/0022243720972106
10. Fadlallah R, Alkhaled L, Brax H, Nasser M, Rajabbik MH, Nass H, et al. Extent of physician-pharmaceutical industry interactions in low- and middle-income countries: A systematic review. Eur J Public Health. (2018) 28:224–30. doi: 10.1093/eurpub/ckx204
11. Sillup GP, Dehshal MH, Namini MT. Pharmaceutical companies and physicians: Assessing their relationship. Int J Healthcare Manag. (2013) 6:276–80. doi: 10.1179/2047971913Y.0000000049
12. Tasnim New Agency. Is “Drug Trade” Institutionalized Between Some Doctors Iranian Pharmaceutical Companies? (2020). Available online at: https://www.tasnimnews.com/fa/news/1398/10/29/2184490/ (accessed October 10, 2022).
13. Shojaeizadeh N, Keyvanara M, Safaeian L, Karimi S. Identification of financial incentives in irrational use and prescribing of drugs: A qualitative study. Iran J Health Insur. (2019) 2:14–20.
14. Pouragha B, Khabiri R, Pourreza A, Zarei E. Behavior of under the Iranian social security organization-insured persons on utilization of laboratory and imaging services. J Mazandaran Univ Med Sci. (2013) 23:38–47.
15. Ebrahimi A, Zand S, Amiri FB, Shahi F, Jafarian A, Kaviani A. Conflict of interest: Are Iranian breast cancer specialists prone to it? Asian Pacific J Cancer Prev. (2020) 21:1653–8. doi: 10.31557/APJCP.2020.21.6.1653
16. Grundy Q, Habibi R, Shnier A, Mayes C, Lipworth W. Decoding disclosure: Comparing conflict of interest policy among the United States, France, and Australia. Health Pol. (2018) 122:509–18. doi: 10.1016/j.healthpol.2018.03.015
17. Ansari B. Industry payments and physicians prescriptions: Effect of a payment restriction policy. Soc Sci Med. (2021) 278:113942. doi: 10.1016/j.socscimed.2021.113942
18. Fickweiler F, Ward F, Urbach E. Interactions between physicians and the pharmaceutical industry generally and sales representatives specifically and their association with physicians' attitudes and prescribing habits: A systematic review. Br Med J Open. (2017) 7:e016408. doi: 10.1136/bmjopen-2017-016408
19. Fadlallah R, Nas H, Naamani D, El-Jardali F, Hammoura I, Al-Khaled L, et al. Knowledge, beliefs and attitudes of patients and the general public towards the interactions of physicians with the pharmaceutical and the device industry: A systematic review. PLoS ONE. (2016) 11:e0160540. doi: 10.1371/journal.pone.0160540
20. Mejia J, Mejia A, Pestilli F. Open data on industry payments to healthcare providers reveal potential hidden costs to the public. Nat Commun. (2019) 10:4314. doi: 10.1038/s41467-019-12317-z
21. Nakayama DK. In defense of industry-physician relationships. Am Surgeon. (2010) 76:987–94. doi: 10.1177/000313481007600935
22. Anand CL, Dweik RA. Physician interactions with industry: striking the right balance. J Natl Comprehens Cancer Netw. (2021) 1:1–3. doi: 10.6004/jnccn.2021.7100
23. Kang JS. Ethics and industry interactions: Impact on specialty training, clinical practice, and research. Rheumat Dis Clin. (2020) 46:119–33. doi: 10.1016/j.rdc.2019.09.007
24. King M, Bearman PS. Gifts and influence: Conflict of interest policies and prescribing of psychotropic medications in the United States. Soc Sci Med. (2017) 172:153–62. doi: 10.1016/j.socscimed.2016.11.010
25. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. Br Med J. (2021) 372:n160. doi: 10.1136/bmj.n160
26. Donaldson MS, Capron AM. Patient Outcomes Research Teams: Managing Conflict of Interest. Washington, DC: National Academies Press (US) (1991).
27. Ozieranski P, Martinon L, Jachiet P-A, Mulinari S. Accessibility and quality of drug company disclosures of payments to healthcare professionals and organisations in 37 countries: A European policy review. Br Med J Open. (2021) 11:e053138. doi: 10.1136/bmjopen-2021-053138
28. Rose SL, Sah S, Dweik R, Schmidt C, Mercer M, Mitchum A, et al. Patient responses to physician disclosures of industry conflicts of interest: A randomized field experiment. Organ Behav Hum Decis Process. (2021) 166:27–38. doi: 10.1016/j.obhdp.2019.03.005
29. Moriarty F, Larkin J, Fahey T. Payments reported by the pharmaceutical industry in Ireland from 2015 to 2019: An observational study. Health Pol. (2021) 125:1297–304. doi: 10.1016/j.healthpol.2021.07.016
30. Mulinari S, Martinon L, Jachiet P-A, Ozieranski P. Pharmaceutical industry self-regulation and non-transparency: Country and company level analysis of payments to healthcare professionals in seven European countries. Health Pol. (2021) 125:915–22. doi: 10.1016/j.healthpol.2021.04.015
31. Brown SR. Physicians should refuse pharmaceutical industry gifts. Am Fam Physician. (2021) 104:348–50.
32. Kanter GP, Carpenter D, Lehmann LS, Mello MM. US nationwide disclosure of industry payments and public trust in physicians. J Am Med Assoc Netw Open. (2019) 2:e191947. doi: 10.1001/jamanetworkopen.2019.1947
33. Garattini L, Padula A. Conflict of interest disclosure: Striking a balance? Eur J Health Econ. (2019) 20:633–6. doi: 10.1007/s10198-018-1028-5
34. Martínez DP. Ethics in medical relationships with the pharmaceutical industry. Revista Colombiana de Cardiología. (2019) 26:60–2. doi: 10.1016/j.rccar.2019.04.002
35. Zezza MA, Bachhuber MA. Payments from drug companies to physicians are associated with higher volume and more expensive opioid analgesic prescribing. PLoS ONE. (2018) 13:e0209383. doi: 10.1371/journal.pone.0209383
36. Mulinari S, Ozieranski P. Disclosure of payments by pharmaceutical companies to healthcare professionals in the UK: Analysis of the Association of the British Pharmaceutical Industry's Disclosure UK database, 2015 and 2016 cohorts. Br Med J Open. (2018) 8:e023094. doi: 10.1136/bmjopen-2018-023094
37. Makowska M. Polish physicians' cooperation with the pharmaceutical industry and its potential impact on public health. PLoS ONE. (2017) 12:e0184862. doi: 10.1371/journal.pone.0184862
38. Brax H, Fadlallah R, Al-Khaled L, Kahale LA, Nas H, El-Jardali F, et al. Association between physicians' interaction with pharmaceutical companies and their clinical practices: A systematic review and meta-analysis. PLoS ONE. (2017) 12:e0175493. doi: 10.1371/journal.pone.0175493
39. Larkin I, Ang D, Steinhart J, Chao M, Patterson M, Sah S, et al. Association between academic medical center pharmaceutical detailing policies and physician prescribing. J Am Med Assoc. (2017) 317:1785–95. doi: 10.1001/jama.2017.4039
40. Nissanholtz-Gannot R, Yankellevich A. Regulating the relationship between physicians and pharmaceutical companies: A qualitative and descriptive analysis of the impact of Israeli legislation. Isr J Health Policy Res. (2017) 6:45. doi: 10.1186/s13584-017-0170-3
41. Keller F, Marczewski K, Pavlović D. The relationship between the physician and pharmaceutical industry: Background ethics and regulation proposals. Croat Med J. (2016) 57:398–401. doi: 10.3325/cmj.2016.57.398
42. Gupta S, Nayak R, Sivaranjani R. A study on the interactions of doctors with medical representatives of pharmaceutical companies in a Tertiary Care Teaching Hospital of South India. J Pharmacy Bioallied Sci. (2016) 8:47–51. doi: 10.4103/0975-7406.171695
43. Breault JL, Shenson D, Dugdale LS. Ethics of physician relationships with industry. Hosp Med Clin. (2015) 4:565–80. doi: 10.1016/j.ehmc.2015.06.002
44. Alkhaled L, Kahale L, Nass H, Brax H, Fadlallah R, Badr K, et al. Legislative, educational, policy and other interventions targeting physicians' interaction with pharmaceutical companies: A systematic review. Br Med J Open. (2014) 4:e004880. doi: 10.1136/bmjopen-2014-004880
45. Hwong AR, Qaragholi N, Carpenter D, Joffe S, Campbell EG, Lehmann LS, et al. Systematic review of state and manufacturer physician payment disclosure websites: Implications for implementation of the Sunshine Act. J Law Med Ethics. (2014) 42:208–19. doi: 10.1111/jlme.12136
46. Albersheim SG, Golan A. The physician's relationship with the pharmaceutical industry: Caveat emptor buyer beware! Israel Med Assoc J. (2011) 13:389–93.
47. Immelt SJ, Gaudiani VA, Sade RM. Should the financial link between industry and physician consultants be severed? Ann Thorac Surg. (2011) 92:781–7. doi: 10.1016/j.athoracsur.2011.05.087
48. Grande D. Limiting the influence of pharmaceutical industry gifts on physicians: Self-regulation or government intervention? J Gen Intern Med. (2010) 25:79–83. doi: 10.1007/s11606-009-1016-7
49. Fugh-Berman AJ, Scialli AR, Bell AM. Why lunch matters: Assessing physicians' perceptions about industry relationships. J Continuing Educ Health Professions. (2010) 30:197–204. doi: 10.1002/chp.20081
50. Ross JS, Lackner JE, Lurie P, Gross CP, Wolfe S, Krumholz HM. Pharmaceutical company payments to physicians - Early experiences with disclosure laws in Vermont and Minnesota. J Am Med Assoc. (2007) 297:1216–23. doi: 10.1001/jama.297.11.1216
51. Thomas JM. Self-regulation and the relationship of physicians with the pharmaceutical industry. Virtual Mentor. (2005) 7:504. doi: 10.1001/virtualmentor.2005.7.4.jdsc1-0504
52. Studdert DM, Mello MM, Brennan TA. Financial conflicts of interest in physicians' relationships with the pharmaceutical industry - Self-regulation in the shadow of federal prosecution. N Engl J Med. (2004) 351:1891–900. doi: 10.1056/NEJMlim042229
53. Wager E. How to dance with porcupines: Rules and guidelines on doctors' relations with drug companies. Br Med J. (2003) 326:1196–8. doi: 10.1136/bmj.326.7400.1196
54. Wazana A, Primeau F. Ethical considerations in the relationship between physicians and the pharmaceutical industry. Med J Austr. (2002) 176:118–21. doi: 10.1016/S0193-953X(01)00016-8
55. Wazana A. Physicians and the pharmaceutical industry - Is a gift ever just a gift? J Am Med Assoc. (2000) 283:373–80. doi: 10.1001/jama.283.3.373
56. Coyle SL. Physician-industry relations. Part 2: Organizational issues. Ann Intern Med. (2002) 136:403–6. doi: 10.7326/0003-4819-136-5-200203050-00015
57. Iran Medical Council. General Guidance of Professional Ethics for Medical Professionals and Affiliates of the Medical Council of the Islamic Republic of Iran. (2018). Available online at: https://irimc.org/ (accessed October 10, 2022).
58. Ahmadi F, Zarei E. Prescribing patterns of rural family physicians: A study in Kermanshah Province, Iran. BMC Public Health. (2017) 17:908. doi: 10.1186/s12889-017-4932-1
59. Sah S, Fugh-Berman A. Physicians under the influence: Social psychology and industry marketing strategies. J Law Med Ethics. (2013) 41:665–72. doi: 10.1111/jlme.12076
60. Yazdani S, Azandehi SK, Ghorbani A, Shakerian S. Explaining the process of choosing clinical specialties in general medical graduates: A grounded theory. Electr J General Med. (2018) 15:em89. doi: 10.29333/ejgm/93457
Appendix 1
PubMed
#1 (doctor*[Title]) OR (physician*[Title])
#2 ((drug*[Title]) OR (pharmaceutical [Title])) OR (industry [Title])
#3 (((((((((interact*[Title/Abstract]) OR (relation*[Title/Abstract])) OR (link*[Title/Abstract])) OR (collaboration*[Title/Abstract])) OR (contact [Title/Abstract])) OR (influence*[Title/Abstract])) OR (payment [Title/Abstract])) OR (tie [Title/Abstract])) OR (conflict of interest [Title/Abstract]))
#4 ((((((((control [Title/Abstract]) OR (polic*[Title/Abstract])) OR (strateg*[Title/Abstract])) OR (intervention*[Title/Abstract])) OR (solution*[Title/Abstract])) OR (law [Title/Abstract])) OR (regulat*[Title/Abstract])) OR (recommendation*[Title/Abstract])) OR (legislat*[Title/Abstract]) (((((drug*[Title]) OR (pharmaceutical [Title])) OR (industry [Title])) AND ((doctor*[Title]) OR (physician*[Title]))) AND ((((((((((interact*[Title/Abstract]) OR (relation*[Title/Abstract])) OR (link*[Title/Abstract])) OR (collaboration*[Title/Abstract])) OR (contact [Title/Abstract])) OR (influence*[Title/Abstract])) OR (payment [Title/Abstract])) OR (tie [Title/Abstract])) OR (conflict of interest [Title/Abstract])))) AND (((((((((control [Title/Abstract]) OR (polic*[Title/Abstract])) OR (strateg*[Title/Abstract])) OR (intervention*[Title/Abstract])) OR (solution*[Title/Abstract])) OR (law [Title/Abstract])) OR (regulat*[Title/Abstract])) OR (recommendation*[Title/Abstract])) OR (legislat*[Title/Abstract])) Filters: English, from 2000 – 2022
Filters: English, from 2000 – 2022
WOS
#1 (TI=(doctor*)) OR TI=(physician* )
#2 ((TI=(drug*)) OR TI=(pharmaceutical)) OR TI=(industry)
#3 ((((((((TS=(interact*)) OR TS=(relation* )) OR TS=(link*)) OR TS=(collaboration*)) OR TS=(contact )) OR TS=(influence*)) OR TS=(payment )) OR TS=(tie )) OR TS=(conflict of interest)
#4 ((((((((TS=(control )) OR TS=(polic*)) OR TS=(strateg*)) OR TS=(intervention*)) OR TS=(solution*)) OR TS=(law )) OR TS=(regulat*)) OR TS=(recommendation*)) OR TS=(legislat*)
#5: #1 AND #2 AND #3 AND #4
2000–2022
ProQuest:35
((ti(doctor*) OR ti(physician*)) AND PEER(yes)) AND ((ti(industry) OR ti(drug*) OR ti(pharmaceutical)) AND PEER(yes)) AND ((ab(interact*) OR ab(relation*) OR ab(link*) OR ab(collaboration*) OR ab(contact) OR ab(influence*) OR ab(payment) OR ab(tie) OR ab(conflict of interest)) AND PEER(yes)) AND ((ab(control) OR ab(polic*) OR ab(strateg*) OR ab(intervention*) OR ab(solution*) OR ab(law) OR ab(regulat*) OR ab(recommendation*) OR ab(legislat*)) AND PEER(yes))
2000–2022
Keywords: conflict of interest, medical ethics, pharmaceutical industry, transparency, gift giving, physician-industry interaction
Citation: Zarei E, Ghaffari A, Nikoobar A, Bastami S and Hamdghaddari H (2023) Interaction between physicians and the pharmaceutical industry: A scoping review for developing a policy brief. Front. Public Health 10:1072708. doi: 10.3389/fpubh.2022.1072708
Received: 17 October 2022; Accepted: 19 December 2022;
Published: 12 January 2023.
Edited by:
Morteza Arab-Zozani, Birjand University of Medical Sciences, IranReviewed by:
Demba Sarr, University of Georgia, United StatesJulie Campbell, University of Tasmania, Australia
Copyright © 2023 Zarei, Ghaffari, Nikoobar, Bastami and Hamdghaddari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ehsan Zarei,  emFyZWlfMTk4MEB5YWhvby5jb20=
emFyZWlfMTk4MEB5YWhvby5jb20=
 Ehsan Zarei
Ehsan Zarei Amir Ghaffari
Amir Ghaffari Ali Nikoobar
Ali Nikoobar Shayan Bastami
Shayan Bastami Hasan Hamdghaddari
Hasan Hamdghaddari