
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 01 December 2022
Sec. Occupational Health and Safety
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.1055374
 Yidan Dong1
Yidan Dong1 Ping Jiang1
Ping Jiang1 Xu Jin1
Xu Jin1 Nanyu Jiang1
Nanyu Jiang1 Wenchu Huang1
Wenchu Huang1 Yu Peng1
Yu Peng1 Yuhong Shen2
Yuhong Shen2 Lihua He1*
Lihua He1* Mikael Forsman3,4
Mikael Forsman3,4 Liyun Yang3,4
Liyun Yang3,4Background: Musculoskeletal disorders (MSDs) are critical occupational and social problems. With the improvement of production mechanization and automation, and the widespread application of computers, more occupations are exposed to static postures and load. This study explored the role of inflammation in the association between static postures exposure and MSDs.
Methods: This study adopted a prospective nested case-control design in which 66 lower back MSDs cases and 66 healthy controls were selected from a cohort study of university employees. The personal information, postural load, musculoskeletal symptoms, pressure pain thresholds (PPTs), and inflammatory cytokines were collected. Logistic and linear regressions were used to investigate the association among postural load, inflammatory cytokines, and lower back MSDs. Mediation analysis was used to calculate the mediation effect.
Results: The results of logistic and linear regressions showed that postural load and inflammatory cytokines were positively associated with lower back MSDs (P < 0.05), and postural load was positively associated with inflammatory cytokines (P < 0.05). Further, mediation analysis showed that the mediation effect of postural load on the lower back MSDs through TNF-α was 0.073 (95%CI: 0.025–0.128), and the mediation effect of posture load on the lower back MSDs through IL-6 was 0.098 (95%CI: 0.041–0.179), respectively.
Conclusion: Static postures were associated with the occurrence of MSDs through inflammatory cytokines, and low-level inflammation may be a critical early event in the generation of MSDs. This study may help bridge the gap of potential mechanisms linking static postures to increased risks of MSDs, and provide new evidence for targeted protection against the global increasing MSDs.
Musculoskeletal disorders (MSDs) are major health problems globally (1). For example, data from the Global Burden of Disease Study 2019 showed that about 1.71 billion people worldwide suffer from MSDs, and MSDs are the highest contributors to years of life lived with disability (2). These diseases not only bring severe individual consequences such as disability, poor quality of life, and sickness absence but also impose a major economic burden on society (3). For example, the total cost of lost productivity attributable to MSDs was approximately 240 billion euros in the EU, up to 2% of the gross domestic product (GDP) (3).
Biomechanical factors (e.g., postural load) are considered as risk factors for MSDs (4). Static postures refer to physical exertion in which the same posture or position is held throughout the exertion. These types of exertions usually put a low level of sustained loads or forces on the muscles and tendons. Static postures mainly occur in occupational activities such as sitting, standing, prolonged bent or twisted posture of trunk, neck, or wrists, working with hands above shoulder level, kneeling or squatting posture (5). Prolonged postures can lead to static loading of the muscles and joint tissues and cause discomfort (6). Previous studies have shown that occupational activities requiring static loading of muscles and joint tissues constitute risk factors for the development of MSDs (7, 8). With the improvement of production mechanization and automation, as well as the widespread application of computers, more occupations are exposed to static postures and load (9, 10). Although the association between static postures and MSDs has been observed in humans, most studies are cross-sectional. There is a lack of human longitudinal studies on the causal relationship between static postures and MSDs.
Despite epidemiological evidence for the role of repetition, force, and postural load in the onset and progression of MSDs, complete understanding of these important health problems requires further elucidation of the pathophysiological mechanisms of MSDs. The National Occupational Research Agenda for Musculoskeletal Health emphasizes the need for etiologic research in determining the contribution of biomechanical mechanisms toward the development of tissue injury and MSDs, as well as strategies to reduce their severity (11). However, the mechanisms leading to pathophysiological tissue changes associated with MSDs are still incompletely understood.
The inflammation in musculoskeletal tissues may be an essential element in the pathogenesis of MSDs (12, 13). In a small number of studies, the severity of upper extremity MSDs has been positively associated with serum levels of tumor necrosis factor-alpha (TNF-α), interleukin-1alpha (IL-1α), interleukin-1beta (IL-1β) and interleukin- 6 (IL-6) in patients (14, 15). Freeland et al. have found higher levels of IL-6 and prostaglandin E2 (PGE2) in flexor tendon synovium in patients with carpal tunnel syndrome than in healthy controls (16). In addition, previous studies have also shown increased levels of TNF-α, IL-1α, IL-1β, and IL-6 in serum and muscle tissue in animals with injuries of the upper forelimb caused by excessive repetitive tasks (17, 18). The results of these studies suggested that inflammation may play an essential role in the course of MSDs. Therefore, it is plausible to hypothesize that static postures mediate or aggravate the occurrence of MSDs through inflammatory pathways. In light of the above findings, in this study, we aimed to explore the role of inflammation in the association between static postures exposure and MSDs based on a prospective nested case-control study.
This population-based case-control study came from the cohort study of MSDs among university employees in a university in Beijing, China. This cohort study was conducted from March to August 2021. University employees usually work in a sitting position for a prolonged period. This cohort study recruited 400 healthy participants who had to meet the following criteria: (a) adults aged 18 to 30 years, (b) no history of smoking and drinking, (c) no history of musculoskeletal injuries, rheumatoid arthritis, tumors, tuberculosis, infections or other diseases affecting the musculoskeletal system, (d) no history of respiratory infections, nephritis, hepatitis, and other inflammatory diseases in the past 1 month, (e) no history of taking analgesic and anti-inflammatory drugs in the past 1 month, (f) no symptoms in the neck, shoulders, upper back, lower back, elbows, wrists/hands, hips/thighs, knees, and ankles/feet in the past 3 months. The baseline survey used questionnaires to collect information on general demographic characteristics, occupational characteristics, and musculoskeletal symptoms. In the baseline survey, 383 valid questionnaires were collected. Among them, 109 participants who withdrew from the study and those who did not meet the inclusion criteria of the case-control study were excluded, and 66 participants with lower back MSDs were included in the first follow-up survey 3 months later. Controls were randomly selected from the remaining healthy participants by 1-to-1 matching based on gender and age (±2 years). In this study, cases were taken from the participants who had lower back symptoms such as pain, discomfort, numbness, or limitation of movement in the past 1 month, which lasted for more than 24 h and had no relief after rest, without lower back symptoms caused by menstruation and exercise. Controls were selected from those who had no symptoms in the neck, shoulders, upper back, lower back, elbows, wrists/hands, hips/thighs, knees, and ankles/feet during the past 3 months.
In addition, cases and controls had to meet the following criteria during the follow-up period: (a) no history of smoking and drinking, (b) no history of musculoskeletal injuries, rheumatoid arthritis, tumors, tuberculosis, infections, or other diseases affecting the musculoskeletal system, (c) no history of respiratory infections, nephritis, hepatitis, and other inflammatory diseases, (d) no history of taking analgesic and anti-inflammatory drugs. Finally, 66 cases and 66 controls were included in this study. All participants provided written informed consent before the study began. This study was approved by the Institutional Review Board of Peking University (IRB0000105220087). The flow chart of the nested case-control study is shown in Figure 1.
Information on demographic characteristics (gender, education, age, body mass index (BMI), and physical exercise time per week) and occupational exposure (hours spent per day working, hours spent per day working in a sitting position, hours spent per day using computers, hours spent per day using phones) were obtained through face to face interviews. The postural load of participants exposed to static postures was assessed by Rapid Upper Limb Assessment (RULA) (19). The overall Cronbach's α of RULA in this study was 0.764. RULA is divided into two sections labeled A and B. Section A covers the upper arms, lower arms, and wrists (upper limb score, 1–9 points), and section B covers the neck, trunk, and legs (lower limb score, 1–9 points). The scores for group A and group B postures and the scores for static muscle work and force are added to give an upper limb score and a lower limb score. The two scores are combined in a table to give a grand score (1–7 points). The English version of the instrument can be accessed by clicking on the following link: http://www.rula.co.uk. The grand score is divided into three levels, which are low postural load (1 to 4 points), moderate postural load (5 to 6 points), and high postural load (7 points).
The musculoskeletal symptoms were evaluated by the Nordic Musculoskeletal Questionnaire (NMQ) (20). The overall Cronbach's α in this study was 0.907. Participants were asked if they experienced musculoskeletal symptoms such as ache, pain, or discomfort during the past 1 month and during the past 3 months, by using a body map with nine body regions: neck, shoulders, upper back, low back, elbows, wrists/hands, hips/thighs, knees, and ankles/feet. Furthermore, symptoms in the past 1 month were assessed by self-reported pain duration (< 1 day, 1–7 days, 8–30 days, almost every day), pain frequency (1 time/month, 2–4 times/month, 5–10 times/month, >10 times/month, always symptomatic), and pain intensity (a 0 to 10 Visual Analog Scale (VAS): 0 mark as being painless, 1–3 marks as mild pain, 4–6 marks as moderate pain, 7–9 severe pain, and 10 marks as maximum pain) in each body part.
The pressure pain thresholds (PPTs) of the participants' lumbar multifidus muscles were measured by a hand-held digital force gauge (Wagner FDX 25, USA). The PPT was measured in all subjects in the prone position by trained physiotherapists. The force area of FDX 25 was a 1 cm2 round rubber tip, which was used to apply pressure to 2 cm lateral to the L4/L5 interspinous space (21). The examiners practiced applying pressure at a rate of approximately 1 kg/s. Standardized instructions were read to the participants: “The moment the pressure increases to a point where it first feels uncomfortable or painful, press and releases the button. This means the first onset of discomfort or pain and not the most pressure you can bear”. The PPT measurements were repeated three times with a pause of 10 s between the measurements. The average value of the three measurements was used as the individual's PPT of the lumbar multifidus muscles.
The peripheral blood was sampled between 8:00 a.m. and 9:00 a.m. during each clinic visit. The serum was harvested by centrifuging blood at 3,000 rpm (1,500 g) for 10 min and then stored at −80°C until assayed. The levels of TNF-α, IL-1β, and IL-6 in the serum were determined using ELISA kits (Solarbio Science & Technology Co. Ltd., China), and the PGE2 was determined using ELISA kits (Cusabio Biotech Co. Ltd., China). The procedures were performed according to the manufacturer's instructions. The specimens were examined using FLUOstar Omega (BMG Labtech, Germany). The levels of inflammatory cytokines were divided into low, moderate, and high levels according to tertiles of serum TNF-α, IL-1β, IL-6, and PGE2 in controls.
Descriptive statistics were performed with STATA 13.0 (STATA Corp, TX, USA) to reveal the characteristics of participants by using count, percentage, and median with interquartile range (IQR). The normality of distribution was determined by the Shapiro-Wilk test. The Chi-square (χ2) test and the Wilcoxon test were used to evaluate the differences in demographic, occupational, and clinical characteristics between lower back MSDs cases and controls. Binary logistic regression was used to estimate odd ratios (ORs) with 95% confidence intervals (CI) for the associations of postural load and inflammatory cytokines with lower back MSDs. Linear regression was used to assess the association between postural load and inflammatory cytokines.
The following logistic equation can describe the mediation effect of binary outcomes. Where X is the independent variable, M is the mediator, and Y is the dependent variable. The coefficient c in equation (1) represents the total effect of X on Y when M is not considered, and e1 is the corresponding residual. The coefficient a in equation (2) represents the effect of X on M. The coefficient b in equation (3) represents the effect of M on Y after controlling for X, the coefficient c' represents the direct effect of X on Y after controlling for M, e2 and e3 represent the residuals of M and Y, respectively.
In the mediation model of continuous outcomes, the mediation effect is equal to ab or c-c'. However, for the mediation model of categorical X, continuous M and Y, the regression coefficients are not on the same scale, and a standardized regression coefficient conversion need to be carried out. The conversion method is as follows:
The standardized coefficients and the mediation effect were calculated using MPLUS 7.0 (Muthén and Muthén, CA, USA). The nonparametric percentile bootstrap method with deviation correction was adopted to test the significance of the mediation effect. The number of bootstrap operation samples was set to 1,000, and the confidence level and the confidence interval were taken as 95%.
As is seen in Table 1, the results showed that there was no significant difference between the cases and controls in gender, education, age, BMI, and physical exercise time per week (P > 0.05). In terms of occupational exposure, the hours spent per day working, the hours spent per day working in a sitting position, and the RULA score in the cases were higher than those in the controls (P < 0.05). Regarding clinical characteristics, PPTs and the level of PGE2 in the cases were significantly lower than those in the controls, while the levels of TNF-α, IL-1β, and IL-6 in the cases were significantly higher than those in the controls (P < 0.05).
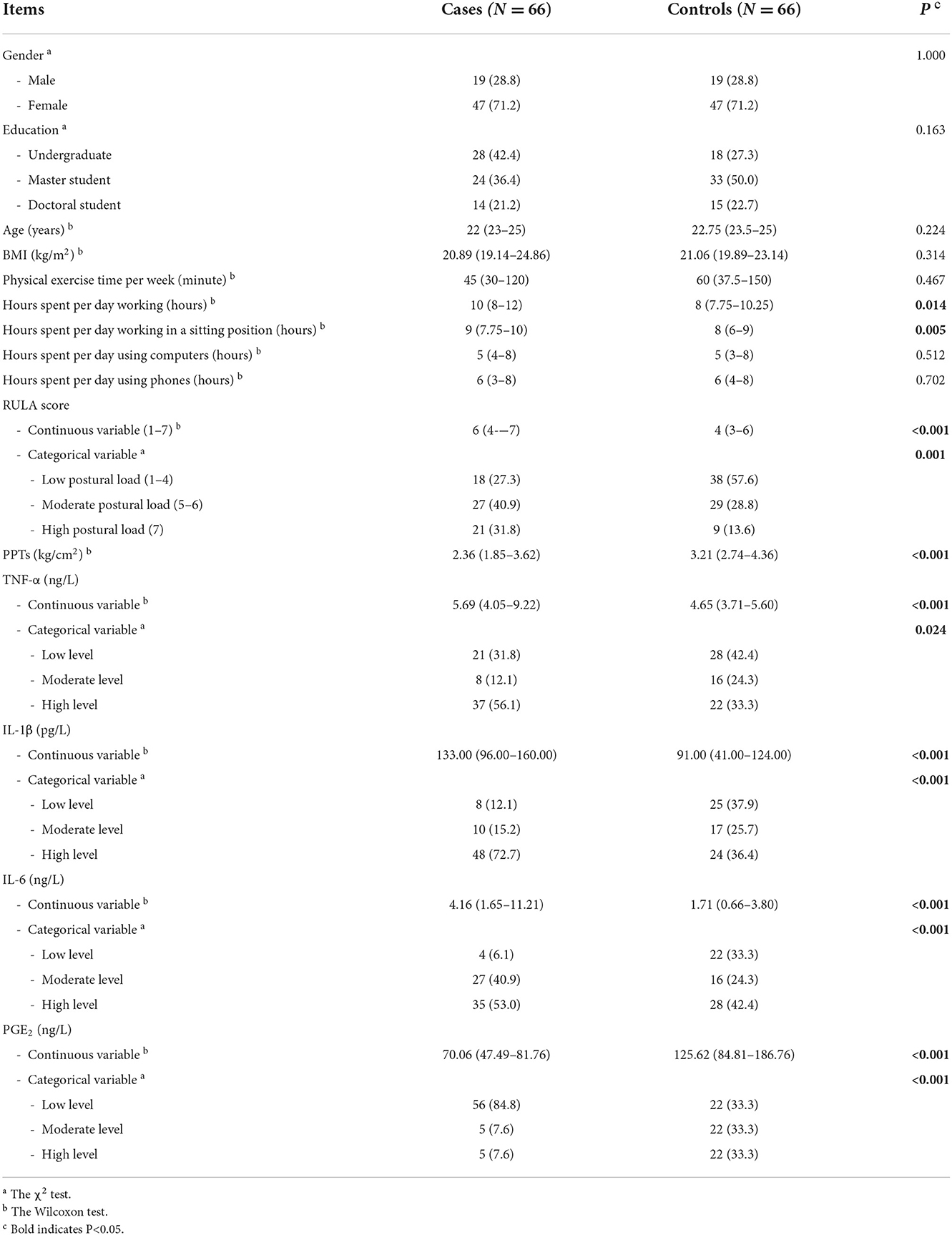
Table 1. Demographic, occupational, and clinical characteristics of lower back MSDs cases and controls (N = 132).
The association of postural load and inflammatory cytokines with lower back MSDs are shown in Figures 2A,B. After adjusting for potential confounding factors, the results of logistic regression indicated that the risk of MSDs in the lower back increased by 1.49 times with an increase of one point of the RULA score. Taking the “low postural load” group as a reference, the risk of lower back MSDs in the “moderate postural load” and “high postural load” groups were 2.37 (95%CI: 0.96–5.86) and 4.27 (95%CI: 1.50–12.10), respectively. After adjusting for potential confounding factors, the results of logistic regression showed that with one unit increase in the levels of TNF-α, IL-1β, and IL-6 in serum, the risk of MSDs in the lower back increased by 1.27 times, 1.02 times and 1.14 times, respectively.
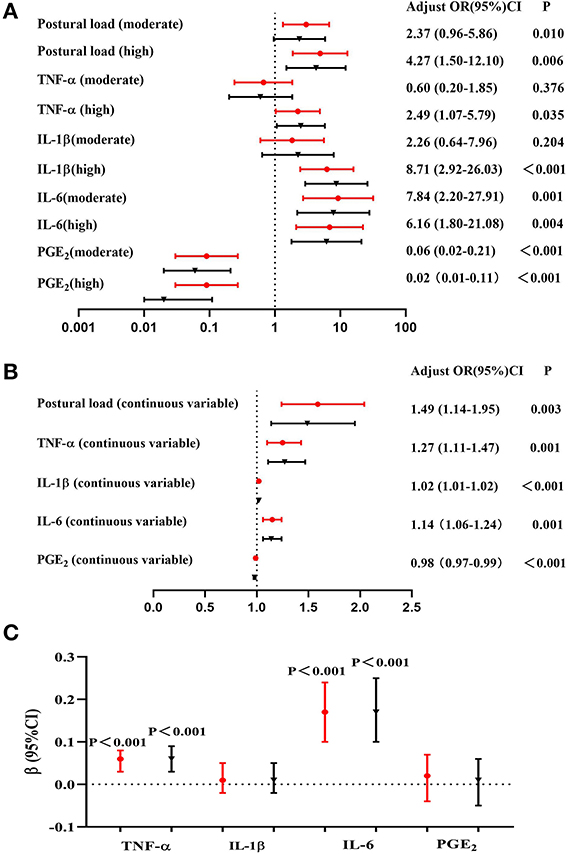
Figure 2. Association among postural load, inflammatory cytokines, and lower back MSDs (N = 132). (A) Association between postural load (categorical variable) and lower back MSDs, (B) Association between postural load (continuous variable) and lower back MSDs, (C) Association between postural load and inflammatory cytokines. The red circle indicates unadjusted models, and the black triangle indicates adjusted models (adjusted for gender, age, BMI, physical exercise time per week, hours spent per day working, hours spent per day working in a sitting position, hours spent per day using computers, and hours spent per day using phones).
The association between postural load and inflammatory cytokines is shown in Figure 2C. The serum TNF-α, IL-1β, IL-6, and PGE2 levels of all participants were transformed to logarithmic variables. After adjusting for potential confounding factors, the results of linear regression showed that the levels of TNF-α and IL-6 were associated with postural load (β = 0.06, P < 0.001 and β = 0.17, P < 0.001, respectively).
After adjusting for gender, age, BMI, physical exercise time per week, hours spent per day working, hours spent per day working in a sitting position, hours spent per day using computers, and hours spent per day using phones, the results of mediation analysis showed that in the mediation model of TNF-α (Figure 3A), the total effect of postural load on the lower back MSDs was 0.269 (95%CI: 0.091–0.421), and the direct effect was 0.196 (95%CI: 0.018–0.360). The mediation effect of postural load on the lower back MSDs through TNF-α was 0.073 (95%CI: 0.025–0.128), accounting for 27.1% of the total effect. In the mediation model of IL-6 (Figure 3B), the total effect of postural load on the lower back MSDs was 0.259 (95%CI: 0.077–0.410), and the mediation effect of postural load on the lower back MSDs through IL-6 was 0.098 (95%CI: 0.041–0.179), accounting for 100% of the total effect.
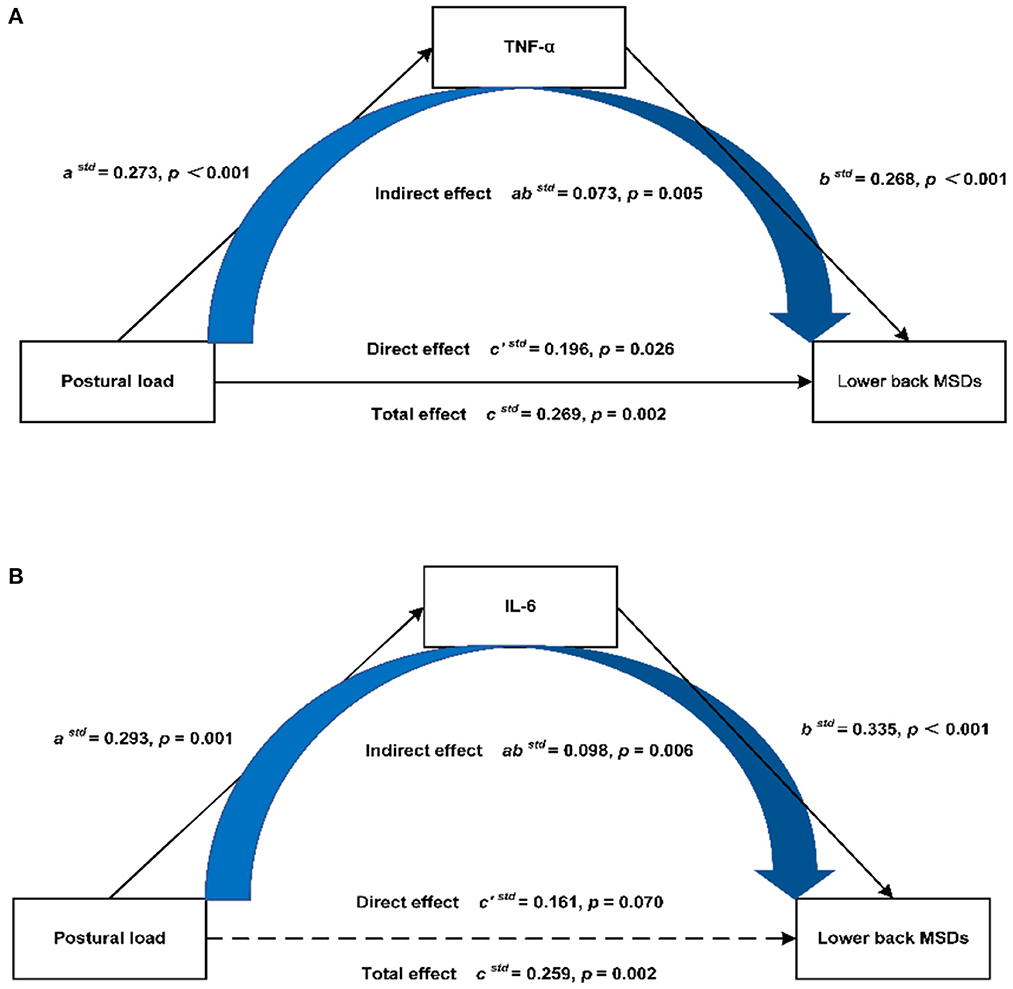
Figure 3. Mediation model of postural load on the lower back MSDs through inflammatory cytokines. (A) Mediation model of postural load on the lower back MSDs through TNF-α, (B) Mediation model of postural load on the lower back MSDs through IL-6.
In this study, we investigated the role of inflammation in the association between static postures exposure and MSDs based on a prospective nested case-control study. The major findings of our study include that: (a) the postural load, PPTs, and serum inflammatory cytokines were associated with lower back MSDs among university employees exposed to static postures, (b) the postural load was associated with inflammatory cytokines among university employees exposed to static postures, c) by the use of mediation analysis, we found that postural load affected lower back MSDs through inflammatory cytokines.
Our study showed a positive correlation between postural load and lower back MSDs among university employees exposed to static postures, which is consistent with previous studies (8, 22). A plausible hypothesis, which has been proposed previously and is supported by our findings, is that long-term static postures may change the internal biomechanical loading level or muscle tissue tolerance level by adjusting the physiologic reaction of the muscles, resulting in the internal biomechanical loading exceeding the tolerance of muscle tissues, which will cause tissue damages and lead to the occurrence of MSDs (23).
Another finding in our study was that lower back MSDs were associated with increased pain sensitivity. A previous systematic review and meta-analysis did not find such a significantly lower pain threshold among office workers with chronic neck pain when compared with healthy controls (24). But there is at least one study with findings in line with our study, that is a study of patients with carpal tunnel syndrome which showed a lower pain threshold than healthy controls (25). Our study also found musculoskeletal pain was associated with an increase in inflammatory cytokines. Previous studies have shown that inflammatory cytokines may be related to pain sensitivity, and inflammation may play an important role in the progression of pain (26, 27). For example, a few studies have indicated that higher plasma levels of pro-inflammatory cytokines TNF-α, IL-6, and IL-8 may be related to higher pain sensitivity (28, 29). The reason that inflammatory cytokines may affect the increase of pain sensitivity may be induced by the sensitization of peripheral nociceptors by pro-inflammatory mediators (30). It has also been shown that pro-inflammatory mediators, like cytokines signal the brain via humoral, vagal, and spinal pathways and induce symptoms of sickness behavior, including pain amplification (29). For example, TNF-α regulates the sensitivity of nociceptive neurons through potassium, sodium, and calcium ion channels, thus possibly participating in the pain pathway (31). IL-1β in addition to inducing inflammatory reaction, also directly activates nociceptors to generate action potentials and induce pain hypersensitivity (32). IL-6 may be involved in microglial and astrocytic activation and in the regulation of neuronal neuropeptide expression, and then induce pain hypersensitivity (33). PGE2 may cause pain by directly exciting nociceptive nociceptors and indirectly stimulating the release of pain-related neuropeptides, such as substance P and calcitonin gene-related peptides from nociceptors and their central and peripheral terminals (34).
Cytokines are proteins involved in many cellular processes, which are the chief stimulators of acute-phase-response proteins and contribute to the development and remediation of signs and symptoms of acute and chronic inflammation, such as the recruitment of immune cells. The increase of pro-inflammatory cytokines in serum indicates a chronic and/or systemic inflammatory reaction in the body. The cytokines TNF-α, IL-1, and IL-6 are intercellular signaling polypeptides produced by most injured cells and activated immune cells, including activated monocytes and macrophages (35). TNF-α and IL-1 can potently stimulate immune and stromal cell production of other cytokines and chemokines as well as most mechanisms of inflammation, including phagocyte proliferation and activation, adhesion, and angiogenesis (35). IL-6, a pleiotropic cytokine, has many pro-inflammatory effects that overlap with those of IL-1 and TNF-α. TNF-α and IL-1β are not influenced by the glycogen content of skeletal muscle and thus are not elevated in serum during exercise unless tissue damage has occurred (36). IL-6 is a tightly regulated cytokine normally not detectable in serum unless there is injury, infection, or cellular stress (12). PGE2 is a factor believed to cause vasodilation, edema, and increased release of inflammatory cytokines. When tissue injury occurs, PGE2 is produced by a large number of inflammatory cells in inflammatory tissue, which plays an essential role in the process of inflammation and pain.
Mediation analysis in the population provided an indication that postural load may affect the occurrence of MSDs through inflammatory cytokines such as TNF-a and IL-6. These new findings showed that there may be the participation of tissue inflammatory and systemic inflammation in the process of MSDs induced by static postures, which is in agreement with previous studies of repetitive tasks and exercise-induced skeletal muscle injuries (17, 18). Elevated systemic inflammation induces a pro-inflammatory response in tissues, such as muscles at the injury site (37). Conversely, inflamed tissues secrete inflammatory cytokines that contribute to systemic inflammation (38). This self-perpetuating cycle could underpin a chronically elevated inflammatory state with profound effects on the physiology and structure of musculoskeletal tissues, including connective tissues (39, 40).
To our best knowledge, this is an innovative study linking static postures and MSDs through a viewpoint of inflammatory pathways. The main strengths of our study are its prospective cohort-based, nested case-control design, which minimized selection bias and information bias. Our study provides evidence for the potential mechanism of increased MSDs risks associated with static postures exposure. Moreover, the increase of inflammatory cytokines may be a critical early event in MSDs progression and intervention target for the primary prevention of MSDs. Further studies are needed to investigate if preventing the occurrence of inflammation contributes to reducing the development of MSDs. However, several limitations should be noted. Firstly, variables may be influenced by subjective factors, and more objective methods such as clinical examination and special tests (such as surface electromyography) should be used in future research (41, 42). Secondly, the sample size of our study is limited, and it is quite advisable to include more occupational populations exposed to static postures to verify the results in future studies.
Long-term static postures are closely related to the occurrence of MSDs through a viewpoint of inflammatory pathways, and low-level inflammation may be a critical early event in the generation of MSDs. Our findings may help bridge the gap of potential mechanisms linking static postures to increased risks of MSDs, and provide new evidence for targeted protection against the globally increasing MSDs. In addition, this study can likely contribute to the prevention of MSDs and improved health of the occupational population.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board of Peking University. The patients/participants provided their written informed consent to participate in this study.
YD, YS, and LH were responsible for the study conceptualization, methodology development and validation, and study supervision. YD, PJ, XJ, NJ, and WH contributed to the conduction of population surveys. YD and PJ contributed to the preparation of samples and molecular biology experiments. YD contributed to the biostatistical analysis and interpretation and drafting of the manuscript. YD, XJ, NJ, WH, MF, and LY contributed to the critical revision of the manuscript for important intellectual content. All authors have read and approved the final version of the manuscript and agreed with the order of presentation of the authors.
This study was supported by Foundation strengthening program of China (2020JCJQJJ238) and National Key Technologies Research and Development Program of China (2016YFC0801700).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. European Agency for Safety Health at Work. Work-related MSDs: prevalence, costs and demographics in the EU. (2019). Available online at: https://osha.europa.eu/en/publications/work-related-musculoskeletal-disorders-prevalence-costs-and-demographics-eu/view (accessed November 15, 2019).
2. Cieza A, Causey K, Kamenov K, Hanson SW, Chatterji S, Vos T. Global estimates of the need for rehabilitation based on the global burden of disease study 2019: A systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:2006–17. doi: 10.1016/S0140-6736(20)32340-0
3. Bevan S. Economic impact of musculoskeletal disorders (msds) on work in europe. Best Pract Res Clin Rheumatol. (2015) 29:356–73. doi: 10.1016/j.berh.2015.08.002
4. da Costa BR, Vieira ER. Risk factors for work-related musculoskeletal disorders: a systematic review of recent longitudinal studies. Am J Ind Med. (2010) 53:285–323. doi: 10.1002/ajim.20750
5. Hildebrandt VH, Bongers PM, van Dijk FJ, Kemper HC, Dul J. Dutch musculoskeletal questionnaire: description and basic qualities. Ergonomics. (2001) 44:1038–55. doi: 10.1080/00140130110087437
6. Pope MH, Goh KL, Magnusson ML. Spine ergonomics. Annu Rev Biomed Eng. (2002) 4:49–68. doi: 10.1146/annurev.bioeng.4.092101.122107
7. De Sio S, Traversini V, Rinaldo F, Colasanti V, Buomprisco G, Perri R, et al. Ergonomic risk and preventive measures of musculoskeletal disorders in the dentistry environment: An umbrella review. PeerJ. (2018) 6:e4154. doi: 10.7717/peerj.4154
8. Waongenngarm P, van der Beek AJ, Akkarakittichoke N, Janwantanakul P. Perceived musculoskeletal discomfort and its association with postural shifts during 4-h prolonged sitting in office workers. Appl Ergon. (2020) 89:103225. doi: 10.1016/j.apergo.2020.103225
9. Baker R, Coenen P, Howie E, Williamson A, Straker L. The short term musculoskeletal and cognitive effects of prolonged sitting during office computer work. Int J Environ Res Public Health. (2018) 15:1678. doi: 10.3390/ijerph15081678
10. Locks F, Gupta N, Hallman D, Birk Jørgensen M, Oliveira AB, Holtermann A. Association between objectively measured static standing and low back pain - a cross-sectional study among blue-collar workers. Ergonomics. (2018) 61:1196–207. doi: 10.1080/00140139.2018.1455900
11. National Institute for Occupational Safety Health. National Occupational Research Agenda for Musculoskeletal Health. (2022). Available online at: https://www.cdc.gov/nora/councils/mus/researchagenda.html (accessed May 23, 2022).
12. Barbe MF, Barr AE. Inflammation and the pathophysiology of work-related musculoskeletal disorders. Brain Behav Immun. (2006) 20:423–9. doi: 10.1016/j.bbi.2006.03.001
13. Barr AE, Barbe MF, Clark BD. Systemic inflammatory mediators contribute to widespread effects in work-related musculoskeletal disorders. Exerc Sport Sci Rev. (2004) 32:135–42. doi: 10.1097/00003677-200410000-00003
14. Carp SJ, Barbe MF, Winter KA, Amin M, Barr AE. Inflammatory biomarkers increase with severity of upper-extremity overuse disorders. Clin Sci. (2007) 112:305–14. doi: 10.1042/CS20060050
15. Riondino S, La Farina F, Martini F, Guadagni F, Ferroni P. Functional impairment in video terminal operators is related to low-grade inflammation. Int Arch Occup Environ Health. (2011) 84:745–51. doi: 10.1007/s00420-010-0605-4
16. Freeland AE, Tucci MA, Barbieri RA, Angel MF, Nick TG. Biochemical evaluation of serum and flexor tenosynovium in carpal tunnel syndrome. Microsurgery. (2002) 22:378–85. doi: 10.1002/micr.10065
17. Fedorczyk JM, Barr AE, Rani S, Gao HG, Amin M, Amin S, et al. Exposure-dependent increases in il-1beta, substance p, ctgf, and tendinosis in flexor digitorum tendons with upper extremity repetitive strain injury. J Orthop Res. (2010) 28:298–307. doi: 10.1002/jor.20984
18. Gao HG, Fisher PW, Lambi AG, Wade CK, Barr-Gillespie AE, Popoff SN, et al. Increased serum and musculotendinous fibrogenic proteins following persistent low-grade inflammation in a rat model of long-term upper extremity overuse. PLoS One. (2013) 8:e71875. doi: 10.1371/journal.pone.0071875
19. McAtamney L, Nigel Corlett E. Rula: a survey method for the investigation of work-related upper limb disorders. Appl Ergon. (1993) 24:91–9. doi: 10.1016/0003-6870(93)90080-s
20. Crawford JO. The Nordic musculoskeletal questionnaire. Occup Med. (2007) 57:300–1. doi: 10.1093/occmed/kqm036
21. Waller R, Smith AJ, O'Sullivan PB, Slater H, Sterling M, Straker LM. Associations between musculoskeletal pain experience and pressure and cold pain sensitivity: a community-based cross-sectional study of young adults in the raine study. Clin J Pain. (2019) 35:56–64. doi: 10.1097/AJP.0000000000000650
22. Rodrigues MS, Leite RDV, Lelis CM, Chaves TC. Differences in ergonomic and workstation factors between computer office workers with and without reported musculoskeletal pain. Work. (2017) 57:563–72. doi: 10.3233/WOR-172582
23. Lanfranchi JB, Duveau A. Explicative models of musculoskeletal disorders (msd): From biomechanical and psychosocial factors to clinical analysis of ergonomics. Eur Rev Appl Psychol. (2008) 58:201–13. doi: 10.1016/j.erap.2008.09.004
24. Nunes AMP, Moita J, Espanha M, Petersen KK, Arendt-Nielsen L. Pressure pain thresholds in office workers with chronic neck pain: a systematic review and meta-analysis. Pain Pract. (2021) 21:799–814. doi: 10.1111/papr.13014
25. Zhou WBS, Meng J, Zhang J. Does low grade systemic inflammation have a role in chronic pain? Front Mol Neurosci. (2021) 14:785214. doi: 10.3389/fnmol.2021.785214
26. Iordanova Schistad E, Kong XY, Furberg AS, Bäckryd E, Grimnes G, Emaus N, et al. A population-based study of inflammatory mechanisms and pain sensitivity. Pain. (2020) 161:338–50. doi: 10.1097/j.pain.0000000000001731
27. Karshikoff B, Lekander M, Soop A, Lindstedt F, Ingvar M, Kosek E, et al. Modality and sex differences in pain sensitivity during human endotoxemia. Brain Behav Immun. (2015) 46:35–43. doi: 10.1016/j.bbi.2014.11.014
28. Wegner A, Elsenbruch S, Maluck J, Grigoleit JS, Engler H, Jäger M, et al. Inflammation-induced hyperalgesia: effects of timing, dosage, and negative affect on somatic pain sensitivity in human experimental endotoxemia. Brain Behav Immun. (2014) 41:46–54. doi: 10.1016/j.bbi.2014.05.001
29. Euteneuer F, Schwarz MJ, Hennings A, Riemer S, Stapf T, Selberdinger V, et al. Depression, cytokines and experimental pain: evidence for sex-related association patterns. J Affect Disord. (2011) 131:143–9. doi: 10.1016/j.jad.2010.11.017
30. Watkins LR, Hutchinson MR, Milligan ED, Maier SF. “Listening” and “talking” to neurons: Implications of immune activation for pain control and increasing the efficacy of opioids. Brain Res Rev. (2007) 56:148–69. doi: 10.1016/j.brainresrev.2007.06.006
31. Czeschik JC, Hagenacker T, Schäfers M, Büsselberg D. Tnf-alpha differentially modulates ion channels of nociceptive neurons. Neurosci Lett. (2008) 434:293–8. doi: 10.1016/j.neulet.2008.01.070
32. Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, et al. Nociceptors are interleukin-1beta sensors. J Neurosci. (2008) 28:14062–73. doi: 10.1523/JNEUROSCI.3795-08.2008
33. Zhang J-M, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. (2007) 45:27–37. doi: 10.1097/AIA.0b013e318034194e
34. Ma W, St-Jacques B, Duarte PC. Targeting pain mediators induced by injured nerve-derived cox2 and pge2 to treat neuropathic pain. Expert Opin Ther Targets. (2012) 16:527–40. doi: 10.1517/14728222.2012.680955
35. Al-Shatti T, Barr AE, Safadi FF, Amin M, Barbe MF. Increase in inflammatory cytokines in median nerves in a rat model of repetitive motion injury. J Neuroimmunol. (2005) 167:13–22. doi: 10.1016/j.jneuroim.2005.06.013
36. Chan MH, Carey AL, Watt MJ, Febbraio MA. Cytokine gene expression in human skeletal muscle during concentric contraction: Evidence that il-8, like il-6, is influenced by glycogen availability. Am J Physiol Regul Integr Comp Physiol. (2004) 287:R322–7. doi: 10.1152/ajpregu.00030.2004
37. Benatti FB, Pedersen BK. Exercise as an anti-inflammatory therapy for rheumatic diseases-myokine regulation. Nat Rev Rheumatol. (2015) 11:86–97. doi: 10.1038/nrrheum.2014.193
38. Lee JH, Jun HS. Role of myokines in regulating skeletal muscle mass and function. Front Physiol. (2019) 10:1–9. doi: 10.3389/fphys.2019.00042
39. Klyne DM, Barbe MF, James G, Hodges PW. Does the interaction between local and systemic inflammation provide a link from psychology and lifestyle to tissue health in musculoskeletal conditions? Int J Mol Sci. (2021) 22:7299–320. doi: 10.3390/ijms22147299
40. Barbe MF, Gallagher S, Popoff SN. Serum biomarkers as predictors of stage of work-related musculoskeletal disorders. J Am Acad Orthop Surg. (2013) 21:644–6. doi: 10.5435/JAAOS-21-10-644
41. Brandt M, Madeleine P, Samani A, Ajslev JZ, Jakobsen MD, Sundstrup E, et al. Effects of a participatory ergonomics intervention with wearable technical measurements of physical workload in the construction industry: cluster randomized controlled trial. J Med Internet Res. (2018) 20:e10272. doi: 10.2196/10272
Keywords: musculoskeletal disorders, lower back pain, inflammatory cytokine, static postures, nested case-control study, university employees
Citation: Dong Y, Jiang P, Jin X, Jiang N, Huang W, Peng Y, Shen Y, He L, Forsman M and Yang L (2022) Association between long-term static postures exposure and musculoskeletal disorders among university employees: A viewpoint of inflammatory pathways. Front. Public Health 10:1055374. doi: 10.3389/fpubh.2022.1055374
Received: 27 September 2022; Accepted: 16 November 2022;
Published: 01 December 2022.
Edited by:
Kun-Shan Wu, Tamkang University, TaiwanReviewed by:
Sakineh Varmazyar, Qazvin University of Medical Sciences, IranCopyright © 2022 Dong, Jiang, Jin, Jiang, Huang, Peng, Shen, He, Forsman and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lihua He, YWxpaGUyMDA5QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.