- 1Institute for Physical Activity and Nutrition, School of Exercise and Nutrition Science, Deakin University, Geelong, VIC, Australia
- 2Transforming Obesity Prevention in CHILDren (TOPCHILD) Collaboration, Sydney, NSW, Australia
- 3Caring Futures Institute, College of Nursing and Health Sciences, Flinders University, Bedford Park, SA, Australia
- 4NHMRC Centre of Research Excellence in Translating Early Prevention of Obesity in Childhood (EPOCH-Translate CRE), Sydney, NSW, Australia
- 5Sydney Nursing School, Faculty of Medicine and Health, University of Sydney and Sydney Local Health District, Sydney, NSW, Australia
- 6Department of Paediatrics, Melbourne Medical School, University of Melbourne, Melbourne, VIC, Australia
Introduction: Early life parent-focused interventions can effectively improve infant and child nutrition and movement (physical activity and sedentary behavior) as well as parents' health behaviors. Scale-up of such interventions to real-world settings is essential for population-wide benefits. When progressing to scale-up, intervention components may be modified to reflect contextual factors and promote feasibility of scale-up. The INFANT program, an efficacious early life nutrition and movement behavioral intervention began as a randomized controlled trial (RCT), was modified after a small-scale translation, and is currently being scaled-up in Victoria, Australia. This study mapped and compared discrete intervention components of both the original RCT and the scaled-up version of INFANT to examine modifications for scaling up.
Methods: Discrete intervention components, specifically the target behaviors (child-related and parent-related behaviors), delivery features and behavior change techniques (BCTs) from the RCT and the scaled-up program were coded and mapped using established frameworks and taxonomies. Publications and unpublished materials (e.g., facilitator notes, handouts, videos, app) were coded. Coding was performed independently in duplicate, with final coding validated in a meeting with interventionists. Interventionists reported the rationale for modifications made.
Results: The INFANT RCT and scaled-up version targeted the same obesity prevention-related nutrition and movement behaviors. Key modified delivery features at scale-up included reduced number of sessions, a broader range of professionals facilitating groups, the addition of a mobile app for parents replacing hard-copy materials and tangible tools (e.g., pedometers), and broadening of content (e.g., early feeding, updated 24-h movement guidelines). BCTs used across the RCT and scale-up sessions were unchanged. However, the BCTs identified in the between-session support materials were almost double for the scale-up compared with the RCT, primarily due to the reduced number of sessions and the app's capacity to include more content.
Conclusions: INFANT is one of few early life nutrition and movement behavioral interventions being delivered at scale. With INFANT as an example, this study provides critical understanding about what and why intervention components were altered as the RCT was scaled-up. Unpacking these intervention modifications provides important insights for scale-up feasibility, outcome effects, and how to optimize implementation strategies for population-level benefits.
Introduction
Establishing optimal nutrition and movement behaviors (physical activity and sedentary behavior) in early life is critical for achieving health and wellbeing benefits that track into adulthood, including preventing overweight and obesity (1, 2). Family and parental-child influences are important for establishing healthy early life nutrition and movement behaviors (2–5). Early childhood family-based behavior change interventions are an important avenue for supporting optimal nutrition and movement behaviors; such interventions have been shown to reduce obesity risk behaviors in children aged 0–5 years (6–8). While there is evidence about what works in controlled research studies, few interventions progress to implementation at scale (9, 10).
Scale-up refers to the expansion of health interventions or innovations tested under research conditions to reach more people and achieve sustained benefits (11). Scale-up is essential for population-level reach and impact, providing opportunity to contribute to widespread improvements in children's health and wellbeing. However, three recent systematic reviews (12–14) of scaled-up obesity, physical activity and nutrition interventions targeting varied populations, report that most scaled-up interventions only achieve 50–75% of original trial effect size. This scale-up “penalty” may reflect the modifications that are made to an intervention when it moves from randomized controlled trial (RCT) to scale-up to make it more feasible to deliver within a given practice and policy context. Scaled-up intervention modifications may include changes to the target audience, target behaviors and delivery features (e.g., who delivers the intervention, how and where it is delivered and at what dose). These modifications may impact the intervention's effective components, which may explain the drop in effect size from RCT to scaled-up delivery. Further research is needed to better understand what and why modifications occur during the scale-up process (15), and how this impacts the effective components used between trial and scaled-up interventions.
Behavior change interventions for preventing childhood obesity are complex and contain multiple components. It is vital to have methods for describing intervention components with consistency, transferability, and specificity (16, 17). Intervention components can be described as the target behaviors, delivery features and Behavior Change Techniques (BCTs). A BCT is “an observable, replicable, and irreducible component of an intervention designed to change behavior and a postulated active ingredient within the intervention” (17). Frameworks, such as BCT taxonomies and intervention reporting templates, provide a standardized approach for describing, identifying, and specifying components of complex behavior change interventions. Deconstructing interventions into their components enables consistent and complete reporting, replicability and an investigation of which components are most likely to contribute effective behavior change.
Describing intervention components can contribute to developing, enhancing, and understanding effective behavior change interventions. Building on previous work examining BCTs used in early childhood obesity prevention interventions in Australia and New Zealand (18), a comprehensive global review is underway to characterize the effective components of early childhood obesity prevention interventions to identify the components used to target infant nutrition, sleep and movement behaviors (19, 20). Other previous systematic reviews have identified BCTs associated with effective interventions related to childhood obesity prevention (21–23). However, all studies and reviews to-date focus on controlled trials; little is known about intervention components of early obesity prevention interventions at scale.
The INFANT (INfant Feeding, Active play and NuTrition) program is the first evidence-based early childhood intervention targeting parents and caregivers aiming to improve child nutrition and movement behaviors to be scaled-up in Australia. INFANT was developed with input from child health experts, health professionals and parents and, in 2008, was delivered as a randomized controlled trial (RCT) with 542 families in Victoria, Australia (24). In the RCT, INFANT consisted of six group sessions with mothers, held over the first 18 months of their child's life, led by a dietitian. The intervention showed positive maternal and child outcomes under controlled conditions, specifically, improved maternal dietary patterns, self-efficacy and knowledge, and improved child diet (fewer sweet snacks and improved dietary quality) and reduced child sedentary time (less television viewing) (25–27). Positive intervention benefits for several targeted child behaviors were sustained up to school age. Children in the intervention group had increased fruit, vegetable and water intake and fewer sweet snacks at 2 years post-intervention (child aged 3.5 years), and fewer sweetened drinks and fewer sweet snacks at 3.5 years post-intervention (child aged 5 years) (28). Further to this, at both follow-up timepoints, improved maternal television viewing knowledge was maintained and associated with less television viewing time among their children (29).
In 2012, INFANT was delivered as a small-scale translation trial, where uptake was high and provided proof of concept for implementation at scale (30, 31). INFANT is currently being scaled-up across Victoria, Australia and evaluated as a hybrid implementation-effectiveness trial in partnership with ten policy, practice, and research partners (32). Implementation support is enhanced through funding from the Victorian Department of Health and evaluation was funded by a National Health and Medical Research Council partnership grant (2019–2024, GNT1161223). The evaluation is in progress and aims to assess real-world implementation, effectiveness and cost-effectiveness of INFANT when delivered at scale.
The aim of this study is to examine how the intervention components (target behaviors, delivery features and BCTs) of INFANT changed from RCT to scale-up, and to explore factors that influenced these modifications. This will provide important new insights into how scale-up may impact the components of behavioral interventions to then better understand, and potentially mitigate, the frequently observed scale-up penalty.
Materials and methods
Study design
In May-July 2022, we undertook systematic mapping and analysis to compare the intervention components, namely target behaviors, delivery features, and BCTs of the INFANT RCT and the INFANT scale-up. This study leverages procedures and intervention coding conducted as part of the Transforming Obesity Prevention for CHILDren (TOPCHILD) Collaboration, specifically coding of the INFANT RCT delivery features, child-related target behaviors and corresponding BCTs (20). Recommendations for best practice application of BCT taxonomies in childhood obesity prevention were applied in the current study methods (e.g., duplicate independent coders) and reporting (e.g., detailed methods, referring to BCTs with the taxonomy number and label) (33) as described below.
Target population, target behaviors, and delivery features
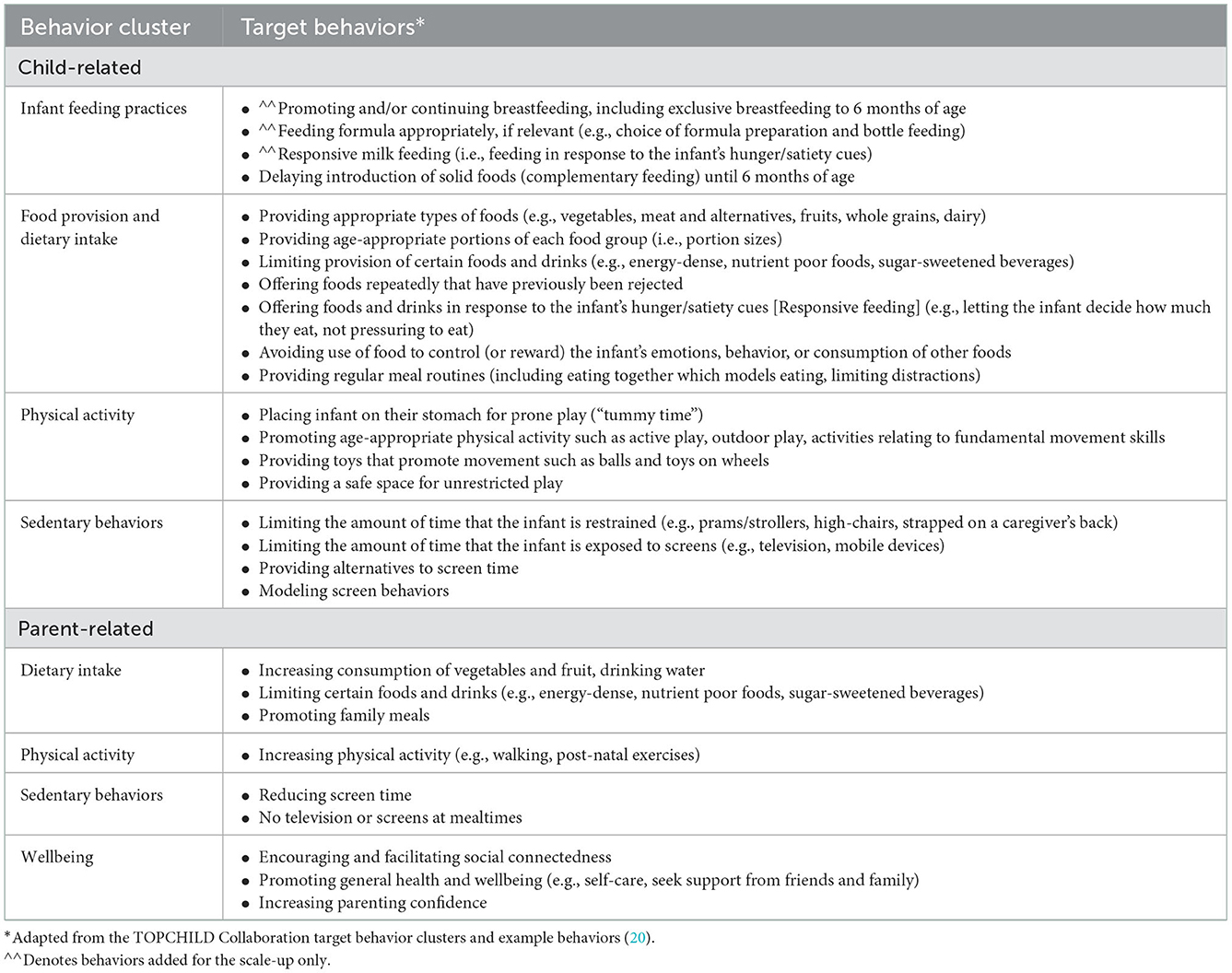
Parents are the target population of the INFANT program, with parental behaviors targeting change in children's health behaviors (i.e., child-related behaviors) and parents' own health behaviors (i.e., parent-related behaviors). The target population and target behaviors used in the INFANT program were identified from published papers and discussion with INFANT program designers (KJC, KDH) and related to parental feeding practices, dietary intake, physical activity, sedentary behavior of both children and parents, as well as parental wellbeing.
Delivery features were coded using pre-specified categories according to the Template for Intervention Description and Replication (TIDieR) checklist for reporting interventions (16). The TIDieR checklist consists of 12 items to aid consistent reporting of interventions. Items describe delivery of the intervention and include intervention name, the rationale or theory underpinning the intervention, intervention materials, procedures, delivery agents, mode of delivery setting/ location of intervention delivery, intervention dose, and any tailoring of the intervention or modifications made during delivery along with plans for maintaining fidelity and actual fidelity (16). The TIDieR checklist is particularly useful for reporting RCTs, yet has scope for enhancements for reporting intervention implementation and scale-up (34). To tailor the TIDieR checklist for this study, we added a column to describe the rationale for changes and an additional item of ‘context' to enable description of the environment in which the intervention was delivered, acknowledging that context is central to implementation (35).
Behavior change technique coding and synthesis
We used the standardized reporting BCT Taxonomy v1 (BCTTv1) from Michie and colleagues (17). This taxonomy consists of 93 techniques, hierarchically clustered within 16 categories, with labels, definitions, and examples. The TOPCHILD Collaboration BCT codebook, with examples of the application of each technique to early childhood obesity prevention interventions, was used to assist with coding (20). BCTs were coded separately for the behaviors targeted across two populations (child-related health behaviors and parent-related behaviors), and for intervention sessions, videos and between-session materials across both the RCT and scale-up versions of the intervention.
All coders (BJJ, SP, SM, KF) completed the open-access University College London BCTTv1 online training (36). BJJ previously undertook further specialized training at University College London Centre for Behavior Change, completing the Behavior Change – Principles and Practice course. BJJ and SP have prior experience applying the BCTTv1 to childhood obesity prevention interventions. SM and KF were less experienced in applying BCTTv1 and consulted regularly with BJJ as an experienced coder. All coders had at least an undergraduate degree in a health-related field. Coders SP and KF had limited previous knowledge of the INFANT intervention; BJJ and SM were familiar with the INFANT RCT from previous projects, and SM was familiar with the INFANT scale-up through coordinating the implementation-effectiveness research project. No coders developed or delivered the RCT or scale-up intervention.
Published and unpublished intervention materials from the RCT and scale-up (such as session facilitator guides, newsletters, handouts, videos, app) were coded (detailed in the Supplementary material). For the RCT, all intervention materials were coded line-by line. For the scale-up, all intervention materials were coded line-by-line except for the mobile phone app for parents. Given the volume of content in the app, SM and KF independently selected a random sample of app content to code across all app features (i.e., articles, activities, push notifications and forum) until several examples were evidenced for the BCT or no evidence of the BCT was present. BJJ and SP independently coded the RCT materials. SM and KF independently coded the scale-up materials. BJJ checked all final coding and was part of the consensus process to discuss areas of ambiguity. An Excel template for “BCT present,” “source material,” and “direct excepts” was used by coders. BCTs were coded as yes/present or no/not present based on the BCT definitions, the codebook and coders' judgements to categorize intervention content. If there was coder uncertainty due to insufficient evidence, the BCT was coded as maybe/unsure. Coders met to discuss and agree upon discrepancies separately for the RCT, then the scale-up. For consistency, SM, BJJ and KF were involved in consensus meetings for both the RCT and scale-up. At this point, BCTs could remain coded as “maybe.”
The level of agreement between independent coders' BCT coding was assessed using prevalence-adjusted and bias-adjusted kappa (PABAK) to account for the high prevalence of negative agreement between coders (i.e., when both agree that a BCT is not present) (37). PABAK agreement values above 0.81 are classified as “excellent/almost perfect,” between 0.61 to 0.80 are classified as “substantial agreement,” 0.41 to 0.60 are “moderate” and below 0.4 are classified as “fair” agreement (38).
Validation meetings
Coding validation with lead interventionists is not commonly undertaken when retrospectively coding BCTs, but is an important step for verification of intervention components that may be unclear from the descriptions in publications and available unpublished intervention materials. A validation process is currently being developed and tested as part of the TOPCHILD Collaboration to clarify any BCTs coded as “maybe” as well as confirm target behaviors and delivery features coded to ensure intervention coding aligned with intervention intent (20); the pilot validation methods were applied in the current study. This involved meeting online to discuss and review the coding with the lead interventionists. BJJ reviewed the RCT coding with leads investigators of INFANT RCT (KDH and KJC). SM, BJJ, and KF reviewed the scale-up coding with lead investigators of INFANT scale-up (RL, PL, KJC, KDH, EDW). BCTs were narratively contrasted and compared between the RCT and the scale-up and discussed with the interventionists to explore the rationale behind any intentional changes made to the intervention during the scale-up process over the 12-year period.
Results
Changes to target population, target behaviors, and delivery features from RCT to scale-up
Enhancements to the INFANT intervention from efficacy testing to scale-up were informed by RCT and small-scale implementation experiences (32). This included end-user evaluation studies with both practitioners (31) and parents (39–41). Decisions regarding planned adjustments for scale-up were made in close consultation with an implementation advisory committee consisting of interventionists and key practice and policy stakeholders. The committee was established prior to INFANT scale-up commencement and continues to meet several times per year to inform implementation and scale-up strategies.
Adjustments were made to the eligible target population for the INFANT intervention from the RCT to the scale-up. In the RCT, the intervention participants were first-time parents of children from 3 months of age. For the scale-up, the target population was expanded to any parents of children (as an option, determined by local sites) with intervention commencement from birth. Expanding beyond first-time parents and caregivers was to increase reach and potential benefits as well as fit adjusted local delivery set-up. Earlier intervention commencement was to enable inclusion of anticipatory breastfeeding information via the mobile app before the first group session.
Target behaviors related to children and parents for the RCT and scaled-up version of the INFANT intervention were largely unchanged (Table 1). Most child-related behaviors related to the primary intervention outcomes in both the RCT and scale-up. The exception was the addition of some infant feeding behaviors in the scale-up version (i.e., promoting breastfeeding, appropriate formula feeding and responsive milk feeding). The RCT was designed to commence at 3 months of age to align with the timing of established first-time parent groups led by community Maternal and Child Health Nurses as part of the free universal healthcare system in Victoria, Australia. In discussion with the interventionists, the advice from practitioners and experts when designing the RCT intervention was that delivering breastfeeding content at around 3 months of age (when the RCT commenced) may isolate or disengage some participants, as feeding mode is likely to be already determined. The addition of the app in the scale-up, allowed the intervention to begin from birth with potential to influence milk feeding decisions and assist with anticipatory guidance for overcoming challenges. Content about breast, formula and mixed feeding, including a new key message “feeding is a learning curve,” was added to the app to support parents before attending sessions and added as a new section on responsive milk feeding in first INFANT group session (held at around 3 months or age). This was informed by a feasibility study of an earlier version of the app that showed the value of providing support to parents from birth on breastfeeding and optimal formula feeding (if not breastfeeding) (42).
Delivery features related to children and parents for the RCT and scaled-up version of the INFANT intervention are described in Table 2 along with the rationale for these changes. Changes to delivery features were informed by small scale translation studies with end users (both parents and practitioners) (30, 31, 41) and were primarily enacted to improve scalability and in response to temporal changes such as mothers' earlier return to work, availability and use of mobile phone apps and changes in preferences for online information.

Table 2. Intervention delivery features of INFANT RCT and scale-up, including rationale for changes.
Behavior change technique coding
BCTs targeting child-related behaviors and parent-related behaviors coded for the RCT and scaled-up version of the INFANT intervention are summarized in Tables 3, 4. Examples of intervention content related to the coded BCTs are presented in the Supplementary material. Of the 93 BCTs in the BCTTv1, the RCT included 20 unique BCTs and the scale-up intervention included 28 unique BCTs targeting children's behaviors (Table 3). The RCT and scale-up intervention feature of group sessions included the same 15 BCTs (see Table 3). The only BCT that was coded for the RCT but not for the scale-up related to the provision of tangible tools between sessions, such as balls for children's physical activity (BCT 12.5 adding objects to the environment). There were nine BCTs coded for the scale-up version of INFANT only, and all were coded from the app.
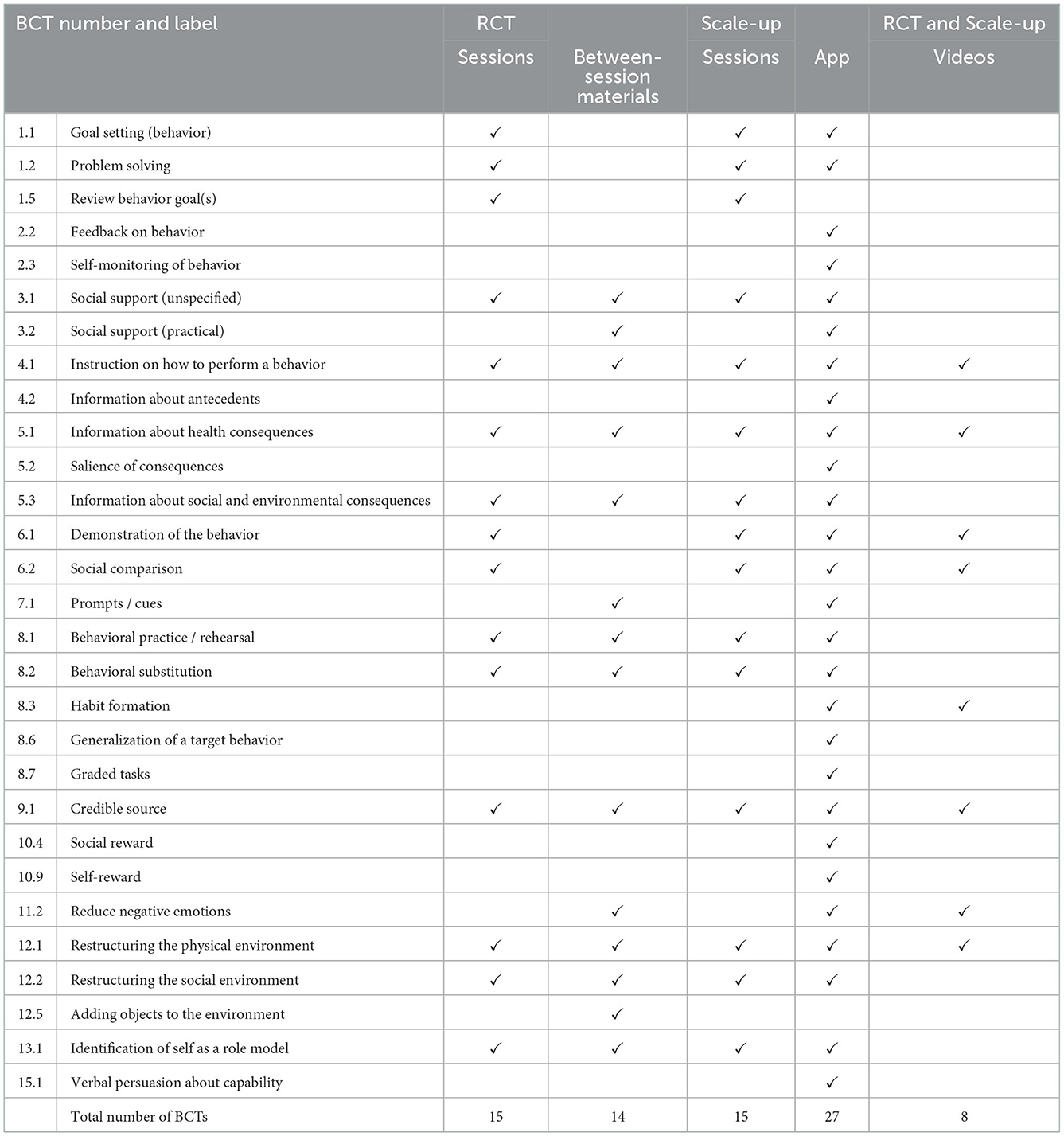
Table 3. A comparison of BCTs in the INFANT RCT and scale-up targeting children's feeding practices, nutrition, physical activity and sedentary behaviors.
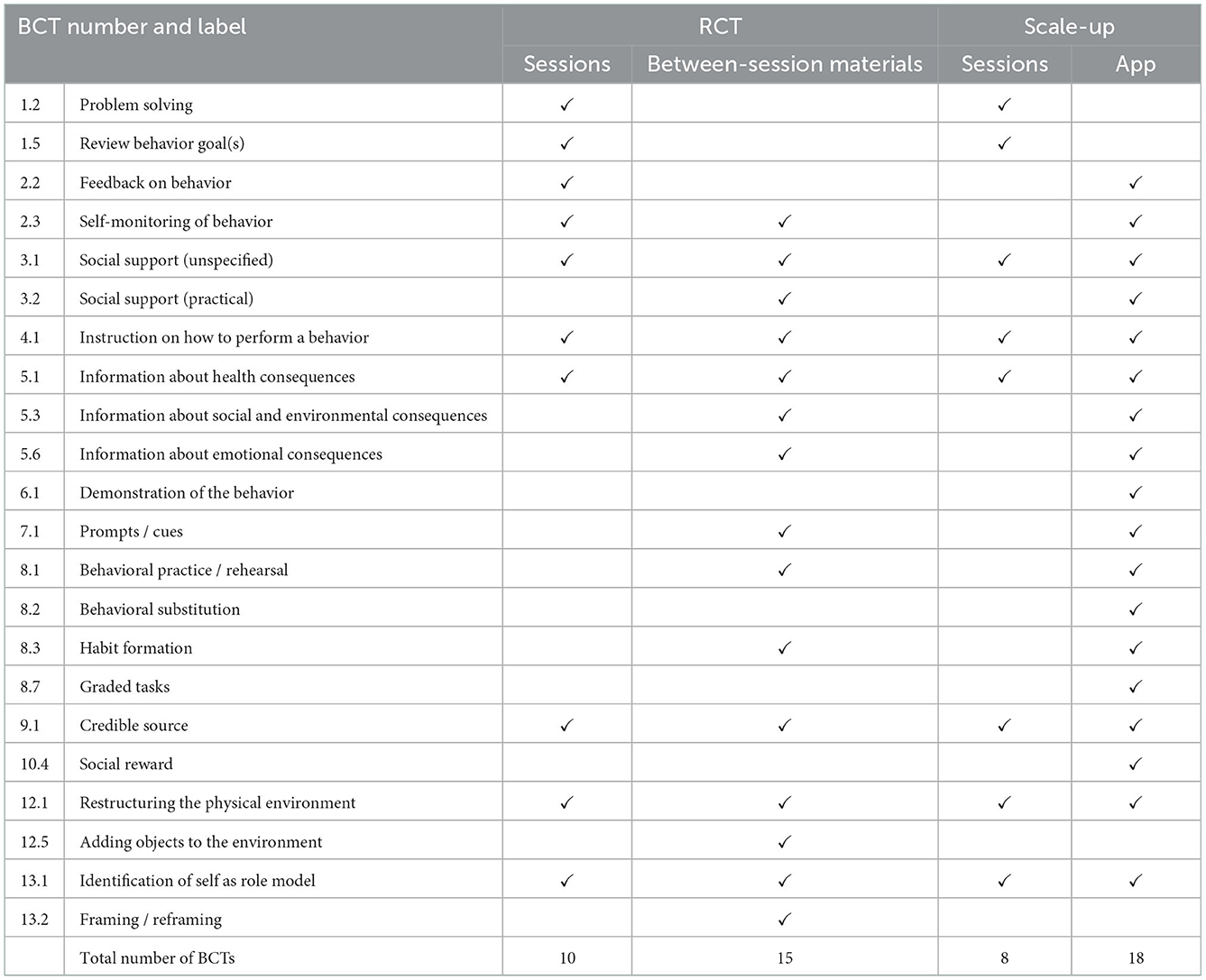
Table 4. A comparison of BCTs in the INFANT RCT and Scale-up targeting parents' own nutrition and physical activity behaviors.
For the parent-focused behaviors, 18 BCTs in the RCT intervention and 20 BCTs in the scale-up intervention were identified (Table 4). The RCT and scale-up intervention feature of group sessions included the same nine BCTs, with two additional BCTs in the RCT sessions (Table 4). The RCT version of INFANT included activities for parents to monitor and receive feedback on their own behaviors e.g., tracking their physical activity and assessing their diets (BCT 2.3 Self-monitoring of behavior and 2.2 Feedback on behavior). These activities were removed from the scale-up session when the session duration was reduced, and were instead included in the scale-up app. Same as for the child-related behaviors, BCT 12.5 (adding objects to the environment) was coded for the RCT but not for the scale-up, based on the provision of tangible tools between sessions, such as pedometers for monitoring parents' steps. The app incorporated four BCTs unique to the scale-up intervention.
Rationale for BCT changes between RCT and scale-up
The differences in BCTs coded for the RCT compared to the scaled-up version of INFANT related to modifications made for intervention scalability. For example, the BCT 12.5 (adding objects to the environment) that was coded for the tangible tools given out to participants during the RCT (e.g., balls, fridge magnets, a pedometer for parents). This BCT was not present for the scale-up as these tools were not given out during the scale-up due to funding and logistics of delivering to sites. Notably, there were more unique BCTs present in the scale-up app, primarily due to the app's scope to include more activities and topics (including milk feeding content) which could be coded from explicitly stated written materials.
BCT coder agreement
BCT inter-coder reliability, measured using PABAK, ranged from 0.76 to 0.89 for child- and parent-related behaviors targeted in all the coded RCT intervention materials (substantial to excellent agreement). Inter-coder reliability for identifying BCTs in the scaled-up version of INFANT ranged from substantial agreement for both child-related and parent-related behaviors targeted in the sessions (0.76 and 0.74, respectively), and moderate to fair agreement for child-related and parent-related behaviors targeted in the mobile app (0.44 and 0.40, respectively).
Discussion
This is the first study to examine the components (target behaviors, delivery features and BCTs) of INFANT, an early life nutrition and movement behavioral intervention from RCT to scale-up. We found few published research studies that presented BCTs of scaled-up behavior change interventions in other disciplines. For example, one study described using BCTs to inform implementation strategies (43) and another examined BCTs in publicly available apps (44). Yet, none explored changes from RCT to scale-up. This study offers an important and unique contribution to the literature, unpacking what and why intervention components, including BCTs, were altered from RCT to scale-up of the INFANT intervention.
Our results highlight that the scaled-up version of the INFANT intervention stayed true to the initial purpose to promote healthy nutrition and movement behaviors in early childhood. The target population and target behaviors were expanded, and the delivery features were adapted for scale-up. The BCT mapping showed that the intervention was largely using the same techniques to change behaviors. The main changes seen in BCTs identified for the RCT vs. scale-up corresponded to modifications made for scalability and in response to temporal changes, such as the enhanced technology available, with these two iterations occurring more than a decade apart. With the exception of new content around milk feeding (breastfeeding, formula feeding and mixed feeding), we utilized app capabilities (push notifications, quizzes providing personal feedback, parent forum) to reinforce messages received in the intervention and the app enabled increased opportunity for BCT inclusions. Unpacking the intervention components lays the foundation for understanding the implementation-effectiveness outcomes as the scale-up of INFANT progresses, as well as exploring the reasons for the potential scale-up “penalty” when moving from RCT to scale-up.
In comparing the intervention components of the INFANT RCT and scaled-up versions, it is important to highlight that the scale-up is currently in progress and therefore the reported data is for the planned, rather than actual, intervention implemented. Scaled-up interventions are much more likely to be modified and adapted than an RCT given that scale-up implementation is led by local delivery organizations and applied to local contexts (45), whereas an RCT is led by interventionists and conducted in a controlled manner. In addition to the planned local adjustments documented in this study (e.g., involvement of varied practitioners as facilitators, varied settings for delivering sessions), there are likely to be unplanned adjustments to the intervention and its delivery according to local contexts, these may include modifications to the sessions to suit local populations (31, 45). The use of an app in the scale-up does offer technological advantages for easily capturing engagement data and ensuring consistency in information to parents, however the group session content and delivery are more open to local modifications. We aim to capture and explore rationale for local adaptations and modifications in the evaluation of INFANT scale-up through documented local implementation plans collected at baseline (prior to implementation) and yearly thereafter, surveys with implementers (collected at baseline, 12 and 24 months), and semi-structured interviews with a purposeful sample of implementers at 12 and 24 months (low/high adaptions, implementers/non-implementers) (32).
Little is known about the associations between intervention components, including BCTs, and outcomes, and assessing this is challenging (46). Previous systematic reviews have identified BCTs associated with effective interventions related to childhood obesity prevention (21–23), yet BCTs identified were inconsistent between reviews. Matvienko-Sikar et al. (22) identified eight BCTs using BCTTv1 associated with effective health professional-delivered infant feeding obesity prevention interventions (1.2 problem solving, 1.5 review behavior goal(s), 2.2 feedback on behavior, 2.7 feedback on outcome(s) of behavior, 3.1 social support (unspecified), 4.1 instruction on how to perform a behavior, 5.1 information about health consequences and 6.1 demonstration of the behavior), of which seven of the eight identified are used in the INFANT RCT and scale-up intervention. However, contrastingly, Anselma et al. (23) found no major differences between identified BCTs in effective vs. non-effective interventions. It should be noted that such reviews have often relied on arbitrary cut-offs for effectiveness and further exploration of effectiveness of BCTs is required using a large sample of interventions; the TOPCHILD Collaboration is seeking to address this gap (20).
There is limited evidence regarding an ideal number of unique BCTs to support behavior change and scarce evidence about the optimal frequency or “dose” of BCTs. It is plausible that a greater number of unique BCTs and repetition throughout an intervention could see greater behavioral outcomes. A systematic review of internet-based behavior change interventions found that the greater number of BCTs used correlated to larger intervention effects (47). Yet, a 2019 publication by JaKa et al. (48) found that the number of unique BCTs used in an obesity prevention intervention was not associated with change in child BMI percentile. No studies were identified that investigate the optimal repetition or dose of BCTs. It was beyond the scope of the current project to capture the frequency of each identified BCT. Future research using the app in the INFANT scale-up could allow for future exploration of BCT dose by using technology to capture metrics of participant engagement with different components and BCTs, and the association with behavioral outcomes. Other intervention factors relating to dose may also have importance related to behavioral outcomes, for example length of sessions or contact-time (48, 49).
Strengths and limitations
The key strengths of this study include best practice methods and reporting for BCT coding (33), including use of standardized coding processes, independent coders, and reporting of coder agreement. Also, we used established frameworks to categorize intervention components (16, 17), and had strong involvement from the RCT and scale-up interventionists. Commonly, BCT assessment relies on often poorly described intervention characteristics in published materials which, in turn, impacts the accuracy of coding (33, 50). This study addressed this issue through access to unpublished material and undertaking a novel validation process with interventionists.
Limitations of this study include coding BCT presence but not frequency. Coding BCT presence is common practice when coding BCTs, however without understanding the frequency of BCTs in an intervention it is not possible to understand dose nor infer relationships with outcomes. Another limitation is the moderate agreement between coders when BCT coding the scale-up intervention app. Moderate inter-coder agreement was seen by others coding childhood obesity prevention and treatment interventions (51), highlighting the complexities of the deductive coding process. In this study, the moderate agreement scores were likely in-part due to the approach undertaken for coding the app; due to the volume of content in the app, the coders randomly selected sections from all features of the app opposed to coding line-by-line and therefore resulted in differing independent results. However, all coders had undertaken the BCT training, used the same supporting resources and followed the same process. All coding was based on intervention materials, and there were thorough coding consensus and validation meetings with an experienced BCCTv1 coder external to the intervention and interventionists (including two interventionists who led the original RCT and remain involved in leading the scale-up) that ensured rigorous debate and accurate resolution of discrepancies.
Implications for future research
While there has been an increase in family-based intervention trials for early childhood obesity prevention and a rise in the unpacking of intervention components to explore “active” elements, there has been no investigations of the intervention components once scaled-up. This is partly due to there being few early childhood obesity prevention trials being scaled-up. Acknowledging the many factors that contribute to whether an intervention progresses to scale-up (52), we recommend that trials that do scale up also assess the discrete intervention components. This study offers a template for other interventions in undertaking such work. Future scale-up of effective early childhood obesity prevention interventions and increased research into unpacking the components of interventions, will allow further examination into the scale-up penalty. An important step for the INFANT research will be to assess the effectiveness of the intervention at scale [currently underway (32)] and compare this with the RCT. Having the intervention components explicitly presented in this study, researchers will be able to consider factors contributing to effective outcomes. Standardized reporting of intervention delivery features with sufficient details, publishing intervention protocols and materials or making them accessible via research groups are essential for accurate understanding of interventions and identification of BCTs (53).
Conclusion
This unique study examined the components (target behaviors, delivery features and BCTs) of INFANT, an early life nutrition and movement behavioral intervention from RCT to scale-up. Findings show that while the scaled-up version of the INFANT intervention had modifications to target behaviors and delivery features for scalability, the techniques identified to change behaviors were largely consistent. Other behavior change interventions enacting scale-up, particularly for early childhood obesity prevention, should consider undertaking similar research to increase understanding and transparency of what and why changes were made to a scaled-up intervention and the active ingredients for changing behavior. Scale-up of early childhood nutrition and movement behavioral interventions is critical for achieving population-level health benefits; this work presents INFANT as an example and lays the foundation for investigating scale-up implementation-effectiveness outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for this study in accordance with the local legislation and institutional requirements. Written informed consent from the program designers was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
SM, PL, RL, KH, ED-W, and KC conceptualized this study. SM administered the project. SM, PL, RL, KH, ED-W, KC, and BJ designed the methodology. SM, BJ, and KF coded the trial, conducted the formal analysis, and presented the data. SM wrote the original draft with input from BJ and KF. All authors contributed to reviewing and editing the manuscript and approved the final version.
Funding
The INFANT research was supported by the National Health and Medical Research Council (GNT425801 and GNT1161223), the Victorian Health Promotion Foundation, and the Victorian Department of Health. SM was supported to lead this study by funding from the Institute for Physical Activity and Nutrition, School of Exercise and Nutrition Science, Deakin University, Australia. BJ was supported by funding from the NHMRC Ideas Grant (GNT1186363). KH was supported by a Heart Foundation Future Leader Fellowship (105929). JS was supported by a NHMRC Leadership Level 2 Investigator Grant (APP1176885). Beyond the peer review process, the funding bodies did not have a role in study design; data management, analysis, nor interpretation; writing of the report; or final authority over the decision to submit study findings for publication.
Acknowledgments
SM, BJ, KH, KC, PL, ED-W, and RL are affiliated with the NHMRC Centre for Research Excellence in Translating the Early Prevention of Obesity in Childhood (CRE EPOCH-Translate, GNT2006999). This study was informed by research from the NHMRC Centre for Research Excellence in the Early Prevention of Obesity in Childhood (EPOCH CRE, GNT1101675). INFANT RCT intervention coding was conducted as part of the Transforming Obesity Prevention for CHILDren (TOPCHILD) Collaboration NHMRC Ideas Grant (GNT1186363). The authors would like to acknowledge Samantha Pryde and Hei In Lau at Flinders University for coding INFANT RCT target behaviors, delivery features and BCTs, and for coding delivery features, respectively.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1026856/full#supplementary-material
References
1. Di Cesare M, Sorić M, Bovet P, Miranda JJ, Bhutta Z, Stevens GA, et al. The epidemiological burden of obesity in childhood: a worldwide epidemic requiring urgent action. BMC Med. (2019) 17:212. doi: 10.1186/s12916-019-1449-8
2. Moore TG, Arefadib N, Deery A, West S. The First Thousand Days: An Evidence Paper. Parkville, Victoria: Centre for Community Child Health, Murdoch Children's Research Institute (2017).
3. Mahmood L, Flores-Barrantes P, Moreno LA, Manios Y. Gonzalez-Gil EM. The influence of parental dietary behaviors and practices on children's eating habits. Nutrients. (2021) 13:1138. doi: 10.3390/nu13041138
4. Rhodes RE, Guerrero MD, Vanderloo LM, Barbeau K, Birken CS, Chaput JP, et al. Development of a consensus statement on the role of the family in the physical activity, sedentary, and sleep behaviours of children and youth. Int J Behav Nutr Phys Act. (2020) 17:74. doi: 10.1186/s12966-020-00973-0
5. Laws R, Adam M, Esdaile E, Love P, Campbell KJ. What works to improve nutrition and food sustainability across the first 2,000 days of life: a rapid review. Nutrients. (2022) 14:731. doi: 10.3390/nu14040731
6. Brown T, Moore TH, Hooper L, Gao Y, Zayegh A, Ijaz S, et al. Interventions for preventing obesity in children. Cochrane Database Sys Rev. (2019) 7:CD001871. doi: 10.1002/14651858.CD001871.pub4
7. Hennessy M, Heary C, Laws R, van Rhoon L, Toomey E, Wolstenholme H, et al. The effectiveness of health professional-delivered interventions during the first 1000 days to prevent overweight/obesity in children: a systematic review [published correction appears in Obes Rev. (2020) 20:1691–707. doi: 10.1111/obr.12924
8. Askie LM, Espinoza D, Martin A, Daniels LA, Mihrshahi S, Taylor R, et al. Interventions commenced by early infancy to prevent childhood obesity-The EPOCH Collaboration: An individual participant data prospective meta-analysis of four randomized controlled trials. Pediatr Obes. (2020) 15:e12618. doi: 10.1111/ijpo.12618
9. Wolfenden L, Ziersch A, Robinson P, Lowe J, Wiggers J. Reducing research waste and improving research impact. Aust N Z J Public Health. (2015) 39:303–4. doi: 10.1111/1753-6405.12467
10. Klaic M, Kapp S, Hudson P, Chapman W, Denehy L, Story D, et al. Implementability of healthcare interventions: an overview of reviews and development of a conceptual framework. Implement Sci. (2022) 17:10. doi: 10.1186/s13012-021-01171-7
11. World Health Organization. Practical guidance for scaling up health service innovations. World Health Organization. (2009). Available online at: https://apps.who.int/iris/handle/10665/44180
12. McCrabb S, Lane C, Hall A, Milat A, Bauman A, Sutherland R, et al. Scaling-up evidence-based obesity interventions: a systematic review assessing intervention adaptations and effectiveness and quantifying the scale-up penalty. Obes Rev. (2019) 20:964–82. doi: 10.1111/obr.12845
13. Sutherland RL, Jackson JK, Lane C, McCrabb S, Nathan NK, Yoong SL, et al. A systematic review of adaptations and effectiveness of scaled-up nutrition interventions. Nutr Rev. (2022) 80:962–79. doi: 10.1093/nutrit/nuab096
14. Lane C, McCrabb S, Nathan N, Naylor PJ, Bauman A, Milat A, et al. How effective are physical activity interventions when they are scaled-up: a systematic review. Int J Behav Nutr Phys Act. (2021) 18:16. doi: 10.1186/s12966-021-01080-4
15. Seidler AL, Johnson BJ, Golley RK, Hunter KE. The complex quest of preventing obesity in early childhood: describing challenges and solutions through collaboration and innovation. Front Endocrinol. (2022) 12:803545. doi: 10.3389/fendo.2021.803545
16. Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. (2014) 348:g1687. doi: 10.1136/bmj.g1687
17. Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. (2013) 46:81–95. doi: 10.1007/s12160-013-9486-6
18. Seidler AL, Hunter KE, Johnson BJ, Ekambareshwar M, Taki S, Mauch CE, et al. Understanding, comparing and learning from the four EPOCH early childhood obesity prevention interventions: a multi-methods study. Pediatr Obes. (2020) 15:e12679. doi: 10.1111/ijpo.12679
19. Hunter KE, Johnson BJ, Askie L, Golley RK, Baur LA, Marschner IC, et al. Transforming Obesity Prevention for CHILDren (TOPCHILD) Collaboration: protocol for a systematic review with individual participant data meta-analysis of behavioral interventions for the prevention of early childhood obesity. BMJ Open. (2022) 12:e048166. doi: 10.1136/bmjopen-2020-048166
20. Johnson BJ, Hunter KE, Golley RK, Chadwick P, Barba A, Aberoumand M, et al. Unpacking the behavioural components and delivery features of early childhood obesity prevention interventions in the TOPCHILD Collaboration: a systematic review and intervention coding protocol. BMJ Open. (2022) 12:e048165. doi: 10.1136/bmjopen-2020-048165
21. Martin J, Chater A, Lorencatto F. Effective behaviour change techniques in the prevention and management of childhood obesity. Int J Obes. (2013) 37:1287–94. doi: 10.1038/ijo.2013.107
22. Matvienko-Sikar K, Toomey E, Delaney L, Flannery C, McHugh S, McSharry J, et al. Behaviour change techniques and theory use in healthcare professional-delivered infant feeding interventions to prevent childhood obesity: a systematic review. Health Psychol Rev. (2019) 13:277–94. doi: 10.1080/17437199.2019.1605838
23. Anselma M, Chinapaw MJM. Kornet-van der Aa DA, Altenburg TM. Effectiveness and promising behavior change techniques of interventions targeting energy balance related behaviors in children from lower socioeconomic environments: a systematic review. PLoS One. (2020) 15:e0237969. doi: 10.1371/journal.pone.0237969
24. Campbell K, Hesketh K, Crawford D, Salmon J, Ball K, McCallum Z. The Infant Feeding Activity and Nutrition Trial (INFANT) an early intervention to prevent childhood obesity: cluster-randomized controlled trial. BMC Public Health. (2008) 8:103. doi: 10.1186/1471-2458-8-103
25. Campbell KJ, Lioret S, McNaughton SA, Crawford DA, Salmon J, Ball K, et al. A parent-focused intervention to reduce infant obesity risk behaviors: a randomized trial. Pediatrics. (2013) 131:652–60. doi: 10.1542/peds.2012-2576
26. Lioret S, Campbell KJ, Crawford D, Spence AC, Hesketh K, McNaughton SA. A parent focused child obesity prevention intervention improves some mother obesity risk behaviors: the Melbourne inFANT program. Int J Behav Nutr Phys Act. (2012) 9:100. doi: 10.1186/1479-5868-9-100
27. Spence AC, McNaughton SA, Lioret S, Hesketh KD, Crawford DA, Campbell KJ. A health promotion intervention can affect diet quality in early childhood. J Nutr. (2013) 143:1672–8. doi: 10.3945/jn.113.177931
28. Hesketh KD, Salmon J, McNaughton SA, Crawford D, Abbott G, Cameron AJ, et al. Long-term outcomes (2 and 35 years post-intervention) of the INFANT early childhood intervention to improve health behaviors and reduce obesity: cluster randomized controlled trial follow-up. Int J Behav Nutr Phys Act. (2020) 17:95. doi: 10.1186/s12966-020-00994-9
29. Delisle Nyström C, Abbott G, Cameron AJ, Campbell KJ, Löf M, Salmon J, et al. Maternal knowledge explains screen time differences 2 and 35 years post-intervention in INFANT. Eur J Pediatr. (2021) 180:3391–8. doi: 10.1007/s00431-021-04134-8
30. Love P, Laws R, Hesketh KD, Campbell KJ. Lessons on early childhood obesity prevention interventions from the Victorian Infant Program. Public Health Res Pract. (2019) 29:2911904. doi: 10.17061/phrp2911904
31. Laws R, Hesketh KD, Ball K, Cooper C, Vrljic K, Campbell KJ. Translating an early childhood obesity prevention program for local community implementation: a case study of the Melbourne InFANT Program. BMC Public Health. (2016) 16:748. doi: 10.1186/s12889-016-3361-x
32. Laws R, Love P, Hesketh KD, Koorts H, Denney-Wilson E, Moodie M, et al. Protocol for an effectiveness-implementation hybrid trial to evaluate scale up of an evidence-based intervention addressing lifestyle behaviors from the start of life: INFANT. Front Endocrinol. (2021) 12:717468. doi: 10.3389/fendo.2021.717468
33. Chakraborty D, Bailey BA, Seidler AL, Yoong S, Hunter KE, Hodder RK, et al. Exploring the application of behaviour change technique taxonomies in childhood obesity prevention interventions: a systematic scoping review. Prev Med Rep. (2022) 29:101928. doi: 10.1016/j.pmedr.2022.101928
34. Cotterill S, Knowles S, Martindale AM, Elvey R, Howard S, Coupe N, et al. Getting messier with TIDieR: embracing context and complexity in intervention reporting. BMC Med Res Methodol. (2018) 18:12. doi: 10.1186/s12874-017-0461-y
35. Dryden-Palmer KD, Parshuram CS, Berta WB. Context, complexity and process in the implementation of evidence-based innovation: a realist informed review. BMC Health Serv Res. (2020) 20:81. doi: 10.1186/s12913-020-4935-y
36. UCL Centre for Behavior Change. BCT Taxonomy V1 Online Training. (2022). Available online at: https://www.bct-taxonomy.com/
37. Byrt T, Bishop J, Carlin JB. Bias, prevalence, and kappa. J Clin Epidemiol. (1993) 46:423–9. doi: 10.1016/0895-4356(93)90018-v
38. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. (1977) 33:159–74.
39. Lunn PL, Roberts S, Spence A, Hesketh KD, Campbell KJ. Mothers' perceptions of Melbourne InFANT Program: informing future practice. Health Promot Int. (2016) 31:614–22. doi: 10.1093/heapro/dav004
40. Spence AC, Hesketh KD, Crawford DA, Campbell KJ. Mothers' perceptions of the influences on their child feeding practices - A qualitative study. Appetite. (2016) 105:596–603. doi: 10.1016/j.appet.2016.06.031
41. Love P, Laws R, Litterbach E, Campbell KJ. Factors influencing parental engagement in an early childhood obesity prevention program implemented at scale. The infant program. Nutrients. (2018) 10:509. doi: 10.3390/nu10040509
42. Laws RA, Denney-Wilson EA, Taki S, Russell CG, Zheng M, Litterbach E-K, et al. Key Lessons and impact of the growing healthy mhealth program on milk feeding, timing of introduction of solids, and infant growth: quasi-experimental study. JMIR Mhealth Uhealth. (2018) 6:e78. doi: 10.2196/mhealth.9040
43. Sutherland R, Campbell E, McLaughlin M, Nathan N, Wolfenden L, Lubans DR, et al. Scale-up of the Physical Activity 4 Everyone (PA4E1) intervention in secondary schools: 24-month implementation and cost outcomes from a cluster randomized controlled trial. Int J Behav Nutr Phys Act. (2021) 18:137. doi: 10.1186/s12966-021-01206-8
44. Ramsey RR, Caromody JK, Voorhees SE, Warning A, Cushing CC, Guilbert TW, et al. A systematic evaluation of asthma management apps examining behavior change techniques. J Allergy Clin Immunol Pract. (2019) 7:2583–91. doi: 10.1016/j.jaip.2019.03.041
45. Movsisyan A, Arnold L, Copeland L, Evans R, Littlecott H, Moore G, et al. Adapting evidence-informed population health interventions for new contexts: a scoping review of current practice. Health Res Policy Syst. (2021) 19:13. doi: 10.1186/s12961-020-00668-9
46. Michie S, West R, Sheals K, Godinho CA. Evaluating the effectiveness of behavior change techniques in health-related behavior: a scoping review of methods used. Transl Behav Med. (2018) 8:212–24. doi: 10.1093/tbm/ibx019
47. Webb TL, Joseph J, Yardley L, Michie S. Using the internet to promote health behavior change: a systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res. (2010) 12:e4. doi: 10.2196/jmir.1376
48. JaKa MM, French SA, Wolfson J. Understanding outcomes in behavior change interventions to prevent pediatric obesity: the role of dose and behavior change techniques. Health Educ Behav. (2019) 46:312–21. doi: 10.1177/1090198118798679
49. Heerman WJ, JaKa MM, Berge JM, Trapl ES, Sommer EC, Samuels LR, et al. The dose of behavioral interventions to prevent and treat childhood obesity: a systematic review and meta-regression. Int J Behav Nutr Phys Act. (2017) 14:157. doi: 10.1186/s12966-017-0615-7
50. Scott C, de Barra M, Johnston M, de Bruin M, Scott N, Matheson C, et al. Using the behaviour change technique taxonomy v1 (BCTTv1) to identify the active ingredients of pharmacist interventions to improve non-hospitalised patient health outcomes. BMJ Open. (2020) 10:e036500. doi: 10.1136/bmjopen-2019-036500
51. JaKa MM, Wood C, Veblen-Mortenson S, Moore SM, Matheson D, Stevens J, et al. Applying the behavior change technique taxonomy to four multicomponent childhood obesity interventions. West J Nurs Res. (2021) 43:468–77. doi: 10.1177/0193945920954782
52. Lee K, van Nassau F, Grunseit A. Scaling up population health interventions from decision to sustainability - a window of opportunity? A qualitative view from policy-makers. Health Res Policy Syst. (2020) 18:118. doi: 10.1186/s12961-020-00636-3
Keywords: behavioral intervention, behavior change techniques (BCTs), early childhood, dietary, physical activity, sedentary behavior, parents and caregivers
Citation: Marshall S, Johnson BJ, Hesketh KD, Campbell KJ, Fraser K, Love P, Denney-Wilson E, Salmon J, McCallum Z and Laws R (2023) Mapping intervention components from a randomized controlled trial to scale-up of an early life nutrition and movement intervention: The INFANT program. Front. Public Health 10:1026856. doi: 10.3389/fpubh.2022.1026856
Received: 24 August 2022; Accepted: 15 December 2022;
Published: 13 January 2023.
Edited by:
Stevo Popovic, University of Montenegro, MontenegroReviewed by:
Katherine Pickard, Emory University, United StatesPaolo Vineis, Imperial College London, United Kingdom
Copyright © 2023 Marshall, Johnson, Hesketh, Campbell, Fraser, Love, Denney-Wilson, Salmon, McCallum and Laws. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachel Laws,  ci5sYXdzQGRlYWtpbi5lZHUuYXU=
ci5sYXdzQGRlYWtpbi5lZHUuYXU=
 Sarah Marshall
Sarah Marshall Brittany J. Johnson
Brittany J. Johnson Kylie D. Hesketh1,4
Kylie D. Hesketh1,4 Penelope Love
Penelope Love Elizabeth Denney-Wilson
Elizabeth Denney-Wilson Jo Salmon
Jo Salmon Rachel Laws
Rachel Laws