- 1Department of Endocrinology, Nanjing Drum Tower Hospital, Nanjing University Medical School, Nanjing, Jiangsu, China
- 2Department of Laboratory Medicine, Nanjing Drum Tower Hospital, Nanjing University Medical School, Nanjing, Jiangsu, China
- 3Department of Biomedicine Statistics, Nanjing Drum Tower Hospital, Nanjing University Medical School, Nanjing, Jiangsu, China
- 4Department of Prevention and Health Care, Nanjing Drum Tower Hospital, Nanjing University Medical School, Nanjing, Jiangsu, China
- 5Department of Infectious Diseases and Jiangsu Key Laboratory for Molecular Medicine, Nanjing Drum Tower Hospital, Nanjing University Medical School, Nanjing, Jiangsu, China
Background: Patients with type 2 diabetes mellitus (T2DM) are at increased risk for COVID-19 related morbidity and mortality. Antibody response to COVID-19 vaccine in T2DM patients is not very clear. The present work aims to evaluate the antibody response to the inactivated SARS-CoV-2 vaccine in this population.
Methods: Two groups of subjects with no history of SARS-CoV-2 infection were included: 63 T2DM patients and 56 non-T2DM controls. Each participant received two doses of inactivated COVID-19 vaccine. IgG antibodies against the nucleocapsid (N) and spike (S) proteins of SARS-CoV-2 (anti-N/S IgG) and receptor binding domain (RBD) proteins (anti-RBD IgG) were quantitatively evaluated by the electrochemiluminescence immunoassays, respectively.
Results: It was observed that the positive rates and titers of anti-N/S IgG and anti-RBD IgG in T2DM patients were significantly lower than those in controls, respectively (anti-N/S: 85.7 vs. 98.2%, P = 0.034; 25.48 vs. 33.58 AU/ml P = 0.011; anti-RBD: 85.7 vs. 96.4%, P = 0.044; 15.45 vs. 22.25 AU/ml, P = 0.019). Compared to non-T2DM subjects, T2DM patients with uncontrolled glycemia showed lower positive antibody rates and titers (anti-N/S IgG: 75% and 13.30 AU/ml; anti-RBD IgG: 75% and 11.91 AU/ml, respectively, all P < 0.05), while T2DM patients with controlled glycemia had similar positive antibody rates and titers (anti-N/S IgG: 94.3% and 33.65 AU/ml; and anti-RBD IgG: 94.3% and 19.82 AU/ml, respectively, all P > 0.05).
Conclusion: In the analysis performed, the data indicate that T2DM patients with uncontrolled glycemia showed a lower level of IgG antibodies compared to non-diabetic controls and individuals with controlled glycemia when immunized with the inactivated COVID-19 vaccine.
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a global healthcare crisis, since as of 30 October 2022, at least 627 million confirmed cases and 6.5 million deaths were reported globally (1). COVID-19 also affects other patients or causes other medical issues (2–7). Compared to healthy individuals, those who had underlying chronic diseases, including hypertension, type 2 diabetes mellitus (T2DM), chronic obstructive pulmonary disease, cerebrovascular or cardiovascular disease, and others have increased fatality rate after infection with COVID-19 (8–10). COVID-19 occurred in diabetic patients is usually more severe than in non-diabetic patients (11–13). Thus, diabetic patients are among the critical subpopulations for prevention of COVID-19 (14, 15).
Diabetic patients are at increased risk for various infections (16), suggesting that the immunity in diabetic patients is to some extents compromised. Studies showed that the antibody response to hepatitis B vaccine is impaired in diabetic patients (17–19). However, the antibody response to influenza vaccines appears to be not impaired in people with T2DM (20, 21). These studies indicate that diabetic patients may present different immune response to different vaccines.
Since December 2020, several COVID-19 vaccines, composed of inactivated SARS-CoV-2, mRNA encoding the full-length spike (S) protein of SARS-CoV-2, viral-vector based vaccine encoding the S protein, or recombinant S proteins, have been applied in human to prevent the pandemic of COVID-19 (22–25). Recently, several studies reported the antibody response to mRNA or viral-vector vaccine against COVID-19 in diabetic patients with inconsistent results (26–29). COVID-19 vaccines composed of inactivated SARS-CoV-2 have been demonstrated to be effective and are also widely used in the world (24, 25, 29–32). However, the immunogenicity of inactivated COVID-19 vaccine in diabetic patients is not very clear (33). The present study aims to evaluate the antibody response to the inactivated SARS-CoV-2 vaccine in this population.
Materials and methods
Participants
China issued the first license for COVID-19 vaccine (Aikewei, Beijing Institute of Biological Products/Sinopharm, Beijing, China) composed of inactivated SARS-CoV-2 on December 30, 2020, and the second inactivated COVID-19 vaccine (CoronaVac, Sinovac Life Sciences, Beijing, China) on February 5, 2021, for emergency use in adult individuals at the age 18–60 years. The recommended vaccination requires two vaccine doses at an interval 2–4 weeks. During the first three-month period of vaccination campaign, the vaccines were mainly used in individuals who were at the frontier lines for controlling the pandemic of COVID-19, such as healthcare worker and other populations at high risk for infection of SARS-CoV-2 (24, 25, 34). Since April 1, 2021, COVID-19 vaccines have been administered among all general populations at the age of 18–60 years, and the vaccines have been then applied in adults over 60 years old and children at the age of 3–17 years. The COVID-19 vaccines initially used in China were mainly composed of inactivated SARS-CoV-2 adsorbed on adsorbed on aluminum hydroxide adjuvant (Aikewei or CoronaVac) (35, 36).
This was a cross-sectional study. Two groups of participants with no history of SARS-CoV-2 infection were included, and each participant received two doses of inactivated COVID-19 vaccines (Aikewei or CoronaVac). The patient group was composed of the individuals with T2DM who were out-patients in the department of endocrinology at Nanjing Drum Tower Hospitals between March 10 and September 24, 2021. The diagnosis of T2DM was based on the criteria (37). The inclusion criteria included: (1) ≥18 years older, (2) immunized with two doses of COVID-19 vaccine composed of inactivated SARS-CoV-2, within 2–10 weeks before recruitment. Patients who met any of following conditions were excluded from the study: (1) with autoimmune disease, (2) with malignant tumor, (3) with history of administration steroid hormones or other immunosuppressive agents within recent 3 months, (4) ongoing medication with any immunosuppressive agent, (5) Type 1 Diabetes, and (6) pregnancy. The control group consisted of age and sex matched subjects who had no history of diabetes and had normal fasting blood glucose; they were healthcare workers in Nanjing Drum Tower Hospital. All the subjects in the control group underwent regular yearly health examinations at least in the last 3 years, and no one showed the fasting blood glucose over 6.4 mMol/L. The inclusion and exclusion criteria were same as those mentioned above. The blood samples were collected between March 10 and September 16, 2021.
This study was approved by the institutional review board (IRB) of Nanjing Drum Tower Hospital (No. 2021-606-02). Written informed consent was obtained from each participant.
Sample size calculation
Considering that the positive rate of anti-RBD IgG was 97% in the non-T2DM subjects based on the results of clinical trials (33, 34) and assumed 80% in the T2DM patients, we calculated that 46 patients per group would be required, with a power of 80% and a type I error rate of 0.05, by using a χ2-test. On the basis of an expected dropout of 10%, we planned to enroll 52 subjects per group.
Blood sample collection
Fasting blood samples were taken by venipuncture from each participant. In addition to the necessary laboratory tests such as clinical biochemistry and glycosylated hemoglobin, serum or plasma samples left over after clinical testing were aliquoted and stored at −30°C.
Detection of anti-SARS-CoV-2 antibody
Two chemo-luminescence immunoassay kits for anti-SARS-CoV-2 antibody, SARS-CoV-2 IgG kit and surrogate neutralization assay kit (iFlash 3000 chemiluminescence immunoassay analyzer, Shenzhen YHLO Biotech, China), were used to measure the levels of anti-SARS-CoV-2 antibodies as described elsewhere (38, 39). The SARS-CoV-2 IgG kit detects total IgG antibodies to the combination of nucleocapsid (N) and S proteins of SARS-CoV-2 (anti-N/S IgG), and the surrogate neutralization assay kit measures the IgG antibody specific to the receptor binding domain (RBD) of the S protein (anti-RBD IgG). The surrogate neutralization activity correlates well with the inhibition of SARS-CoV-2 infection in the cell culture (39). Based on the manufacturer's instructions, the measured results with values ≥10.0 arbitrary units (AU)/ml were considered positive for the antibodies, and the results below 10.0 AU/mL as negative.
Statistical analysis
Categorical data were presented as percentages and continuous data were presented as means ± standard deviation or median (25–75th percentile). The characteristics of participants with and without diabetes were compared by unpaired Student's t′-test for ages, by Mann-Whitney U-test for time interval (days) after the 2nd vaccine dose, and by χ2-test for sex and the positive rates of anti-N/S IgG and anti-RBD IgG. Seropositivity and Clopper-Pearson 95% confidence intervals (CI) were calculated. The antibody levels were compared by Mann-Whitney U-test. The χ2-test was used to compare the seropositivity of anti-N/S IgG and anti-RBD IgG between the subjects without diabetes and diabetic patients with high glycemia or with controlled glycemia. The amount of anti-N/S IgG and anti-RBD IgG in the sera of vaccinated individuals with high glycemia was compared to that in vaccinated individuals without high glycemia by Kruskal-Wallis test. A two-sided P-value of < 0.05 was considered significant. All statistical analyses were conducted using the SPSS 25.0 (version 25.0, SPSS, Chicago, IL, USA).
Results
Participant characteristics
A total of 119 participants who did not have history of SARS-CoV-2 infection were included in this study. Sixty-three subjects who had been diagnosed with T2DM were divided into the patient group and 56 subjects who did not have history of T2DM were divided into the control group. The demographic characteristics and relevant variables of these two groups are presented in Table 1. Overall, there was no statistical significance in these parameters between these two groups.

Table 1. Comparison of demographic characteristics and antibody response between T2DM and non-T2DM participants.
Antibody response to inactivated COVID-19 vaccine in subjects with or without T2DM
Table 1 presents the results of anti-N/S IgG and anti-RBD IgG in the T2DM patients and controls. The positive rate of anti-N/S IgG in the T2DM patients was 85.7% (54/63) (95% CI 74.6, 93.3%), and the positive rate of anti-RBD IgG was also 85.7% (54/63) (95% CI 74.6, 93.3%); these 54 patients were positive for both anti-N/S IgG and anti-RBD IgG. In the controls, 98.2% (55/56) (95% CI 90.4, 100.0%) were positive for anti-N/S IgG and 96.4% (54/56) (95% CI 87.7, 100.0%) were positive for anti-RBD IgG. The positive rates of anti-N/S IgG and anti-RBD IgG in T2DM patients were significantly lower than those in the non-T2DM subjects, respectively (both P < 0.05) (Table 1).
As shown in Figure 1, the median (interquartile range) level of anti-N/S IgG in T2DM patients was significantly lower than that in non-T2DM subjects (25.48 [8.89, 49.14] vs. 33.58 [25.11, 57.39] AU/ml, p = 0.011) (Figure 1A), and similarly, the median level of anti-RBD IgG in T2DM patients was also significantly lower than that in non-T2DM subjects (15.45 [10.44, 24.34] vs. 22.25 [15.25, 32.09] AU/ml, p = 0.019) (Figure 1B).
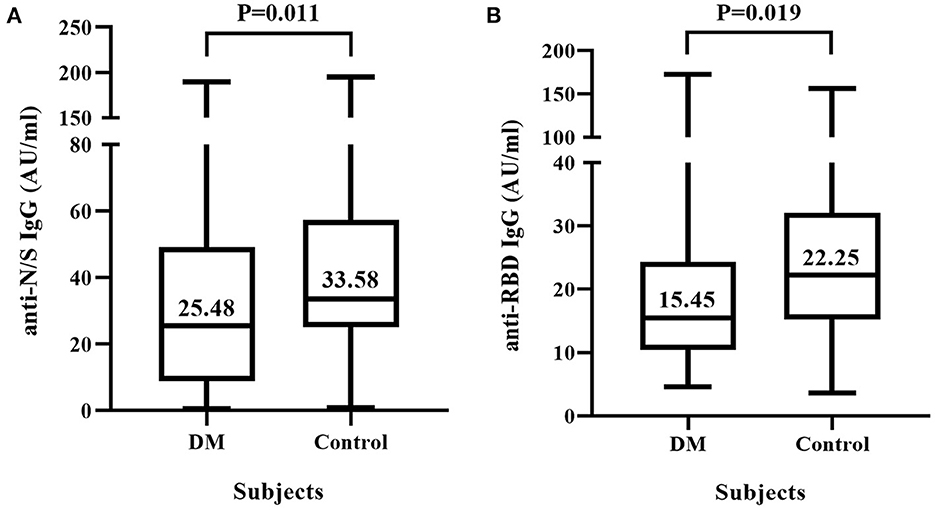
Figure 1. IgG antibody response to inactivated COVID-19 vaccine in diabetic mellitus (DM) patients and non-DM controls. Sixty-three DM patients and 56 non-DM subjects were each vaccinated with two doses of COVID-19 vaccine composed of inactivated SARS-CoV-2. (A) Titers of IgG antibody against the nucleocapsid (N) and spike (S) proteins of SARS-CoV-2 (anti-N/S IgG). (B) Titers of IgG antibody against receptor binding domain (RBD) of S protein (anti-RBD IgG).
Anti-N/S IgG and anti-RBD IgG antibodies in T2DM patients with controlled and uncontrolled glycemia
To further clarify whether the antibody response to COVID-19 vaccine is influenced by the uncontrolled glycemia, we compared the positive rates and the levels of anti-N/S IgG and anti-RBD IgG between T2DM patients who had controlled glycemia and those who had uncontrolled glycemia. Figure 2A shows that the positive rates of anti-N/S IgG and anti-RBD IgG in the T2DM patients with fasting blood glucose ≥7 mMol/L were both 75.0% (95% CI 55.1, 89.3%), significantly lower than the rates (94.3% [95% CI 80.8, 99.3%]) in the patients with fasting blood glucose <7 mMol/L (P = 0.030), and lower than those (anti-N/S IgG 98.2% and anti-RBD IgG 96.4%) in the non-T2DM individuals (P = 0.003 and 0.009, respectively). However, compared to non-diabetic controls, T2DM patients with fasting blood glucose <7 mMol/L had similar positive rate for anti-N/S IgG (94.3 vs. 98.2%, P = 0.676) and for anti-RBD IgG (94.3.0 vs. 96.4%, P = 1.000) (Figure 2A).
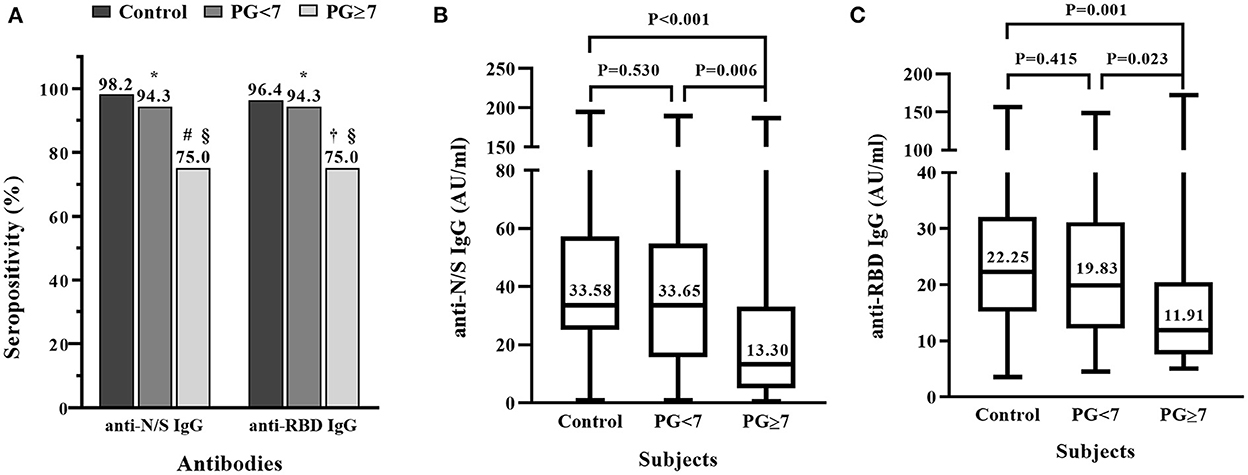
Figure 2. Comparison of IgG antibody response to inactivated COVID-19 vaccine in patients with diabetic mellitus (DM) who had controlled or uncontrolled fasting blood glucose and non-DM subjects. Thirty-five DM patients who had plasma glucose (PG) <7 mMol/L, 28 DM patients who had PG ≥7 mMol/L, and 56 non-DM subjects were each vaccinated with two doses of inactivated COVID-19 vaccine. (A) Positive rates of IgG antibody against the combination of nucleocapsid (N) and spike (S) proteins (anti-N/S IgG), and against receptor binding domain (RBD) (anti-RBD IgG). *P > 0.05, compared to control; #P = 0.003, compared to control;†P = 0.009, compared to control; §P = 0.030, comparison of diabetic patients with PG <7 and ≥7 mMol/L. (B) Comparison of titers of anti-N/S IgG between controls and diabetic patients with PG <7 and ≥7 mMol/L, respectively. (C) Comparison of titers of anti-RBD IgG between controls and diabetic patients with PG <7 and ≥7 mMol/L, respectively.
The comparison of antibody titers between the T2DM patients with controlled and uncontrolled glycemia and the non-T2DM individuals showed that the median levels of anti-N/S IgG and anti-RBD IgG in the patients with fasting blood glucose ≥7 mMol/L were much lower than those in the patients with fasting blood glucose <7 mMol/L, respectively (anti-N/S IgG: 13.30 vs. 33.65 AU/ml, P = 0.006; anti-RBD IgG: 11.91 vs. 19.83 AU/ml. P = 0.023), and significantly lower than those in the non-T2DM individuals (anti-N/S IgG: 13.30 vs. 33.58 AU/ml, P < 0.001; anti-RBD: 11.91 vs. 22.25 AU/ml. P = 0.001) (Figures 2B,C). However, the titers of anti-N/S IgG and anti-RBD IgG between the patients with fasting blood glucose <7 and non-T2DM subjects were comparable, respectively (anti-N/S IgG: 33.65 vs. 33.58 AU/ml, P = 0.530; anti-RBD IgG: 19.83 vs. 22.25 AU/ml, P = 0.415) (Figures 2B,C).
Discussion
In the present study, we revealed that antibody response to COVID-19 vaccine composed of inactivated SARS-CoV-2 in T2DM patients was lower than that in non-T2DM subjects, and the antibody response in T2DM patients with uncontrolled glycemia was lower than that in T2DM patients with controlled glycemia. The data indicate that diabetic patients have reduced antibody response to inactivated COVID-19 vaccine, particularly in the patients with uncontrolled glycemia.
The participants included in this study were vaccinated with COVID-19 vaccine composed of inactivated SARS-CoV-2. Thus, the vaccinees were able to produce antibodies to all viral proteins of SARS-CoV-2. We used two types of assays to measure the antibody responses. One assay contains a combination of the N and S proteins of SARS-CoV-2, which can detect antibodies directed against both the N and S proteins. And the other assay contains the RBD domain only, which can detect antibodies specifically directed against RBD. Anti-RBD IgG antibodies are proved to be neutralizing against SARS-CoV-2 (39, 40). In the present study, 98.2% (55/56) and 96.4% (54/56) of the non-DM subjects showed anti-N/S IgG positive and anti-RBD positive, respectively after a full vaccination with two doses at an interval of 2–4 weeks (Table 1 and Figure 2), which is in agreement with the results in the clinical trials (33, 34). Thus, our data in the present study added more evidence that the inactivated COVID-19 vaccine efficiently elicited the non-neutralizing (anti-N) as well as neutralizing (anti-RBD) antibodies.
Diabetic patients are usually considered to be to some contents immunocompromised in both innate and adaptive immune responses. One of the common complications among diabetic patients is various infections (41). Clinical observations showed that COVID-19 patients who had underlying diabetes have an increased risk of severe disease and mortality (42, 43). This may be explained by the impaired antibody responses to the natural SARS-CoV-2 infection in diabetic patients (44), although others reported that diabetic patients with COVID-19 had same antibody responses as non-diabetic COVID-19 patients (45). In diabetic patients who were vaccinated with mRNA or viral vector-based COVID-19 vaccine, the antibody titers are relatively lower than that in non-diabetic subjects (26, 27). In our present study, we also observed that the antibody response to inactivated COVID-19 vaccine in diabetic patients was lower than that in subjects who had no diabetes. The impaired antibody response to inactivated COVID-19 vaccine was mainly seen in diabetic patients who had uncontrolled glycemia, whereas diabetic patients who had controlled glycemia showed similar antibody response as the non-diabetic subjects did (Figure 2). Our finding is in agreement with what reported by Marfella et al. that diabetic patients with poor glycemic control showed a weak immunity to mRNA vaccines (mRNA-BNT162b2 and mRNA-1273 vaccine) or a viral vector-based vaccine (ChAdOx1-S) (27). This suggests that uncontrolled glycemia may inhibit the immune response to COVID-19 vaccines. Indeed, compared to diabetic patients with controlled glycemia, those with poor glycemic control are at the increased risk of various infections (46).
The reduced antibody response (the seroconversion rate and antibody titers) to COVID-19 vaccine in diabetic patients observed in this study suggests that the protective efficacy and duration of protection against COVID-19 may be relatively lower, particularly in the patients with uncontrolled glycemia. Therefore, the issue of whether diabetic subjects with uncontrolled glycemia require more doses of COVID-19 vaccine, or a relatively shorter interval to receive booster immunization, to obtain the optimized protective efficacy merits further study. Alternatively, to have the full efficacy of the vaccine, diabetic patients with uncontrolled glycemia may delay the vaccination until their glycemia is controlled. Nevertheless, breakthrough infection occurred in diabetic patients who had already received COVID-19 vaccination is prone to have more severe COVID-19 than non-diabetic patients (47). Thus, other preventive measures, such as social distance and face masking, are still critical in diabetic subjects, even after COVID-19 vaccination.
There are several limitations in our study. First, it was single center study and the sample size was small. Second, the participants in this study received inactivated COVID-19 vaccines prepared by two manufacturers and we did not compare the antibody responses between these two inactivated vaccines. Third, although anti-RBD is considered to be neutralizing antibodies, we did not directly measure the neutralizing antibody response. Fourth, because of the limited number of patients with type 1 DM in the study period, we did not evaluate the antibody response to inactivated COVID-19 vaccines in such patients. Whether they have impaired immune response to COVID-19 vaccines requires further investigation.
In conclusion, after vaccinated with two doses of inactivated COVID-19 vaccines, T2DM patients with uncontrolled glycemia developed significantly lower anti-RBD IgG antibody levels than non-T2DM subjects and T2DM patients with controlled glycemia. Our results indicate that the vaccination schedule against COVID-19 requires further investigation to optimize the protective efficacy of COVID-19 vaccines.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board (IRB) of Nanjing Drum Tower Hospital (No. 2021-606-02). The patients/participants provided their written informed consent to participate in this study.
Author contributions
YC, PS, ZH, and Y-HZ: conceptualized and designed the study. PS, YT, and WZ: performed the laboratory work. YC, YB, and ZH: recruited the study subjects. BX: participated in the design and performed the statistical analysis. YC and PS: drafted the manuscript. ZH and Y-HZ: critically revised the manuscript. All authors contributed to the interpretation of the results and agreed to the submitted version of the manuscript.
Funding
This work was supported by a grant for the Key Laboratory from the Jiangsu Provincial Health Commission (Grant Number: XK201607) and grants from the Health Commission of Nanjing City (Grant Number: YKK17059; ZKX20021), China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. WHO. Weekly Epidemiological Update on COVID-19. (2022). Available online at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19–2-november (accessed November 2, 2022).
2. Nieto-Calvache AJ, Padilla I, Tabares-Blanco MF, López-Girón MC, Vergara Galliadi LM. Fear is the path to the dark side: Unsafe delivery, one of the consequences of fear of the SARS-CoV-2 pandemic, a case report. Maternal-Fetal Med. (2021) 3:292–4. doi: 10.1097/FM9.0000000000000112
3. Zhao Y, Zou L, Dong MH, Liu XX, Liu YL, Zhu JW, et al. Challenges for obstetricians and the countermeasures of COVID-19 epidemic. Maternal-Fetal Med. (2020) 2:68–71. doi: 10.1097/FM9.0000000000000046
4. Yang D, Luo C, Feng X, Qi W, Qu S, Zhou Y, et al. Changes in obesity and lifestyle behaviours during the COVID-19 pandemic in Chinese adolescents: a longitudinal analysis from 2019 to 2020. Pediatr Obes. (2022) 17:e12874. doi: 10.1111/ijpo.12874
5. Wu AJ, Aris IM, Hivert MF, Rocchio C, Cocoros NM, Klompas M, et al. Association of changes in obesity prevalence with the COVID-19 pandemic in youth in Massachusetts. JAMA Pediatr. (2022) 176:198–201. doi: 10.1001/jamapediatrics.2021.5095
6. Loh C, Weihe P, Kuplin N, Placzek K, Weihrauch-Blüher S. Diabetic ketoacidosis in pediatric patients with type 1- and type 2 diabetes during the COVID-19 pandemic. Metabolism. (2021) 122:154842. doi: 10.1016/j.metabol.2021.154842
7. Plotzker RES, Sowunmi S, Eckert V, Barnes E, Ngo V, Stockman LJ, et al. Second and third trimester fetal death in the setting of COVID-19: a California 2020 case series. Maternal-Fetal Med. (2022) 4:127–9. doi: 10.1097/FM9.0000000000000128
8. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. (2020) 323:2052–9. doi: 10.1001/jama.2020.6775
9. Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. (2020) 180:1345–55. doi: 10.1001/jamainternmed.2020.3539
10. Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. (2020) 14:395–403. doi: 10.1016/j.dsx.2020.04.018
11. Dispinseri S, Lampasona V, Secchi M, Cara A, Bazzigaluppi E, Negri D, et al. Robust neutralizing antibodies to SARS-CoV-2 develop and persist in subjects with diabetes and COVID-19 pneumonia. J Clin Endocrinol Metab. (2021) 106:1472–81. doi: 10.1210/clinem/dgab055
12. Gregory JM, Slaughter JC, Duffus SH, Smith TJ, LeStourgeon LM, Jaser SS, et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic's impact in type 1 and type 2 diabetes. Diabetes Care. (2021) 44:526–32. doi: 10.2337/dc20-2260
13. Fadlyana E, Rusmil K, Tarigan R, Rahmadi AR, Prodjosoewojo S, Sofiatin Y, et al. A phase III, observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of SARS-CoV-2 inactivated vaccine in healthy adults aged 18-59 years: an interim analysis in Indonesia. Vaccine. (2021) 39:6520–8. doi: 10.1016/j.vaccine.2021.09.052
14. Katulanda P, Dissanayake HA, Ranathunga I, Ratnasamy V, Wijewickrama PSA, Yogendranathan N, et al. Prevention and management of COVID-19 among patients with diabetes: an appraisal of the literature. Diabetologia. (2020) 63:1440–52. doi: 10.1007/s00125-020-05164-x
15. Ceriello A. Diabetes, SARS-CoV-2/COVID-19 vaccines and glycemic control: call for data. Diabetes Res Clin Pract. (2021) 174:108741. doi: 10.1016/j.diabres.2021.108741
16. Pearson-Stuttard J, Blundell S, Harris T, Cook DG, Critchley J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. (2016) 4:148–58. doi: 10.1016/S2213-8587(15)00379-4
17. Schillie SF, Spradling PR, Murphy TV. Immune response of hepatitis B vaccine among persons with diabetes: a systematic review of the literature. Diabetes Care. (2012) 35:2690–7. doi: 10.2337/dc12-0312
18. Zitt E, Sprenger-Mähr H, Knoll F, Neyer U, Lhotta K. Vitamin D deficiency is associated with poor response to active hepatitis B immunisation in patients with chronic kidney disease. Vaccine. (2012) 30:931–5. doi: 10.1016/j.vaccine.2011.11.086
19. Jackson S, Lentino J, Kopp J, Murray L, Ellison W, Rhee M, et al. Immunogenicity of a two-dose investigational hepatitis B vaccine, HBsAg-1018, using a toll-like receptor 9 agonist adjuvant compared with a licensed hepatitis B vaccine in adults. Vaccine. (2018) 36:668–74. doi: 10.1016/j.vaccine.2017.12.038
20. Frasca D, Diaz A, Romero M, Mendez NV, Landin AM, Ryan JG, et al. Young and elderly patients with type 2 diabetes have optimal B cell responses to the seasonal influenza vaccine. Vaccine. (2013) 31:3603–10. doi: 10.1016/j.vaccine.2013.05.003
21. Sheridan PA, Paich HA, Handy J, Karlsson EA, Schultz-Cherry S, Hudgens M, et al. The antibody response to influenza vaccination is not impaired in type 2 diabetics. Vaccine. (2015) 33:3306–13. doi: 10.1016/j.vaccine.2015.05.043
22. Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. (2021) 385:875–84. doi: 10.1056/NEJMoa2107715
23. Thompson MG, Burgess JL, Naleway AL, Tyner H, Yoon SK, Meece J, et al. Prevention and attenuation of COVID-19 with the Bnt162b2 and Mrna-1273 vaccines. N Engl J Med. (2021) 385:320–9. doi: 10.1056/NEJMc2113575
24. Zheng Y, Shen P, Xu B, Chen Y, Luo Y, Dai Y, et al. COVID-19 vaccination coverage among healthcare workers in obstetrics and gynecology during the first three months of vaccination campaign: a cross-sectional study in Jiangsu province, China. Hum Vaccin Immunother. (2021) 17:4946–53. doi: 10.1080/21645515.2021.1997297
25. Xu B, Gao X, Zhang X, Hu Y, Yang H, Zhou YH. Real-world acceptance of COVID-19 vaccines among healthcare workers in perinatal medicine in China. Vaccines. (2021) 9:704. doi: 10.3390/vaccines9070704
26. Ali H, Alterki A, Sindhu S, Alahmad B, Hammad M, Al-Sabah S, et al. Robust antibody levels in both diabetic and non-diabetic individuals after BNT162b2 MRNA COVID-19 vaccination. Front Immunol. (2021) 12:752233. doi: 10.3389/fimmu.2021.752233
27. Marfella R, D'Onofrio N, Sardu C, Scisciola L, Maggi P, Coppola N, et al. Does poor glycaemic control affect the immunogenicity of the COVID-19 vaccination in patients with type 2 diabetes: the caveat study. Diabetes Obes Metab. (2022) 24:160–5. doi: 10.1111/dom.14547
28. Lustig Y, Sapir E, Regev-Yochay G, Cohen C, Fluss R, Olmer L, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. (2021) 9:999–1009. doi: 10.1016/S2213-2600(21)00220-4
29. Sauré D, O'Ryan M, Torres JP, Zuniga M, Santelices E, Basso LJ. Dynamic IgG seropositivity after rollout of coronavac and BNT162b2 COVID-19 vaccines in Chile: a sentinel surveillance study. Lancet Infect Dis. (2022) 22:56–63. doi: 10.1016/S1473-3099(21)00479-5
30. Mallapaty S. Who approval of Chinese CoronaVac Covid vaccine will be crucial to curbing pandemic. Nature. (2021) 594:161–2. doi: 10.1038/d41586-021-01497-8
31. Desai D, Khan AR, Soneja M, Mittal A, Naik S, Kodan P, et al. Effectiveness of an inactivated virus-based SARS-CoV-2 vaccine, BBV152, in India: a test-negative, case-control study. Lancet Infect Dis. (2022) 22:349–56. doi: 10.1016/S1473-3099(21)00674-5
32. Ella R, Reddy S, Blackwelder W, Potdar V, Yadav P, Sarangi V, et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet. (2021) 398:2173–84. doi: 10.1101/2021.06.30.21259439
33. Lee CH, Gray V, Teo JMN, Tam AR, Fong CH, Lui DT, et al. Comparing the B and T cell-mediated immune responses in patients with type 2 diabetes receiving mRNA or inactivated COVID-19 vaccines. Front Immunol. (2022) 13:1018393. doi: 10.3389/fimmu.2022.1018393
34. Cheng Y, Li T, Zheng Y, Xu B, Bi Y, Hu Y, et al. Self-reported adverse events among Chinese healthcare workers immunized with COVID-19 vaccines composed of inactivated SARS-CoV-2. Hum Vaccin Immunother. (2022) 18:2064134. doi: 10.1080/21645515.2022.2064134
35. Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CoRV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. (2021) 21:39–51. doi: 10.1016/S1473-3099(20)30831-8
36. Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. (2021) 21:181–92. doi: 10.1016/S1473-3099(20)30843-4
37. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. (2021) 44:S15-s33. doi: 10.2337/dc21-S002
38. Beppu H, Fukuda T, Kawanishi T, Yasui F, Toda M, Kimura H, et al. Hemodialysis patients with coronavirus disease 2019: reduced antibody response. Clin Exp Nephrol. (2022) 26:170–7. doi: 10.1007/s10157-021-02130-8
39. Tenbusch M, Schumacher S, Vogel E, Priller A, Held J, Steininger P, et al. Heterologous prime-boost vaccination with Chadox1 Ncov-19 and BNT162b2. Lancet Infect Dis. (2021) 21:1212–3. doi: 10.1016/S1473-3099(21)00420-5
40. Robbiani DF, Gaebler C, Muecksch F, Lorenzi JCC, Wang Z, Cho A, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. (2020) 584:437–42. doi: 10.1038/s41586-020-2456-9
41. Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Risk of Infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care. (2018) 41:513–21. doi: 10.2337/dc17-2131
42. Liu W, Tao ZW, Wang L, Yuan ML, Liu K, Zhou L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J. (2020) 133:1032–8. doi: 10.1097/CM9.0000000000000775
43. Mantovani A, Byrne CD, Zheng MH, Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. (2020) 30:1236–48. doi: 10.1016/j.numecd.2020.05.014
44. Pal R, Sachdeva N, Mukherjee S, Suri V, Zohmangaihi D, Ram S, et al. Impaired anti-SARS-CoV-2 antibody response in non-severe COVID-19 patients with diabetes mellitus: a preliminary report. Diabetes Metab Syndr. (2021) 15:193–6. doi: 10.1016/j.dsx.2020.12.035
45. Lampasona V, Secchi M, Scavini M, Bazzigaluppi E, Brigatti C, Marzinotto I, et al. Antibody response to multiple antigens of SARS-CoV-2 in patients with diabetes: an observational cohort study. Diabetologia. (2020) 63:2548–58. doi: 10.1007/s00125-020-05284-4
46. Critchley JA, Carey IM, Harris T, DeWilde S, Hosking FJ, Cook DG. Glycemic control and risk of infections among people with type 1 or type 2 diabetes in a large primary care cohort study. Diabetes Care. (2018) 41:2127–35. doi: 10.2337/dc18-0287
Keywords: T2DM patients, COVID-19, vaccination, inactivated SARS-CoV-2, impaired antibody response
Citation: Cheng Y, Shen P, Tao Y, Zhang W, Xu B, Bi Y, Han Z and Zhou Y-H (2022) Reduced antibody response to COVID-19 vaccine composed of inactivated SARS-CoV-2 in diabetic individuals. Front. Public Health 10:1025901. doi: 10.3389/fpubh.2022.1025901
Received: 23 August 2022; Accepted: 23 November 2022;
Published: 08 December 2022.
Edited by:
Fuqiang Cui, Peking University, ChinaReviewed by:
Elizabeth De Gaspari, Adolfo Lutz Institute, BrazilRoyana Singh, Banaras Hindu University, India
Copyright © 2022 Cheng, Shen, Tao, Zhang, Xu, Bi, Han and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi-Hua Zhou, emdyMDNzdW1tZXJAMTI2LmNvbQ==; Zhen Han, MTM5NTE2MjIyMTBAMTM5LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yandong Cheng1†
Yandong Cheng1† Biyun Xu
Biyun Xu Yan Bi
Yan Bi Yi-Hua Zhou
Yi-Hua Zhou