- Department of Environmental, Occupational, and Geospatial Health Sciences, CUNY Graduate School of Public Health and Health Policy, The City University of New York, New York, NY, United States
Intermittent fasting (IF), time-restricted eating (TRE) and fasting-mimicking diets (FMD) are gaining popularity as weight loss programs. As such, the timing and frequency of meals have been recognized as essential contributors to improving cardiometabolic health and a role as adjuvant therapy in cancer. Randomized controlled trials suggested that the weight loss associated with IF is due to a reduced energy intake due to time restriction. Although the supervised TRE clinical trials documented the dietary caloric intake, many free-living studies focused on the timing of meals without a complete characterization of the dietary intake, caloric density, or macronutrient composition. It is possible that both caloric-restriction diets and time-restriction protocols could work synergistically or additively to improve metabolic health outcomes. Like personalized medicine, achieving precision nutrition mandates the provision of the right nutrients to the right patient at the right time. To accomplish this goal, future studies need to evaluate the benefits of IF and TRE. Randomized controlled trials were conducted in different populations, ethnic groups, ages, geographic distribution, physical activity levels, body composition and in patients with obesity, diabetes, and cardiovascular diseases. Also, it is crucial to analyze the dietary composition and caloric density as related to circadian rhythm and timing of meals. It is conceivable that IF and TRE may contribute to precision nutrition strategies to achieve optimal health. However, more research is needed to evaluate IF and TRE effects on health outcomes and any side effects.
Introduction
Fasting is defined as voluntary abstinence from food and drinks for a specific period of time (1). Metabolically, fasting could be categorized into a post-absorptive state, fasting and starvation. In the post-absorptive state, where no glucose or other nutrients are ingested, glycogenolysis, which is the breakdown of glycogen in the liver, provides glucose to the blood and tissues. Approximately after 18–24 h. Without food, the hepatic glycogen is depleted, and gluconeogenesis, the synthesis of glucose from non-carbohydrates sources such as fat, lactate and eventually amino acids, occurs, leading to the de novo synthesis of glucose for energy production. It has been suggested that fasting has a multitude of health benefits [reviewed in (1, 2)]. Therefore, various forms of intermittent fasting and time-restricted eating have been developed over the last decade.
This perspective summarizes some of the current protocols in fast-mimicking diets. Intermittent fasting (IF) and time-restricted eating (TRE) refer to predetermined timing in pausing or abstaining from eating followed by ingestion of food (3, 4). In this approach, macronutrients providing calories are consumed in a time-specific window. Most free-living clinical studies do not restrict caloric consumption at the time-restricted window, which is considered an advantage to improving adherence as no calorie count is needed. On the other hand, traditional comprehensive weight management programs encompass caloric restriction, either as a low-calorie diet (LCD) or a very low-calorie diet (VLCD) under particular circumstances (5, 6), in addition to physical activity and behavior modification (7).
Furthermore, if co-morbidities exist or at a specific Body Mass Index (BMI), pharmacological drugs and weight loss surgery may be added to the low-calorie diet, physical activity, and behavior modification protocols (8–11). However, the timing and frequency of meals have been recognized as essential contributors to improving cardiometabolic health, weight loss, and other health benefits, which will be discussed in the following paragraphs.
Intermittent fasting
Refers to a dietary strategy that alternates feeding days with fasting days (12). The most common methods are alternating days of fasting (ADF) with one-day feasting and 1-day fasting or a 5:2 diet with 5 days of feasting and 2 days of fasting (either two consecutive fasting days or two separate days within the week). IF protocols do not restrict feeding during the feasting days, where people are allowed to eat normally or ad libitum and then abstain from eating for one or 2 days (Figure 1); however, some protocols allow for up to 500 kcal per day on fasting days (13).
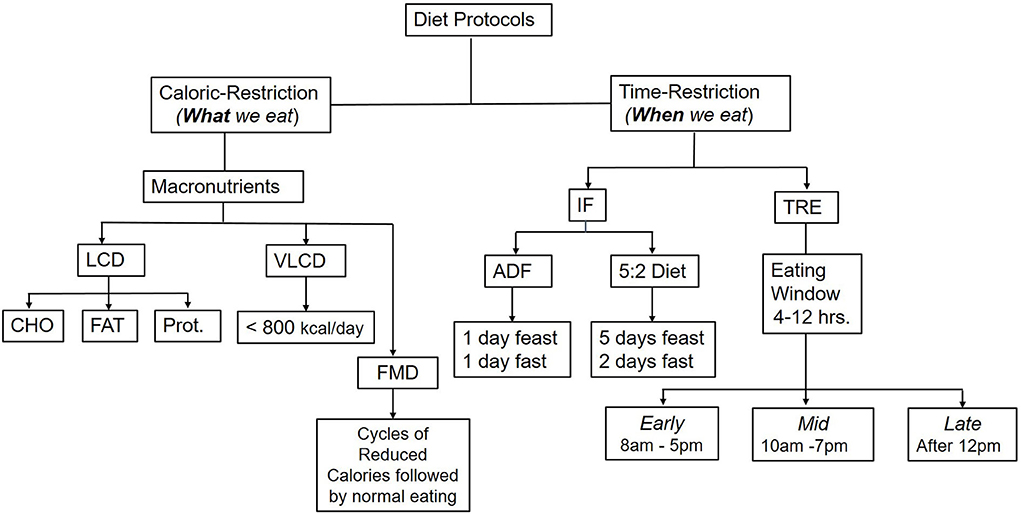
Figure 1. Common caloric-restriction and time-restriction diet protocols. Comprehensive weight management programs include a low-calorie diet (LCD), physical activity and behavior modifications. In addition, pharmacological drugs or weight loss (bariatric surgery) may be added if co-morbidities exist or at a certain high BMI. LCD could be either a low carbohydrate (CHO) diet, low fat (FAT), or under specific circumstances, low protein (Prot.). A very low-calorie diet (VLCD), which provides only 800 kcal/day, is used only with medical supervision. Time-restricted diets include Intermittent fasting (IF), Time-restricted eating (TRE), and Fasting-mimicking diets (FMN). TRE could be either at the earlier part of the day, starting from 8 AM to 5 PM, or midday with delayed breakfast from 10 AM to 7 PM, or late from 12 PM−8 PM or a variation of these times as a personal preference.
Time-restricted eating
Refers to restricting the time when meals are consumed within 24 h. Most TRE studies to date ranged from 4–12 h of eating window without caloric restriction. Some investigators consider TRE a chrono-nutritional strategy (14, 15). The flexibility of the TRE protocols allows for maintaining individual eating pattern preferences, which may facilitate adherence to and compliance with the diet protocol. In addition, alternating feeding and fasting cycles following the circadian rhythm, i.e., eating during the active phase and fasting during the resting phase, may also have a favorable effect on nutrient metabolism, hormonal regulation and physiological processes and thereby may improve cardiometabolic health.
Fasting-mimicking diets
FMD differs from the IF in that it allows for reduced calorie intake instead of complete abstinence of food during the fasting period FMD constitutes periodic cycles of consecutive days consuming a reduced-calorie diet followed by eating ad libitum (15, 16). FMD could be a plant-based diet, low in protein and sugar content. FMD has emerged as another dietary modification that could be beneficial in cardiometabolic diseases and weight loss programs (16–18), as well as an adjuvant intervention in addition to cancer therapy (19–22). Since FMD does not include restricting eating for a specific period of time but instead just reduced caloric intake, it will not be discussed further in this report.
Protocols for IF and TRE are gaining popularity as weight loss programs, health benefits, and interventions in chronic diseases (Figure 1). They are suggested to help individuals achieve a clinically significant weight loss (>5% from baseline) (23), which would be relevant to improving cardiometabolic health biomarkers (24, 25). Some scholars consider IF and TRE different strategies (14). As such, TRE focuses on the timing of meals and their relation to circadian rhythm, hormonal, and metabolite profile within 24 h period, while IF does not affect the chronobiology as it does not restrict feeding during the feasting days (14, 26, 27).
There are also religious, ethical, and spiritual practices of fasting. For example, there are fasting practices in Judaism and Christianity. For example, fasting (abstaining from food) on Yom Kippur includes a full day from the previous day to the following evening; the orthodox Jews also abstain from drinking water for approximately 25 h (28). The literature also describes Ramadan fasting for practicing Muslims as an intermittent fasting strategy for weight loss; however, fasting in Ramadan is different than intermittent fasting as it requires a consecutive 29–30 days of fasting from sunrise to sunset with nothing per oral (NPO), including both food and beverages while IF, alternative day fasting, and 5/2 strategies allow for drinking water or zero calorie beverages or black coffee alternative during the fasting day (0–500 kcal per day). When designing diet strategies, however, it is crucial to consider the feasibility, adherence, and sustainability over time. If these strategies interfere with socialization, family time, dining on special occasions, cultural, ethnic, religious, or social eating patterns, they might not be sustainable or tolerable. These factors could affect weight loss maintenance to sustain the weight loss. Incorporating behavior modification and cognitive behavioral therapy strategies such as goal setting, self-monitoring, problem-solving, and portion size control are also encouraged as adjuvants to TRE, IF, and FMD (29–31).
Types of studies of intermittent fasting and time-restricted eating diets
Preclinical studies
Intermittent fasting
Animal models have been used as a model for glucose management in diabetes [reviewed in (28)]. For example, Tikoo et al. (32) reported that alternative day fasting did not affect the body weight in Sprague-Dawley rats compared to the control but improved the laboratory values of creatinine, albumin, and albumin improved the elevated blood pressure, and reduced type 1 diabetes induced nephropathy. In mice, Liu and colleagues reported that alternative-day fasting improved glucose tolerance, restored the pancreatic islet of Langerhans autophagy response, and enhanced beta cell survival (33).
Time-restricted eating
In vitro studies showed that periodic fasting might protect from oxidative damage, decreasing tumor growth and proliferation (34, 35). In animal models, TRE was investigated in mice where food availability was regulated during the 24 h circadian rhythm. The mice gained less weight, had a better blood glucose profile, longer life span, healthier muscle, and better cardiometabolic health when food was available only during a shorter window compared to the energy-matched mice fed ad-lib throughout the day (36–38). Mitchel and colleagues conducted a study in genetically homogenous male [n = 292 C57BL/6J (B6) mice comparing feeding ad libitum (AL) to either a 30% calorie restriction diet (CR) or single-meal feeding diet (MF)]. In the MF diet (caloric intake matched to the AL diet), the mice consumed the meal quickly, inducing self-imposed fasting (21 h), which led to significant improvement in health and decreased mortality rate as evidenced by the Kaplan-Meier survival analysis. Using a multiple comparison procedure, the main lifespan extension was 11% in MF and 28% in the CR group compared to the AL diet (39).
It is important to note that mice are nocturnal animals, and active eating time is at night. Similarly, studies in Drosophila showed that caloric restriction increased the lifespan and decreased age-related deterioration in cardiac function (40). The underpinning mechanisms of these findings involved several signaling pathways. Such pathways include mTOR (mechanistic Target of Rapamycin), which regulates autophagy (auto cell degradation for recycling of nutrients) and energy metabolic pathways (41), also AMPK (adenosine monophosphate kinase) and the circadian clock downstream targets.
Furthermore, mice fed a high-fat diet restricted to the active phase (in the dark) reduced fat mass and body weight and improved glycemic index compared to mice fed the same calories ad libitum (38).
Taken together, Findings from both IF and TRE suggest a beneficial effect of time-restricted feeding in murine models. However, the limitations in the translation of animal studies to humans lay in their different feeding patterns, where mice are nocturnal feeders. In contrast, human eats mainly during the day and under photopic light. Also, the duration of studies in animal models and humans varies significantly. Nevertheless, the animal model studies provided a proof-of-concept to be confirmed in human studies where the caloric intake was matched in Time-restricted feedings compared to the control subject fed ad lib (42, 43).
Clinical studies
Intermittent fasting
Randomized controlled trials in human suggested that the weight loss associated with intermittent fasting are due to a reduction in calories and energy intake range between 10–30% compared to baseline (44–46). It has been shown to effectively achieve clinically significant weight loss (i.e., reduction of 5% body weight) in the short term. Strategies for intermittent fasting include a period of fasting alternating with feeding. Alternate Day Fasting (ADF) includes fasting for 1 day (0–500 kcal/day), followed by ad libitum eating for a day (Figure 1). During the fasting days, people could consume water or zero-calorie beverages. The other common type is the 5:2 diet which includes 5 days of eating ad libitum followed by 2 days of fasting. The 2 days could be either consecutive or separate days within a week. Although an individual could overeat during feasting days, there is a net deficit due to the alternating fasting days. However, it could be argued that adherence to and compliance with fasting days could be challenging to sustain and implement long-term.
Time restricted eating
Investigators tried various timed feeding to optimize the timing for maximal health outcome benefits. The clinical trials included early TRF from 8 AM to 5 PM, mid-TRE ending at 7 PM, or late TRE beginning at 12 PM. In a robust supervised randomized controlled crossover feeding study, Sutton et al. showed that timed feeding for 6 h (8 AM−2 PM) with the intake of 33% of the individual's daily energy requirements containing 50% carbohydrates (3 meals/day), improved insulin sensitivity, beta cell functions, and metabolic health, compared to the control group which was allowed to consume the same calories (33% daily energy requirements, 50% carbohydrates) for 12 h (8 AM-8 PM) tailored to the individual preferences (43). However, the study was conducted for 5 weeks in only 8 individuals who completed the study from 938 assessed for initial eligibility due to difficulty in recruitment for a supervised eating study. Similar findings were obtained from other short-term studies (26, 47), confirming the role of circadian glucose homeostasis rhythm in the cardiometabolic benefits of TRE. However, studies conducted in free-living conditions have altered both the caloric intake and timing of meals making it challenging to differentiate the independent effect of timing of food intake vs. caloric density's impact on metabolic health.
Furthermore, many studies did not quantify the dietary intake or discretionary calories or only provided baseline and final intervention data without dietary analysis (48–55). On the other hand, systematic reviews and meta-analyses of randomized controlled trials in patients with obesity found that fasting for 2–3 days (5:2 diet) led to lower body fat content and more weight loss than the matched controls who only had caloric restriction without time restriction (13, 56). However, studies with ADF and TRE, which restrict timed eating within 24 h. did not reveal similar findings (51, 57). Based on the current evidence, it appears that both caloric restriction and timing of meals are critical to weight loss and that time-restricted feeding has an independent beneficial effect on weight loss and improving insulin sensitivity in individuals with prediabetes (43).
System biology benefits of IF and TRE
Weight management
Studies have shown that IF and TRE have cardiometabolic benefits aligned with the circadian rhythm, independent of their weight loss outcomes. In a symposium review (58), Cienfuegos and colleagues attributed the cardiometabolic benefits of TRE to circadian rhythm and biological clocks, which affected glucose regulation, beta cell responsiveness, body composition, and body composition body weight, reduction of oxidative stress and metabolic switch (24, 59–62). As such, since fasting deprives the body of glucose, and after glycogen stores are depleted (within 12 h of fasting), the energy production is shifted to alternative energy sources such as fatty acids and ketone bodies, leading to the metabolic switch flipping (63). In addition, studies have shown that IF improves glucose regulation and decrease inflammatory biomarkers in the blood (64). Other mechanisms include augmented autophagy due to the downregulation of mTOR because the caloric restriction activates autophagy to eliminate damaged cellular content and recycle healthy cellular components (65, 66). IF studies, including ADF (45, 67–71) and the 5:2 diet (4, 72) studies found a 4–8% weight loss compared to the control in the short term. However, Long-term studies are required to directly compare the health benefits of IF and TRE in the same population for the same duration compared to the control group. More research would be warranted to address the sustainability and maintenance of weight loss and the metabolic differences between IF and TRE.
Cardiometabolic benefits
Several clinical trials provided evidence that IF improved cardiometabolic biomarkers such as reducing LDL-C, BP, TG, insulin resistance, and HbA1C (24, 64, 73). Also, with IF protocols, people with insulin resistance and pre-diabetes have improved metabolic profiles and have lost weight (56). Further, studies have shown in T1D (74) and T2D (75) that IF is also effective in weight loss. However, other investigators reported that the time restriction benefits are not superior to the caloric restriction in the cardiometabolic outcome (44, 51, 76, 77).
Inflammation and oxidative stress
Most clinical trials to date that measured the markers of inflammation, such as IL-6, TNF, and C-Reactive protein, did not show an effect of IF either ADF (57, 71) or 5:2 diet (78) or TRE (43, 79–81) on the inflammatory markers. However, combined TRE and exercise have been shown to improve the inflammatory markers, possibly due to the exercise component (82). On the other hand, RCTs have shown that IF (ADF, 5:2 diet) and TRE reduce the oxidative stress markers (4, 43, 79, 83), which might explain the improved insulin sensitivity (61, 62).
The microbiome abundance and composition
IF and TRE can alter the microbiome composition and the host circadian rhythm or biological clock, thereby improving cardiometabolic health (15). However, individual variations of the host-microbe interactions may alter the host's response to diet. These variations may include differences in the microbial diversity, short-chain fatty acid (SCFA) composition, diversity of the microbiota, species abundance, phylum level, ratio of Firmicutes/Bacteroidetes, and microbial and host metabolic functions, which are altered in individuals with cardiometabolic diseases and may serve as discriminatory or predictive biomarkers (84, 85). It is also postulated that the gut microbiome modulates the energy metabolism diurnal rhythm by regulating the genes that control lipid uptake and adipose tissue thermogenesis during timed eating (86). Also, the microbiome can produce SCFA and ketone bodies that may be used as an energy source during fasting (87). Further, the feeding-fasting cycles affect the metabolic pathways, metabolites, and microbiome composition, which may drive the cardiometabolic advantage of IF and TRE. Other benefits of the IF on the microbiome include a possible role in reducing microvascular and macrovascular complications of diabetes (88) and brain health, as shown in animal models (89). However, more research is needed to confirm these findings in humans.
Cancer and time-restricted diets
Tumor cells require nutrients and oxygen to grow and proliferate. Diet has been shown to play a role in tumor initiation and progression (90, 91). As such, diet modification can restrict nutrient availability to the tumor microenvironment or alter the tumor's metabolic vulnerabilities. Therefore, timed feeding may be targeted for inhibiting tumor growth or as an adjuvant for anticancer drugs (92). Studies in preclinical models show that nutrients play a role in tumor initiation, progression, cancer metabolism and survival (90, 93, 94). Moreover, recent findings using IF, TRE and FMD suggest that dietary interventions may be utilized as adjuvants to cancer therapy (19, 90, 92, 95, 96). Like personalized medicine, achieving precision nutrition mandates the provision of the right nutrients to the right patient at the right time. To do so, future studies need to analyze the tumor utilization of nutrients and the biochemical processes involved in the digestion and absorption of macronutrients and micronutrients.
Potential side effects and contradictions
The contraindications for IF and TRE are like contradictions to dieting for weight loss in general. For example, IF and TRE are contraindicated in people with eating disorders or underweight (below 18.5 kg/m2). Also, IF and TRE are not recommended for pregnant women or lactating women and children under 12 years old (73). Clinicians should assess and monitor individuals for adverse effects frequency. When IF protocols are started, researchers should allow an adjustment period to allow participants to adapt to the protocol. For example, headache is reported, possibly due to dehydration, but subsides with increasing water intake (73). There was speculation about susceptibility to eating disorders, but evaluation of clinical trials of ADF and TRE did not show evidence of binge eating or purging behavior (97, 98). Also, there is no evidence of changed thyroid hormone levels (82), but the resting metabolic rate might be decreased with the 5–7% weight reduction following IF (57, 99). However, more research is needed to evaluate IF, FMD, and TRE's effects on health outcomes and the possible side effects.
Challenges and limitations
Although the supervised TRE clinical trials documented the dietary caloric intake in the treatment and control groups, many free-living studies focused on the timing of meals without a complete characterization of the dietary intake in terms of caloric density and macronutrient intake. Additionally, many studies have not reported the body composition analysis, macronutrient distribution between meals, and numbers of meals and snacks within the timed-eating window; these limitations hinder comparing the time-restricted eating strategies with the traditional caloric-restriction standardized weight loss programs that account for total caloric intake (Figure 1). Furthermore, most studies to date were short-term, ranging between 8–12 weeks; thus, the long-term impact of the IF and TRE has not been thoroughly studied and is primarily undetermined (100). Moreover, the long-term success of weight loss and weight loss maintenance is not known yet and remains to be seen. Importantly, future studies should expand to include different populations, various ethnic groups, various personal dietary preferences, and discrepancies in individual responses to IF and determine the feasibility of such protocols, accessibility of meals, and long-term adherence to the diet. The assumption is that since people have less time to eat, they will eat fewer calories as it is hard to fit a large volume in a short period of time; however, this has not been confirmed by empirical data.
Recommendations
It would be informative to document the dietary composition, meal composition, intake of discretionary calories, and caloric density in addition to the timing of meals to differentiate the effects of timing of feeding vs. caloric restriction, diet composition, or macronutrient intake. These factors (caloric restriction and time restriction) could work synergistically or with an additive effect to improve metabolic health outcomes. For example, 3-consecutive days' diet records, including 2 weekdays and 1 weekend, should be analyzed throughout the studies. A more detailed analysis would help determine possible synergism between diet quality such as macronutrients, micronutrients, dietary pattern, food groups, supplements and herbs, the amount of processed food intake and the timing of meals and snacks. To translate the recent advances in the IF and TRE research into practical applications, it would be worthwhile to incorporate the diet quality of what we eat with insights from the timing of when we eat it. Since the goal of studying TRE and IF is to provide precision nutrition recommendations, future directions should include studying the best timing window and diet composition and quality of diet, including the intakes of whole grain, plant-based diet, limiting ultra-processed food, and portion control. Furthermore, the benefits of IF and TRE should be evaluated by RCTs in different populations, ethnic groups, ages, geographic differences, physical activity levels, body composition, BMI, and in patients with obesity, type 1 or type 2 diabetes, metabolic syndrome, and cardiovascular disease. It is possible that individualized, tailored IF and TRE protocols may be adopted for personalized precision nutrition that increases compliance, tolerability, and sustainability to achieve optimal health outcomes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary files, further inquiries can be directed to the corresponding author.
Author contributions
GS researched, reviewed the literature, wrote the paper, and prepared the figure.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Patterson RE, Sears DD. Metabolic effects of intermittent fasting. Annu Rev Nutr. (2017) 37:371–93. doi: 10.1146/annurev-nutr-071816-064634
2. Golbidi S, Daiber A, Korac B, Li H, Essop MF, Laher I. Health benefits of fasting and caloric restriction. Curr Diab Rep. (2017) 17:123. doi: 10.1007/s11892-017-0951-7
3. Halberg N, Henriksen M, Soderhamn N, Stallknecht B, Ploug T, Schjerling P, et al. Effect of intermittent fasting and refeeding on insulin action in healthy men. J Appl Physiol. (2005) 99:2128–36. doi: 10.1152/japplphysiol.00683.2005
4. Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes. (2011) 35:714–27. doi: 10.1038/ijo.2010.171
5. Capstick F, Brooks BA, Burns CM, Zilkens RR, Steinbeck KS, Yue DK. Very low calorie diet (VLCD): a useful alternative in the treatment of the obese NIDDM patient. Diabetes Res Clin Pract. (1997) 36:105–11. doi: 10.1016/S0168-8227(97)00038-7
6. Mathus-Vliegen EM, Balance Study G. Long-term maintenance of weight loss with sibutramine in a GP setting following a specialist guided very-low-calorie diet: a double-blind, placebo-controlled, parallel group study. Eur J Clin Nutr. (2005) 59:S31–8. doi: 10.1038/sj.ejcn.1602172
7. Kirkpatrick CF, Bolick JP, Kris-Etherton PM, Sikand G, Aspry KE, Soffer DE, et al. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: a scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J Clin Lipidol. (2019) 13:689–711 e681. doi: 10.1016/j.jacl.2019.08.003
8. Treatment of obesity in adults. Council on scientific affairs. JAMA. (1988) 260:2547–51. doi: 10.1001/jama.260.17.2547
9. Cheng FW, Garay JL, Handu D. Weight management interventions for adults with overweight or obesity: an evidence analysis center scoping review. J Acad Nutr Diet. (2021) 121:1855–65. doi: 10.1016/j.jand.2020.07.022
10. Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. Reviewers of the AACEOCPG: American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for medical care of patients with obesity. Endocr Pract. (2016) 22:1–203. doi: 10.4158/EP161365.GL
11. Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. (2014) 129:S102–38. doi: 10.1161/01.cir.0000437739.71477.ee
12. Keenan S, Cooke MB, Chen WS, Wu S, Belski R. The effects of intermittent fasting and continuous energy restriction with exercise on cardiometabolic biomarkers, dietary compliance, and perceived hunger and mood: secondary outcomes of a randomised, controlled trial. Nutrients. (2022) 14:3071. doi: 10.3390/nu14153071
13. Patikorn C, Roubal K, Veettil SK, Chandran V, Pham T, Lee YY, et al. Intermittent fasting and obesity-related health outcomes: an umbrella review of meta-analyses of randomized clinical trials. JAMA Netw Open. (2021) 4:e2139558. doi: 10.1001/jamanetworkopen.2021.39558
14. Parr EB, Devlin BL, Hawley JA. Perspective: time-restricted eating-integrating the what with the when. Adv Nutr. (2022) 13:699–711. doi: 10.1093/advances/nmac015
15. Ratiner K, Shapiro H, Goldenberg K, Elinav E. Time-limited diets and the gut microbiota in cardiometabolic disease. J Diabetes. (2022) 14:377–93. doi: 10.1111/1753-0407.13288
16. Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. (2015) 22:86–99. doi: 10.1016/j.cmet.2015.05.012
17. Fanti M, Mishra A, Longo VD, Brandhorst S. Time-restricted eating, intermittent fasting, and fasting-mimicking diets in weight loss. Curr Obes Rep. (2021) 10:70–80. doi: 10.1007/s13679-021-00424-2
18. Choi IY, Piccio L, Childress P, Bollman B, Ghosh A, Brandhorst S, et al. A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep. (2016) 15:2136–46. doi: 10.1016/j.celrep.2016.05.009
19. Brandhorst S. Fasting and fasting-mimicking diets for chemotherapy augmentation. Geroscience. (2021) 43:1201–16. doi: 10.1007/s11357-020-00317-7
20. Salvadori G, Zanardi F, Iannelli F, Lobefaro R, Vernieri C, Longo VD. Fasting-mimicking diet blocks triple-negative breast cancer and cancer stem cell escape. Cell Metab. (2021) 33:2247–59 e2246. doi: 10.1016/j.cmet.2021.10.008
21. Caffa I, Spagnolo V, Vernieri C, Valdemarin F, Becherini P, Wei M, et al. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature. (2020) 583:620–4. doi: 10.1038/s41586-020-2502-7
22. Vernieri C, Ligorio F, Zattarin E, Rivoltini L, de Braud F. Fasting-mimicking diet plus chemotherapy in breast cancer treatment. Nat Commun. (2020) 11:4274. doi: 10.1038/s41467-020-18194-1
23. Williamson DA, Bray GA, Ryan DH. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity. (2015) 23:2319–20. doi: 10.1002/oby.21358
24. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. (2019) 381:2541–51. doi: 10.1056/NEJMra1905136
25. Duregon E, Pomatto-Watson L, Bernier M, Price NL, de Cabo R. Intermittent fasting: from calories to time restriction. Geroscience. (2021) 43:1083–92. doi: 10.1007/s11357-021-00335-z
26. Parr EB, Devlin BL, Lim KHC, Moresi LNZ, Geils C, Brennan L, et al.s Time-Restricted eating as a nutrition strategy for individuals with type 2 diabetes: a feasibility study. Nutrients. (2020) 12:3228. doi: 10.3390/nu12113228
27. Corley BT, Carroll RW, Hall RM, Weatherall M, Parry-Strong A, Krebs JD. Intermittent fasting in type 2 diabetes mellitus and the risk of hypoglycaemia: a randomized controlled trial. Diabet Med. (2018) 35:588–94. doi: 10.1111/dme.13595
28. Visioli F, Mucignat-Caretta C, Anile F, Panaite SA. Traditional and medical applications of fasting. Nutrients. (2022) 14:433. doi: 10.3390/nu14030433
29. Hartmann-Boyce J, Aveyard P, Piernas C, Koshiaris C, Velardo C, Salvi D, et al. Cognitive and behavioural strategies for weight management in overweight adults: results from the Oxford Food and Activity Behaviours (OxFAB) cohort study. PLoS ONE. (2018) 13:e0202072. doi: 10.1371/journal.pone.0202072
30. Kelley CP, Sbrocco G, Sbrocco T. Behavioral modification for the management of obesity. Prim Care. (2016) 43:159–75. doi: 10.1016/j.pop.2015.10.004
31. Teixeira PJ, Carraca EV, Marques MM, Rutter H, Oppert JM, De Bourdeaudhuij I, et al. Successful behavior change in obesity interventions in adults: a systematic review of self-regulation mediators. BMC Med. (2015) 13:84. doi: 10.1186/s12916-015-0323-6
32. Tikoo K, Tripathi DN, Kabra DG, Sharma V, Gaikwad AB. Intermittent fasting prevents the progression of type I diabetic nephropathy in rats and changes the expression of Sir2 and p53. FEBS Lett. (2007) 581:1071–8. doi: 10.1016/j.febslet.2007.02.006
33. Liu H, Javaheri A, Godar RJ, Murphy J, Ma X, Rohatgi N, et al. Intermittent fasting preserves beta-cell mass in obesity-induced diabetes via the autophagy-lysosome pathway. Autophagy. (2017) 13:1952–68. doi: 10.1080/15548627.2017.1368596
34. Cheng CW, Adams GB, Perin L, Wei M, Zhou X, Lam BS, et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell. (2014) 14:810–23. doi: 10.1016/j.stem.2014.04.014
35. Di Biase S, Lee C, Brandhorst S, Manes B, Buono R, Cheng CW, et al. Fasting-mimicking diet reduces HO-1 to promote T cell-mediated tumor cytotoxicity. Cancer Cell. (2016) 30:136–46. doi: 10.1016/j.ccell.2016.06.005
36. Long H, Panda S. Time-restricted feeding and circadian autophagy for long life. Nat Rev Endocrinol. (2022) 18:5–6. doi: 10.1038/s41574-021-00600-3
37. Chaix A, Manoogian ENC, Melkani GC, Panda S. Time-restricted eating to prevent and manage chronic metabolic diseases. Annu Rev Nutr. (2019) 39:291–315. doi: 10.1146/annurev-nutr-082018-124320
38. Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. (2012) 15:848–60. doi: 10.1016/j.cmet.2012.04.019
39. Mitchell SJ, Bernier M, Mattison JA, Aon MA, Kaiser TA, Anson RM, et al. Daily fasting improves health and survival in male mice independent of diet composition and calories. Cell Metab. (2019) 29:221–8 e223. doi: 10.1016/j.cmet.2018.08.011
40. Gill S, Le HD, Melkani GC, Panda S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science. (2015) 347:1265–9. doi: 10.1126/science.1256682
41. Panda S. Circadian physiology of metabolism. Science. (2016) 354:1008–15. doi: 10.1126/science.aah4967
42. Chen S, Han R, Liu H. A bibliometric and visualization analysis of intermittent fasting. Front Public Health. (2022) 10:946795. doi: 10.3389/fpubh.2022.946795
43. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. (2018) 27:1212–21 e1213. doi: 10.1016/j.cmet.2018.04.010
44. Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. (2017) 177:930–8. doi: 10.1001/jamainternmed.2017.0936
45. Varady KA, Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Haus JM, et al. Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr J. (2013) 12:146. doi: 10.1186/1475-2891-12-146
46. Klempel MC, Kroeger CM, Varady KA. Alternate day fasting (ADF) with a high-fat diet produces similar weight loss and cardio-protection as ADF with a low-fat diet. Metabolism. (2013) 62:137–43. doi: 10.1016/j.metabol.2012.07.002
47. Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the Circadian clock, aging, and autophagy in humans. Nutrients. (2019) 11:1234. doi: 10.3390/nu11061234
48. Chow LS, Manoogian ENC, Alvear A, Fleischer JG, Thor H, Dietsche K, et al. Time-restricted eating effects on body composition and metabolic measures in humans who are overweight: a feasibility study. Obesity. (2020) 28:860–9. doi: 10.1002/oby.22756
49. Hutchison AT, Regmi P, Manoogian ENC, Fleischer JG, Wittert GA, Panda S, et al. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity. (2019) 27:724–32. doi: 10.1002/oby.22449
50. Zeb F, Wu X, Chen L, Fatima S, Haq IU, Chen A, et al. Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br J Nutr. (2020) 123:1216–26. doi: 10.1017/S0007114519003428
51. Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med. (2020) 180:1491–9. doi: 10.1001/jamainternmed.2020.4153
52. Schroder JD, Falqueto H, Manica A, Zanini D, de Oliveira T, de Sa CA, et al. Effects of time-restricted feeding in weight loss, metabolic syndrome and cardiovascular risk in obese women. J Transl Med. (2021) 19:3. doi: 10.1186/s12967-020-02687-0
53. Anton SD, Lee SA, Donahoo WT, McLaren C, Manini T, Leeuwenburgh C, et al. The effects of time restricted feeding on overweight, older adults: a pilot study. Nutrients. (2019) 11:1500. doi: 10.3390/nu11071500
54. Kesztyus D, Vorwieger E, Schonsteiner D, Gulich M, Kesztyus T. Applicability of time-restricted eating for the prevention of lifestyle-dependent diseases in a working population: results of a pilot study in a pre-post design. Ger Med Sci. (2021) 19:Doc04. doi: 10.21203/rs.2.20835/v1
55. Kesztyus D, Cermak P, Gulich M, Kesztyus T. Adherence to time-restricted feeding and impact on abdominal obesity in primary care patients: results of a pilot study in a pre-post design. Nutrients. (2019) 11:2854. doi: 10.3390/nu11122854
56. Wang X, Li Q, Liu Y, Jiang H, Chen W. Intermittent fasting versus continuous energy-restricted diet for patients with type 2 diabetes mellitus and metabolic syndrome for glycemic control: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. (2021) 179:109003. doi: 10.1016/j.diabres.2021.109003
57. Trepanowski JF, Kroeger CM, Barnosky A, Klempel M, Bhutani S, Hoddy KK, et al. Effects of alternate-day fasting or daily calorie restriction on body composition, fat distribution, and circulating adipokines: secondary analysis of a randomized controlled trial. Clin Nutr. (2018) 37:1871–8. doi: 10.1016/j.clnu.2017.11.018
58. Cienfuegos S, Corapi S, Gabel K, Ezpeleta M, Kalam F, Lin S, et al. Effect of Intermittent fasting on reproductive hormone levels in females and males: a review of human trials. Nutrients. (2022) 14:2343. doi: 10.3390/nu14112343
59. Cienfuegos S, McStay M, Gabel K, Varady KA. Time restricted eating for the prevention of type 2 diabetes. J Physiol. (2022) 600:1253–64. doi: 10.1113/JP281101
60. Park SJ, Yang JW, Song YJ. The effect of four weeks dietary intervention with 8-hour time-restricted eating on body composition and cardiometabolic risk factors in young adults. Nutrients. (2021) 13:2164. doi: 10.3390/nu13072164
61. Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. (2006) 440:944–8. doi: 10.1038/nature04634
62. Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. (2011) 50:567–75. doi: 10.1016/j.freeradbiomed.2010.12.006
63. Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA, Mainous AG, et al. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity. (2018) 26:254–68. doi: 10.1002/oby.22065
64. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. (2017) 39:46–58. doi: 10.1016/j.arr.2016.10.005
65. Ferreira-Marques M, Carvalho A, Cavadas C, Aveleira CA. PI3K/AKT/MTOR and ERK1/2-MAPK signaling pathways are involved in autophagy stimulation induced by caloric restriction or caloric restriction mimetics in cortical neurons. Aging. (2021) 13:7872–82. doi: 10.18632/aging.202805
66. Weichhart T. mTOR as regulator of lifespan, aging, and cellular senescence: a mini-review. Gerontology. (2018) 64:127–34. doi: 10.1159/000484629
67. Cho AR, Moon JY, Kim S, An KY, Oh M, Jeon JY, et al. Effects of alternate day fasting and exercise on cholesterol metabolism in overweight or obese adults: a pilot randomized controlled trial. Metabolism. (2019) 93:52–60. doi: 10.1016/j.metabol.2019.01.002
68. Catenacci VA, Pan Z, Ostendorf D, Brannon S, Gozansky WS, Mattson MP, et al. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity. (2016) 24:1874–83. doi: 10.1002/oby.21581
69. Parvaresh A, Razavi R, Abbasi B, Yaghoobloo K, Hassanzadeh A, Mohammadifard N, et al. Modified alternate-day fasting vs. calorie restriction in the treatment of patients with metabolic syndrome: a randomized clinical trial. Complement Ther Med. (2019) 47:102187. doi: 10.1016/j.ctim.2019.08.021
70. Razavi R, Parvaresh A, Abbasi B, Yaghoobloo K, Hassanzadeh A, Mohammadifard N, et al. The alternate-day fasting diet is a more effective approach than a calorie restriction diet on weight loss and hs-CRP levels. Int J Vitam Nutr Res. (2021) 91:242–50. doi: 10.1024/0300-9831/a000623
71. Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Varady KA. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity. (2013) 21:1370–9. doi: 10.1002/oby.20353
72. Fitzgerald KC, Bhargava P, Smith MD, Vizthum D, Henry-Barron B, Kornberg MD, et al. Intermittent calorie restriction alters T cell subsets and metabolic markers in people with multiple sclerosis. EBioMedicine. (2022) 82:104124. doi: 10.1016/j.ebiom.2022.104124
73. Varady KA, Cienfuegos S, Ezpeleta M, Gabel K. Clinical application of intermittent fasting for weight loss: progress and future directions. Nat Rev Endocrinol. (2022) 18:309–21. doi: 10.1038/s41574-022-00638-x
74. Overland J, Toth K, Gibson AA, Sainsbury A, Franklin J, Gauld A, et al. The safety and efficacy of weight loss via intermittent fasting or standard daily energy restriction in adults with type 1 diabetes and overweight or obesity: a pilot study. Obesity Medicine. (2018) 12:13–7. doi: 10.1016/j.obmed.2018.11.001
75. Carter S, Clifton PM, Keogh JB. Effect of intermittent compared with continuous energy restricted diet on glycemic control in patients with type 2 diabetes: a randomized noninferiority trial. JAMA Netw Open. (2018) 1:e180756. doi: 10.1001/jamanetworkopen.2018.0756
76. Liu D, Huang Y, Huang C, Yang S, Wei X, Zhang P, et al. Calorie restriction with or without time-restricted eating in weight loss. N Engl J Med. (2022) 386:1495–504. doi: 10.1056/NEJMoa2114833
77. Cioffi I, Evangelista A, Ponzo V, Ciccone G, Soldati L, Santarpia L, et al. Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: a systematic review and meta-analysis of randomized controlled trials. J Transl Med. (2018) 16:371. doi: 10.1186/s12967-018-1748-4
78. Schubel R, Nattenmuller J, Sookthai D, Nonnenmacher T, Graf ME, Riedl L, et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: a randomized controlled trial. Am J Clin Nutr. (2018) 108:933–45. doi: 10.1093/ajcn/nqy196
79. Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Wiseman E, Pavlou V, et al. Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab. (2020) 32:366–78 e363. doi: 10.1016/j.cmet.2020.06.018
80. Gabel K, Hoddy KK, Haggerty N, Song J, Kroeger CM, Trepanowski JF, et al. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Healthy Aging. (2018) 4:345–53. doi: 10.3233/NHA-170036
81. Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. (2016) 14:290. doi: 10.1186/s12967-016-1044-0
82. Moro T, Tinsley G, Pacelli FQ, Marcolin G, Bianco A, Paoli A. Twelve months of time-restricted eating and resistance training improves inflammatory markers and cardiometabolic risk factors. Med Sci Sports Exerc. (2021) 53:2577–85. doi: 10.1249/MSS.0000000000002738
83. Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. (2007) 42:665–74. doi: 10.1016/j.freeradbiomed.2006.12.005
84. Ferrocino I, Pellegrini M, D'Eusebio C, Goitre I, Ponzo V, Fadda M, et al. The effects of time-restricted eating on metabolism and gut microbiota: a real-life study. Nutrients. (2022) 14:2569. doi: 10.3390/nu14132569
85. Huang Y, Wang Z, Ma H, Ji S, Chen Z, Cui Z, et al. Dysbiosis and implication of the gut microbiota in diabetic retinopathy. Front Cell Infect Microbiol. (2021) 11:646348. doi: 10.3389/fcimb.2021.646348
86. Kuang Z, Wang Y, Li Y, Ye C, Ruhn KA, Behrendt CL, et al. The intestinal microbiota programs diurnal rhythms in host metabolism through histone deacetylase 3. Science. (2019) 365:1428–34. doi: 10.1126/science.aaw3134
87. Crawford PA, Crowley JR, Sambandam N, Muegge BD, Costello EK, Hamady M, et al. Regulation of myocardial ketone body metabolism by the gut microbiota during nutrient deprivation. Proc Natl Acad Sci U S A. (2009) 106:11276–81. doi: 10.1073/pnas.0902366106
88. Beli E, Yan Y, Moldovan L, Vieira CP, Gao R, Duan Y, et al. Restructuring of the gut microbiome by intermittent fasting prevents retinopathy and prolongs survival in db/db mice. Diabetes. (2018) 67:1867–79. doi: 10.2337/db18-0158
89. Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, et al. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer's disease. Neurobiol Dis. (2007) 26:212–20. doi: 10.1016/j.nbd.2006.12.019
90. Taylor SR, Falcone JN, Cantley LC, Goncalves MD. Developing dietary interventions as therapy for cancer. Nat Rev Cancer. (2022) 22:452–66. doi: 10.1038/s41568-022-00485-y
91. Guo R, Zhang Y, Turdi S, Ren J. Adiponectin knockout accentuates high fat diet-induced obesity and cardiac dysfunction: role of autophagy. Biochim Biophys Acta. (2013) 1832:1136–48. doi: 10.1016/j.bbadis.2013.03.013
92. Kanarek N, Petrova B, Sabatini DM. Dietary modifications for enhanced cancer therapy. Nature. (2020) 579:507–17. doi: 10.1038/s41586-020-2124-0
93. Arcidiacono D, Dedja A, Giacometti C, Fassan M, Nucci D, Francia S, et al. Hyperinsulinemia promotes esophageal cancer development in a surgically-induced duodeno-esophageal reflux murine model. Int J Mol Sci. (2018) 19:1198. doi: 10.3390/ijms19041198
94. Hopkins BD, Goncalves MD, Cantley LC. Obesity and cancer mechanisms: cancer metabolism. J Clin Oncol. (2016) 34:4277–83. doi: 10.1200/JCO.2016.67.9712
95. Vernieri C, Fuca G, Ligorio F, Huber V, Vingiani A, Iannelli F, et al. Fasting-mimicking diet is safe and reshapes metabolism and antitumor immunity in patients with cancer. Cancer Discov. (2022) 12:90–107. doi: 10.1158/2159-8290.CD-21-0030
96. Valdemarin F, Caffa I, Persia A, Cremonini AL, Ferrando L, Tagliafico L, et al. Safety and feasibility of fasting-mimicking diet and effects on nutritional status and circulating metabolic and inflammatory factors in cancer patients undergoing active treatment. Cancers. (2021) 13:4013. doi: 10.3390/cancers13164013
97. Hoddy KK, Kroeger CM, Trepanowski JF, Barnosky AR, Bhutani S, Varady KA. Safety of alternate day fasting and effect on disordered eating behaviors. Nutr J. (2015) 14:44. doi: 10.1186/s12937-015-0029-9
98. Varady KA, Gabel K. Safety and efficacy of alternate day fasting. Nat Rev Endocrinol. (2019) 15:686–7. doi: 10.1038/s41574-019-0270-y
99. Sundfor TM, Svendsen M, Tonstad S. Effect of intermittent vs. continuous energy restriction on weight loss, maintenance and cardiometabolic risk: a randomized 1-year trial. Nutr Metab Cardiovasc Dis. (2018) 28:698–706. doi: 10.1016/j.numecd.2018.03.009
Keywords: intermittent fasting (IF), time-restricted eating (TRE), fasting-mimicking diets (FMD), precision nutrition, alternative day modified fasting (ADMF)
Citation: Soliman GA (2022) Intermittent fasting and time-restricted eating role in dietary interventions and precision nutrition. Front. Public Health 10:1017254. doi: 10.3389/fpubh.2022.1017254
Received: 11 August 2022; Accepted: 10 October 2022;
Published: 28 October 2022.
Edited by:
Santiago Navas-Carretero, University of Navarra, SpainReviewed by:
Spyridon N. Karras, Aristotle University of Thessaloniki, GreeceAmrendra Mishra, University of Southern California, United States
Copyright © 2022 Soliman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ghada A. Soliman, ghada.soliman@sph.cuny.edu
 Ghada A. Soliman
Ghada A. Soliman