- 1School of Health Policy & Management, Nanjing Medical University, Nanjing, China
- 2Center for Global Health, Nanjing Medical University, Nanjing, China
- 3School of Medicine and Health Management, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 4School of International Pharmaceutical Business, China Pharmaceutical University, Nanjing, China
- 5The Research Center of National Drug Policy and Ecosystem, China Pharmaceutical University, Nanjing, China
Objectives: To explore whether a societal preference for orphan drugs exists in Chinese general public and to quantitatively measure the personal trade-off between essential attributes of orphan drugs through a discrete choice experiment.
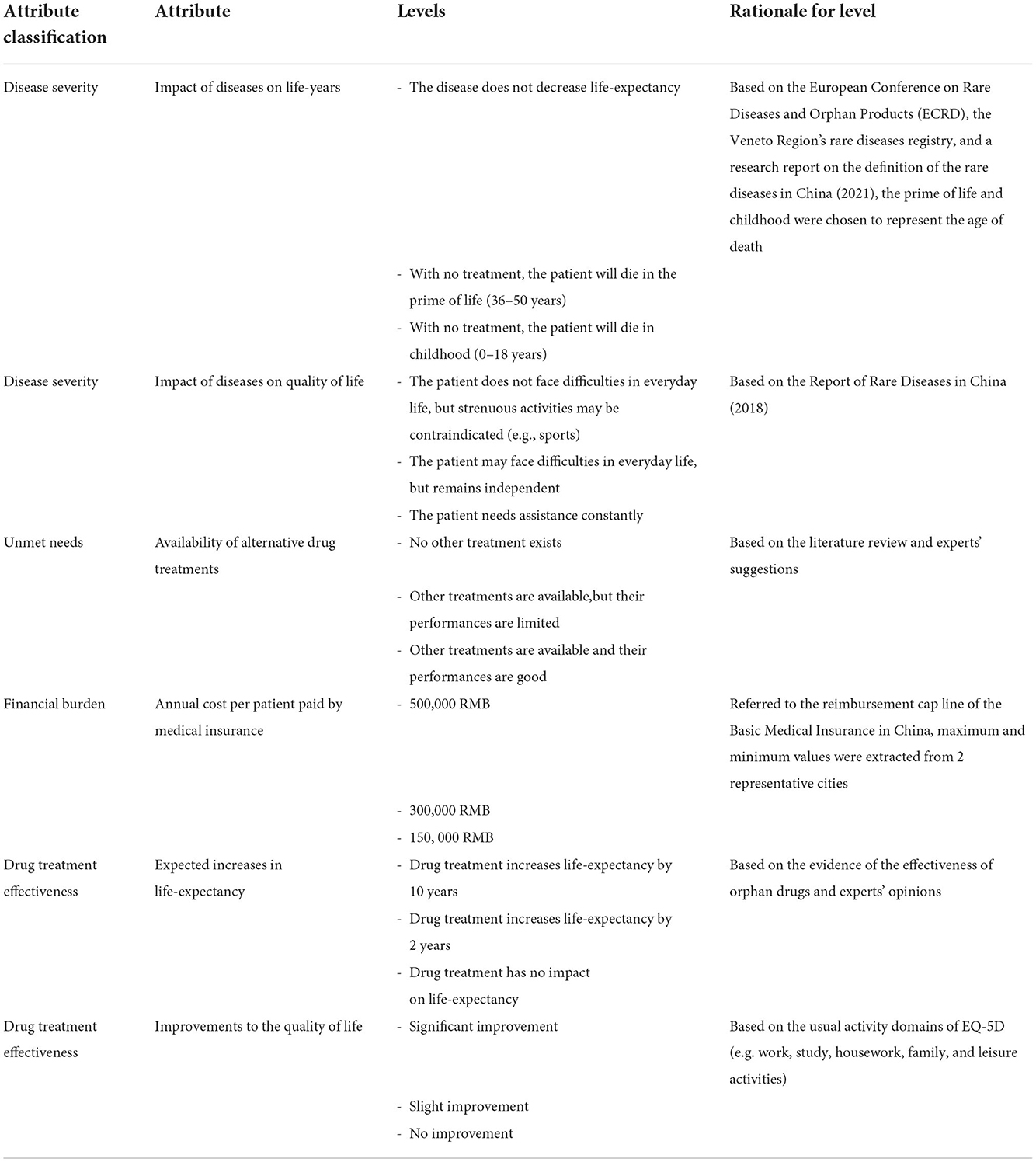
Methods: A labeled discrete choice experiment was employed to measure public preference. Six attributes (impact of diseases on life-years, impact of diseases on quality of life, availability of alternative drug treatments, annual cost per patient paid by medical insurance, expected increases in life-expectancy, and improvements to the quality of life) were identified through a literature review, experts' suggestions, and stakeholders' semi-structured interviews, then refined through a pre-survey. The current study used a D-efficient design to yield 27 choice sets divided into three blocks with nine questions containing the labeled treatment (either orphan drugs or common drugs). Information on sociodemographic characteristics and individual preferences was collected through a web-based questionnaire using convenience sampling. A mixed logit model was used to test societal preferences for orphan drugs over common drugs, while a binary logit model was used to measure the relative importance of each attribute in orphan drug access for the National Reimbursement Drug List and its willingness to pay.
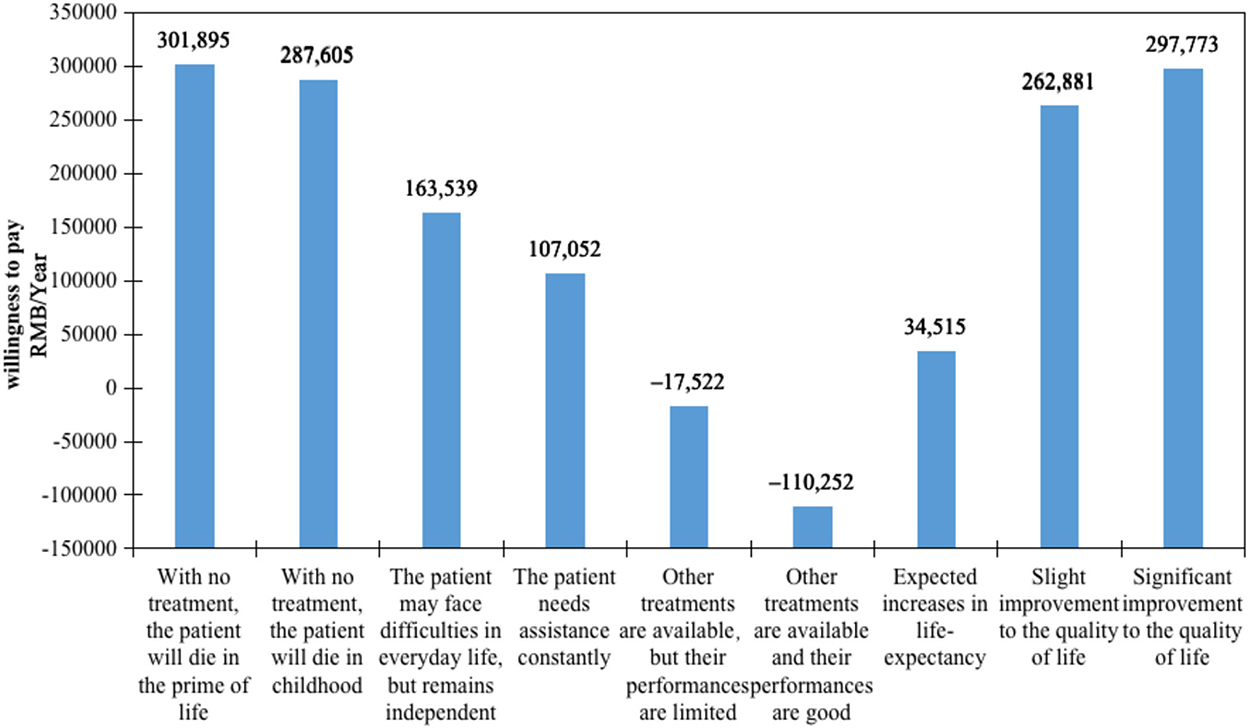
Results: A total of 323 persons participated in this study. Respondents largely had indifferent attitudes toward orphan drugs and common drugs. The binary logit model results showed that 5 of the 6 attributes were significant, except for the availability of alternative drug treatments. The most impacted factor was the annual cost per patient paid by medical insurance (β = −1.734, odds ratio [OR] = 0.177). Among non-economic attributes, the impact of diseases on life-years—with no treatment, the patient will die in the prime of life (β = 0.523, OR = 1.688, willingness to pay = 301,895)—was most concerning, followed by significant improvements to the quality of life (β = 0.516, OR = 1.676, willingness to pay = 297,773).
Conclusion: The general public in China does not value rarity as a sufficient reason to justify special consideration in funding orphan drugs. When making orphan drug coverage decisions, the public prioritized the annual cost, disease severity, and drug effects.
Introduction
Different from common diseases, rare diseases often have a low disease prevalence, difficulties in patient diagnosis and recruitment, and high treatment costs (1). However, because of the large size of the Chinese population, the low incidence of all rare diseases has resulted in a large number of patients. In China, the number of patients with rare diseases is >20 million and growing at a rate of 200,000 per year (2), placing an increasingly great disease burden on the society (3).
Medical insurance coverage is one of the most efficient ways to improve the accessibility of orphan drugs (4). As the resource is limited, however, policy-makers often face a dilemma between maximizing the total health benefits of society and achieving health equity while considering the minority group when making an orphan drug reimbursement decision (5). To resolve the conflict therein, priorities should be constructed to rank all alternative health technologies. Health technology assessment is the general approach to assess the value of drugs and medical devices. Traditionally, a cost-effectiveness analysis is used to test whether the extra cost paid for 1 quality-adjusted life-year (QALY) gained is under the willingness to pay (WTP) threshold. As for orphan drugs, their prices are usually high and the clinical evidence is weak despite the difficulty of conducting clinical trials. Through a cost-effectiveness approach, orphan drugs commonly have no chance of being prioritized over common disease drugs.
Rarity itself is not the criterion that leads to a prioritized reimbursement decision for orphan drugs (6). The value of orphan drugs is impacted by many value dimensions aside from cost and health outcome, such as unmet health needs, the severity of the disease, and the availability of alternative treatments (7). Although no consensus has yet been reached regarding the way to assess orphan drugs' value, multi-criteria appraisal methods have been applied in orphan drug reimbursement decision-making instead of cost-effectiveness approaches. The multi-criteria decision analysis (MCDA) method encompasses a group of assessment tools implied to assess the value of health technologies impacted by multi-dimensional factors. A number of attempts have been made to build or test these tools, such as reflective MCDA in the Catalan Health Service (8), the Evidence and Value: Impact of Decision-making (9), and other MCDA frameworks (10, 11), in different regions and from different perspectives to appraise orphan drugs. Among all the MCDA types, the discrete choice experiment (DCE) is one of the most commonly used methods to elicit individual preferences in the step of criteria weight measurement and becoming increasingly popular (12). Choice experiments could be suitable to reveal general preferences in health care priority settings (13) and can be completed by respondents themselves online (14).
The drug price negotiation is the way for brand name drugs (including most orphan drugs) to be covered by the Basic Medical Insurance in China. In the official document disclosed by the National Healthcare Security Administration in June 2022, price negotiation for orphan drugs was encouraged. However, no value assessment framework specific to orphan drugs has yet been established in China, which has provoked a wide discussion about the rationality of the medical insurance access mechanism. The question of what criteria are appropriate and acceptable for the general public should be considered when making a national health care reimbursement decision (15). This is especially the case for health technology, such as orphan drugs, because a single rare disease patient could consume the same amount of a medical resource needed by multiple common disease patients. As payers and beneficiaries of the medical insurance fund, the public is an orphan drug–reimbursement stakeholder. The societal preferences for orphan drugs have been measured in several western countries (16–18). Without other differences, no societal preference for rarity has been found across studies. The preference varied with the survey scenario setting, which reflected reimbursement policy and funding situation in each country, changed (16). No consensus has been reached on the list of orphan drug attributes and levels to be included in the trade-off study (18–20). As the heterogeneity of policy and subjectivity of preference, these studies only shed light on the value of research population and would not reflect preferences in other countries.
The objective of this study was to explore whether a societal preference for orphan drugs exists in China and to quantitatively measure the personal trade-off between essential attributes of orphan drugs in the Chinese general public.
Methods
DCE is a quantitative research method designed to reveal stated preferences, which forces respondents to consider a trade-off between ≥2 alternatives in hypothetical scenarios. It allows the assessment of relative importance and the WTP for selected attributes by including a cost attribute (21). DCEs have been widely applied and have offered a great deal of priority-setting information in the health care sector (22, 23). By offering a detailed description of competing scenarios, DCE technique makes it easier to obtain the true preferences and leads to more precise weights than other weighting methods used in MCDA (24). Compared to other stated preference revealed methods, such as best-worst scaling (BWS), the stability, continuity and acceptability of DCE are better (25, 26).
DCE can be presented as labeled (such as orphan drugs vs. common drugs) or generic (such as treatment 1 vs. treatment 2). In the present study, a labeled DCE was employed because there is no official definition of rare diseases in China, so an attribute of orphan drugs (i.e., the rarity of the diseases) could not be accurately presented to the public. Unlike the generic experiment, the labeled experiment brands each alternative, containing information that is difficult to measure and will influence respondent's choices.
Attributes and their levels
The use of qualitative research in DCE is an important step when selecting and determining the attributes and their corresponding levels (27). A 3-step approach was used to select attributes for the present study. First, an initial set of potential attributes and levels of orphan drugs was derived from a literature review. Some high-quality research reports of rare diseases and orphan drugs in China were also considered, such as Report of rare diseases in China (2018) (28) and the China rare disease drug accessibility report (2019) (29). Second, three experts on orphan drugs were invited to review the potential attributes and levels list. The experts were asked to check the comprehensiveness and rationality of selected attributes and levels as well as the accuracy of the description. Third, semi-structured interviews were conducted with 13 stakeholders, including rare disease patients, clinicians, specialists of orphan drug policy, policy-makers, and people working in the orphan drug industry. A refined list (shown in Supplementary material) was offered to the stakeholders, asking them to rate all attributes according to the importance in medical insurance access decision-making using a 10-point scale. Potential attributes and levels were not limited by the preliminary list. Each expert was encouraged to recommend any additional attributes he or she found relevant. The final rank of potential attributes was created after this round of reviews; the six most important attributes were finally incorporated, and the wording of all potential attributes and levels was further revised according to interview results and research team discussion (Table 1).
Experimental design
The experiment design employed Ngene version 1.1 (ChoiceMetrics, Sydney, Australia) to define the combination of levels for the choice set and avoid the logically impossible ones. The experiment was conducted among the general population following the good practice guidelines of DCE (30, 31) in two steps: a pre-survey and a formal survey. To improve the clarity of the survey and questionnaire feasibility for respondents, 135 eligible respondents were recruited for the pre-survey to test the questionnaire and collect a priori information about the value of the attributes. The pre-survey was generated using an orthogonal main effects design. Feedback from the pre-survey led to further wording adjustment of the questionnaire; in this way, the questionnaire was updated to its final version. A D-efficient design was incorporated in the formal survey, and the results of the pre-survey were adopted as prior values. A D-efficient design was used to minimize standard errors and parameter prior variances in DCE (32).
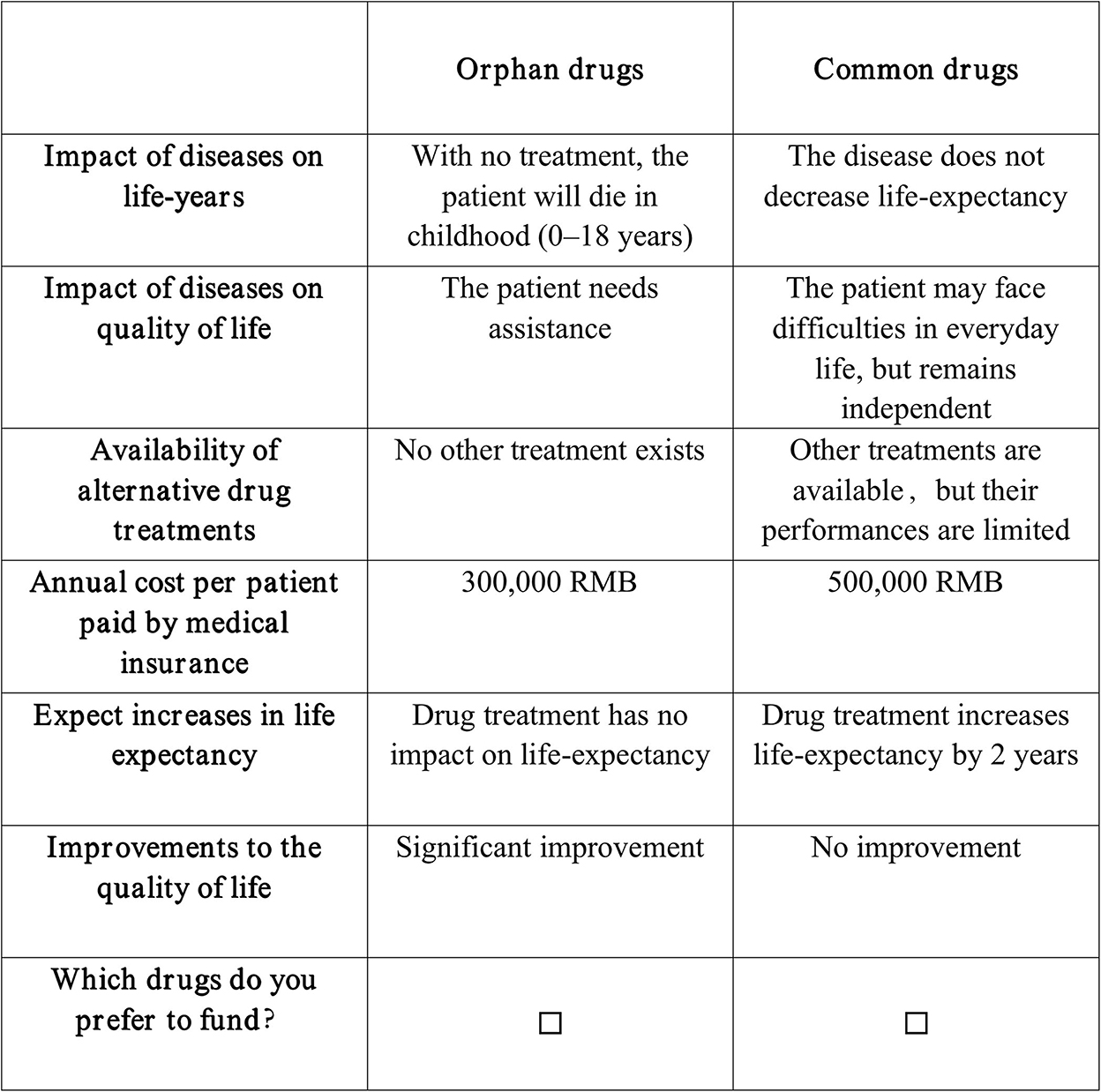
The final number of choice situations was limited to 27 situations, which were divided into three blocks with nine questions in each and consisted of the labeled orphan drugs and common drugs. The blocking method was used to limit the burden of respondents, which also satisfied efficient design criteria (31). The choice set did not represent any specific existing drugs. The survey did not offer the opt-out option because the options in our DCE were a fair reflection of medical insurance drug access in China. Furthermore, a final choice set (the 10th choice set), which was the same as the second choice set, was added. The repeated choice set was used as an internal consistency check of the questionnaire to ensure that respondents were engaged in the experiment and taking it seriously. The 10th choice set was subsequently dropped from the final statistical analysis. Figure 1 shows an example of the choice set as presented to the respondents.
Study sample and survey administration
According to the International Society for Pharmacoeconomics and Outcomes Research Task Force Report on DCE construction, the sample size of DCE is decided by the object of the study and the number of attributes and levels (33). When blocking a DCE questionnaire into different versions, >20 participants per version are rarely required to estimate reliable models (34). It was reported that the mean sample size for conjoint analysis studies in health care published between 2005 and 2008 was 259, with nearly 40% of DCEs having sample sizes of 100–300 respondents (35). The following formula used to calculate the minimum sample size in DCE was also considered: n = (500*c)/(t*a), where “c” represents the largest number of levels for an attribute, “t” represents the number of choice sets in a block, and “a” represents the number of alternatives (36). While there was limited consensus on appropriate sample sizes for DCE studies, a sample size of 300 participants in total and 100 participants per version would be sufficient for a reliable statistical analysis.
A convenience sampling method was used in this study and those who met all the following criteria were included: (1) Chinese citizens aged ≥18 years, (2) individuals with no diagnosis of a rare disease, (3) individuals who participated in the Basic Medical Insurance in China, and (4) individuals without cognitive impairment who were willing to sign the informed consent form and finish the questionnaire on their own.
If the participants had no prior knowledge or cognitive basis, their preferences were assumed to be unstable and constructed rather than just revealed in the process of answering choice-related questions (37). As the funding issue of orphan drugs is beyond the common sense of the public, the questionnaire started with a brief introduction of rare diseases and orphan drugs as well as the disease burden of both rare diseases and common diseases. Socioeconomic characteristics of the respondents (e.g., sex, age, education) were collected. A profile that clearly defined all attributes and levels and an example of the choice set were shown to respondents. Each respondent was asked to make reimbursement trade-offs between the paired alternatives. Before making decisions, the respondents were informed that the Basic Medical Insurance funding could not cover all drugs because of limited health care budgets. They were asked to place themselves in a hypothetical situation as decision-makers and to prioritize the drugs that should be reimbursed. It was specified that choosing an alternative meant that resources could not be allocated to the other one. The 10 choice sets were presented to the respondents, following the description of the hypothetical situation.
Statistical methods
Descriptive statistics were used to present the demographic characteristics of the respondents. The random utility model provided the theoretical underpinning for analysis of the DCE data (5). In a choice set, it assumed that individuals chose a certain alternative that yielded a higher utility to them over the other one. Utility was calculated using the following formula:
Where Unjt represents the utility respondent n obtained from alternative j on choice set t, which was composed of a systematic unit (Vnjt) and a random unit (εnjt); Xnjt represents the explanatory vector of the attribute; and β represents the coefficient vector of the corresponding preference value, the size and significance of which is the weight of each level of each attribute. In other words, the coefficients of the model can be interpreted to define the relative importance that the respondents gave to the movement of any given attribute from the reference level to a different level (38). Apart from the attributes and levels, there was an alternative specific constant, which reflected whether the public had a preference for the label of orphan drugs or common drugs.
The DCE data were analyzed using a mixed logit model and binary logit model. The mixed logit model was used to test whether the Chinese public preferred orphan drugs over common drugs and whether the listed attributes were important factors for drug funding decisions, while the binary logit model was used to measure the relative importance of each attribute in orphan drug access for National Reimbursement Drug List (NRDL) and its WTP. The response variable was a binary (0/1) dependent discrete variable where “1” represented the alternative chosen and “0” represented the one not chosen. Except for the continuous variables of attributes, including the annual cost per patient paid by medical insurance and expected increases in life-expectancy, other attributes were considered as categorical variables. An example of the categorical variable code is listed as follows: a = reference case [0], b = 1, and c = 2. Furthermore, the WTP was also calculated, which represented the marginal utility respondents were willing to pay for a particular change in attribute level. Finally, an exclusion criterion was applied to remove the questionnaires completed in ≤60 s. All statistical analyses were performed using STATA version 16.0 (StataCorp LLC, College Station, TX, USA).
Results
Respondent characteristics
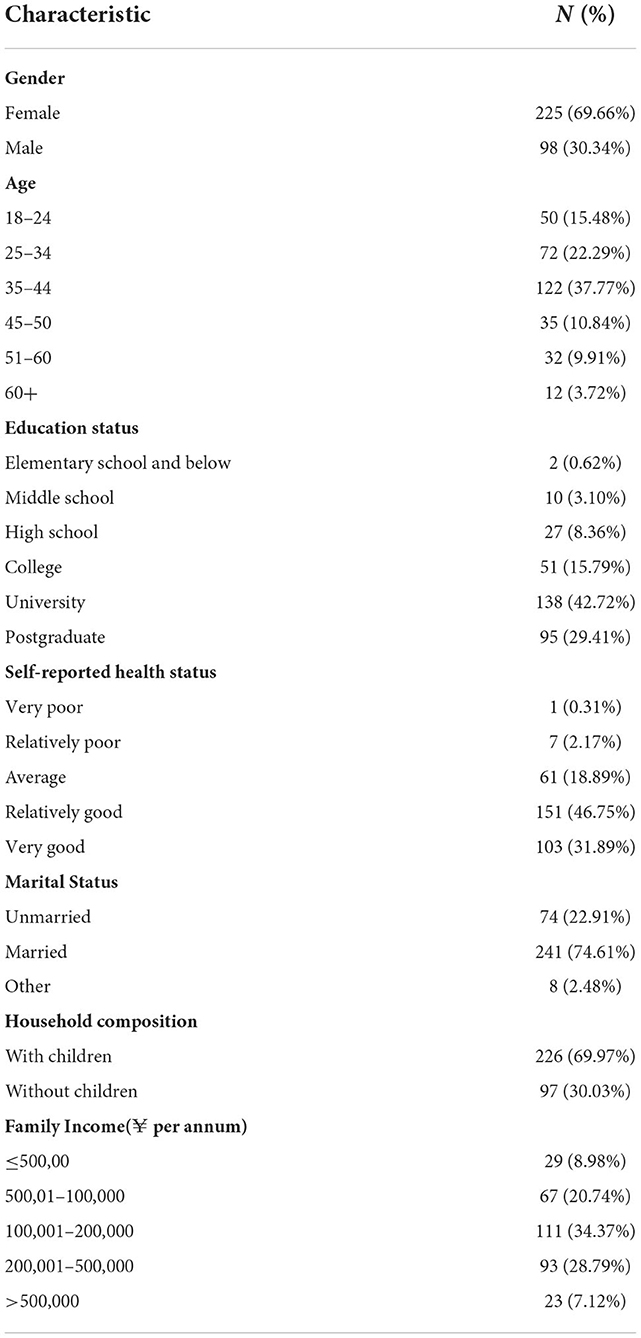
A total of 412 individuals participated in the formal survey, 346 questionnaires were initially considered valid (valid response rate, 83.98%), but 323 questionnaires were finally included in the analysis after excluding those completed in ≤60 s. The sociodemographic characteristics of the study sample are presented in Table 2. Women represented 69.66% of the respondents, and 37.77% of the respondents were aged 35–44 years. A Bachelor's degree or higher was held by 72.13% of the respondents, and 78.64% of the respondents self-reported that they were healthy. A relatively large portion of participants were married (74.61%) and most of participants had children (69.97%). The largest group (34.37%) had a family income of 100,001–200,000 RMB per year.
DCE results
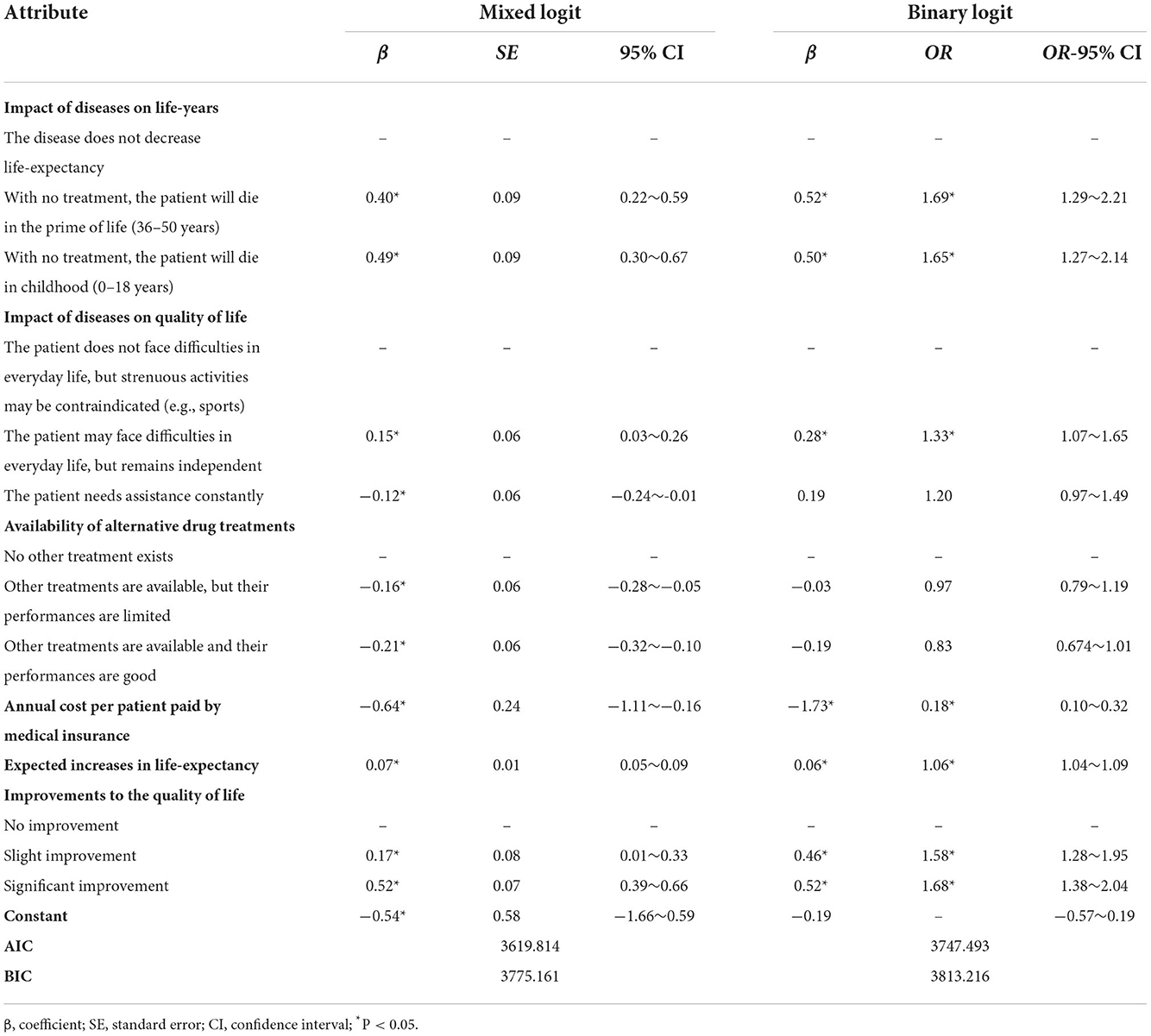
The results of the mixed logit model are presented in Table 3. A positive coefficient means that the corresponding level has a higher utility than the reference level, and the probability of being chosen would be higher in this context. The estimated means for orphan drugs (β = −0.536, p = 0.351) was not statistically significant, meaning that respondents had no preference to fund orphan drugs over common drugs considering only the rarity of the disease when controlling for the defined attributes differences. The estimated coefficients were all statistically significant (p < 0.05), indicating that the 6 selected attributes in the experiment were all important factors influencing the respondents' reimbursement choice between orphan drugs and common drugs. As excepted, the public preferred to reimburse the drugs that treated severe diseases rather than moderate diseases, that had no other alternative treatments available rather than had alternatives, that were cheaper rather than expensive, and that produced more clinical outcomes rather than were less effective.
The results of binary logit model are shown in Table 3. The results showed that the coefficients were significant (p < 0.05) for five out of the six attributes, including the impact of diseases on life-years, impact of diseases on quality of life, annual cost per patient paid by medical insurance, expected increases in life-expectancy, and improvements to the quality of life. Meanwhile, societal preference was not influenced by whether there were other available treatments or not.
The annual cost per patient paid by medical insurance significantly impacted participants' choices [β = −1.734, odds ratio (OR) = 0.177]. With a higher cost, the possibility of being chosen was lower. It can be clearly seen that the disease's impact on life-years was the most impacted non-economic factor. The participants were 1.688 [95% confidence interval (CI) = 1.290–2.207, β = 0.523] times more likely to fund orphan drugs that treated rare disease patients who would die in the prime of life than those that treated rare diseases with no impact on life-years. Additionally, the participants were sensitive to drug improvements to the quality of life. A significant improvement increased the odds of preferring to fund an orphan drug by 1.676 times (95% CI = 1.378–2.038, β = 0.516) compared to no improvement. All else being equal, the odds of successfully listing an orphan drug in NRDL increased by 1.062 (95% CI = 1.039–1.085, β = 0.060) for 1 additional year of survival and by 1.328 (95% CI = 1.071–1.646, β = 0.284) for treating a patient who had difficulties in daily life but remained independent. The results revealed that the respondents would not want to prioritize the Basic Medical Insurance fund on orphan drugs according to the unavailability of alternative treatments or the insurance annual cost of the drug. There was a preference toward drugs that treated severe rare diseases or that had a good effect on the improvement of life-years or quality of life.
Results of WTP
The WTP values shown in Figure 2 indicate the rate at which participants' trade-off of drug costs increased according to gains in other criteria. The comparison of each attribute is presented in economic value form, representing the relative importance of each attribute level. The WTP analysis demonstrated that the impact of diseases on life-years was the most valued attribute. Participants were willing to spend 301,895 RMB per year for rare disease patients who would die in the prime of life with no treatments and 287,605 RMB per year for rare disease patients who would die in childhood rather than patients whose life-years would not decrease. Drug improvements to the quality of life were revealed to be another essential attribute. All else being equal, participants were willing to pay 297,773 RMB to improve patients' quality of life significantly. Moreover, the WTP for 1 year of life expectancy gained was 34,515 RMB.
Discussion
To our knowledge, this paper is the first study to quantitatively investigate individual preferences of the Chinese public and their trade-offs to have the Basic Medical Insurance fund orphan drugs. As the study was conducted on a convenience sample of the general public in China, we consider it to be a pilot MCDA study. The preferences revealed by this study could be used as a priori information for future Bayesian designing of DCEs. The present research showed that public had an indifferent attitude toward orphan drugs and common drugs. This finding aligned with other preferences revealed in studies conducted in other countries (16), which found no evidence of a preference to fund high-cost treatments for rare diseases on the basis of rarity alone. This was also in line with National Institute for Health and Clinical Excellence (NICE)'s evaluation philosophy, which highlights that the “rule of rescue” would not be applicable in an economic evaluation (39), and the criteria for being a highly specialized technology include not only the rarity of the disease but also the severity of the disease and the effectiveness of the drug as well (40). There has always been debated about implementing a higher WTP threshold for orphan drugs (41). In China, no WTP threshold is set specifically for orphan drugs right now and, according to the results of the present study, no evidence exists supporting its necessity. To get an orphan drug listed for reimbursement, multiple criteria should be considered rather than just rarity alone.
Despite all this, slightly more (57%) respondents were observed to prefer funding orphan drugs over common drugs. The first possible explanation for this trend is that the Chinese public has a tendency for altruism—in other words, some subjects were willing to sacrifice part of their own payoff to help people in need (42). Meanwhile, altruism is also an inherent quality in Chinese culture. This point had been demonstrated by the feedback from some of the respondents. The second possible reason was that the results of a prosocial behavior study designed in a laboratory experiment might be more positive than those from a field experiment (43).
Furthermore, this study measured the relative importance of the orphan drug value elements to society. The DCE results suggested that all these attributes mattered significantly from the societal perspective, except for the availability of alternative drug treatments. The results of the experiment offered some hints on priority setting for orphan drugs to be listed in NRDL. Attention should be paid to the annual cost per patient paid by medical insurance, which outweighed all other influences. This finding was consistent with findings of other DCEs conducted from the perspective of decision-makers and patients in European countries (38, 44). There was great evidence that the general public would prioritize orphan drugs that treat severe diseases as well as confer great health gains (17). The top 2 WTP values revealed that the public were willing to pay for orphan drugs that treated diseases from which patients would die in the prime of their life or that could significantly improve patients' quality of life, highlighting the importance of disease severity and drug effect. These two criteria are often related to QALY and presented as quality of life and life-years. Another DCE study have indicated the significance of disease severity and drug effect from different population and in different countries (19). Some studies even argued that these two criteria should be prioritized over cost (20). The availability of alternative drug treatments is a debatable attribute. In the present study, the public showed no preference toward it, yet this was a key factor emphasized by experts in the semi-structured interviews in this study. A similar study from the perspective of the U.K. public also revealed the same result as ours (18), while other studies have demonstrated that the existence of alternative treatments (unmet needs) is an influential factor of societal preferences (19, 45). The difference might result from disparate perspectives of thinking and difficulty in understanding the attribute for the general public. A study also found this disparity existed between the general public and the experts, which was attributed to differences in knowledge and scope (46).
The present study has some limitations. First, due to the coronavirus disease 2019 pandemic, the experiment was conducted online instead of face-to-face. Although it has been proved effective to reveal individual preferences by an online method, the face-to-face method would still have been preferred so that the researchers could catch all feedback from respondents and provide aid when respondents found it difficult to understand. Second, the sample employed in the present study might not perfectly represent the general public in China. However, the results of this study as a pilot study are still considered to have a certain degree of generalizability.
All the evidence gathered herein indicated the necessity to consider multi-level criteria in the orphan drug value assessment process in China. Societal preferences should not be the only evidence used to resolve complex ethic issues regarding orphan drug priority setting. National health resource allocation decisions should be deliberative, taking both multi-stakeholders' preferences and ethical principles into consideration. To establish a specific value assessment framework for orphan drugs, future studies should be conducted in broader samples and multiple stakeholders, including but not limited to the general public, patients, physicians, decision-makers, policy researchers, and people working in the orphan drug industry. Meanwhile, future experiments should explore better DCE designs. Interviewer-dominated face-to-face experiments should be implemented to obtain less biased results. Different colors could be set to different levels, reducing the complexity in the DCE choice task (47). Lastly, more comprehensive criteria should be considered in future studies, such as the quality of clinical evidence and uncertainty of drug and opportunity costs (48). However, the key aspect is to control the increasing complexity of the trade-off task caused by the increasing number of attributes and levels.
Conclusion
To conclude, the results of the present study shed light on the possibility of constructing a MCDA framework using the DCE method to assess the value of orphan drugs, deviating from the traditional cost-effectiveness analysis process. The general public in China does not value rarity as a sufficient reason to justify special consideration in funding orphan drugs. When making coverage decisions, the public prioritized the annual cost, disease severity, and drug effects.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
ST contributed to collection and assembly of data, data analysis, and manuscript writing. YW and YT contributed to data analysis and manuscript review. RJ and MC contributed to manuscript review. HC contributed to data collection, manuscript review, and administrative support. FY contributed to study design, manuscript writing, and administrative support. All authors approved the final version of the manuscript before its submission.
Funding
The present study was supported by the National Natural Science Foundation of China (Nos. 71874086 and 72174093), the Philosophy and Social Science Research Foundation for University of Jiangsu Province (No. 2019SJA0295), and the Excellent Innovation Team of the Philosophy and Social Sciences in the Universities and Colleges of Jiangsu Province The Public Health Policy and Management Innovation Research Team.
Acknowledgments
We acknowledge the support from Yuzhe Zhang, Linguo Li in designing the interview outline. We are also grateful to stakeholders and respondents for participating in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1005453/full#supplementary-material
Abbreviations
QALY, Quality-adjusted life-year: WTP, Willingness to pay; MCDA, Multi-criteria decision analysis; DCE, Discrete choice experiment; NRDL, National Reimbursement Drug List; NICE, National Institute for Health and Clinical Excellence.
References
1. Gong S, Wang Y, Pan X, Zhang L, Huang R, Chen X, et al. The availability and affordability of orphan drugs for rare diseases in China. Orphanet J Rare Dis. (2016) 11:1–12. doi: 10.1186/s13023-016-0392-4
2. Yan X, Dong D, He S. Webster C. Examining trans-provincial diagnosis of rare diseases in china: the importance of healthcare resource distribution and patient. Mobility. (2020) 12:5444. doi: 10.3390/su12135444
3. Cai X, Yang H, Genchev GZ, Lu H, Yu G. Analysis of economic burden and its associated factors of twenty-three rare diseases in Shanghai. Orphanet J Rare Dis. (2019) 14:1–10. doi: 10.1186/s13023-019-1168-4
4. Zamora B, O'Neill FMP, Mestre-Ferrandiz J, Garau M. Comparing access to orphan medicinal products in Europe. Orphanet J Rare Dis. (2019) 14:1–2. doi: 10.1186/s13023-019-1078-5
5. Drummond M, Towse A. Orphan drugs policies: a suitable case for treatment. European Journal of Health Economics. (2014) 15:335–40. doi: 10.1007/s10198-014-0560-1
6. Council NC. Nice Citizens Council Report: Ultra Orphan Drugs London. (2004). Available online at: https://www.nice.org.uk/niceMedia/pdf/Citizens_Council_Ultraorphan.pdf (accessed May 5, 2020).
7. Annemans L, Aymé S, Le Cam Y, Facey K, Gunther P, Nicod E, et al. Recommendations from the European working group for value assessment and funding processes in rare diseases (Orph-Val). Orphanet J Rare Dis. (2017) 12:1–15. doi: 10.1186/s13023-017-0601-9
8. Guarga L, Badia X, Obach M, Fontanet M, Prat A, Vallano A, et al. Implementing reflective multicriteria decision analysis (Mcda) to assess orphan drugs value in the catalan health service (Catsalut). Orphanet J Rare Dis. (2019) 14:1–9. doi: 10.1186/s13023-019-1121-6
9. Radaelli G, Lettieri E, Masella C, Merlino L, Strada A, Tringali M. Implementation of eunethta core model® in lombardia: the vts framwork. Int J Technol Assess Health Care. (2014) 30:105–12. doi: 10.1017/S0266462313000639
10. Schey C, Krabbe PFM, Postma MJ, Connolly MP. Multi-criteria decision analysis (Mcda): Testing a proposed Mcda framework for orphan drugs. Orphanet J Rare Dis. (2017) 12:1–9. doi: 10.1186/s13023-016-0555-3
11. Kolasa K, Zwolinski KM, Zah V, Kaló Z, Lewandowski T. Revealed preferences towards the appraisal of orphan drugs in Poland - multi criteria decision analysis. Orphanet J Rare Dis. (2018) 13:1–14. doi: 10.1186/s13023-018-0803-9
12. Whitty JA, Lancsar E, Rixon K, Golenko X, Ratcliffe J, A. Systematic Review of Stated Preference Studies Reporting Public Preferences for Healthcare Priority Setting. The Patient - Patient-Centered Outcomes Research. (2014) 7:365–86. doi: 10.1007/s40271-014-0063-2
13. Krabbe PFM, Devlin NJ, Stolk EA, Shah KK, Oppe M, Hout Bv, et al. Multinational evidence of the applicability and robustness of discrete choice modeling for deriving Eq-5d-5l health-state values. Medical Care. (2014) 52:935–43. doi: 10.1097/MLR.0000000000000178
14. Mulhern B, Longworth L, Brazier J, Rowen D, Bansback N, Devlin N, et al. Binary choice health state valuation and mode of administration: head-to-head comparison of online and Capi. Value Health. (2013) 16:104–13. doi: 10.1016/j.jval.2012.09.001
15. Rawlins M, Barnett D, Stevens A. Pharmacoeconomics: nice's approach to decision-making. Br J Clin Pharmacol. (2010) 70:346–9. doi: 10.1111/j.1365-2125.2009.03589.x
16. Desser AS, Gyrd-Hansen D, Olsen JA, Grepperud S, Kristiansen IS. Societal views on orphan drugs: cross sectional survey of norwegians aged 40 to 67. BMJ. (2010) 341:c4715-c. doi: 10.1136/bmj.c4715
17. Dragojlovic N, Rizzardo S, Bansback N, Mitton C, Marra CA, Lynd LD. Challenges in measuring the societal value of orphan drugs: insights from a canadian stated preference survey. Patient Cent. Outcomes Res. (2015) 8:93–101. doi: 10.1007/s40271-014-0109-5
18. Bourke SM, Plumpton CO, Hughes DA. Societal preferences for funding orphan drugs in the united kingdom: an application of person trade-off and discrete choice experiment methods. Value Health. (2018) 21:538–46. doi: 10.1016/j.jval.2017.12.026
19. Toumi M, Millier A, Cristeau O, Thokagevistk-Desroziers K, Dorey J, Aballéa S. Social preferences for orphan drugs: a discrete choice experiment among the french general population. Front Med. (2020) 7:323. doi: 10.3389/fmed.2020.00323
20. Mentzakis E, Stefanowska P, Hurley J, A. Discrete choice experiment investigating preferences for funding drugs used to treat orphan diseases: an exploratory study. Health Econ Policy Law. (2011) 6:405–33. doi: 10.1017/S1744133110000344
21. Manipis K, Mulhern B, Haywood P, Viney R, Goodall S. Estimating the willingness-to-pay to avoid the consequences of foodborne illnesses: a discrete choice experiment. Eur J Health Econ. (2022) 8:1–2. doi: 10.1007/s10198-022-01512-3
22. Botha W, Donnolley N, Shanahan M, Norman RJ, Chambers GM. Societal preferences for fertility treatment in Australia: a stated preference discrete choice experiment. J Med Econ. (2018) 22:95–107. doi: 10.1080/13696998.2018.1549055
23. Zhu J, Li J, Zhang Z, Li H, Cai L. Exploring Determinants of health provider choice and heterogeneity in preference among outpatients in beijing: a labelled discrete choice experiment. BMJ Open. (2019) 9:e023363. doi: 10.1136/bmjopen-2018-023363
24. Nemeth B, Molnar A, Bozoki S, Wijaya K, Inotai A, Campbell JD, et al. Comparison of weighting methods used in multicriteria decision analysis frameworks in healthcare with focus on low- and middle-income countries. J Comp Effect Res. (2019) 8:195–204. doi: 10.2217/cer-2018-0102
25. Himmler S, Soekhai V, van Exel J, Brouwer W. What works better for preference elicitation among older people? Cognitive burden of discrete choice experiment and case 2 best-worst scaling in an online setting. J Choice Modell. (2021) 38:100265. doi: 10.1016/j.jocm.2020.100265
26. Huynh E, Coast J, Rose J, Kinghorn P, Flynn T. Values for the icecap-supportive care measure (Icecap-Scm) for use in economic evaluation at end of life. Soc Sci Med. (2017) 189:114–28. doi: 10.1016/j.socscimed.2017.07.012
27. Coast J, Al-Janabi H, Sutton EJ, Horrocks SA, Vosper AJ, Swancutt DR, et al. Using qualitative methods for attribute development for discrete choice experiments. Health Econ. (2012) 21:730–41. doi: 10.1002/hec.1739
28. Jie D, Lin W. (2018). Report of Rare Diseases in China. Beijing: China Medical Science and Technology Press.
29. Iqvia, C. China Rare Disease Drug Accessibility Report. (2019). Available online at: https://xw.qq.com/cmsid/20211206a01a5l00 (accessed November 24, 2021).
30. John FP, Bridges A, Brett H, Deborah M, Andrew L, Lisa AP, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value in Health. (2011) 14:403–13. doi: 10.1016/j.jval.2010.11.013
31. Mangham LJ, Hanson K, McPake B. How to do (or not to do) designing a discrete choice experiment for application in a low-income country. Health Policy Plan. (2009) 24:151–8. doi: 10.1093/heapol/czn047
32. Szinay D, Cameron R, Naughton F, Whitty JA, Brown J, Jones A. Understanding uptake of digital health products: methodology tutorial for a discrete choice experiment using the bayesian efficient design. J Med Internet Res. (2021) 23:e32365. doi: 10.2196/32365
33. Reed F, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier D. Constructing experimental designs for discrete-choice experiments: report of the ispor conjoint analysis experimental design good research practices task force. Value Health. (2013) 16:3–13. doi: 10.1016/j.jval.2012.08.2223
34. Emily Lancsar JL. Conducting discrete choice experiments to inform healthcare decision making: a user's guide. Pharmacoeconomics. (2008) 26:661–77. doi: 10.2165/00019053-200826080-00004
35. Marshall D, Bridges JF, Hauber B, Cameron R, Donnalley L, Fyie K, et al. Conjoint analysis applications in health - how are studies being designed and reported? an update on current practice in the published literature between 2005 and 2008. Patient. (2010) 3:249–56. doi: 10.2165/11539650-000000000-00000
36. De Bekker-Grob EW, Donkers B, Jonker MF, Stolk EA. Sample size requirements for discrete-choice experiments in healthcare: a practical guide. Pat Cent Outcomes Res. (2015) 8:373–84. doi: 10.1007/s40271-015-0118-z
37. Shiell A, Seymour J, Hawe P, Cameron S. Are preferences over health states complete? Health Econ. (2000) 9:47–55. doi: 10.1002/(SICI)1099-1050(200001)9:1<47::AID-HEC485>3.0.CO;2-L
38. López-Bastida J, Ramos-Goñi JM, Aranda-Reneo I, Taruscio D, Magrelli A, Kanavos P. Using a stated preference discrete choice experiment to assess societal value from the perspective of patients with rare diseases in Italy. Orphanet J Rare Dis. (2019) 14:1–7. doi: 10.1186/s13023-019-1126-1
39. Nice. Interim Process and Methods of the Highly Specialised Technologies Programme: Updated to Reflect 2017 Changes. (2017). Available online at: https://www.nice.org.uk/Media/Default/About/What-We-Do/Nice-Guidance/Nice-Highly-Specialised-Technologies-Guidance/Hst-Interim-Methods-Process-Guide-May-17.Pdf (accessed June 21, 2022).
40. Nice. The Principles That Guide the Development of Nice Guidance and Standards. (2020). Available online at: https://www.nice.org.uk/about/who-we-are/our-principles (accessed June 21, 2022).
41. Balijepalli C, Gullapalli L, Druyts E, Yan K, Desai K, Barakat S, et al. Can standard health technology assessment approaches help guide the price of orphan drugs in Canada? A review of submissions to the Canadian agency for drugs and technologies in health common drug review. Clin Outcomes Res. (2020) 12:445–57. doi: 10.2147/CEOR.S264589
42. Andreoni J, Miller J. Giving According to Garp: an experimental test of the consistency of preferences for altruism. Econometrica. (2002) 70:737–53. doi: 10.1111/1468-0262.00302
43. List JA. The behavioralist meets the market: measuring social preferences and reputation effects in actual transactions. J. Polit. Econ. (2006) 114:1–37. doi: 10.1086/498587
44. López-Bastida J, Ramos-Goñi JM, Aranda-Reneo I, Trapero-Bertran M, Kanavos P, Rodriguez Martin B. Using a stated preference discrete choice experiment to assess societal value from the perspective of decision-makers in Europe. Does it work for rare diseases? Health Policy. (2019) 123:152–8. doi: 10.1016/j.healthpol.2018.11.015
45. Baltussen R, Marsh K, Thokala P, Diaby V, Castro H, Cleemput I, et al. Multicriteria decision analysis to support health technology assessment agencies: benefits, limitations, and the way forward. Value Health. (2019) 22:1283–8. doi: 10.1016/j.jval.2019.06.014
46. Campolina AG, Suzumura EA, Hong QN, de Soárez PC. Multicriteria Decision analysis in health care decision in oncology: a systematic review. Expert Rev Pharmacoecon Outcomes Res. (2022) 22:365–80. doi: 10.1080/14737167.2022.2019580
47. Jonker MF, Donkers B, Bekker-Grob Ed, Stolk EA. Attribute level overlap (and color coding) can reduce task complexity, improve choice consistency, and decrease the dropout rate in discrete choice. Exp. Health Econ. (2019) 28:350–63. doi: 10.1002/hec.3846
Keywords: orphan drugs, discrete choice experiment, societal preference, willingness to pay, China
Citation: Tan S, Wang Y, Tang Y, Jiang R, Chen M, Chen H and Yang F (2022) Societal preferences for funding orphan drugs in China: An application of the discrete choice experiment method. Front. Public Health 10:1005453. doi: 10.3389/fpubh.2022.1005453
Received: 28 July 2022; Accepted: 28 November 2022;
Published: 12 December 2022.
Edited by:
Mihajlo (Michael) Jakovljevic, Hosei University, JapanCopyright © 2022 Tan, Wang, Tang, Jiang, Chen, Chen and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haihong Chen, Y2hlbmhhaWhvbmdAbmptdS5lZHUuY24=; Fan Yang, eWFuZ2ZhbjUxMkBuam11LmVkdS5jbg==
 Shuoyuan Tan
Shuoyuan Tan Yu Wang1,2
Yu Wang1,2 Yuqing Tang
Yuqing Tang Rong Jiang
Rong Jiang Mingsheng Chen
Mingsheng Chen Haihong Chen
Haihong Chen Fan Yang
Fan Yang