
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 09 November 2022
Sec. Aging and Public Health
Volume 10 - 2022 | https://doi.org/10.3389/fpubh.2022.1003505
This article is part of the Research Topic Aging and Chronic Disease: Public Health Challenge and Education Reform View all 37 articles
 Na Wu1,2†
Na Wu1,2† Xiangyu Zhai3†
Xiangyu Zhai3† Mofan Feng2
Mofan Feng2 Jie Li1
Jie Li1 Ning Yu1
Ning Yu1 Fengwei Zhang1
Fengwei Zhang1 Dong Li4
Dong Li4 Jianying Wang1
Jianying Wang1 Lei Zhang1
Lei Zhang1 Yi Shi2*
Yi Shi2* Guang He2*
Guang He2* Guang Ji5*
Guang Ji5* Baocheng Liu1*
Baocheng Liu1*Aging is accompanied by changes in physiology over time, which remains the largest risk of chronic diseases. The aim of this study was to explore the gender-specific bidirectional relations between the risk of chronic diseases and serum traits in a 3-year longitudinal study. A hierarchical non-linear model with random effects was used to assess the temporal patterns of anthropometric and serum traits from 2017 to 2019 among 2,338 participants. To assess the directional effect between the risk of chronic diseases and serum traits, a bivariate cross-lagged panel model (CLPM) was used to estimate the structural relations of repeatedly measured variables at three different time points. Candidate SNPs were analyzed and genotyped in MassARRAY Analyzer 4 platforms. In this study, metabolic syndrome (MS) score increased with aging in females, whereas the fatty liver disease (FLD) index decreased with aging in males; the MS score was negatively correlated with TB in females, and FLD index was positively related to urea in males; CLPM showed that the MS score predicted total bilirubin (TB) in females, and urea predicted the FLD index in males. Additionally, rs2292354 in G protein-coupled receptor kinase interactor 2 (GIT2) was associated with the MS score and TB in aged females. Our study suggests the potential gender-specific causal associations between development in MS and increase in TB level in females, and rise in urea level and improved FLD index in males. The SNP rs2292354 we investigated might be a biomarker for predicting MS in the elderly Chinese Han population.
By the year 2050, the population aged over 60 years old is estimated to increase by nearly 0.4 billion in China (1). Aging is accompanied by changes in physiology over time, which remains the largest risk of chronic diseases, such as neurodegenerative diseases, cardiovascular diseases, metabolic syndrome (MS) and non-alcoholic fatty liver (NAFLD) (2). As major public health problems, these chronic diseases have led to a cumulative burden on society. Thus, it is urgently required to explore the key mechanisms of aging and age-related chronic diseases.
Age-related impairment of endocrine function results from phenotypic alterations of different cell types, such as endothelial cells, cytokine, adipocytes and hepatocytes (3). The mechanism of MS during aging is likely driven by those phenotypic changes. Evidence has shown that bilirubin can function as an antioxidant by reducing reactive oxygen species and suppressing the oxidative activity of nicotinamide adenine dinucleotide phosphate, resulting in oxidative stress alleviation (4), which is involved in the pathogenesis and development of MS (5). In recent cross-sectional studies, Hwang and Kim observed an inverse relationship between total bilirubin (TB) and MS in Korean women (6); Li et al. (7) confirmed the negative association between bilirubin and MS incidence among Chinese men; Zhong et al. (8) found that TB was negatively associated with MS among the aged Chinese women. Although the association of TB with MS appears to be reported a lot, it still lacks the support from longitudinal studies, which might be helpful to clarify the inconsistency between females and males, and further provide the causality clues. Notably, the urea cycle plays an essential role in NAFLD progression (9). The conversion process from nitrogen into urea has been disrupted, especially in the elderly population with chronic diseases; the bidirectional relation between fatty liver and urea is seldom mentioned.
As we all know, genetic factors play essential roles in the occurrence and progression of chronic diseases. While the genetic susceptibility in the aging population with chronic diseases was seldom mentioned.
Our objective was to evaluate whether TB and urea levels change with aging and predict the later development of the MS and NAFLD in the aged females and males by using longitudinal studies. In addition, candidate genes related to chronic diseases in aging population was also investigated.
Participants were recruited from the outpatient registration pool of those who participated in annual health checks from 2014 to 2019 at the Zhangjiang area of Pudong District Health Care Service Centers, Shanghai, China. The study followed the Helsinki Declaration. A standard protocol has been developed by the Shanghai Innovation Center of Traditional Chinese Medicine Health Service and approved by the Shanghai University of Traditional Chinese Medicine Ethics Committee. Consent was obtained from all subjects. Participants with age over 60 years, who live in Shanghai, can complete measurements and informed consent were included in the inclusion criteria. This study excluded participants with mental disorders, malignant tumors, or incomplete medical records. During the investigation, six male subjects with age < 60 years were excluded, resulting in a total of 2,338 (female, n = 1,303; male, n = 1,035) Chinese elderly subjects with complete data overlapped in 2017, 2018, and 2019 (Figure 1). To elucidate the association between genetic variants and NAFLD in the elderly Chinese Han population, we did a sub-analysis of SNPs in 2017.
Collection of information such as age, gender, alcohol consumption, smoking and medical history were collected by questionnaire (Supplementary Table 1). Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Electronic sphygmomanometers were used to measure blood pressure (Bio-space, Cheonan, South Korea). Blood pressure was measured by electronic sphyg-momanometers (Biospace, Cheonan, South Korea). Waist and hip circumference were reliably measured using a non-stretch tape by the trained professional. Blood samples from the antecubital vein after fasting overnight were collected in the morning. Fasting glucose, alanine transaminase (ALT), aspartate transaminase (AST), total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglyceride (TG), hemoglobin, hemameba, erythrocyte, urea, uric acid, total bilirubin, creatinine and alpha-fetoprotein (AFP) were measured using the biochemistry analyzer (Hitachi, Tokyo, Japan). The tumor marker carcinoma embryonic antigen (CEA) was quantitatively determined by an electro-chemiluminescence immunoassay (ECLIA).
Genomic DNA was extracted from venous blood leukocytes using the EZ1 DNA Blood 350 μL kit (Qiagen) according to the manufacturer's instructions for genotyping. Seven SNPs related to NAFLD relevant traits, including rs2071518 in cellular communication network factor 3 (CCN3), rs12409877 in leptin receptor (LEPR), rs10770141 in tyrosine hydroxylase (TH), rs4430796 in hepatocyte nuclear factor 1-beta (HNF1B), rs2292354 in G protein-coupled receptor kinase interactor 2 (GIT2), rs5186 in angiotensin II receptor type 1 (AGTR1) and rs2206277 in transcription factor AP-2 beta (TFAP2B) from NCBI database of SNP database (www.ncbi.nlm.nih.gov/SNP) were analyzed, and genotyped by matrix-assisted laser desorption/ionization time-off light mass spectrometer in MassARRAY Analyzer 4 platforms (Sequenom, San Diego, CA). Probes and primers were determined with online Assay Design Suite version 2.0 software. Polymerase chain reaction was performed according to the instructions of the manufacturers. More detailed information about primers and polymerase chain reaction conditions is available upon request.
Shapiro-Wilk test was used to check the normality of the data using IBM SPSS Statistics (version 26.0). If data were not normally distributed, their natural logarithms were used. Clinical data were presented as mean and standard deviation. Categorical data were calculated as percentages. FLD index was calculated by the following formula: FLD index = BMI + TG + 3 × (ALT/AST ratio) + 2 × Hyperglycemia (presence of Hyperglycemia, 1; absence of Hyperglycemia, 0) (10). Hyperglycemia was defined as fasting glucose ≥6.1 mmol/L and/or 2-h glucose ≥7.8 mmol/L and/or a previous clinical diagnosis of type 2 diabetes (11). MS score was calculated by the following formula: MS score = 2*waist/height + fasting glucose/5.6 + TG/1.7 + SBP/130 - HDL/1.02 (male) or 1.28 (female) (12).
A hierarchical non-linear model with random effects was used to assess the temporal patterns of anthropometric and serum traits from 2017 to 2019 (MLwin 2.26 software, Multiple Project, Institute of Education, University of London, UK). Age was entered as the explanatory variable in the form of polynomial spline functions to explain the change of target variables over time. Additionally, to determine the longitudinal associations of the FLD index or MS score with serum traits, this hierarchical model analysis was also used with urea and TB as independent variables and FLD index or MS score as an outcome variable.
To assess the directional effect between MS score and TB in females or FLD index and urea in males from 2017 to 2019, a bivariate cross-lagged panel model (CLPM) was used to estimate the structural relations of repeatedly measured variables at three different time points. The auto-regressive part of the model indicates the temporal stability of the variables from one-time point to the next. Meanwhile, CLPM was used to assess reciprocal relationships between the variables at consecutive time points, that is, MS score and TB in females and FLD index and urea in males during the follow-up. Structural equation modeling was conducted by Lavaan in R software (13).
For the sub-analysis, allelic and genotypic distributions and Hardy-Weinberg equilibrium (HWE) were analyzed with the online software SHEsis (http://analysis.bio-x.cn/myAnalysis.php) (14). The association between each SNP with NAFLD in five genetic models (codominant, dominant, recessive, over-dominant and log-additive models, respectively) was analyzed by using “SNPassoc” R package (15). P < 0.05 was considered statistically significant.
Characteristics of the study participants are shown in Table 1. The average ages of females and males were 71 (in 2017) and 73 years old (in 2019).
For anthropometric traits, waistline and SBP increased significantly over time, while DBP showed the opposite trend (p < 0.001 for all) and BMI showed no significant trend both in females (Figure 2) and males (Figure 3); For serum traits, albumin, erythrocyte, hemoglobin, ALT, TC, LDL and urea decreased steadily with aging, AST and HDL showed no significant trend in both females and males, while TB and glucose increased with aging only in females (p < 0.05 for all) (Supplementary Figures 1, 2). Notably, MS score increased with aging in females (p < 0.001), whereas the FLD index decreased with aging in males (p < 0.001).
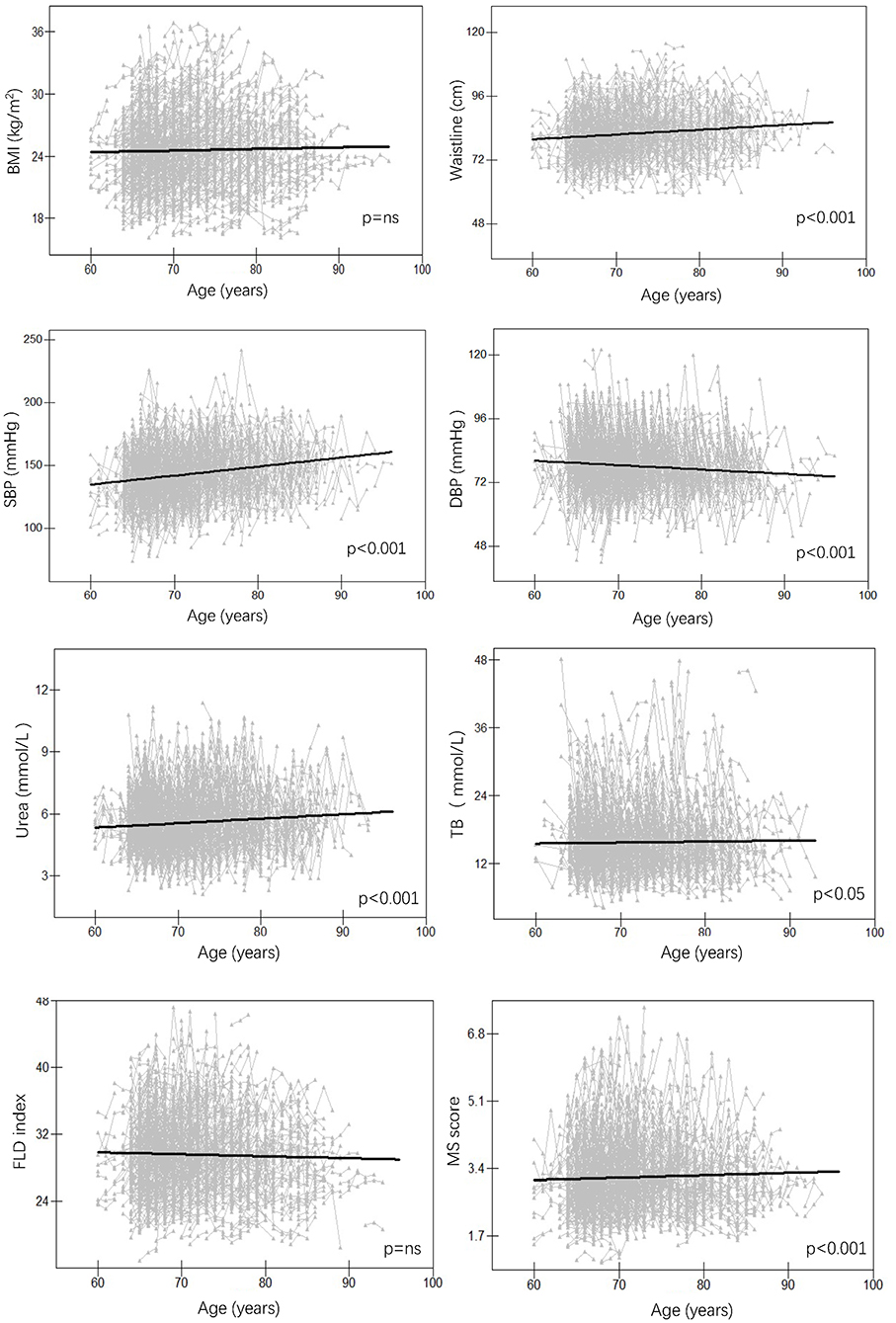
Figure 2. Longitudinal change pattern in females. BMI, body mass index; DBP, diastolic blood pressure; FLD index, fatty liver disease index; MS score, metabolic syndrome score; SBP, systolic blood pressure; TB, total bilirubin.

Figure 3. Longitudinal change pattern in males. BMI, body mass index; DBP, diastolic blood pressure; FLD index, fatty liver disease index; MS score, metabolic syndrome score; SBP, systolic blood pressure; TB, total bilirubin.
To further explore the mechanism differing in females and males, we assessed the longitudinal association between MS score or FLD index and serum traits in females and males. We found that MS score was negatively correlated with TB in females (p < 0.001), and FLD index was positively related to urea in males (p < 0.05) (Figure 4). Notably, no significant associations were found between MS score and urea in females or FLD index and TB in males.
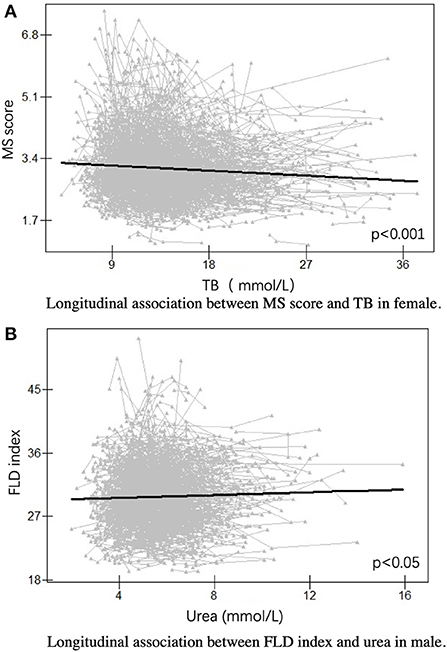
Figure 4. Longitudinal association in females and males. (A) Longitudinal association between MS score and TB in female. (B) Longitudinal association between FLD index and urea in male. FLD index, fatty liver disease index; MS score, metabolic syndrome score; TB, total bilirubin.
In females, the CLPM showed that MS score predicted subsequent MS score at each time point (p < 0.001), and similar patterns were observed between the repeated measurements of TB (p < 0.001). The CLPM also showed that the MS score in 2017 predicted TB in 2018 (β = 0.048, p = 0.016), while TB in 2017 did not predict the MS score in 2018 (β = −0.002, p = 0.906) (Figure 5).
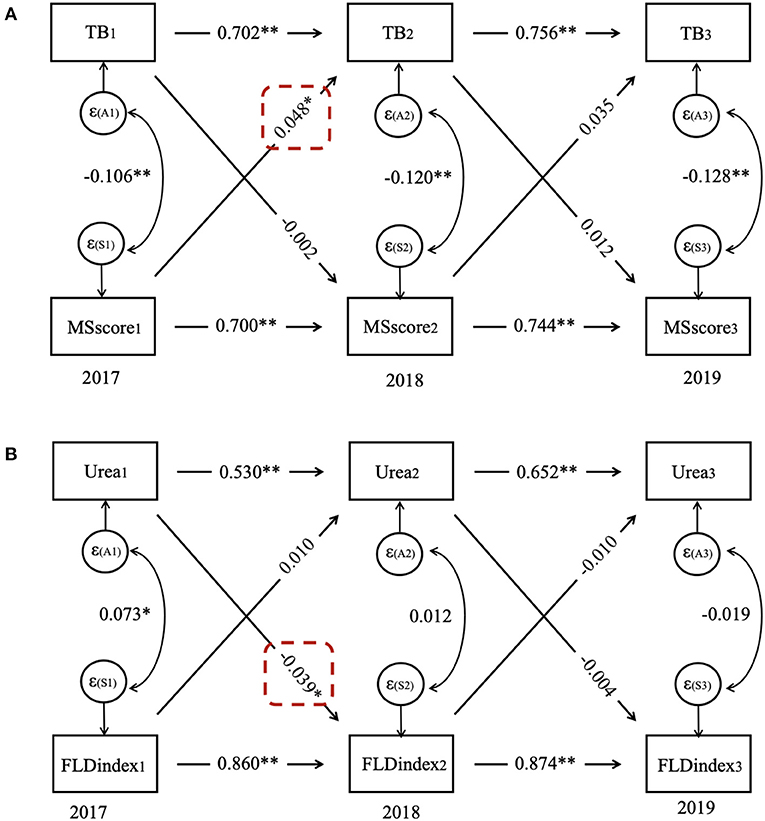
Figure 5. Cross-lagged panel model for (A) TB and MS score in females and (B) urea and FLD index in males. FLD index, fatty liver disease index; MS score, metabolic syndrome score; TB, total bilirubin.
In males, the CLPM presented that the FLD index predicted subsequent FLD index at each time point (p < 0.001), the same as repeated measurements of urea (p < 0.001). Moreover, the CLPM also showed that urea in 2017 predicted the FLD index in 2018 (β = −0.039, p = 0.015), whereas the FLD index in 2017 did not predict urea in 2018 (β = 0.010, p = 0.718).
As genetic factors play essential roles in the occurrence and progression of NAFLD, we also conducted the SNPs test among 732 participants (NAFLD, n = 479; control, n = 253) in 2017 (Supplementary Table 2). There was no significant difference in age between NAFLD and control groups. The BMI of NAFLD patients was much higher than the controls (p < 0.001). The detailed information on seven SNPs is presented in Table 2.
All the SNPs met HWE (p > 0.05). The allele frequencies of rs2071518, rs12409877, rs10770141, and rs4430796, and the genotype frequencies of rs2071518, rs10770141, rs2292354, rs5186, and rs2206277 were significantly different between NAFLD and controls (p < 0.05) (Table 3).
As the longitudinal association between MS score and TB in females, FLD index and urea in males were different; here, we also checked the association of seven SNPs with NAFLD in females and males, respectively. In females, for rs12409877, A/G-G/G genotype under the dominant model (OR = 1.87, 95%CI = 1.07–3.26, p = 0.029) and A/G genotype under the overdominant model (OR = 1.90, 95%CI = 1.07–3.37, p = 0.031) were statistically related to increased risk of NAFLD, as well as the log-additive model (OR = 1.68, 95%CI = 1.03–2.76, p = 0.041), even after adjusting for age and BMI (FDR1 < 0.05); for rs10770141, A/G genotype under the codominant model (OR = 1.67, 95%CI = 0.94–2.96, p = 0.022) and A/G-A/A genotype under the dominant model (OR = 1.81, 95%CI = 1.03–3.17, p = 0.040) were significantly associated with increased risk of NAFLD, as well as the log-additive model (OR=1.89, 95%CI=1.11-3.22, p=0.022); for rs2292354, there was a significant association between G/A genotype under the overdominant model and the increased risk of NAFLD (OR = 1.54, 95%CI = 1.11–3.22, p = 0.022) (Table 4). Notably, we also analyzed the association between these SNPs, the MS score, and TB. We found that rs2292354 was significantly related to MS score in the dominant and overdominant genetic model (p = 0.025 and p = 0.023, respectively), as well as TB in the codominant and overdominant genetic model (p = 0.034 and p = 0.019, respectively) (Supplementary Table 3).
In males, for rs2071518, T/C and T/T genotype under the codominant model (OR = 1.99, 95%CI = 1.17–3.39; OR = 1.17, 95%CI = 0.27–5.02, FDR = 0.049), T/C-T/T genotype under the dominant model (OR = 1.90, 95%CI = 1.14–3.17, FDR = 0.036) and T/C genotype under the overdominant model (OR = 1.98, 95%CI = 1.17–3.36, FDR = 0.036) were significantly related to increased risk of NAFLD, as well as the log-additive model (OR = 1.62, 95%CI = 1.03–2.53, FDR = 0.049), even after adjusting age and BMI (FDR1 < 0.05); for rs2206277, C/T and T/T genotype under the codominant model (OR = 0.85, 95%CI = 0.52-1.39; OR = 0.09, 95%CI = 0.01–0.71, FDR = 0.017) and T/T genotype under the recessive model (OR = 0.10, 95%CI = 0.01–0.75, FDR = 0.010) were significantly associated with the decreased risk of NAFLD, as well as the log-additive model (OR = 0.63, 95%CI = 0.42–0.95, FDR = 0.043), even after adjusting age and BMI (p1 < 0.05) (Table 5). No significant associations between above SNPs and FLD index or urea were found (Supplementary Table 3).
In the present study, we observed that the MS score increased with aging in females, and FLD index decreased with aging in males; further, the longitudinal negative association between MS score and TB in females, and positive association between FLD index and urea in males were found; additionally, it suggests potential causal associations between development in MS and increase of TB level in females, and rise in urea level and improved FLD index in males by CLPM. Additionally, this gender-specific distinction might be explained by the genetic variants.
Aging is accompanied with changes in body composition and blood traits. Waistline seems a better indicator to determine the health risk associated with obesity in the elderly (16). Lee et al. (17) reported that waistline was superior to BMI as a predictor of hypertension, dyslipidemia and type II diabetes in females and males. Our study also showed that waistline decreased with aging rather than BMI. Further, as cardiomyocyte has a limited capacity for regeneration and repair, evidence has shown that cardiac output decreases with aging (18). Instead, SBP increased with aging in our study, which was highly associated with arterial stiffness (19). In addition, decreased serum albumin, erythrocyte, hemoglobin, ALT, AST, and TC were strongly associated with aging and could reflect the inflammation, metabolic demand and several pathological conditions, including NAFLD, non-alcoholic steatohepatitis, fibrosis and hepatocyte carcinoma (20–27). Our results were consistent with the previous studies. Notably, TC and LDL levels declined with aging in both men and women, but HDL levels did not change much or even slightly increase with aging. This was supported by the finding from a cross-sectional study (28), and this phenomenon might suggest that people with longer life expectancy need maintain a high HDL concentration. All these physiological changes directly or indirectly led to the occurrence of MS and NAFLD with aging.
Aging seems to underlie many of the most prevalent chronic diseases, such as MS and NAFLD. In the present longitudinal study, we used MS score to quantify MS (12) as this continuous quantitative trait could reflect the changing pattern with aging and easily compare across studies and populations. TB was suggested as a biomarker to monitor the resistance against chronic diseases and successful aging (29). Kang et al. (30) found that individuals in the highest bilirubin quartile had a 41% reduced risk of coronary atherosclerosis compared with individuals in the lowest bilirubin quartile. Temme et al. (31) observed that the risk of cancer mortality decreased as bilirubin increased, and the effects were retained in the females but with no significance. Of note, Ong et al. (32) showed that the older females had higher TB levels, and the prevalence of self-reported cardiovascular diseases also tended to decrease with higher TB. These were in line with our results. Although TB increased with aging, MS score was negatively associated with TB in females. Besides, the MS score predicted TB level in females through CLPM. This finding might help answer the cause-and-effect relationship between MS and TB. Combined with the Kao' finding, which showing that estrogen receptor signaling could facilitate bilirubin metabolism (33), it may imply the importance of estrogen between TB and MS, especially for the elderly female population. This finding may also help us explain the link between TB and MS only occurring in the females. FLD index was also applied to reflect the extent of NAFLD (10) in our study. And FLD index declined with aging and urea changed in vice versa; urea predicted FLD index in males. Aging is the most common cause of NAFLD progression and is associated with changes in urea metabolism (9, 34). Our study provided more evidence on the causal link between NAFLD and urea.
For the above gender-specific pattern, it might be explained through genetic susceptibility. As genetic variants play essential roles in the occurrence and progression of chronic diseases, such as MS and NAFLD, here we found different associations between SNPs and the risk of NAFLD in females and males. And rs12409877 in LEPR, rs10770141 in TH and rs2292354 in GIT2 were significantly associated with the increased risk of NAFLD in females; rs2071518 in CNN3 and rs2206277 in TFAP2B were statistically related to the risk of NAFLD in males. Regarding the distinctive finding after gender stratification, we investigated SNPs' role in regulating NAFLD-related traits, such as TB and urea. It showed that rs2292354 in GIT2 gene potentially regulated TB levels and MS in aging females. And this was supported by the following studies, GIT2 was identified as a hub gene to connect with the aging process and aging-related diseases (35); as metabolic status influences aging, Martin et al. (36) deleted GIT2 and found it altered transcriptomic signatures of the hypothalamus, which affects type II diabetes and metabolic pathways; GIT2 is also highly responsive to oxidative stress (37); TB, as the end product of heme degradation, can improve the endothelial function of MS through inhibiting oxidative stress. All these clues suggested genetic variants in GIT2 might play critical roles in the susceptibility of MS. Considering the possible sexual dimorphism, our results also highlighted the importance of consideration of gender in exploring risk factors for the progression in chronic diseases.
The main strength of this study is that longitudinal analysis was conducted in a homogenous, regionally representative cohort of aging females and males, and chronological patterns of anthropometric and blood traits were displayed. CLPM analysis was used to investigate the direction of associations between MS score and TB in females, and FLD index and urea in males, which allows for examining temporal associations better than logistic regression analysis and provides some clues to prevent the chronic diseases. Additionally, the associations of rs2292354 in GIT2 with MS score and TB supported the gender-specific pattern in females in genetics. The limitation of this study is that the associations between MS score and TB in females, and FLD index and urea in males were required regardless of the effects of the hormone, while hormones might profoundly affect the chronic diseases in aging. In addition, since multiple factors would affect the gene variation, more research in different ethnic groups and regions with larger sample size are needed to verify the current result.
Our study suggests the potential causal associations between development in MS and increase in TB level in females, and rise in urea level and improved FLD index in males. Moreover, the associations of rs2292354 in GIT2 with MS score and TB in females were found. The SNP rs2292354 we investigated might be a biomarker for predicting MS in the elderly Chinese Han population.
The original contributions presented in the study are included in the article/Supplementary materials, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Ethics Committee of Shanghai University of Traditional Chinese Medicine. All participants provided informed written consent prior to the study. The patients/participants provided their written informed consent to participate in this study.
BL, GH, and YS designed research. NW, JL, NY, FZ, DL, and JW conducted research. NW and XZ analyzed and interpreted data. NW wrote the paper. BL, GJ, GH, LZ, and YS reviewed the manuscript critically. All authors have read and agreed to the published version of the manuscript.
3-year action plan for Shanghai (project number: ZY (2021-2023)-0211), National Natural Science Foundation of China (81973730), Local Colleges Faculty Constitution of Shanghai MSTC 2022 (22010504300), Shanghai Collaborative Innovation Center for Chronic Disease Prevention and Health Services (2021 Science and Technology 02-37). Shanghai Health Commission for Traditional Chinese Medicine Research (2022QN014).
We would like to thank all participants in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1003505/full#supplementary-material
1. Zeng Y. Towards deeper research and better policy for healthy aging –using the unique data of chinese longitudinal healthy longevity survey. China Economic J. (2012) 5:131–49. doi: 10.1080/17538963.2013.764677
2. Tchkonia T, Kirkland JL. Aging, cell senescence, and chronic disease: emerging therapeutic strategies. JAMA. (2018) 320:1319–20. doi: 10.1001/jama.2018.12440
3. Kubben N, Misteli T. Shared molecular and cellular mechanisms of premature ageing and ageing-associated diseases. Nat Rev Mol Cell Biol. (2017) 18:595–609. doi: 10.1038/nrm.2017.68
4. Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. (1987) 235:1043–6. doi: 10.1126/science.3029864
5. Rani V, Deep G, Singh RK, Palle K, Yadav UC. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. (2016) 148:183–93. doi: 10.1016/j.lfs.2016.02.002
6. Hwang HJ, Kim SH. Inverse relationship between fasting direct bilirubin and metabolic syndrome in Korean adults. Clin Chim Acta. (2010) 411:1496–501. doi: 10.1016/j.cca.2010.06.003
7. Li XH, Lin HY, Guan LY, Peng H, Wen MM, Cao YQ, et al. Direct bilirubin levels and risk of metabolic syndrome in healthy Chinese men. Biomed Res Int. (2017) 2017:9621615. doi: 10.1155/2017/9621615
8. Zhong P, Sun DM, Wu DH Li TM, Liu XY, Liu HY. Serum total bilirubin levels are negatively correlated with metabolic syndrome in aged Chinese women: a community-based study. Braz J Med Biol Res. (2017) 50:e5252. doi: 10.1590/1414-431x20165252
9. De Chiara F, Heebøll S, Marrone G, Montoliu C, Hamilton-Dutoit S, Ferrandez A, et al. Urea cycle dysregulation in non-alcoholic fatty liver disease. J Hepatol. (2018) 69:905–15. doi: 10.1016/j.jhep.2018.06.023
10. Fuyan S, Jing L, Wenjun C, Zhijun T, Weijing M, Suzhen W, et al. Fatty liver disease index: a simple screening tool to facilitate diagnosis of nonalcoholic fatty liver disease in the Chinese population. Dig Dis Sci. (2013) 58:3326–34. doi: 10.1007/s10620-013-2774-y
11. Li Y, He Y, Qi L, Jaddoe VW, Feskens EJ, Yang X, et al. Exposure to the Chinese famine in early life and the risk of hyperglycemia and type 2 diabetes in adulthood. Diabetes. (2010) 59:2400–6. doi: 10.2337/db10-0385
12. Soldatovic I, Vukovic R, Culafic D, Gajic M, Dimitrijevic-Sreckovic V. siMS score: simple method for quantifying metabolic syndrome. PLoS ONE. (2016) 11:e0146143. doi: 10.1371/journal.pone.0146143
13. Rosseel Y. lavaan: an R package for structural equation modeling. J Stat Softw. (2012) 48:1–36. doi: 10.18637/jss.v048.i02
14. Shi YY, He L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. (2005) 15:97–8. doi: 10.1038/sj.cr.7290272
15. González JR, Armengol L, Solé X, Guinó E, Mercader JM, Estivill X, et al. SNPassoc: an R package to perform whole genome association studies. Bioinformatics. (2007) 23:644–5. doi: 10.1093/bioinformatics/btm025
16. Bosello O, Vanzo A. Obesity paradox and aging. Eat Weight Disord. (2021) 26:27–35. doi: 10.1007/s40519-019-00815-4
17. Lee CM, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol. (2008) 61:646–53. doi: 10.1016/j.jclinepi.2007.08.012
18. Chiong M, Wang ZV, Pedrozo Z, Cao DJ, Troncoso R, Ibacache M, et al. Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis. (2011) 2:e244. doi: 10.1038/cddis.2011.130
19. Wu S, Jin C, Li S, Zheng X, Zhang X, Cui L, et al. Aging, arterial stiffness, and blood pressure association in Chinese adults. Hypertension. (2019) 73:893–9. doi: 10.1161/HYPERTENSIONAHA.118.12396
20. Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. (2004) 17:432–7. doi: 10.1111/j.0894-0959.2004.17603.x
21. Kosmachevskaya OV, Topunov AF. Alternate and additional functions of erythrocyte hemoglobin. Biochemistry (Mosc). (2018) 83:1575–93. doi: 10.1134/S0006297918120155
22. Sookoian S, Pirola CJ. Liver enzymes, metabolomics and genome-wide association studies: from systems biology to the personalized medicine. World J Gastroenterol. (2015) 21:711–25. doi: 10.3748/wjg.v21.i3.711
23. Teté S, Tripodi D, Rosati M, Conti F, Maccauro G, Saggini A, et al. Endothelial cells, cholesterol, cytokines, and aging. Int J Immunopathol Pharmacol. (2012) 25:355–63. doi: 10.1177/039463201202500205
24. Tarantino G, Barrea L, Capone D, Citro V, Mosca T, Savastano S. Hematocrit values predict carotid intimal-media thickness in obese patients with non-alcoholic fatty liver disease: a cross-sectional study. Front Endocrinol. (2018) 9:203. doi: 10.3389/fendo.2018.00203
25. Seo E, Kang H, Choi H, Choi W, Jun HS. Reactive oxygen species-induced changes in glucose and lipid metabolism contribute to the accumulation of cholesterol in the liver during aging. Aging Cell. (2019) 18:e12895. doi: 10.1111/acel.12895
26. De Vincentis A, Costanzo L, Vespasiani-Gentilucci U, Picardi A, Bandinelli S, Ferrucci L. Association between non-invasive liver fibrosis scores and occurrence of health adverse outcomes in older people. Dig Liver Dis. (2019) 51:1330–6. doi: 10.1016/j.dld.2019.01.017
27. Sun M, Wang W, Liu X, Wang Y, Cui H, Liu S, et al. Total cholesterol, alanine aminotransferase and the risk of primary liver cancer: a population-based prospective study. Medicine. (2021) 100:e25746. doi: 10.1097/MD.0000000000025746
28. Walter M. Interrelationships among HDL metabolism, aging, and atherosclerosis. Arterioscler Thromb Vasc Biol. (2009) 29:1244–50. doi: 10.1161/ATVBAHA.108.181438
29. Wagner KH, Wallner M, Mölzer C, Gazzin S, Bulmer AC, Tiribelli C, et al. Looking to the horizon: the role of bilirubin in the development and prevention of age-related chronic diseases. Clin Sci. (2015) 129:1–25. doi: 10.1042/CS20140566
30. Kang SJ, Kim D, Park HE, Chung GE, Choi SH, Choi SY, et al. Elevated serum bilirubin levels are inversely associated with coronary artery atherosclerosis. Atherosclerosis. (2013) 230:242–8. doi: 10.1016/j.atherosclerosis.2013.06.021
31. Temme EH, Zhang J, Schouten EG, Kesteloot H. Serum bilirubin and 10-year mortality risk in a Belgian population. Cancer Causes Control. (2001) 12:887–94. doi: 10.1023/A:1013794407325
32. Ong KL, Allison MA, Cheung BM, Wu BJ, Barter PJ, Rye KA. The relationship between total bilirubin levels and total mortality in older adults: the United States National Health and Nutrition Examination Survey (NHANES) 1999-2004. PLoS One. (2014) 9:e94479. doi: 10.1371/journal.pone.0094479
33. Kao TL, Chen YL, Kuan YP, Chang WC, Ho YC, Yeh S, et al. Estrogen-estrogen receptor alpha signaling facilitates bilirubin metabolism in regenerating liver through regulating cytochrome P450 2A6 expression. Cell Transplant. (2017) 26:1822–9. doi: 10.1177/0963689717738258
34. Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of non-alcoholic fatty liver disease and nonalcoholic steatohepatitis in the united states and the rest of the world. Clin Liver Dis. (2016) 20:205–14. doi: 10.1016/j.cld.2015.10.001
35. Chadwick W, Martin B, Chapter MC, Park SS, Wang L, Daimon CM, et al. GIT2 acts as a potential keystone protein in functional hypothalamic networks associated with age-related phenotypic changes in rats. PLoS ONE. (2012) 7:e36975. doi: 10.1371/journal.pone.0036975
36. Martin B, Chadwick W, Janssens J, Premont RT, Schmalzigaug R, Becker KG, et al. GIT2 Acts as a systems-level coordinator of neurometabolic activity and pathophysiological aging. Front Endocrinol. (2015) 6:191. doi: 10.3389/fendo.2015.00191
Keywords: aging, gender-specific, chronic diseases, longitudinal study, bidirectional relation
Citation: Wu N, Zhai X, Feng M, Li J, Yu N, Zhang F, Li D, Wang J, Zhang L, Shi Y, He G, Ji G and Liu B (2022) The gender-specific bidirectional relations between chronic diseases and total bilirubin/urea in the elderly population: A 3-year longitudinal study. Front. Public Health 10:1003505. doi: 10.3389/fpubh.2022.1003505
Received: 26 July 2022; Accepted: 25 October 2022;
Published: 09 November 2022.
Edited by:
Xuan Li, University of Mississippi Medical Center, United StatesReviewed by:
Catherine Lynn T. Silao, University of the Philippines Manila, PhilippinesCopyright © 2022 Wu, Zhai, Feng, Li, Yu, Zhang, Li, Wang, Zhang, Shi, He, Ji and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baocheng Liu, YmFvY2hsaXVfbGFiQDE2My5jb20=; YmFvY2hsaXVAc2h1dGNtLmVkdS5jbg==; Guang Ji, amdAc2h1dGNtLmVkdS5jbg==; Guang He, aGVndWFuZ2Jpb3hAMTYzLmNvbQ==; Yi Shi, eWlzaGlAc2p0dS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.