- 1Acute Infectious Disease Control and Prevention Institute, Jiangsu Provincial Center for Disease Control and Prevention, Nanjing, China
- 2School of Public Health, Nanjing Medical University, Nanjing, China
- 3Institute of Microbiology, Jiangsu Provincial Center for Disease Control and Prevention, Nanjing, China
Background: At present, the global sever acute respiratory syndrome coronavirus 2 (SARS-CoV-2) situation is still grim, and the risk of local outbreaks caused by imported viruses is high. Therefore, it is necessary to monitor the genomic variation and genetic evolution characteristics of SARS-CoV-2. The main purpose of this study was to detect the entry of different SARS-CoV-2 variants into Jiangsu Province, China.
Methods: First, oropharyngeal swabs were collected from 165 patients (55 locally confirmed cases and 110 imported cases with confirmed and asymptomatic infection) diagnosed with SARS-CoV-2 infection in Jiangsu Province, China between January 2020 and June 2021. Then, whole genome sequencing was used to explore the phylogeny and find potential mutations in genes of the SARS-CoV-2. Last, association analysis among clinical characteristics and SARS-CoV-2 Variant of Concern, pedigree surveillance analysis of SARS-COV-2, and single nucleotide polymorphisms (SNPs) detection in SARS-COV-2 samples was performed.
Results: More men were infected with the SARS-CoV-2 when compared with women. The onset of the SARS-CoV-2 showed a trend of younger age. Moreover, the number of asymptomatic infected patients was large, similar to the number of common patients. Patients infected with Alpha (50%) and Beta (90%) variants were predominantly asymptomatic, while patients infected with Delta (17%) variant presented severe clinical features. A total of 935 SNPs were detected in 165 SARS-COV-2 samples. Among which, missense mutation (58%) was the dominant mutation type. About 56% of SNPs changes occurred in the open reading frame 1ab (ORF1ab) gene. Approximately, 20% of SNP changes occurred in spike glycoprotein (S) gene, such as p.Asp501Tyr, p.Pro681His, and p.Pro681Arg. In total, nine SNPs loci in S gene were significantly correlated with the severity of patients. It is worth mentioning that amino acid substitution of p.Asp614Gly was significantly positively correlated with the clinical severity of patients. The amino acid replacements of p.Ser316Thr and p.Lu484Lys were significantly negatively correlated with the course of disease.
Conclusion: Sever acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may further undergo a variety of mutations in different hosts, countries, and weather conditions. Detecting the entry of different virus variants of SARS-CoV-2 into Jiangsu Province, China may help to monitor the spread of infection and the diversity of eventual recombination or genomic mutations.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first detected in Wuhan, China, in December of 2019 (1), has spread throughout the world by travel and community-based contacts (2, 3). The SARS-CoV-2 is a pleomorphic, enveloped, positive-sense, and single-stranded RNA virus (4, 5). It has four structural proteins, such as nucleocapsid (N), membrane “matrix” (M), spike (S), and envelope (E). The assembly of these proteins into the infectious virion results in distinct infectivity of these coronaviruses (5–7). Clinically, the majority of SARS-CoV-2 vaccine candidates are based on the S protein (8), which is the major target of neutralizing antibodies (9, 10). Based on the clinical characteristics, patients with SARS-CoV-2 are classified as mild, moderate, severe, and critical (11). Signs and symptoms of SARS-CoV-2 include fatigue, shortness of breath, cough and chest pain, sore throat, respiratory distress, invasive lung lesions, pneumonia with prominent fever, anosmia, and diarrhea (12–18). For severe SARS-CoV-2 disease, major risk factors include age, male sex, smoking, obesity, and comorbid chronic conditions (such as, hypertension, type 2 diabetes mellitus, and others) (19–21). Now, the global SARS-CoV-2 situation is still grim, and the risk of local outbreaks caused by imported viruses is high. Therefore, it is important to monitor the genomic variation and genetic evolution characteristics of SARS-CoV-2. In this study, we collected oropharyngeal swabs from 165 patients (55 locally confirmed cases and 110 imported cases with confirmed and asymptomatic infection) diagnosed with SARS-CoV-2 infection in Jiangsu Province, China between January 2020 and June 2021 to detect the entry of different SARS-CoV-2 variants into Jiangsu Province. Our study could help to monitor the spread of infection and the diversity of genomic mutations in SARS-CoV-2.
Materials and Methods
Collection of Samples
Oropharyngeal swabs were collected from 165 patients (locally confirmed cases and imported cases with confirmed and asymptomatic infection) diagnosed with SARS-CoV-2 infection in Jiangsu Province between January 2020 and June 2021. For imported cases, these patients became ill during the 14-day quarantine period, indicating that they were infected abroad. The relevant clinical characteristics of these patients were recorded. According to the SARS-CoV-2 Diagnosis and Treatment Protocol (Trial Version 7) of the National Health Commission, PRC, for course distribution, patients were divided into the following four groups: (1) mild type: the clinical symptoms were mild, and no pneumonia was observed on imaging; (2) common type: patients with fever, respiratory symptoms, and other imaging manifestations of pneumonia, but no dyspnea or other complications; (3) severe type: patients conformed to any of the following: (1) the occurrence of shortness of breath, breathing rate ≥ 30 times/min; (2) at rest, oxygen saturation ≤ 93%; (3) arterial partial pressure of oxygen (PaO2)/oxygen concentration (FiO2) ≤ 300 mmHg; (4) patients with significant progression > 50% in lung imaging within 24–48 h; (4) asymptomatic type: patients with a positive nucleic acid testing (NAT) result but without any relevant clinical symptoms and radiological changes of the lung during quarantine. All procedures conducted in this study involving human materials were approved by the Ethics Committee of Novel Coronavirus sequence analysis in Jiangsu province (JSJK2021-B016-01). Moreover, all subjects gave written informed consent.
Whole Genome Sequencing
Total viral RNA was extracted from 200 μl oropharyngeal swabs using the QIAamp Viral RNA Mini Kit (Qiagen, Germany) according to the instructions, and stored at −80°C for further use. Genome-wide specific amplification of total viral RNA was performed using ULSEN® Ultra-sensitive Novel Coronavirus Whole-genome capture Kit (Beijing MicroFuture, V-090418-1, China). PCR products were purified using Agencourt AMPure XP kit (Beckman Coulter, CA, USA). DNA sequencing library was constructed according to the steps of NexteraXT DNA Library Preparation Kit (Illumina, CA, USA). The library was diluted into 100 pmol/μl. The whole genome was deeply sequenced using iseq100 sequencer of Illumina Sequencing platform. The original sequencing data were assembled into a complete sequence using 2019nCOV automatic analysis software of Beijing Micro Future Company. Nucleotide variation sites were compared with reference genome SARS-CoV-2 Wuhan-Hu-1 (GenBank: MN908947.3) using CLC Genomic Workbench 12.0. Mafft software was used for multiple sequence comparison analysis of data from this study (165 cases), public database data on 108 cases (from public SARS-CoV-2 database GISAID, 9 clades and 6 continents), and reference sequences. The comparison results were analyzed by using the MEGA-X software. Neighbor-joining algorithm and Bootstrap were used to construct the phylogenetic tree. Ggtree software was used to mark and beautify the phylogenetic tree. Based on the results of the multi-sequence alignment in the previous step, the assembled virus genome sequence was compared with the reference sequence. The annotation information of loci was based on the general feature format (GFF) annotation information of MN908947.3 and the SNPEFF software. Statistical summaries of variation information and annotation information were performed by using custom Python scripts. For each mutation locus, emphasis was placed on the S gene sequence alignment, single nucleotide polymorphism/insertion/deletion (SNP/INDEL) mutation locus detection, and gene function annotation analysis. Clinical disease progression phenotypes of patients with SARS-CoV-2 were analyzed and summarized and referred to the database of outbreak.info.
Results
Basic Clinical Characteristics of the Research Object
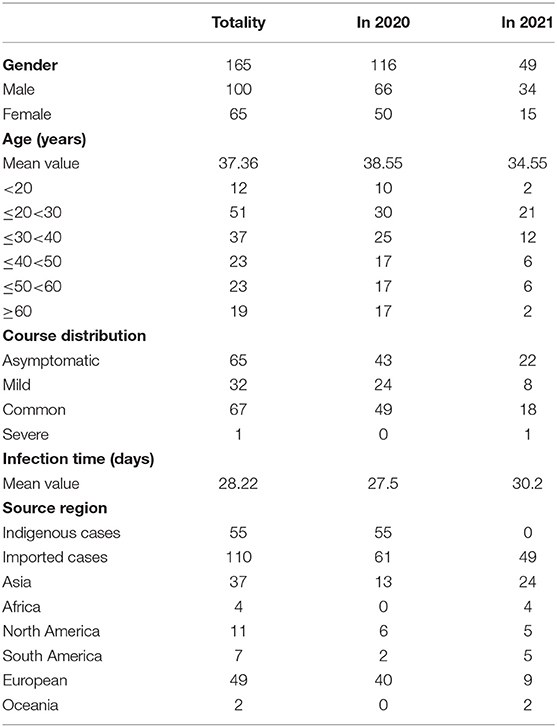
Clinical trials of 165 patients with SARS-CoV-2 are shown in Table 1. There were 100 male patients and 65 female patients. Although the number of patients with SARS-CoV-2 decreased in 2021 as compared with that in 2020, there were still more men than women. The onset age of the patients with SARS-CoV-2 in 2021 was 34.55 years, which was younger than that in 2020 (38.55 years). This suggested that the onset of the SARS-CoV-2 showed a trend of younger age. For course distribution, the number of asymptomatic infected patients was large, similar to the number of common patients. The mean infection time of SARS-CoV-2 was 28.22. The infection time in 2021 was 30.2, which was a little longer than that in 2020 (27.5). There were 55 indigenous cases. It is noted that 110 cases came from abroad, such as 49 (49%) cases from Europe and 37 (34%) cases from Asia.
Association Between SARS-CoV-2 Variant of Concern and Clinical Characteristics
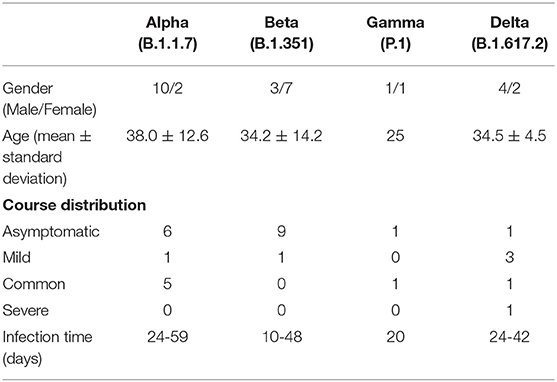
In addition to GISAID database, there are Pangolin classification A/B main branch, and the descendant branch B.X.X. As shown in Table 2, B.1.1.7 (Alpha), B.1.351 (Beta), and P.1 (Gamma) variants did not appear until March 2021. The B.1.617.2 (Delta) variant did not appear until May 2021. Alpha and Beta variants were mainly from patients in Asia, and Gamma and Delta variants were from patients in South America and Europe. According to the epidemiological characteristics in Table 3, patients infected with Alpha (50%) and Beta (90%) variants were predominantly asymptomatic, while patients infected with Delta (17%) presented severe clinical features.
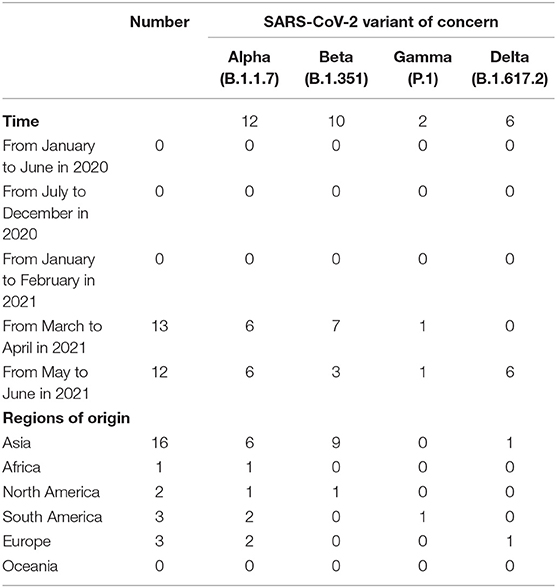
Table 2. Genotype (Pangolin) of SARS-CoV-2 patients at different times and in different regions of origin.
Pedigree Surveillance of SARS-CoV-2
The phylogenetic tree was constructed to analyze the mutation regularity and transmission mode of virus evolution to provide the theoretical basis for tracing virus origin and determining virus transmission chain. The 165 sequences described in this study were combined with a phylogenetic tree of 108 genomic datasets (9 clades, 6 continents, and selected 2 cases). According to the GISAID classification, phylogenetic analysis showed that the virus samples from 37 patients were located in clade GR and GRY, 15 patients were located in clade V, 10 patients were from clade S, and 6 patients were located in clade L (Figure 1). Other virus samples were scattered in different clades. It is reported that the high predominance of the clades GR is mainly related to the occurrence of the amino acid substitution p.Asp614Gly that improved viral fitness (22, 23). The global prevalence of variant Alpha has generated clade GRY from clade GR.
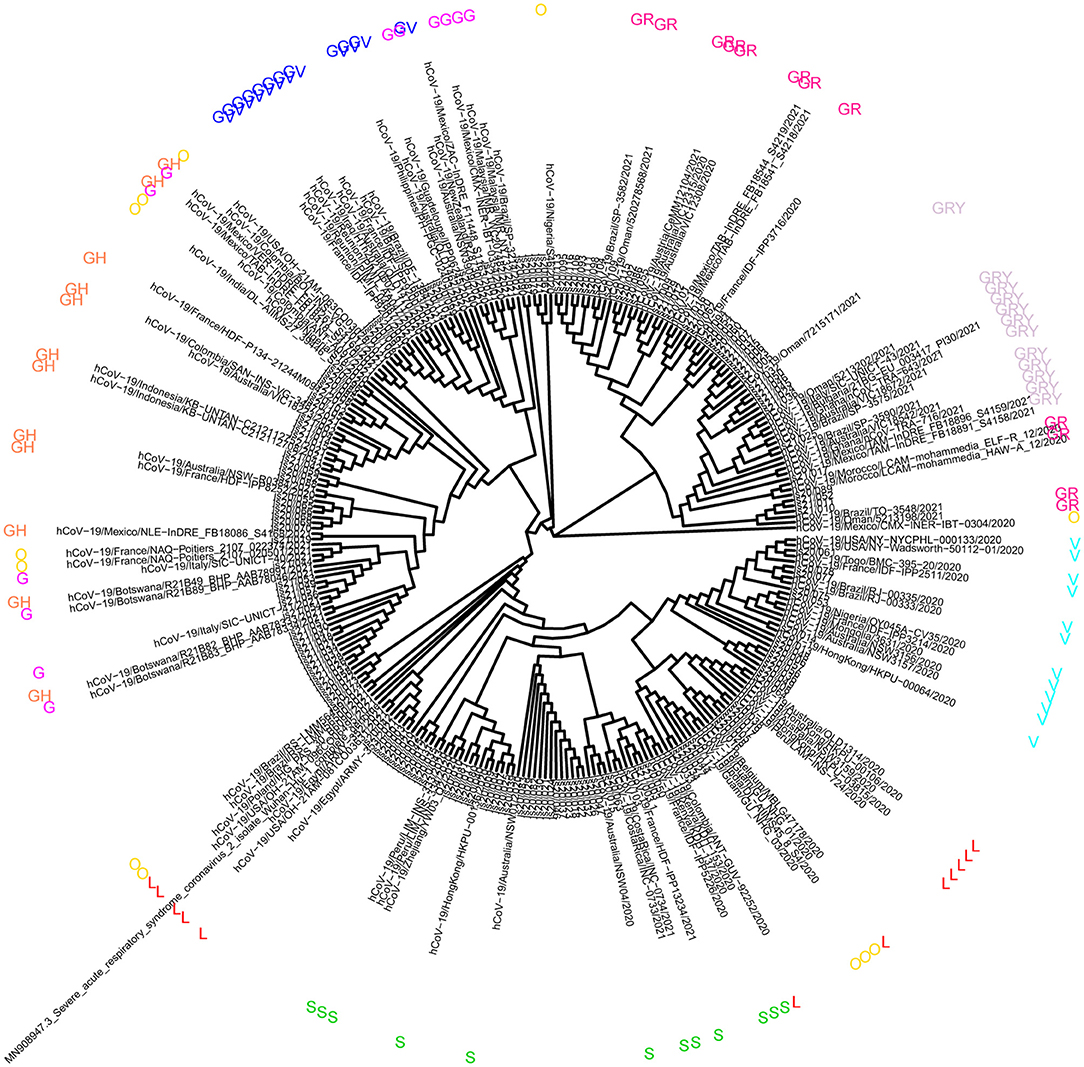
Figure 1. Analysis of phylogeny relationship between the severe acute respiratory symptom of coronavirus 2 (SARSCoV-2) genomes of Coronaviridae of GISAID in Jiangsu Province, China. An interactive view of phylogeny was plotted using Interactive Tree Of Life (iTOL).
Molecular Analysis of SARS-CoV-2 Samples
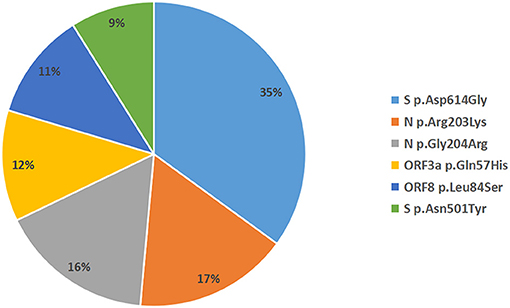
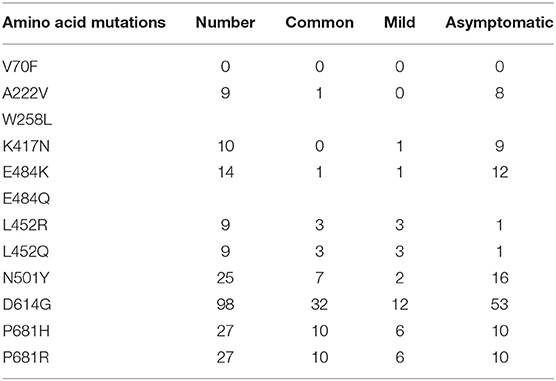
A total of 935 single nucleotide polymorphisms (SNPs) were detected in 165 SARS-COV-2 samples, of which 58% of nucleic acid changes resulted in amino acid substitution (missense mutations) (Supplementary Table 1). In the missense mutation, 303 (56%) SNP changes occurred in the open reading frame 1ab (ORF1ab) gene and 108 (20%) SNP changes occurred in the S gene (Figure 2). As showed in Figure 3, two amino acid substitutions occurred in N gene, such as p.Gli204Arg substitution in 46 SARS-COV-2 samples (16%) and p.Arg203Lys in 46 SARS-COV-2 samples (17%). The p.Glin57His substitution in open reading frame 3a (ORF3a) and the p.Leu84Ser substitution in open reading frame 8 (ORF8) was found in 33 (12%) and 32 (11%) SARS-COV-2 samples, respectively. In S gene, 98 SARS-COV-2 samples (35%) had p.Asp614Gly substitution and 25 SARS-COV-2 samples (9%) had p.Asp501Tyr substitution. In addition to the above mutations, amino acid substitutions of p.Pro681His and p.Pro681Arg were found in the S gene in 27 and 27 SARS-COV-2 samples, respectively (Table 4). In addition, Pearson's correlation coefficient was used to analyze the correlation between SNP changes in the S gene and the severity of patients. Interestingly, 9 SNPs loci in S gene were found to be significantly correlated with severity of patients (Supplementary Table 2). Among which, the amino acid substitution of p.Asp614Gly (cor = 0.29, p < 0.001) was significantly positively correlated with the clinical severity of patients. The amino acid replacements of p.Ser316Thr (cor = −0.16, p = 0.04) and p.Lu484Lys (cor = −0.28, p < 0.001) were significantly negatively correlated with the course of disease.
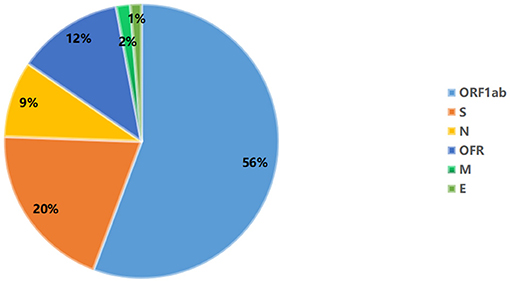
Figure 3. Distribution of amino acid mutation in spike (S), nucleocapsid (N), open reading frame 3a (ORF3a), and open reading frame 3a (ORF8) gene.
Discussion
According to the basic clinical characteristics of infected patients in Jiangsu Province, China, we found that the onset age of the patients with SARS-CoV-2 in 2021 was 34.55 years, which was younger than that in 2020 (38.55). This suggested that the onset of the SARS-CoV-2 showed a trend of younger age. Moreover, we found that there were still more male patients than female patients, even though the number of SARS-CoV-2 patients decreased in 2021 compared with that in 2020. A study has reported the association between SARS-COV-2 infection and transmission and gender (24). In addition, Jin et al. found that gender could be an important factor affecting the SARS-COV-2 mortality (25). It is assumed that gender differences in susceptibility toward SARS-CoV-2 may be associated with the immune response (26). The data of 10 European countries on the distribution of SARS-COV-2 cases by gender showed that women of working age outnumbered infected men (27). Interestingly, it is reported that male sex is one of the major risk factors for severe SARS-CoV-2 disease (19–21). This is indicated that men could be more likely to develop SARS-CoV-2 than women. In addition, mean infection time of SARS-CoV-2 was 28.22. The infection time in 2021 was 30.2, which was a little longer than that in 2020 (27.5). Furthermore, the number of asymptomatic infected patients was large, similar to common patients. SARS-CoV-2 causes asymptomatic infections. Asymptomatic infection is defined as an individual with a positive NAT result but without any relevant clinical symptoms and radiological changes of the lung during quarantine. It is found that the number of SARS-CoV-2 infections is still rising rapidly, and asymptomatic infections could play roles in transmission (28). The higher asymptomatic infection of SARS-CoV-2 could be associated with the high replication efficiency of the virus (29, 30). Therefore, it is urgent to timely detect the source of asymptomatic infections to provide scientific data as a reference to fight against SARS-CoV-2. Among all patients, we found that 110 cases came from abroad, such as 49 (49%) cases from Europe and 37 (34%) cases from Asia. Thus, it is also important to strictly control the importation of the virus abroad.
Based on the novel coronavirus genome information currently published worldwide, mutation rate of SARS-CoV-2 is about 1~2 nucleotides/month. On February 25, 2021, the WHO first proposed the definition of SARS-COV-2 Variant of Concern. As of 24 June 2021, 4 SARS-COV-2 Variant of Concern have been defined, such as B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), and B.1.617.2 (Delta). Variant of Concern, Alpha, is first detected in southeast England in September 2020 and spread to become the dominant lineage in the United Kingdom. Alpha variant has been spreading to at least 114 countries worldwide. The Alpha variant is characterized by 17 mutations. The Beta variant was isolated from an oropharyngeal swab from a patient in the Ugu district, South Africa in November 2020. The emergence of the Beta variant includes several mutations within the structural and nonstructural proteins (31). It is found that variant Beta is purported to be more transmissible (32). The Gamma variant (acquired 17 mutations) emergence occurred around mid-November 2020 in Brazil and was preceded by a period of faster molecular evolution. Gamma variant is purported to be more transmissible (33). In April 2021, the Delta variant was identified in India and classified on May 11, 2021 as a Variant of Concern due to its fast spreading and potential immune escape (34). The Delta variant has been suggested to be more transmissible than the Alpha variant, which is more transmissible than the wild-type SARS-CoV-2 virus (35, 36). Delta variant has now been detected across the Europe, such as the United Kingdom. In our study, we found that Alpha, Beta, and Gamma variants did not appear until March 2021. Delta variant did not appear until May 2021. Tracing its origin, we found that Alpha and Beta variants were mainly from patients in Asia, and Gamma and Delta variants were from patients in South America and Europe. It is worth mentioning that patients infected with Alpha and Beta variants were predominantly asymptomatic, while patients infected with Delta variant presented severe clinical features. The abovementioned variants have increased transmissibility or harmful changes in epidemic characteristics, increased virulence, or clinical manifestations. Therefore, increased surveillance and strict control of the importation of the virus remain urgent.
The SARS-CoV-2 genome shows the typical structure of coronavirus, 5' Untranslated regions (UTR), genes of ORF1ab, ORF3a, ORF8, N, S, E, Membrane protein (M) and 3' UTR containing poly (A) tail, and several unidentified unstructured ORFs (37). It is noted that the mutation rate is fastest at the ORF1ab, ORF3a, and ORF8, N and S genes (38, 39). In the present study, a total of 935 SNPs were detected in 165 SARS-COV-2 samples in Jiangsu Province, China. Missense mutation (58%) was the dominant mutation type. Among which, 56% of SNP changes occurred in ORF1ab gene. ORF1ab, encodes a large polyprotein pp1ab, is proteolytically cleaved into 16 non-structural proteins critical for viral replication (40). In addition, 20% of SNP changes occurred in the S gene, such as p.Asp501Tyr, p.Pro681His, and p.Pro681Arg. It is worth mentioning that p.Asp614Gly variant was significantly positively correlated with the clinical severity of patients. The p.Ser316Thr and p.Lu484Lys variants were significantly negatively correlated with the course of disease. Around September 2020, genetic variants carrying the p.Asp501Tyr substitution on the S protein of SARS-CoV-2 were first detected in the United Kingdom, and spread to elsewhere globally, such as Brazil, South Africa, and the United States (41–44). The p.Asp501Tyr substitution reduces the neutralization capacity of antibodies generated against wild type virus (45–47). Detection of p.Asp501Tyr mutation flags samples that may harbor mutations associated with immune evasion and increased transmissibility. The p.Pro681His mutation is the characteristic of the new SARS-CoV-2 variants from the United Kingdom and Nigeria. The p.Pro681His mutation has unique and emerging characteristics with a significant exponential increase in worldwide frequency. Previous SARS-CoV-2 studies suggest that the p.Pro681His mutation is associated with enhanced systemic infection and increased membrane fusion (48–50). The p.Pro681Arg mutation, was first identified in Uganda in September 2020, is designated a variant of interest in Africa and with subsequent spread to other countries. The p.Pro681Arg mutation causes a small increase in proteolytic processing that might have an effect on viral replication, transmissibility, or pathogenic properties (51). The variant p.Asp614Gly is associated with increased viral load in patients with SARS-CoV-2 (52–54), which may significantly enhance the infectivity and transmissibility of the virus. The mutation p.Lu484Lys, first identified in March 2020, has now been identified in the United Kingdom fast-spreading variant, prompting fears that the virus is evolving further and could become resistant to vaccines (55). The p.Lu484Lys mutation is associated with the humoral immune response evasion (56), which may affect antibody neutralization. This indicated that abovementioned variants in S gene are associated with poor clinical outcome of patients with SARS-CoV-2.
Conclusion
Our study provided crucial epidemiological data on the clinical features, variation characteristics of SARS-CoV-2 in Jiangsu Province, China. These findings could help to assess the burden of viral infection in patients. Timely and accurate monitoring of the spreading of infection and the diversity of genomic mutations in SARS-CoV-2 are needed to alleviate the burden of these diseases. There are limitations to our study. First, the small number of samples may bring some errors, therefore, larger numbers of samples are further needed to minimize the errors. Second, potential biological function of variants in genes is further needed to investigate. Anyway, our findings could have certain reference value for the evaluation of patients with SARS-CoV-2 and clinical treatment.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
All procedures conducted in this study involving human materials were approved by the Ethics Committee of Novel Coronavirus sequence analysis in Jiangsu province (JSJK2021-B016-01). Moreover, all subjects gave written informed consent. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SW, LZ, and CB: conception and design. LZ, CB, and LC: administrative support. SW, ZL, JF, HF, HY, FD, KZ, and LC: provision of materials and samples. SW, XZ, ZL, JF, HF, HY, FD, HH, and JP: data collection and collation. SW and XZ: data analysis and interpretation. All author read and approved the final manuscript.
Funding
This study was funded by the Social Development Project of Jiangsu Province (BE2021739, provided by LZ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2021.791600/full#supplementary-material
References
1. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. (2020) 20:533–4. doi: 10.1016/S1473-3099(20)30120-1
2. Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. (2020) 395:689–97. doi: 10.1016/S0140-6736(20)30260-9
3. Zhu H, Wei L, Niu P. The novel coronavirus outbreak in Wuhan, China. Global Health Res Policy. (2020) 5:6. doi: 10.1186/s41256-020-00135-6
4. The new scope of virus taxonomy: partitioning the virosphere into 15 hierarchical ranks. Nat Microbiol. (2020) 5:668–74. doi: 10.1038/s41564-020-0709-x
5. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. (2020) 395:565–74. doi: 10.1016/S0140-6736(20)30251-8
6. Rey FA, Lok SM. Common features of enveloped viruses and implications for immunogen design for next-generation vaccines. Cell. (2018) 172:1319–34. doi: 10.1016/j.cell.2018.02.054
7. Kang S, Yang M, Hong Z, Zhang L, Huang Z, Chen X, et al. Crystal structure of SARS-CoV-2 nucleocapsid protein RNA binding domain reveals potential unique drug targeting sites. Acta pharmaceutica Sinica B. (2020) 10:1228–38. doi: 10.1016/j.apsb.2020.04.009
8. Le TT, Cramer JP, Chen R, Mayhew S. Evolution of the COVID-19 vaccine development landscape. Nat Rev Drug Disc. (2020) 19:667–8. doi: 10.1038/d41573-020-00151-8
9. Yong SEF, Anderson DE, Wei WE, Pang J, Chia WN, Tan CW, et al. Connecting clusters of COVID-19: an epidemiological and serological investigation. Lancet Infect Dis. (2020) 20:809–15. doi: 10.1016/S1473-3099(20)30273-5
10. Zohar T, Alter G. Dissecting antibody-mediated protection against SARS-CoV-2. Nat Rev Immunol. (2020) 20:392–4. doi: 10.1038/s41577-020-0359-5
11. Jin A, Yan B, Hua W, Feng D, Xu B, Liang L, et al. Clinical characteristics of patients diagnosed with COVID-19 in Beijing. Biosafety Health. (2020) 2:104–11. doi: 10.1016/j.bsheal.2020.05.003
12. Hung LS. The SARS epidemic in Hong Kong: what lessons have we learned? J Royal Soc Med. (2003) 96:374–8. doi: 10.1258/jrsm.96.8.374
13. Chen J, Qi T, Liu L, Ling Y, Qian Z, Li T, et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. (2020) 80:e1–6. doi: 10.1016/j.jinf.2020.03.004
14. Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Military Med Res. (2020) 7:11. doi: 10.1186/s40779-020-00240-0
15. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. (2020) 382:1199–207. doi: 10.1056/NEJMoa2001316
16. Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. (2020) 395:514–23. doi: 10.1016/S0140-6736(20)30154-9
17. Brann DH, Tsukahara T. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. (2020) 6:eabc5801. doi: 10.1126/sciadv.abc5801
18. Tay MZ, Poh CM, Rénia L. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. (2020) 20:363–74. doi: 10.1038/s41577-020-0311-8
19. Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. (2020) 53:368–70. doi: 10.1016/j.jmii.2020.03.005
20. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
21. Garibaldi BT, Fiksel J. Patient trajectories among persons hospitalized for COVID-19: a cohort study. Ann Int Med. (2021) 174:33–41. doi: 10.7326/M20-3905
22. Brufsky A. Hyperglycemia, hydroxychloroquine, and the COVID-19 pandemic. J Med Virol. (2020) 92:770–5. doi: 10.1002/jmv.25887
23. Plante JA, Liu Y, Liu J, Xia H. Spike mutation D614G alters SARS-CoV-2 fitness. Nature. (2021) 592:116–21. doi: 10.1038/s41586-020-2895-3
24. Saghazadeh A, Rezaei N. Immune-epidemiological parameters of the novel coronavirus - a perspective. Exp Rev Clin Immunol. (2020) 16:465–70. doi: 10.1080/1744666X.2020.1750954
25. Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. (2020) 8:152. doi: 10.3389/fpubh.2020.00152
26. Aleksova A, Gagno G, Sinagra G, Beltrami AP. Effects of SARS-CoV-2 on cardiovascular system: the dual role of angiotensin-converting enzyme 2 (ACE2) as the virus receptor and homeostasis regulator-review. Int J Mol Sci. (2021) 22:4526. doi: 10.3390/ijms22094526
27. Shoaib MH, Ahmed FR, Sikandar M, Yousuf RI, Saleem MT. A journey from SARS-CoV-2 to COVID-19 and beyond: a comprehensive insight of epidemiology, diagnosis, pathogenesis, and overview of the progress into its therapeutic management. Front Pharmacol. (2021) 12:576448. doi: 10.3389/fphar.2021.576448
28. Al-Tawfiq JA. Asymptomatic coronavirus infection: MERS-CoV and SARS-CoV-2 (COVID-19). Travel Med Infect Dis. (2020) 35:101608. doi: 10.1016/j.tmaid.2020.101608
29. V'Kovski P, Kratzel A, Steiner S, Stalder H. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. (2021) 19:155–70. doi: 10.1038/s41579-020-00468-6
30. Rossi GA, Sacco O, Mancino E, Cristiani L, Midulla F. Differences and similarities between SARS-CoV and SARS-CoV-2: spike receptor-binding domain recognition and host cell infection with support of cellular serine proteases. Infection. (2020) 48:665–9. doi: 10.1007/s15010-020-01486-5
31. Tegally H, Wilkinson E, Giovanetti M, Iranzadeh A, Oliveira TD. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. mSystems. (2021) 6:e0035321. doi: 10.1101/2020.12.21.20248640
32. Mwenda M, Saasa N, Sinyange N, Busby G, Chipimo PJ, Hendry J, et al. Detection of B.1.351 SARS-CoV-2 variant strain - Zambia, December 2020. MMWR Morbid Mortal Week Rep. (2021) 70:280–2. doi: 10.15585/mmwr.mm7008e2
33. Francisco RDS Jr., Benites LF, Lamarca AP, de Almeida LGP, Hansen AW, Gularte JS, et al. Pervasive transmission of E484K and emergence of VUI-NP13L with evidence of SARS-CoV-2 co-infection events by two different lineages in Rio Grande do Sul, Brazil. Virus Res. (2021) 296:198345. doi: 10.1016/j.virusres.2021.198345
34. Singh J, Rahman SA, Ehtesham NZ, Hira S, Hasnain SE. SARS-CoV-2 variants of concern are emerging in India. Nat Med. (2021) 27:1131–3. doi: 10.1038/s41591-021-01397-4
35. Davies NG, Abbott S. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. (2021) 372:eabg3055. doi: 10.1126/science.abg3055
36. Leung K, Shum MH, Leung GM, Lam TT, Wu JT. Early transmissibility assessment of the N501Y mutant strains of SARS-CoV-2 in the United Kingdom, October to November 2020. Euro Surveil. (2021) 26:2002106. doi: 10.2807/1560-7917.ES.2020.26.1.2002106
37. Weiss SR, Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev. (2005) 69:635–64. doi: 10.1128/MMBR.69.4.635-664.2005
38. Laha S, Chakraborty J, Das S, Manna SK, Biswas S, Chatterjee R. Characterizations of SARS-CoV-2 mutational profile, spike protein stability and viral transmission. Infect Genet Evol. (2020) 85:104445. doi: 10.1016/j.meegid.2020.104445
39. Popa A, Genger JW. Genomic epidemiology of superspreading events in Austria reveals mutational dynamics and transmission properties of SARS-CoV-2. Sci Transl Med. (2020) 12:eabe2555. doi: 10.1126/scitranslmed.abe2555
40. Chan JF, Kok KH. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. (2020) 9:221–36. doi: 10.1080/22221751.2020.1719902
41. Tang JW, Tambyah PA, Hui DS. Emergence of a new SARS-CoV-2 variant in the UK. J Infect. (2021) 82:e27–8. doi: 10.1016/j.jinf.2020.12.024
42. Tang JW, Toovey OTR, Harvey KN, Hui DDS. Introduction of the South African SARS-CoV-2 variant 501Y.V2 into the UK. J Infect. (2021) 82:e8–10. doi: 10.1016/j.jinf.2021.01.007
43. Claro IM, da Silva Sales FC, Ramundo MS, Candido DS, Silva CAM, de Jesus JG, et al. Local transmission of SARS-CoV-2 Lineage B.1.1.7, Brazil, December 2020. Emerg Infect Dis. (2021) 27:970–2. doi: 10.3201/eid2703.210038
44. Galloway SE, Paul P, MacCannell DR, Johansson MA, Brooks JT, MacNeil A, et al. Emergence of SARS-CoV-2 B.1.1.7 lineage - United States, December 29, 2020-January 12, 2021. MMWR Morbid Mortal Weekly Rep. (2021) 70:95–9. doi: 10.15585/mmwr.mm7003e2
45. Mahase E. Covid-19: Novavax vaccine efficacy is 86% against UK variant and 60% against South African variant. BMJ. (2021) 372:n296. doi: 10.1136/bmj.n296
46. Wu K, Werner AP, Moliva JI, Koch M, Choi A, Stewart-Jones GBE, et al. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. bioRxiv [Preprint]. (2021). doi: 10.1101/2021.01.25.427948
47. Starr TN, Greaney AJ. Prospective mapping of viral mutations that escape antibodies used to treat COVID-19. bioRxiv. (2021) 371:850–4. doi: 10.1126/science.abf9302
48. Huang Y, Yang C, Xu XF, Xu W, Liu SW. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta pharmacologica Sinica. (2020) 41:1141–9. doi: 10.1038/s41401-020-0485-4
49. Wang Q, Qiu Y, Li JY, Zhou ZJ, Liao CH, Ge XY. A unique protease cleavage site predicted in the spike protein of the novel pneumonia coronavirus (2019-nCoV). potentially related to viral transmissibility. Virologica Sinica. (2020) 35:337–9. doi: 10.1007/s12250-020-00212-7
50. Yarmarkovich M, Warrington JM, Farrel A, Maris JM. Identification of SARS-CoV-2 vaccine epitopes predicted to induce long-term population-scale immunity. Cell Rep Med. (2020) 1:100036. doi: 10.1016/j.xcrm.2020.100036
51. Tada T, Zhou H, Dcosta BM, Samanovic MI, Landau NR. The Spike Proteins of SARS-CoV-2 B.1.617 and B.1.618 variants identified in india provide partial resistance to vaccine-elicited and therapeutic monoclonal antibodies. bioRxiv [Preprint]. (2021). doi: 10.1101/2021.05.14.444076
52. Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, et al. Tracking changes in SARS-CoV-2 Spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. (2020) 182:812–27.e19. doi: 10.1016/j.cell.2020.06.043
53. McNamara RP, Caro-Vegas C, Landis JT, Moorad R, Pluta LJ, Eason AB, et al. High-density amplicon sequencing identifies community spread and ongoing evolution of SARS-CoV-2 in the Southern United States. Cell Rep. (2020) 33:108352. doi: 10.1016/j.celrep.2020.108352
54. Volz E, Hill V, McCrone JT, Price A, Jorgensen D, O'Toole Á, et al. Evaluating the effects of SARS-CoV-2 spike mutation D614G on transmissibility and pathogenicity. Cell. (2021) 184:64–75.e11. doi: 10.1016/j.cell.2020.11.020
55. Wise J. Covid-19: The E484K mutation and the risks it poses. BMJ. (2021) 372:n359. doi: 10.1136/bmj.n359
Keywords: SARS-CoV-2, pharyngeal swabs, whole genome sequencing, single nucleotide polymorphisms, spike glycoprotein, clinical characteristics, patients severity SARS-CoV-2, patients severity
Citation: Wang S, Zou X, Li Z, Fu J, Fan H, Yu H, Deng F, Huang H, Peng J, Zhao K, Cui L, Zhu L and Bao C (2021) Analysis of Clinical Characteristics and Virus Strains Variation of Patients Infected With SARS-CoV-2 in Jiangsu Province—A Retrospective Study. Front. Public Health 9:791600. doi: 10.3389/fpubh.2021.791600
Received: 08 October 2021; Accepted: 29 November 2021;
Published: 24 December 2021.
Edited by:
Jessica L. Jones, United States Food and Drug Administration, United StatesReviewed by:
Luigi Santacroce, University of Bari Aldo Moro, ItalyHamid Asadzadeh, Shahid Beheshti University of Medical Sciences, Iran
Copyright © 2021 Wang, Zou, Li, Fu, Fan, Yu, Deng, Huang, Peng, Zhao, Cui, Zhu and Bao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: LiGuo Zhu, emh1bGdAanNjZGMuY24=; Changjun Bao, YmFvMjAwMF9jbkAxNjMuY29t
†These authors have contributed equally to this work
 Shenjiao Wang1†
Shenjiao Wang1† Jianguang Fu
Jianguang Fu LiGuo Zhu
LiGuo Zhu Changjun Bao
Changjun Bao