- 1Department of Central Laboratory, Guangzhou Twelfth People's Hospital, Guangzhou, China
- 2Key Laboratory of Occupational Environment and Health, Guangzhou Twelfth People's Hospital, Guangzhou, China
- 3Guangzhou Occupational Disease Prevention and Treatment Hospital, Guangzhou, China
- 4Department of Medical Intensive Care Unit (MICU), Guangdong Women and Children Hospital, Guangzhou, China
Background: Parkinson's disease (PD) is an increasing challenge to public health. Tracking the temporal trends of PD burden would inform health strategies.
Methods: Data of PD burden was obtained from the Global Burden of Disease 2019. Trends in the incidence, prevalence, and years lived with disability (YLDs) of PD were estimated using the annual percentage change (EAPC) and age-standardized rate (ASR) from 1990 to 2019. The EAPCs were calculated with ASR through a linear regression model.
Results: The overall ASR of the incidence, prevalence, and YLDs of PD increased from 1990 to 2019, and their EAPCs were 0.61 (95% confidence interval [CI]: 0.58–0.65), 0.52 (95% CI: 0.43–0.61), and 0.53 (95% CI: 0.44–0.62). The largest number of PD patients was seen in the groups aged more than 65 years, and the percentage rapidly increased in the population aged more than 80 years. Upward trends in the ASR of PD were observed in most settings over the past 30 years. Incident trends of ASR increased pronouncedly in the United States of America and Norway, in which the respective EAPCs were 2.87 (95% CI: 2.35–3.38) and 2.14 (95% CI: 2.00–2.29). Additionally, the largest increasing trends for prevalence and YLDs were seen in Norway, with the respective EAPCs of 2.63 (95% CI: 2.43–2.83) and 2.61 (95% CI: 2.41–2.80). However, decreasing trends in PD appeared in about 30 countries, particularly Italy and the Republic of Moldova.
Conclusions: Increasing trends in the burden of PD were observed globally, and in most regions and countries from 1990 to 2019. Our findings suggested that the control and management of PD should be strengthened, especially when considering the aging tendency of the population.
Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disorder (observed only less than Alzheimer's disease), which is characterized by progressive motor symptoms over time (1). In recent years, PD has undergone the fastest growth in prevalence and disability among neurological disorders, and it has become one of the leading causes of disability worldwide (2).
The Global Burden of Disease (GBD) study reported that incident cases of PD were 1.02 million in 2017 (3). There were 6.1 million PD patients reported in 2016 globally, and the age-standardized rate (ASR) of prevalence increased by 21.7% from 1990 to 2016 (4). Years lived with disability (YLDs) is an index measuring the average lifespan of incident cases until rehabilitation or death, and the disability due to that status. YLDs is a widely used index evaluating the health loss caused by PD. Age-standardized rates of YLDs caused by PD increased pronouncedly at 8.9% from 1990 to 2007, and it increased 1.0% during 2007–2017 (3). Studies predicted that the burden of PD would grow substantially in future decades (5, 6). For example, it was estimated that there would be 4.94 million PD patients in China by 2030, which would account for half of the total PD patients worldwide (7). The rapid development of PD has placed a substantial burden on society, individuals, and health system (8–10). Especially, the disability caused by PD dramatically reduced individual health-related quality of life (HRQL), and placed substantial expenses to health system, including drug, rehabilitation, and caregiving (11, 12). However, welfare and health systems in many countries were not prepared for the problems brought by the population aging (13).
Rapid changes in the PD burden have emphasized the necessity for tracking the changing trends in a timely manner, which could inform the development of health strategies. The GBD studies had comprehensively assessed the burden of diseases, injuries, and risk factors over time, which has provided an opportunity to track the temporal trends of diseases. Therefore, this study aimed to estimate the trends of the PD burden at the national, regional, and global levels from 1990 to 2019 using the updated GBD data.
Materials and Methods
Data Source
The data on PD was analyzed using the Global Health Data Exchange (GHDx) query tool (http://ghdx.healthdata.org/gbd-results-tool). According to the GBD instructions, data on the PD burden, including the incidence, prevalence, and YLDs, were extracted for sexes, age groups, regions, and countries/territories from 1990 to 2019. A comprehensive overview of the PD burden was presented at the global, regional, and national levels, covering 21 geographic regions and 204 countries/territories. The sociodemographic index (SDI) is a compound index that indicates the association between social development and health outcomes. These regions and countries were classified into SDI quintiles, including low, low-middle, middle, high-middle, and high. Human Development Index (HDI) is a measurement system for evaluating the levels of social and economic development and individual human development. It was downloaded from the United Nations Development Program (http://hdr.undp.org/en/data).
Statistical Analysis
The estimated annual percentage change (EAPC) and ASR facilitated the horizontal comparison of the changing trends of disease burden among countries, and vertically compared over a long-term interval, which are commonly used indexes in public health research (14, 15). Age standardization is necessary when the data exist for different age structures in multiple populations over time. The ASR (per 100,000 population) was calculated as follows:
In the formula, ai corresponds to the age-specific rate in the ith age group; w corresponds to the number of people in the corresponding ith age group among the standard population; A corresponds to the number of age groups.
EAPC is a widely used index that describes the trend of ASR, and it was calculated as following. First, the natural logarithm of the ASR was estimated to be linearly regressed with time, and the formula was as follows:
In where y was equal to the natural logarithm of the ASR, and x corresponded to the calendar year. The EAPC and its 95% confidence interval (CI) were estimated using a linear regression model. The judgments of trends were the follows: (1) an increasing trend of ASR was found when both the EAPC value and its 95% CI > 0; (2) a decreasing trend of ASR was found when both the EAPC value and 95% CI <0; and (3) any other trends meant the ASR was stable over time. The associations were evaluated between SDI and ASRs in 2019 among regions to find the potential influential factors of ASR. Furthermore, the ASR in 1990 represented the baseline level of disease burden, and the HDI in 2019 could reflect the level and accessibility of health sources in various countries. Therefore, aim to explore the influential factors of EAPC, a Pearson correlation analysis was used to estimate the associations between EAPC and ASR in 1990, and HDI in 2019, respectively. Collation and analysis of data were conducted using R version 3.6.2 (Institute for Statistical Computing, Vienna, Austria). A p < 0.05 was considered to be statistically significant.
Results
Trends in the PD Incidence
Globally, the incident number of PD was 1,081.72 × 103 (95% uncertainty interval [UI]: (953.26 × 103-1,211.20 × 103) in 2019, which increased 159.73% since 1990. The overall age-standardized incidence rate (ASIR) was 13.43/100,000 in 2019, and it increased with an annual average of 0.61% from 1990 to 2019 (EAPC = 0.61, 95% CI: 0.58–0.65) (Table 1 and Figure 1). Compared to female patients, male patients had a larger incident number, and a higher increasing trend in ASIR (EAPC = 0.80, 95% CI: 0.75–0.85). Among the age groups, the high incident numbers of PD were observed in the patients aged over 65 years, and the largest increasing percentage occurred in the age group of over 80 years (221.67%) (Supplementary Table 1 and Figure 2A).
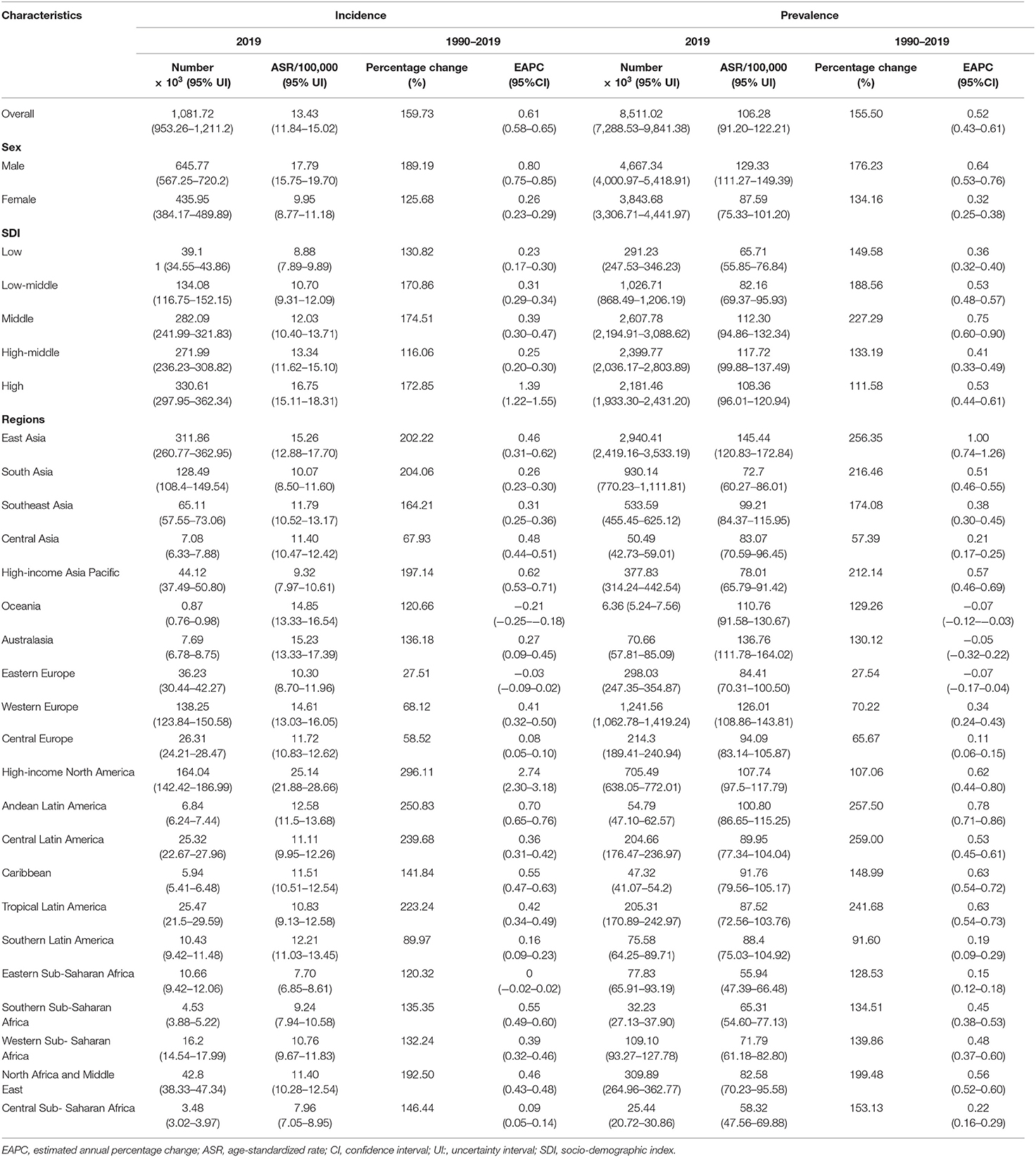
Table 1. The changes in incidence and prevalence of Parkinson's disease worldwide, and in sexes, SDI areas, and regions, 1990–2019.
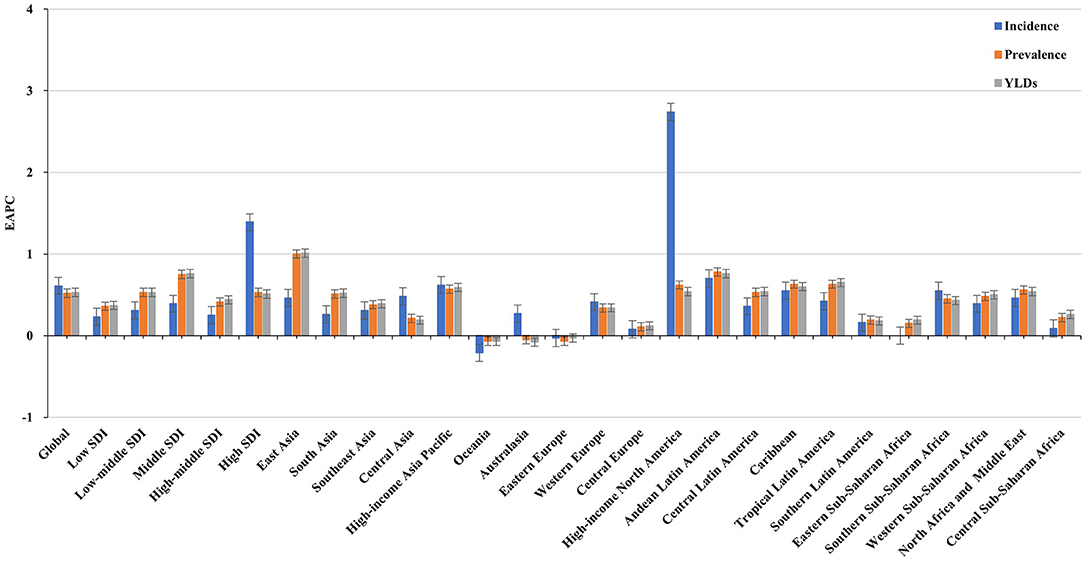
Figure 1. Trends in the ASR of incidence, prevalence, and YLDs of Parkinson's disease in global, SDI areas and geographic regions from 1990 to 2019. ASR, age-standardized rate; SDI, sociodemographic index; YLDs, years lived with disability.
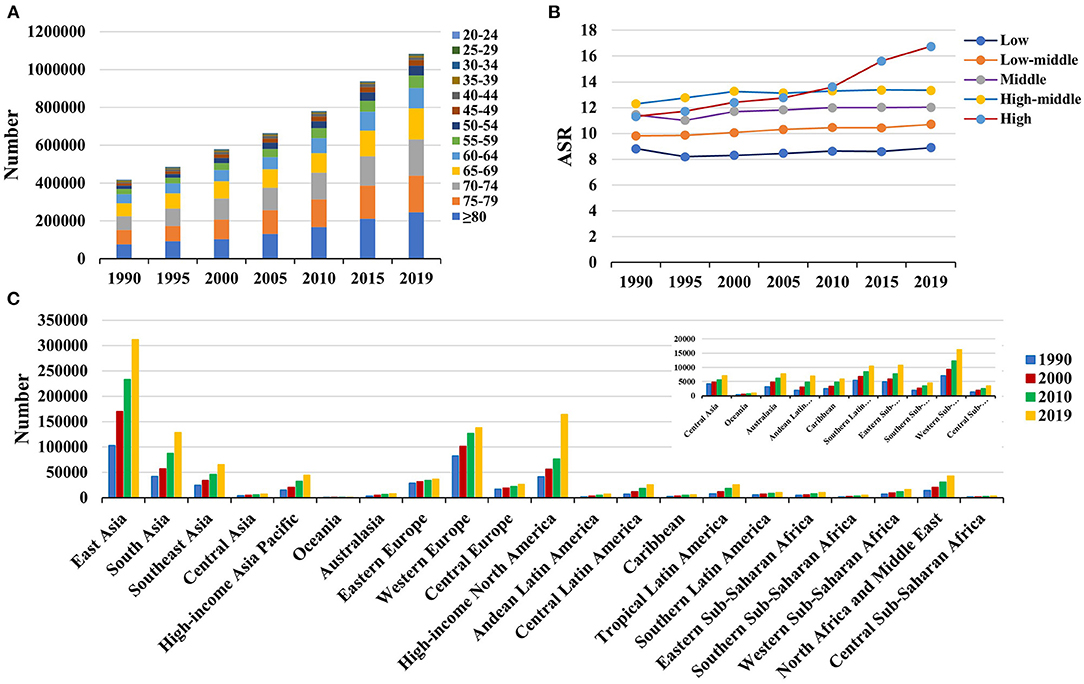
Figure 2. The distribution of Parkinson's disease incidence in age groups, SDI areas and geographic regions from 1990 to 2019. (A) was the incident number in age groups; (B) was the ASIR in SDI areas; (C) was the incident number in geographical regions. ASIR, age-standardized incidence rate; SDI, sociodemographic index.
Increasing trends in the ASIR of PD occurred in all SDI areas, particularly the high SDI area (EAPC = 1.39, 95% CI: 1.22–1.55). Among 21 geographic regions, East Asia had the largest incident number in 2019 (311.86 × 103), but Oceania had the lowest one (0.87 × 103). The increasing percentage of incidence rose from 27.51% in Eastern Europe to 296.11% in high-income North America. The ASIR varied from Eastern Sub-Saharan Africa (7.70/100,000) to high-income North America (25.14/100,000) in 2019. Eighteen regions presented upward trends in the ASIR, and the largest one occurred in high-income North America (EAPC = 2.74, 95% CI: 2.30–3.18), while downward trend only appeared in Oceania (EAPC = −0.21, 95%CI: −0.25 to −0.18) (Table 1 and Figures 1, 2B,C). A positive association was demonstrated between ASIRs and SDI in 2019 among regions (ρ = 0.43, p < 0.001; Figure 3A).
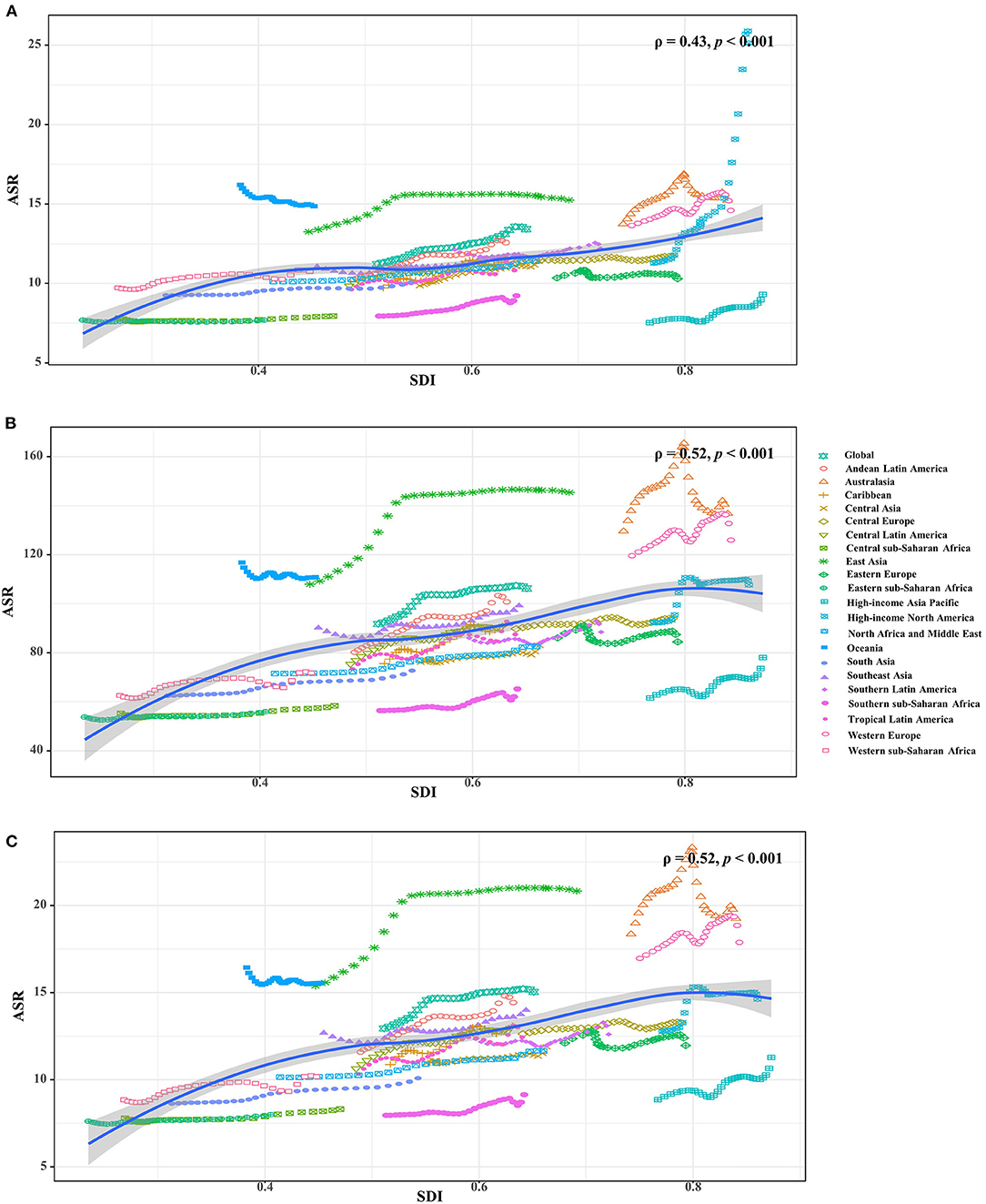
Figure 3. The associations between ASRs of Parkinson's disease and SDI in 2019 among regions. (A–C) were that of incidence, prevalence and YLDs, respectively. The points correspond the ASRs of the locations in the years from 1990 to 2019. The association was calculated with Pearson correlation analysis. ASR, age-standardized rate; SDI, socio-demographic index; YLDs, years lived with disability.
The ASIR of PD was heterogeneous among 204 countries/territories, ranging from 7.04/100,000 in Madagascar to 26.44/100,000 in the United States of America in 2019. The largest increasing percentages in incident numbers were seen in Qatar (796.51%) and the United Arab Emirates (854.71%) in 1990–2019, but the decreasing percentages occurred in Tokelau (−8.34%) and Niue (−6.81%). Increasing trends in the ASIR were seen in 150 countries/territories, and the largest ones occurred in the United States of America and Norway, with the respective EAPCs of 2.87 (95% CI: 2.35–3.38) and 2.14 (95% CI: 2.00–2.29). However, decreasing trends were seen in 35 countries, particularly the Republic of Moldova (EAPC = −1.26, 95% CI: −1.33 to −1.19) (Supplementary Table 2 and Figures 4A–C).
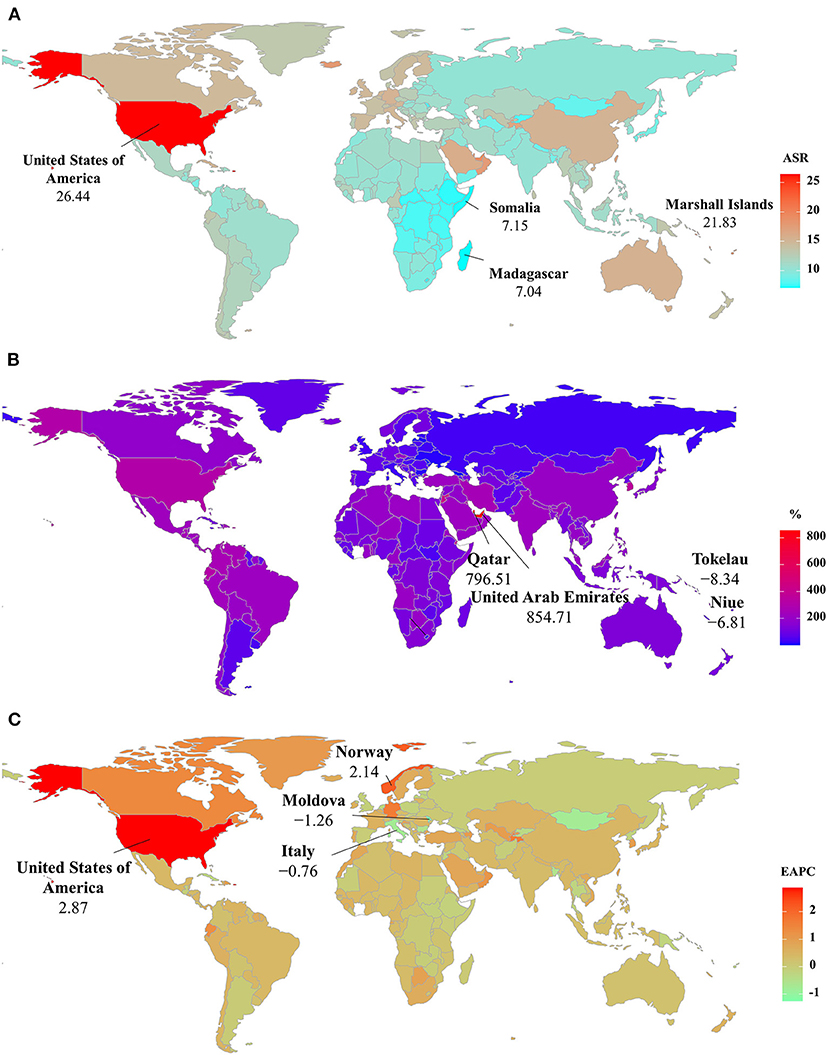
Figure 4. The distribution of ASRs, percentage changes, and EAPCs of Parkinson's disease incidence at the national level. (A) was the ASR in 2019; (B) was the percentage changes in number between 2000 and 2019; (C) was the EAPCs in countries/territories, respectively. Countries/territories with an extreme value were annotated. ASR, age-standardized rate; EAPC, estimated annual percentage change.
Trends in the PD Prevalence
The global prevalent number of PD increased 155.50% from 1990, and reached 8,511.02 × 103 (95% UI: 7,288.53 × 103-9,841.38 × 103) in 2019. The overall ASR of prevalence was 106.28/100,000 in 2019, and showed an upward trend from 1990 to 2019 (EAPC = 0.52, 95%CI: 0.43–0.61) (Table 1 and Figure 1). Compared with female patients, male patients had a higher prevalence, and showed a larger increasing trend (EAPC = 0.64, 95% CI: 0.53–0.76). Among the age groups, the pronounced high prevalent numbers occurred in patients aged over 65 years (more than 1,000 × 103), and the largest increasing percentage occurred in the age group of over 80 years (203.85%) (Supplementary Table 1 and Supplementary Figure 1A).
All SDI areas presented increasing trends in the ASR of PD prevalence, and the most pronounced one was seen in the middle SDI area (EAPC = 0.75, 95% CI: 0.60–0.90). At the regional level, the largest prevalent number in 2019 appeared in East Asia (2940.41 × 103), while the lowest one occurred in Oceania (6.36 × 103). The increasing percentage of prevalence varied from 27.54% in Eastern Europe to 256.90% in Central Latin America. In 2019, the ASR of prevalence ranged from 55.94/100,000 in Eastern Sub-Saharan Africa to 145.44/100,000 in East Asia. Trends in the ASR of prevalence increased in most regions, particularly East Asia (EAPC = 1.00, 95% CI: 0.74–1.26). Whereas, only Oceania had a minor downward trend (Table 1, Figure 1, and Supplementary Figures 1B,C). A positive association was observed between the ASRs and SDI in 2019 among regions (ρ = 0.52, p < 0.001; Figure 3B).
Among 204 countries/territories, the ASR of PD prevalence in 2019 varied from 49.51/100,000 in Somalia to 158.20/100,000 in the Northern Mariana Islands. During 1990–2019, the percentages of the prevalent numbers increased pronouncedly in Qatar and the United Arab Emirates (811.57 and 901.04 9%, respectively), but declined in only four countries, particularly Niue (−4.12%) and Tokelau (−3.32%). Increasing trends in the ASR of prevalence occurred in 164 countries/territories, and the largest one was observed in Norway (EAPC = 2.63, 95% CI: 2.43–2.83), followed by Canada and Germany. However, decreasing trends were observed in 21 countries, particularly Italy and the Republic of Moldova, in which the respective EAPCs were −1.06 (95% CI: −1.35 to −0.77) and −0.99 (95%CI: −1.07 to −0.92) (Supplementary Table 3 and Figures 5A–C).
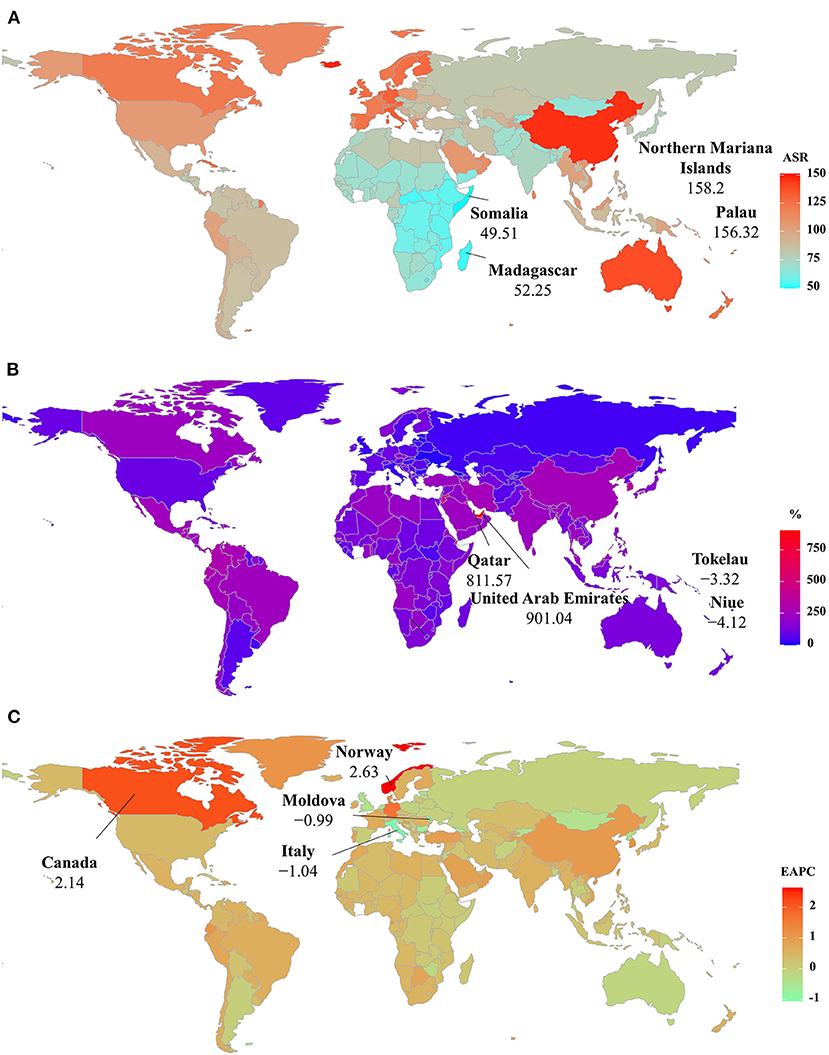
Figure 5. The distribution of ASRs, percentage changes, and EAPCs of Parkinson's disease prevalence at the national level. (A) was the ASR in 2019; (B) was the percentage changes in number between 2000 and 2019; (C) was the EAPCs in countries/territories, respectively. Countries/territories with an extreme value were annotated. ASR, age-standardized rate; EAPC, estimated annual percentage change.
Trends in YLDs Caused by PD
In 2019, the number of YLDs caused by PD was 1210.09 × 103 (95% UI: 841.17 × 103-1640.68 × 103) globally, with an increase of 154.73% since 1990. The overall ASR of YLDs reported an increasing trend from 1990 to 2019, with the EAPC of 0.53 (95% CI: 0.44–0.62) (Table 2 and Figure 1). Male patients had a higher burden, and undertook a larger increasing trend than female patients (EAPC = 0.65, 95% CI: 0.54–0.77). Among the age groups, the highest YLD number was seen in the age group of over 80 years (303.81 × 103), and the percentage increased pronouncedly among the patients aged 45 years old (Supplementary Table 1 and Supplementary Figure 2A).
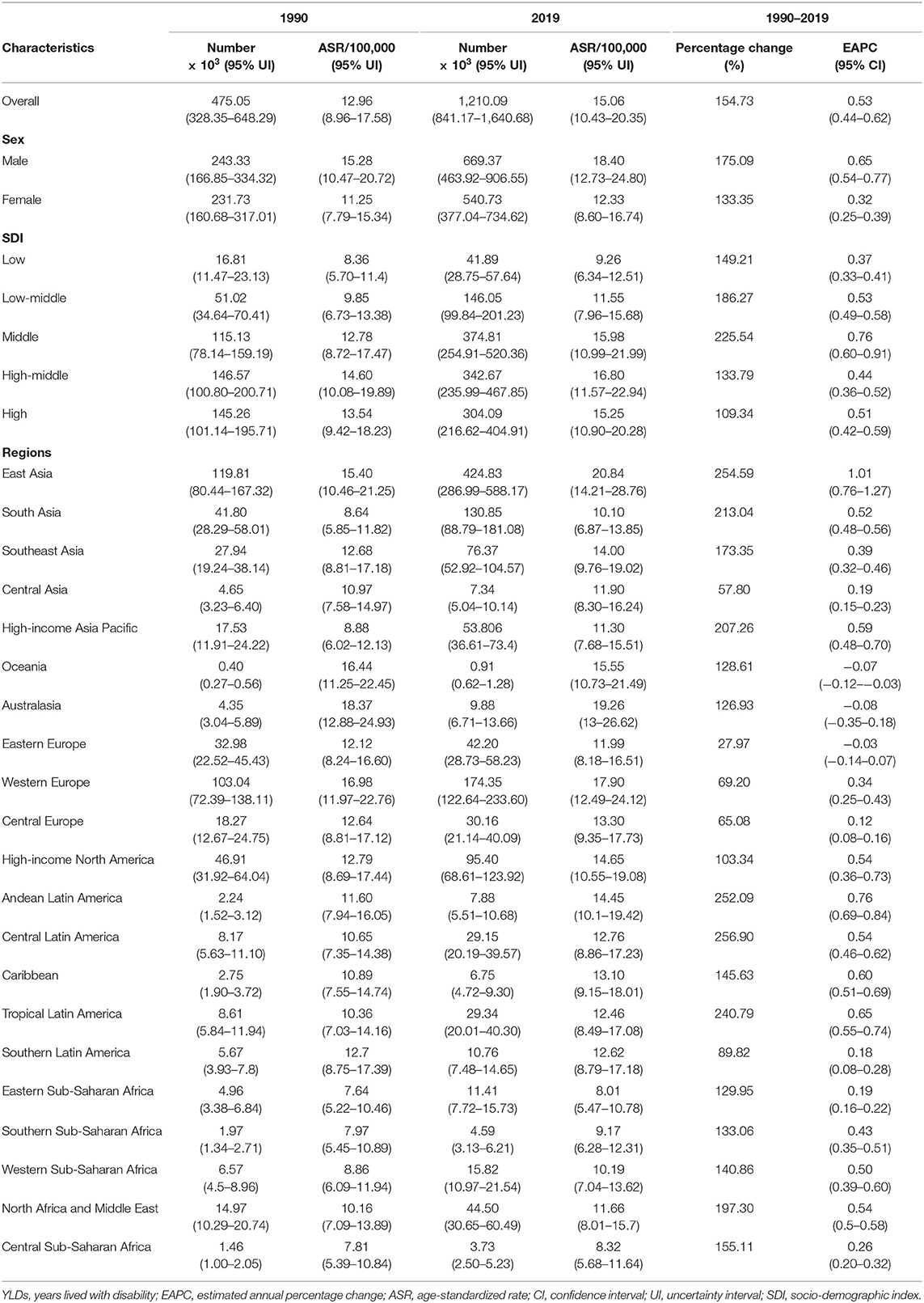
Table 2. The changes in YLDs of Parkinson's disease worldwide, and in sexes, SDI areas, and regions, 1990–2019.
The upward trend in the ASR of YLDs occurred in all SDI areas from 1990 to 2019, particularly the middle one (EAPC = 0.76, 95% CI: 0.60–0.91). In terms of geographic regions, the largest number of YLDs was seen in East Asia (424.83 × 103), while the lowest one was in Oceania (0.91 × 103). The percentage of YLDs number increased from 27.97% in Eastern Europe to 256.90% in Central Latin America during 1990–2019. The ASR of YLDs ranged from 8.01/100,000 in Eastern Sub–Saharan Africa to 20.84/100,000 in East Asia. Increasing trends in the ASR of YLDs occurred in most regions, particularly East Asia (EAPC = 1.01, 95% CI: 0.76–1.27). However, a minor decreasing trend was found in Oceania (Table 2, Figure 1, and Supplementary Figures 2B,C). The ASRs of YLDs were positively associated with SDI among the regions in 2019 (ρ = 0.52, p < 0.001; Figure 3C).
At the national levels, the ASR of YLDs varied across 204 countries/territories, ranging from 7.10/100,000 in Somalia to 22.20/100,000 in the Northern Mariana Islands in 2019. The number of YLDs increased significantly in the United Arab Emirates and Qatar from 1990 to 2019, with the respective percentages of 907.82 and 807.27%. In contrast, decreasing trends occurred in four countries, particularly Niue (−4.83%) and Tokelau (−3.61%). Trends in the ASR of YLDs increased in 123 countries/territories, particularly Norway and Canada, in which the respective EAPCs were 2.61 (95% CI: 2.41–2.80) and 2.13 (95% CI: 1.47–2.79). On the other hand, trends declined in 22 countries/territories, and the most pronounced one occurred in Italy (EAPC = −1.01, 95%CI: −1.30 to −0.72), followed by the Republic of Moldova and Bulgaria (Supplementary Table 4 and Figures 6A–C).
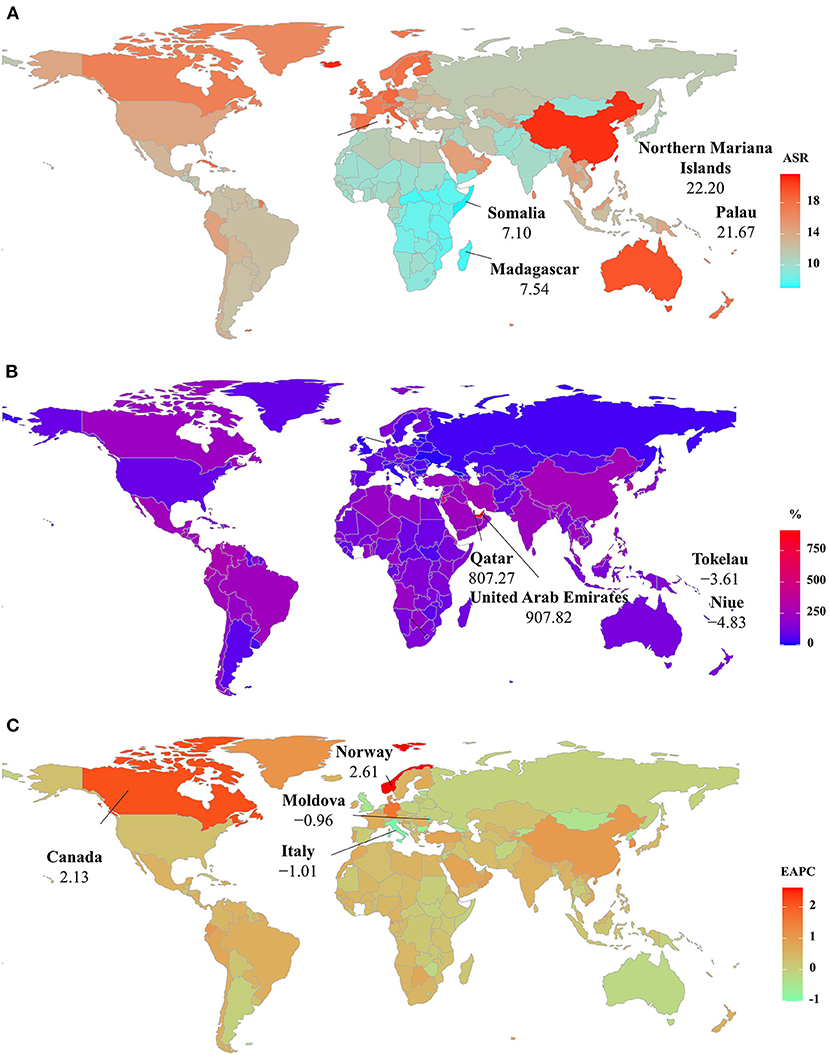
Figure 6. The distribution of ASRs, percentage changes, and EAPCs of YLDs caused by Parkinson's disease at the national level. (A) was the ASR in 2019; (B) was the percentage changes in number between 2000 and 2019; (C) was the EAPCs in countries/territories, respectively. Countries/territories with an extreme value were annotated. YLDs, years lived with disability, ASR, age-standardized rate; EAPC, estimated annual percentage change.
Analysis of the Influential Factors of EAPC
The ASR in 1990 served as the disease reservoir at baseline, and the HDI reflected the level of human development, and the availability of health resources in settings, including regions and countries. The EAPCs of the incidence, prevalence, and YLDs had a negative association with the corresponding ASRs in 1990 (ρ = −0.25, p < 0.001; ρ = −0.29, p < 0.001; ρ = −0.31, p < 0.001, respectively; Figures 7A–C). Meanwhile, only the EAPCs of incidence had a positive association with HDI in 2019 (ρ = 0.20, p = 0.01; Supplementary Figure 3), which further explained that the trends of the ASIR increased pronouncedly in the high SDI regions and countries from 1990 to 2019.
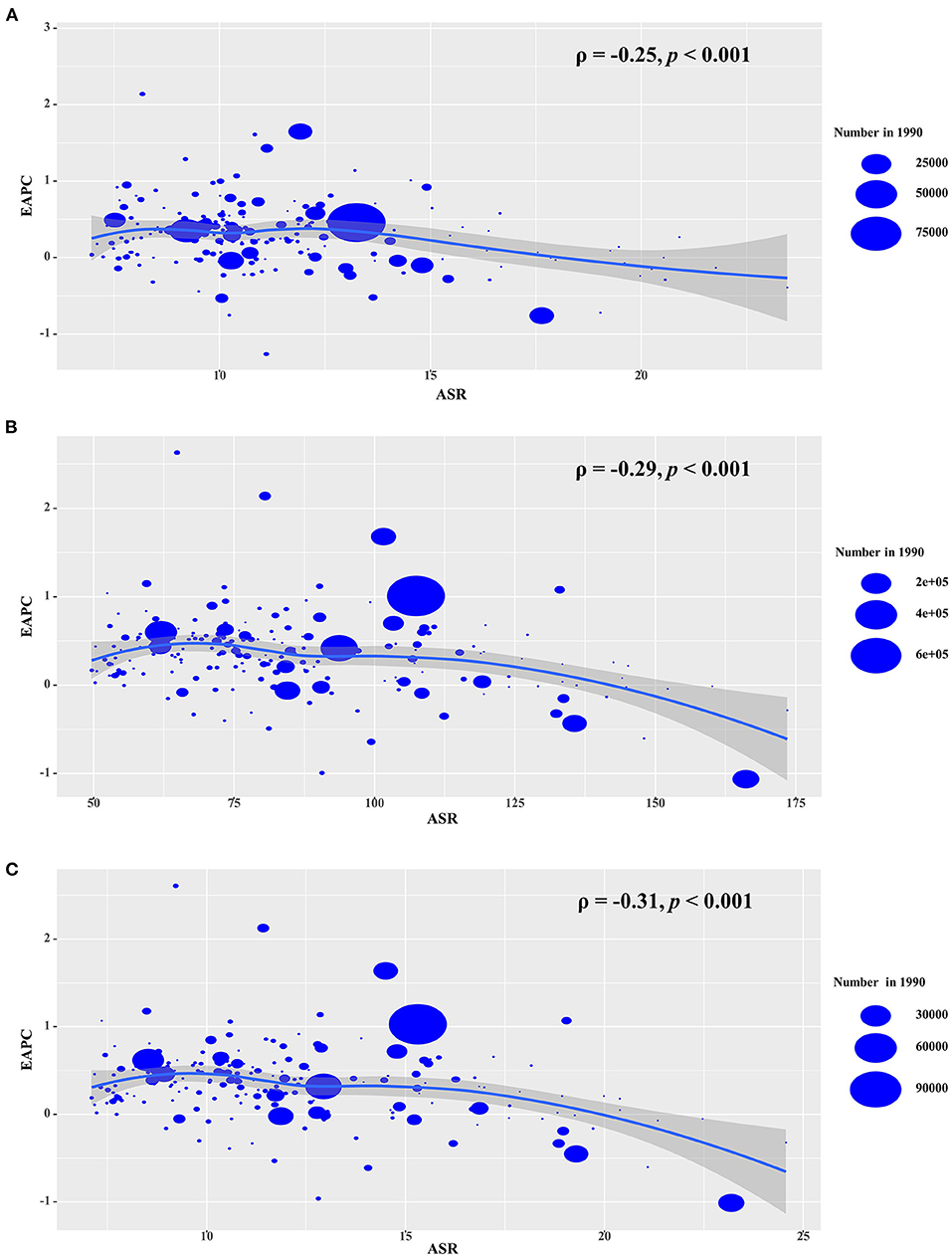
Figure 7. The association s between EAPCs and ASRs in 1990 at the national level. EAPCs of incidence (A), prevalence (B), YLDs (C) had negative associations with ASR in 1990, respectively. The association was calculated with Pearson correlation analysis. The size of circle is increased with the numbers in 1990. EAPC, estimated annual percentage change; ASR, age-standardized rate; YLDs, years lived with disability.
Discussion
Different from previous GBD studies, the present study adopted EAPC and ASR to evaluate the changing trends of PD burden over the past 30 years. The results showed that EAPCs and ASRs of the PD burden varied among countries, and most of them showed upward trends from 1990 to 2019.
The rising incident trends of PD were similar to results from previous studies (2, 4, 16), which might be related to population growth and aging (16, 17), genetic predisposition (18), lifestyle changes, and environmental pollution (19–21). Meanwhile, the improved diagnostic methods also led to better identifications of incidence (22, 23). A larger burden and the upward trends of ASIR in males was possibly due to higher occupational factors, unhealthy lifestyles, and so on (24–26). Furthermore, estrogen had potential neuroprotective effects for females (27). The PD incidence was closely related to age, and the pronounced increasing proportions were seen in the groups over 65 years old, supporting the idea that this disease mainly depends on aging trends of ASIR increased pronouncedly in high SDI areas, in where there had a larger aging population (28), decreased total fertility rates (29), and sound health systems (30, 31). The above would also explain the most pronounced upward trends in high SDI countries, including the United States of America, Norway, and Germany. Furthermore, low fertility rates were also a potential reason for the upward trends in these European countries (32). Except for the sheer size of the population, occupational exposure and industrial pollution expedited the increasing trends in low and middle SDI areas (33–36). On the other hand, the decreasing trends of ASIR in Italy might be due to the Mediterranean diet, which has been demonstrated to be related to reduced risk for PD (37–39).
PD is characterized as chronic and non-fatal, and its rising prevalence trend is highly associated with the aging population, which contributed to an increase of about 22% in the ASR of PD prevalence from 1990 to 2016 (16). Because of the advances in treatment and management (40, 41), it was predicted that the PD patients' life would be prolonged by about 3 years, and the ASR of prevalence would increase by 12% over 20 years. Additionally, better estimation methods and studies also potentially promoted the changes in prevalence (42–44), e.g., door-to-door surveys. Among SDI quintiles, middle SDI area had the largest increasing trend in ASR of prevalence, in where there had the largest increase in the number of PD patients (16). Compared to other regions, East Asia presented the largest increasing trends in ASR for prevalence, which was probably driven by population growth and aging (45). Additionally, substantial progress in the health system and social insurance has been achieved (46). The prevalence trends were highly in line with the morbidity of PD, which explained the changing trends among countries. The reason for the decreasing trends of incidence and prevalence in Moldova was due to the rebuilding health and welfare systems (47). Old Moldovans suffered from poverty and lack of health resources, and their low life expectancy was the equivalent of that in the European Union in the 1990's (48).
YLDs reflects the sum of years that an individual lived with disability caused by the non-fatal disease or injury. PD itself is not fatal, and its symptoms generally develop slowly over years. Disability is a critical characteristic of PD, especially in older patients, emphasizing the advantage and necessity of YLDs for the PD study. The rising trend of YLDs was based on the increasing prevalence and prolonged life expectancy of PD patients. Meanwhile, early onset PD with high genetic susceptibility (49, 50), longer disease duration (51), and low adherence to treatment (52, 53), could prolong the course of disability. Some PD is accompanied by stroke (54), dementia (55), and dysphagia (56), which also contributes to the increase in disability. The high prevalence of disability caused by PD was found in low- and middle-income countries, but increasing recognition should strengthen management and access to resources (57). For example, Zhao suggested that it was necessary to carry out genetic testing in early-onset PD patients, particularly those with a family history (58). On the other hand, the minor decreasing trends of YLDs in Oceania were probably explained by a diagnosis of PD at older ages (peaked at 85 years) (59). The pronounced rising trends of YLDs in many high-income countries, including Norway, Canada, and Germany, probably relate to the remarkable increase in disability in later life (60), and complications such as depression, anxiety, and stroke (61, 62). Lower burden of PD was observed in the Mediterranean countries, and decreasing trends in the ASR occurred in Italy and Spain. The decreasing trend of YLDs in Italy benefited from the Mediterranean diet with protective effects on longevity and age-related diseases (63–65). The increasing trends indicated that current strategies were still ill-prepared for the population aging. The findings indicated that active strategies should be adopt to cope with the problems caused by PD, including healthcare and security systems, civil health promotion plan, and healthy lifestyle (66–68).
There were several limitations in this study. First, the quality and quantity of data determined the accuracy and robustness of GBD estimates, which was probably impacted by the potential bias derived from the miscoding and misclassification of disease. Parkinson's disease is accompanied by clinical challenges, including difficulties in diagnosis, misdiagnosis, interference, and so on. Second, the diagnosis and definition of PD had been refined in countries over time, which was the main source of potential bias. Third, the lack of relevant research or data meant we could not fully explain the reason for changing trends in some countries.
In conclusion, the present study provided a comprehensive overview of PD burden and its trends in the incidence, prevalence, and YLDs at the global, regional, and national levels during 1990–2019. Pronounced increasing trends of PD burden ware observed worldwide, and in most regions and countries, indicating that PD is an increasing challenge to global health. The findings highlighted that more effective strategies are needed to decrease the burden of PD, particularly YLDs, when facing with the rapidly growth of aging populations.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
ZO: project administration and drafting. JP: data analysis and validation. DD and DY: data analysis and visualization. ST and HN: data collection and collation. ZW: supervision and drafting and editing. All authors contributed to the article and approved the submitted version.
Funding
Guangzhou Health Science and Technology Major Project (Grant No.: 2021A031003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thanks to the Institute for Health Metrics and Evaluation (IHME), Washington University, and the Global Burden of Disease study collaborations.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2021.776847/full#supplementary-material
Abbreviations
PD, Parkinson's disease; YLDs, years lived with disability; GBD, Global Burden of Disease; ASR, Age-standardized rate; EAPC, Estimated annual percentage change; UI, Uncertainty interval; CI, Confidence interval; GHDx, Global Health Data Exchange; SDI, Socio-demographic index; HDI, Human Development Index.
References
1. Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nat Rev Dis Primers. (2017) 3:17013. doi: 10.1038/nrdp.2017.13
2. GBD Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. (2017) 16:877–97. doi: 10.1016/S1474-4422(17)30299-5
3. GBD Disease Incidence, Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1789–858. doi: 10.1016/S0140-6736(18)32279-7
4. GBD Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2019) 18:459–80. doi: 10.1016/S1474-4422(18)30499-X
5. Wanneveich M, Moisan F, Jacqmin-Gadda H, Elbaz AJP. Projections of prevalence, lifetime risk, and life expectancy of Parkinson's disease (2010-2030) in France. Mov Disord. (2018) 33:1449–55. doi: 10.1002/mds.27447
6. Kowal SL, Dall TM, Chakrabarti R, Storm MV. The current and projected economic burden of Parkinson's disease in the United States. Mov Disord. (2013) 28:311–8. doi: 10.1002/mds.25292
7. Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. (2007) 68:384–6. doi: 10.1212/01.wnl.0000247740.47667.03
8. Huse DM, Schulman K, Orsini L, Castelli-Haley J, Kennedy S. Burden of illness in Parkinson's disease. Mov Disord. (2005) 20:1449–54. doi: 10.1002/mds.20609
9. Vossius C, Larsen JP, Janvin C. The economic impact of cognitive impairment in Parkinson's disease. Mov Disord. (2011) 26:1541–4. doi: 10.1002/mds.23661
10. Weintraub D, Comella CL. Parkinson's disease–Part 1: Pathophysiology, symptoms, burden, diagnosis, and assessment. Am J Manag Care. (2008) 14:S40–8. doi: 10.1097/s10198-019-01100
11. Scheife RT, Schumock GT, Burstein A, Gottwald MD, Luer MS. Impact of Parkinson's disease and its pharmacologic treatment on quality of life and economic outcomes. Am J Health Syst Pharm. (2000) 57:953–62. doi: 10.1093/ajhp/57.10.953
12. Dowding CH, Shenton CL, Salek SS. A review of the health-related quality of life and economic impact of Parkinson's disease. Drugs Aging. (2006) 23:693–721. doi: 10.2165/00002512-200623090-00001
13. Raggi A, Leonardi M. Burden and cost of neurological diseases: a European North-South comparison. Acta Neurol Scand. (2015) 132:16–22. doi: 10.1111/ane.12339
14. Ou ZJ, Yu DF, Liang YH, He WQ, Li YZ, Zhang MY, et al. Analysis of the Global Burden of Disease study highlights the trends in death and disability-adjusted life years of leukemia from 1990 to 2017. Cancer Commun. (2020) 40:598–610. doi: 10.1002/cac2.12094
15. Hankey BF, Ries LA, Kosary CL, Feuer EJ, Merrill RM, Clegg LX, et al. Partitioning linear trends in age-adjusted rates. Cancer Causes Control. (2000) 11:31–5. doi: 10.1023/a:1008953201688
16. GBD Parkinson's Disease Collaborators. Global, regional, and national burden of Parkinson's disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2018) 17:939–53. doi: 10.1016/S1474-4422(18)30295-3
17. Bach JP, Ziegler U, Deuschl G, Dodel R, Doblhammer-Reiter G. Projected numbers of people with movement disorders in the years 2030 and 2050. Mov Disord. (2011) 26:2286–90 doi: 10.1002/mds.23878
18. Wirdefeldt K, Adami HO, Cole P, Trichopoulos D. Epidemiology and etiology of Parkinson's disease: a review of the evidence. Eur J Epidemiol. (2011) 26(suppl. 1):S1–58. doi: 10.1007/s10654-011-9581-6
19. Doiron M, Dupre N, Langlois M, Provencher P. Smoking history is associated to cognitive impairment in Parkinson's disease. Aging Ment Health. (2017) 21:322–6. doi: 10.1080/13607863.2015.1090393
20. Kasdagli MI, Katsouyanni K, Dimakopoulou K. Air pollution and Parkinson's disease: A systematic review and meta-analysis up to 2018. Int J Hyg Environ Health. (2019) 222:402–9. doi: 10.1016/j.ijheh.2018.12.006
21. Zuber M. Parkinson's disease and environmental factors. Rev Epidemiol Sante Publique. (1991) 39:373–87.
22. Alcalay RN, Kehoe C, Shorr E, Battista R, Hall A, Simuni T, et al. Genetic testing for Parkinson disease: current practice, knowledge, and attitudes among US and Canadian movement disorders specialists. Genet Med. (2020) 22:574–80. doi: 10.1038/s41436-019-0684-x
23. Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A. Accuracy of clinical diagnosis of Parkinson disease: A systematic review and meta-analysis. Neurology. (2016) 86:566–76. doi: 10.1212/WNL.0000000000002350
24. Cui L, Hou NN, Wu HM, Zuo X, Lian YZ, Zhang CN, et al. Prevalence of Alzheimer's disease and Parkinson's disease in China: an updated systematical analysis. Front Aging Neurosci. (2020) 12:603854. doi: 10.3389/fnagi.2020.603854
25. Savica R, Grossardt BR, Bower JH, Ahlskog JE. Time trends in the incidence of Parkinson disease. JAMA Neurol. (2016) 73:981–9. doi: 10.1001/jamaneurol.2016.0947
26. Torti M, Fossati C, Casali M, De Pandis MF, Grassini P, Radicati FG, et al. Effect of family history, occupation and diet on the risk of Parkinson disease: A case-control study. PLoS ONE. (2020) 15:e0243612. doi: 10.1371/journal.pone.0243612
27. Wooten GF, Currie LJ, Bovbjerg VE, Lee JK. Are men at greater risk for Parkinson's disease than women? J Neurol Neurosurg Psychiatry. (2004) 75:637–9. doi: 10.1136/jnnp.2003.020982
28. Vlaar T, Kab S, Schwaab Y, Frery N, Elbaz A. Association of Parkinson's disease with industry sectors: a French nationwide incidence study. Eur J Epidemiol. (2018) 33:1101–11. doi: 10.1007/s10654-018-0399-3
29. GBD Population Fertility Collaborators. Population and fertility by age and sex for 195 countries and territories, 1950-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1995–2051. doi: 10.1016/S0140-6736(18)32278-5
30. Feigin VL, Vos T, Alahdab F, Amit AML, Barnighausen TW. Burden of neurological disorders across the US from 1990-2017: A global burden of disease study. JAMA Neurol. (2021) 78:165–76. doi: 10.1001/jamaneurol.2020.4152
31. Fukunaga H. Support of home care and rehabilitation. Nihon Rinsho. (2004) 62:1729–32. doi: 10.1016/nihonrinsho.2004.04.114
32. He M, Zhou W, Liu K, Wang X, Liu C, Shi F, et al. The prevalence of male rotating shift work correlates with reduced total fertility rate: an ecological study of 54,734 reproductive-aged males in 35 European countries between 2000 and 2015. Chronobiol Int. (2021) 38:1072–82. doi: 10.1080/07420528.2021.1907396
33. Muthane UB, Ragothaman M. Epidemiology of Parkinson's disease and movement disorders in India: problems and possibilities. J Assoc Physicians India. (2007) 55:719–24.
34. Yu Z, Wei F, Zhang X, Wu M, Lin H, Shui L, et al. Air pollution, surrounding green, road proximity and Parkinson's disease: A prospective cohort study. Environ Res. (2021) 197:111170. doi: 10.1016/j.envres.2021.111170
35. Weisskopf MG, Weuve J, Nie H, Saint-Hilaire MH, Sudarsky L, Simon DK, et al. Association of cumulative lead exposure with Parkinson's disease. Environ Health Perspect. (2010) 118:1609–13.doi: 10.1289/ehp.1002339
36. Azizova TV, Bannikova MV, Grigoryeva ES, Rybkina VL. Occupational exposure to chronic ionizing radiation increases risk of Parkinson's disease incidence in Russian Mayak workers. Int J Epidemiol. (2020) 49:435–47. doi: 10.1093/ije/dyz230
37. Yin W, Lof M, Pedersen NL, Sandin S. Mediterranean dietary pattern at middle age and risk of Parkinson's disease: a swedish cohort study. Mov Disord. (2021) 36:255–60. doi: 10.1002/mds.28314
38. Sofi F, Macchi C, Abbate R, Gensini GF. Mediterranean diet and health status: an updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. (2014) 17:2769–82. doi: 10.1017/S1368980013003169
39. Gao X, Chen H, Fung TT, Logroscino G, Schwarzschild MA, Hu FB, et al. Prospective study of dietary pattern and risk of Parkinson disease. Am J Clin Nutr. (2007) 86:1486–94. doi: 10.1093/ajcn/86.5.1486
40. Goetz CG, Poewe W, Rascol O. Evidence-based medical review update: pharmacological and surgical treatments of Parkinson's disease: 2001 to 2004. Mov Disord. (2005) 20:523–39. doi: 10.1002/mds.20464
41. Gottwald MD, Bainbridge JL, Dowling GA, Aminoff M. New pharmacotherapy for Parkinson's disease. Ann Pharmacother. (1997) 31:1205–17.
42. Anderson DW, Rocca WA, de Rijk MC, Grigoletto F, Melcon MO, Breteler MM, et al. Case ascertainment uncertainties in prevalence surveys of Parkinson's disease. Mov Disord. (1998) 13:626–32. doi: 10.1002/mds.870130403
43. von Campenhausen S, Bornschein B, Wick R, Botzel K, Sampaio C, Poewe W, et al. Prevalence and incidence of Parkinson's disease in Europe. Eur Neuropsychopharmacol. (2005) 15:473–90. doi: 10.1016/j.euroneuro.2005.04.007
44. Muangpaisan W, Hori H. Systematic review of the prevalence and incidence of Parkinson's disease in Asia. J Epidemiol. (2009) 19:281–93. doi: 10.2188/jea.je20081034
45. Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu S, et al. Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the Global burden of disease study 2013. Lancet. (2016) 387:251–72. doi: 10.1016/S0140-6736(15)00551-6
46. Zhang D, Shi L, Tian F. Care utilization with China's new rural cooperative medical scheme: updated evidence from the China health and retirement longitudinal study 2011-2012. Int J Behav Med. (2016) 23:655–63. doi: 10.1007/s12529-016-9560-0
47. Turcanu G, Domente S, Buga M. Republic of Moldova health system review. Health Syst Transit. (2012) 14:1–151. doi: 10.1002/hst.23211662
48. Cojocari T. Aging in moldova: a country with orphan older adults. Gerontologist. (2018) 58:797–804. doi: 10.1093/geront/gny055
49. Kaiyrzhanov R, Aitkulova A, Vandrovcova J, Murphy D, Zharkinbekova N, Shashkin C, et al. A glimpse of the genetics of young-onset Parkinson's disease in Central Asia. Mol Genet Genomic Med. (2021) 9:e1671. doi: 10.1002/mgg3.1671
50. Kumar S, Yadav N, Pandey S, Muthane UB, Govindappa ST, Abbas MM, et al. Novel and reported variants in Parkinson's disease genes confer high disease burden among Indians. Parkinsonism Relat Disord. (2020) 78:46–52. doi: 10.1016/j.parkreldis.2020.07.014
51. Louis ED, Marder K, Cote L, Tang M. Mortality from Parkinson disease. Arch Neurol. (1997) 54:260–4. doi: 10.1001/archneur.1997.00550150024011
52. Dodel R, Tinelli M, Deuschl G, Petersen G, Oertel W. The economic benefit of timely, adequate, and adherence to Parkinson's disease treatment: the Value of Treatment Project 2. Eur J Neurol. (2021) 28:707–16. doi: 10.1111/ene.14584
53. Schroder S, Kuessner D, Arnold G, Zollner Y, Jones E. Do neurologists in Germany adhere to the national Parkinson's disease guideline? Neuropsychiatr Dis Treat. (2011) 7:103–10. doi: 10.2147/NDT.S8895
54. Naess-Schmidt ET, Christiansen DH, Ostgard RD, Andersen NV, Nielsen JF. Incidence of free of charge physiotherapy in a danish national cohort of stroke, Parkinson's disease, multiple sclerosis and rheumatoid arthritis patients. Clin Epidemiol. (2020) 12:23–9. doi: 10.2147/CLEP.S223000
55. Buter TC, van den Hout A, Matthews FE, Larsen JP, Brayne C. Dementia and survival in Parkinson disease: a 12-year population study. Neurology. (2008) 70:1017–22. doi: 10.1212/01.wnl.0000306632.43729.24
56. Lopez-Liria R, Vega-Ramirez FA, Aguilar-Parra JM, Trigueros-Ramos R, Morales-Gazquez MJ. Treatment of dysphagia in Parkinson's disease: a systematic review. Int J Environ Res Public Health. (2020) 17:4104. doi: 10.3390/ijerph17114104
57. Thakur KT, Albanese E, Giannakopoulos P, Jette N, Linde M, Prince MJ, et al. Neurological disorders. In: Patel D, Chisholm T, editors. Mental, Neurological, and Substance Use Disorders: Disease Control Priorities, Third Edition (Volume 4). Washington, DC (2016).
58. Zhao Y, Qin L, Pan H, Liu Z, Jiang L, He Y, et al. The role of genetics in Parkinson's disease: a large cohort study in Chinese mainland population. Brain. (2020) 143:2220–34. doi: 10.1093/brain/awaa167
59. Myall DJ, Pitcher TL, Pearson JF, Dalrymple-Alford JC, Anderson TJ. Parkinson's in the oldest old: Impact on estimates of future disease burden. Parkinsonism Relat Disord. (2017) 42:78–84. doi: 10.1016/j.parkreldis.2017.06.018
60. Spiers GF, Kunonga TP, Beyer F, Craig D, Hanratty B. Trends in health expectancies: a systematic review of international evidence. BMJ Open. (2021) 11:e045567. doi: 10.1136/bmjopen-2020-045567
61. Skeie GO, Muller B, Haugarvoll K, Larsen JP. Parkinson disease: associated disorders in the Norwegian population based incident ParkWest study. Parkinsonism Relat Disord. (2013) 19:53–5. doi: 10.1016/j.parkreldis.2012.07.003
62. Beyer MK, Larsen JP. Gray matter atrophy in Parkinson disease with dementia and dementia with Lewy bodies. Neurology. (2007) 69:747–54. doi: 10.1212/01.wnl.0000269666.62598.1c
63. Daniels LB, Antonini P, Marino R, Rizzo M, Navarin S, Lucibello SG, et al. Cardiovascular health of nonagenarians in southern Italy: a cross-sectional, home-based pilot study of longevity. J Cardiovasc Med. (2020) 21:89–98. doi: 10.2459/JCM.0000000000000910
64. Perez-Lopez FR, Chedraui P, Haya J. Effects of the Mediterranean diet on longevity and age-related morbid conditions. Maturitas. (2009) 64:67–79. doi: 10.1016/j.maturitas.2009.07.013
65. Angeloni C, Malaguti M, Barbalace MC. Bioactivity of olive oil phenols in neuroprotection. Int J Mol Sci. (2017) 18:2230. doi: 10.3390/ijms18112230
66. Barbabella F, Cela E, Di Matteo C, Socci M, Lamura G, Checcucci P, et al. New multilevel partnerships and policy perspectives on active ageing in italy: a national plan of action. Int J Environ Res Public Health. (2020) 17:24. doi: 10.3390/ijerph17249585
67. Fang EF, Xie C, Schenkel JA, Wu C, Long Q, Cui H, et al. A research agenda for ageing in China in the 21st century (2nd edition): Focusing on basic and translational research, long-term care, policy and social networks. Ageing Res Rev. (2020) 64:101174. doi: 10.1016/j.arr.2020.101174
Keywords: Parkinson's disease, global burden of disease, estimated annual percentage change, age-standardized rate, years lived with disability
Citation: Ou Z, Pan J, Tang S, Duan D, Yu D, Nong H and Wang Z (2021) Global Trends in the Incidence, Prevalence, and Years Lived With Disability of Parkinson's Disease in 204 Countries/Territories From 1990 to 2019. Front. Public Health 9:776847. doi: 10.3389/fpubh.2021.776847
Received: 16 September 2021; Accepted: 15 November 2021;
Published: 07 December 2021.
Edited by:
Tissa Wijeratne, The University of Melbourne, AustraliaReviewed by:
Narimasa Kumagai, Seinan Gakuin University, JapanMijanur Rahman, University of Wollongong, Australia
Copyright © 2021 Ou, Pan, Tang, Duan, Yu, Nong and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi Wang, emhpX3dhbmdAb3V0bG9vay5jb20=
†These authors have contributed equally to this work
 Zejin Ou
Zejin Ou Jing Pan2,3†
Jing Pan2,3† Zhi Wang
Zhi Wang