
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 26 November 2021
Sec. Aging and Public Health
Volume 9 - 2021 | https://doi.org/10.3389/fpubh.2021.771584
This article is part of the Research Topic Aging and Health in China View all 21 articles
Background: Literature shows that olfactory impairment (OI) is associated not only with neurodegenerative diseases (NDDs), but also with increased mortality. In this study, we analyzed data collected from the prospective phase of the 10-year follow-up of the Shanghai Aging Study (SAS) to explore the mediation effect of NDDs on the OI-mortality relationship.
Methods: We analyzed data collected from the prospective phase of the 10-year follow-up of the SAS. We included 1,811 participants aged 60 years or older who completed both an olfactory identification test and a cognitive assessment at baseline (2010–2011). Survival status of the participants from baseline to December 31, 2019 was obtained from the local mortality surveillance system. We used the four-way decomposition method to attribute effects to interaction and mediation and to explore the mediation effect of NDDs on the OI-mortality relationship.
Results: The four-way decomposition method revealed a statistically significant association of OI with death. Overall, 43% higher risk for death was associated with OI [excess relative risk (ERR) = 0.43, 95% CI: 0.06–0.80, p = 0.023]. Excluding the mediation from NDDs and interaction between OI and NDDs, the controlled direct effect of OI on death was even higher in NDDs participants, with an ERR of 77% (95% CI: 0.00–1.55, p = 0.050). Statistically significant association was found for failure to identify coffee (ERR = 0.77, 95% CI: 0.18–1.36, p = 0.010) and marginally significant associations were found for failure to identify cinnamon (ERR = 0.33, 95% CI: −0.02–0.68, p = 0.068) and rose (ERR = 0.33, 95% CI: −0.01–0.67, p = 0.054) with death.
Conclusion: OI was associated with the long-term mortality in older adults and the association was even stronger in those with NDDs. Failure to identify coffee or rose was associated with a higher mortality risk, and the association was mediated by NDDs.
About 50–70% of people aged 65 years or older exhibit olfactory impairment (OI) (1). OI has been observed in patients with neurodegenerative diseases (NDDs) such as Alzheimer's disease (2), Parkinson's disease (PD) (3), or progressive supranuclear palsy (4); therefore, OI might be an early biomarker for a broad spectrum of NDDs. For example, in the neuropathological progression of PD, the olfactory bulb and the anterior olfactory nucleus are one of the first lesions within the central nervous system during the pathogenesis of PD. The lesions would continue to extend into more remote olfactory sites during the three to four pathological stages onward (5). Thus, olfactory impairment might be a manifestation and a potential diagnostic marker for early PD (6). Published literature indicates that OI is associated not only with NDDs, but also with increased mortality. A few longitudinal studies have shown an excessive relative risk for mortality as high as 20–112% in the elderly with OI, adjusted for age, sex, and other covariates (7, 8). Some studies have demonstrated the OI-mortality relationship in the middle-aged group (9, 10). In addition, some evidence indicates that the NDD is one of the leading causes of death and probably would overtake cancer by 2040 as the top one killer (11). Therefore, the NDD was considered as a possible mediator in the relationship between OI and mortality and explains the relationship partly (8, 12, 13).
However, the question arises as whether OI could proceed with the onset of neuropathological lesions or simply cause or potentiate by the neuropathologies (14)? Furthermore, cognitive performance at baseline may also tangle with the relationship (15), which makes the question even more complicated. So far, few studies have addressed the question and the findings remain controversial because of the diverse study design and data analysis method. In this study, we analyzed data collected from the prospective phase of the 10-year follow-up of the Shanghai Aging Study (SAS) to explore the mediation effect of NDDs on the OI-mortality relationship.
The SAS is a population-based prospective cohort study conducted among the older adults residing in a community of downtown Shanghai, China. The study design and recruitment process of the cohort have been described in detail elsewhere (16). A total of 1,811 participants aged 60 years or older (mean age = 70 years) who completed both an olfactory identification test and a cognitive assessment at baseline (2010–2011) were included in this study.
Baseline data collection has been described in detail previously (17). Information on demographics and lifestyle of the participants including age, sex, education, smoking, and alcohol drinking were collected via an interviewer-administered questionnaire. The body mass index (BMI) of each participant was calculated by using his/her height and weight measured by a research nurse. Physical activity was measured in metabolic equivalent (MET) value. Information on previous and/or current chronic diseases was inquired based on the medical records. Apolipoprotein E (APOE) genotyping was conducted by using the blood or saliva samples of the participants and APOE-ε4 allele positive was defined as the presence of at least one ε4 allele.
Olfactory function was assessed by using the Sniffin' Sticks Screening Test-12 (SSST-12), which consists of 12 odors (orange, leather, cinnamon, peppermint, banana, lemon, liquorice, coffee, cloves, pineapple, rose, and fish) presenting on felt-tip sticks (18).
At baseline, participants were invited for a clinical interview. Cognitive function was evaluated by using a battery of neuropsychological tests including the Mini-Mental State Examination (MMSE), Conflicting Instructions Task, Modified Common Object Sorting Test, Auditory Verbal Learning Test, and Renminbi (Chinese currency) Test. The normative data and detailed description of the assessment battery were reported elsewhere (19). A panel of experts reached a consensus for diagnosis of dementia and mild cognitive impairment (MCI) based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders, the 4th edition, and the Peterson criteria (20, 21). PD, Huntington's disease, and other NDDs were reported by the participants and confirmed with their medical records.
Survival statuses of the participants from baseline to December 31, 2019 were obtained from the mortality surveillance system of the local Centers for Disease Control and Prevention, which is responsible for verifying the date of death and causes of death from the death certificate (22).
The continuous variables were presented as mean with standard deviation (SD) and the categorical variables were presented as count and percentage (%). In this study, OI was defined as the total SSST-12 score < 8 (the median of the SSST-12 scores). The Pearson's chi-squared test was used to test the differences between the OI and non- or mild OI groups for categorical variables and the Student's t-test and the Mann–Whitney U test were used for continuous variables.
The directed acyclic graph in Figure 1 illustrates the hypothetical association paths between OI and the survival status of the participants mediated by NDDs. The four-way decomposition method was used to attribute effects to interaction and mediation (23). Association between OI and death was evaluated by using the Cox proportional hazards regression model. The proportional hazards assumption was tested on the basis of Schoenfeld residuals. Association between OI and the mediator, i.e., NDDs was assessed by using the logistic regression model. To exclude the multicollinearity between the independent variables included in the logistic regression models, the bidirectional stepwise variable selection method was implemented before the mediation and interaction analysis.

Figure 1. Directed acyclic graph for the association of olfactory impairment (OI) with mortality, mediated by neurodegenerative diseases (NDDs), adjusted for the associations of potential confounders with OI, NDDs, and mortality.
The total effect of OI on death in excess relative risk (ERR) scale, i.e., a hazard ratio (HR) from the Cox proportional hazards regression model minus one, was decomposed into four components: controlled directed effect (CDE) due to OI only, mediated main effect or pure indirect effect (PIE) due to mediation only, reference interaction (IntRef) due to interaction only, and mediated interaction (IntMed) due to mediation and interaction (24). The relationship of the four components is shown in Figure 2. The effect decomposition analysis was conducted for both the OI and failure to identify individual odors.
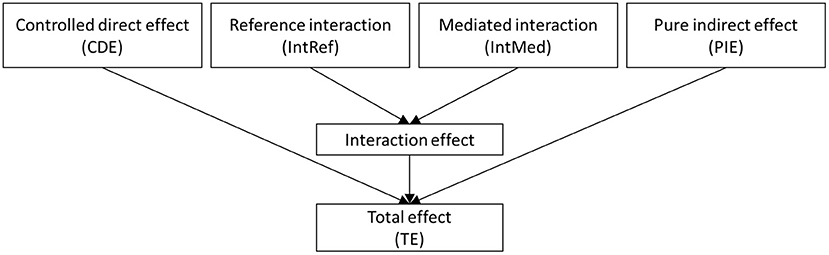
Figure 2. The four-way decomposition method encompasses both the components for mediation and interaction.
All the statistical analyses were conducted in Stata 16.1 (StataCorp LLC, College Station, Texas, USA). A two-sided p-value was considered as statistically significant and a 95% CI was provided for each estimated effect.
In the total 1,811 participants recruited in the SAS cohort, 662 participants were identified as OI at baseline, 378 participants were diagnosed as NDDs (29 dementia, 5 PD, and 344 MCI), and 258 participants died during an averagely 9.4 years of follow-up. Baseline characteristics of the participants and the outcomes during the follow-up are shown in Table 1. Compared to the non-OI group, the OI group was older, less educated, with a higher proportion of hypertension, stroke, chronic bronchitis, the lower MMSE score, higher activities of daily living (ADL) score, lower total cholesterol (TC) and low-density lipoprotein (LDL), and a higher proportion of failure to identify individual odors, NDDs, and death (Table 1).
No statistically significant association was found between OI and death in the general Cox proportional hazards regression model (HR = 1.25, 95% CI: 0.90–1.72, p = 0.181); however, OI was statistically significantly associated with ND [odds ratio (OR) = 2.30, 95% CI: 1.80–2.94, p < 0.001] (Table 2). The four-way decomposition method revealed a statistically significant association of OI with death. Overall, 43% higher risk for death was associated with OI (ERR = 0.43, 95% CI: 0.06–0.80, p = 0.023). Excluding the mediation from NDDs and interaction between OI and NDDs, the controlled direct effect of OI on death was even higher in NDDs participants, with an ERR of 77% (95% CI: 0.00–1.55, p = 0.050) (Table 2).
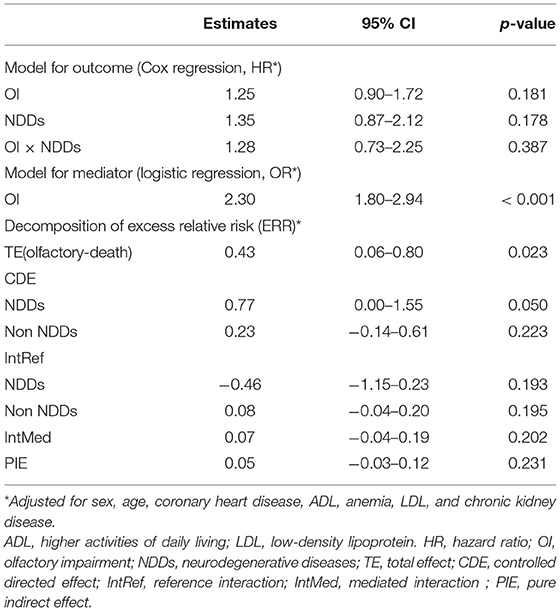
Table 2. Results of the four-way decomposition analysis for the association between OI and mortality.
Statistically significant association was found for failure to identify coffee (ERR = 0.77, 95% CI: 0.18–1.36, p = 0.010) and marginally significant associations were found for failure to identify cinnamon (ERR = 0.33, 95% CI: −0.02–0.68, p = 0.068) and rose (ERR = 0.33, 95% CI: −0.01–0.67, p = 0.054) with death (Figure 3). No statistically significant interaction was found between failure to identify the three individual odors and NDDs. Interestingly, statistically significant mediation by NDDs was found for failure to identify coffee and rose with PIEs of 0.07 (95% CI: 0.00–0.14, p = 0.039) and 0.09 (95% CI: 0.02–0.16, p = 0.016), respectively (Figure 3). However, the mediation from NDDs only contributed to a small part (9.1%) of the association of failure to identify coffee with death, while a considerable mediation (27.2% of total ERR) from NDDs was found in the association between failure to identify rose and death. Besides, the controlled direct effect of failure to identify coffee on death was statistically marginally significant, with ERRs of 0.82 (95% CI: −0.12–1.77, p = 0.089) and 0.64 (95% CI: −0.06–1.34, p = 0.071) for NDDs and non-NDDs participants, respectively. No statistically significant association was found for failure to identify any other odor and death (Figure 3).
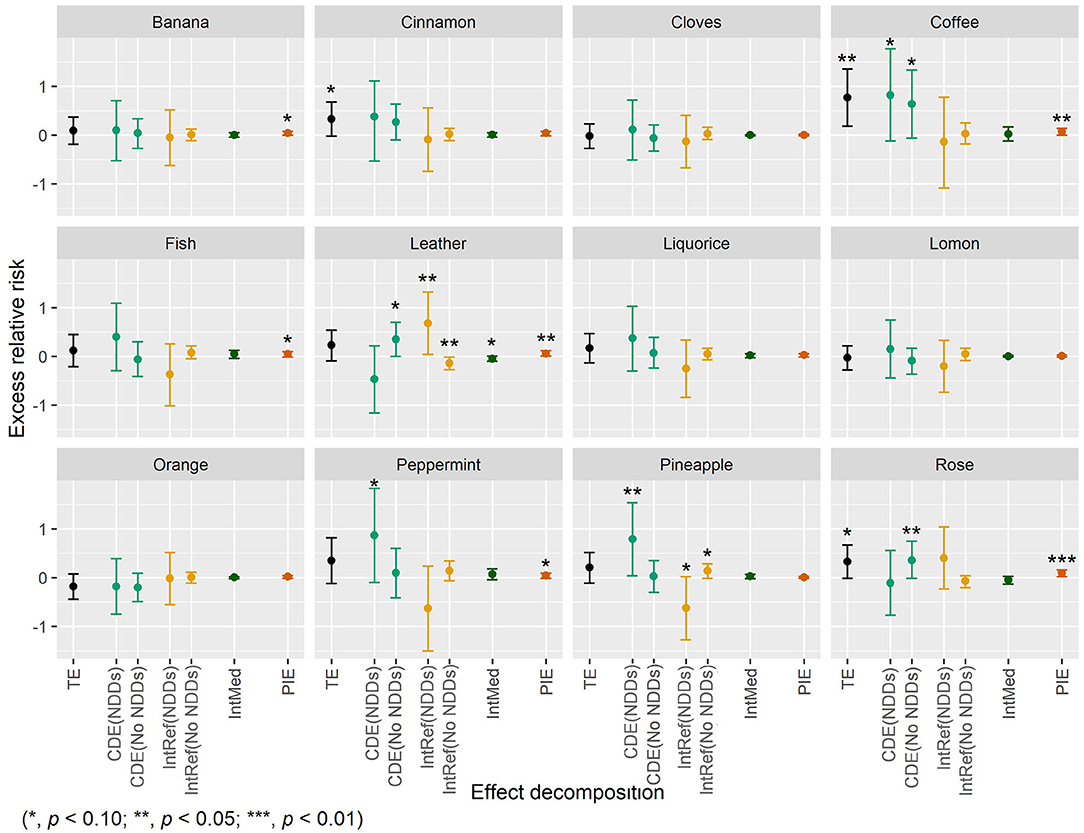
Figure 3. The four-way decomposition method of excess relative risk of mortality for individual odors.
Olfactory dysfunction is reported as the only sensory, which has been associated with mortality, when compared with hearing or visual impairment (2). Although the OI-mortality relationship has been identified independently from incidence of dementia (7), current findings support the relationships between OI, NDDs, and mortality risk and some studies have further shown that cognitive function might be a potential mediator in the relationship (3, 12, 13). Devanand et al. found that the OI-mortality association was weaker when controlled for dementia, yet still statistically significant (13). Liu et al. showed that dementia could account for 22% of the higher 10-year mortality linked to poor olfactory function (3). Our findings have confirmed that OI was associated with mortality in older adults during a 9.4-year follow-up and the association was even stronger in the NDDs participants.
We also found that failure to identify certain odors, especially coffee or rose, was associated with a higher mortality risk and the association was mediated by NDDs. Failure to identify a specific odor-like coffee was also suggestive of reduced survival in another study (25); however, failure to smell rose has not been reported for this potential connection. The mediation of NDDs on the associations of coffee is weak. One hypothetical explanation for the absence or weak mediation of NDDs might be that OI happened, while the clinical cognitive function is not yet affected and, thus, undiagnosed (26); postmortem markers of NDDs existing in the brains of subjects without a diagnosis of NDDs have supported this speculation (27).
There are several strengths in this study. First, although a causal relationship between OI and mortality and the mediation by NDDs cannot be established in the observational study, the study design and the sequential happening of the exposure, mediator, and outcome have ruled out a reversed conclusion. Second, cognitive function at baseline has been controlled in this study. Third, abundant demographic and medical historical variables collected in the SAS offered us a unique opportunity to use the four-way decomposition method to attribute effects to interaction and to assess mediation. The four-way decomposition methods used in published related literature were essentially special cases of the four-way decomposition. The four-way decomposition method could provide maximum insight into how much of an effect is mediated by a potential mediator in an exposure-mediation-outcome causal pathway (23).
Limitations of this study cannot be ignored. First, besides the complicated diagnosis procedure of cognitive impairment, we defined participants with other NDDs only based on their medical records, not used standard inclusion/exclusion criteria. Some participants might have NDDs, but they ignored that and did not go to see doctors. So, the NDDs in this study might be underestimated. On the other hand, there may be misdiagnosis existed because NDDs diagnoses on the medical records were made by doctors from different levels of hospitals. Second, NDDs include a large spectrum of disorders with remarkable differences. In this study, it is not appropriate to take dementia, MCI, and PD into account as the homogeneous NDD. Third, the instrument we used to assess the olfactory function only has 12 sticks for 12 odors. This limited us in seeking odors that were more sensitive than these 12. Fourth, the SSST-12 evaluated the olfactory identification, which cannot completely present the olfactory function. Together with the odor discrimination, detection thresholds should be administered to define the olfactory impairment. Fifth, this study was an observational study with relatively small sample size. The association and the mediation effects resulting from the statistical analysis only could reflect the phenomenon or clue. Future studies should focus on the biological mechanism, which could deeply explain our findings.
This study indicated that OI was associated with the long-term mortality in older adults and the association was even stronger in those with NDDs. Failure to identify coffee or rose was associated with a higher mortality risk and the association was mediated by NDDs. This study result suggests a potential signal for predicting survival in the elderly, especially those with NDDs. The findings need to be further confirmed with larger cohort studies or with interventional design.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Shanghai Aging Study was approved by the Medical Ethical Committee of Huashan Hospital, Fudan University, Shanghai, China (approval number: 2009-195). The patients/participants provided their written informed consent to participate in this study.
YC and DD contributed to the design of the study, data analysis and interpretation, and drafting of the manuscript. ZX contributed drafting of the manuscript and data analysis. ZX, WW, and QZ contributed to the data collection of the study. All authors contributed to the critical revision of the manuscript for important intellectual content.
DD and QZ were supported by grants from the Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX01), the ZJ Lab, National Natural Science Foundation of China (81773513), and the State Key Laboratory of Neurobiology and Frontiers Center for Brain Science of Ministry of Education, Fudan University. The funding agencies had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Doty RL, Kamath V. The influences of age on olfaction: a review. Front Psychol. (2014) 5:20. doi: 10.3389/fpsyg.2014.00020
2. Silva MME, Mercer PBS, Witt MCZ, Pessoa RR. Olfactory dysfunction in Alzheimer's disease systematic review and meta-analysis. Dement Neuropsychol. (2018) 12:123–32. doi: 10.1590/1980-57642018dn12-020004
3. Doty RL. Olfactory dysfunction in Parkinson disease. Nat Rev Neurol. (2012) 8:329–39. doi: 10.1038/nrneurol.2012.80
4. Silveira-Moriyama L, Hughes G, Church A, Ayling H, Williams DR, Petrie A, et al. Hyposmia in progressive supranuclear palsy. Movement Disord. (2010) 25:570–7. doi: 10.1002/mds.22688
5. Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. (2004) 318:121–34. doi: 10.1007/s00441-004-0956-9
6. Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. (2003) 24:197–211. doi: 10.1016/s0197-458000065-9
7. Schubert CR, Fischer ME, Pinto AA, Klein BEK, Klein R, Tweed TS, et al. Sensory impairments and risk of mortality in older adults. J Gerontol A Biol Sci Med Sci. (2017) 72:710–5. doi: 10.1093/gerona/glw036
8. Liu B, Luo Z, Pinto JM, Shiroma EJ, Tranah GJ, Wirdefeldt K, et al. Relationship between poor olfaction and mortality among community-dwelling older adults: a cohort study. Ann Intern Med. (2019) 170:673–81. doi: 10.7326/M18-0775
9. Gopinath B, Sue CM, Kifley A, Mitchell P. The association between olfactory impairment and total mortality in older adults. J Gerontol A Biol Sci Med Sci. (2012) 67:204–9. doi: 10.1093/gerona/glr165
10. Ekstrom I, Sjolund S, Nordin S, Nordin Adolfsson A, Adolfsson R, Nilsson LG, et al. Smell loss predicts mortality risk regardless of dementia conversion. J Am Geriatr Soc. (2017) 65:1238–43. doi: 10.1111/jgs.14770
11. Gammon K. Neurodegenerative disease: brain windfall. Nature. (2014) 515:299–300. doi: 10.1038/nj7526-299a
12. Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS ONE. (2014) 9:e107541. doi: 10.1371/journal.pone.0107541
13. Devanand DP, Lee S, Manly J, Andrews H, Schupf N, Masurkar A, et al. Olfactory identification deficits and increased mortality in the community. Ann Neurol. (2015) 78:401–11. doi: 10.1002/ana.24447
14. Doty RL. Olfactory dysfunction in neurodegenerative diseases: is there a common pathological substrate? Lancet Neurol. (2017) 16:478–88. doi: 10.1016/S1474-4422(17)30123-0
15. Wilson RS, Yu L, Bennett DA. Odor identification and mortality in old age. Chem Senses. (2011) 36:63–7. doi: 10.1093/chemse/bjq098
16. Ding D, Zhao QH, Guo QH, Meng HJ, Wang B, Yu PM, et al. The Shanghai aging study: study design, baseline characteristics, and prevalence of dementia. Neuroepidemiology. (2014) 43:114–22. doi: 10.1159/000366163
17. Ding D, Xiao Z, Liang X, Wu W, Zhao Q, Cao Y. Predictive value of odor identification for incident dementia: the Shanghai aging study. Front Aging Neurosci. (2020) 12:266. doi: 10.3389/fnagi.2020.00266
18. Wolfensberger M. Sniffin'Sticks: a new olfactory test battery. Acta oto-laryngologica. (2000) 120:303–6. doi: 10.1080/000164800750001134
19. Ding D, Zhao Q, Guo Q, Meng H, Wang B, Luo J, et al. Prevalence of mild cognitive impairment in an urban community in China: a cross-sectional analysis of the Shanghai aging study. Alzheimers Dement. (2015) 11:300–9 e2. doi: 10.1016/j.jalz.2013.11.002
20. American Psychiatrie Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington DC: American Psychiatrie Publishing (1994). p. 143–7.
21. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. (2004) 256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x
22. Ding D, Zhao Q, Guo Q, Liang X, Luo J, Yu L, et al. Progression and predictors of mild cognitive impairment in Chinese elderly: a prospective follow-up in the Shanghai aging study. Alzheimers Dement. (2016) 4:28–36. doi: 10.1016/j.dadm.2016.03.004
23. VanderWeele TJ. A unification of mediation and interaction: a 4-way decomposition. Epidemiology. (2014) 25:749–61. doi: 10.1097/EDE.0000000000000121
24. Discacciati A, Bellavia A, Lee JJ, Mazumdar M, Valeri L. Med4way: a Stata command to investigate mediating and interactive mechanisms using the four-way effect decomposition. Int J Epidemiol. (2018) doi: 10.1093/ije/dyy236
25. Laudisio A, Navarini L, Margiotta DPE, Fontana DO, Chiarella I, Spitaleri D, et al. The Association of Olfactory Dysfunction, Frailty, and Mortality Is Mediated by Inflammation: Results from the InCHIANTI Study. J Immunol Res. (2019). doi: 10.1155/2019/3128231
26. Van Regemorter V, Hummel T, Rosenzweig F, Mouraux A, Rombaux P, Huart C. Mechanisms Linking Olfactory Impairment and Risk of Mortality. Front Neurosci. (2020) 14:140. doi: 10.3389/fnins.2020.00140
Keywords: olfactory, neurodegenerative disease, mortality, prospective, elderly
Citation: Cao Y, Xiao Z, Wu W, Zhao Q and Ding D (2021) Is Olfactory Impairment Associated With 10-year Mortality Mediating by Neurodegenerative Diseases in Older Adults? The Four-Way Decomposition Analysis. Front. Public Health 9:771584. doi: 10.3389/fpubh.2021.771584
Received: 06 September 2021; Accepted: 26 October 2021;
Published: 26 November 2021.
Edited by:
Qiushi Feng, National University of Singapore, SingaporeReviewed by:
Carla Masala, University of Cagliari, ItalyCopyright © 2021 Cao, Xiao, Wu, Zhao and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ding Ding, ZGluZ2RpbmdAaHVhc2hhbi5vcmcuY24=; Yang Cao, eWFuZy5jYW9Ab3J1LnNl
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.