
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Public Health, 16 December 2021
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 9 - 2021 | https://doi.org/10.3389/fpubh.2021.751451
This article is part of the Research TopicInsights in Coronavirus Disease (COVID-19) - Surveillance, Prevention and TreatmentView all 84 articles
 Ronan Lordan1*
Ronan Lordan1* Samantha Prior2
Samantha Prior2 Elizabeth Hennessy1
Elizabeth Hennessy1 Amruta Naik3
Amruta Naik3 Soumita Ghosh1
Soumita Ghosh1 Georgios K. Paschos1
Georgios K. Paschos1 Carsten Skarke1
Carsten Skarke1 Kayla Barekat1
Kayla Barekat1 Taylor Hollingsworth1
Taylor Hollingsworth1 Sydney Juska1
Sydney Juska1 Liudmila L. Mazaleuskaya1
Liudmila L. Mazaleuskaya1 Sarah Teegarden1
Sarah Teegarden1 Abigail L. Glascock1,4
Abigail L. Glascock1,4 Sean Anderson1
Sean Anderson1 Hu Meng1
Hu Meng1 Soon-Yew Tang1
Soon-Yew Tang1 Aalim Weljie1,5
Aalim Weljie1,5 Lisa Bottalico1,5
Lisa Bottalico1,5 Emanuela Ricciotti1,5
Emanuela Ricciotti1,5 Perla Cherfane1
Perla Cherfane1 Antonijo Mrcela1
Antonijo Mrcela1 Gregory Grant1,6
Gregory Grant1,6 Kristen Poole7
Kristen Poole7 Natalie Mayer1
Natalie Mayer1 Michael Waring8
Michael Waring8 Laura Adang9
Laura Adang9 Julie Becker10
Julie Becker10 Susanne Fries1
Susanne Fries1 Garret A. FitzGerald1,5
Garret A. FitzGerald1,5 Tilo Grosser1,5*
Tilo Grosser1,5*During the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, providing safe in-person schooling has been a dynamic process balancing evolving community disease burden, scientific information, and local regulatory requirements with the mandate for education. Considerations include the health risks of SARS-CoV-2 infection and its post-acute sequelae, the impact of remote learning or periods of quarantine on education and well-being of children, and the contribution of schools to viral circulation in the community. The risk for infections that may occur within schools is related to the incidence of SARS-CoV-2 infections within the local community. Thus, persistent suppression of viral circulation in the community through effective public health measures including vaccination is critical to in-person schooling. Evidence suggests that the likelihood of transmission of SARS-CoV-2 within schools can be minimized if mitigation strategies are rationally combined. This article reviews evidence-based approaches and practices for the continual operation of in-person schooling.
The impact of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic on a generation of children can be anticipated to be extensive and long-lasting. In addition to the health consequences of coronavirus disease 2019 (COVID-19), the economic repercussions have brought financial insecurity to families and communities. Indeed, pre-existing social and health disparities has disproportionally affected many communities. School closures to reduce the number of person-to-person contacts and viral spread in the population (1) have caused prolonged interruptions to in-person learning and affected children's education and well-being. The benefit of formal education also is in the social, emotional, and behavioral dimensions that are difficult to replace in a distance learning environment. Therefore, enabling continuous in-person education is a central goal within the public health response to the pandemic.
Operating schools for in-person learning requires a dynamic approach as community spread fluctuates and more transmissible (and potentially more virulent) strains emerge (2–4). Key concerns include health risks to members of the school community and their families; disruption of learning, social, emotional and physical well-being associated with school closures, remote or hybrid learning, and quarantine periods; and the potential contribution of schools to sustaining viral spread in the population.
COVID-19 is predicted to evolve into an endemic disease in regions with infection and vaccination rates that confer inadequate protective population-level immunity (5, 6). Recurrent outbreaks among those who are not yet or are no longer immune are characteristic of many infectious diseases, including the common cold or influenza. Akin to their role in the infection dynamics of these seasonal respiratory infections, schools may become reservoirs of SARS-CoV-2 due to their dense social setting if sufficient protective measures are not taken to limit the propagation of natural infections among susceptible individuals (7). Vaccination against SARS-CoV-2 markedly lessens, if not eliminates, most health concerns associated with in-person schooling for adults and children who are immunized—even in regions where highly transmissible viral variants predominate (8–14). However, those in a school community who are not yet eligible for vaccination, who have not had an opportunity to be vaccinated, or who choose to delay or forgo vaccination remain at risk.
The susceptibility of unvaccinated school-aged children to SARS-CoV-2 infection and this population's likelihood of spreading the virus has been widely discussed since lower rates of reported infections in children than adults were noted (15). However, the cause of this age-based disparity is likely multifactorial. Hypotheses include decreased exposure (16), missed asymptomatic cases (17), and a lower biological susceptibility to being infected (15, 18, 19). The interplay of these factors may contribute to heterogeneity in reports on pediatric susceptibility to SARS-CoV-2 infection. While some contact tracing studies estimated a reduced infection risk of children and adolescents (15, 20–24), others found no difference when compared with adults (25, 26). Similarly, although some seroprevalence studies found SARS-CoV-2 antibodies as a marker of past infection in fewer children than adults, others observed no or only small effects of age (27). Additionally, evidence of potential differences in the risk of SARS-CoV-2 infection between younger and older school-aged children are not uniformly supported and may relate more to study design than age-dependent biology (15, 20–26). For example, a large surveillance testing study in Austria found no differences in the infection rates among 6–10 and 11–14 year olds, suggesting that primary and middle school students have similar risks of infection (28).
There is more clarity regarding children's ability to spread the virus. Minor age-based differences between nasopharyngeal viral loads exist, but they do not suggest that children are substantially less infectious than adults (29). Indeed, data from India provide evidence that children and adolescents of all ages are effective transmitters of the disease (30). This is supported by reports of increased prevalence of SARS-CoV-2 in children, particularly among adolescents. For example, during the second pandemic wave in the UK (November 2020), the highest prevalence of SARS-CoV-2 positive tests was obtained from adolescents who attended school (31). This was in the context of the more transmissible virus variant Alpha (B.1.1.7) (31). When the highly transmissible Delta (B.1.617.2) variant began to spread in the British population (June 2021), this age group again showed the highest infection rate in the population (32) and it appeared that some case clusters were associated with schools (4). Similarly, a rapid increase of infections and hospitalizations in children and adolescents was observed in the U.S. when Delta began to spread in July 2021 (14, 33). By October 2021 ~25% of newly reported COVID-19 infections occurred in children under the age of 18 years, an age group that makes up 22 % of the population in the U.S. (34). These findings are in accordance with a contact tracing study that saw the highest probability of transmission among students aged 10–19 years (35). Because of our evolving understanding of how susceptible to infection with increasingly transmissible virus variants children are and how they contribute to the spread of SARS-CoV-2, schools should not rely on an assumed natural protection of (and by) children. Mitigation plans for all age groups continue to be necessary to curb the spread of virus variants in schools.
While the age dependency of infection risk is controversial, there is strong evidence that children who are infected with SARS-CoV-2 have a substantially lower risk of severe outcomes than adults. Most are diagnosed with asymptomatic or minimally symptomatic infections (36–41) or experience a mild form of respiratory disease that will typically resolve within a week or two (25, 42–46). Increasing evidence suggests that the airway epithelium of children may have a better ability to sense infection with SARS-CoV-2 and may mount a more pronounced immune response than adults, resulting in faster viral clearance (47–49). However, even in mild cases, individuals can experience persistent symptoms for weeks following a SARS-CoV-2 infection. These prolonged post-acute sequelae of SARS-CoV-2 infection, referred to as “PASC” or “long-COVID,” have only begun to be studied in children. As in adults, symptoms include fatigue, dyspnea, chest pain, cardiac palpitations, myalgia, gastrointestinal issues, headache, anosmia, and issues with memory and concentration (50, 51). Preliminary studies suggest that the most common persistent symptoms in children are fatigue and cough, although longstanding insomnia, headache, and pain were also frequently reported (52–54). One study found that ~40 % of children diagnosed with COVID-19 reported at least one persistent symptom 2 months post infection (55). Another study estimated that 10% of children may still experience symptoms 3 months post infection (56). In a large, matched cohort study, the incidence rate of health problems was ~30% higher in the COVID-19 cohort than in matched controls at least 3 months after infection (57). Symptoms including fatigue, cough, throat or chest pain, headache, fever, and abdominal pain occurred 1.45 to 2.28 times more frequently in the COVID-19 than in the control cohort. Another study that focused on severely ill children that were hospitalized with COVID-19 estimated that 25% may experience persistent symptoms 5 months post discharge with higher risk in those with pre-existing allergic diseases. Some symptoms persisted even longer (58). The impact of this persistent symptomatology on academic participation and performance is still to be determined.
Despite its overall rarity, children can become severely ill with COVID-19 (59, 60) and just as in adults, racial, ethnic, and socioeconomic disparity in disease severity is evident (25, 61). Exact rates of severe disease are difficult to estimate because of the large number of undetected infections and prophylactic admission to hospitals, which result in an overestimate in unadjusted health statistics of COVID-19 severity (17). Considering these limitations, a reported cumulative (October 2021) hospitalization rate of 0.8% of all laboratory confirmed infections in the pediatric U.S. population represents a conservative estimate of the rate of severe disease (62). Notably, over one quarter had a pre-existing condition such as obesity, type I diabetes, asthma, and neurodevelopmental disorders (63). Youths (12–17 years) and young children (0–4 years) were ~2–3 times more frequently hospitalized than 5–11 year old children (64). Approximately one in four children admitted to a hospital in the U.S. received intensive care (14, 65). In Germany where the hospitalization rate of 1–17 year olds with a documented SARS-CoV-2 infection has also been ~1%, it was determined that between 1% and 10% of children admitted to a hospital received intensive care (66).
A rare, severe, delayed consequence of COVID-19 is multisystem inflammatory syndrome in children (MIS-C), also referred to as pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) (67–73). MIS-C is characterized by persistent fever and systemic inflammation, involving at least two organs (i.e., lungs, heart, kidneys, skin, blood, gastrointestinal tract, and brain), weeks after infection with SARS-CoV-2. As of November, 2021, the Centers for Disease Control and Prevention (CDC) report a median age of 9 years (interquartile range 5–13 years) for MIS-C in the U.S. (74). Initial estimates suggest an MIS-C incidence of ~2 per 100,000 children and adolescents in the U.S. population during the first year of the pandemic (68) before the number of infected children rose steeply when the Delta variant began to spread. During the same period (pre Delta) ~3,500 COVID-19 infections per 100,000 children and adolescents were laboratory confirmed (34). Because the number of total infections including undetected infections can be expected to be markedly higher (17), a rough estimate might be that between 1 in 1,000 and 1 in 10,000 infected children and adolescents have developed MIS-C in the U.S.. An analysis of MIS-C reports in seven U.S. states during the first months of the pandemic estimated that ~3 in 10,000 infected children developed MIS-C (75, 76). German registry data found rates of 7 in 1,000 and 4 in 10,000 for 1–10 and 12–17 year old children and youths with documented infections suggesting that the case rate is similar in both regions (66). Emerging data indicates that while many children with MIS-C require intensive care, most recover fully or with minimal residual functional impairment (70). However, as of November, 2021, the CDC's national registry lists 46 deaths among 5,217 patients (~0.9 %) meeting the MIS-C case definition (75).
An infection fatality rate (IFR) can be estimated as a risk for death among infected individuals. This value accounts for both laboratory confirmed and imputed undetected infections. The IFR of the H1N1 pandemic 2009 influenza is estimated to be <1 in 100,000 infected school-aged children (77, 78), and the fatality rate of measles may approximate 1–2 in 1,000 infected school-aged children (79). In school-aged children with SARS-CoV-2, an IFR of 1–3 in 100,000 has been estimated (80, 81). The IFR may be higher in children under the age of 4 years (80, 81). In comparison, the estimated IFR for adults aged 35 to 44 years ranges from 40 to 75 in 100,000 infected persons and the IFR for adults aged 65 to 74 years ranges from 1,075 to 1,670 in 100,000 infected persons (80).
At the population level, COVID-19 ranked among the 10 leading causes of death among U.S. children throughout most of 2021 (82). A mortality rate of ~0.2 in 100,000 children has been attributed to COVID-19 during the first year of the pandemic (83, 84). Children aged 3–10 had the lowest COVID-19 related mortality (85). Notably, the COVID-19 related mortality was about two-fold higher than the typical mortality rate of influenza in school-aged children in prior years (range 0.10–0.13 in 100,000) (85, 86). However, comparison of COVID-19 related mortality with historical influenza data is complicated, because COVID-19 deaths occurred despite unprecedented protective public health measures. There were 144 to 198 annual pediatric influenza deaths in the U.S. in the 3 years prior to the 2020/21 season (October to May), during which only one influenza-related pediatric death occurred (87). This suggests that the public health measures instituted, including school closures and hybrid learning, may have had broad impact on reducing the transmission of other viruses. In contrast to the isolated pediatric death from influenza in the 2020/21 season, over 220 pediatric COVID-19 deaths were reported in the U.S. during the same time (34, 62). These observations highlight inherent differences in infection dynamics between these two diseases.
As public health mitigation measures are lifted in increasingly vaccinated populations, it remains unclear how this will impact COVID-19 related morbidity and mortality in school age children. Additionally, there is an unmet need to understand the frequency and severity of PASC in children. The emergence of more transmissible (and potentially more virulent) variants may also affect disease burden in the pediatric population. Moreover, the ethical considerations regarding phasing out protective measures for children who are age-eligible but remain unvaccinated are different from those in the adult population. Because of these evolving uncertainties, protecting students should remain a priority, even when many adults and age-eligible students have been immunized.
Since the beginning of the pandemic, education has rotated between remote and in-person learning. However, even when in-person learning was offered quarantine and isolation periods for COVID-19 infections and exposures affected as many as 20% of students during pandemic waves in both Spain and China (3, 88, 89). Shifts between modes of learning have led to negative social, emotional, educational, and physical consequences not only through reduced physical presence in school buildings, but also through loss of essential services for children provided by schools (90–92). Many receive their main source of nutrition in schools. For others school is a place of refuge and protection. For example, child maltreatment was underreported by an estimated 27% in the U.S. during the pandemic, likely because it went unnoticed or unreported due to lack of face-to-face interactions with teachers or other school personnel during school closures and remote programs (93). Many schools were unable to provide mental and behavioral health services to students while working remotely, which may have contributed to under recognition of child maltreatment and more generally increases in stress and anxiety (94, 95).
The impact of the extended school closures during the pandemic remains to be fully appreciated, but students in the Netherlands showed little progress while learning from home during the first lockdown (96). In the U.S., it was projected that students would have ~40–50% of the learning gains in mathematics and 60–70% of the learning gains in reading relative to a typical school year, although the top third of students may have been impacted less (97). Learning loss was reported to be more prevalent for students of color and for socioeconomically disadvantaged students (96, 98). Moreover, students with disabilities were less likely to have had access to educational support (99).
Despite being a necessary public health response to the pandemic, even limited quarantine measures and temporary school closures in response to infections and outbreaks in the school community can have negative consequences— perhaps most for children of working parents. Remote learning may disadvantage students further if the household is not digitally competent (88). Academic outcomes are thought to be similarly affected by transitions to distance learning as they are by absenteeism (100). Home confinement for just 8–10 days can measurably impair emotion regulation and increase symptoms of anxiety and depression (101). Increased screen time can negatively affect sleep patterns and limit exercise, which impacts both physical and mental health (102). Perhaps most fundamentally, interruptions to daily in-person interactions with classmates, and a network of caring adults may potentially cause damaging isolation-related effects such as anxiety and depression (101, 103). Disadvantaged children, such as refugees, migrants, and those with cognitive and/or physical disabilities are likely to have faced greater exclusion from learning (104). Therefore, minimizing disruption of continuous in-person learning by encouraging vaccination and implementing proactive infection control measures that identify potentially infected individuals early and limit the likelihood of spread is a central goal of school operation plans.
At its peak, COVID-19-related school closures, which were based on the response plans to influenza pandemics, affected an estimated 1.5 billion or 90% of the world's learners (105, 106). Estimating the contribution of specific interventions to overall mitigation during countrywide general lockdowns is challenging. However, retrospective modeling analyses suggest that schools may have played an important role in disseminating infections into the population during the initial wave of SARS-CoV-2 before mitigation measures were implemented (1, 16, 107). Indeed, large viral outbreaks among students and teachers, which spread to family members and other close contacts outside of school, have been documented when preventive measures were insufficient or were insufficiently followed (38, 108–112).
Early in the pandemic, in person schooling was associated with increased rates of infection (113). In Sweden, family members of students and teachers who were physically present in school buildings had higher rates of infections than family members of students and teachers who engaged in remote learning (113). As our experiences evolved, it was found that the risk for in-school transmission correlated with community exposure risk (109, 114–121). National and regional contact tracing and surveillance data collected in Europe, Australia, and Singapore during periods of low viral circulation (summer/fall 2020) found that clusters of cases or documented outbreaks were infrequent despite variable degrees of mitigation measures (109, 114–121). Most infections appeared to have been acquired outside of school. Employees were found to be at a higher risk of seeding SARS-CoV-2 infections than children in U.K. schools (114). Random COVID-19 testing among Austrian school students and staff showed that the number of infections detected in schools increased with the surging incidence of infections in the broader community (28). Similarly, widened viral circulation in a region coincided with a growing number of outbreaks in Australian schools (122). Conversely, an increasing vaccination rate in the adult population was associated with a substantial decrease in cases in children and teenagers in Israel before they became age-eligible for vaccination (123). This direct relationship between the infection dynamics in schools and the surrounding population highlights the importance for safe school operation of reducing the infections in the community.
The question of how much in-person learning contributed to the dissemination of infections into the regional population is difficult to address. Two analyses of German incidence data found that SARS-CoV-2 transmission in the population decreased following school reopening after the summer break 2020 under conditions of low baseline viral circulation. This was hypothesized to relate to reduced travel as the summer break ended and more widespread adoption of infection mitigation behaviors by the population (124, 125). In Michigan and Washington, a temporal association between re-opening schools (fall 2020) and infections in the broader population was observed only in regions with high baseline infection activity (126). Where incidence rates were low prior to the begin of in-person learning, no increase in viral transmission in the population was observed (126, 127). Similar observations were made in schools in Sicily (128). Moreover, a nationwide analysis showed no evidence of increased hospitalizations for COVD-19 in U.S. regions with low baseline disease activity where schools re-opened for hybrid or full in-person learning (129). However, an impact of in-person schooling could not be excluded in counties with high baseline disease activity (129). Indeed, a more recent nationwide analysis of U.S. counties found an overall increase of new COVID-19 cases and deaths in communities in temporal association with school opening from April to December, 2020 (130). In counties where teaching resumed in-person but without a mask mandate in schools, the rise in case numbers and deaths was steeper and more pronounced than in counties where masks were required (130).
Overall, these data suggest that the infection dynamics in schools and in the surrounding population are in dynamic equilibrium (Figure 1). This illustrates the importance of continued suppression of viral transmission in the broader population while the vaccination rate increases through effective public health measures such as mask wearing and limits on social gatherings. Adults play a particular role in the infection of children with ~70% of pediatric cases being secondary to an adult case (131). However, young children are not yet age-eligible for vaccination in many regions in the world, or supply shortages limit availability of the SARS-CoV-2 vaccines for those who are eligible. Therefore, the interplay between community spread and outbreaks in schools supports a strategy of prioritizing older adults for vaccination where supply is limited, not only because they are most at risk but because mitigating their role in community spread can also be expected to render in-person learning safer (123). However, this still requires mitigation of transmission within schools to minimize the risk of larger outbreaks. Adults bare the greater responsibility to monitor their actions within the community to prevent the spread of SARS-CoV-2 among children (132).
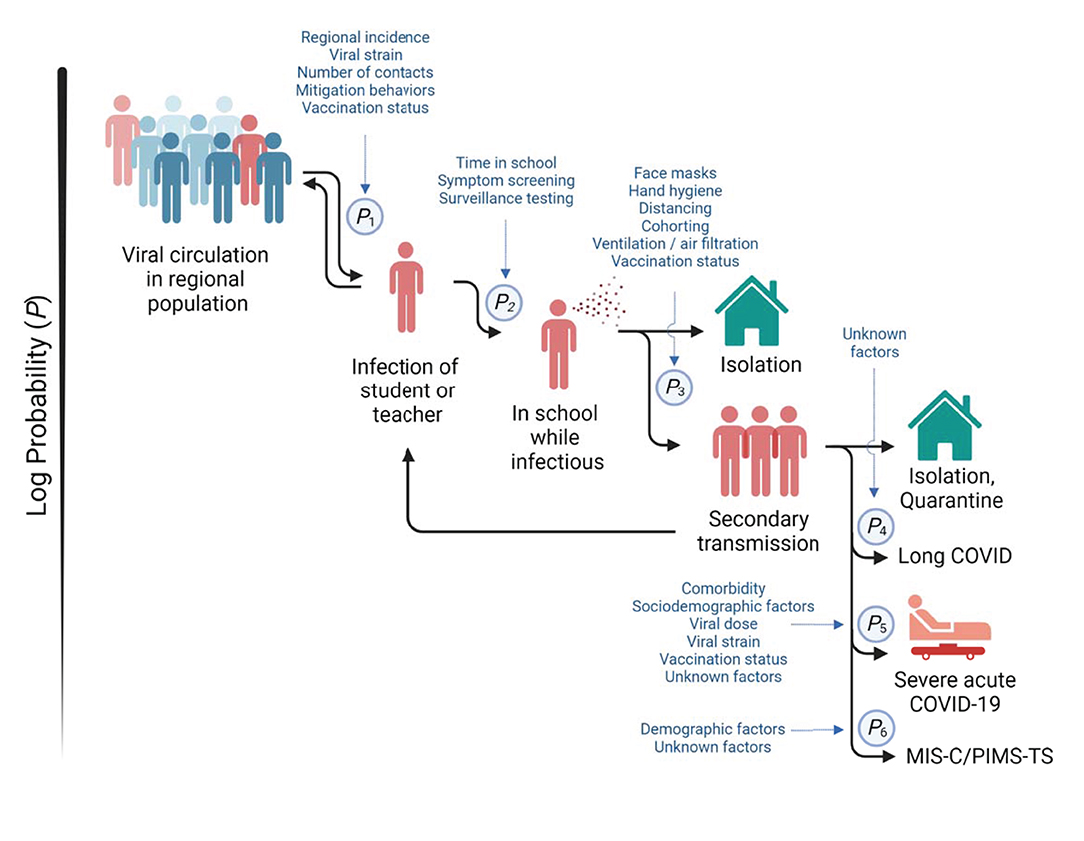
Figure 1. Infection dynamics in schools. Multiple factors and mitigation measures (in blue) modify the probabilities of (P1) infection of students or teachers outside of school, (P2) the import of infection into school, (P3) the spread among students and teachers, (P4) development of prolonged post-acute sequelae of SARS-CoV-2 infection (“long COVID”), (P5) progression to severe acute COVID-19, and (P6) development of multisystem inflammatory syndrome in children (MIS-C), also referred to as pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS). Viral circulation in the regional population is a key determinant of infection risk of students and teachers. Secondary transmission within schools can amplify infections in the regional population.
Emerging evidence suggests that mitigation measures in schools are effective in maintaining low levels of SARS-CoV-2 transmission among students and teachers (secondary transmission) (133, 134). A study in primary schools, nurseries, and kindergartens in Germany determined that asymptomatic spread among these age groups was low when mitigation measures including mask wearing, and classroom ventilation were appropriately applied (135). An Australian study in schools that practiced hand hygiene, physical distancing, reduced mixing of students and reduced after-school activities, but not mask wearing or enhanced classroom ventilation or filtration, found 1 secondary infection case per 9 infectious students who attended school (15). A German analysis of contact tracing data in schools with a 50% reduction of classroom occupancy through hybrid learning, regular room ventilation, limited contacts between cohorts, exclusion with minimal symptoms, but no mask wearing and no distancing in classrooms estimated a frequency of 1 secondary case per 12 infectious students who attended school (136). A report on Norwegian schools that used similar mitigation measures but no masking in classrooms, found no secondary cases among 13 students who attended school while considered infectious (120). A study conducted in Wisconsin observed 1 secondary case per 20 infectious students in schools that consistently practiced mask wearing, had cohort sizes of ≤ 20 students, limited contacts between cohorts, but had no symptom screening program (137). Similarly, school districts in North Carolina detected 1 secondary infection per 24 community-acquired infections in schools that practiced mask wearing, physical distancing, hand hygiene, and reduced cohort mixing (138). Collectively, these studies which were conducted before the Delta variant became dominant, indicate that instituting basic mitigation strategies can enable in-person schooling with a low risk of secondary SARS-CoV-2 transmission. However, the impact of more transmissible virus variants such as Delta remains less understood and can be expected to require additional preventive measures.
Presently, no single intervention, including vaccination, completely prevents viral transmission. However, combined mitigation strategies may be synergistically beneficial (133, 139–141). In safety research this concept of risk management is sometimes described as the “Swiss Cheese Model”, where multiple defensive layers are combined with the understanding that each individual layer has holes. The combination of multiple layers provides safety through redundancy (142). Evidence based choices need to be made as to which mitigation strategies to combine to adapt to changing risk levels and maximize learning.
Given the association between cases in schools with burden of disease in the community, the most effective approach to enable safe in-person learning is to focus on broader public health measures that suppress viral spread in the population (Figure 1). The SARS-CoV-2 vaccines are effective in preventing severe disease and reduce the likelihood of infection and transmission of the virus (123, 143). As such, widespread vaccination of the eligible population is a potent tool in reducing viral spread in the unvaccinated, younger population. Household members are the primary source of infection of school-aged children, and extracurricular activities are a source of infection among adolescents (131, 140, 144). Thus, the entire community of those who interact with unvaccinated children can contribute significantly to protecting schools by getting vaccinated.
Daily symptom-based surveillance combined with aggressive diagnostic testing for COVID-19 infections is effective in reducing the likelihood of having infectious students in schools (140, 145). As many symptomatic students who are infected with SARS-CoV-2 present with mild symptoms, such as rhinorrhea, cough, sore throat, or headache (146), there should be a low threshold for diagnostic COVID-19 testing. Additionally, daily health screening affords the opportunity to gain insights into the overall risk profile of the larger school community including household members by asking about potential contacts of students with symptomatic or laboratory confirmed infected persons.
Silent transmission by individuals who have not yet developed symptoms (“presymptomatic”) or who will never notice symptoms (“persistently asymptomatic”) contribute to the difficulty in containing the pandemic. Surveillance testing approaches in schools are used for identifying pre- / asymptomatic persons who are carrying the virus (147). Most reliable and sensitive diagnostic tests detect the SARS-CoV-2 genome using reverse transcriptase polymerase chain reaction (RT-PCR) (148, 149). Comparative studies suggest that saliva based diagnostic testing accuracy may be similar to that of nasopharyngeal swabs (150). However, slow turn-around times to results of tests based on nucleic acid amplification may not allow for timely isolation of an infected person who has no symptoms. Rapid antigen tests detect viral proteins in a sample of either saliva or a swab of the nose or throat using a lateral flow method similar to pregnancy tests. These tests provide an opportunity to identify pre- or asymptomatic carriers in real-time (151). Although they are generally less sensitive than RT-PCR tests, rapid antigen tests are effective for detecting individuals with high viral loads who would be considered presently infectious (149, 152–154). Saliva-based rapid tests are easy to perform at home or in school, but their sensitivity appears to be substantially lower than those that use nasal swabs (155, 156). Concerns related to surveillance testing include both false negative (the test is reported as negative, but the virus is present) and false positive (the test is reported as positive, but the virus is not present) results (157). The consequences of a false positive test (i.e., unnecessary isolation) can be limited by confirmatory testing using RT-PCR. Regular, for example weekly or more frequent testing reduces the impact of false negative test results (158, 159). Importantly, given the time course of viral load in throat and nose, the sensitivity of the test is less important for effective surveillance testing than the frequency of testing and turnaround time of result reporting (158).
While limited studies have systematically examined surveillance testing in schools, even a testing frequency as low as every 2 weeks appears to provide some balance to the false negative vs. false positive detection rate among students (160, 161). However, because a school wide testing-based surveillance system can identify some, but not all pre- / asymptomatic individuals, even frequent testing should not be relied on as the only strategy to enable in-person schooling. Additionally, school wide testing-based surveillance is not feasible everywhere considering the complex logistics and the costs, particularly in less affluent regions. Despite these hurdles, nationwide surveillance testing for schools has been successfully implemented in many countries, (162, 163), and is being planned or piloted in others (164–166).
An approach to reducing disruptions of learning when COVID-19 positive individuals have been identified in schools is to quarantine only close unvaccinated contacts, i.e., immediate classroom neighbors of students presumed infectious, instead of quarantining all of the unvaccinated students in a learning cohort. Typically, this is combined with proactive COVID-19 testing among the cohort. Another approach is to allow even close unvaccinated contacts of a student presumed infectious to remain in the classroom but require them to test frequently (“test-to-stay”). Currently, there is little empirical data in support of either approach. However, computational simulation suggests that only quarantining close contacts of an infected student rather than the entire class cohort, would not be predicted to increase the probability of large outbreaks in a school as long as SARS-CoV-2 infections in the school community are infrequent and adequate mitigation procedures are in place (i.e., face masks, ventilation, distancing where possible) (161, 167).
In addition to limiting individual health risks by decreasing the likelihood of illness, hospitalization, and death (8–14), COVID-19 vaccines provide a potent additional layer of protection for the school community by reducing the likelihood of infection. For example, the mRNA BNT162b2 COVID-19 vaccine (Pfizer-BioNTech, Comirnaty) has been estimated to reduce the risk of infection with the Delta variant by approximatively four-fold in adults and 10-fold in adolescents (13, 168). Although breakthrough infections can occur in vaccinated individuals (169), emerging evidence suggests that their infectiousness even with the Delta variant might be reduced (170). However, this effect declines with time and so a third (booster) vaccine dose may be needed. A recent study performed in Sweden observed that immunization extended protection from infection to family members who are not vaccinated (171). For example, 2 immunized family members reduced the infection risk for the non-immune family members by 75 to 86%, whereas 3 immunized family members reduced the infection risk for the non-immunized family members by 91% to 94%. Therefore, it can be expected that the likelihood of COVID-19 outbreaks will decline substantially in increasingly vaccinated school communities (i.e., teachers, staff, students, families).
SARS-CoV-2 is spread through liquid particles that are generated by expiratory activities such as breathing, speaking, coughing, and sneezing (172). Liquid particles vary in size by orders of magnitude, ranging from larger droplets to smaller particles dispersed as aerosols. Large droplets (e.g., >50–100 μm) can settle quickly out of the air onto surfaces, so that individuals who are farther away from the droplet source are less likely to inhale them. Viral transmission by large exhaled droplets and secreted respiratory fluids is the basis for physical distancing and hand hygiene recommendations. While 1.8 m (6 foot) separation in classrooms was recommended for U.S. school reopening in fall 2020 and 1.5 m (5 foot) for European schools, more recent observational data suggest that a 0.9 m (3 foot) separation may be sufficient when students are seated and wear facemasks in classrooms (173). The impact of more transmissible variants on distancing requirements remains to be determined. Incorporating instruction on handwashing into the curriculum generally reduces the transmission of infectious diseases in schools (174). Antibacterial adjuvants to soap do not have greater efficacy at preventing infection and may be associated with antibiotic resistance (175). Hand sanitizers are an alternative, but they are not considered as effective as hand washing (176) and are an ingestion risk (177).
Unlike droplets, aerosols (e.g., 10−4 to 10 μm) can stay airborne for hours or until the air is exchanged (178). The risk for virus transmission while outdoors is much lower than indoors because moving air disperses and dilutes aerosol concentrations quickly (179). An important component of mitigation measures targeting indoor air quality is to reduce the release of aerosols by wearing facemasks and by limiting activities indoors that produce high concentrations of aerosols, such as shouting, singing, gym exercise, playing of wind instruments, or having large numbers of students in a classroom (180–183). Well-fitted face masks worn over mouth and nose effectively reduce viral transmission by reducing both emission and inhalation of viral particles (184, 185). Guidance surrounding what age groups should be wearing masks varies (186), but in the U.S., masking is generally recommended for children over 2 years old (187, 188) whereas the World Health Organization (WHO) recommends masks for children over 5 years old (189). A recent study in France found that while many mild inconveniences were reported by parents of children wearing masks, overall, children tend to tolerate them well (190). While wearing face masks does not impair the respiratory function of children (186), even in those as young as 2 years old (191), they reduce the capability to recognize emotional expressions and some mask types can impair speech intelligibility (192–194). Adults interacting with children should be aware of these limitations and compensate where possible. In how far the impact of facemasks on visual and auditory cues may affect language development in young children has not yet been systematically examined.
Additionally, ventilation and filtration strategies can remove virus particles from the air. The effectiveness of ventilation by opening windows or expelling indoor air with fans can be monitored by measuring CO2 levels as a proxy of the cumulative expiratory activity by the occupants (195), which has been a strategy considered by school authorities in Ireland (196). To supplement ventilation, or when it is not practical, air filtration systems should be used. Coronaviruses are found associated with water vapors, proteins, and salts in aerosolized particles often >1.0 μm, which can be effectively removed by filtration (19). Filters for heating, ventilation, and air conditioning (HVAC) systems with a minimum efficiency reporting value (MERV) of 13 have been suggested as a potential air purifying strategy in schools (197, 198). Standalone portable air cleaners that have high efficiency particulate air (HEPA) filters, which are capable of filtering 0.3 μm particles at 99.97% efficiency can supplement or replace centrally located filters (199). Schools that implemented measures to improve ventilation by keeping windows and doors open or using fans had a 35% lower incidence and schools that combined ventilation with filtration had a 48% lower incidence than schools that did not focus on indoor air quality (133).
For most respiratory viruses, the risk of infection depends on the number of viral particles a person inhales, or the viral inhaled dose, which is a function of breathing rate, inhaled concentration, and time of exposure (200). The dose of viral exposure may be proportional to the severity COVID-19 (201–204). It is plausible that prevention of transmission in schools is due to multiple layers of mitigation synergistically minimizing the viral dose students and teachers are exposed to rather than by eliminating exposure entirely.
Many of the infections in a population are spread by a disproportionately small number of people (205). Studies estimate that only 10–20% of coronavirus patients are responsible for 80% of all new infections of the early virus strains (206, 207). While more transmissible strains may spread more widely, outbreaks of viruses with such a hyperdispersed distribution are often driven by super-spreading events. Many super-spreading events involve crowded indoor places, loud speaking or singing, and inadequate use of protective measures such as face masks and physical distancing. The distribution of the sizes of reported outbreaks in schools reflects such a hyperdispersed distribution. Often, no or few new secondary infections are observed following exposures in school, while larger or very larger outbreaks are less frequent (167, 208). Banning larger group gatherings is a practice that avoids super-spreading and is highly effective in suppressing viral spread (1). Avoiding student mixing by creating protective (classroom) cohorts may allow clusters of infection to be more easily contained through limited quarantine measures. However, while this is feasible in early childhood, elementary and middle schools, a typical high school rotation structure creates much more complex networks of person-to-person contacts, which are predicted to result in a higher risk of outbreaks (161). Indeed, a study in Catalonia which was conducted in schools that practiced cohorting found that the secondary attack rates were higher in high school students than in preschool students (208). However, even in situations where protective cohorts are difficult to implement, a focus on limiting longer and closer interactions to members of the same group of individuals whenever feasible may still provide some benefit (209).
Alternating (“hybrid”) in-school and remote-learning models reduce the size of the protective cohort. Computational modeling suggests that weekly rotations (i.e., 5 days in school, 5 days remote) or groups of students rotating 2–3 days in school every week limit the number of contacts between students and teachers to reduce the likelihood of transmission events in schools (161, 210). Existing out-of-school social networks among students may guide such cohort formation and lead to a more effective reduction of contacts (211). However, whether these predictions translate into measurable effects on secondary cases in schools remains to be tested. At the population level, no difference in the incidence rates between hybrid and full in-person learning were apparent in Michigan (126). Therefore, limiting the educational benefit of full in-person instruction by implementing hybrid models should be weighed carefully against mitigating measures such as mask wearing, maximizing distance between individuals and room ventilation / air filtration. Since reduction of in-school time may disadvantage younger students disproportionately, this mitigation tool should be reserved primarily for older middle and high school students.
Societies have struggled worldwide to balance the obligation to provide quality education with minimizing the infection risk. However, the role of the broader community in preventing infections in schools has been a largely neglected consideration. To protect the school communities, there must be a collective acceptance for continuing general public health measures such as wearing masks in dense social settings, limiting contacts, activities with elevated risks of viral spread, and to avail of vaccination and testing. While the risk of severe illness and death related to COVID-19 are low in school-aged children, it is not negligible, and marked uncertainties regarding the long-term sequelae exist. The role of school in community spread and the negative impact of disruptions to in-person schooling by quarantine measures remain strong arguments to focus on protecting schools. Children deserve safe access to education. To achieve this, schools need a dynamic mitigation plan (Figure 1), community investment in public health measures, and the systematic collection of data to assess which strategies are most effective.
RL and TG: conceptualization, visualization, investigation, writing—original draft, and writing—review & editing. SP: conceptualization, investigation, writing—original draft, and writing—review & editing. EH, AN, SG, GP, CS, KB, TH, SJ, LM, ST, AG, SA, HM, S-YT, AW, LB, ER, PC, AM, and GG: investigation and writing—original draft. KP and NM: writing and reviewing & editing. MW: investigation and writing—review & editing. SF: conceptualization, investigation, and writing—review & editing. GF: conceptualization, writing—review & editing, and funding acquisition. All authors contributed to the article and approved the submitted version.
This work was supported by a Grant (UL1TR001878) from the National Center for Advancing Translational Science. Dr. Bottalico was supported by National Institutes of Health Grants K12GM081259 University of Pennsylvania Postdoctoral Opportunities in Research and Teaching and T32ES01985 Translational Research Training Program in Environmental Health Sciences. Dr. Skarke is the McNeill Fellow of Translational Medicine and Therapeutics. Dr. FitzGerald is the McNeil Professor of Translational Medicine and Therapeutics.
LA serves on the medical advisory board of the MLD Foundation, CureMLD and “Don't Forget Morgan”. She is a consultant for Orchard Therapeutics, Biogen, and Takeda. She receives compensation and/or research funding for these roles. Additionally, she serves as an uncompensated member of the Board of Trustees and the scientific COVID-19 advisory committee of the Waldorf School of Philadelphia. GF is a chief scientific advisor for the journal Science Translational Medicine and is a senior advisor to Calico Laboratories and receives compensation for both roles. TG serves as an editor for the journal Circulation Genomic and Precision and receives compensation from the American Heart Association for this work. He was an uncompensated member of the Board of Trustees of the Waldorf School of Philadelphia and serves on the school's scientific COVID-19 advisory committee without compensation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Brauner JM, Mindermann S, Sharma M, Johnston D, Salvatier J, Gavenčiak T, et al. Inferring the effectiveness of government interventions against COVID-19. Science. (2021) 371: Eabd9338. doi: 10.1126/science.abd9338
2. Gurdasani D, Alwan NA, Greenhalgh T, Hyde Z, Johnson L, McKee M, et al. School reopening without robust COVID-19 mitigation risks accelerating the pandemic. Lancet. (2021) 397:1177–8. doi: 10.1016/S0140-6736(21)00622-X
3. Llupià A, Borràs-Santos A, Guinovart C, Utzet M, Moriña D, Puig J SARS-CoV-2 SARS-CoV-2 transmission in students of public schools of catalonia (Spain) after a month of reopening. PLoS ONE. (2021) 16:E0251593. doi: 10.1371/journal.pone.0251593
4. Torjesen I COVID-19: Delta variant is now UK's most dominant strain and spreading through schools. BMJ. (2021) 373:N1445. doi: 10.1136/bmj.n1445
5. Veldhoen M, Simas JP. Endemic SARS-CoV-2 will maintain post-pandemic immunity. Nat. Rev. Immunol. (2021) 21:131–2. doi: 10.1038/s41577-020-00493-9
6. Murray CJL, Piot P. The potential future of the COVID-19 pandemic: Will SARS-CoV-2 become a recurrent seasonal infection? JAMA. (2021) 325:1249–50. doi: 10.1001/jama.2021.2828
7. Basta NE, Chao DL, Halloran ME, Matrajt L, Longini IM Jr. Strategies for pandemic seasonal influenza vaccination of schoolchildren in the United States. Am J Epidemiol. (2009) 170: 679–86. doi: 10.1093/aje/kwp237
8. Self WH, Tenforde MW, Rhoads JP, Gaglani M, Ginde AA, Douin DJ, et al. Comparative effectiveness of Moderna, Pfizer-BioNtech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions — United States March–August 2021. MMWR Morb Mortal Wkly Rep. (2021) 70:1337–43. doi: 10.15585/mmwr.mm7038e1
9. Thomas SJ, Moreira ED, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med. (2021) 385:1761–73. doi: 10.1056/NEJMoa2110345
10. Tenforde MW, Self WH, Naioti EA, Ginde AA, Douin DJ, Olson SM, et al. Sustained effectiveness of Pfizer-BioNtech and Moderna vaccines against COVID-19 associated hospitalizations among adults—United States March–July 2021. Morbid Mortal Week Rep. (2021) 70:1156. doi: 10.15585/mmwr.mm7034e2
11. Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. NE J Med. (2021) 385:585–594. doi: 10.1056/NEJMoa2108891
12. Olson SM, Newhams MM, Halasa NB, Price AM, Boom JA, Sahni LC, et al. Effectiveness of Pfizer-BioNtech mRNA vaccination against COVID-19 hospitalization among persons aged 12–18 Years—United States June–September 2021. MMWR. Morbid Mortal Week Rep. (2021) 70:. doi: 10.15585/mmwr.mm7003e1
13. Reis BY, Barda N, Leshchinsky M, Kepten E, Hernán MA, Lipsitch M, et al. Effectiveness of BNT162b2 vaccine against Delta variant in adolescents. N Engl J Med. 10.1056/NEJMc2114290. (2021) doi: 10.1056/NEJMc2114290
14. Delahoy MJ, Ujamaa D, Whitaker M, O'Halloran A, Anglin O, Burns E, et al. Hospitalizations associated With COVID-19 among children and adolescents—COVID-NET, 14 States March 1, 2020–August 14, 2021. Morbid Mortal Week Rep. (2021) 70:1255. doi: 10.15585/mmwr.mm7036e2
15. Viner RM, Mytton OT, Bonell C, Melendez-Torres GJ, Ward J, Hudson L, et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: A systematic review and meta-analysis. JAMA Pediatr. (2021) 175:143–56. doi: 10.1001/jamapediatrics.2020.4573
16. Auger KA, Shah SS, Richardson T, Hartley D, Hall M, Warniment A, et al. Association between statewide school closure and COVID-19 incidence and mortality in the US. JAMA. (2020) 324:859–70. doi: 10.1001/jama.2020.14348
17. Hippich M, Holthaus L, Assfalg R, Zapardiel-Gonzalo J, Kapfelsperger H, Heigermoser M, et al. A public health antibody screening indicates a 6-fold higher SARS-CoV-2 exposure rate than reported cases in children. Med. (2020) 10:3. doi: 10.1016/j.medj.2020.10.003
18. Spielberger BD, Goerne T, Geweniger A, Henneke P, Elling R. Intra-household and close-contact SARS-CoV-2 transmission among children - A systematic review. Front Pediatr. (2021) 9:613292. doi: 10.3389/fped.2021.613292
19. Meng B, Kemp SA, Papa G, Datir R, Ferreira IATM, Marelli S, et al. Recurrent Emergence of SARS-CoV-2 spike deletion H69/V70 and its role in the Alpha variant B.1.1.7. Cell Rep. (2021) 35:109292.
20. Li W, Zhang B, Lu J, Liu S, Chang Z, Peng C, et al. Characteristics of household transmission of COVID-19. Clinical Infectious Diseases 10.1093/cid/Ciaa450. (2020) doi: 10.1093/cid/ciaa450
21. Mizumoto K, Omori R, Nishiura H. Age specificity of cases and attack rate of novel coronavirus disease (COVID-19). MedRxiv. (2020) doi: 10.1101/2020.03.09.20033142
22. Jing Q-L, Liu M-J, Zhang Z-B, Fang L-Q, Yuan J, Zhang A-R, et al. Household Secondary Attack Rate of COVID-19 and Associated Determinants in Guangzhou China: a Retrospective Cohort study. Lancet Infect Dis. (2020) 20:1141–1150. doi: 10.1016/S1473-3099(20)30471-0
23. Dattner I, Goldberg Y, Katriel G, Yaari R, Gal N, Miron Y, et al. The role of children in the spread of COVID-19: Using household data from Bnei Brak Israel, to estimate the relative susceptibility and infectivity of children. PLoS Comput Biol. (2021) 17:E1008559. doi: 10.1371/journal.pcbi.1008559
24. Davies NG, Klepac P, Liu Y, Prem K, Jit M, Pearson CAB, et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat. Med. (2020) 26:1205–211. doi: 10.1038/s41591-020-0962-9
25. Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, et al. COVID-19 in children and adolescents in Europe: A multinational, multicentre cohort study. Lancet Child Adolesc Health. (2020) 4:653–61. doi: 10.1016/S2352-4642(20)30177-2
26. Hyde Z. COVID-19, children schools: Overlooked at risk. Med J Aust. (2020) 213: 444–446. doi: 10.5694/mja2.50823
27. Irfan O, Li J, Tang K, Wang Z, Bhutta ZA. Risk of infection and transmission of SARS-CoV-2 among children and adolescents in households, communities and educational settings: A systematic review and meta-analysis. J Glob Health. (2021) 11:05013–05013. doi: 10.7189/jogh.11.05013
28. Willeit P, Krause R, Lamprecht B, Berghold A, Hanson B, Stelzl E, et al. Prevalence of RT-qPCR-detected SARS-CoV-2 infection at schools: First results from the Austrian School-SARS-CoV-2 prospective cohort study. Lancet Regional Health Eur. (2021) 5:100086. doi: 10.1016/j.lanepe.2021.100086
29. Jones TC, Biele G, Mühlemann B, Veith T, Schneider J, Beheim-Schwarzbach J, et al. Estimating infectiousness throughout SARS-CoV-2 infection course. Science. (2021) 21:523. doi: 10.1126/science.abi5273
30. Laxminarayan R, Wahl B, Dudala SR, Gopal K, Mohan BC, Neelima S, et al. Epidemiology and transmission dynamics of COVID-19 in two Indian states. Science. (2020) 370:691–697. doi: 10.1126/science.abd7672
31. Riley S, Walters CE, Wang H, Eales O, Ainslie KEC, Atchison C, et al. REACT-1 round 7 updated report: Regional heterogeneity in changes in prevalence of SARS-CoV-2 infection during the second national COVID-19 lockdown in England. MedRxiv 10.1101/2020.12.15.20248244. (2020) 2020.12.15.20248244. doi: 10.1101/2020.12.15.20248244
32. Elliott P, Haw D, Wang H, Eales O, Walters CE, Ainslie KEC, et al. Exponential growth, high prevalence of SARS-CoV-2, and vaccine effectiveness associated with the Delta variant. Science. (2021) Eabl9551. doi: 10.1126/science.abl9551
33. Siegel DA, Reses HE, Cool AJ, Shapiro CN, Hsu J, Boehmer TK, et al. Trends in COVID-19 cases emergency department visits, and hospital admissions among children and adolescents aged 0-17 years - United States August 2020-August 2021. MMWR Morb Mortal Wkly Rep. (2021) 70:1249–54. doi: 10.15585/mmwr.mm7036e1
34. American American Academy of Pediatrics Children's Hospital Association Children COVID-19: State Data, Report,. A Joint Report From the American Academy of Pediatrics the Children's Hospital Association - Summary of Publicly Reported Data From 49 States NYC. DC PR, and HU (2021). Available online at: https://www.aap.org/en/pages/2019-novel-coronavirus-COVID-19-infections/children-and-COVID-19-state-level-data-report/ (accessed November 1, 2021).
35. Park YJ, Choe YJ, Park O, Park SY, Kim Y-M, Kim J, et al. Contact tracing during coronavirus disease outbreak South Korea, 2020. Emerg Infect Dis J. (2020) 26:2465. doi: 10.3201/eid2610.201315
36. Byambasuren O, Cardona M, Bell K, Clark J, McLaws M-L, Glasziou P, et al. Shedding of viable virus in asymptomatic SARS-CoV-2 carriers. Off. J Assoc Med. Microbiol Infect Dis Can. (2020) 6:19–21. doi: 10.3138/jammi-2020-0030
37. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernán MA, Pérez-Olmeda M, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): A nationwide, population-based seroepidemiological study. Lancet. (2020) 396:535–44. doi: 10.1016/S0140-6736(20)31483-5
38. Fontanet A, Tondeur L, Grant R, Temmam S, Madec Y, Bigot T, et al. SARS-CoV-2 infection in schools in a Northern French city: A retrospective serological cohort study in an area of high transmission france January to April 2020. Euro Surveill. (2021) 26:1695. doi: 10.2807/1560-7917.ES.2021.26.15.2001695
39. Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS-CoV-2 infection in Children. N Engl J Med. (2020) 382:1663–5. doi: 10.1056/NEJMc2005073
40. Parri N, Lenge M, Buonsenso D. Children with COVID-19 in pediatric emergency departments in Italy. N Engl J Med. (2020) 383:187–90. doi: 10.1056/NEJMc2007617
41. Rando HM, MacLean AL, Lee AJ, Lordan R, Ray S, Bansal V, et al. Pathogenesis symptomatology, and transmission of SARS-CoV-2 through analysis of viral genomics and structure. MSystems. (2021) 6:E0009521. doi: 10.1128/mSystems.00095-21
42. Liguoro I, Pilotto C, Bonanni M, Ferrari ME, Pusiol A, Nocerino A, et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr. (2020) 179:1029–1046. doi: 10.1007/s00431-020-03684-7
43. Zimmermann P, Curtis N. Why Is COVID-19 less severe in children? a review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Archiv Dis Childhood. (2021) 106:429–49. doi: 10.1136/archdischild-2020-320338
44. Ludvigsson JF Systematic Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. (2020) 109:1088–95. doi: 10.1111/apa.15270
45. Shekerdemian LS, Mahmood NR, Wolfe KK, Riggs BJ, Ross CE, McKiernan CA, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatrics. (2020) 174:868–73. doi: 10.1001/jamapediatrics.2020.1948
46. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA. (2020) 323:1239–42. doi: 10.1001/jama.2020.2648
47. Loske J, Röhmel J, Lukassen S, Stricker S, Magalhães VG, Liebig J, et al. Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children. Nat. Biotechnol. (2021) 1037:9. doi: 10.1038/s41587-021-01037-9
48. Pierce CA, Sy S, Galen B, Goldstein DY, Orner E, Keller MJ, et al. Natural mucosal barriers and COVID-19 in children. JCI Insight. (2021) 6:21. doi: 10.1172/jci.insight.148694
49. Pierce CA, Preston-Hurlburt P, Dai Y, Aschner CB, Cheshenko N, Galen B, et al. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci Translat Med. (2020) 12:Eabd5487. doi: 10.1126/scitranslmed.abd5487
50. Rando HM, Bennett TD, Byrd JB, Bramante C, Callahan TJ, Chute CG, et al. Challenges in defining long COVID: Striking differences across literature electronic health records, and patient-reported information. MedRxiv. (2021) doi: 10.1101/2021.03.20.21253896
51. Brackel CLH, Lap CR, Buddingh EP, van Houten MA, van der Sande LJTM, Langereis EJ, et al. Pediatric long-COVID: an overlooked phenomenon? Pediatr. Pulmonol. (2021) 56:2495–2502. doi: 10.1002/ppul.25521
52. Say D, Crawford N, McNab S, Wurzel D, Steer A, Tosif S. Post-Acute COVID-19 outcomes in children with mild and asymptomatic disease. Lancet Child Adolesc Health. (2021) 5:e22–E23. doi: 10.1016/S2352-4642(21)00124-3
53. Poline J, Gaschignard J, Leblanc C, Madhi F, Foucaud E, Nattes E, et al. Systematic severe acute respiratory syndrome coronavirus 2 screening at hospital admission in children: A french prospective multicenter study. Clin. Infect. Dis. (2020) 10:44. doi: 10.1093/cid/ciaa1044
54. Office for National Statistics. Updated Estimates of the Prevalence of Long COVID Symptoms, United Kingdom (2021). Available online at: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/adhocs/12788updatedestima tesoftheprevalenceoflongcovidsymptoms (accessed November 4, 2021).
55. Buonsenso D, Munblit D, De Rose C, Sinatti D, Ricchiuto A, Carfi A, et al. Preliminary evidence on long COVID in children. Acta Paediatr. (2021) 110:2208–11. doi: 10.1111/apa.15870
56. Ayoubkhani D, Gaughan C, Jenkins J. Update on Long COVID Prevalence Estimate (2021). Available online at: overnment/uploads/system/uploads/attachment_data/file/962830/s1079-ons-update-on-long-covid-prevalence-estimate.pdf (accessed on December 2, 2021).
57. Roessler M, Tesch F, Batram M, Jacob J, Loser F, Weidinger O, et al. Post COVID-19 in children, adolescents, adults: results of a matched cohort study including more than 150,000 individuals with COVID-19. MedRxiv. (2021) doi: 10.1101/2021.10.21.21265133
58. Osmanov IM, Spiridonova E, Bobkova P, Gamirova A, Shikhaleva A, Andreeva M, et al. Risk factors for long covid in previously hospitalized Children Using the ISARIC global follow-up protocol: A prospective cohort study. Euro Respirat J. (2021) 210:1341. doi: 10.1183/13993003.01341-2021
59. Castagnoli R, Votto M, Licari A, Brambilla I, Bruno R, Perlini S, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: A systematic review. JAMA Pediatrics. (2020) 174:882–9. doi: 10.1001/jamapediatrics.2020.1467
60. Rimensberger PC, Kneyber MCJ, Deep A, Bansal M, Hoskote A, Javouhey E, et al. Caring for critically ill children with suspected or proven coronavirus disease 2019 infection: Recommendations by the Scientific Sections' Collaborative of the European Society of Pediatric and Neonatal Intensive Care*. Pediatr. Crit. Care Med. (2021) 1:22:. doi: 10.1097/PCC.0000000000002599
61. Centers for Disease Control Prevention, . COVID-19 Laboratory-Confirmed Hospitalizations. Available online at: https://gis.cdc.gov/grasp/covidnet/COVID19_5.html (accessed November 23, 2021).
62. American Academy of Pediatrics Children COVID-19. State -Level Data Report (2021). Available online at: https://Services.aap.org/en/Pages/2019-Novel-Coronavirus-Covid-19-Infections/Children-and-Covid-19-State-Level-Data-Report/ (accessed November 1, 2021).
63. Kompaniyets L, Agathis NT, Nelson JM, Preston LE, Ko JY, Belay B, et al. Underlying medical conditions associated with severe COVID-19 illness among children. JAMA Netw Open. (2021) 4:E2111182–E2111182. doi: 10.1001/jamanetworkopen.2021.11182
64. Havers FP, Whitaker M, Self JL, Chai SJ, Kirley PD, Alden NB, et al. Hospitalization of adolescents aged 12–17 years with laboratory-confirmed COVID-19—COVID-NET, 14 States March 1, 2020–April 24, 2021. Morb Mortal Wkly Rep. (2021) 70:21. doi: 10.15585/mmwr.mm7023e1
65. Preston LE, Chevinsky JR, Kompaniyets L, Lavery AM, Kimball A, Boehmer TK, et al. Characteristics and disease severity of US children and adolescents diagnosed with COVID-19. JAMA Netw Open. (2021) 4:E215298–E215298. doi: 10.1001/jamanetworkopen.2021.5298
66. Robert Koch. Institut Epidemiologisches Bulletin (2021). Available online at: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2021/Ausgaben/23_21.pdf?__Blob=PublicationFile (accessed June 15, 2021).
67. Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. (2020) 383:334–46. doi: 10.1056/NEJMoa2021680
68. Belay ED, Abrams J, Oster ME, Giovanni J, Pierce T, Meng L, et al. Trends in geographic and temporal distribution of us children with multisystem inflammatory syndrome during the COVID-19 pandemic. JAMA Pediatric. (2021) 21:630. doi: 10.1001/jamapediatrics.2021.0630
69. Feldstein LR, Tenforde MW, Friedman KG, Newhams M, Rose EB, Dapul H, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. (2021) 325:1074–87. doi: 10.1001/jama.2021.2091
70. Penner J, Abdel-Mannan O, Grant K, Maillard S, Kucera F, Hassell J, et al. 6-Month multidisciplinary follow-up and outcomes of patients with pediatric inflammatory multisystem syndrome (PIMS-TS) at a uk tertiary pediatric hospital: a retrospective cohort study. Lancet Child Adolescent Health 10.1016/S2352-4642(21)00138-3 (2021). doi: 10.1016/S2352-4642(21)00138-3
71. Flood J, Shingleton J, Bennett E, Walker B, Amin-Chowdhury Z, Oligbu G, et al. Pediatric Multisystem inflammatory syndrome temporally associated with SARS-CoV-2 (PIMS-TS): Prospective, national surveillance United Kingdom and Ireland, 2020. Lancet Reg Health Eur. (2021) 3:100075–100075. doi: 10.1016/j.lanepe.2021.100075
72. Augustin M, Schommers P, Stecher M, Dewald F, Gieselmann L, Gruell H, et al. Post-COVID Syndrome in non-hospitalized patients with COVID-19: a longitudinal prospective cohort study. The Lancet Regional Health—Europe. (2021) 6:. doi: 10.1016/j.lanepe.2021.100122
73. Rando HM, Wellhausen N, Ghosh S, Lee AJ, Dattoli AA, Hu F, et al. Identification and development of therapeutics for COVID-19. MSystems. (2021) 0:E00233–21. doi: 10.1128/mSystems.00233-21
74. Jones J. Centers for Disease Control Prevention Epidemiology of COVID-19 in Children Aged 5-11 Years (2021). Available online at: https://www.cdc.gov/Vaccines/Acip/Meetings/Downloads/Slides-2021-11-2-3/03-COVID-Jefferson-508.pdf (accessed November 8, 2021).
75. Centers for Disease Control Prevention COVID Data Tracker. Health Department-Reported Cases of Multisystem Inflammatory Syndrome in Children (MIS-C) in the United States (2021). Available online at: https://Covid.cdc.gov/Covid-Data-Tracker/#mis-National-Surveillance (accessed October 29, 2021).
76. Payne AB, Gilani Z, Godfred-Cato S, Belay ED, Feldstein LR, Patel MM, et al. Incidence of multisystem inflammatory syndrome in children among us persons infected with SARS-CoV-2. JAMA Network Open. (2021) 4:E2116420. doi: 10.1001/jamanetworkopen.2021.16420
77. Wong JY, Wu P, Nishiura H, Goldstein E, Lau EHY, Yang L, et al. infection fatality risk of the pandemic A(H1N1)2009 virus in Hong Kong. Am J Epidemiol. (2013) 177:834–40. doi: 10.1093/aje/kws314
78. Hadler JL, Konty K, McVeigh KH, Fine A, Eisenhower D, Kerker B, et al. Case fatality rates based on population estimates of influenza-like illness due to novel H1N1 Influenza: New York City May-June 2009. PLoS ONE. (2010) 5:E11677. doi: 10.1371/journal.pone.0011677
79. Atkinson WL, Orenstein WA, Krugman S. The Resurgence of Measles in the United States, 1989-1990. Annu Rev Med. (1992) 43:451–63. doi: 10.1146/annurev.me.43.020192.002315
80. O'Driscoll M, Ribeiro Dos Santos G, Wang L, Cummings DAT, Azman AS, Paireau J, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. (2021) 590:140–5. doi: 10.1038/s41586-020-2918-0
81. Levin AT, Hanage WP, Owusu-Boaitey N, Cochran KB, Walsh SP, Meyerowitz-Katz G. Assessing the age specificity of infection fatality Rates for COVID-19: Systematic review, meta-analysis, and public policy implications. Euro J Epidemiol. (2020) 35:1123–38. doi: 10.1007/s10654-020-00698-1
82. Ortaliza J, Orgera K, Krutika A, Cox C. Peterson-KFF Health System Tracker: COVID-19 Continues to be a Leading Cause of Death in the U.S. in September 2021 (2021). Available online at: https://www.Healthsystemtracker.org/Brief/Covid19-and-Other-Leading-Causes-of-Death-in-the-Us/ (accessed November 4, 2021).
83. Bhopal SS, Bagaria J, Olabi B, Bhopal R. Children and young people remain at low risk of COVID-19 mortality. Lancet Child Adolesc Health. (2021) 5:e12–E13. doi: 10.1016/S2352-4642(21)00066-3
84. Smith C, Odd D, Harwood R, Ward J, Linney M, Clark M, et al. Deaths in children and young people in england following SARS-CoV-2 infection during the first pandemic year: A national study using linked mandatory child death reporting data. MedRxiv. (2021) doi: 10.1101/2021.07.07.21259779
85. Khera N, Santesmasses D, Kerepesi C, Gladyshev VN. COVID-19 mortality rate in children is U-shaped. Aging. (2021) 13:19954–62. doi: 10.18632/aging.203442
86. Shang M, Blanton L, Brammer L, Olsen SJ, Fry AM. Influenza-associated pediatric deaths in the United States, 2010–2016. Pediatrics. (2018) 141:E20172918. doi: 10.1542/peds.2017-2918
87. Centers, for Disease Control Prevention Weekly U,.S. Influenza Surveillance Report, 2021, . Available online at: https://www.cdc.gov/flu/weekly/index.htm (accessed December 2, 2021).
88. Asanov I, Flores F, McKenzie D, Mensmann M, Schulte M. Remote-learning, time-use, and mental health of ecuadorian high-school students during the COVID-19 quarantine. World Develop. (2021) 138:105225. doi: 10.1016/j.worlddev.2020.105225
89. Li W, Zhang Y, Wang J, Ozaki A, Wang Q, Chen Y, et al. Association of home quarantine and mental health among teenagers in Wuhan China during the COVID-19 pandemic. JAMA Pediatrics. (2021) 175:313–316. doi: 10.1001/jamapediatrics.2020.5499
90. Verlenden JV, Pampati S, Rasberry C, Liddon N, Hertz M, Kilmer G, et al. Association of children's mode of school instruction with child and parent experiences and well-being during the COVID-19 pandemic - COVID experiences survey United States October 8-November 13, 2020. Morb Mortal Wkly Rep. (2021) 70:369–76. doi: 10.15585/mmwr.mm7011a1
91. Van Lancker W, Parolin Z. COVID-19, school closures, and child poverty: a social crisis in the making. Lancet Public Health. (2020) 5:E243–4. doi: 10.1016/S2468-2667(20)30084-0
92. Tang S, Xiang M, Cheung T, Xiang Y-T. Mental health and its correlates among children and adolescents during COVID-19 school closure: The importance of parent-child discussion. J Affect Disorder. (2021) 279:353–60. doi: 10.1016/j.jad.2020.10.016
93. Baron EJ, Goldstein EG, Wallace CT. Suffering in silence: How COVID-19 school closures inhibit the reporting of child maltreatment. J Public Econ. (2020) 190:104258. doi: 10.1016/j.jpubeco.2020.104258
94. Davis CR, Grooms J, Ortega A, Rubalcaba JA-A, Vargas E. Distance learning and parental mental health during COVID-19. Education Res. (2021) 50:61–64. doi: 10.3102/0013189X20978806
95. Phelps C, Sperry LL. Children and the COVID-19 pandemic. Psychol Trauma:Theory Res Pract Policy. (2020) 12:S73–S75. doi: 10.1037/tra0000861
96. Engzell P, Frey A, Verhagen MD. Learning loss due to school closures during the COVID-19 pandemic. Proceed Nat Acad Sci. (2021) 118:E2022376118. doi: 10.1073/pnas.2022376118
97. Kuhfeld M, Soland J, Tarasawa B, Johnson A, Ruzek E, Liu J. Projecting the potential impact of COVID-19 school closures on academic achievement. Educat Res. (2020) 49:549–565. doi: 10.3102/0013189X20965918
98. Dorn E, Hancock B, Sarakatsannis J, Viruleg E. COVID-19 Learning Loss - Disparities Grow Students Need Help (2020). Available online at: https://www.Mckinsey.com/Industries/Public-and-Social-Sector/Our-Insights/Covid-19-and-Learning-Loss-Disparities-Grow-and-Students-Need-Help?utm_Medium=Email&_Hsmi=102339642&_Hsenc=P2ANqtz-__YLVm-YZgDiCNzWECEnsdW3rZ72QX4sMT5AJSKx_OqSGZ9gp74AFa6oqjPVlfcPWY_QB5FRc5KOG27abiuQiMReSwUQ&utm_Content=102339642&utm_Source=hs_Email# (accessed June 14, 2021).
99. Woolf N. The Impact of the COVID-19 Pandemic on Student Learning Social-Emotional Development (2021). Available online at: https://Insidesel.com/2020/11/19/the-Impact-of-the-Covid-19-Pandemic-on-Student-Learning-and-Social-Emotional-Development/ (accessed June 14, 2021).
100. Santibañez L, Guarino CM. The effects of absenteeism on academic and social-emotional outcomes: lessons for COVID-19. Educat Res. (2021) 0013189X21994488. doi: 10.3102/0013189X21994488
101. Pizarro-Ruiz JP, Ordóñez-Camblor N. Effects of COVID-19 confinement on the mental health of children and adolescents in Spain. Scientific Rep. (2021) 11:11713. doi: 10.1038/s41598-021-91299-9
102. Balram A. How Online Learning can Affect Student Health (2020). Available online at: https://www.Jhunewsletter.com/Article/2020/04/how-Online-Learning-Can-Affect-Student-Health (accessed June 14, 2021).
103. Yard E, Radhakrishnan L, Ballesteros MF, Sheppard M, Gates A, Stein Z, et al. Emergency department visits for suspected suicide attempts among persons aged 12–25 years before and during the COVID-19 pandemic — United States January 2019–May 2021. Morb Mortal Wkly Rep. (2021) 21:704. doi: 10.15585/mmwr.mm7024e1
104. Buonsenso D, Roland D, De Rose C, Vásquez-Hoyos P, Ramly B, Chakakala-Chaziya JN, et al. Schools closures during the COVID-19 pandemic: a catastrophic global situation. Pediatric Infect Dis J. (2021) 40:3052. doi: 10.1097/INF.0000000000003052
105. Cauchemez S, Ferguson NM, Wachtel C, Tegnell A, Saour G, Duncan B, et al. Closure of schools during an influenza pandemic. Lancet Infect Dis. (2009) 9:473–81. doi: 10.1016/S1473-3099(09)70176-8
106. UNESCO, . 1.3 Billion Learners Are Still Affected by School or University Closures, as Educational Institutions Start Reopening Around the World, Says UNESCO (2020). Available online at: https://en.Unesco.org/News/13-Billion-Learners-Are-Still-Affected-School-University-Closures-Educational-Institutions (accessed June 16, 2021).
107. Li Y, Campbell H, Kulkarni D, Harpur A, Nundy M, Wang X, et al. The temporal association of introducing and lifting non-pharmaceutical interventions with the time-varying reproduction number (r) of SARS-CoV-2: a modeling study across 131 countries. Lancet Infect Dis. (2021) 21:193–202. doi: 10.1016/S1473-3099(20)30785-4
108. Stein-Zamir C, Abramson N, Shoob H, Libal E, Bitan M, Cardash T, et al. A large COVID-19 outbreak in a high school 10 days after schools' reopening israel May 2020. Eurosurveillance. (2020) 25:2001352. doi: 10.2807/1560-7917.ES.2020.25.29.2001352
109. Otte im Kampe E, Lehfeld A-S Buda S, Buchholz U, Haas W. Surveillance of COVID-19 school outbreaks Germany March to August 2020. Eurosurveillance. (2020) 25:2001645. doi: 10.2807/1560-7917.ES.2020.25.38.2001645
110. Gold JA. Clusters of SARS-CoV-2 infection among elementary school educators and students in one school district—Georgia December 2020–January 2021. Morb Mortal Wkly Rep. (2021) 70:e4. doi: 10.15585/mmwr.mm7008e4
111. Torres JP, Piñera C, De La Maza V, Lagomarcino AJ, Simian D, Torres B, et al. Severe acute respiratory syndrome coronavirus 2 antibody prevalence in blood in a large school community subject to a coronavirus disease 2019 outbreak: A cross-sectional study. Clin Infect Dis. (2021) 73:E458–E465. doi: 10.1093/cid/ciaa955
112. Lam-Hine T, McCurdy SA, Santora L, Duncan L, Corbett-Detig R, Kapusinszky B, et al. Outbreak associated With SARS-CoV-2 B. 1.617. 2 (Delta) variant in an elementary school—Marin County California May–June 2021. Morbid Mortal Week Rep. (2021) 70:1214. doi: 10.15585/mmwr.mm7035e2
113. Vlachos J, Hertegård EB, Svaleryd H. the effects of school closures on SARS-CoV-2 among parents and teachers. Proceed Nat Acad Sci. (2021) 118:E2020834118. doi: 10.1073/pnas.2020834118
114. Ismail SA, Saliba V, Lopez Bernal J, Ramsay ME, Ladhani SN. SARS-CoV-2 infection and transmission in educational settings: a prospective, cross-sectional analysis of infection clusters and outbreaks in England. Lancet Infect Dis. (2021) 21:344–353. doi: 10.1016/S1473-3099(20)30882-3
115. European Center for Disease Control COVID-19 in Children and the Role of School Settings in COVID-19 Transmission. Stockholm Sweden (2020). Available online at: https://www.ecdc.europa.eu/en/publications-data/children-and-school-settings-covid-19-transmission (accessed December 2, 2021).
116. Macartney K, Quinn HE, Pillsbury AJ, Koirala A, Deng L, Winkler N, et al. Transmission of SARS-CoV-2 in Australian educational settings: a prospective cohort study. Lancet Child AdolescHealth. (2020) 4:807–16. doi: 10.1016/S2352-4642(20)30251-0
117. Lewis T. Schools Can Open Safely During COVID, the Latest Evidence Shows (2021). Available online at: https://www.Scientificamerican.com/Article/Schools-Can-Open-Safely-During-Covid-the-Latest-Evidence-Shows/b (accessed June 14, 2021).
118. Heavey L, Casey G, Kelly C, Kelly D, McDarby G. No evidence of secondary transmission of COVID-19 from children attending school in Ireland, 2020. Euro Surveill. (2020) 25:903. doi: 10.2807/1560-7917.ES.2020.25.21.2000903
119. Yung CF, Kam K-Q, Nadua KD, Chong CY, Tan NWH, Li J, et al. Novel Coronavirus 2019 Transmission Risk in Educational Settings. Clin. Infect. Dis. 10.1093/cid/Ciaa794. (2020) doi: 10.1093/cid/ciaa794
120. Brandal LT, Ofitserova TS, Meijerink H, Rykkvin R, Lund HM, Hungnes O, et al. Minimal transmission of SARS-CoV-2 from pediatric COVID-19 cases in primary schools, Norway, August to November 2020. Euro Surveill. (2021) 26:2002011. doi: 10.2807/1560-7917.ES.2020.26.1.2002011
121. Buonsenso D, De Rose C, Moroni R, Valentini P. SARS-CoV-2 infections in italian schools: preliminary findings after 1 month of school opening during the second wave of the pandemic. Front Pediatrics. (2021) 8:894. doi: 10.3389/fped.2020.615894
122. Russell F, Ryan KE, Snow K, Danchin M, Mulholland K, Goldfeld S. COVID-19 in Victorian Schools: An Analysis of Child-Care School Outbreak Data Eviidence-Based Recommendations for Opening Schools Keeping Them Open Murdoch Children's Research Institute the University of Melbourne Melbourne Australia (2020). Available online at: https://www.dhhs.vic.gov.au/sites/default/files/documents/202009/Report-summary-COVID-19-in-victorian-schools-pdf.pdf (accessed December 2, 2021).
123. Milman O, Yelin I, Aharony N, Katz R, Herzel E, Ben-Tov A, et al. Community-level evidence for SARS-CoV-2 vaccine protection of unvaccinated individuals. Nat. Med. (2021) 7:5. doi: 10.1038/s41591-021-01407-5
124. Isphording IE, Lipfert M, Pestel N. Does re-opening schools contribute to the spread of SARS-CoV-2? Evidence from staggered summer breaks in Germany. J Public Econ. (2021) 198:104426. doi: 10.1016/j.jpubeco.2021.104426
125. von Bismarck-Osten C, Borusyak K, SchÃNberg U. The Role of Schools in Transmission of the SARS-CoV-2 Virus: Quasi-Experimental Evidence From Germany - CReAM Discussion Paper Series 2022 (2020). Available online at: https://Ideas.Repec.org/p/crm/Wpaper/2022.Html (accessed June 16, 2021).
126. Goldhaber D, Imberman SA, Strunk KO, Hopkins B, Brown N, Harbatkin N, et al. To what extent does in-person schooling contribute to the spread of COVID-19? Evidence From Michigan and Washington. National Bureau Economic Res. (2021) 28455:74. Available online at: https://caldercenter.org/sites/default/files/CALDER%20WP%20247-1220-3.pdf
127. Mossong J, Mombaerts L, Veiber L, Pastore J, Coroller GL, Schnell M, et al. SARS-CoV-2 Transmission in educational settings during an early summer epidemic wave in Luxembourg, 2020. BMC Infect Dis. (2021) 21:417. doi: 10.2139/ssrn.3696896
128. Battisti M, Kourtellos A, Maggio G. School Openings Affect Local COVID-19 Diffusion. (2021). Available online at: https://voxeu.org/article/school-openings-affect-local-covid-19-diffusion (accessed December 2, 2021).
129. Harris DN. The Effects of School Reopenings on COVID-19 Hospitalizations (2021). Available online at: https://www.Reachcentered.org/Publications/the-Effects-of-School-Reopenings-on-Covid-19-Hospitalizations (accessed June 14, 2021).
130. Chernozhukov V, Kasahara H, Schrimpf P. The association of opening K−12 schools with the spread of COVID-19 in the United states: County-level panel data analysis. Proceed Nat Acad Sci. (2021) 118:E2103420118. doi: 10.1073/pnas.2103420118
131. Soriano-Arandes A, Gatell A, Serrano P, Biosca M, Campillo F, Capdevila R, et al. Household severe acute respiratory syndrome coronavirus 2 transmission and children: A network prospective study. Clin Infect. Dis. (2021) 73:E1261–9. doi: 10.1093/cid/ciab228
132. Buonsenso D, Valentini P. Children and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission: a step closer to better understanding and evidence-based interventions? Clin Infect Dis. (2021) 73:E1270–2. doi: 10.1093/cid/ciab559
133. Gettings J, Czarnik M, Morris E, Haller E, Thompson-Paul AM, Rasberry C, et al. Mask use and ventilation improvements to reduce COVID-19 incidence in elementary schools - Georgia November 16-December 11, 2020. Morb Mortal Wkly Rep. (2021) 70:779–84. doi: 10.15585/mmwr.mm7021e1
134. Vlopp KG, Kraut BH, Ghosh S, Neatherlin J. Minimal SARS-CoV-2 transmission after implementation of a comprehensive mitigation strategy at a school - New Jersey August 20-November 27, 2020. Morb Mortal Wkly Rep. (2021) 70:377–81. doi: 10.15585/mmwr.mm7011a2
135. Hoch M, Vogel S, Kolberg L, Dick E, Fingerle V, Eberle U, et al. Weekly SARS-CoV-2 sentinel surveillance in primary schools kindergartens, and nurseries, Germany June–November 2020. Emerg. Infect. Dis. (2021) 27:2192–6. doi: 10.3201/eid2708.204859
136. Ehrhardt J, Ekinci A, Krehl H, Meincke M, Finci I, Klein J, et al. Transmission of SARS-CoV-2 in children aged 0 to 19 years in childcare facilities and schools after their reopening in may 2020, Baden-Württemberg Germany. Eurosurveillance. (2020) 25:2001587. doi: 10.2807/1560-7917.ES.2020.25.36.2001587
137. Falk A, Benda A, Falk P, Steffen S, Wallace Z, Høeg TB. COVID-19 cases and transmission in 17 K−12 schools — wood county wisconsin august 31–November 29, 2020. MMWR Morb Mortal Wkly Rep. (2021) 31:7004. doi: 10.15585/mmwr.mm7004e3
138. Zimmerman KO, Akinboyo IC, Brookhart MA, Boutzoukas AE, McGann K, Smith MJ, et al. Incidence and secondary transmission of SARS-CoV-2 infections in schools. Pediatrics (2021) 20:E2020048090. doi: 10.1542/peds.2020-048090
139. Goyal A, Reeves DB, Thakkar N, Famulare M, Cardozo-Ojeda EF, Mayer BT, et al. Slight reduction in SARS-CoV-2 exposure viral load due to masking results in a significant reduction in transmission with widespread implementation. Scientific Rep. (2021) 11:11838. doi: 10.1038/s41598-021-91338-5
140. Lessler J, Grabowski MK, Grantz KH, Badillo-Goicoechea E, Metcalf CJE, Lupton-Smith C, et al. Household COVID-19 risk and in-person schooling. Science. (2021) 372:1092–1097. doi: 10.1126/science.abh2939
141. Lordan R, FitzGerald GA, Grosser T. Reopening schools during COVID-19. Science. (2020) 369:1146–1146. doi: 10.1126/science.abe5765
142. Reason J. Human error: Models and management. BMJ. (2000) 320:768–770. doi: 10.1136/bmj.320.7237.768
143. Levine-Tiefenbrun M, Yelin I, Katz R, Herzel E, Golan Z, Schreiber L, et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat. Med. (2021) 27:790–2. doi: 10.1038/s41591-021-01316-7
144. Posfay-Barbe KM, Wagner N, Gauthey M, Moussaoui D, Loevy N, Diana A, et al. COVID-19 in children and the dynamics of infection in families. Pediatrics. (2020) 146:E20201576. doi: 10.1542/peds.2020-1576
145. Theuring S, Thielecke M, van Loon W, Hommes F, Hülso C, von der Haar A, et al. SARS-CoV-2 infection and transmission in school settings during the second COVID-19 wave: a cross-sectional study Berlin Germany November 2020. Eurosurveillance. (2021) 26:2100184. doi: 10.2807/1560-7917.ES.2021.26.34.2100184
146. Wang Z, Zhou Q, Wang C, Shi Q, Lu S, Ma Y, et al. Clinical characteristics of children with COVID-19: a rapid review and meta-analysis. Ann Transl Med. (2020) 8:620–620. doi: 10.21037/atm-20-3302
147. Paltiel AD, Zheng A, Walensky RP. Assessment of SARS-CoV-2 screening strategies to permit the safe reopening of college campuses in the United States. JAMA Network Open. (2020) 3:E2016818. doi: 10.1001/jamanetworkopen.2020.16818
148. Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. (2020) 323:2249–51. doi: 10.1001/jama.2020.8259
149. Mina MJ, Andersen KG. COVID-19 Testing: One size does not fit all. Science. (2021) 371:126–7. doi: 10.1126/science.abe9187
150. Butler-Laporte G, Lawandi A, Schiller I, Yao M, Dendukuri N, McDonald EG, et al. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Intern Med. (2021) 181:353–60. doi: 10.1001/jamainternmed.2020.8876
151. Revollo B, Blanco I, Soler P, Toro J, Izquierdo-Useros N, Puig J, et al. Same-Day SARS-CoV-2 Antigen test screening in an indoor mass-gathering live music event: a randomized controlled trial. Lancet Infect Dis. (2021) 268:1. doi: 10.1016/S1473-3099(21)00268-1
152. Weissleder R, Lee H, Ko J, Pittet MJ. COVID-19 diagnostics in context. Sci Transl Med. (2020) 12:Eabc1931. doi: 10.1126/scitranslmed.abc1931
153. Pekosz A, Parvu V, Li M, Andrews JC, Manabe YC, Kodsi S, et al. Antigen-based testing but not real-time polymerase chain reaction correlates with severe acute respiratory syndrome coronavirus 2 viral culture. Clin Infect Dis. (2021) 10:1706. doi: 10.1093/cid/ciaa1706
154. Dinnes J, Deeks JJ, Berhane S, Taylor M, Adriano A, Davenport C, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. (2021) 3:Cd013705. doi: 10.1002/14651858.CD013705
155. Uwamino Y, Nagata M, Aoki W, Nakagawa T, Inose R, Yokota H, et al. Accuracy of rapid antigen detection test for nasopharyngeal swab specimens and saliva samples in comparison with RT-PCR and viral culture for SARS-CoV-2 detection. J Infect Chemother. (2021) 27:1058–62. doi: 10.1016/j.jiac.2021.04.010
156. Brümmer LE, Katzenschlager S, Gaeddert M, Erdmann C, Schmitz S, Bota M, et al. Accuracy of novel antigen rapid diagnostics for SARS-CoV-2: a living systematic review and meta-analysis. PLoS Med. (2021) 18:E1003735. doi: 10.1371/journal.pmed.1003735
157. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection - challenges and implications. N Engl J Med. (2020) 383:e38. doi: 10.1056/NEJMp2015897
158. Larremore DB, Wilder B, Lester E, Shehata S, Burke JM, Hay JA, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv. (2021) 7:Eabd5393. doi: 10.1126/sciadv.abd5393
159. Bershteyn A, Kim H-Y, McGillen J, Braithwaite RS. Which policies most effectively reduce SARS-CoV-2 transmission in schools? MedRxiv. (2020) doi: 10.1101/2020.11.24.20237305
160. Lanier WA, Babitz KD, Collingqood A, Graul MF, Dickson S, Cunningham L, et al. COVID-19 testing to sustain in-person instruction and extracurricular activities in high schools — Utah November 2020–March 2021. Morb Mortal Wkly Rep. (2021) 70:785–791. doi: 10.15585/mmwr.mm7021e2
161. McGee RS, Homburger JR, Williams HE, Bergstrom CT, Zhou AY. Model-driven mitigation measures for reopening schools during the COVID-19 pandemic. Proceed Nat Acad Sci. (2021) 118:E2108909118. doi: 10.1073/pnas.2108909118
162. Couzin-Frankel Schools Have Begun Mass Testing for COVID-19. But hurdles and uncertainties remain: scientific unknowns, access, and logistics are barriers to routine screening. Science. (2021) 21:9118. doi: 10.1126/Science.Acx9118
163. The New York Times. Germany Makes Rapid Virus Tests a Key to Everyday Freedoms (2021). Available online at: https://www.Nytimes.com/2021/06/09/World/Europe/Germany-Covid-Rapid-Antigen-Tests.Html (accessed October 22, 2021).
164. Irish Examiner,. Children Piloted for Rapid Antigen Test Use (2021). Available online at: https://www.Independent.ie/Irish-News/Children-Piloted-for-Rapid-Antigen-Test-Use-40979053.Html (accessed October 22, 2021).
165. NSW Government,. Rapid Antigen Testing (2021). Available online at: https://www.nsw.gov.au/Covid-19/Stay-Safe/Testing/how-Testing-Works/Rapid-Antigen-Testing (accessed October 22, 2021).
166. CBC. As Schools Roll out Rapid Testing for COVID-19, Different Approaches Emerge Across Regions (2021). Available online at: https://www.cbc.ca/News/Schools-Rapid-Test-Rollout-1.6201575 (accessed October 22, 2021).
167. Tupper P, Colijn C. COVID-19 in schools: Mitigating classroom clusters in the context of variable transmission. PLoS Comput Biol. (2021) 17:E1009120. doi: 10.1371/journal.pcbi.1009120
168. Scobie HM, Johnson AG, Suthar AB, Severson R, Alden NB, Balter S, et al. Monitoring Incidence of COVID-19 cases hospitalizations, and deaths, by vaccination status - 13 U.S. Jurisdictions April 4-July 17, 2021. MMWR Morbid Mortal Week Rep. (2021) 70:1284–1290. doi: 10.15585/mmwr.mm7037e1
169. Brown CM, Vostok J, Johnson H, Burns M, Gharpure R, Sami S, et al. Outbreak of SARS-CoV-2 infections including COVID-19 vaccine breakthrough infections associated with large public gatherings - Barnstable County Massachusetts July 2021. MMWR Morbid Mortal Week Rep. (2021) 70:1059–1062. doi: 10.15585/mmwr.mm7031e2
170. Levine-Tiefenbrun M, Yelin I, Alapi H, Katz R, Herzel E, Kuint J, et al. Viral loads of Delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nat Med. (2021) 21:4. doi: 10.1038/s41591-021-01575-4
171. Nordström P, Ballin M, Nordström A. Association between risk of COVID-19 infection in nonimmune individuals and COVID-19 immunity in their family members. JAMA Int Med. (2021) 21:5814. doi: 10.1001/jamainternmed.2021.5814
172. Zhang R, Li Y, Zhang AL, Wang Y, Molina MJ. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proceed Nat Acad Sci. (2020) 117:14857–63. doi: 10.1073/pnas.2009637117
173. van den Berg P, Schechter-Perkins EM, Jack RS, Epshtein I, Nelson R, Oster E, et al. Effectiveness of 3 vs. 6 ft of physical distancing for controlling spread of coronavirus disease 2019 among primary and secondary students and staff: a retrospective statewide cohort study. Clin Infect Dis. (2021) 21:230. doi: 10.1093/cid/ciab230
174. Niffenegger JP. Proper handwashing promotes wellness in child care. J Pediatric Health Care. (1997) 11:26–31. doi: 10.1016/S0891-5245(97)90141-3
175. Giuliano CA, Rybak MJ. Efficacy of triclosan as an antimicrobial hand soap and its potential impact on antimicrobial resistance: A focused review. Pharmacotherapy J Hum Pharmacol Drug Therapy. (2015) 35:328–36. doi: 10.1002/phar.1553
176. Morton JL, Schultz AA Healthy Healthy Hands: Use of alcohol gel as an adjunct to handwashing in elementary school children. J. Sch. Nurs. (2004) 20:161–7. doi: 10.1177/10598405040200030601
177. Mahmood A, Eqan M, Pervez S, Alghamdi HA, Tabinda AB, Yasar A, et al. COVID-19 and frequent use of hand sanitizers; human health and environmental hazards by exposure pathways. Sci Total Environ. (2020) 742:140561. doi: 10.1016/j.scitotenv.2020.140561
178. Stadnytskyi V, Bax CE, Bax A, Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proceed Nat Acad Sci. (2020) 117:11875–7. doi: 10.1073/pnas.2006874117
179. Bulfone TC, Malekinejad M, Rutherford GW, Razani N. Outdoor transmission of SARS-CoV-2 and other respiratory viruses: A systematic review. J Infect Dis. (2021) 223:550–61. doi: 10.1093/infdis/jiaa742
180. Asadi S, Wexler AS, Cappa CD, Barreda S, Bouvier NM, Ristenpart WD. Aerosol emission and superemission during human speech increase with voice loudness. Scientific Rep. (2019) 9:2348. doi: 10.1038/s41598-019-38808-z
181. Miller SL, Vance M, Hertzberg J, Toohey D. Statement of Work: Aerosol Generation From Playing Band Instruments Singing Performing Risk of Infectious Disease Transmission (2020). Available online at: https://aec-music.eu/userfiles/File/Newsitems/2020/Statement_of_Work.pdf (accessed December 2, 2021).
182. Jimenez JL. 2020_06_COVID-19_Airborne_Transmission_Estimator (2020). Available online at: https://Docs.Google.com/Spreadsheets/d/16K1OQkLD4BjgBdO8ePj6ytf-RpPMlJ6aXFg3PrIQBbQ/Edit#gid=519189277 (accessed October 20, 2021).
183. National Federation of State High School Associations. Preliminary Results of Performing Arts Aerosol Study Depict Hopeful Outlook for Future Music Activities (2020). Available online at: https://www.Nfhs.org/Articles/Preliminary-Results-of-Performing-Arts-Aerosol-Study-Depict-Hopeful-Outlook-for-Future-Music-Activities/ (accessed October 20, 2021).
184. Prather KA, Wang CC, Schooley RT. Reducing transmission of SARS-CoV-2. Science. (2020) 368:1422–4. doi: 10.1126/science.abc6197
185. Howard J, Huang A, Li Z, Tufekci Z, Zdimal V, van der Westhuizen H-M, et al. An Evidence Review of Face Masks Against COVID-19. Proceed Nat Acad Sci. (2021) 118:E2014564118. doi: 10.1073/pnas.2014564118
186. Eberhart M, Orthaber S, Kerbl R. The impact of face masks on children-a mini review. Acta Paediatrica. (2021) 110:1778–3. doi: 10.1111/apa.15784
187. Mayo Clinic. Benefits of Kids Wearing Masks in School (2021). Available online at: https://Newsnetwork.Mayoclinic.org/Discussion/Benefits-of-Kids-Wearing-Masks-in-School/ (accessed October 20, 2021).
188. Centers for Disease Control Prevention. Your Guide to Masks (2021). Available online at: https://www.cdc.gov/Coronavirus/2019-Ncov/Prevent-Getting-Sick/About-Face-Coverings.Html (accessed October 22, 2021).
189. The World Health Organization. Coronavirus Disease (COVID-19): Children and Masks. (2020). Available online at: https://www.who.int/News-Room/q-a-Detail/q-a-Children-and-Masks-Related-to-Covid-19 (accessed October 22, 2021).
190. Assathiany R, Salinier C, Béchet S, Dolard C, Kochert F, Bocquet A, et al. Face masks in young children during the COVID-19 pandemic: Parents' and pediatricians' point of View. Front Pediatric. (2021) 9:676718–676718. doi: 10.3389/fped.2021.676718
191. Lubrano R, Bloise S, Testa A, Marcellino A, Dilillo A, Mallardo S, et al. Assessment of respiratory function in infants and young children wearing face masks during the COVID-19 pandemic. JAMA Netw Open. (2021) 4:E210414–E210414. doi: 10.1001/jamanetworkopen.2021.0414
192. Marini M, Ansani A, Paglieri F, Caruana F, Viola M. The impact of facemasks on emotion recognition, trust attribution and re-identification. Scien Rep. (2021) 11:5577. doi: 10.1038/s41598-021-84806-5
193. Palmiero AJ, Symons D, Morgan JW, Shaffer RE. Speech intelligibility assessment of protective facemasks air-purifying respirators. J. Occup. Environ Hyg. (2016) 13:960–968. doi: 10.1080/15459624.2016.1200723
194. Chládková K, Podlipský VJ, Nudga N, Šimáčková Š. The McGurk effect in the time of pandemic: age-dependent adaptation to an environmental loss of visual speech cues. Psychon Bull Rev. (2021) 28:992–1002. doi: 10.3758/s13423-020-01852-2
195. Peng Z, Jimenez JL. Exhaled CO2 as a COVID-19 infection risk proxy for different indoor environments and activities. Environ Sci Technol Lett. (2021) 8:392–7. doi: 10.1021/acs.estlett.1c00183
196. Government of Ireland Department of Education. Practical Steps for the Deployment of Good Ventilation Practices in Schools V3 May 2021. (2021). Available online at: https://Assets.gov.ie/85177/D9643a37-5254-483e-A72e-D2a08ae36d46.pdf (accessed June 14, 2021).
197. Dietz L, Horve PF, Coil DA, Fretz M, Eisen JA, Van Den Wymelenberg K. 2019 Novel Coronavirus (COVID-19) Pandemic: Built Environment Considerations To Reduce Transmission. MSystems. (2020) 5:E00245–20. doi: 10.1128/mSystems.00245-20
198. Jones E, Young A, Clevenger K, Salimifard P, Wu E, Lahaie Luna M. Healthy Schools: Risk Reduction Strategies for Reopening Schools. Harvard T.H. Chan School of Public Health Healthy Buildings program (2020). Available online at: https://schools.forhealth.org/wp-content/uploads/sites/19/2020/06/Harvard-Healthy-Buildings-Program-Schools-For-Health-Reopening-Covid19-June2020.pdf (accessed December 2, 2021).
199. Allen JG, Ibrahim AM. Indoor air changes and potential implications for SARS-CoV-2 transmission. JAMA. (2021) 325:2112–3. doi: 10.1001/jama.2021.5053
200. Yezli S, Otter JA. Minimum infective dose of the major human respiratory and enteric viruses transmitted through food and the environment. Food Environ Virol. (2011) 3:1–30. doi: 10.1007/s12560-011-9056-7
201. Zhang X, Chen X, Chen L, Deng C, Zou X, Liu W, et al. The evidence of SARS-CoV-2 infection on ocular surface. Ocul Surf. (2020) 18:360–2. doi: 10.1016/j.jtos.2020.03.010
202. Cereda D, Tirani M, Rovida F, Demicheli V, Ajelli M, Poletti P, et al. The Early Phase of the COVID-19 Outbreak in Lombardy Italy. arXiv:2003.09320 (2020).
203. Imai M, Iwatsuki-Horimoto K, Hatta M, Loeber S, Halfmann PJ, Nakajima N, et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc Natl Acad Sci USA. (2020) 117:16587–95.
204. Guallar MP, Meiriño R, Donat-Vargas C, Corral O, Jouvé N, et al. Inoculum at the time of SARS-CoV-2 exposure and risk of disease severity. Int J Infect Dis. (2020) 97:290–2. doi: 10.1016/j.ijid.2020.06.035
205. Sneppen K, Nielsen BF, Taylor RJ, Simonsen L. Overdispersion in COVID-19 increases the effectiveness of limiting nonrepetitive contacts for transmission control. Proceed Nat Acad Sci. (2021) 118:E2016623118. doi: 10.1073/pnas.2016623118
206. Leclerc QJ, Fuller NM, Knight LE, CMMID COVID-19 Working Group, Funk S. What settings have been linked to SARS-CoV-2 transmission clusters? Wellcome Open Res. (2020) 15:98. doi: 10.12688/wellcomeopenres.15889.2
207. Adam DC, Wu P, Wong JY, Lau EHY, Tsang TK, Cauchemez S, et al. Clustering and superspreading potential of SARS-CoV-2 Infections in Hong Kong. Nat Med. (2020) 26:1714–19. doi: 10.1038/s41591-020-1092-0
208. Alonso S, Alvarez-Lacalle E, Català M, López D, Jordan I, García-García JJ, et al. Age-dependency of the propagation rate of coronavirus disease 2019 inside school bubble groups in catalonia Spain. Pediatr Infect Dis J. (2021) 40:955–961. doi: 10.1097/INF.0000000000003279
209. Block P, Hoffman M, Raabe IJ, Dowd JB, Rahal C, Kashyap R, et al. Social network-based distancing strategies to flatten the COVID-19 curve in a post-lockdown world. Nat Hum Behav. (2020) 4:588–96. doi: 10.1038/s41562-020-0898-6
210. Landeros A, Ji X, Lange K, Stutz TC, Xu J, Sehl ME, et al. An examination of school reopening strategies during the SARS-CoV-2 pandemic. PLoS ONE. (2021) 16:E0251242. doi: 10.1371/journal.pone.0251242
Keywords: COVID-19, SARS-CoV-2, education, vaccination, post-acute sequelae of SARS-CoV-2 infection (PASC), pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS), multisystem inflammatory syndrome in children (MIS-C)
Citation: Lordan R, Prior S, Hennessy E, Naik A, Ghosh S, Paschos GK, Skarke C, Barekat K, Hollingsworth T, Juska S, Mazaleuskaya LL, Teegarden S, Glascock AL, Anderson S, Meng H, Tang S-Y, Weljie A, Bottalico L, Ricciotti E, Cherfane P, Mrcela A, Grant G, Poole K, Mayer N, Waring M, Adang L, Becker J, Fries S, FitzGerald GA and Grosser T (2021) Considerations for the Safe Operation of Schools During the Coronavirus Pandemic. Front. Public Health 9:751451. doi: 10.3389/fpubh.2021.751451
Received: 01 August 2021; Accepted: 18 November 2021;
Published: 16 December 2021.
Edited by:
Marc Jean Struelens, Université Libre de Bruxelles, BelgiumReviewed by:
Danilo Buonsenso, Catholic University of the Sacred Heart, ItalyCopyright © 2021 Lordan, Prior, Hennessy, Naik, Ghosh, Paschos, Skarke, Barekat, Hollingsworth, Juska, Mazaleuskaya, Teegarden, Glascock, Anderson, Meng, Tang, Weljie, Bottalico, Ricciotti, Cherfane, Mrcela, Grant, Poole, Mayer, Waring, Adang, Becker, Fries, FitzGerald and Grosser. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ronan Lordan, cm9uYW4ubG9yZGFuQHBlbm5tZWRpY2luZS51cGVubi5lZHU=; Tilo Grosser, dGlsb0BwZW5ubWVkaWNpbmUudXBlbm4uZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.