- 1Department of Pharmacy, Peking University Third Hospital, Beijing, China
- 2Institute for Drug Evaluation, Peking University Health Science Center, Beijing, China
- 3Department of Pharmacy, Aviation General Hospital, Beijing, China
Objectives: The aim of this systematic review is to assess the published cost-effectiveness analyses of aprepitant for patients with chemotherapy-induced nausea and vomiting (CINV).
Methods: A systematic literature search was performed on PubMed, EMbase, the Cochrane Library, CNKI, WANFANG DATA, and CBM database. The date of publication is up to January 2019. Two reviewers independently reviewed titles, abstracts, and articles sequentially to select studies for data abstraction based on the inclusion and exclusion criteria. Disagreements were resolved and reviewers reached a consensus. The quality of the included studies was assessed according to the 24-item checklist of the consolidated health economic evaluation reporting standards (CHEERS). The costs reported by the included studies were converted to US dollars via purchasing power parities (PPP) in the year 2019 using the CCEMG–EPPI–Certer Cost Converter.
Results: Thirteen articles were included based on the inclusion criteria for cost-effectiveness analysis and cost-utility analysis. Twelve studies were rated as good quality and one as a moderate quality based on the CHEERS checklist. Eight studies compared aprepitant plus 5-hydroxytryptamine-3 receptor antagonist (5-HT3RA) and dexamethasone with the standard regimen (5-HT3RA and dexamethasone). It was concluded that aprepitant plus standard regimen was a cost-effective strategy for preventing CINV. Only one study that compared aprepitant plus 5-HT3RA with 5-HT3RA, concluded that the addition of aprepitant reduced the incidence of severe nausea, and it might also provide an economic benefit in the overall management. Four studies that compared aprepitant with other antiemetic drugs concluded that aprepitant is a cost-effective strategy for preventing CINV compared with metoclopramide. However, netupitan + palonosetron and olanzapine are cost-effective compared with aprepitant.
Conclusion: This study is the first systematic evaluation of adding aprepitant to standard regimens for patients with CINV. Most economic evaluations of antiemetic medications are reported to be of good quality. Adding aprepitant to standard regimens is found to be a cost-effective strategy for preventing CINV.
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is a common side effect of chemotherapy. The prevalence of CINV has been estimated to be as high as 70–80% without appropriate antiemetic prophylaxis (1). Patients who receive highly emetogenic chemotherapy (HEC) and moderately emetogenic chemotherapy (MEC) are the major populations who suffer from nausea and vomiting (2). CINV can be classified as the following: acute (occurs within the first 24 h after chemotherapy initiation) and delayed (occurs within 24–120 h postchemotherapy) events.
Aprepitant, a neurokinin-1 receptor antagonist (NK-1RA), has been showing effectiveness in preventing CINV. Adding to standard antiemetic regimens (a 5-hydroxytryptamine-3 receptor antagonist (5-HT3RA) and/or a glucocorticoid), aprepitant has been proved to lead to a further decrease in the incidence of CINV than the standard regimen alone (3–6).
Currently, the guidelines of the National Comprehensive Cancer Network (NCCN) (7), the American Society of Clinical Oncology (ASCO) (8), and the Multinational Association of Supportive Care in Cancer (MASCC)/European Society of Medical Oncology (ESMO) (9) endorsed the use of NK-1RAs plus as a standard regimen in patients who received HEC for preventing CINV. However, the ASCO and MASCC/ESMO guidelines did not recommend NK-1RA for MEC patients. In contrast, the NCCN guideline recommended that an NK-1RA should be added to a standard regimen for patients with additional risk factors or previous treatment failure with a standard regimen alone. As for the Chinese guideline (10), NK-1RA was recommended for MEC patients based on particular situations.
Chemotherapy-induced nausea and vomiting can significantly affect the adherence of patients to cancer treatments and impair the quality of life (11). Uncontrolled CINV can also increase health care expenditure and resource utilization (12). Although optimal antiemetic prophylaxis, according to the emetogenic risk of chemotherapy, is important for patients to continue their cancer treatment, the increased financial burden is a concern for aprepitant, which is a costly antiemetic agent. Gomez et al. (11) reported that socioeconomic barriers associated with NK1RA therapy affected suboptimal adherence to guideline recommendations for antiemetic prophylaxis. While many studies have reported on the cost-effectiveness of aprepitant for treating CINV, a systematic review of economic evaluations of aprepitant is currently lacking. Therefore, it is necessary to conduct a comprehensive systematic evaluation and analysis of the existing economic research evidence of aprepitant, assess the cost-effectiveness of adding aprepitant to standard regimens for patients with CINV, and provide support for clinical rational drug use and medical insurance decision-making.
Methods
A systematic review was conducted following the preferred reporting items for systematic reviews and metaanalyses (PRISMA) guidelines (12). It was registered on the International Prospective Register for systematic reviews (No. CRD 42020152060).
Search Methods for Identification of Studies
A literature search was performed using the following databases: PubMed, Embase, the Cochrane Library, and three Chinese databases (China National Knowledge Infrastructure [CNKI], WANFANG DATA, and Chinese Biomedical Literature Database [CBM]). The search time was from the date of establishment of the databases to January 2019. The following search terms were used, “aprepitant,” “emend,” “cost,” “effectiveness,” “utility,” “benefit,” “economic,” “expenses,” and “pharmacoeconomic.”
Criteria for Considering Studies
Types of Participants
Patients diagnosed with malignant tumors by histopathology and/or cytology who received HEC or MEC.
Types of Interventions
Aprepitant plus 5HT3RA with or without dexamethasone for the prevention of CINV.
Types of Comparators
The following comparisons were acceptable for evaluation:
·Aprepitant regimen (aprepitant, 5-HT3RA and dexamethasone) vs. standard regimen (5-HT3RA and dexamethasone);
·Aprepitant plus 5-HT3RA vs. 5-HT3RA;
·Aprepitant vs. other antiemetic drugs.
Types of Outcomes
We evaluated the incremental cost-effectiveness ratio (ICER) measure as the primary outcome, and incremental effectiveness and incremental cost measures as the secondary outcome.
Types of Studies
Pharmacoeconomic studies were included if: (1) full texts were published in any language; (2) economic evaluations (including cost-effectiveness, cost-utility, cost-minimization, cost-benefit analyses, and cost analysis). Exclusion criteria were as follows: review articles, editorials and opinions, letters, research protocols, conference abstracts, notes and books.
Selection of Included Studies
Articles retrieved from the literature search were independently screened based on the title and abstract by two authors (TTQ and PM). Studies that did not meet the criteria were excluded. After the initial screening, two researchers (TTQ, PM) independently assessed the full texts of eligible citations. The list of included studies was reached by a consensus. Any disagreements were resolved by discussion or by consulting with a senior author (SDZ).
Data Extraction
Data extraction was performed using predesigned data extraction tables in Microsoft Excel. For all studies, the following information: authors, published year, country, type of model, perspective, model details (time horizon, discount rate), source of funding, sensitivity or uncertainty analysis, incremental effectiveness and costs, and ICER were extracted.
Reporting Quality Assessment
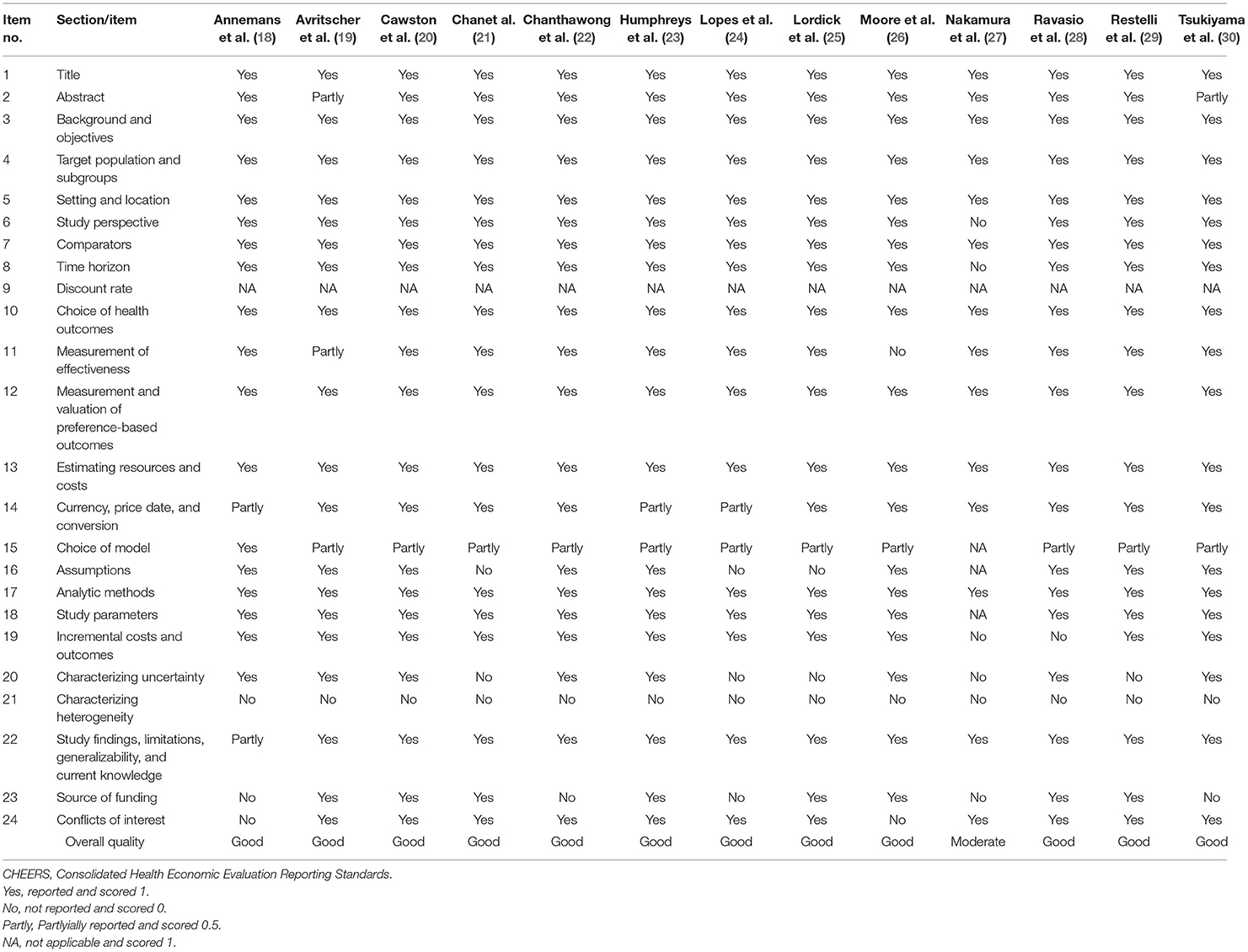
The quality of the pharmacoeconomic studies was assessed by a 24-item checklist of the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement, which was used as a checklist to rate the quality of reporting in the included papers. The CHEERS statement of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force is a guideline intended to improve reporting of the economic evaluation (13, 14). The quality of the included studies was evaluated by the answers to the questions, which were “yes” (reported and scored 1) or “no” (not reported and scored 0) or “partly” (partially reported and scored 0.5) or “NA”(not applicable and scored 1). The studies were separated into four quality categories. Those studies that fulfilled 100% of the items were classified as excellent quality; those that fulfilled between 75 and 100% of the items were classified as good quality; those that fulfilled between 50 and 75% of the items were classified as moderate quality; and those that fulfilled ≤50% of the items were classified as low quality (15, 16).
Strategy for Data Synthesis
To facilitate the comparison of ICERs, all costs were converted into US dollars via purchasing power parities (PPP) in the year 2019. PPP was defined as the rates of currency conversion that eliminate the differences in price levels between countries. PPP conversion factors were obtained from the Organization for Economic Co-Operation and Development Stat database (17). We converted the original cost estimates to the target currency and price year.
Results
Selection of Studies
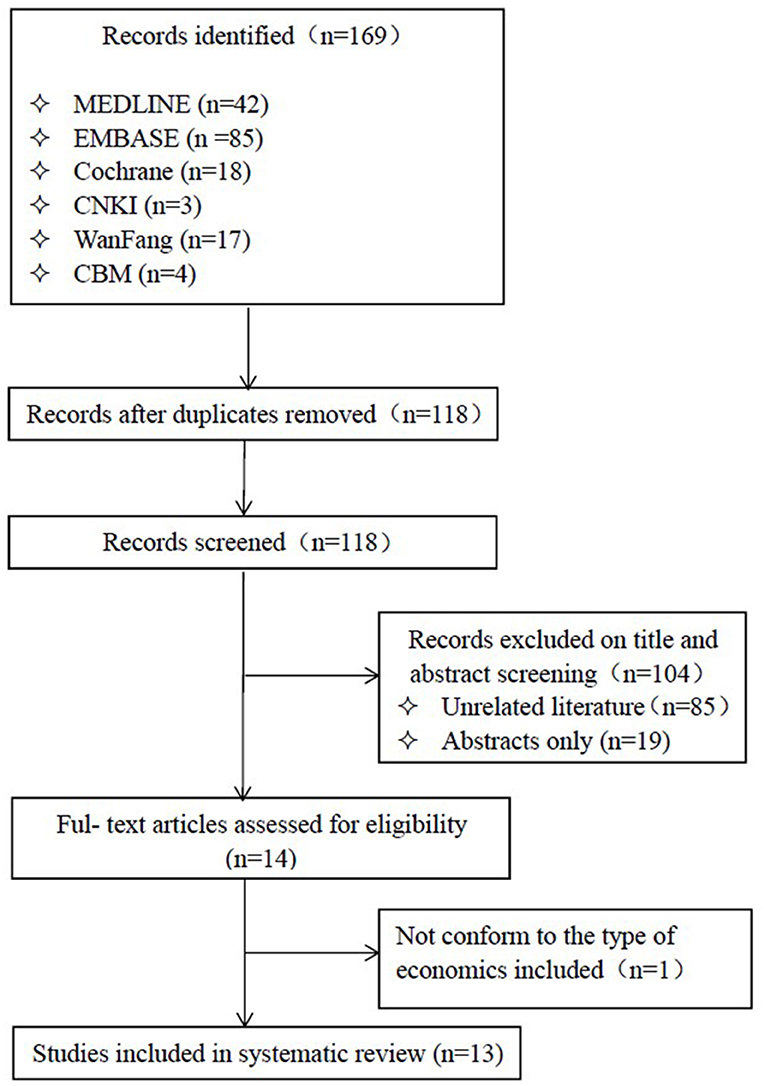
After a thorough search of the databases, we acquired 169 articles, of which 104 were excluded based on the title and abstract screening. A total of 13 published studies (18–30) were selected for final inclusion (Figure 1) after reviewing.
General Characteristics of the Included Studies
Table 1 describes the general characteristics of the included studies. Of the 13 studies, eight (18, 21–26, 30) conducted both cost-effectiveness analysis (CEA) and cost-utility analysis (CUA), two studies conducted CUA (19, 20), one study conducted CEA (27), one study conducted cost-consequences analysis (CCA) (28), and one study conducted both CUA and budget impact analysis (29).
Ten of the 13 economic studies included data of clinical outcomes from randomized controlled trials. The clinical data of Chanthawong et al. (22) and Cawston et al. (20) came from systematic review and metaanalysis. Nakamura et al. (27) acquired data from a retrospective analysis of direct medical costs of National Hospital Organization Nagoya Medical Center between January 2009 and December 2013.
Of the 13 studies included, eight of used the decision analytical model (18, 21–25, 28, 30), four used the Markov model (19, 20, 26, 29), and one study did not use model analysis (27).
Eleven studies were conducted from a payer perspective, of which eight were performed from a public payer perspective (e.g., National Health Service, National Health Insurance system, and health-care system) (18, 20, 21, 23, 24, 28–30). In contrast, one study used a patient and statutory health insurance perspective (25), and two studies used the perspective of the payer, but did not describe it specifically (19, 26). One study used a societal perspective (22) and one study (27) did not mention it.
The time horizon for two studies (18, 19) was four cycles (21 days in each cycle), for another eight studies (20–25, 28, 30) time horizon was 5 days, for one study (26) it was five cycles (28 days in each cycle), time horizon of one study (29) was at least six cycles (5 days in each cycle) when CUA was conducted and 5 years when budget impacted analyses, and one study (27) did not mention the time horizon.
Among the included studies, the most common comparison was the aprepitant triple regimen (aprepitant+5-HT3RA+glucocorticoid) vs. standard regimen (5-HT3RA+glucocorticoid) with or without placebo (18, 19, 21, 24–26, 28, 30), the other comparison was aprepitant +5HT3RA vs. aprepitant (27), and the comparison of positive comparators such as netupitan + palonosetron (PAL) (NEPA) (20, 29), metoclopramide (23), and olanzapine (22). Most studies reported ICERs as cost-effectiveness evaluation outcomes.
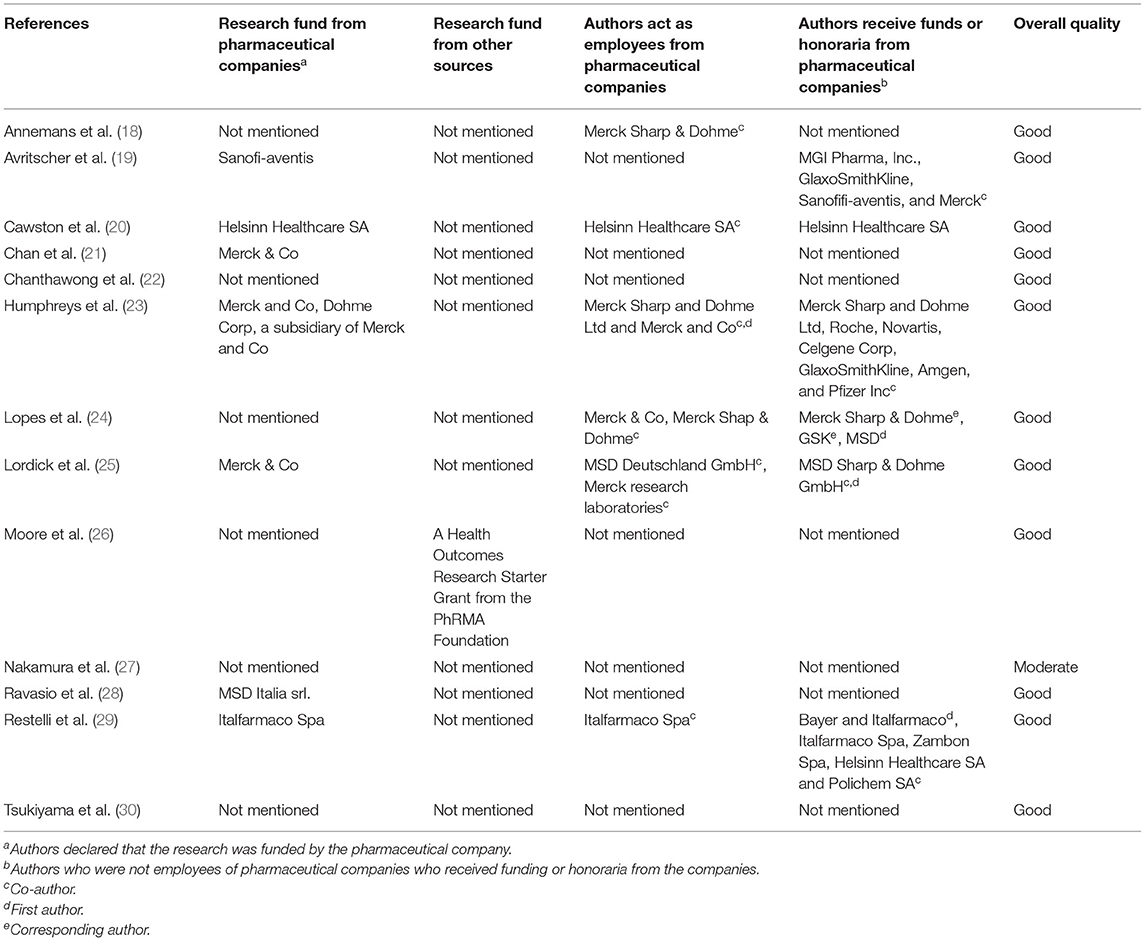
Three of the studies were funded by Merck & Co (21, 23, 25), one was funded by Sanofi-aventis (19), one was funded by Helsinn Healthcare SA (20), one was funded by MSD Italia srl (28), one was funded by Italfarmaco Spa (29), and one was supported by a Health Outcomes Research Starter Grant from the PhRMA Foundation (26). Two studies (18, 24) did not disclose the funding source, although some authors of these studies were employees from pharmaceutical companies, and some authors received funding or honoraria from pharmaceutical companies. The remaining three studies (22, 27, 30) did not mention any funding information at all.
Quality of the Included Studies
Twelve studies were of good quality based on the CHEERS checklist (18–26, 28–30), one was of moderate quality (27). The results of the quality assessment of the included studies are shown in Table 2.
We found that the included studies did not report several items on the CHEERS checklist. One of the studies (27) did not mention the perspective of the research, and five studies (21, 24, 25, 27, 29) did not state the results of uncertainty analyses, both the items on the CHEERS checklist that should be reported in the abstracts of economic evaluations.
Two studies used the perspective of the payer but did not describe it specifically (19, 26), and one study (27) did not mention the perspective nor did it use the model analysis.
Reasons for the choice of the economic model were not reported in most of the studies. Two studies did not perform a sensitivity analysis (21, 27). Even some studies that achieved good quality ratings did not meet the checklist criterion for characterizing heterogeneity. The roles of the funders play in the identification, design, conduct, and reporting of the analysis were not reported in all the included studies.
We also synthesized the results of quality rating and the funding information (Table 3). However, no relationship between sources of funding and the quality of the included studies was identified in this review.
Economic Evaluation and Sensitivity Analysis Results
The economic outcomes of the included studies are summarized in Tables 4–6.
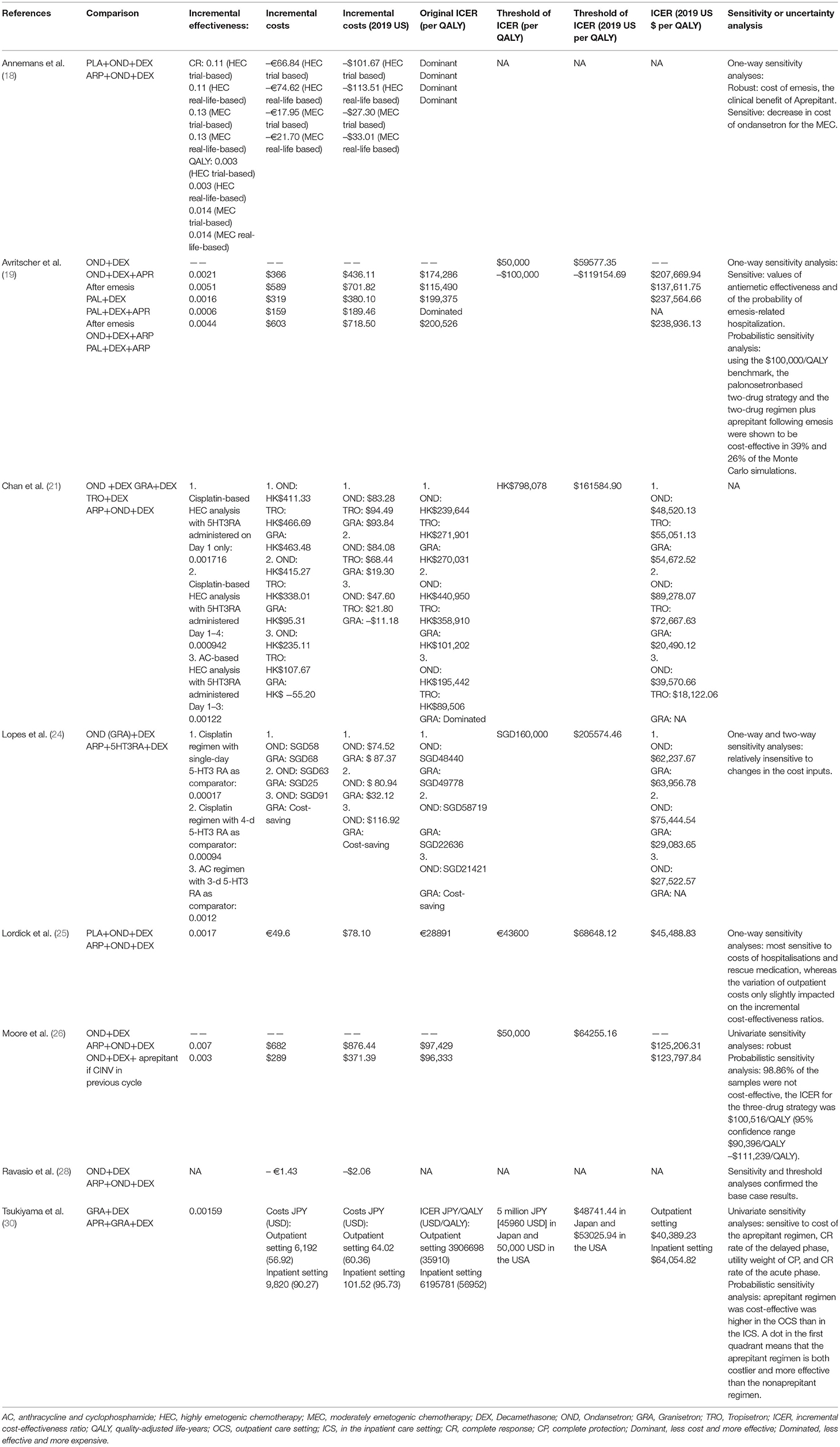
Table 4. Summary of economic evaluation outcomes comparing aprepitant regimen (aprepitant, 5-HT3RA and dexamethasone) vs. standard regimen (5-HT3RA and dexamethasone).
Aprepitant Regimen vs. Standard Regimen
Eight studies compared aprepitant regimen (aprepitant, 5-HT3RA, and dexamethasone) vs. standard regimen (5-HT3RA and dexamethasone), two of which used placebo (18, 25). The drugs of 5-HT3RA are ondansetron (OND), granisetron (GRA), PAL, and tropisetron (TRO). Six studies used the Decision analytical model (18, 21, 24, 25, 28, 30), and two used the Markov model (19, 26).
Eight studies concluded that aprepitant regimen was cost-effective compared with standard regimen. Among these eight studies, three were conducted in Europe [Belgium (18), Germany (25), and Italy (28)], two were from North America (USA (19, 26)), and three were from Asia [Hong Kong (21), Singapore (24), and Japan (30)].
Table 4 summarized the comparisons of case-based ICER values adjusted to year 2019 US$ values, and the results of the sensitivity analyses. Among them, sensitivity analysis was not performed in one study (21). One of the eight studies (28) did not analyze quality-adjusted life years (QALYs), but it conducted cost-consequence analysis, and the remaining seven studies all analyzed QALYs. Two studies (18, 19) showed that the ICER of aprepitant ranged from US$207,669.94 per QALY to US$ 238,936.13(adjusted to the year 2019 value) for a 21-day time horizon. One study (26) showed that the ICER of aprepitant was US$125,206.31 per QALY (adjusted to year 2019 value) for a 28-day time horizon. The other studies (21, 24, 25, 30) indicated that the ICER of aprepitant ranged from US$ 18,122.06 per QALY to US$ 89,278.07 per QALY (adjusted to year 2019 value) for a 5-day time horizon.
Two studies, Chan et al. (21) and Lopes et al. (24), were developed under the following three scenarios:
Scenario 1. Patients receiving cisplatin-based chemotherapy who received the aprepitant-containing regimen were compared with a standard regimen in which the 5HT3RA was administered on day 1 only;
Scenario 2. Patients receiving cisplatin-based chemotherapy who received the aprepitant-containing regimen were compared with a standard regimen in which the 5HT3RA was administered on days 1–4;
Scenario 3. Patients receiving an AC-based chemotherapy who received the aprepitant-containing regimen were compared with a standard regimen in which the 5HT3RA was administered on days 1–3.
These two studies (21, 24) came to the same conclusion that aprepitant-containing regimen was associated with higher acquisition costs but lower costs relating to patient emesis-related management, hospitalization, and use of rescue medication.
The results of eight studies suggested that adding aprepitant to standard regimen (5HT3RA+dexamethasone) was a cost-effective strategy for preventing CINV.
Aprepitant Plus 5-HT3RA vs. 5-HT3RA
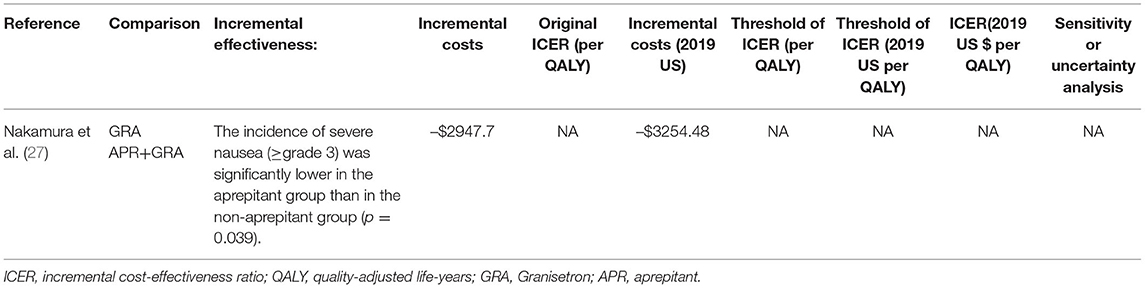
Only one study, Nakamura et al. (27), compared aprepitant plus 5-HT3RA with a 5-HT3RA (GRA) (Table 5). This study did not mention the perspective of the research and time horizon. It did not use model analysis and did not perform a sensitivity analysis. The study data was from a retrospective analysis of direct medical costs. It also conducted cost-effectiveness analysis. The research was of moderate quality according to the 24 questions of the CHEERS checklist. The guidelines recommend the treatment of CINV by comparing the aprepitant triple regimen with the standard regimen. Aprepitant plus 5-HT3RA vs. 5-HT3RA was not common. In the studies of Nakamura et al. (27), the total mean cost per patient during hospitalization was USD 19,052.33 (adjusted to the year 2019 value) in the aprepitant group and USD 22306.81(adjusted to the year 2019 value)in the non-aprepitant group. Although this difference was not statistically significant (p = 0.077), it indicated that the use of aprepitant reduced the total medical expense by USD 3,254.48 (adjusted to the year 2019 value) per patient. This lower cost in the aprepitant group was due to the shorter hospitalization period and reduced costs for transfusion and infection treatment. This study indicated that the addition of aprepitant for CINV prophylaxis during high-dose chemotherapy (HDCT) reduced the incidence of severe nausea and might also provide an economic benefit in the overall management of HDCT.
Aprepitant vs. Other Antiemetic Drugs
Table 6 summarized the economic results and the sensitivity analysis results of the comparison of aprepitant with other antiemetic drugs.
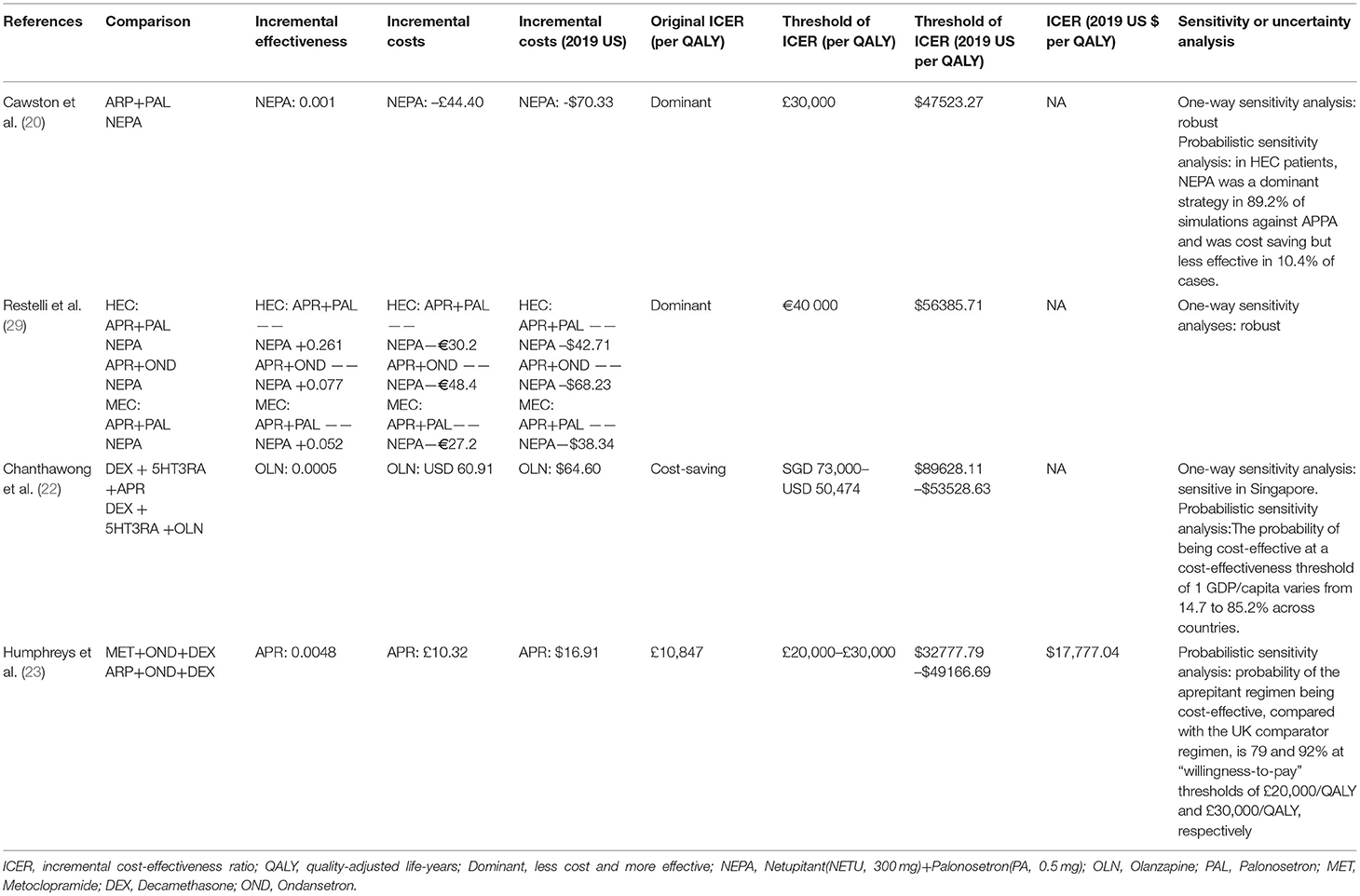
Table 6. Summary of economic evaluation outcomes comparing aprepitant versus other antiemetic drugs.
Aprepitant vs. NEPA
Netupitan + palonosetron is a fixed-dose combination of netupitant (NETU, 300 mg), a new NK-1RA with a long half-life period of 90 h, and PAL (0.5 mg) (41).
Cawston et al. (20) showed that in HEC patients, the NEPA strategy was more effective than APPA (QALDs of 4.263 vs. 4.053; incremental emesis-free, and CINV-free days of +0.354 and +0.237, respectively) and costed less ($126.73 vs. $196.43) (adjusted to the year 2019 value). The result showed that NEPA is the dominant strategy. NEPA was cost-effective for MEC patients, cumulating in an estimated 0.182 extra QALDs at an incremental cost of $10.53 (adjusted to the year 2019 value) compared with PA.
Restelli et al. (29) showed that NEPA is more effective and less expensive (dominant) compared with Aprepitant (APR) + PAL (for HEC and MEC) and APR + OND (for HEC). The use of NEPA would lead to a 5-year cost decrease of $89.8 million (60.2 million for HEC and $29.5 million for MEC) (adjusted to the year 2019 value).
The results of two studies (20, 29) suggest that NEPA is cost-effective for preventing CINV associated with HEC and MEC.
Aprepitant vs. Olanzapine
Studies have reported the advantages of olanzapine, an atypical antipsychotic drug, in improving the control of acute and delayed CINV. Chanthawong et al. (22) switched aprepitant to olanzapine and yielded additional 0.0005 QALY with a cost saving of USD 64.60 in Singapore (adjusted to the year 2019 value). This study suggests that the use of olanzapine as part of standard antiemetic regimen is cost-effective for the prevention of CINV in patients receiving HEC.
Aprepitant vs. Metoclopramide
Humphreys et al. (23) showed that 5 days after chemotherapy, 64% of patients who received the aprepitant regimen [aprepitant + OND + Decamethasone (DEX)] and 47% of those who received the UK comparator regimen (metoclopramide+OND+DEX) had a complete response to antiemetic therapy (no emesis and no rescue antiemetic therapy). A mean of $60.82 (adjusted to the year 2019 value) (78%) of the cost of aprepitant was offset by reduced health care resource utilization costs. The predicted gain in QALYs of the aprepitant regimen was 0.0048. The ICER of aprepitant, relative to the UK comparator, was $17777.04/QALY, which is well below the threshold commonly accepted in the UK($32777.79–$49166.69/QALY) (adjusted to the year 2019 value). The results of this study suggest that aprepitant is cost-effective for preventing CINV associated with chemotherapy for patients with breast cancer in the UK health care setting.
Discussion
Quality of the Economic Evaluations
We systematically searched for, assessed, and summarized the available literature on the cost-effectiveness of aprepitant in patients with CINV. The quality assessment of reviewed articles indicated that most articles were of good and moderate quality. Some studies did not report the reasons for choosing the time horizon or discount rate, and no included studies had a subgroup analysis. We found that only one study (22) in this review used a societal perspective; most studies considered a payer perspective. Societal perspective is the gold standard of pharmacoeconomic studies because it incorporates all costs and health outcomes, although other perspectives may be better for some decision-making situations (42, 43).
The role of funders in the identification, design, implementation, and reporting of research is critical to ensure that readers can reliably detect any potential bias. We found that seven of the included studies received funding from pharmaceutical companies. One study (26) was supported by a Health Outcomes Research Starter Grant from the PhRMA Foundation. Two studies (18, 24) did not disclose the funding source, but some authors were employees from pharmaceutical companies and some authors received funding or honoraria from pharmaceutical companies. Three studies (22, 27, 30) did not mention funding information at all. We found no relationship between the funding sources and the quality of the included studies that were identified in this review.
Evidence for Cost-Effectiveness
Ten of the 13 economic studies were highly consistent. The aprepitant regimen (APR+5-HT3RA+DEX) is more economical than the standard regimen (5-HT3RA+DXE), APR+5-HT3RA is more economical than 5-HT3RA, and APR+5-HT3RA+DEX is cost-effective for preventing CINV when compared with MET+ 5-HT3RA+DEX. Although aprepitant brings higher drug costs, the costs associated with vomiting management (such as patient management, hospitalization, and costs associated with the use of rescue drugs) are lower in the aprepitant group. Therefore, the aprepitant triple regimen is a cost-saving strategy.
The other three studies were Cawston et al. (20), Restelli et al. (29) on NEPA economics research and Chanthawong et al. (22) on olanzapine. NEPA is recommended for HEC by the NCCN, ASCO, and the MASCC/ ESMO, but as the drug is not yet on the market in China, it is not recommended in the 2014 edition of the Guidelines for the Prevention and Treatment of Vomiting Related to Cancer Therapy (7–10).
Chanthawong et al. (22) showed that compared with triplet antiemetic regimen, switching aprepitant to olanzapine increased QALY and saved costs. The use of olanzapine as part of standard antiemetic regimen is cost-effective for the prevention of CINV in patients receiving HEC in multiple SEA countries. The 2019 version of NCCA recommends two options for olanzapine for HEC, one is in combination with PAL and dexamethasone, and the other is in combination with NK1RA, 5HT3RA, and dexamethasone (7). The 2016 version of ESMO/MASCC recommends that olanzapine seems to be useful in the prophylaxis of delayed nausea [superior to (fos)aprepitant] and equal to (fos)aprepitant in the prevention of acute symptoms. Olanzapine may be considered with a 5-HT3 RA plus dexamethasone, particularly when nausea is an issue, but when using the 10-mg dose, patient sedation may be a concern [MASCC level of confidence: low; MASCC level of consensus: low; ESMO level of evidence II; ESMO grade of recommendation: B] (9). The ASCO considers that olanzapine lacks high-quality efficacy and safety studies, and hence is not recommended (8). There is no recommendation for olanzapine in China guidelines (10), and there are no indications for preventing CINV in the olanzapine instructions. Olanzapine should be used with caution in older people (44). The use of olanzapine for CINV did not reach the consensus of national guidelines. Therefore, even if olanzapine showed economic advantages when compared with aprepitant, the advantages and disadvantages should be considered in clinical decision-making. More studies are needed to analyze olanzapine or NEPA compared with aprepitant.
Strengths and Limitations
This study has several strengths. First, this review is the first comprehensive synthesis of the evidence of cost-effectiveness for aprepitant in preventing CINV. Second, this review includes all published cost-effectiveness studies of aprepitant, and adjusts all cost-related values of different time and countries to 2019 dollars for better comparison.
This study also has several limitations. First, because of heterogeneity in the methodology (e.g., different types of economic models, time horizon, and perspective) and data sources (e.g., effectiveness and safety data, and costing data) of economic evaluations, it is impossible to combine the data (45). Since it is difficult to compare different economic evaluations and reach an overall conclusion regarding the results, reasoning and conducting quantitative analysis (metaanalysis) is impossible. Thus, the explicit and precise estimation of the reported indicators was not possible, so this issue should be considered for using and generalizing the results.
Second, we analyzed the results of economic assessments conducted in different countries with different health care systems and reimbursement mechanisms, and most studies did not use real-world data. Methods such as cost-benefit thresholds, budgeting, and reimbursement should be taken into account, and so the interpretation of the results should be cautious.
Conclusions
This is the first systematic review of the cost-effectiveness of aprepitant for people with CINV. Based on the available literature, we drew a conclusion that in patients with CINV, aprepitant as an add-on treatment may represent a cost-effective option compared with standard regimen.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
TQ and PM are responsible for the main research and writing of the article. TS is responsible for modifying the article. SZ is the manager of this research and also the corresponding writer. All authors contributed to the conception and design of this work, and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wiser W, Berger A. Practical management of chemotherapy-induced nausea and vomiting. Oncology. (2005) 19:637–45.
2. Navari RM. Management of chemotherapy-induced nausea and vomiting: focus on newer agents and new uses for older agents. Drugs. (2013) 73:249–62. doi: 10.1007/s40265-013-0019-1
3. Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin—The Aprepitant Protocol 052 Study Group. J Clin Oncol. (2003) 21:4112–9. doi: 10.1200/JCO.2003.01.095
4. Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, Julie Ma G, Eldridge K, Hipple A, et al. Addition of neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting, results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer. (2003) 97:3090–8. doi: 10.1002/cncr.11433
5. Schmoll HJ, Aapro MS, Poli-Bigelli S, Kim HK, Park K, Jordan K, et al. Comparison of an aprepitant regimen with a multiple-day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high-dose cisplatin treatment. Ann Oncol. (2006) 17:1000–6. doi: 10.1093/annonc/mdl019
6. Warr DG, Hesketh PJ, Gralla RJ, Muss HB, Herrstedt J, Eisenberg PD, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol. (2005) 23:2822–30. doi: 10.1200/JCO.2005.09.050
7. Ettinger DS, Berger MJ, Ashton J, Barbour S, Bergsbaken J, Brandt D, et al. Antiemetics NCCN Clinical Practice Guidelines in Oncology Version1.2021[EB/OL]. National Comprehensive Cancer Network: Antiemesis 2021 v1. (2020). Available online at: http://www.nccn.org/patients (accessed December 23, 2020).
8. Hesketh PJ, Bohlke K, Lyman GH, Basch E1, Chesney M1, Clark-Snow RA, et al. Antiemetics: American Society Of Clinical Oncology Focused Guideline Update. J Clin Oncol. (2016) 34:381–6. doi: 10.1200/JCO.2015.64.3635
9. Roila F, Molassiotis A, Herrstedt J, Aapro M4, Gralla RJ5, Bruera E, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. (2016) 27(Suppl. 5):v119–33. doi: 10.1093/annonc/mdw270
10. Yu SY, Yin JL, Qin SK, Wang YJ, Chan Y, Shen L, et al. Tumor treatment related vomiting prevention guide (2014 edition). Chinese Clin Oncol. (2014) 19:263–73.
11. Gomez DR, Liao KP, Giordano S, Nguyen H, Smith BD, Elting LS. Adherence to national guidelines for antiemesis prophylaxis in patients undergoing chemotherapy for lung cancer: a populationbased study. Cancer. (2013) 119:1428–36. doi: 10.1002/cncr.27899
12. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
13. Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health. (2013) 16:231–50. doi: 10.1016/j.jval.2013.02.002
14. Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Pharmacoeconomics. (2013) 31:361–7. doi: 10.1007/s40273-013-0032-y
15. Hamberg-van Reenen HH, Proper KI, van den Berg M. Worksite mental health interventions: a systematic review of economic evaluations. Occup Environ Med. (2012) 69:837–45. doi: 10.1136/oemed-2012-100668
16. Mihalopoulos C, Chatterton ML. Economic evaluations of interventions designed to prevent mental disorders: a systematic review. Early Interv Psychiatry. (2015) 9:85–92. doi: 10.1111/eip.12156
17. Organisation for Economic Co-operation and Development(OECD). Prices and Purchasing Power Parities (PPP): PPs and Exchange Rates. (2019). Available online at: http://stats.oecd.org/Index.aspx?datasetcode=SNA_TABLE4# (accessed June 15, 2019).
18. Annemans L, Strens D, Lox E, Petit C, Malonne H. Cost-effectiveness analysis of aprepitant in the prevention of chemotherapy-induced nausea and vomiting in Belgium. Support Care Cancer. (2008) 16:905–15. doi: 10.1007/s00520-007-0349-1
19. Avritscher EB1, Shih YC, Sun CC, Gralla RJ, Grunberg SM, Xu Y, et al. Cost-utility analysis of palonosetron-based therapy in preventing emesis among breast cancer patients. J Support Oncol. (2010) 8:242–51. doi: 10.1016/j.suponc.2010.09.027
20. Cawston H, Bourhis F, Eriksson J, Ruffo P, D'Agostino P, Turini M, et al. NEPA, a new fixed combination of netupitant and palonosetron, is a cost-effective intervention for the prevention of chemotherapy-induced nausea and vomiting in the UK. Drugs Context. (2017) 6:212298. doi: 10.7573/dic.212298
21. Chan SL1, Jen J, Burke T, Pellissier J. Economic analysis of aprepitant-containing regimen to prevent chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy in Hong Kong. Asia Pac J Clin Oncol. (2014) 10:80–91. doi: 10.1111/ajco.12170
22. Chanthawong S, Lim YH, Subongkot S, Chan A, Andalusia R, Ahmad Bustamam RS, et al. Cost-effectiveness analysis of olanzapine-containing antiemetic therapy for managing highly emetogenic chemotherapy in Southeast Asia: a multinational study. Support Care Cancer. (2019) 27:1109–19. doi: 10.1007/s00520-018-4400-1
23. Humphreys S, Pellissier J, Jones A. Cost-effectiveness of an aprepitant regimen for prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer in the UK. Cancer Manag Res. (2013) 5:215–24. doi: 10.2147/CMAR.S44539
24. Lopes G, Burke T, Pellissier J, Zhang XH, Dedhiya S, Chan A. Aprepitant for patients receiving highly emetogenic chemotherapy: an economic analysis for Singapore. Value Health Reg Issues. (2012) 1:66–74. doi: 10.1016/j.vhri.2012.03.002
25. Lordick F, Ehlken B, Ihbe-Heffinger A, Berger K, Krobot KJ, Pellissier J, et al. Health outcomes and cost-effectiveness of aprepitant in outpatients receiving antiemetic prophylaxis for highly emetogenic chemotherapy in Germany. Eur J Cancer. (2007) 43:299–307. doi: 10.1016/j.ejca.2006.09.019
26. Moore S, Tumeh J, Wojtanowski S, Flowers C. Cost-effectiveness of aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with highly emetogenic chemotherapy. Value Health. (2007) 10:23–31. doi: 10.1111/j.1524-4733.2006.00141.x
27. Nakamura A, Kojima Y, Miyazawa K, Matsumoto S, Iida H, Nagai H. Clinical impact of aprepitant in patients receiving high-dose chemotherapy prior to autologous peripheral blood stem cell transplantation: a cost-effectiveness analysis. Oncology. (2017) 93:302–8. doi: 10.1159/000479032
28. Ravasio R, Rosti G. Cost-consequence analysis of aprepitant compared to standard therapy (5-HT3 + corticosteroids) for the prevention of highly emetogenic chemotherapy-induced nausea and vomit. Global Regional Health Technol Assess. (2015) 2:89–96. doi: 10.5301/GRHTA.5000195
29. Restelli U, Saibene G, Nardulli P, Di Turi R, Bonizzoni E, Scolari F, et al. Cost-utility and budget impact analyses of the use of NEPA for chemotherapy-induced nausea and vomiting prophylaxis in Italy. BMJ Open. (2017) 7:e015645. doi: 10.1136/bmjopen-2016-015645
30. Tsukiyama I, Hasegawa S, Ikeda Y, Takeuchi M, Tsukiyama S, Kurose Y, et al. Cost-effectiveness of aprepitant in Japanese patients treated with cisplatin-containing highly emetogenic chemotherapy. Cancer Sci. (2018) 109:2881–8. doi: 10.1111/cas.13736
31. Herrstedt J, Muss HB, Warr DG, Hesketh PJ, Eisenberg PD, Raftopoulos H, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and emesis over multiple cycles of moderately emetogenic chemotherapy. Cancer. (2005) 104:1548–55. doi: 10.1002/cncr.21343
32. Eisenberg P, Figueroa-Vadillo J, Zamora R, Charu V, Hajdenberg J, Cartmell A, et al. Palonosetron Study Group. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer. (2003) 98:2473–82. doi: 10.1002/cncr.11817
33. Gralla R, Lichinitser M, Van Der Vegt S, Sleeboom H, Mezger J, Peschel C, et al. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol. (2003) 14:1570–7. doi: 10.1093/annonc/mdg417
34. Grunberg SM, Dugan M, Muss H, Wood M, Burdette-Radoux S, Weisberg T, et al. Effectiveness of a single-day three-drug regimen of dexamethasone, palonosetron, and aprepitant for the prevention of acute and delayed nausea and vomiting caused by moderately emetogenic chemotherapy. Support Care Cancer. (2009) 17:589–94. doi: 10.1007/s00520-008-0535-9
35. Rapoport BL, Jordan K, Boice JA, Taylor A, Brown C, Hardwick JS, et al. Aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with a broad range of moderately emetogenic chemotherapies and tumor types: a randomized, doubleblind study. Support Care Cancer. (2010) 18:423–31. doi: 10.1007/s00520-009-0680-9
36. de Wit R, Herrstedt J, Rapoport B, Carides AD, Guoguang-Ma J, Elmer M, et al. The oral NK1 antagonist aprepitant, given with standard antiemetics provides protection against nausea and vomiting over multiple cycles of cisplatin-based chemotherapy: a combined analysis of two randomized, placebo-controlled phase III clinical trials. Eur J Cancer. (2004) 40:403–10. doi: 10.1016/j.ejca.2003.08.028
37. Hesketh PJ, Rossi G, Rizzi G, Palmas M, Alyasova A, Bondarenko I, et al. Efficacy and safety of NEPA, an oral combination of netupitant and Palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol. (2014) 25:1340–6. doi: 10.1093/annonc/mdu110
38. Aapro M, Rugo H, Rossi G, Rizzi G, Borroni ME, Bondarenko I, et al. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and Palonosetron, for prevention of chemotherapyinduced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol. (2014) 25:1328–33. doi: 10.1093/annonc/mdu101
39. Gralla RJ, Bosnjak SM, Hontsa A, Balser C, Rizzi G, Rossi G, et al. A phase III study evaluating the safety and efficacy of NEPA, a fixed-dose combination of netupitant and Palonosetron, for prevention of chemotherapyinduced nausea and vomiting over repeated cycles of chemotherapy. Ann Oncol. (2014) 25:1333–9. doi: 10.1093/annonc/mdu096
40. Takahashi T, Hoshi E, Takagi M, Katsumata N, Kawahara M, Eguchi K. Multicenter, phase II, placebo-controlled, double-blind, randomized study of aprepitant in Japanese patients receiving high-dose cisplatin. Cancer Sci. (2010) 101:2455–61. doi: 10.1111/j.1349-7006.2010.01689.x
41. Keating GM. Netupitant/Palonosetron: a review in the prevention of chemotherapy- induced nausea and vomiting. Drugs. (2015) 75:2131–41. doi: 10.1007/s40265-015-0512-9
42. Weinstein MC. Principles of cost-effective resource allocation in health care organizations. Int J Technol Assess Health Care. (1990) 6:93–103. doi: 10.1017/S0266462300008953
43. Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. (2016) 316:1093–103. doi: 10.1001/jama.2016.12195
44. Morita T, Tei Y, Shishido H, Inoue S. Olanzapine-induced delirium in a terminally ill cancer patient. J Pain Symptom Manage. (2004) 28:102–3. doi: 10.1016/j.jpainsymman.2004.04.008
Keywords: aprepitant, nausea, vomiting, cost, effectiveness
Citation: Qiu T, Men P, Sun T and Zhai S (2021) Cost-Effectiveness of Aprepitant in Preventing Chemotherapy-Induced Nausea and Vomiting: A Systematic Review of Published Articles. Front. Public Health 9:660514. doi: 10.3389/fpubh.2021.660514
Received: 26 May 2021; Accepted: 26 July 2021;
Published: 25 August 2021.
Edited by:
Mihajlo (Michael) Jakovljevic, Hosei University, JapanReviewed by:
Joseph Bubalo, Oregon Health and Science University, United StatesMoawia Elhassan, University of Gezira, Sudan
Copyright © 2021 Qiu, Men, Sun and Zhai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suodi Zhai, emhhaXN1b2RpQDE2My5jb20=
†These authors have contributed equally to this work
 Tingting Qiu
Tingting Qiu Peng Men
Peng Men Tong Sun
Tong Sun Suodi Zhai
Suodi Zhai