- 1Department of Mathematics and Computer Science, Eindhoven University of Technology, Eindhoven, Netherlands
- 2Computer, Electrical, and Mathematical Sciences and Engineering Division, King Abdullah University of Science and Technology, Thuwal, Saudi Arabia
- 3Prince Mohammad Bin Salman College, King Abdullah Economic City, Saudi Arabia
Background: Kidney Exchange Programs can play an important role to increase access to the life saving and most cost-effective treatment for End Stage Renal Disease. The rise of national KEPs in Europe brings a need for standardized performance reporting to facilitate the development of an international evidence base on program practices.
Methods: We systematically searched and reviewed the literature to extract kidney exchange program performance measures. Reported measures were initially categorized as structure, process, and outcome measures. Expert feedback was used to redefine categories and extend the set of measures to be considered. Using the Delphi method and a panel of 10 experts, the resulting measures were subsequently classified as mandatory (Base set), optional (Extended set), or deleted.
Results: Out of the initial 1,668 articles identified by systematic literature search, 21 European publications on kidney exchange programs were included to collect performance measures, accompanied by three national program reports. The final measurement categories were Context, Population, Enrollment, Matching, Transplantation, and Outcomes. The set of performance measures resulting from the literature review was modified and classified as mandatory or optional. The resulting Base set and Extended set form the kidney exchange program reporting standard.
Conclusions: The evidence-based and consensus-based kidney exchange program reporting standard can harmonize practical and scientific reporting on kidney exchange programs, thus facilitating the advancement of national programs. In addition, the kidney exchange program reporting standard can promote and align cross-national programs.
Introduction
With a mortality number of 1.2 million, Chronic Kidney Disease (CKD) is the 11th most common cause of death globally, and ranks 13th in Europe (1). It is a progressive disease of which End Stage Renal Disease (ESRD) is the last stage. The relative contribution of ESRD to European mortality is increasing, and currently stands at 1.58% in Europe, while CKD accounts for 1.06% of the total burden of disease in Europe (1). The default treatment for ESRD is dialysis (2). Dialysis incurs higher costs and lower quality of life than transplantation (2). In many European countries, transplantation programs have emanated from deceased donor programs. They have been complemented by living donor programs to promote access and quality of transplantation (3, 4). Live donation traditionally has been restricted to family members or close friends of a patient donating one of their two kidneys. Unfortunately, even when a living donor is available, transplantation may not be feasible because of incompatibility between the patient and the donor.
Over the past two decades, Kidney Exchange Programs (KEPs) have emerged in many countries to promote the benefits of living donor kidney donation. They particularly service pairs consisting of a patient and a living donor willing to donate to the patient for whom transplant is not feasible because the donor is not compatible with the patient. The KEPs “exchange” donors among patients so that patients are matched with compatible donors after which corresponding transplants take place.
Across the world, the design and developments of KEPs have varied considerably. The variations often are solutions to resolve country specific challenges such as small population size (Iceland) geography (Australia) and pre-existing deceased donor programs (Spain) (3, 4). In addition, the variations have arisen from challenges posed by differences in legislation. For example, countries such as Finland and Germany legally forbid living donation to recipients with whom the donor doesn't have a close relationship, whereas in other countries, such as France and Switzerland, altruistic donation is not legally allowed (3, 4).
Another main difference among KEPs arises from differences in national governance models. In many countries, national governance is limited to providing regulation for KEPs. The regulatory frameworks then may govern single center KEPS, such as in the Czech Republic and Slovakia, or KEPs between a small number of centers, such as in Poland. In the USA, less than half of the 250 living donor transplant centers participate in the nationwide KEP administered by UNOS, the organization which manages the nation's organ transplant system under contract with the federal government. In addition, the National Kidney Registry and the Alliance for Paired Donation operate nationwide and regional and single-center KEPs exist. At the other end of the governance spectrum, a national KEP has naturally emerged in the UK with its nationwide public health system. We refer to (3, 5) for a more detailed discussion of KEPS in Europe and across the globe, including in Australia, Canada and South Korea.
The dynamics of the emergent KEPs and their contextual differences have not only resulted in a variety of KEPs but have also brought along a variety of KEP performance measures reported. While this variety of reporting practices promotes novel approaches and viewpoints, it hampers comparability of practices and performance, and ultimately the development of an evidence base on KEP performance, as is beneficial for existing and newly emerging KEPs and most importantly for patients suffering from ESRD. Our research aims to synthesize reported measures and develop a consensus-based set of reporting standards.
Our research focusses on reporting for national KEPs. Moreover, we limit the scope to European KEPs. The reasons for limiting the scope to nationally coordinated KEPs in Europe are 2-fold. First, it serves to limit contextual differences, which complicate consensus and harmonization of standards. European countries and health systems differ essentially from other countries reporting on KEPS, such as Australia, China, India, Iran, Japan, Korea, and the USA, which translate to differences in KEPS and in KEP reporting priorities and practices. Second, Europe is presently witnessing various initiatives for cross-national KEPs (6), which especially call for harmonization of performance measures and reporting among European KEPs. These initiatives include bilateral KEPs between two countries, such as the Czech-Austrian kidney exchange (6) or KEPs involving multiple countries such as the Scandia Transplant Kidney Exchange Program (3).
To accomplish our research aim, we address two research questions. First, we seek to comprehensively map present scientific and practical reporting by European national KEPs. On this basis, we then proceed to develop unified reporting standards for European KEPs. The resulting Kidney Exchange Program Reporting Standards (KEPREPS) consist of a Base Set of essential, mandatory measures and an Extended set of optional measures, valuable to report for some programs. The presented work is developed within the EU COST Action 15210 entitled European Network for Collaboration on Kidney Exchange Programs (ENCKEP).
Methods
The method section contains two main parts. First is a systematic review and synthesis of European literature on Kidney Exchange Programs. The results of this literature review served as the starting point for the second stage of developing a reporting standard. In this stage, we solicited and processed several rounds of expert feedback following the Delphi method to derive consensus on the Kidney Exchange Program Reporting Standards (KEPREPS) reporting standard. The details of both methods are specified below.
Systematic Literature Review
Data on KEP performance measures were extracted from peer-reviewed English language scientific journals and conference proceedings and (annual) reports from Europe's three largest kidney exchange programs: The Netherlands, Spain, and The United Kingdom. A publication was considered 'European' if it reported data from a European KEP or if the first author was affiliated with a European institution.
After extensive consultation with an expert librarian, we included articles from Embase, Medline, Web of Science, and Cochrane matching the following query:
[(kidney OR donor* OR transplant* OR graft*) AND (exchange*OR pair*-exchange* OR pair*-donation* OR sharing)]
OR
[(pair*-exchange* OR pair*-donation* OR ((exchange*OR sharing OR chain) AND (donor* OR donation* OR kidney*))] AND (renal* OR kidney*)]
In the first round, the first and third author screened the title and abstract of all articles and excluded the articles for which both agreed on exclusion. In a second round, the same two authors considered the full texts of the non-excluded articles, again deciding to exclude only if both authors agreed on exclusion. Differences in assessment on exclusion were resolved by consensus.
Next, all included articles were screened by the same two authors to collect all performance measures reported. Again, the two authors ensured full consensus on each of the reported measures for each of the included articles. Lastly, the first author included all performance measures explicitly included in the annual reports of the British, Dutch, and Spanish KEPs.
Measure Categories
The measures extracted from the literature were subsequently categorized from two perspectives. Firstly, we distinguished different types of measures, using Donabedian's seminal Structure-Process-Outcome framework (7). This resulted in three initial categories of KEP measures-structure measures, process measures, and outcome measures. A fourth set of measures on the population participating in the KEP was added to ensure all measures were categorized. The second perspective regarded the frequency of reporting, which we interpreted to indicate the relevance of the measures. Hence, based on the reporting frequency, we provided an initial classification of measures. Commonly reported measures were initially classified as mandatory, less commonly, but still regularly reported measures were initially classified as optional, and the remainder as exceptional.
Base Set and Extended Set
The systematic review results were presented to the large group of ENCKEP participants, representing 28 predominantly European countries. This large audience of representatives provided feedback and proposed additional measures.
Based on this initial expert feedback, the categories were redefined and formed the input for a procedure following the Delphi method with a set of 10 volunteering expert participants of ENCKEP (8). The experts in the Delphi panel responded to two questionnaire rounds. During the first questionnaire round, the experts categorized measures into three groups: Base set, Extended set, and Other. The Base Set consisted of measures that should be reported by every KEP. The role of the Extended set was to incorporate important but non-essential measures, while the category “Other” accommodated non-essential measures to be excluded from the standard.
The experts were also given the opportunity to provide motivation for their answers and make additional comments. Based on the results of the first round, we calculated the average score for each measure granting 3/2/1 points for Base Set/Extended Set/Other and rounded the score for each measure to the nearest integer. This rounded score was then converted into an initial classification of each measure into Base set (score ≥ 2.5), Extended set (2.5 ≥ score ≥ 1.5), or Other (score <1.5).
In the second round, we presented the resulting Base set and Extended set and asked each expert to agree or disagree. The experts were requested to motivate any disagreement to facilitate further improvement or finalize decision making on the classification. A third round would have been conducted in case of a lack of consensus but was not necessary. The resulting classification formed the targeted reporting standard KEPREPS.
Results: Systematic Literature Review
The systematic literature search yielded 1,668 articles. After scanning of titles and abstracts, 346 were included. After reading of the full text (thus eliminating conference papers and posters), 116 articles remained, 21 of which were regarded as European. To this set, we added three publicly available annual reports of major European KEP programs.
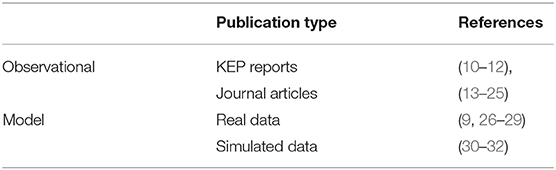
The review distinguishes observational empirical studies and (simulation) model studies. No controlled experimental studies have been reported. There are 16 observational studies, including three recent annual reports from the aforementioned KEPs, and 8 model studies. With one exception (9), the observational studies are quantitative studies. The (simulation) model studies used real-life data, generated data, or a combination of both. Table 1 categorizes the included publications.
The majority of the included studies originate in the Netherlands (13/24) and consider the long-running Dutch KEP. By contrast, only 2 UK documents are included, despite the large size of the UK program. Seven publications are from 2005 to 2009, 9 from 2010 to 2014, the remaining eight from 2015 onwards. Detailed information per included article, including the reported measures, is available as Supplementary Material.
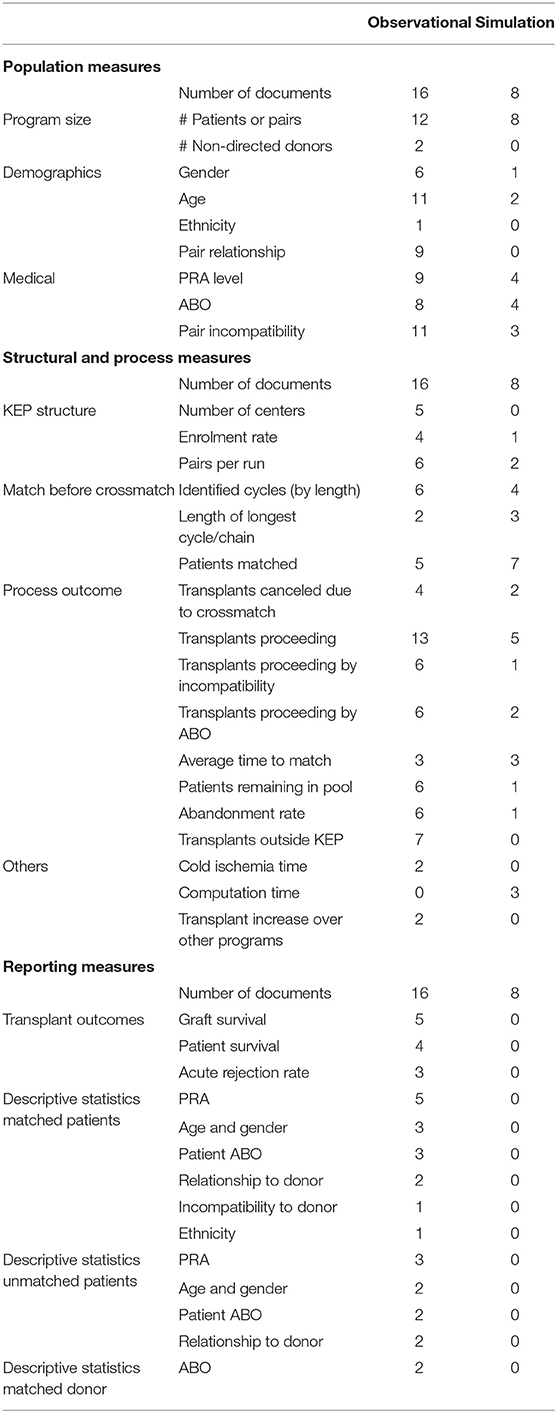
Population Measures
Population measures are the measures describing the donors and patients involved in the kidney exchange program. This data can be further divided into three main categories, information regarding program size, demographic data, and medical data.
Program Size
Program size measures are commonly reported. For instance, 12 out of 16 observational studies report the number of patients participating in the KEP, as do seven out of eight model studies. Of the model studies, five publications report on computational challenges in relation to program size. Papers not reporting on program size typically have a specific, different focus, such as validation of virtual crossmatch procedures (9) or KEP transplant outcomes compared to living-related transplant outcomes (13).
Demographics
Demographic data is commonly reported in the observational literature. Age (10/16), the relationship between donor and patient (8/16) and gender (5/16) information is provided often, mainly in papers describing functioning KEP programs. Ethnicity is only mentioned once (14).
In the simulation literature, demographic data is nearly absent. Within this literature, population data is focused on characteristics with a direct impact on the kidney exchange graph.
Clinical Population Measures
The composition of the KEP pool with regards to ABO and immunological characteristics can have a large impact on the overall and individual outcomes. These measures also shed light on patient enrolment causes.
In the observational literature, the type of incompatibility (ABOi, positive crossmatch) within a pair is reported in 11 out of 16 papers. ABO information for donors or candidates (7/16) and patient PRA (8/16) are also commonly reported. In some cases, this information is given by the type of incompatibility. ABO information for patients is often limited to the number of blood type O patients. Reporting on incompatibility types is often in combination with subsequent reporting on outcomes, e.g., transplant probability per incompatibility type (see below).
Simulation papers have less commonly reported on the ABO and PRA typing (3/8) and type of incompatibility (2/8) explicitly. However, an additional two papers refer to the data simulator they employ, which addressed these measures too. Table 2 summarizes the reporting of population measures.
Structure Measures
There is little reporting on structural characteristics. Five (observational) studies report on the number of transplant centers involved. Four studies report on the spread of transplants over the transplant centers, three of which are model studies.
Process Measures
As depicted in Table 2, five studies report on enrolment rates, only one of which is a model study. For the matching process, ten studies report on cycle lengths and numbers before HLA crossmatching, six of which are observational. This topic may have received more interest from model studies because cycle length received considerable attention in the scientific literature in relation to computational complexity. Likewise, three of the five studies which report the length of the longest cycle and/or chain are model studies.
The process (outcome) measure receiving the most attention is the number of transplants. As much as 13 of the 18 observational studies and five out of eight model studies report the total number of transplants proceeding. Seven of these studies distinguished ABO incompatible and crossmatch incompatible pairs, one of which was a model study. Six studies reported transplants per blood type. Two studies reported on the number of blood type identical transplants, and one study reported on ABO incompatible transplants.
A closely related process measure is the number of matched patients before crossmatch. This measure is reported by 12 studies and by all but one of the model studies. A next closely related measure is the number of transplants canceled because of negative crossmatch. This measure is reported by six studies, only two of which are model studies.
Eight studies (of which six observational) report the average number of patients included per match run, and six studies (of which three are observational) report the average time until being matched. This average can be the overall average but may also distinguish blood types and highly sensitized patients. Seven studies report on the number of pairs remaining in the pool, of which six are observational. The same set of seven studies also reported on the abandonment rate. Seven observational studies reported on the number of patients who received a transplant outside of the exchange program. Several other less frequently reported measures are provided in Table 2.
Outcome Measures
In comparison to process or population measures, outcome measures have received less attention. The most frequently reported outcome measures are graft survival rate (five observational studies), patient survival rate (four observational studies), and acute rejection rates (three observational studies). The only qualitative study included reported on psychological outcomes, such as psychological distress and complaints, and the need for support.
Five observational studies-four of which are Dutch-report on descriptive statistics for matched and unmatched patients. All these studies report on PRA levels of matched patients and four for unmatched patients. The age and gender of donors and recipients (matched patients), as well as recipient ABO type, are reported by three studies. Less frequently reported outcome measures can be found in Table 2.
Results: Toward a Reporting Standard
The above results were discussed with a broad expert panel of ENCKEP participants who proposed additional measures. The discussion led to a revised categorization. The three resulting main categories are: Context Information, Process Measures, and Outcome Measures. The Context Information is subdivided into measures on the program, on participating individuals (recipients and donors) and on pairs. The process measures are partitioned into three sequential subcategories: Enrolment, Matching, and Transplantation.
For each of the categories, measures were classified as essential (Base Set), important but non-essential (Extended Set), or not important (Other) by a Delphi panel of 10 experts from France, Hungary, Italy, The Netherlands, Portugal, Spain, Switzerland, and United Kingdom. In a second Delphi round with the same panel (except one expert), the averaged classifications (see methods section) were proposed to the panel for approval.
The resulting classification, as presented in Tables 3, 4, was approved nearly unanimously. We refer to the Appendix for a total of 16 exceptions and expert reservations. Sometimes, disagreement or reservation was because of legal considerations (collecting data on ethnicity is not allowed) or regulatory conventions (reporting measures for the living donor program as a whole). Five of the measures involved were from the Base Set. In each of these cases, one expert disagreed. For varying reasons, the corresponding items were kept in the Base Set. For the items in the Extended Set, we judged minor disagreement was not problematic as reporting of items in the Extended Set is optional anyway.

Table 3. Base set of context information, donor/recipient/pair attributes, and performance measures suggested to report by every KEP.
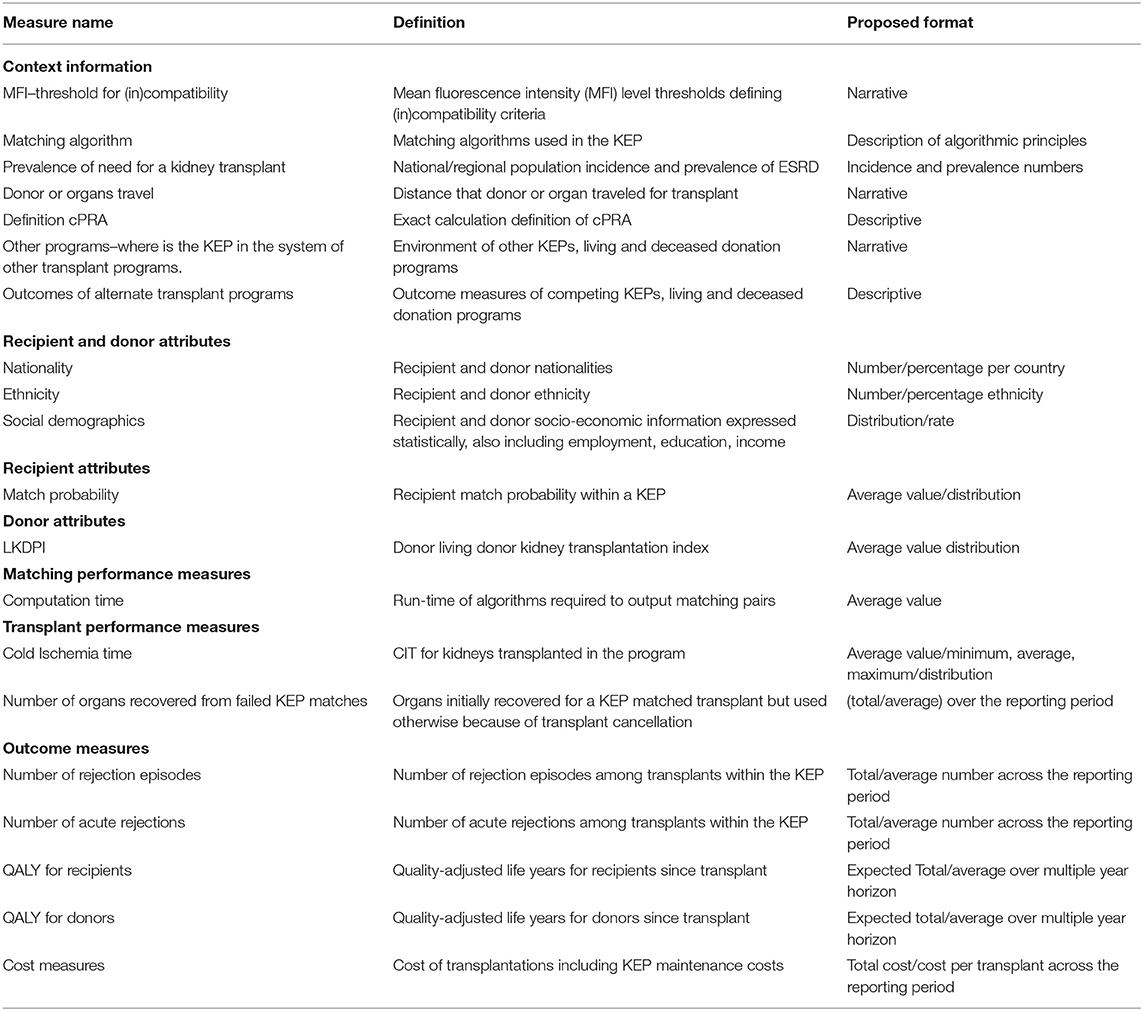
Table 4. Extended Set of context information, donor/recipient/pair attributes, and performance measures to report by KEPs.
Table 3 shows the resulting Base Set. Among the added context measures is the definition of incompatibility, considered as necessary context information to interpret reporting on other measures. From the European perspective, participant participation in the Eurotransplant Acceptable Mismatch Program (33) (or similar program for highly sensitized candidates) was also considered essential. For recipient and donor attributes, the Base Set contained blood type, gender, cPRA, and age. Experts also judged reporting on relationship and type of incompatibility for enrolled pairs to be mandatory.
The Base Set process and outcome measures are presented in Table 3 Experts classified all proposed matching measures as essential (Base Set) except for computation time. In addition to the measures from the review, measures on KEP transplants as a percentage of the total living donor program, and the total increase in donation caused by the KEP are included in the Base Set. While not frequently reported in the systematic review, outcome performance measures on patient, donor, and graft survival were also selected as Base Set measures.
Toward a Guideline on Reporting: Extended Set
Table 4 shows all context information, process, and outcome measures considered to be important but not essential and, therefore, to be included in the Extended Set. Most of the context information, including MFI thresholds, matching algorithm, organ/donor travel, and cPRA definitions, ended up in the Extended Set. The same goes for nationality, ethnicity, social demographics, donor's LKDPI (34), and recipient's match probability. Many of these measures were not in the systematic literature review results. The Extended Set also contained additional outcome measures relating to quality-adjusted life years (QALYs) for donors/recipients, number of rejections, and cost measures.
Discussion
The results presented above provide a Reporting Standard for European KEPs based on systematic literature review and expert opinion collected from a panel of practitioners and scientists from a variety of European countries and KEPs. The literature review made clear that not all existing European KEPs have reported on performance in the scientific literature. Moreover, it is remarkable that more than half of the publications are from The Netherlands, as the UK and Spain have larger KEPs but only modestly contributed to the European evidence base in the form of peer-reviewed scientific publications.
Living donor transplantation, as promoted by KEPs, is evidenced to be the most cost-effective treatment for End Stage Renal Disease (35–37). Given the importance of cost-effectiveness in current health policy and decision making, the lack of KEP reporting on cost measures is remarkable, as is the fact that outcome measures (effects) are among the least reported. However, measures on cost and effects (outcomes) have been included in the Base Set of KEPREPS upon expert suggestion and approval. Measures of equity and fairness, e.g., in relation to PRA level, blood type, and ethnicity, have received little attention in the European literature and are not included.
The main and intuitive proxy for outcomes in KEPs has typically been the process indicator number of transplants, which is indeed the most reported measure. It signals that much of the reporting and assessment of KEPs has focused on the process rather than on the outcomes. This may be due to the need to focus on mastering and improving the operations of KEPs in the initial years of developing KEPs. It may alternatively be explained by the view that the relation between processes and outcomes are well-understood, and hence the outcome performance can be measured and managed by the process measures, such as the number of transplants. In any case, the process measures-subdivided into enrolment, matching, and transplantation measures-form the largest set of measures in KEPREPS and are pre-dominantly included in the Base Set.
The process and outcome measures require information on the context to be appreciated. Factors such as age, sensitization, blood type distribution, et cetera, are important to interpret process and outcome performance. Hence, the experts have considerably expanded the collection of measures providing context information, compared to the measures found by systematic literature review. Most of these measures are seen as important but not essential and are included in the Extended Set.
The Base set and Extended set of KEPREPS together facilitate unified scientific and practical reporting on KEPs. Moreover, KEPREPS serves to enhance the practical relevance of model studies, which so far have differed quite substantially in their reporting, hampering their usefulness to inform and improve practice. In view of the importance presently attached to (health) outcomes and the difficulties faced globally to sustainably finance health systems, we believe that the inclusion of costs and outcomes in KEPREPS are especially valuable. Cost and health outcome measures have been hardly reported in scientific literature thus far. Hence the evidence base for the cost-effectiveness of KEPs is lacking, while a sound evidence base provides legitimacy to policy advancements of KEPS and especially larger coordinated KEPS which appear to be more effective than single center KEPS (23). Adoption of the KEPREPS standards by researchers and policy makers can therefore contribute to reducing the burden of ESRD while saving cost as the alternative of Dialysis is more expensive (38). More so, if KEPREPS may serve as a basis for reporting on model studies and the practice of emerging cross-national collaborations between KEPs (3) and future research expands it from a European standard to a global standard.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
BM: literature review, coordinating expert panel work, and writing. MM: writing and synthesis of results. JK: literature review and writing. All authors contributed to the article and approved the submitted version.
Funding
The authors acknowledge travel reimbursements by the COST program of the European Union (CA15210–European Network for Collaboration on Kidney Exchange Programmes). Article processing charges were funded by King Abdullah University of Science and Technology (KAUST).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are very thankful to the members of the expert panel: Peter Biro, Lisa Burnapp, Karine Hadaya, Antonio Nicolò, María Valentin, and five others. Furthermore, we thank the attendees of the ENCKEP Workshop in Glasgow. Finally, we thank Wichor Bramer for his help with the literature search.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2021.623966/full#supplementary-material
References
1. Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2018. Seattle, WA: Institute for Health Metrics and Evaluation (2018).
2. Axelrod DA, Schnitzler MA, Xiao H, Irish W, Tuttle-Newhall E, Chang SH, et al. An economic assessment of contemporary kidney transplant practice. Am J Transplant. (2018) 18:1168–76. doi: 10.1111/ajt.14702
3. Biró P, Haase-Kromwijk B, Andersson T, Ásgeirsson EI, Baltesová T, Boletiset I, et al. Building kidney exchange programmes in Europe—an overview of exchange practice and activities. Transplantation. (2019) 103:1514–22. doi: 10.1097/TP.0000000000002432
4. Glorie K, Haase-Kromwijk B, van de Klundert J, Wagelmans A, Weimar W. Allocation and matching in kidney exchange programs. Transpl Int. (2014) 27:333–43. doi: 10.1111/tri.12202
5. Ferrari P, Weimar W, Johnson R. Kidney paired donation: principles, protocols and programs. Nephrol Dial Transpl. (2015) 30:1276–85. doi: 10.1093/ndt/gfu309
6. Böhmig G, Fronek J, Slavcev A, Fischer G, Berlakovich G, Viklicky O. Czech-Austrian kidney paired donation: first European cross-border living donor kidney exchange. Transpl Int. (2017) 30:638–9. doi: 10.1111/tri.12945
7. Donabedian A. The quality of care. How can it be assessed? JAMA. (1988) 260:1743–8. doi: 10.1001/jama.1988.03410120089033
9. Böhmig G, Fidler S, Christiansen F, Fischer G, Ferrari P. Transnational validation of the Australian algorithm for virtual crossmatch allocation in kidney paired donation. Hum Immunol. (2013) 74:500–5. doi: 10.1016/j.humimm.2013.01.029
10. Organización Nacional de Trasplantes. Actividad de Donación y Trasplante Renal España 2018. (2018).
12. NHS Blood and Transplant. Organ Donation and Transplantation Activity Report 2018/2019. NHS Blood and Transplant (2019).
13. Tuncer M, Tekin S, Yücetin L, Sengül A, Demirbas A. Comparison of paired exchange kidney transplantations with living related kidney transplantations. Transpl Proc. (2012) 44:1626–7. doi: 10.1016/j.transproceed.2012.05.045
14. Roodnat J, Zuidema W, Van De Wetering J, De Klerk M, Erdman RAM, Massey EK, et al. Altruistic donor triggered domino-paired kidney donation for unsuccessful couples from the kidney-exchange program. Am J Transplant. (2010) 10:821–7. doi: 10.1111/j.1600-6143.2010.03034.x
15. De Klerk M, Keizer M, Claas F, Witvliet M, Haase-Kromwijk B, Weimar W. The Dutch national living donor kidney exchange program. Am J Transplant. (2005) 5:2302–5. doi: 10.1111/j.1600-6143.2005.01024.x
16. Keizer K, De Klerk M, Haase-Kromwijk B, Weimar W. The Dutch algorithm for allocation in living donor kidney exchange. Transplant Proc. (2005) 37:589–91. doi: 10.1016/j.transproceed.2004.12.096
17. De Klerk M, Haase-Kromwijk B, Claas F, Witvliet M, Weimar W. Living donor kidney exchange for both ABO-incompatible and crossmatch positive donor-recipient combinations. Transplant Proc. (2006) 38:2793–5. doi: 10.1016/j.transproceed.2006.08.157
18. De Klerk M, Witvliet M, Haase-Kromwijk B, Claas F, Weimar W. A highly efficient living donor kidney exchange program for both blood type and crossmatch incompatible donor-recipient combinations. Transplantation. (2006) 82:1616–20. doi: 10.1097/01.tp.0000250906.66728.8d
19. Kranenburg L, Zuidema W, Vanderkroft P, Duivenvoorden H, Weimar W, Passchier J, et al. The implementation of a kidney exchange program does not induce a need for additional psychosocial support. Transpl Int. (2007) 20:432–9. doi: 10.1111/j.1432-2277.2007.00461.x
20. De Klerk M, Witvliet M, Haase-Kromwijk B, Claas F, Weimar W. Hurdles, barriers, and successes of a national living donor kidney exchange program. Transplantation. (2008) 86:1749–53. doi: 10.1097/TP.0b013e3181908f60
21. De Klerk M, Witvliet M, Haase-Kromwijk B, Weimar W, Claas F. A flexible national living donor kidney exchange program taking advantage of a central histocompatibility laboratory: the Dutch model. Clin Transpl. (2008):69–73.
22. Johnson R, Allen J, Bradley J, Rudge C. Early experience of paired living kidney donation in the United Kingdom. Transplantation. (2008) 86:1672–7. doi: 10.1097/TP.0b013e3181901a3d
23. Gumber M, Kute V, Goplani K, Shah PR, Patel HV, Vanikar AV, et al. Transplantation with kidney paired donation to increase the donor pool: a single-center experience. Transplant Proc. (2011) 43:1423–14. doi: 10.1016/j.transproceed.2011.02.016
24. Kaçar S, Eroglu A, Tilif S, Güven B, Okçuoglu Kadioglu Z. A novel experience in living donor renal transplantation: voluntary exchange kidney transplantation. Transplant Proc. (2013) 45:2106–10. doi: 10.1016/j.transproceed.2012.10.032
25. Poldervaart R, Laging M, Royaards T, Kal-van Gestel JA, van Agteren M, de Klerk M, et al. Alternative living kidney donation programs boost genetically unrelated donation. J Transplant. (2015) 2015. doi: 10.1155/2015/748102
26. de Klerk M, Van Der Deijl W, Witvliet M, Haase-Kromwijk B, Claas F, Weimar W. The optimal chain length for kidney paired exchanges: an analysis of the Dutch program. Transplant Int. (2010) 23:1120–5. doi: 10.1111/j.1432-2277.2010.01114.x
27. Glorie K, de Klerk M, Wagelmans A, van de Klundert JJ, Zuidema WC, Claas FHJ, et al. Coordinating unspecified living kidney donation and transplantation across the blood-type barrier in kidney exchange. Transplantation. (2013) 96:814–20. doi: 10.1097/TP.0b013e3182a132b7
28. Bofill M, Calderón M, Castro F, Acebo ED, Delgado P, Garcia M, et al. The Spanish kidney exchange model: study of computation-based alternatives to the current procedure. In: Artificial Intelligence in Medicine, AIME 2017. Vienna: Springer International Publishing (2017). p. 272–7. doi: 10.1007/978-3-319-59758-4_31
29. Glorie K, van de Klundert J, Wagelmans A. Kidney exchange with long chains: an efficient pricing algorithm. M&SOM-Manuf Serv Oper Manag. (2014) 16:498–512. doi: 10.1287/msom.2014.0496
30. Santons N, Tubertini P, Viana A, Pedroso J. Kidney exchange simulation and optimization. J Oper Res Soc. (2017) 68:1521–32. doi: 10.1057/s41274-016-0174-3
31. Pedroso J. Maximizing expectation on vertex-disjoint cycle packing. In: International Conference on Computational Science and Its Applications. Berlin: Spinger-Verlag (2014). p. 32–46. doi: 10.1007/978-3-319-09129-7_3
32. Klimentova X, Pedroso J, Viana A. Maximizing expectation of the number of transplants in kidney exchange programmes. Comput Oper Res. (2016) 73:1–11. doi: 10.1016/j.cor.2016.03.004
33. Heidt S, Witvliet M, Haasnoot G, Claas F. The 25th anniversary of the Eurotransplant acceptable mismatch program for highly sensitized patients. Transpl Immunol. (2015) 33:51–7. doi: 10.1016/j.trim.2015.08.006
34. Massie A, Leanza J, Fahmy L, Chow EKH, Desai NM, Luo X, et al. A risk index for living donor kidney transplantation. Am J Transplant. (2016) 16:2077–84. doi: 10.1111/ajt.13709
35. Klarenbach S, Barnieh L, Gill J. Is living kidney donation the answer to the economic problem of end-stage renal disease? Seminars Nephrol. (2009) 29:533–8. doi: 10.1016/j.semnephrol.2009.06.010
36. Smith C, Woodward R, Cohen D, Singer GG, Brennan DC, Lowell JA, et al. Cadaveric versus living donor kidney transplantation: a medicare payment analysis. Transplantation. (2000) 69:311. doi: 10.1097/00007890-200001270-00020
37. McFarlane P. Should patients remain on intensive hemodialysis rather than choosing to receive a kidney transplant? Semin Dial. (2010) 23:516–9. doi: 10.1111/j.1525-139X.2010.00740.x
Keywords: kidney exchange, Living donation, reporting standard, end stage renal disease (ESRD), kidney exchange program
Citation: Smeulders B, Mankowski MA and van de Klundert J (2021) Kidney Exchange Program Reporting Standards: Evidence-Based Consensus From Europe. Front. Public Health 9:623966. doi: 10.3389/fpubh.2021.623966
Received: 30 October 2020; Accepted: 15 January 2021;
Published: 11 February 2021.
Edited by:
Jonathan Ling, University of Sunderland, United KingdomReviewed by:
Mustafa Sevinc, Sişli Hamidiye Etfal Education and Research Hospital, TurkeyStephen Michael Sammut, University of Pennsylvania, United States
Copyright © 2021 Smeulders, Mankowski and van de Klundert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bart Smeulders, Yi5zbWV1bGRlcnNAdHVlLm5s
 Bart Smeulders
Bart Smeulders Michal A. Mankowski
Michal A. Mankowski Joris van de Klundert
Joris van de Klundert