
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Public Health , 15 December 2020
Sec. Exposome
Volume 8 - 2020 | https://doi.org/10.3389/fpubh.2020.550753
 Yacong Bo1†
Yacong Bo1† Yongjian Zhu2†
Yongjian Zhu2† Yuchang Tao3
Yuchang Tao3 Xue Li1,4
Xue Li1,4 Desheng Zhai1
Desheng Zhai1 Yongjun Bu1
Yongjun Bu1 Zhongxiao Wan3
Zhongxiao Wan3 Ling Wang3
Ling Wang3 Yuming Wang5
Yuming Wang5 Zengli Yu1*
Zengli Yu1*Background: There is no study that has systematically investigated the breadth and validity of the associations of folate and multiple health outcomes. We aimed to evaluate the quantity, validity, and credibility of evidence regarding associations between folate and multiple health outcomes by using umbrella review of meta-analysis.
Methods: We searched the MEDLINE, EMBASE, and Cochrane Library databases from inception to May 20, 2018, to identify potential meta-analyses that examined the association of folate with any health outcome. For each included meta-analysis, we estimated the summary effect size and their 95% confidence interval using the DerSimonian and Laird random-effects model. We used the AMSTAR 2 (A Measurement Tool to Assess Systematic Reviews) to assess methodological quality and the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation working group classification) to assess the quality of evidence for each outcome included in the umbrella review.
Results: Overall, 108 articles reporting 133 meta-analyses of observational studies and 154 meta-analyses of randomized controlled trials (RCTs) were included in the study. Among them, 108 unique exposure–outcome–population triplets (referred to as unique meta-analyses hereafter) of RCTs and 87 unique meta-analyses of observational studies were reanalyzed. Beneficial effects of folate were observed in the all-cause mortality rate and in a number of chronic diseases, including several birth/pregnancy outcomes, several cancers, cardiovascular disease and metabolic-related outcomes, neurological conditions, and several other diseases. However, adverse effects of folate were observed for prostate cancer, colorectal adenomatous lesions, asthma or wheezing, and wheezing as an isolated symptom and depression.
Conclusions: Current evidence allows for the conclusion that folate is associated with decreased risk of all-cause mortality and a wide range of chronic diseases. However, folate may be associated with an increased risk of prostate cancer. Further research is warranted to improve the certainty of the estimates.
Folate, which mediates the transfer of one-carbon units in methylation and biosynthesis of nucleotides, has been well-established to play important roles in the processes of DNA synthesis, stability, repair, and methylation (1). It has been known for more than 2 decades that folic acid supplements during a woman's pregnancy can reduce the risk of neural tube malformation. Since then, numerous studies have investigated the effects of folate on a wide range of health outcomes, including the all-cause and cause-specific mortality, cancer outcomes, cardiovascular disease (CVD), diabetes, neurocognitive disorders, and pregnancy and birth outcomes. It is also noteworthy that folate supplement has been becoming popular worldwide, although evidence regarding the associations between folate and various health outcomes is still inconclusive.
Given this, a systematic assessment of the credibility of the published evidence will provide important implications for folate in both clinical practice and public health. Previous original or meta-analysis studies of the health effects of folate usually focused on a single health outcome (e.g., neural tube malformation). We therefore carried out the current umbrella review of existing published data on the associations between folate exposure and diverse health outcomes. In addition, we aimed to describe the magnitude, direction, and significance of the suggested associations; evaluate the potential biases; and identify which studies produced the highest-quality evidence.
The umbrella review method, which synthesizes information from meta-analyses both of observational studies and randomized controlled trials (RCTs) on multiple health outcomes associated with a particular exposure, could provide an instructive panorama for public health interventions (2, 3). We conducted this umbrella review of folate and multiple health outcomes by systematically searching for meta-analyses in which folate was part or all of the exposure of interest. Meanwhile, we excluded those systematic reviews without meta-analyses.
The MEDLINE, EMBASE, and Cochrane Library databases were searched from inception to May 20, 2018 to identify meta-analyses that examined the association between folate and any health outcome. The detailed search strategies are presented in Supplementary Table 1. The titles, abstracts, and full texts of potentially eligible articles were screened by two researchers independently. Disagreements were arbitrated by a third researcher.
Articles with meta-analyses were included if they met the following inclusion criteria:
(1) Meta-analyses of either observational (i.e., cohort, case-control, and cross-sectional studies) or interventional studies (i.e., RCTs)
(2) Evaluating the association of folate (folate intake, folate supplementation, and folate concentration) with any health outcome
(3) The included population aged 18 years or older
(4) Published in peer-reviewed journals in English.
We excluded meta-analyses that evaluated the effects of genetic polymorphisms related to folate metabolism on health outcomes, animal research, and laboratory studies. If an article presented separate meta-analysis for more than one health outcome, we included each of these separately. For meta-analyses of observational studies, if more than one meta-analysis addressed the same research question, the one with the largest number of prospective cohort studies was included.
Two investigators independently extracted information from eligible meta-analyses. For each meta-analysis, the following information was extracted: first author's last name, year of publication, number of studies included, populations, health outcomes of interest, study designs, exposure of folate, effect sizes [odds ratio (OR), risk ratio (RR), hazard ratio (HR), or mean difference (MD)], and the corresponding 95% confidence intervals (CIs), and types of effect model used in the meta-analysis (fixed or random). In addition, we also extracted number of cases and controls (for case–control studies), events and participant/person-years (for cohort studies), or number of subjects in interventional and control groups (for RCTs). For each original study included in each meta-analysis, the following data were extracted for further reevaluation: the effect estimates (OR, RR, HR, or MD) with 95% CI, number of cases, total number of participants, and study design.
Summary effects and 95% CIs for each meta-analysis were reanalyzed by using a DerSimonian and Laird random-effects model to be consistent with the method widely used in the included meta-analyses. For any associations with p < 0.05, the following metrics were further estimated: the 95% prediction interval to evaluate the uncertainty for the effect that would be expected in a new original study (4, 5); the between-study heterogeneity (defined as significant for I2 ≥ 50% and p < 0.05); the excess significance test to assess whether the observed number (O) of studies with significant results (positive studies) was larger than the expected number (E) (6); and the presence of small-study effect by using Egger regression asymmetry test (significance threshold p < 0.10) (7).
For overlapping outcomes that were examined both in meta-analyses of RCTs and those of observational studies, we examined whether the observed direction and statistical significance were consistent between the two study types.
We used the updated AMSTAR 2 (A Measurement Tool to Assess Systematic Reviews) to evaluate the methodological quality of the included meta-analyses. Compared with the original AMSTAR tool, the AMSTAR 2 emphasizes the risk-of-bias assessment in study design and heterogeneity and is a reliable and valid tool for quality assessment of meta-analyses of both interventional and observational research (8). The AMSTAR 2 includes 16 items for evaluating the methodological quality of systematic reviews/meta-analyses, with each item scoring 0 or 1. The methodological quality of each individual meta-analysis was then classified as high, moderate, low, or critically low accordingly.
The Grading of Recommendations, Assessment, Development, and Evaluation working group classification (GRADE) was used to assess the quality of evidence for those meta-analyses included in the umbrella review (9, 10). The GRADE categorizes evidence from systematic reviews and meta-analyses into the levels of high, moderate, low, or very low. In the GRADE approach, RCTs start as high-quality evidence, and observational studies start as low-quality evidence. Other factors may then upgrade or downgrade the quality level. For example, unexplained heterogeneity or high probability of publication bias may downgrade the quality of evidence, whereas a large effect or dose-response gradient may upgrade it. Two reviewers independently assessed the included studies, and a third reviewer settled disagreements.
The flow of study selection is presented in Figure 1. We initially identified 1,975 unduplicated articles. After considering the inclusion and exclusion criteria, 108 articles were finally included in the study. Among them, 133 meta-analyses of observational studies were reported in 62 articles (11–72), and 154 meta-analyses of RCTs were reported in 51 articles (13, 20, 28, 47, 62, 73–118). Another five articles reported both meta-analyses with observational studies and RCTs (13, 20, 28, 47, 62). As a result, a total of 195 unique health outcomes classified into eight health fields (i.e., all-cause and cause-specific mortality rates, cancer outcomes, cardiovascular outcomes, birth outcomes, pregnancy outcomes, neurocognitive disorders, and other outcomes) were reported (Supplementary Figure 1).
As shown in Supplementary Table 2, the median number of meta-analyses with observational studies included in each outcome was 7 (range, 2–36), and the median numbers of participants/case numbers were 43,063 (range, 635–59,514,473) and 3,463 (range, 11–147,424), respectively. Twenty-one outcomes were reported in more than one meta-analysis.
After excluding 46 duplicated meta-analyses, we further analyzed 87 unique exposure–outcome–population triplets (referred to as unique meta-analyses hereafter) of observational studies with a wide range of outcomes (Supplementary Table 3): all-cause and cause-specific mortality (n = 3), birth outcomes (n = 28), cancer-related outcomes (n = 45), cardiovascular outcomes (n = 2), neurocognitive disorders (n = 5), pregnancy outcomes (n = 3), and other outcomes (n = 1). Figures 2, 3 show the summarized results of these 87 unique meta-analyses. Overall, 35 of the 87 (40.2%) meta-analyses reported nominally significant pooled results (p < 0.05).
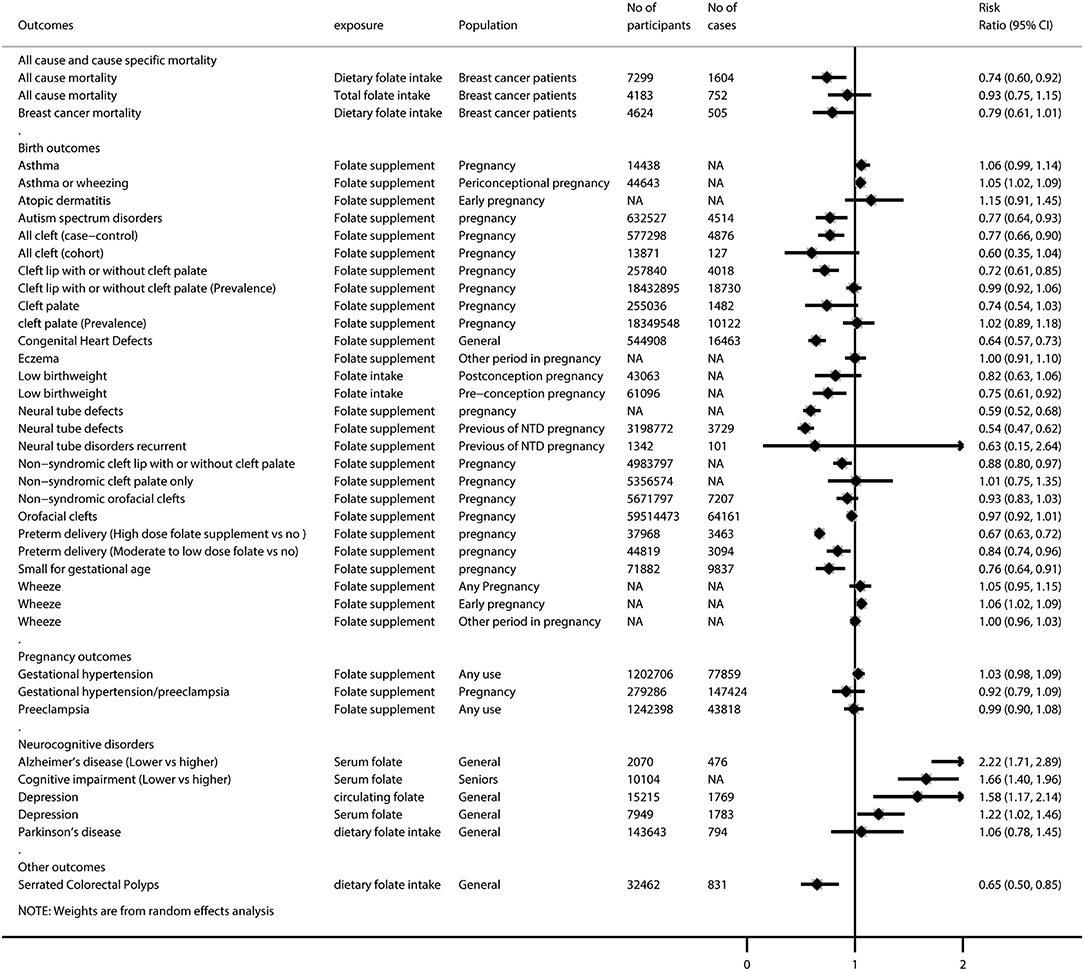
Figure 2. Summary random-effects estimates of cancer outcomes and cardiovascular outcomes reported in meta-analyses of observational studies.
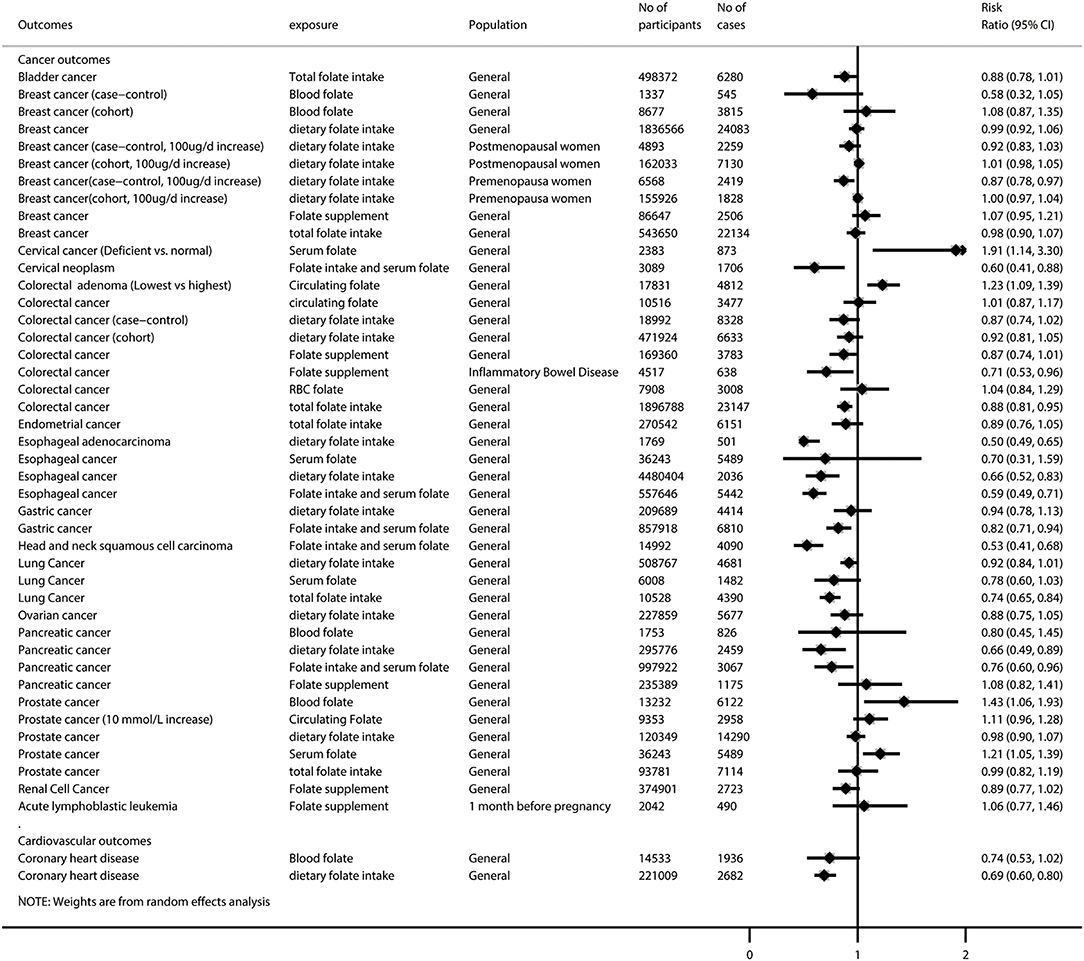
Figure 3. Summary random-effects estimates of all-cause and cause-specific mortality, birth outcomes, pregnancy outcomes, neurocognitive disorders, and other outcomes reported in meta-analyses of observational studies.
Of these 87 unique meta-analyses, 10 (11.5%) were with statistical significance of p < 10−6, 7 (8.0%) had a 95% prediction interval excluding the null, 60 (69.0%) had more than 1,000 cases (or more than 20,000 participants for continuous outcomes), 16 (18.4%) had neither evidence of excess significance bias (p > 0.10) nor small-study effects (p > 0.10), and 40 (46.0%) had no large heterogeneity (I2 < 50% and p > 0.05).
Supplementary Table 4 provides a breakdown of the AMSTAR 2 scores for the meta-analyses representing each outcome. None of the 87 meta-analyses was rated at the high methodological level, and 3 (3.4%) were rated as moderate, leaving 31 (35.6%) as low and 53 (60.9%) as critically low. Regarding the GRADE classification for evidence level, 4 of the 87 meta-analyses (4.6%) were rated as high-quality evidence for the corresponding outcomes, 21 (24.1%) were rated as moderate, 12 (13.8%) were rated as low, and 50 (57.5%) were rated as very low quality (Supplementary Table 5).
Among the 25 meta-analyses with high or moderate GRADE classification, we found that folate intake was associated with lower risks of low birth weight (during preconception), esophageal adenocarcinoma, gastric cancer, head and neck squamous cell carcinoma, pancreatic cancer, coronary heart disease, and serrated colorectal polyps (among adults undergoing endoscopic investigation of the colorectal), but it did not show a significant association with low birth weight (during post-conception pregnancy), colorectal cancer, lung cancer, and Parkinson disease. Folate supplementation was associated with lower risks of non-syndromic cleft lip with or without cleft palate and small for gestational age, but it did not show a significant association with non-syndromic cleft palate, wheezing, acute lymphoblastic leukemia, and gestational hypertension/preeclampsia. A higher level of circulating folate was associated with lower risks of cervical cancer, colorectal adenoma, and Alzheimer disease, but it did not show a significant association with lung cancer and coronary heart disease (Supplementary Figure 2). Interestingly, we found that circulating folate did not show a significant association with prostate cancer, but higher serum folate was associated with increased risk of prostate cancer.
As shown in Supplementary Table 6, the median number of meta-analyses of RCTs included in each outcome was 5.5 (range, 2–25), and the median numbers of participants and cases were 3,113 (range, 28–82,723) and 653 (range, 3–39,923), respectively. More than one meta-analysis was reported for 17 outcomes.
After removing 46 duplicated meta-analyses, we further analyzed 108 unique meta-analyses of RCTs for the associations of folate with all-cause and cause-specific mortality (n = 2), birth outcomes (n = 17), cancer-related outcomes (n = 14), cardiovascular outcomes (n = 29), diabetes-related outcomes (n = 9), endothelial function (n = 5), neurocognitive disorders (n = 5), pregnancy outcomes (n = 8), and other outcomes (n = 19). The summarized results of these 108 unique meta-analyses are presented in Figures 4, 5. Overall, 31 (28.7%) meta-analyses showed nominally significant pooled results (p < 0.05). Among the 31 meta-analyses, 6 were for birth outcomes, 3 were for cardiovascular outcomes, 5 were for diabetes-related outcomes, 1 was for neurocognitive disorders, 3 were for pregnancy outcomes, and 11 were for other outcomes, suggesting that folate supplementation was associated with a decreased risk of these aforementioned diseases. However, two meta-analyses for cancer-related outcomes reported pooled results with p-values lower than 0.05, suggesting that folate supplementation was associated with increased risks of colorectal adenomatous lesion and prostate cancer.
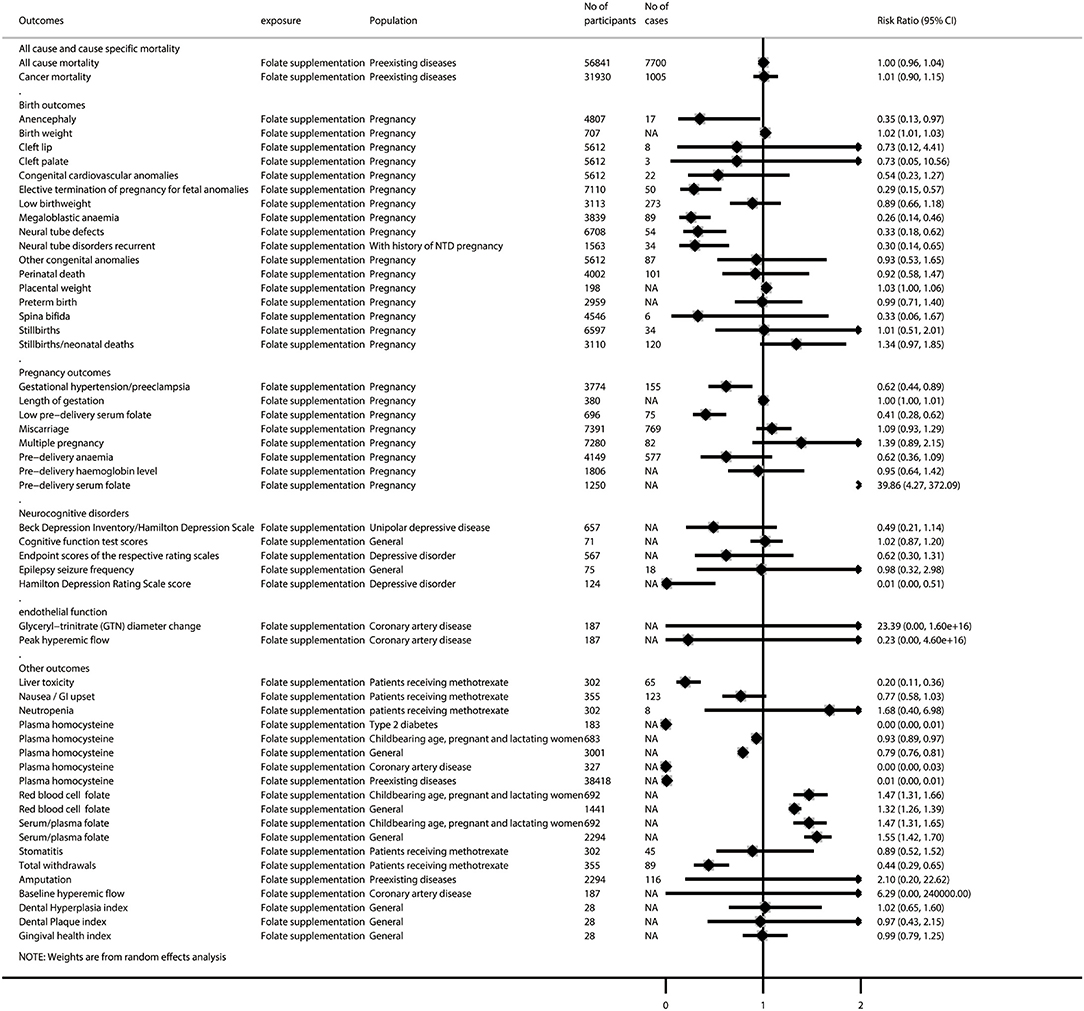
Figure 4. Summary random-effects estimates of all-cause and cause-specific mortality, birth outcomes, pregnancy outcomes, neurocognitive disorders, endothelial function, and other outcomes reported in meta-analyses of randomized controlled trials.
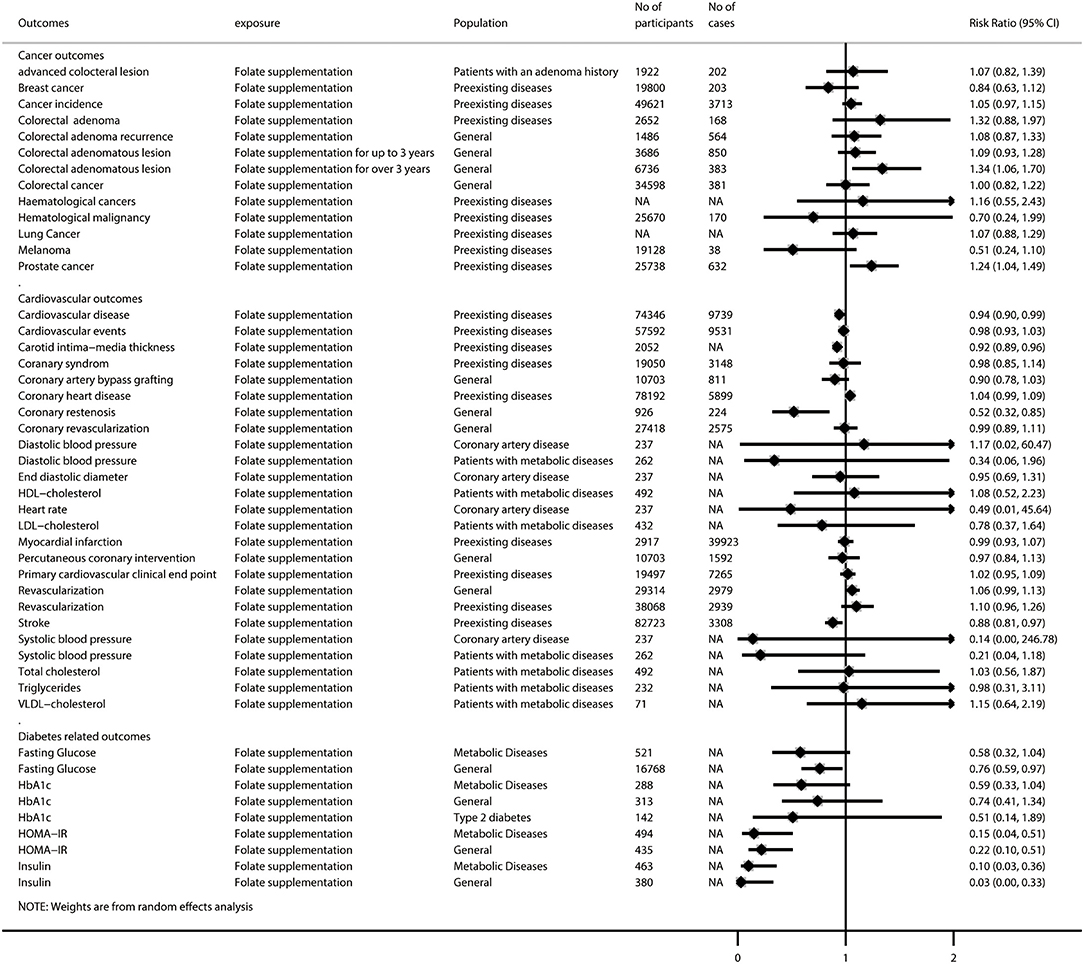
Figure 5. Summary random-effects estimates of cancer outcomes, cardiovascular outcomes, and diabetes-related outcomes reported in meta-analyses of randomized controlled trials.
As shown in Supplementary Table 7, 25 of the 108 meta-analyses (23.1%) showed statistical significance (p < 0.01); the 95% prediction interval excluded the null in 6 (5.6%), 14 (13.0%) had more than 1,000 cases (or more than 20,000 participants for continuous outcomes), 8 (7.4%) had no evidence of excess significance bias (p > 0.10) or small-study effects (p > 0.10), and 49 (45.4%) showed no great heterogeneity (I2 < 50% and p > 0.05).
Supplementary Table 8 presents a breakdown of the AMSTAR 2 scores for the meta-analyses representing each outcome. None of the 108 meta-analyses was rated at a high methodological level, and 14 (13.0%) were rated as moderate, leaving 36 (33.3%) as low and 58 (53.7%) as critically low. In terms of evidence quality for each outcome, 10 of the 108 meta-analyses (9.3%) were rated as high, 24 (22.2%) were rated as moderate, 22 (20.4%) were rated as low, and 52 (48.1%) were rated as very low quality by the GRADE classification (Supplementary Table 9).
Among the 34 meta-analyses with high or moderate GRADE classification, we found that folate supplementation was associated with decreased risk of elective termination of pregnancy for fetal anomalies; megaloblastic anemia; neural tube defects; CVD (among those with preexisting diseases); liver toxicity (patients receiving methotrexate); gestational hypertension/preeclampsia; low predelivery serum folate; decreased scores on the Hamilton Depression Rating Scale and levels of plasma homocysteine (both among patients with type 2 diabetes and the general population); increased levels of birth weight, red blood cell folate, and serum/plasma folate; and increased risk of prostate cancer (among those with preexisting diseases). However, we did not find any significant association between folate supplementation and the all-cause mortality rate (among those with preexisting diseases), cancer mortality rate (among those with preexisting diseases), low birth weight, preterm birth, stillbirths/neonatal deaths, cancer incidence (among those with preexisting diseases), colorectal adenomatous lesion, colorectal cancer, coronary artery bypass grafting, diastolic blood pressure (among patients with coronary artery disease), end-diastolic diameter (among patients with coronary artery disease), myocardial infarction (among those with preexisting diseases), amputation, gingival health index, miscarriage, or multiple pregnancy (Supplementary Figure 3).
One hundred eighty (92.3%) unique meta-analyses examined only observational studies (n = 77) or RCTs (n = 103), so the evidence from those meta-analyses could not be compared between observational and randomized studies.
Five outcomes from 15 meta-analyses were investigated by meta-analyses of both observational studies (n = 10) and RCTs (n = 5) (Supplementary Table 10): cleft palate, neural tube defects, recurrence of neural tube defects, colorectal cancer, and gestational hypertension/preeclampsia. Between meta-analyses of observational studies and those of RCTs, the direction of the association/effect and level of statistical significance were concordant for cleft palate, neural tube defects, and the effects of different folate exposure [dietary folate intake (both case–control and cohort studies), red blood cell folate, circulating folate, and folate supplementation] on colorectal cancer. The direction of the association/effect but not the level of statistical significance was concordant for gestational hypertension/preeclampsia and the recurrence of neural tube defects among women with a previous pregnancy with indicators of neural tube defects. In addition, the pooled results of the effect of total folate intake on colorectal cancer from observational studies were also discordant with those from RCTs both in direction and the level of significance.
In this study, we first provided an overview and appraisal of the relationships between folate exposure and a wide range of health outcomes. We found that folate is more often associated with benefit than harm for a range of health outcomes across multiple measures of exposure, including folate intake, folate supplementation, and folate concentration. Overall, we observed the beneficial effects of folate intake/level/supplementation on all-cause mortality and a number of chronic diseases, including cancers, CVD, and metabolic-related outcomes, as well as several birth outcomes. However, adverse effects of supplemented/serum folate were observed on prostate cancer, colorectal adenomatous lesion, asthma or wheezing, and wheezing as an isolated symptom.
The beneficial effects of folate on the aforementioned health outcomes might be explained by a number of plausible mechanisms. First, folate is the cofactor for methionine synthase, which catalyzes the conversion of homocysteine, and folate levels are therefore inversely associated with homocysteine levels (119, 120). Hyperhomocysteinemia has been found to be associated with higher risks of some birth/pregnancy outcomes (121–123), cancers (124–126), CVD (127), and neurological conditions (128, 129). Second, the polymorphisms of 5,10-methylenetetrahydrofolate reductase, which are critical junctions in the folate-metabolizing pathway via their role of guiding folate metabolites to the DNA methylation pathway and away from the DNA synthesis pathway, may modulate the susceptibility of subjects to several birth/pregnancy outcomes (130–132), cancers (133, 134), CVD (135), and neurological conditions (136).
The evidence on the association of folate with the all-cause mortality rate in the general population remains controversial. Several studies have demonstrated that folate supplementation could reduce the risk of CVD-related death, which might be attributable to serum homocysteine reduction (137). In contrast, Ebbing et al. reported that folate treatment was associated with increased risks of cancer outcomes and all-cause mortality in patients with ischemic heart disease (138). Excess folic acid intake may stimulate the growth of established neoplasms in experimental animals (139). As such, establishing the appropriate range of folate dosage might be crucial to balance the benefits against the risks and allow us to more accurately study the associations of folate with all-cause or cause-related mortality.
Other than the reasons mentioned above, the associations between folate and cancers may also be explained by two further mechanisms: (1) folate deficiency may induce complete transformation of deoxyuridylate monophosphate to deoxythymidylate monophosphate, which induces mis-incorporation of uracil into DNA and leads to chromosomal breaks and mutations (140, 141); and/or (2) folate deficiency may cause abnormal methylation of DNA, leading to alterations in expression of critical protooncogenes and tumor suppressor genes (142, 143). Experiments in vivo on mice and dogs have suggested that increased folate intake altered DNA methylation and in turn reduced the risks of cancers (144, 145).
Studies have suggested that folate can also prevent and reverse endothelial dysfunction (146, 147), which is an important risk factor for CVD (148, 149). Folate may improve the bioavailability of nitric oxide (NO) by increasing endothelial NO synthase coupling and NO production and by directly scavenging superoxide radicals (150, 151). By enhancing NO bioavailability, folate may improve endothelial function, thereby preventing or reversing the progression of CVD (147).
In contrast to its many beneficial effects, we observed adverse effects of folate on prostate cancer, colorectal adenomatous lesion, asthma or wheezing, and wheezing as an isolated symptom. For the adverse effect of folate on increased risk of prostate cancer/asthma, we found that the significant associations were both driven by one individual study. And the removal of these two influential studies from the respective meta-analysis resulted in non-significant results. However, the assessment of New Castle–Ottawa scale suggested that both of these two studies were with low risks of bias (i.e., scored 7–9 out of 10, data not shown). We thus speculate that the inconsistent findings across the included studies may be ascribed to the heterogeneity of population and study design. Further meta-analyses with larger sample size are warranted to verify these associations. For the association between folate and increased risk of colorectal adenomatous lesion, the most likely explanation is that undiscovered early precursor lesions might have existed in the mucosa of these patients, and folate could have accelerated the proliferation and growth of these paraneoplastic lesions.
We found high-quality evidence that folate supplementation was associated with a lower risk of several birth/pregnancy outcomes (neural tube defects, megaloblastic anemia, elective termination of pregnancy for fetal anomalies, small for gestational age, non-syndromic cleft lip with or without cleft palate, gestational hypertension/preeclampsia, and low predelivery serum folate), decreased scores on the Hamilton Depression Rating Scale (in a population with depressive disorder) and levels of plasma homocysteine, and increased serum/plasma folate. Although the meta-analyses of these outcomes might still be subject to potential biases, such as those without a preregistered protocol and the presence of high heterogeneity (for outcomes of small for gestational age and serum/plasma folate), our results are encouraging enough to verify the recommendation that women of child-bearing age should take folate supplementation to prevent adverse birth/pregnancy outcomes.
We found moderate-quality evidence from meta-analyses of observational studies that serum folate was associated with a higher risk of prostate cancer, which is consistent with the high-quality evidence from meta-analyses of RCT that folate supplementation was associated with increased risk of prostate cancer. The potential mechanism of folate in the development of this cancer is unclear. In vitro models using human prostate tissue have shown enhanced proliferation of tumor cells under conditions of elevated folate concentrations (152). Elsewhere, mice with transgenic adenoma of mouse prostate (TRAMP) that were fed a folate-depleted diet had lower cellular proliferation than mice with TRAMP fed a normal or high-folate diet (153).
In this umbrella review, the specific trends of relationships between folate and increased risks of neurocognitive disorders (such as cognitive impairment, Alzheimer disease, and depression) were also observed. The proposed mechanisms through which folate affects these diseases include suppression of DNA methylation and reduction of tetrahydrobiopterin levels, hyperhomocysteinemia, and excessive mis-incorporation of uracil into DNA (154). In contrast to the evidence that folate supplementation reduced the risk of stroke, the effect of folate on improving cognitive function or slowing cognitive decline in healthy or cognitively impaired older individuals was inconclusive (155). Prospective studies are strongly warranted to cover this knowledge gap.
This umbrella review has several strengths. First, we are the first to summarize the evidence for the associations between folate intake/levels and a wide range of health-related outcomes by incorporating information from published meta-analyses of observational studies or RCTs. Second, we used systematic methods that included a robust search strategy of three scientific literature databases and independent study selection and extraction by two investigators. When possible, we repeated each meta-analysis with a standardized approach that included the use of random-effects analysis and produced measures of heterogeneity and publication bias to allow better comparison across outcomes. We also used standard approaches to assess the quality of methods (AMSTAR 2) and the quality of evidence (GRADE) of the included meta-analyses.
Our study should also be interpreted cautiously with several limitations. First, the credibility assessment method was based on established tools for observational evidence, which are susceptible to bias and uncertainty. Another limitation of the umbrella review approach is the use of existing meta-analyses. Meta-analyses are known to have important limitations, such as limited coverage of the literature search, quality of included studies, and selective outcome reporting.
Our umbrella review found high- and moderate-quality evidence for the effect of folate on health outcomes such as mortality, cancers, CVD, and metabolic-related outcomes, as well as several birth outcomes. Therefore, our results support the current recommendation of daily folate supplementation for preventing adverse birth/pregnancy outcomes, cardiovascular and metabolic disease, and other disease. Further RCTs with large sample sizes are warranted to confirm these observed findings and to study the concentration–response relationships between folate exposure and health outcomes.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
ZY was the project lead for the current study. YBo and YZ searched databases and screened the articles. YBo and YT extracted the data. YBo, XL, and YZ conducted statistical analysis. YBo wrote the manuscript. DZ, YBu, ZW, LW, and ZY reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China No. 21577119. The funder was not involved in the design of the study and collection, analysis and interpretation of data, and in writing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2020.550753/full#supplementary-material
1. Plumptre L, Masih SP, Ly A, Aufreiter S, Sohn KJ, Croxford R, et al. High concentrations of folate and unmetabolized folic acid in a cohort of pregnant Canadian women and umbilical cord blood. Am J Clin Nutr. (2015) 102:848–57. doi: 10.3945/ajcn.115.110783
2. Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. (2015) 13:132–40. doi: 10.1097/XEB.0000000000000055
3. Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, Mitra A, et al. Obesity and gynaecological and obstetric conditions: umbrella review of the literature. BMJ. (2017) 359:j4511. doi: 10.1136/bmj.j4511
4. Jackson D, Bowden J. A re-evaluation of the 'quantile approximation method' for random effects meta-analysis. Stat Med. (2009) 28:338–48. doi: 10.1002/sim.3487
5. Higgins JP. Commentary: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. (2008) 37:1158–60. doi: 10.1093/ije/dyn204
6. Ioannidis JP, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials. (2007) 4:245–53. doi: 10.1177/1740774507079441
7. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
8. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008
9. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. (2011) 64:383–94. doi: 10.1016/j.jclinepi.2010.04.026
10. Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015
11. Badovinac RL, Werler MM, Williams PL, Kelsey KT, Hayes C. Folic acid-containing supplement consumption during pregnancy and risk for oral clefts: a meta-analysis. Birth Defects Res A Clin Mol Teratol. (2007) 79:8–15. doi: 10.1002/bdra.20315
12. Bailie L, Loughrey MB, Coleman HG. Lifestyle risk factors for serrated colorectal polyps: a systematic review and meta-analysis. Gastroenterology. (2017) 152:92–104. doi: 10.1053/j.gastro.2016.09.003
13. Blencowe H, Cousens S, Modell B, Lawn J. Folic acid to reduce neonatal mortality from neural tube disorders. Int J Epidemiol. (2010) 39(Suppl. 1):i110–21. doi: 10.1093/ije/dyq028
14. Burr NE, Hull MA, Subramanian V. Folic acid supplementation may reduce colorectal cancer risk in patients with inflammatory bowel disease: a systematic review and meta-analysis. J Clin Gastroenterol. (2017) 51:247–53. doi: 10.1097/MCG.0000000000000498
15. Chen P, Li C, Li X, Li J, Chu R, Wang H. Higher dietary folate intake reduces the breast cancer risk: a systematic review and meta-analysis. Br J Cancer. (2014) 110:2327–38. doi: 10.1038/bjc.2014.155
16. Chuang SC, Rota M, Gunter MJ, Zeleniuch-Jacquotte A, Eussen SJ, Vollset SE, et al. Quantifying the dose-response relationship between circulating folate concentrations and colorectal cancer in cohort studies: a meta-analysis based on a flexible meta-regression model. Am J Epidemiol. (2013) 178:1028–37. doi: 10.1093/aje/kwt083
17. Collin SM, Metcalfe C, Refsum H, Lewis SJ, Zuccolo L, Smith GD, et al. Circulating folate, vitamin B12, homocysteine, vitamin B12 transport proteins, and risk of prostate cancer: a case-control study, systematic review, and meta-analysis. Cancer Epidemiol Biomarkers Prev. (2010) 19:1632–42. doi: 10.1158/1055-9965.EPI-10-0180
18. Crider KS, Cordero AM, Qi YP, Mulinare J, Dowling NF, Berry RJ. Prenatal folic acid and risk of asthma in children: a systematic review and meta-analysis. Am J Clin Nutr. (2013) 98:1272–81. doi: 10.3945/ajcn.113.065623
19. Dai WM, Yang B, Chu XY, Wang YQ, Zhao M, Chen L, et al. Association between folate intake, serum folate levels and the risk of lung cancer: a systematic review and meta-analysis. Chin Med J. (2013) 126:1957–64. doi: 10.3760/cma.j.issn.0366-6999.20130391
20. Dean SV, Lassi ZS, Imam AM, Bhutta ZA. Preconception care: nutritional risks and interventions. Reprod Health. (2014) 11(Suppl. 3):S3. doi: 10.1186/1742-4755-11-S3-S3
21. Du L, Wang Y, Zhang H, Zhang H, Gao Y. Folate intake and the risk of endometrial cancer: a meta-analysis. Oncotarget. (2016) 7:85176–84. doi: 10.18632/oncotarget.13211
22. Fan C, Yu S, Zhang S, Ding X, Su J, Cheng Z. Association between folate intake and risk of head and neck squamous cell carcinoma: an overall and dose-response PRISMA meta-analysis. Medicine. (2017) 96:e8182. doi: 10.1097/MD.0000000000008182
23. Feng Y, Wang S, Chen R, Tong X, Wu Z, Mo X. Maternal folic acid supplementation and the risk of congenital heart defects in offspring: a meta-analysis of epidemiological observational studies. Sci Rep. (2015) 5:8506. doi: 10.1038/srep08506
24. Gilbody S, Lightfoot T, Sheldon T. Is low folate a risk factor for depression? A meta-analysis and exploration of heterogeneity. J Epidemiol Commun Health. (2007) 61:631–7. doi: 10.1136/jech.2006.050385
25. He H, Shui B. Folate intake and risk of bladder cancer: a meta-analysis of epidemiological studies. Int J Food Sci Nutr. (2014) 65:286–92. doi: 10.3109/09637486.2013.866641
26. Heine-Broring RC, Winkels RM, Renkema JM, Kragt L, van Orten-Luiten AC, Tigchelaar EF, et al. Dietary supplement use and colorectal cancer risk: a systematic review and meta-analyses of prospective cohort studies. Int J Cancer. (2015) 136:2388–401. doi: 10.1002/ijc.29277
27. Hodgetts VA, Morris RK, Francis A, Gardosi J, Ismail KM. Effectiveness of folic acid supplementation in pregnancy on reducing the risk of small-for-gestational age neonates: a population study, systematic review and meta-analysis. BJOG. (2015) 122:478–90. doi: 10.1111/1471-0528.13202
28. Hua X, Zhang J, Guo Y, Shen M, Gaudet L, Janoudi G, et al. Effect of folic acid supplementation during pregnancy on gestational hypertension/preeclampsia: a systematic review and meta-analysis. Hypertens Pregnancy. (2016) 35:447–60. doi: 10.1080/10641955.2016.1183673
29. Imdad A, Yakoob MY, Bhutta ZA. The effect of folic acid, protein energy and multiple micronutrient supplements in pregnancy on stillbirths. BMC Public Health. (2011) 11(Suppl. 3):S4. doi: 10.1186/1471-2458-11-S3-S4
30. Johnson CY, Little J. Folate intake, markers of folate status and oral clefts: is the evidence converging? Int J Epidemiol. (2008) 37:1041–58. doi: 10.1093/ije/dyn098
31. Kennedy DA, Stern SJ, Moretti M, Matok I, Sarkar M, Nickel C, et al. Folate intake and the risk of colorectal cancer: a systematic review and meta-analysis. Cancer Epidemiol. (2011) 35:2–10. doi: 10.1016/j.canep.2010.11.004
32. Kim DH, Smith-Warner SA, Spiegelman D, Yaun SS, Colditz GA, Freudenheim JL, et al. Pooled analyses of 13 prospective cohort studies on folate intake and colon cancer. Cancer Causes Control. (2010) 21:1919–30. doi: 10.1007/s10552-010-9620-8
33. Larsson SC, Giovannucci E, Wolk A. Folate intake, MTHFR polymorphisms, and risk of esophageal, gastric, and pancreatic cancer: a meta-analysis. Gastroenterology. (2006) 131:1271–83. doi: 10.1053/j.gastro.2006.08.010
34. Larsson SC, Giovannucci E, Wolk A. Folate and risk of breast cancer: a meta-analysis. JNCI. (2007) 99:64–76. doi: 10.1093/jnci/djk006
35. Lewis SJ, Harbord RM, Harris R, Smith GD. Meta-analyses of observational and genetic association studies of folate intakes or levels and breast cancer risk. J Natl Cancer Inst. (2006) 98:1607–22. doi: 10.1093/jnci/djj440
36. Li B, Lu Y, Wang L, Zhang CX. Folate intake and breast cancer prognosis: a meta-analysis of prospective observational studies. Eur J Cancer Prev. (2015) 24:113–21. doi: 10.1097/CEJ.0000000000000028
37. Li C, Chen P, Hu P, Li M, Li X, Guo H, et al. Folate intake and MTHFR polymorphism C677T is not associated with ovarian cancer risk: evidence from the meta-analysis. Mol Biol Rep. (2013) 40:6547–60. doi: 10.1007/s11033-013-2686-0
38. Lin HL, An QZ, Wang QZ, Liu CX. Folate intake and pancreatic cancer risk: an overall and dose-response meta-analysis. Public Health. (2013) 127:607–13. doi: 10.1016/j.puhe.2013.04.008
39. Liu M, Cui LH, Ma AG, Li N, Piao JM. Lack of effects of dietary folate intake on risk of breast cancer: an updated meta-analysis of prospective studies. Asian Pac J Cancer Prev. (2014) 15:2323–8. doi: 10.7314/APJCP.2014.15.5.2323
40. Liu W, Zhou H, Zhu Y, Tie C. Associations between dietary folate intake and risks of esophageal, gastric and pancreatic cancers: an overall and dose-response meta-analysis. Oncotarget. (2017) 8:86828–42. doi: 10.18632/oncotarget.18775
41. Liu Y, Yu Q, Zhu Z, Zhang J, Chen M, Tang P, et al. Vitamin and multiple-vitamin supplement intake and incidence of colorectal cancer: a meta-analysis of cohort studies. Med Oncol. (2015) 32:434. doi: 10.1007/s12032-014-0434-5
42. Liu YX, Wang B, Wan MH, Tang WF, Huang FK, Li C. Meta-analysis of the relationship between the metholenetetrahydrofolate reductase C677T genetic polymorphism, folate intake and esophageal cancer. Asian Pac J Cancer Prev. (2011) 12:247–52.
43. Mao B, Li Y, Zhang Z, Chen C, Chen Y, Ding C, et al. One-carbon metabolic factors and risk of renal cell cancer: a meta-analysis. PLoS ONE. (2015) 10:e0141762. doi: 10.1371/journal.pone.0141762
44. Michelakos T, Kousoulis AA, Katsiardanis K, Dessypris N, Anastasiou A, Katsiardani KP, et al. Serum folate and B12 levels in association with cognitive impairment among seniors: results from the VELESTINO study in Greece and meta-analysis. J Aging Health. (2013) 25:589–616. doi: 10.1177/0898264313482488
45. Millacura N, Pardo R, Cifuentes L, Suazo J. Effects of folic acid fortification on orofacial clefts prevalence: a meta-analysis. Public Health Nutr. (2017) 20:2260–8. doi: 10.1017/S1368980017000878
46. Milne E, Royle JA, Miller M, Bower C, de Klerk NH, Bailey HD, et al. Maternal folate and other vitamin supplementation during pregnancy and risk of acute lymphoblastic leukemia in the offspring. Int J Cancer. (2010) 126:2690–9. doi: 10.1002/ijc.24969
47. Moazzen S, Dolatkhah R, Tabrizi JS, Shaarbafi J, Alizadeh BZ, de Bock GH, et al. Folic acid intake and folate status and colorectal cancer risk: a systematic review and meta-analysis. Clin Nutr. (2017) 37(6 Pt A):1926–34. doi: 10.1016/j.clnu.2017.10.010
48. Myung SK, Ju W, Kim SC, Kim H. Vitamin or antioxidant intake (or serum level) and risk of cervical neoplasm: a meta-analysis. BJOG. (2011) 118:1285–91. doi: 10.1111/j.1471-0528.2011.03032.x
49. Park YM, Youn J, Cho CH, Kim SH, Lee JE. Circulating folate levels and colorectal adenoma: a case-control study and a meta-analysis. Nutr Res Pract. (2017) 11:419–29. doi: 10.4162/nrp.2017.11.5.419
50. Petridou ET, Kousoulis AA, Michelakos T, Papathoma P, Dessypris N, Papadopoulos FC, et al. Folate and B12 serum levels in association with depression in the aged: a systematic review and meta-analysis. Aging Ment Health. (2016) 20:965–73. doi: 10.1080/13607863.2015.1049115
51. Price AJ, Travis RC, Appleby PN, Albanes D, Barricarte Gurrea A, Bjorge T, et al. Circulating folate and Vitamin B12 and risk of prostate cancer: a collaborative analysis of individual participant data from six cohorts including 6875 Cases and 8104 Controls. (2016) Eur Urol. 70:941–51. doi: 10.1016/j.eururo.2016.05.024
52. Sanjoaquin MA, Allen N, Couto E, Roddam AW, Key TJ. Folate intake and colorectal cancer risk: a meta-analytical approach. Int J Cancer. (2005) 113:825–8. doi: 10.1002/ijc.20648
53. Shen L. Associations between B Vitamins and Parkinson's Disease. Nutrients. (2015) 7:7197–208. doi: 10.3390/nu7095333
54. Shen L, Ji HF. Associations between homocysteine, folic acid, Vitamin B12 and Alzheimer's disease: insights from meta-analyses. J Alzheimers Dis. (2015) 46:777–90. doi: 10.3233/JAD-150140
55. Tio M, Andrici J, Cox MR, Eslick GD. Folate intake and the risk of upper gastrointestinal cancers: a systematic review and meta-analysis. J Gastroenterol Hepatol. (2014) 29:250–8. doi: 10.1111/jgh.12446
56. Tio M, Andrici J, Cox MR, Eslick GD. Folate intake and the risk of prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. (2014) 17:213–9. doi: 10.1038/pcan.2014.16
57. Tio M, Andrici J, Eslick GD. Folate intake and the risk of breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. (2014) 145:513–24. doi: 10.1007/s10549-014-2969-8
58. Wang M, Li K, Zhao D, Li L. The association between maternal use of folic acid supplements during pregnancy and risk of autism spectrum disorders in children: a meta-analysis. Mol Autism. (2017) 8:51. doi: 10.1186/s13229-017-0170-8
59. Wang R, Zheng Y, Huang JY, Zhang AQ, Zhou YH, Wang JN. Folate intake, serum folate levels, and prostate cancer risk: a meta-analysis of prospective studies. BMC Public Health. (2014) 14:1326. doi: 10.1186/1471-2458-14-1326
60. Wang T, Zhang HP, Zhang X, Liang ZA, Ji YL, Wang G. Is folate status a risk factor for asthma or other allergic diseases? Allergy Asthma Immunol Res. (2015) 7:538–46. doi: 10.4168/aair.2015.7.6.538
61. Wang ZM, Zhou B, Nie ZL, Gao W, Wang YS, Zhao H, et al. Folate and risk of coronary heart disease: a meta-analysis of prospective studies. Nutr Metab Cardiovasc Dis. (2012) 22:890–9. doi: 10.1016/j.numecd.2011.04.011
62. Wien TN, Pike E, Wisloff T, Staff A, Smeland S, Klemp M. Cancer risk with folic acid supplements: a systematic review and meta-analysis. BMJ Open. (2012) 2:e000653. doi: 10.1136/bmjopen-2011-000653
63. Xu A, Cao X, Lu Y, Li H, Zhu Q, Chen X, et al. A meta-analysis of the relationship between maternal folic acid supplementation and the risk of congenital heart defects. Int Heart J. (2016) 57:725–728. doi: 10.1536/ihj.16-054
64. Yang X, Chen H, Du Y, Wang S, Wang Z. Periconceptional folic acid fortification for the risk of gestational hypertension and pre-eclampsia: a meta-analysis of prospective studies. Matern Child Nutr. (2016) 12:669–79. doi: 10.1111/mcn.12209
65. Zhang Q, Wang Y, Xin X, Zhang Y, Liu D, Peng Z, et al. Effect of folic acid supplementation on preterm delivery and small for gestational age births: a systematic review and meta-analysis. Reprod Toxicol. (2017) 67:35–41. doi: 10.1016/j.reprotox.2016.11.012
66. Zhang YF, Shi WW, Gao HF, Zhou L, Hou AJ, Zhou YH. Folate intake and the risk of breast cancer: a dose-response meta-analysis of prospective studies. PLoS ONE. (2014) 9:e100044. doi: 10.1371/journal.pone.0100044
67. Zhang YF, Zhou L, Zhang HW, Hou AJ, Gao HF, Zhou YH. Association between folate intake and the risk of lung cancer: a dose-response meta-analysis of prospective studies. PLoS ONE. (2014) 9:e93465. doi: 10.1371/journal.pone.0093465
68. Zhao Y, Guo C, Hu H, Zheng L, Ma J, Jiang L, et al. Folate intake, serum folate levels and esophageal cancer risk: an overall and dose-response meta-analysis. Oncotarget. (2017) 8:10458–69. doi: 10.18632/oncotarget.14432
69. Zhou X, Meng Y. Association between serum folate level and cervical cancer: a meta-analysis. Arch Gynecol Obstet. (2016) 293:871–7. doi: 10.1007/s00404-015-3852-5
70. Ni Y, Du J, Yin X, Lu M. Folate intake, serum folate, and risk of esophageal cancer: a systematic review and dose-response meta-analysis. Eur J Cancer Prev. (2018) 28:173–80. doi: 10.1097/CEJ.0000000000000441
71. Jahanbin A, Shadkam E, Miri HH, Shirazi AS, Abtahi M. Maternal folic acid supplementation and the risk of oral clefts in offspring. J Craniofac Surg. (2018) 29:e534–41. doi: 10.1097/SCS.0000000000004488
72. Yang L, Jiang L, Bi M, Jia X, Wang Y, He C, et al. High dose of maternal folic acid supplementation is associated to infant asthma. Food Chem Toxicol. (2015) 75:88–93. doi: 10.1016/j.fct.2014.11.006
73. Homocysteine Lowering Trialists' Collaboration. Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr. (2005) 82:806–12. doi: 10.1093/ajcn/82.4.806
74. Baggott JE, Oster RA, Tamura T. Meta-analysis of cancer risk in folic acid supplementation trials. Cancer Epidemiol. (2012) 36:78–81. doi: 10.1016/j.canep.2011.05.003
75. Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA. (2006) 296:2720–6. doi: 10.1001/jama.296.22.2720
76. Berti C, Fekete K, Dullemeijer C, Trovato M, Souverein OW, Cavelaars A, et al. Folate intake and markers of folate status in women of reproductive age, pregnant and lactating women: a meta-analysis. J Nutr Metab. (2012) 2012:470656. doi: 10.1155/2012/470656
77. Carroll C, Cooper K, Papaioannou D, Hind D, Tappenden P, Pilgrim H, et al. Meta-analysis: folic acid in the chemoprevention of colorectal adenomas and colorectal cancer. Aliment Pharmacol Ther. (2010) 31:708–18. doi: 10.1111/j.1365-2036.2010.04238.x
78. Clarke R, Armitage J. Vitamin supplements and cardiovascular risk: review of the randomized trials of homocysteine-lowering vitamin supplements. Semin Thromb Hemost. (2000) 26:341–8. doi: 10.1055/s-2000-8101
79. de Bree, van Mierlo LA, Draijer R. Folic acid improves vascular reactivity in humans: a meta-analysis of randomized controlled trials. Am J Clin Nutr. (2007) 86:610–7. doi: 10.1093/ajcn/86.3.610
80. de-Regil LM, Pena-Rosas JP, Fernandez-Gaxiola AC, Rayco-Solon P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. (2015) 12:Cd007950. doi: 10.1002/14651858.CD007950.pub3
81. Duffy ME, Hoey L, Hughes CF, Strain JJ, Rankin A, Souverein OW, et al. Biomarker responses to folic acid intervention in healthy adults: a meta-analysis of randomized controlled trials. Am J Clin Nutr. (2014) 99:96–106. doi: 10.3945/ajcn.113.062752
82. Fekete K, Berti C, Trovato M, Lohner S, Dullemeijer C, Souverein OW, et al. Effect of folate intake on health outcomes in pregnancy: a systematic review and meta-analysis on birth weight, placental weight and length of gestation. Nutr J. (2012) 11:75. doi: 10.1186/1475-2891-11-75
83. Fife J, Raniga S, Hider PN, Frizelle FA. Folic acid supplementation and colorectal cancer risk: a meta-analysis. Colorectal Dis. (2011) 13:132–7. doi: 10.1111/j.1463-1318.2009.02089.x
84. Figueiredo JC, Mott LA, Giovannucci E, Wu K, Cole B, Grainge MJ, et al. Folic acid and prevention of colorectal adenomas: a combined analysis of randomized clinical trials. Int J Cancer. (2011) 129:192–203. doi: 10.1002/ijc.25872
85. Huo Y, Qin X, Wang J, Sun N, Zeng Q, Xu X, et al. Efficacy of folic acid supplementation in stroke prevention: new insight from a meta-analysis. Int J Clin Pract. (2012) 66:544–51. doi: 10.1111/j.1742-1241.2012.02929.x
86. Ibrahim EM, Zekri JM. Folic acid supplementation for the prevention of recurrence of colorectal adenomas: metaanalysis of interventional trials. Med Oncol. (2010) 27:915–8. doi: 10.1007/s12032-009-9306-9
87. Lassi ZS, Salam RA, Haider BA, Bhutta ZA. Folic acid supplementation during pregnancy for maternal health and pregnancy outcomes. Cochrane Database Syst Rev. (2013) Cd006896. doi: 10.1002/14651858.CD006896.pub2
88. Lee M, Hong K-S, Chang S-C, Saver JL. Efficacy of homocysteine lowering therapy with folic acid in stroke prevention: a meta-analysis. Stroke. (2010) 41:1205–12. doi: 10.1161/STROKEAHA.109.573410
89. Li Y, Huang T, Zheng Y, Muka T, Troup J, Hu FB. Folic acid supplementation and the risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. J Am Heart Assoc. (2016) 15:e003768. doi: 10.1161/JAHA.116.003768
90. McRae MP. High-dose folic acid supplementation effects on endothelial function and blood pressure in hypertensive patients: a meta-analysis of randomized controlled clinical trials. J Chiropr Med. (2009) 8:15–24. doi: 10.1016/j.jcm.2008.09.001
91. Miller ER 3rd, Juraschek S, Pastor-Barriuso R, Bazzano LA, Appel LJ, Guallar E. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am J Cardiol. (2010) 106:517–27. doi: 10.1016/j.amjcard.2010.03.064
92. Myung SK, Ju W, Cho B, Oh SW, Park SM, Koo BK, et al. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: systematic review and meta-analysis of randomised controlled trials. BMJ. (2013) 346:f10. doi: 10.1136/bmj.f10
93. Qin T, Du M, Du H, Shu Y, Wang M, Zhu L. Folic acid supplements and colorectal cancer risk: meta-analysis of randomized controlled trials. Sci Rep. (2015) 5:12044. doi: 10.1038/srep12044
94. Qin X, Cui Y, Shen L, Sun N, Zhang Y, Li J, et al. Folic acid supplementation and cancer risk: a meta-analysis of randomized controlled trials. Int J Cancer. (2013) 133:1033–41. doi: 10.1002/ijc.28038
95. Qin X, Fan F, Cui Y, Chen F, Chen Y, Cheng X, et al. Folic acid supplementation with and without vitamin B6 and revascularization risk: a meta-analysis of randomized controlled trials. Clin Nutr. (2014) 33:603–12. doi: 10.1016/j.clnu.2014.01.006
96. Qin X, Huo Y, Langman CB, Hou F, Chen Y, Matossian D, et al. Folic acid therapy and cardiovascular disease in ESRD or advanced chronic kidney disease: a meta-analysis. Clin J Am Soc Nephrol. (2011) 6:482–8. doi: 10.2215/CJN.05310610
97. Qin X, Xu M, Zhang Y, Li J, Xu X, Wang X, et al. Effect of folic acid supplementation on the progression of carotid intima-media thickness: a meta-analysis of randomized controlled trials. Atherosclerosis. (2012) 222:307–13. doi: 10.1016/j.atherosclerosis.2011.12.007
98. Ranganathan LN, Ramaratnam S. Vitamins for epilepsy. Cochrane Database Syst Rev. (2005) 2:CD004304. doi: 10.1002/14651858.CD004304.pub2
99. Saccone G, Berghella V. Folic acid supplementation in pregnancy to prevent preterm birth: a systematic review and meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. (2016) 199:76–81. doi: 10.1016/j.ejogrb.2016.01.042
100. Schefft C, Kilarski LL, Bschor T, Kohler S. Efficacy of adding nutritional supplements in unipolar depression: a systematic review and meta-analysis. Eur Neuropsychopharmacol. (2017) 27:1090–109. doi: 10.1016/j.euroneuro.2017.07.004
101. Schwingshackl L, Boeing H, Stelmach-Mardas M, Gottschald M, Dietrich S, Hoffmann G, et al. Dietary supplements and risk of cause-specific death, cardiovascular disease, and cancer: a systematic review and meta-analysis of primary prevention trials. Adv Nutr. (2017) 8:27–39. doi: 10.3945/an.116.013516
102. Shea B, Swinden MV, Tanjong Ghogomu E, Ortiz Z, Katchamart W, Rader T, et al. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev. (2013) 5:CD000951. doi: 10.1002/14651858.CD000951.pub2
103. Sudchada P, Saokaew S, Sridetch S, Incampa S, Jaiyen S, Khaithong W. Effect of folic acid supplementation on plasma total homocysteine levels and glycemic control in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. (2012) 98:151–8. doi: 10.1016/j.diabres.2012.05.027
104. Tabrizi R, Lankarani KB, Akbari M, Naghibzadeh-Tahami A, Alizadeh H, Honarvar B, et al. The effects of folate supplementation on lipid profiles among patients with metabolic diseases: a systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Syndr. (2018) 12:423–30. doi: 10.1016/j.dsx.2017.12.022
105. Taylor MJ, Carney SM, Goodwin GM, Geddes JR. Folate for depressive disorders: systematic review and meta-analysis of randomized controlled trials. J Psychopharmacol. (2004) 18:251–6. doi: 10.1177/0269881104042630
106. Tian T, Yang KQ, Cui JG, Zhou LL, Zhou XL. Folic acid supplementation for stroke prevention in patients with cardiovascular disease. Am J Med Sci. (2017) 354:379–87. doi: 10.1016/j.amjms.2017.05.020
107. van Dijk M, Pot GK. The effects of nutritional interventions on recurrence in survivors of colorectal adenomas and cancer: a systematic review of randomised controlled trials. Eur J Clin Nutr. (2016) 70:566–73. doi: 10.1038/ejcn.2015.210
108. Vollset SE, Clarke R, Lewington S, Ebbing M, Halsey J, Lonn E, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50 000 individuals. Lancet. (2013) 381:1029–36. doi: 10.1016/S0140-6736(12)62001-7
109. Wald DS, Kasturiratne A, Simmonds M. Effect of folic acid, with or without other B vitamins, on cognitive decline: meta-analysis of randomized trials. Am J Med. (2010) 123:522–7.e2. doi: 10.1016/j.amjmed.2010.01.017
110. Wang X, Qin X, Demirtas H, Li J, Mao G, Huo Y, et al. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet. (2007) 369:1876–82. doi: 10.1016/S0140-6736(07)60854-X
111. Yang HT, Lee M, Hong KS, Ovbiagele B, Saver JL. Efficacy of folic acid supplementation in cardiovascular disease prevention: an updated meta-analysis of randomized controlled trials. Eur J Intern Med. (2012) 23:745–54. doi: 10.1016/j.ejim.2012.07.004
112. Yi X, Zhou Y, Jiang D, Li X, Guo Y, Jiang X. Efficacy of folic acid supplementation on endothelial function and plasma homocysteine concentration in coronary artery disease: a meta-analysis of randomized controlled trials. Exp Ther Med. (2014) 7:1100–10. doi: 10.3892/etm.2014.1553
113. Zhao M, Wu G, Li Y, Wang X, Hou FF, Xu X, et al. Meta-analysis of folic acid efficacy trials in stroke prevention: insight into effect modifiers. Neurology. (2017) 88:1830–8. doi: 10.1212/WNL.0000000000003909
114. Zhou YH, Tang JY, Wu MJ, Lu J, Wei X, Qin YY, et al. Effect of folic acid supplementation on cardiovascular outcomes: a systematic review and meta-analysis. PLoS ONE. (2011) 6:e25142. doi: 10.1371/journal.pone.0025142
115. Roberts E, Carter B, Young AH. Caveat emptor: folate in unipolar depressive illness, a systematic review and meta-analysis. J Psychopharmacol. (2018) 32:377–384. doi: 10.1177/0269881118756060
116. Akbari M, Tabrizi R, Lankarani KB, Heydari ST, Karamali M, Kashanian M, et al. The effects of folate supplementation on diabetes biomarkers among patients with metabolic diseases: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res. (2018) 50:93–105. doi: 10.1055/s-0043-125148
117. Zhao JV, Schooling CM, Zhao JX. The effects of folate supplementation on glucose metabolism and risk of type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Ann Epidemiol. (2018) 28:249–57.e1. doi: 10.1016/j.annepidem.2018.02.001
118. Hsu CY, Chiu SW, Hong KS, Saver JL, Wu YL, Lee JD, et al. Folic acid in stroke prevention in countries without mandatory folic acid food fortification: a meta-analysis of randomized controlled trials. J Stroke. (2018) 20:99–109. doi: 10.5853/jos.2017.01522
119. Javadi L, Pourghassem Gargari B, Salekzamani S, Yousefzadeh R. Folate and homocysteine levels and their association with dietary intakes in Iranian patients infected with Helicobacter pylori: a case-control study. Acta Med Iran. (2015) 53:162–7.
120. Serapinas D, Boreikaite E, Bartkeviciute A, Bandzeviciene R, Silkunas M, Bartkeviciene D. The importance of folate, vitamins B6 and B12 for the lowering of homocysteine concentrations for patients with recurrent pregnancy loss and MTHFR mutations. Reprod Toxicol. (2017) 72:159–3. doi: 10.1016/j.reprotox.2017.07.001
121. Limpach A, Dalton M, Miles R, Gadson P. Homocysteine inhibits retinoic acid synthesis: a mechanism for homocysteine-induced congenital defects. Exp Cell Res. (2000) 260:166–74. doi: 10.1006/excr.2000.5000
122. Smedts HP, van Uitert EM, Valkenburg O, Laven JS, Eijkemans MJ, Lindemans J, et al. A derangement of the maternal lipid profile is associated with an elevated risk of congenital heart disease in the offspring. Nutr Metab Cardiovasc Dis. (2012) 22:477–85. doi: 10.1016/j.numecd.2010.07.016
123. Gaiday AN, Tussupkaliyev AB, Bermagambetova SK, Zhumagulova SS, Sarsembayeva LK, Dossimbetova MB, et al. Effect of homocysteine on pregnancy: A systematic review. Chem Biol Interact. (2018) 293:70–6. doi: 10.1016/j.cbi.2018.07.021
124. Chiang FF, Wang HM, Lan YC, Yang MH, Huang SC, Huang YC. High homocysteine is associated with increased risk of colorectal cancer independently of oxidative stress and antioxidant capacities. Clin Nutr. (2014) 33:1054–60. doi: 10.1016/j.clnu.2013.11.007
125. Lin J, Lee IM, Song Y, Cook NR, Selhub J, Manson JE, et al. Plasma homocysteine and cysteine and risk of breast cancer in women. Cancer Res. (2010) 70:2397–405. doi: 10.1158/0008-5472.CAN-09-3648
126. Zhang D, Wen X, Wu W, Guo Y, Cui W. Elevated homocysteine level and folate deficiency associated with increased overall risk of carcinogenesis: meta-analysis of 83 case-control studies involving 35,758 individuals. PLoS ONE. (2015) 10:e0123423. doi: 10.1371/journal.pone.0123423
127. Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. (2015) 14:6. doi: 10.1186/1475-2891-14-6
128. Clarke R, Bennett D, Parish S, Lewington S, Skeaff M, Eussen SJ, et al. Effects of homocysteine lowering with B vitamins on cognitive aging: meta-analysis of 11 trials with cognitive data on 22,000 individuals. Am J Clin Nutr. (2014) 100:657–66. doi: 10.3945/ajcn.113.076349
129. Hu Q, Teng W, Li J, Hao F, Wang N. Homocysteine and Alzheimer's disease: evidence for a causal link from mendelian randomization. J Alzheimers Dis. (2016) 52:747–56. doi: 10.3233/JAD-150977
130. Yin M, Dong L, Zheng J, Zhang H, Liu J, Xu Z. Meta analysis of the association between MTHFR C677T polymorphism and the risk of congenital heart defects. Ann Hum Genet. (2012) 76:9–16. doi: 10.1111/j.1469-1809.2011.00687.x
131. Wu H, Zhu P, Geng X, Liu Z, Cui L, Gao Z, et al. Genetic polymorphism of MTHFR C677T with preterm birth and low birth weight susceptibility: a meta-analysis. Arch Gynecol Obstet. (2017) 295:1105–18. doi: 10.1007/s00404-017-4322-z
132. Zhang Y, He X, Xiong X, Chuan J, Zhong L, Chen G, et al. The association between maternal methylenetetrahydrofolate reductase (MTHFR) C677T and A1298C polymorphism and birth defects and adverse pregnancy outcomes. Prenat Diagn. (2018) 39:3–9. doi: 10.1002/pd.5396
133. Yi K, Yang L, Lan Z, Xi M. The association between MTHFR polymorphisms and cervical cancer risk: a system review and meta analysis. Arch Gynecol Obstet. (2016) 294:579–88. doi: 10.1007/s00404-016-4037-6
134. Liu W, Li Y, Li R, Han X, Ma Y, Liu B, et al. Association of MTHFR A1298C polymorphism with breast cancer and/or ovarian cancer risk: an updated meta-analysis. Afr J Tradit Complement Altern Med. (2016) 13:72–86. doi: 10.21010/ajtcam.v13i5.11
135. Nassereddine S, Kassogue Y, Korchi F, Habbal R, Nadifi S. Association of methylenetetrahydrofolate reductase gene (C677T) with the risk of hypertension in Morocco. BMC Res Notes. (2015) 8:775. doi: 10.1186/s13104-015-1772-x
136. Rai V. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism and Alzheimer disease risk: a meta-analysis. Mol Neurobiol. (2017) 54:1173–86. doi: 10.1007/s12035-016-9722-8
137. Sonawane K, Zhu Y, Chan W, Aguilar D, Deshmukh AA, Suarez-Almazor ME. Association of serum folate levels with cardiovascular mortality among adults with rheumatoid arthritis. JAMA Netw Open. (2020) 3:e200100. doi: 10.1001/jamanetworkopen.2020.0100
138. Ebbing M, Bønaa KH, Nygård O, Arnesen E, Ueland PM, Nordrehaug JE, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. (2009) 302:2119–26. doi: 10.1001/jama.2009.1622
139. Ulrich CM, Potter JD. Folate and cancer–timing is everything. JAMA. (2007) 297:2408–9. doi: 10.1001/jama.297.21.2408
140. Duthie SJ, Narayanan S, Brand GM, Pirie L, Grant G. Impact of folate deficiency on DNA stability. J Nutr. (2002) 132(8 Suppl):2444s–9s. doi: 10.1093/jn/132.8.2444S
141. Wei Q, Shen H, Wang LE, Duphorne CM, Pillow PC, Guo Z, et al. Association between low dietary folate intake and suboptimal cellular DNA repair capacity. Cancer Epidemiol Biomarkers Prev. (2003) 12:963–9. doi: 10.1023/A:1026300619747
142. Blount BC, Mack MM, Wehr CM, MacGregor JT, Hiatt RA, Wang G, et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci USA. (1997) 94:3290–5. doi: 10.1073/pnas.94.7.3290
143. Choi SW, Mason JB. Folate and carcinogenesis: an integrated scheme. J Nutr. (2000) 130:129–32. doi: 10.1093/jn/130.2.129
144. Xiao SD, Meng XJ, Shi Y, Hu YB, Zhu SS, Wang CW. Interventional study of high dose folic acid in gastric carcinogenesis in beagles. Gut. (2002) 50:61–4. doi: 10.1136/gut.50.1.61
145. Gonda TA, Kim YI, Salas MC, Gamble MV, Shibata W, Muthupalani S, et al. Folic acid increases global DNA methylation and reduces inflammation to prevent Helicobacter-associated gastric cancer in mice. Gastroenterology. (2012) 142:824–833.e7. doi: 10.1053/j.gastro.2011.12.058
146. Moat SJ, Lang D, McDowell IF, Clarke ZL, Madhavan AK, Lewis MJ, et al. Folate, homocysteine, endothelial function and cardiovascular disease. J Nutr Biochem. (2004) 15:64–79. doi: 10.1016/j.jnutbio.2003.08.010
147. Stanhewicz AE, Kenney WL. Role of folic acid in nitric oxide bioavailability and vascular endothelial function. Nutr Rev. (2017) 75:61–70. doi: 10.1093/nutrit/nuw053
148. Konukoglu D, Uzun H. Endothelial dysfunction and hypertension. Adv Exp Med Biol. (2017) 956:511–40. doi: 10.1007/5584_2016_90
149. Sun HJ, Hou B, Wang X, Zhu XX, Li KX, Qiu LY. Endothelial dysfunction and cardiometabolic diseases: role of long non-coding RNAs. Life Sci. (2016) 167:6–11. doi: 10.1016/j.lfs.2016.11.005
150. Chalupsky K, Kracun D, Kanchev I, Bertram K, Gorlach A. Folic acid promotes recycling of tetrahydrobiopterin and protects against hypoxia-induced pulmonary hypertension by recoupling endothelial nitric oxide synthase. Antioxid Redox Signal. (2015) 23:1076–91. doi: 10.1089/ars.2015.6329
151. Stanhewicz AE, Alexander LM, Kenney WL. Folic acid supplementation improves microvascular function in older adults through nitric oxide-dependent mechanisms. Clin Sci. (2015) 129:159–67. doi: 10.1042/CS20140821
152. Petersen LF, Brockton NT, Bakkar A, Liu S, Wen J, Weljie AM, et al. Elevated physiological levels of folic acid can increase in vitro growth and invasiveness of prostate cancer cells. BJU Int. (2012) 109:788–95. doi: 10.1111/j.1464-410X.2011.10437.x
153. Bistulfi G, Foster BA, Karasik E, Gillard B, Miecznikowski J, Dhiman VK, et al. Dietary folate deficiency blocks prostate cancer progression in the TRAMP model. Cancer Prev Res. (2011) 4:1825–34. doi: 10.1158/1940-6207.CAPR-11-0140
154. Vlachos GS, Scarmeas N. Dietary interventions in mild cognitive impairment and dementia. Dialogues Clin Neurosci. (2019) 21:69–82. doi: 10.31887/DNC.2019.21.1/nscarmeas
Keywords: folate, meta-analysis, umbrella review, multiple health outcomes, chronic diseases
Citation: Bo Y, Zhu Y, Tao Y, Li X, Zhai D, Bu Y, Wan Z, Wang L, Wang Y and Yu Z (2020) Association Between Folate and Health Outcomes: An Umbrella Review of Meta-Analyses. Front. Public Health 8:550753. doi: 10.3389/fpubh.2020.550753
Received: 10 April 2020; Accepted: 06 November 2020;
Published: 15 December 2020.
Edited by:
Vittorio Perduca, Université de Paris, FranceReviewed by:
Ersilia Lucenteforte, University of Pisa, ItalyCopyright © 2020 Bo, Zhu, Tao, Li, Zhai, Bu, Wan, Wang, Wang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zengli Yu, eXV6ZW5nbGkyMDE3QDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.