- 1Department of Epidemiology and Biostatistics, School of Public Health, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
- 2Department of Clinical Pharmacy, School of Pharmacy, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
Background: Cardiovascular disease (CVD) is the most prevalent complication and the leading cause of death among patients with diabetes mellitus (DM). Type 2 diabetes mellitus (T2DM) patients have a 2- to 4-fold increased risk of CVD. There is a scarcity of data about the magnitude of CVD among patients with diabetes in Ethiopia. This study aimed to assess the prevalence and associated factors of CVD among T2DM patients at selected hospitals of Harari regional state of Ethiopia.
Methods: This hospital-based retrospective data review was conducted among T2DM patients on follow-up in the diabetes clinics of selected hospitals of Harari regional state. The records of T2DM patients who have been diagnosed between January 1, 2013, and December 31, 2017, were reviewed from March to April 2018. Data were collected by using structured checklists from all necessary documents of T2DM patients. Statistical analysis was done using STATA 14.1. Bivariate and multivariate logistic regressions were used to identify factors associated with CVD.
Result: The records of 454 T2DM patients were extracted from three government hospitals in Harari regional state. Their age was ranging from 15 to 86 years with a mean age (±SD) of 45.39 (14.76). The overall prevalence of CVD among T2DM patients was 42.51%, composed of hypertensive heart diseases (38.99%), heart failure (6.83%), and stroke (2.20%). The final multivariate logistic regression model revealed that age older than 60 years [adjusted odds ratio (AOR) = 3.22; 95% CI: 1.71–6.09], being physically inactive (AOR = 1.45; 95 CI: 1.06–2.38), drinking alcohol (AOR = 2.39; 95% CI: 1.17–6.06), hypertension (AOR = 2.41; 95% CI: 1.52–3.83), body mass index >24.9 kg/m2 (AOR = 1.81; 95% CI: 1.07–3.07), and experiencing microvascular diabetic complications (AOR = 3.62; 95% CI: 2.01–6.53) were significantly associated with the odds of having CVD.
Conclusion: The prevalence of CVD was high and associated with advanced age, physical inactivity, drinking alcohol, higher body mass index, hypertension, and having microvascular complications. Health care workers should educate T2DM patients about healthy lifestyles like physical activity, weight reduction, blood pressure control, and alcohol secession, which can reduce the risk of CVD.
Introduction
Type 2 diabetes mellitus (T2DM) is a progressive and chronic metabolic disorder that is characterized by insulin resistance and functional failure of pancreatic beta cells (1). The prevalence of T2DM has been increasing intensely over the past few decades, with the highest rates of growth being seen in Sub-Saharan Africa (2, 3).
Cardiovascular disease (CVD), which involves heart and blood vessels, includes coronary heart disease (CHD), cerebrovascular disease, peripheral arterial disease, deep vein thrombosis, and pulmonary embolism (4, 5). It is the main cause of complications and morbidity among patients with T2DM globally (6, 7). Among T2DM patients, CVD risk was estimated to be 2- to 4-fold higher than the non-diabetic population (8, 9).
The natural history of T2DM is a slow process and may last even a decade; it might be initially presented with macroangiopathy, particularly CHD (10–13). The effect of T2DM on cardiovascular manifestations varies based on the specific cardiovascular outcome. Atherosclerosis, the major cause of macrovasculature, is the result of metabolic syndrome in diabetes patients (14–16). Similarly, alterations of small vessels in the brain, heart, and peripheral vasculature are contributing to the development of CVD and mortality (15). Another reason for the occurrence of CVD is inflammation, as immune response occasionally resulted in a detrimental effect. Even though DM is characterized by low-level inflammation, there is evidence showing that the immune activation preceding insulin resistance in diabetic and pre-diabetic states increases cardiovascular risk in T2DM processes (17). In addition to the impact of DM, modifiable and non-modifiable factors are contributing to the causation of CVD (18, 19).
Although microvascular complications have a significant role in the prognosis of T2DM, CVDs are the leading cause of morbidity and mortality among patients living with T2DM (6, 20). More than 70% of hospitalizations for chronic complications of diabetes are attributable to CVD (21, 22). The risk of morbidity and mortality caused by CVD in diabetes patients increases with the long duration of the diabetes (23–25).
Even though epidemiological studies have demonstrated an association between CVD and blood glucose levels, studies that indicate the magnitude of CVD and associated factors among diabetes patients in Harari region are limited. Thus, we aimed to assess the prevalence and associated factors of CVD among T2DM patients in hospitals of Harari regional state of Ethiopia.
Materials and Methods
Study Area and Period
This study was conducted among T2DM patients on follow-up in the diabetes clinics of government hospitals of Harari regional state. Harari is the smallest of the nine states of Ethiopia located in the eastern part of the country and surrounded by the east Hararghe zone of Oromia regional state. In the region, there are two public hospitals, one federal police hospital, two private hospitals, and eight health centers. This study was conducted in two public hospitals and one federal police hospital, namely, Hiwot Fana Specialized University Hospital (HFSUH), Jugal General Hospital (JGH), and Federal Harar Police Hospital (FHPH).
HFSUH is the teaching hospital for Haramaya University and comprehensive hospital for East Ethiopia (including Harari region, some parts of Somali region, and eastern Hararghe zone of Oromia) that is expected to serve about 5.8 million people in the eastern part of Ethiopia (26). Similarly, JGH is the oldest hospital in the country. FHPH is the government hospital serving police communities and their families. Hospitals are serving diabetes patients under the established chronic follow-up clinics. Patients' records from January 2013 to December 2017 were extracted in March to April 2018.
Study Design
Hospital-based retrospective data review was conducted on records of T2DM patients at government hospitals of Harari regional state of Ethiopia.
Population and Selection Criteria
Records of T2DM patients who were diagnosed after January 1, 2013, and before December 31, 2017, were included, but patients with no baseline records were excluded. Additionally, patients with body mass index (BMI) <18.5 kg/m2, end-stage renal diseases, transplanted organs or on dialysis, and/or other diagnosed chronic diseases like human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS), chronic obstructive pulmonary disease (COPD), or chronic liver disease (cirrhosis) were also excluded, as these factors lead to the immune deficiency.
Sampling Technique and Sample Size Determination
The sample size was calculated by Epi Info version 7 using single and double population proportion formula taking 95% confidence level, 80% power, and 5% precision. For the first objective, the calculated sample size was 376, which was based on the 42.6% prevalence of CVD in North India during 2011–2014 (27). For the second objective, the maximum sample was found for the obesity (≥30 kg/m2) (N = 334). By adding the 20% for incomplete records for CVD, the minimum sample size calculated was 454. Patients were selected by simple random sampling from the registry of the follow-up using computer-generated numbers. Patient identification number (medical record identifier) was randomized by using Excel to select an individual patient.
Operational Definition
Cardiovascular Disease
CVD comprises the major disorders of the heart and the arterial circulation supplying the heart, brain, and peripheral tissues. Thus, CVD will be considered if the patient had at least one but not limited to hypertensive heart diseases, heart failure, or stroke (4, 28).
Physical Activity
classified based on the occupational status of the patients (29). Physical activities were merged and regrouped into three categories: both moderate and high physical activity are leveled as “physically active” and those whose score is within the range of sedentary life are termed as “physically inactive.”
Hypertension (HTN)
defined as systolic blood pressure >139 mmHg and/or diastolic blood pressure >89 mmHg.
Controlled Blood Pressure
defined as systolic blood pressure between 120 and 139 mmHg and diastolic blood pressure between 65 and 85 mmHg (30, 31).
Controlled Blood Glucose
Glycemic status was categorized as good glycemic control if average (3-months average) fasting blood glucose (FBG) is 80–130 mg/dl (4.4–7.2 mmol/L) and poor control if FBG was >130 mg/dl (>7.2 mmol/L) (32).
Body Mass Index
The BMI is reclassified as normal if BMI is between 18.5 and 24.9 kg/m2 (18.5–24.9 kg/m2) and above normal if BMI is above 24.9 kg/m2 (33).
Data Collection Methods
The data were collected by using structured checklists from T2DM patients' documents including DM registration book, electronic information databases, patient card, and follow-up records. Data were collected by health officers and nurses working in the respective hospitals but not at diabetes clinics. All the filled extraction sheets were checked for completeness and consistency by supervisors and investigators to ensure the quality of data. We also cross-checked the data entry and clarified any missing data.
Data Management and Analysis
Statistical analysis was done using STATA 14.1. The risks of CVD and sociodemographic characteristics were summarized using proportion and mean with standard deviations. Outcome variable is determined if a patient experienced at least one type of CVD (coronary artery diseases, hypertensive heart diseases, stroke, heart failure, or any other else). To determine the factors for CVD, bivariate and multivariate logistic regressions were fitted, and variables were selected using the acyclic graph model selection (Figure 1). The final optimal model was selected based on Akaike information criterion (AIC) (36). Hosmer and Lemeshow test was fitted to test model fitness, and appropriate methods of multicollinearity test between independent variables were applied.
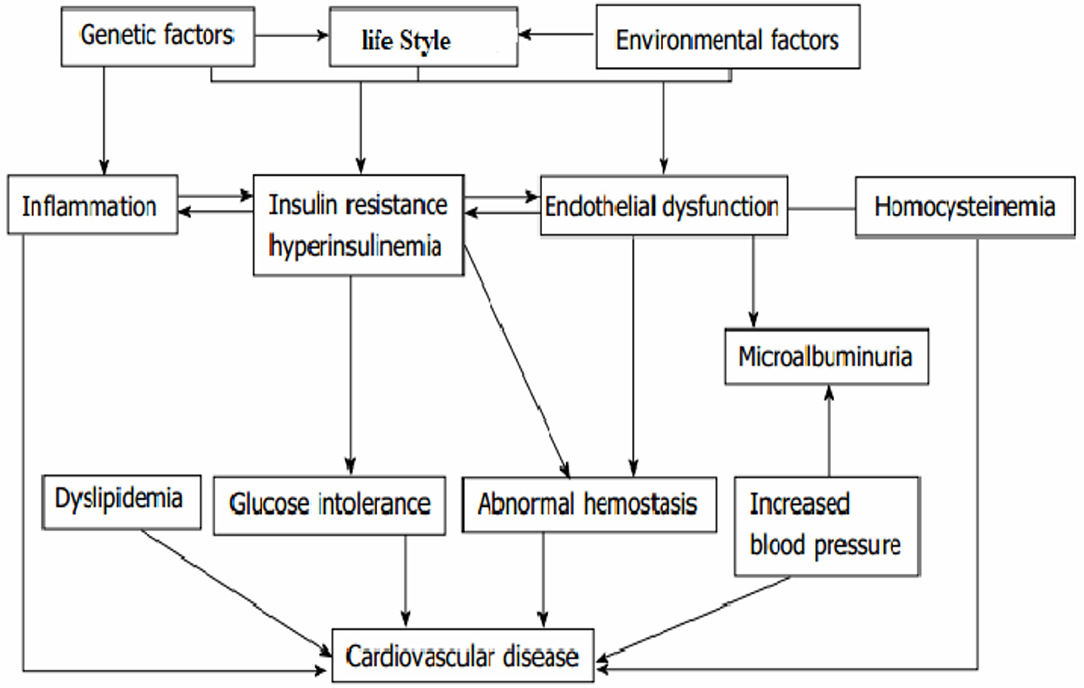
Figure 1. Conceptual framework for the causation and risk factors of cardiovascular disease in type 2 diabetes. Source [different kinds of literature (28, 34, 35)].
Results
Sociodemographic Characteristics
Total records of 454 T2DM patients were extracted from three government hospitals: 230 (50.66%) HFSUH, 111 (24.45%) JGH, and 113 (24.89%) FHPH. Two hundred fifty-nine (57%) were males. The age ranges from 15 to 86 years with mean (±SD) of 45.39 (14.76). The median age was 50 years (q1:30, q3:60). The majority (74.67%) of the patients where older than 40 years, and only 44 (9.69%) were younger than 30 years. The majority (73.84%) of the patients were urban dwellers and 389 (85.68%) were currently married. Regarding the occupation, 186 (40.97%) were civil servants, while 177 (39%) had private work (Table 1).
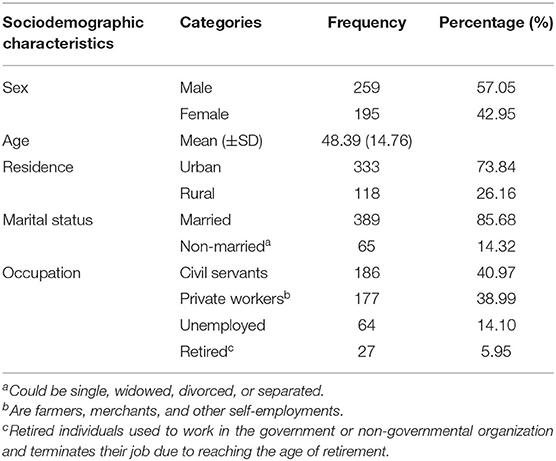
Table 1. Sociodemographic characteristics of type 2 diabetes patients in government hospitals of the Harari region, Eastern Ethiopia.
Clinical Characteristics and Risks of Cardiovascular Complication
Most (93.17%) patients started their DM follow-up immediately after diagnosis, but 6.83% delayed from 1 month to a year. Metformin alone (38.55%) and metformin in combination with glibenclamide (49.56%) were the commonly utilized therapies. From the total, 115 (25.39%) had a family history of DM. Majority (68.81%) of the patients' FBS level was above 130 mg/dl, while only 128 patients (28.19%) have a controlled FBS level.
Regarding the complications of DM, 75 (16.52%) patients have experienced acute complications of DM, either diabetic ketoacidosis or hyperglycemic hyperosmolar state. Microvascular complications were seen among 84 (18.50%) patients. Retinopathy was the major (47.62%) microvascular complication followed by nephropathy (27.38%) and neuropathy (25%). Other complications were developing foot complications (21%) and non-diabetic kidney diseases (15.20%).
Overall, 193 (42.51%, 95% CI: 38.02–47.13) patients were diagnosed with CVD. Majority of them were diagnosed with hypertensive heart diseases (38.99%) and heart failure (6.83%), and the remaining experienced stroke (2.20%). The prevalence of hypertensive heart diseases was 28% (95% CI: 21–35) for younger than 40 years, 40% (95% CI: 33–46) for 40–60 years, and 49% (95% CI: 52–64) for older than 60 years patients. The magnitude of heart failure was 2.5, 8.5, and 4.4% for age <40, 40–60, and above 60 years patients, respectively. At least one risk factor for CVD was recorded among 272 (59.91%). From the total study participants, 274 (60.35%) were hypertensive. From 274 hypertensive patients, 166 (60.6%) of them were with uncontrolled blood pressure. Moreover, 124 (27.31%) were physically inactive, 28 (6.17%) were active smokers, 34 (7.49%) were alcoholics, and 83 (18.28%) were obese (Table 2).
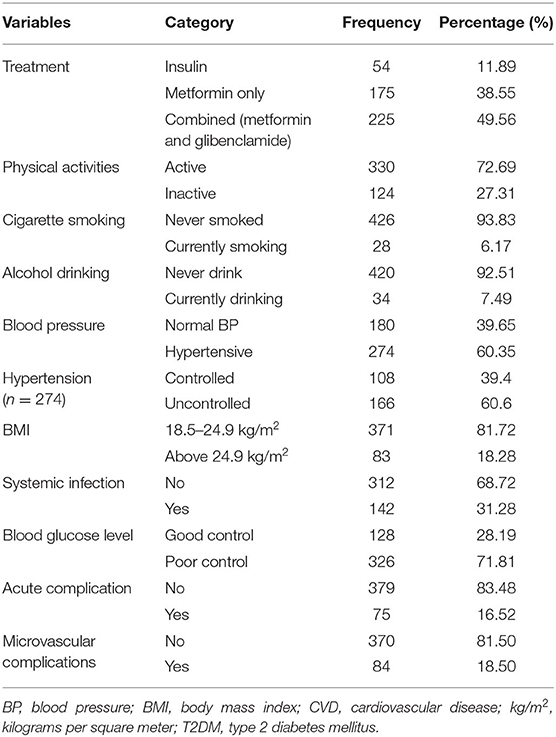
Table 2. Treatment categories and magnitude of CVD risk factors among T2DM patients in government hospitals of Ethiopia, from 2013 to 2017.
In bivariate logistic regression, age, place of residence, physical activity, drinking alcohol, smoking, level of blood pressure, BMI, history of infection, and microvascular complications are significantly associated with CVD. However, in multivariate logistic regression, the significant association was not seen for age between 40 and 60 years, place of residence, smoking, and history of infection.
In the final multivariate logistic regression model, age older than 60, being physically inactive, drinking alcohol, HTN, BMI > 24.9 kg/m2, and experiencing microvascular DM complications were significantly and positively associated with CVD.
Patients with age older than 60 years were having three times [adjusted odds ratio (AOR) = 3.22; 95% CI: 1.71–6.09] higher chance of experiencing CVD as compared with those aged <40 years. The odds of developing CVD was more than two times (AOR = 2.39; 95% CI: 1.17–6.06) higher among adults who consumed alcohol compared with T2DM patients who did not drink alcohol at all. Moreover, physically inactive T2DM patients were having 45% higher odds of developing CVD (AOR =1.45; 95 CI: 1.06–2.38) when compared to the physically active patients.
On the other hand, the odds of developing CVD was 1.81 times (AOR = 1.81; 95% CI: 1.07–3.07) higher among overweight (BMI > 24.9 kg/m2) patients compared to patients who had normal weight (BMI 18.5–24.9 kg/m2). Similarly, the likelihood of acquiring CVD among hypertensive patients is more than two times (AOR = 2.41; 95% CI: 1.52–3.83) higher than those with normal blood pressure. Patients who had a history of microvascular DM complications had three times (AOR = 3.62; 95% CI: 2.01–6.53) the likelihood of developing CVD compared with T2DM patients who did not experience microvascular complications of DM (Table 3).
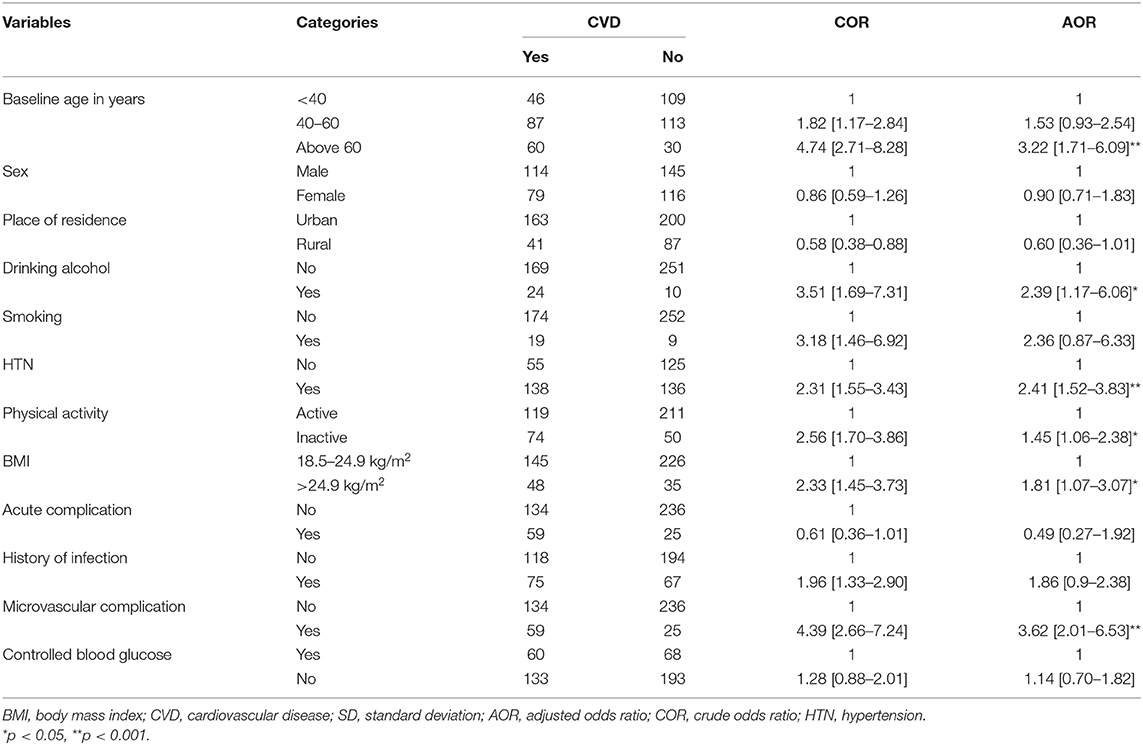
Table 3. Factors associated with cardiovascular disease among type 2 diabetes patients in government hospitals of the Harari region, Eastern Ethiopia, from 2013 to 2017 (N = 454).
Discussion
Identification of potentially modifiable associated factors of CVD is an initial step to prevent and control CVD and its outcome among T2DM patients. In this study, the overall prevalence of CVD was 42.5% (95% CI: 38%, 47%). Specific complications account for 39% hypertensive heart diseases, 7% heart failure, and 2.2% stroke. This prevalence is higher than the pooled prevalence (32%) reported in a systematic literature review of studies in the period of 2007–2017, though the specific complications are lower in the current study (37). It is also higher than those of studies conducted in Iraq (38) and Scotland (39). This discrepancy might be due to the difference in sample size, follow-up, and treatment protocol between the countries. The difference in subtypes of CVD with Malik et al. (39) and Einarson et al. (37) might be due to the wide range of age composition (younger population) in this study.
In this study, the prevalence of the CVD among T2DM is associated with the presence of older age, being physically inactive, drinking alcohol, BMI > 24.9 kg/m2, HTN, and presence of microvascular complications.
Patients older than 60 years have more than three times the likelihood of developing CVD than those who are younger than 40 years. The prevalence of HTN is also seen increased with age. This result is comparable to the result of studies conducted in Pakistan (40), Taiwan (41), and Sweden (42), as all of these studies revealed older age is the important predictor of a cardiovascular event in T2DM. Aging can cause changes in the heart and blood vessels that may increase a person's risk of developing CVD. Moreover, there is a high prevalence of atherosclerosis and arteriosclerosis due to the progression of diabetes in advanced age (43).
The likelihood of CVD in the female is lowered by 10% compared to male T2DM patients, though it is not statistically significant. There is heterogeneity between studies regarding the difference of risk of CVD among the male and female. Many studies indicated that the females are more likely to develop CVD (44–46). To the opposite of the former evidence from a review by Al-Salameh et al. (47), which showed that regardless of CVD being more prevalent in female in the absence of T2DM, the disparity disappears for the T2DM patients and the CVD is not barely associated with the sex difference but it is influenced by BMI above normal (overweight or obesity) and high prevalence of HTN after the age of 60–65 years in women. Hence, the reason for the non-significant association of sex and CVD in this study might be due to the higher levels of risk factors (obesity, microvascular diabetes complications, and age).
Physical inactivity is positively associated with the development of CVD among T2DM patients in this study. It is in line with the study conducted in Ethiopia among T2DM patients that indicated physical inactivity is significantly associated with the chronic complications of T2DM (48). Previous studies have consistently showed the protective effect of physical activity in diabetic patients at any level of other risk factors for CVD in diabetic patients (29, 40, 49, 50). This might be the reason for the conclusion of lifestyle modification that includes the exercise as one of the milestones of diabetes treatment and prevention of its complications (51).
Drinking alcohol is positively associated with the prevalence of CVD. The likelihood of CVD is increased by about 3-fold among alcohol consumers. Similar evidence was reported in a systematic review and meta-analysis of 20 studies (52) and individual study in southwest Ethiopia (48). Alcoholism (heavy or moderate drinking) might accelerate the development of coronary arterial diseases (CADs), as it causes systemic HTN, valvular diseases, cardiomyopathies, rhythm disturbances, and many non-cardiac problems, such as anemia, infection, and tumors (53–55). Though the risk is increased with the dose of intake and types of beverage, alcohol drinking is the risk for CVD in any individual (56).
The association between smoking and CVD is not significant in the current study. This finding is in line with a similar study conducted in Saidu Teaching Hospital, Pakistan (40). But most previous studies were reporting that smoking habit is the risk factor for the CVD, as it alters the process of controlling blood glucose level (57, 58). In INTERHEART study from 52 countries, smoking was among the reported potentially modifiable risk factors of heart diseases (59). The discrepancy might be due to the low proportion of smokers among the study population of the current study.
In the current study, there was an increment of CVD among hypertensive patients by more than two times. This study was in line with the study conducted in Sweden, as it showed that the risk of CVD is increased with the high blood pressure (31). The result from CVD research using linked bespoke studies and electronic health records (CALIBER) indicated that people with HTN had a higher lifetime risk of overall CVD and developed CVD 5 years earlier than those with normal blood pressure (30). HTN is also reported as a risk factor for CVD in published articles (31, 40–42, 60–63) through causing hypertensive heart diseases (64).
The likelihood of CVD is increased by 14% among individuals with uncontrolled blood glucose level compared to patients with controlled blood glucose. But this association was not significant statistically. To the opposite of our result, there was evidence of strong association between hyperglycemia and CVD (40, 63, 65–67). Hyperglycemia is the principal cause of microvasculopathy but also appears to play an important role in causation of macrovasculopathy (40, 68). The risk of cardiovascular complications might be increased in the long term (more than 10 years' duration); hyperglycemia as long-standing hyperglycemia induces the toxicity of endothelial cell and alters its function (11, 69, 70). The discrepancy might be due to the short period of follow-up in our study. Since the follow-up was limited to 5 years, it is rare to find the association between the uncontrolled blood glucose and CVD.
In the present study, the likelihood of developing CVD is increased by 81% among patients whose BMI is >24.9 kg/m2 compared to those whose BMI is between 18.5 and 24.9 kg/m2. A similar result was reported by a systematic review of 57 individual studies—the positive relationship between obesity and increased prevalence rates of CVD (37). This might be due to the nature of T2DM, as the majority of individuals suffering from T2DM are obese and suffer from metabolic syndrome, which could be a risk factor for the major cardiovascular events (71).
Patients who had a history of microvascular complications had an increased likelihood of developing CVD than their counterparts. Mainly recorded microvascular complications were retinopathy and nephropathy. Similarly, published studies reported the association between microvascular complication and CVD (70, 72–74). Diabetic vascular complications are a continuum and depend on each other (74). In addition to endothelial cell-dependent vascular injury mechanisms, endothelial cell-independent vascular dysfunction is leading to BK channelopathy and vascular complications in T2DM (75).
Finally, this study has a number of limitations. Since this study was a retrospective document review in a resource-limited setting, there was no recorded HbA1c, as it is not routinely available, blood cholesterol level, diet, family history, and time at CVD diagnoses for most of the patients. The coronary artery diseases, the most common types of heart disease, were not reported in the records of the patients. Moreover, the criteria used to diagnose the type of CVD are not specifically registered, and the lifetime burden of risk factors was difficult to determine.
Conclusion
The prevalence of CVD was high and associated with advanced age, being physically inactive, drinking alcohol, BMI higher than 24.9 kg/m2, being hypertensive, and having microvascular complications.
Recommendation
To reduce the risk of CVD in T2DM, helping the patients to attain a healthy lifestyle by encouraging physical activity, weight reduction, and cessation of alcohol drinking during the follow-up is the essential step. In addition to this, we recommend a multifactorial intervention aimed at achieving recommended levels of critical indicators (blood pressure, blood cholesterol, microvascular complications, and treatment at early stage).
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by University of Gondar College of Medical and Health Science Ethical Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LR contributed to the proposal development, data curation, investigation, formal analysis, methodology, project administration, writing the original draft, and writing, review, and editing. AT contributed to the proposal development, investigation, methodology, writing the original draft, and writing, review, and editing. YA contributed to the proposal development, investigation, methodology, writing the original draft, and writing, review, and editing. The manuscript was also developed through the active participation of all authors. All authors read and approved the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the respective hospital administrators, hospital staff working at chronic follow-up clinics, and the data collectors for their willingness and unreserved contribution in this study.
References
1. Nolan CJ, Ruderman NB, Kahn SE, Pedersen O, Prentki MJD. Insulin resistance as a physiological defense against metabolic stress: implications for the management of subsets of type 2 diabetes. Diabetes. (2015) 64:673–86. doi: 10.2337/db14-0694
2. Bandello F, Zarbin MA, Lattanzio R, Zucchiatti I (editors). Management of diabetic retinopathy. Dev Ophthalmol. (2017) 60:1–5. doi: 10.1159/000459641
3. Mensah GA. Descriptive epidemiology of cardiovascular risk factors and diabetes in Sub-Saharan Africa. Prog Cardiovasc Dis. (2013) 56:240–50. doi: 10.1016/j.pcad.2013.10.014
4. WHO. Definition of Cardiovascular Diseases. (2017). Available online at: http://www.euro.who.int/en/health-topics/noncommunicable-diseases/cardiovascular-diseases/cardiovascular-diseases2/definition-of-cardiovascular-diseases
5. Birkeland KI, Jørgensen ME, Carstensen B, Persson F, Gulseth HL, Thuresson M, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (Cvd-real nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. (2017) 5:709–17. doi: 10.1016/S2213-8587(17)30258-9
6. Laakso M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes Care. (1999) 48:937–42. doi: 10.2337/diabetes.48.5.937
7. O'Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (interstroke): a case-control study. Lancet Diabetes. (2016) 388:761–75. doi: 10.1016/S0140-6736(16)30506-2
8. Gu K, Cowie CC, Harris MI. Diabetes and decline in heart disease mortality in Us adults. JAMA. (1999) 281:1291–7. doi: 10.1001/jama.281.14.1291
9. Haffner SJ, Cassells H. Hyperglycemia as a cardiovascular risk factor. Am J Med. (2003) 115:6–11. doi: 10.1016/j.amjmed.2003.09.009
10. Laakso M. Cardiovascular disease in type 2 diabetes: challenge for treatment and prevention. J Intern Med. (2001) 249:225–35. doi: 10.1046/j.1365-2796.2001.00789.x
11. Kitada M, Zhang Z, Mima A, King GL. Molecular mechanisms of diabetic vascular complications. J Diabetes Invest. (2010) 1:77–89. doi: 10.1111/j.2040-1124.2010.00018.x
12. Kosiborod M, Gomes MB, Nicolucci A, Pocock S, Rathmann W, Shestakova MV, et al. Vascular complications in patients with type 2 diabetes: prevalence and associated factors in 38 countries (the Discover Study Program). Cardiovasc Diabetol. (2018) 17:150. doi: 10.1186/s12933-018-0787-8
13. Mbanya J-C, Sobngwi E. Diabetes in Africa. Diabetes microvascular and macrovascular disease in Africa. J Cardiovasc Risk. (2003) 10:97–102. doi: 10.1177/174182670301000204
14. Dinesh Shah A, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, et al. Type 2 diabetes and incidence of a wide range of cardiovascular diseases: a cohort study in 1.9 million people. Lancet. (2015) 385(Suppl.1):S86. doi: 10.1016/S0140-6736(15)60401-9
15. Dokken BB. The pathophysiology of cardiovascular disease and diabetes: beyond blood pressure and lipids. Diabetes Spectrum. (2008) 21:160–5. doi: 10.2337/diaspect.21.3.160
16. Meigs JB, Mittleman MA, Nathan DM, Tofler GH, Singer DE, Murphy-Sheehy PM, et al. Hyperinsulinemia, hyperglycemia, and impaired hemostasis: the framingham offspring study. JAMA. (2000) 283:221–8. doi: 10.1001/jama.283.2.221
17. Festa A, D'Agostino R Jr, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the insulin resistance atherosclerosis study (Iras). Circulation. (2000) 102:42–7. doi: 10.1161/01.CIR.102.1.42
18. Lin JS, Evans CV, Johnson E, Redmond N, Burda BU, Coppola EL, et al. Nontraditional Risk Factors in Cardiovascular Disease Risk Assessment: A Systematic Evidence Report for the U.S. Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality (US) (2018). doi: 10.1001/jama.2018.4242
19. Singh P, Singh S, Pandi-Jain GS. Effective heart disease prediction system using data mining techniques. Int J Nanomed. (2018) 13:121–4. doi: 10.2147/IJN.S124998
20. Kannel WB, McGee DL. Diabetes and cardiovascular disease: the framingham study. JAMA. (1979) 241:2035–8. doi: 10.1001/jama.1979.03290450033020
21. Micha R, Peñalvo JL, Cudhea F, Imamura F, Rehm CD, Mozaffarian D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA. (2017) 317:912–24. doi: 10.1001/jama.2017.0947
23. Branch M, German C, Bertoni A, Yeboah J. Incremental risk of cardiovascular disease and/or chronic kidney disease for future Ascvd and mortality in patients with type 2 diabetes mellitus: accord trial. J Diabetes Complications. (2019) 33:468–72. doi: 10.1016/j.jdiacomp.2019.04.004
24. Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. (2018) 34:575–84. doi: 10.1016/j.cjca.2017.12.005
25. Hayward RA, Reaven PD, Wiitala WL, Bahn GD, Reda DJ, Ge L, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2015) 372:2197–206. doi: 10.1056/NEJMoa1414266
26. Sisay H. Nursing Process and Factors Affecting Its Utilization in Governmental Hospitals in Harari and Dire Dawa regions (Doctoral dissertation), Haramaya University, Dire Dawa.
27. Bhatti GK, Bhadada SK, Vijayvergiya R, Mastana SS, Bhatti JS. Metabolic syndrome and risk of major coronary events among the urban diabetic patients: North Indian diabetes and cardiovascular disease study—Nidcvd-2. J Diabetes Complications. (2016) 30:72–8. doi: 10.1016/j.jdiacomp.2015.07.008
28. Roger VL, Sidney S, Fairchild AL, Howard VJ, Labarthe DR, Shay CM, et al. Recommendations for cardiovascular health and disease surveillance for 2030 and beyond: a policy statement from the American Heart Association. Circulation. (2020) 141:e104–19. doi: 10.1161/CIR.0000000000000756
29. Hu G, Jousilahti P, Barengo NC, Qiao Q, Lakka TA, Tuomilehto J. Physical activity, cardiovascular risk factors, and mortality among finnish adults with diabetes. Diabetes Care. (2005) 28:799–805. doi: 10.2337/diacare.28.4.799
30. Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, et al. Blood pressure and incidence of 12 cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet Diabetes. (2014) 383:1899–911. doi: 10.1016/S0140-6736(14)60685-1
31. Afghahi H, Svensson MK, Pirouzifard M, Eliasson B, Svensson A-M. Blood pressure level and risk of major cardiovascular events and all-cause of mortality in patients with type 2 diabetes and renal impairment: an observational study from the Swedish National Diabetes Register. Diabetologia. (2015) 58:1203–11. doi: 10.1007/s00125-015-3548-1
32. Ivanov TD, Ivanova MJK. American Diabetes Association. Standards of medical care in diabetes-−2017. Kidneys. (2017) 6:47–63. doi: 10.22141/2307-1257.6.1.2017.93784
33. World Health Organization. Bmi Classification, 2014. c2020–02. Available online at: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
34. Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes. (2014) 5:444–70. doi: 10.4239/wjd.v5.i4.444
35. Stern MPJD. Diabetes and cardiovascular disease: The “Common Soil” hypothesis. Perspect Diabetes. (1995) 44:369–74. doi: 10.2337/diab.44.4.369
36. Bozdogan H. Akaike's information criterion and recent developments in information complexity. J Math Psychol. (2000) 44:62–91. doi: 10.1006/jmps.1999.1277
37. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. (2018) 17:83. doi: 10.1186/s12933-018-0728-6
38. Mansour AA, Ajeel NA. Atherosclerotic cardiovascular disease among patients with type 2 diabetes in Basrah. World J Diabetes. (2013) 4:82–7. doi: 10.4239/wjd.v4.i3.82
39. Malik MO, Govan L, Petrie JR, Ghouri N, Leese G, Fischbacher C, et al. Ethnicity and risk of cardiovascular disease (Cvd): 4.8 years follow-up of patients with type 2 diabetes living in Scotland. Diabetologia. (2015) 58:716–25. doi: 10.1007/s00125-015-3492-0
40. Akhtar S, Asghar N. Risk factors of cardiovascular disease in district Swat. J Pak Med Assoc. (2015) 65:1001–4.
41. Kornelius E, Chiou J-Y, Yang Y-S, Lu Y-L, Peng C-H, Huang C-N. The diabetes shared care program and risks of cardiovascular events in type 2 diabetes. Am J Med. (2015) 128:977–85. doi: 10.1016/j.amjmed.2015.03.025
42. Rawshani A, Rawshani A, Franzén S, Sattar N, Eliasson B, Svensson A-M, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. (2018) 379:633–44. doi: 10.1056/NEJMoa1800256
43. Zhou K, Donnelly LA, Morris AD, Franks PW, Jennison C, Palmer CN, et al. Clinical and genetic determinants of progression of type 2 diabetes: a direct study. Diabetes Care. (2014) 37:718–24. doi: 10.2337/dc13-1995
44. Ballotari P, Venturelli F, Greci M, Giorgi Rossi P, Manicardi V. Sex differences in the effect of type 2 diabetes on major cardiovascular diseases: results from a population-based study in Italy. Int J Endocrinol. (2017) 2017: 6039356. doi: 10.1155/2017/6039356
45. Gouni-Berthold I, Berthold HK, Mantzoros CS, Böhm M, Krone WJDc. Sex disparities in the treatment and control of cardiovascular risk factors in type 2 diabetes. Diabetes Care. (2008) 31:1389-91. doi: 10.2337/dc08-0194
46. Eeg-Olofsson K, Cederholm J, Nilsson P, Zethelius B, Nunez L, Gudbjörnsdóttir S, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia. (2009) 52:65. doi: 10.1007/s00125-008-1190-x
47. Al-Salameh A, Chanson P, Bucher S, Ringa V, Becquemont L editors. Cardiovascular disease in type 2 diabetes: a review of sex-related differences in predisposition and prevention. Mayo Clin Proc. (2019) 94:287–308. doi: 10.1016/j.mayocp.2018.08.007
48. Tamiru S, Alemseged F. A risk factors for cardiovascular diseases among diabetic patients in Southwest Ethiopia. Ethiop J Health Sci. (2010) 20:2. doi: 10.4314/ejhs.v20i2.69438
49. Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. (2004) 141:421–31. doi: 10.7326/0003-4819-141-6-200409210-00007
50. Tanasescu M, Leitzmann MF, Rimm EB, Hu FB. Physical activity in relation to cardiovascular disease and total mortality among men with type 2 diabetes. Circulation. (2003) 107:2435–9. doi: 10.1161/01.CIR.0000066906.11109.1F
51. Praet S, Van Loon L. Exercise: the brittle cornerstone of type 2 diabetes treatment. Diabetologia. (2008) 51:398–401. doi: 10.1007/s00125-007-0910-y
52. Baliunas DO, Taylor BJ, Irving H, Roerecke M, Patra J, Mohapatra S, et al. Alcohol as a risk factor for type 2 diabetes: a systematic review and meta-analysis. J Diabetes Care. (2009) 32:2123–32. doi: 10.2337/dc09-0227
53. Klatsky AL. Alcohol, cardiovascular diseases and diabetes mellitus. Pharmacol Res. (2007) 55:237–47. doi: 10.1016/j.phrs.2007.01.011
54. Polsky S, Akturk HK. Alcohol consumption, diabetes risk, and cardiovascular disease within diabetes. Curr Diab Rep. (2017) 17:136. doi: 10.1007/s11892-017-0950-8
55. Wahid A, Manek N, Nichols M, Kelly P, Foster C, Webster P, et al. Quantifying the association between physical activity and cardiovascular disease and diabetes: a systematic review and meta-analysis. J Am Heart Assoc. (2016) 5:e002495. doi: 10.1161/JAHA.115.002495
56. Piano MR, Mazzuco A, Kang M, Phillips SA. Cardiovascular consequences of binge drinking: an integrative review with implications for advocacy, policy, and research. Alcohol Clin Exp Res. (2017) 41:487–96. doi: 10.1111/acer.13329
57. Balakumar P, Maung-U K, Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol Res. (2016) 113:600–9. doi: 10.1016/j.phrs.2016.09.040
58. Sasso FC, Chiodini P, Carbonara O, De Nicola L, Conte G, Salvatore T, et al. High cardiovascular risk in patients with type 2 diabetic nephropathy: the predictive role of albuminuria and glomerular filtration rate. The Nid-2 prospective cohort study. Nephrol Dial Transplant. (2012) 27:2269–74. doi: 10.1093/ndt/gfr644
59. Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the interheart study): case-control study. Lancet. (2004) 364:937–52. doi: 10.1016/S0140-6736(04)17018-9
60. Miller IM, Ellervik C, Yazdanyar S, Jemec GB. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. (2013) 69:1014–24. doi: 10.1016/j.jaad.2013.06.053
61. Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, et al. For the field study investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9,795 people with type 2 diabetes mellitus (the field [fenofibrate intervention and event lowering in diabetes] study): randomised controlled trial. Lancet. (2005) 366:1849–61. doi: 10.1016/S0140-6736(05)67667-2
62. Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. (2015) 3:105–13. doi: 10.1016/S2213-8587(14)70219-0
63. Vazquez-Benitez G, Desai JR, Xu S, Goodrich GK, Schroeder EB, Nichols GA, et al. Preventable major cardiovascular events associated with uncontrolled glucose, blood pressure, and lipids and active smoking in adults with diabetes with and without cardiovascular disease: a contemporary analysis. Diabetes Care. (2015) 38:905–12. doi: 10.2337/dc14-1877
64. Tefera YG, Abegaz TM, Abebe TB, Mekuria AB. The changing trend of cardiovascular disease and its clinical characteristics in Ethiopia: hospital-based observational study. Vasc Health Risk Manage. (2017) 13:143. doi: 10.2147/VHRM.S131259
65. Reaven PD, Emanuele NV, Wiitala WL, Bahn GD, Reda DJ, McCarren M, et al. Intensive glucose control in patients with type 2 diabetes-−15-years follow-up. N Engl J Med. (2019) 380:2215–24. doi: 10.1056/NEJMoa1806802
66. Freemantle N, Danchin N, Calvi-Gries F, Vincent M, Home PD. Relationship of glycaemic control and hypoglycaemic episodes to 4-years cardiovascular outcomes in people with type 2 diabetes starting insulin. Diabetes Obes Metab. (2016) 18:152–8. doi: 10.1111/dom.12598
67. Kamuhabwa AR, Charles E. Predictors of poor glycemic control in type 2 diabetic patients attending public hospitals in Dar Es Salaam. Drug Healthcare Patient Saf . (2014) 6:155–65. doi: 10.2147/DHPS.S68786
68. Cardoso CRL, Leite NC, Moram CBM, Salles GF. Long-term visit-to-visit glycemic variability as predictor of micro- and macrovascular complications in patients with type 2 diabetes: the Rio de Janeiro type 2 diabetes cohort study. Cardiovasc Diabetol. (2018) 17:33. doi: 10.1186/s12933-018-0677-0
69. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circulation. (2010) 107:1058–70. doi: 10.1161/CIRCRESAHA.110.223545
70. van Wijngaarden RPT, Overbeek JA, Heintjes EM, Schubert A, Diels J, Straatman H, et al. Relation between different measures of glycemic exposure and microvascular and macrovascular complications in patients with type 2 diabetes mellitus: an observational cohort study. Diabetes Ther. (2017) 8:1097–109. doi: 10.1007/s13300-017-0301-4
71. Scheen AJ. Pathophysiology of type 2 diabetes. Acta Clinica Belgica. (2003) 6:58. doi: 10.1179/acb.2003.58.6.001
72. Gimeno-Orna JA, Faure-Nogueras E, Castro-Alonso FJ, Boned-Juliani B. Ability of retinopathy to predict cardiovascular disease in patients with type 2 diabetes mellitus. Am J Cardiol. (2009) 103:1364–7. doi: 10.1016/j.amjcard.2009.01.345
73. Seferovic JP, Bentley-Lewis R, Claggett B, Diaz R, Gerstein HC, Køber LV, et al. Retinopathy, neuropathy, and subsequent cardiovascular events in patients with type 2 diabetes and acute coronary syndrome: analysis from elixa and importance of duration of diabetes. Circulation. (2016) 134(Suppl.1):A19191. doi: 10.1155/2018/1631263
74. Chawla A, Chawla R, Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: distinct or continuum? Indian J Endocrinol Metabol. (2016) 20:546. doi: 10.4103/2230-8210.183480
75. Lu T, Lee H-C. Impaired Vascular BK Channel Function in Type 2 Diabetes Mellitus, Medical Complications of Type 2 Diabetes, Croniger C, editor. IntechOpen. (2011). doi: 10.5772/22077. Available online at: https://www.intechopen.com/books/medical-complications-of-type-2-diabetes/impaired-vascular-bk-channel-function-in-type-2-diabetes-mellitus
Keywords: type 2 diabetes mellitus, prevalence, cardiovascular diseases, Ethiopia, hospitals, Harar
Citation: Regassa LD, Tola A and Ayele Y (2021) Prevalence of Cardiovascular Disease and Associated Factors Among Type 2 Diabetes Patients in Selected Hospitals of Harari Region, Eastern Ethiopia. Front. Public Health 8:532719. doi: 10.3389/fpubh.2020.532719
Received: 05 February 2020; Accepted: 21 December 2020;
Published: 05 February 2021.
Edited by:
Charumathi Sabanayagam, Singapore Eye Research Institute (SERI), SingaporeReviewed by:
Avinash Ravipati, University of Oklahoma Health Sciences Center, United StatesAbdurezak Ahmed Abdela, Addis Ababa University, Ethiopia
Copyright © 2021 Regassa, Tola and Ayele. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lemma Demissie Regassa, ZXNyYWVsZGVtaXNzQGdtYWlsLmNvbQ==
 Lemma Demissie Regassa
Lemma Demissie Regassa Assefa Tola
Assefa Tola Yohanes Ayele
Yohanes Ayele