- 1Department of Population and Health, University of Cape Coast, Cape Coast, Ghana
- 2College of Public Health, Medical and Veterinary Sciences, James Cook University, Townsville, QLD, Australia
Introduction: Globally, HIV testing and counseling is considered a key cost-effective component of HIV prevention and treatment. This study sought to use Anderson's model of health service utilization to assess the uptake of HIV testing services by sexually active men in Ghana.
Materials and Methods: Data were from the 2014 Ghana Demographic and Health Survey. Both bivariate and multivariate analysis were conducted. The multivariate analysis results are presented as Adjusted Odds ratios (AORs) with 95% confidence intervals (CI). Statistical significance was declared at p < 0.05.
Results: A total of 3,052 sexually active men aged 15–59 were included in the analysis. Of these, 25.4% tested for their HIV status. Men aged 30–39 (AOR = 2.715, CI = 1.458, 5.054), those with higher level of education (AOR = 3.566,CI = 2.309, 5.509), married (AOR = 1.50, CI = 1.167, 1.931), and men in Upper East (AOR = 2.625, CI = 1.608, 4.285) had higher odds of HIV testing uptake than their counter parts aged 15–19, those with no formal education unmarried and those in Western Region, respectively. However, men with no religion (AOR = 0.606, CI = 0.376, 0.975) and those who belong to the Mole-Dagbani ethnic group (AOR = 0.633, CI = 0.429, 0.934) had lower odds of HIV testing uptake compared to those who are Christians, and Akans, respectively. Men who have subscribed to health insurance (AOR = 1.896, 95% CI = 1.361, 2.643), those in the rich wealth quintile (AOR = 1.896, CI = 1.361, 2.643), those who read newspaper (AOR = 1.552, CI = 1.198, 2.012), listened to radio (AOR = 1.530, CI = 1.087, 2.153) at least once a week, and men who experienced discharge from their penis (AOR = 1.056, CI = 1.200, 1.515) had higher odds of HIV testing uptake.
Conclusion: Uptake of HIV testing among Ghanaian men is relatively low. There is the need for a concerted effort by various stakeholders to strengthen current efforts to target younger and unmarried men, men with low level of education, those who do not profess any religious affiliation and men belonging to Mole-Dagbani ethnic group.
Introduction
Ghana has been seemingly successful in fighting against HIV, evident in the decline of the prevalence of the illness among the Ghanaian population. For instance, from 2003 to 2014, the prevalence of HIV among women aged 15–49 declined from 2.1 to 2.0%. A similar reduction of HIV prevalence was observed in the case of men of the same age group, with a decline of 2.8 to 1.1% in the same period of time (1). However, in order to meet the Joint United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90 target by 2020, Ghana needs to work on the reduction further (2). This requires, among other things, an expansion of HIV testing services, so as to ensure that HIV-infected people are diagnosed and made aware of their status. In fact, some research attest to the effectiveness of HIV testing as a strategy for reducing HIV-induced deaths and illnesses (3, 4). Besides, the World Health Organization (WHO) and Center for Disease Control (CDC) also recognize the important roles played by HIV testing in fighting against the HIV pandemic (5, 6). For instance, when couples get to know their HIV status, they adopt strategies to avoid mother-to-child transmissions (7, 8). Also, when individuals get to know their HIV status, it helps them to get access to some medical and social support such as the antiretroviral therapy and emotional support needed to cope with HIV (9, 10).
Previous research reports low HIV testing among the general population in some low- and middle-income countries. In sub-Saharan Africa, the prevalence ranges from 69.9% in Malawi (11), 59% in South Africa (12), 25.1% in Tanzania (13), 23% in Uganda (10), and 22.7% in Ghana (14). Such studies have also reported low uptake of HIV testing services among males than among females, largely due to the emphasis placed on HIV testing among women prenatal care (15), who tend to use healthcare services more than men (16, 17). In the case of Ghana, an earlier study by Nyarko and Sparks (14) revealed a similar trend. This, therefore, highlights the need for further investigation of individual and contextual factors associated with uptake of HIV testing services among the general population in Ghana (18). Previous research on the Ghanaian situation has focused principally on women (13, 19–21), men sleeping with men (22), and young people (23). Generally, some studies have revealed an association between HIV testing among men and variables such as age (11, 12, 14, 24, 25), place of residence (11, 12, 14), marital status (11, 12, 14, 26), health insurance subscription (11), wealth status (27), educational level (11, 12, 14, 24, 26, 27), age at first sexual debut (11, 12), number of sex partners (25), condom use (24), religion (26), and media exposure (25).
In the present study, the factors associated with the uptake of HIV testing services among Ghanaian men were assessed. In Ghana, a patriarchal society, men usually lead the decision making process and control economic resources in households, which is likely to influence the control of economic resources that are significant for HIV prevention and care (11). It is, therefore, necessary to investigate the factors influencing the uptake of HIV testing in this population, as such factors will be needful in developing strategies to increase HIV testing among men in Ghana. For instance, findings from this study will reveal the specific category of men to be targeted with more efforts in order to improve HIV testing uptake. In fact, a similar study by Nyarko and Sparks (14) employed the 2003, 2008, and 2014 Ghana Demographic and health survey to explore the predictors of HIV testing among men in Ghana. The present study, however, differs from that study, since it focuses specifically on the 2014 version of the GDHS and also situates the study within an empirical model. This current study also considered only sexually active men. With this, it is hoped that the present study will extend the body of knowledge on HIV testing and counseling among men in Ghana and provide a better understanding of the phenomenon. The findings will augment existing efforts and strategies steering progress in the uptake of HIV testing among men in Ghana (14).
Conceptual Framework
Evidence indicates that theory-based research is crucial in predicting human behaviors including utilization of sexual and reproductive health services such as HIV Testing (28). The study is underpinned by the Health Care Utilization Model originally propounded by Anderson and Newman (29). Over the years, the model has gone through successive modifications. For example, in 2000, Gelberg et al. modified the theory to include some challenges that impede healthcare access of vulnerable populations (30). There are three key elements in the model: predisposing, enabling, and need-for-care factors which either expedite or hinder the utilization of services by individuals (31). Predisposing factors include demographic characteristics, social structural variables, and an individual's basic beliefs, attitudes, and knowledge pertaining to health services (31). Enabling factors include resources available, whether individually or in a community (32). Need factors include the illnesses, conditions, and health statuses requiring health services. The model has been applied in various fields such as sociology, medicine, public health, and psychology. Specifically, it has been used to examine health care services utilization, such as HIV testing among young women in Trinidad and Tobago (33), American women in midlife (34), rural American cocaine users (35), primary care use among HIV-positive Haitian immigrants in Florida (36), racial/ethnic differences in HIV testing (32), and HIV testing service use among men in Haiti (37). Despite its wider application in different disciplines, some scholars have critiqued the model. Wilson et al. (38) were, for instance, of the view that the model does not pay attention to cultural dimensions and social interactions. Andersen (39), however, argued that need in itself is a social construct. In spite of the criticisms, the model is considered appropriate for this study because it is a multilevel theory and has been applied in various settings and disciplines.
Materials and Methods
Description of the Survey and Sampling
Data from the current, 2014 version of the Ghana Demographic and Health Survey (GDHS) were used for the study. The GDHS is conducted in five-year intervals nationwide, with specific focus on demographic and health issues, including HIV testing. Ghana Statistical Service and the Ghana Health Service are responsible for the conduct of the survey, with technical support from ICF International through MEASURE DHS. The sampling of participants for the survey is done at two stages. In the first stage, 427 Enumeration Areas(EAs) were chosen with probability proportional to the size of the EA with independent selection in each sampling stratum. The EA constitute the number of residential households inhabiting in the EA computed with the 2010 Population and Housing Census. A household listing operation was carried out in all the selected EAs, and the resulting lists of households served as a sampling frame for the selection of households in the second stage. To minimize the task of household listing for EAs with more than 200 households, each large EA was segmented. Only one segment was selected for the survey with probability proportional to the segment size. Household listing was conducted only in the selected segment. Therefore, a 2014 GDHS cluster is either an EA or a segment of an EA [1, p. 317–319]. At the second level of sampling, a fixed number of 30 households per cluster was selected with an equal probability systematic selection from the newly created household listing from January to March 2014. The survey interviewers visited and interviewed only the selected households. No replacements or changes of the selected households were allowed during data collection, in order to prevent bias. All women age 15–49 who were usual members of the selected households or who spent the night before the survey in the selected households were eligible for the female survey. In half of the selected households, all men age 15–59 who were usual members of the households or who spent the night before the survey in the households were eligible for the male survey. In all, the survey was conducted in 12,810 households, (6,480 urban and 6,330 in rural) [1, p. 317–319]. The total sample for the 2014 GDHS was expected to be 10,214 among women aged 15–49 (5,098 in urban and 5,116 in rural areas), and 4,175 completed interviews with men age 15–49 (2,061 in urban areas and 2,114 in rural areas). However, 4,388 men aged 15–59 were successfully interviewed. By extension, this implies a response rate of 95%. The present study featured 3,052 sexually active men because they had complete information on HIV testing, which the study is interested in. Detailed description of the methodology employed in the 2014 GDHS has been provided in the final report (1) which is also available online at https://dhsprogram.com/publications/publication-FR307-DHS-Final-Reports.cfm. Strengthening the Reporting of Observational Studies in Epidemiology' (STROBE) statement was followed in conducting this study and the writing of the manuscript.
Study Variables
Outcome Variable
In the present study, self-reported previous HIV testing was used as the outcome variable. The survey obtained information on this by asking this question: Have you ever tested for HIV? I considered HIV testing as a binary variable and categorized the responses into “Yes” or “No.” In the “Yes” category are those who reported ever testing for HIV at least once before the time of the survey. On the other hand, those who had never gone for HIV testing before fell under the “No” category.
Independent Variables
The independent variables were selected and grouped based on three reasons: first, conclusion from previous studies (11, 12, 14, 18, 19, 37, 40, 41) that have found them to have an association with HIV testing among men; second, the conceptual framework (Figure 1); and finally, their availability in the 2014 GDHS dataset.
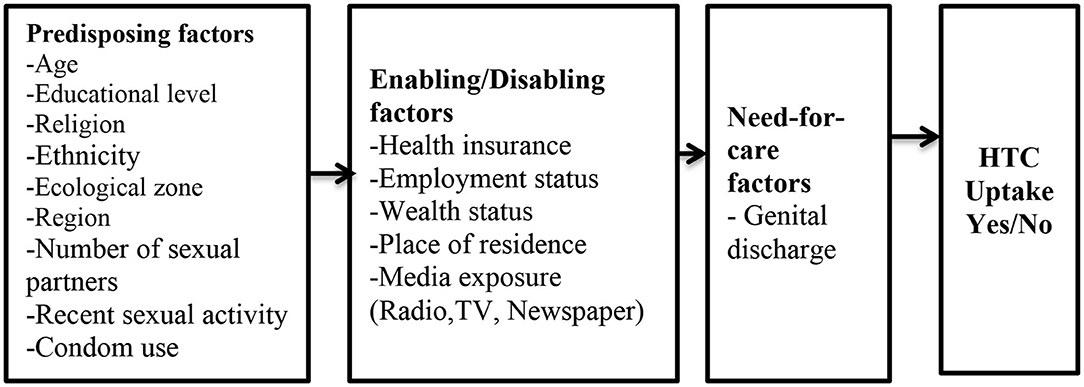
Figure 1. Conceptual Framework. Source: Anderson and Newman (29).
Predisposing Factors
Predisposing factors were categorized as follow: age (15–19, 20–29, 30–39, and 40 years or older), educational level (no education, primary, secondary, and higher), religious affiliation (Christianity, Islam, Traditionalist, and No religion), marital status (married and not married), ethnicity(Akan, Ga/Adangbe, Ewe, Mole–Dagbani, and Other), region (Western, Central, Greater Accra, Volta, Eastern, Ashanti, Brong-Ahafo, Northern, Upper East, and Upper West), zone (middle, northern and coastal), number of sex partners (1 and 2 or more), recent sexual activity (active and not active), and condom use during recent sexual activity with partner (yes and no).
Enabling Factors
These include place of residence (rural and urban), employment status (working and not working), wealth index (poor, middle, and rich), health insurance subscription (yes and no), frequency of reading newspaper/magazine (not at all, less than once a week and at least once a week), frequency of watching television (not at all, less than once a week and at least once a week), and frequency of listening to radio (not at all, less than once a week and at least once a week).
Need-For-Care
The need-for-care factor was captured as: genital discharge in the past 12 months (no and yes).
Statistical Analyses
The analyses were done in three steps. At the initial stage, descriptive statistics (frequencies and percentages) was employed to describe the socio-demographic features of the men under investigation (see Table 1). Afterwards, bivariate analysis featuring Chi-square (χ2) was used to investigate the association between HIV testing and the independent variables. The variables that emerged statistically significant (p < 0.05) were transported to the next level, where multivariable logistic regression analysis was conducted to examine determinants of previous HIV testing. In the logistic regression modeling, three hierarchical logistic models were developed based on the categorization of the independent variables into predisposing factors, enabling factors, and need-for-care factors (see Table 2). The results were presented as adjusted odds ratios [AORs] with their respective 95% confidence intervals (CIs) indicating the level of precision. Prior to the regression analysis, multicollinearity among the variables were checked using variance inflation factor and there was no evidence of collinearity among the variables. STATA version 14.0 for Mac OS was used to conduct all the analysis and used the svy command to account for the complex sampling design. Weighting was also applied. Furthermore, the “svylogitgof” command was used to check for the fit of the logistic regression models, which showed no evidence of lack of fit of the models in significantly predicting HIV testing uptake among men in Ghana.
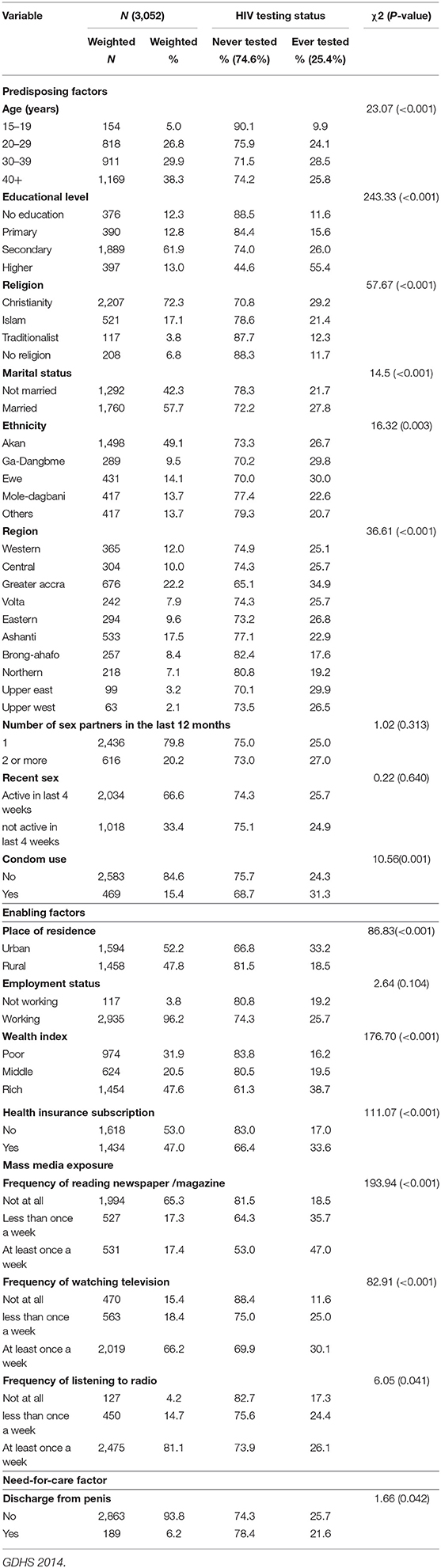
Table 1. HIV testing uptake by different background characteristics among sexually active men in Ghana.
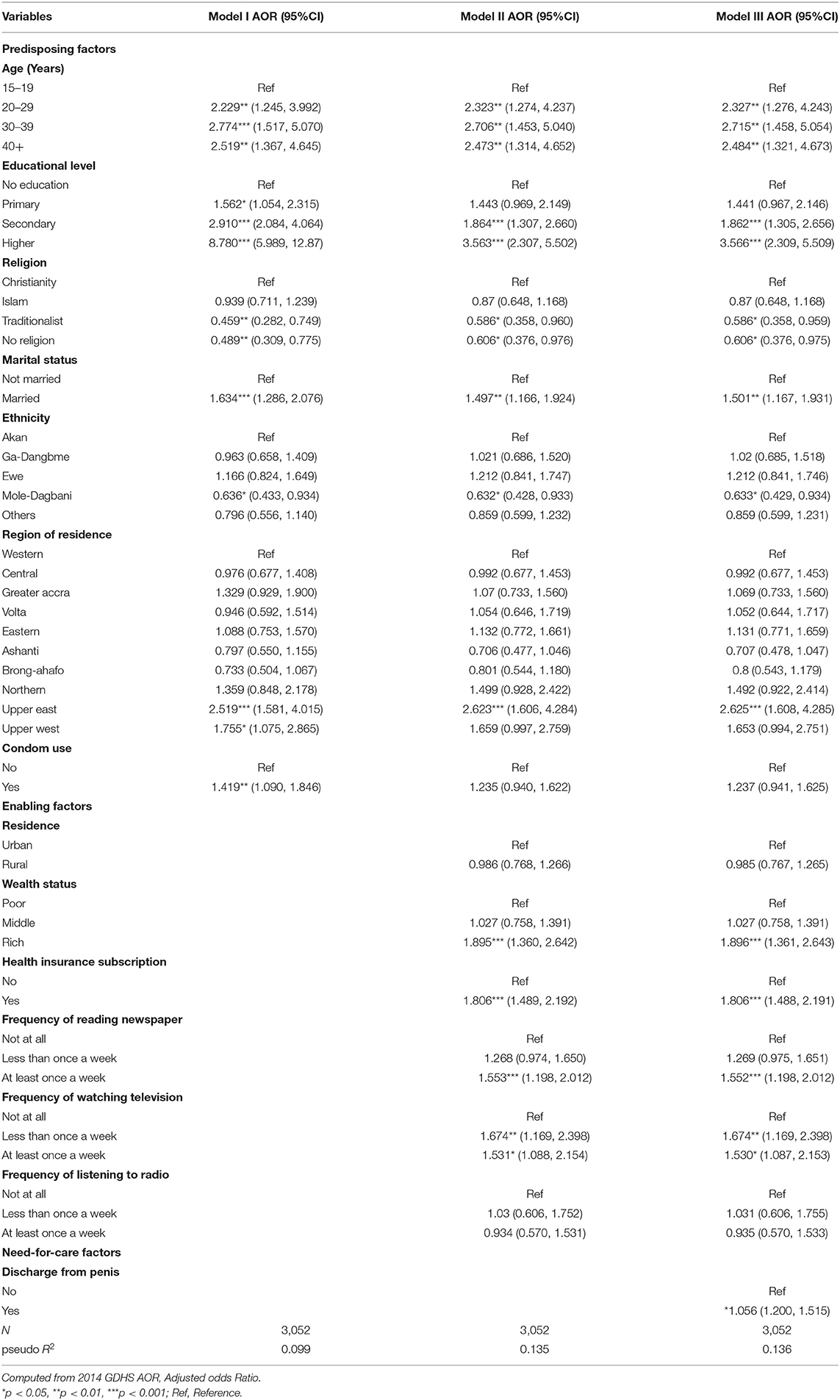
Table 2. Multivariable logistic regression analyses of factors associated with HIV testing among sexually active men in Ghana.
Ethical Considerations
Ethical clearance was obtained from the Institutional Review Board of ICF International and Ethical Review Committee of Ghana Health Service. Demographic and Health Survey also anonymised all data before making them accessible to the public. Permission to use the data was obtained from MEASURE DHS, which is a USAID–funded project that assists and funds population and health surveys in countries worldwide.
Results
Characteristics of the Study Population and HIV Testing Uptake
Table 1 presents the weighted profile of men in the analysis sample by their HIV testing category. Of the 3,052 men interviewed, only about a quarter (25.6%) of them had tested for their HIV status. The age of the participants ranged from 15 to 59 years. Men aged 40 and above represented 38.3% of the sample. The majority (61.9%) of the respondents had secondary level of education. A greater percentage of the respondents were Christians (72.3%), married (57.7%), working (96.2%) and did not use condom (84.6%). Almost half (49.1%) of the sample were Akans. In terms of regional variations, men in the greater Accra region constituted 22.2% of the sample. Almost eighty percent of the men who participated in the study had one sexual partner in the past 12 months preceding the survey.
Bivariate Analysis
The bivariate analysis showed that the predisposing factors associated with HIV testing are age, educational level, religion, marital status, ethnicity, region, and condom use. The enabling factors associated with HIV testing were place of residence, household wealth index, health insurance subscription, frequency of reading newspaper, frequency of watching television and frequency of listening to radio. Finally, the need-for-care factor associated with HIV testing was discharge from penis (see Table 1).
Determinants of HIV Testing Uptake Among Sexually Active Men in Ghana
The determinants of HIV testing among men in Ghana are presented in Table 2. The predisposing factors such as age, educational level, religion, marital status, ethnicity, and region were found to be significantly associated with HIV testing. With age, it was found that men aged 30–39 had higher odds (AOR = 2.715, 95% CI = 1.458, 5.054) of HIV testing uptake, compared to men aged 15–19. HIV testing uptake increased with level of education. Specifically, men with higher level of education had 3.6 higher odds (AOR = 3.566, 95% CI = 2.309, 5.509) of HIV testing, compared to those with no formal education. With religion, it was shown that men with no religion (AOR = 0.606, 95% CI = 0.376, 0.975) had lower odds of HIV testing uptake, compared to those who are Christians. The enabling factors that were significantly associated with HIV testing were health insurance subscription and media exposure (reading newspaper and watching television). Specifically, men who had subscribed to health insurance had higher odds of HIV testing uptake (AOR = 1.896, 95% CI = 1.361, 2.643), compared to those who had not subscribed to national health insurance scheme. With media exposure, those who read newspaper (AOR = 1.552, 95% CI = 1.198, 2.012) and listened to radio (AOR = 1.530, 95% C I = 1.087, 2.153) at least once a week had higher odds of HIV testing uptake, compared to those who did not read newspaper and those who did not watch television. With the need-for-care factor, men who experienced discharge from their penis had higher odds (AOR = 1.056, 95% CI = 1.200, 1.515) for HIV testing uptake, compared to those who did not experience any discharge from their penis.
Discussion
This study sought to assess the uptake of HIV testing services among sexually active men in Ghana. It was found from the study that only a quarter (25.4%) of sexually active men in Ghana had ever tested for their HIV status. The predisposing factors such as age, educational level, religion, marital status, ethnicity, and region demonstrated statistically significant association with HIV testing. The enabling factors that were significantly associated with HIV testing were health insurance subscription and media exposure (reading newspaper and watching television). With the need-for-care factors, men who experienced discharge from their penis had higher odds for HIV testing uptake, compared to those who did not experience any discharge from their penis.
The prevalence recorded in this study is similar to what has been found in previous studies in Ghana, other African settings and other low-and middle-income countries. For example, prevalence rate of 22.7, 23, 25.1, and 59% were found in Ghana (14), Uganda, (10), Tanzania (24), and South Africa (12), respectively. However, this current finding is lower than what was found in Zambia (61%) and Malawi (69%) by Hensen et al. (26) and Mandiwa and Namondwe (11), respectively. The low uptake of HIV testing reported in the present study could be attributed to the perception of low risk of transmission, mistrust of health professionals, and fear of HIV-related stigma, as well as the belief that once they look healthy and not engaging in any sexual activity, there is no need for HIV testing (14). The lower rate of men being tested for their HIV status indicates the need for prevention efforts to also target men since the predominant mode of HIV transmission is through heterosexual intercourse.
The study revealed a significant association between age and HIV testing among sexually active men in Ghana. With age, relative to adolescents, sexually active men who were aged 30–39 were more likely to test for HIV. Previous studies in Malawi (11), Haiti (37), South Africa (42) and Sierra Leone (43) reported similar findings. This finding could be due to the fact that adolescents might not have the courage to test for their HIV and AIDS status.
Educational attainment was found to be significantly associated with the odds of HIV testing. Specifically, the odds of HIV testing increased with the increase in educational attainment. This association is consistent with several previous studies (11, 14, 25, 43, 44). This finding is not surprising, as higher educational attainment has the likelihood of exposing individuals to health information, such as the importance of knowing one's HIV status. People with lower educational attainment, on the other hand, are less likely to get access to such information (11).
In line with some previous studies (14, 37, 45, 46), religious affiliation also showed significant association with HIV testing, with non-religious people showing less likelihood for HIV testing. This finding is suggestive of the role of religion in the fight against HIV/AIDS. USAID has attempted to use religious leaders as a channel to promote HIV testing. This finding may therefore be suggestive of the effectiveness of the efforts of UNAID in that regard (47). A study in Tanzania by Mbago (48), however, reported contrary findings. The differences in finding could be due to differences in socio-cultural settings and research methodologies adopted.
There was also a significant association between region of residence and HIV testing among Ghanaian men, with residents of Upper West Region being most likely to test for HIV. The findings concur with what has been reported in Ghana (14), Ethiopia (44), Malawi (11), and Mozambique (49). This finding suggests an influence of sociocultural differences that characterize different geographical regions on HIV testing (11). In the case of Ghana, northern Ghana has often been the focus of some non-governmental organizations aimed at improving the health of people. Corollary to the relationship between region of residence and HIV testing is the finding that ethnicity has a significant association with HIV testing among the studied population. Specifically, Mole-Dagbani men were less probable to go for HIV testing. A plausible explanation might be that there are strong cultural beliefs concerning certain procedures among this ethnic group, which might obstruct the uptake of these services. Nonetheless, there is a need for a further exploration of this relationship between HIV testing and ethnicity, perhaps through the use of qualitative methods, to reveal the reasons behind such an association.
Additionally, relative to unmarried men, married men were more probable to go for HIV testing. Previous studies in Kenya (50), Malawi (11), Haiti (37), and Mozambique (49) reported similar findings. This finding could be due to the fact that HIV testing is usually part of the preparations toward marriage (11). Evidently, in some African countries including Ghana, there are instances of mandatory premarital HIV testing (51), and this might explain the higher likelihood of HIV testing among married men reported in the present study. In addition, this difference in likelihood of HIV testing between married and non-married men seems to give credence to the assumption that marriage leads to changes in sexual behavior (37, 52).
In terms of household wealth, higher odds of HIV testing were reported among rich men than among poor men. This finding confirms previous studies in Ghana (14), Haiti (37), Uganda (53), Zimbabwe (46), and Burkina Faso (25). Relatedly, men who had subscribed to NHIS were more likely to test for HIV, compared to their counterparts who has not subscribed to NHIS. Previous studies in Haiti (37) and Malawi (11) similarly reported higher odds of HIV testing among men with health insurance. In line with the conceptual framework, household wealth and health insurance subscription are enabling factors to increase access to HIV testing services. In Ghana, clients with NHIS subscription are allowed free and regular access to HIV testing. Similarly, NHIS subscription increases men's use of health services which brings them into frequent contact with healthcare providers, which is likely to increase their likelihood of HIV testing (11).
Just like what was reported by some previous studies (14, 23, 25), mass media exposure also increased the likelihood of HIV testing among the studied men. In line with the conceptual framework (29), need-for-care factors such as discharge from penis increased the odds of service uptake. As rational beings, people with various symptoms of certain health conditions may put up behaviors to get rid of such conditions. It is, therefore, not surprising that men who experienced discharge from their penis had higher odds of testing for their HIV status.
Strength and Limitations of the Study
Although this study has brought to bear some factors that determine HIV testing among sexually active men in Ghana that could have implications for policy, the limitations are not far-fetched. First, only variables that were collected in the GDHS were considered. As such, other important variables (such as sexual orientation) that may affect HIV testing but were not available could not be examined (11). Second, the cross-sectional nature of the GDHS makes it impossible to draw causal inferences but only associations (37). Third, there is the possibility of social desirability bias since the responses were self-reported (11, 37). Also, only data from the 2014 Ghana Demographic and Health Survey was used and as a result does not allow comparisons and evolution analysis. Despite these limitations, the study has its strength from the relatively large dataset and high response rate. The use of a nationally representative survey and the use of stratified two-stage sampling technique made it possible to obtain samples that are highly representative of the target population. The study also employed a behavioral model to guide the selection and interpretations of the findings. The large sample size and the national representativeness of the data, therefore, make the conclusions in this study more generalisable and valid.
Conclusion
The prevalence of sexually active men who have ever tested for their HIV status was 25.4 percent, which is relatively low. The predisposing factors associated with HIV testing were age, educational level, religion, marital status, ethnicity, and region. The enabling factors for HIV status testing were wealth status, health insurance subscription, and media exposure (reading newspaper, watching television) while the need-for-care factor related to HIV testing is experiencing discharge from penis. There is the need for a concerted effort by various stakeholders to strengthen current efforts to target younger and unmarried men, men with low level of education, those who do not profess any religion and men belonging to Mole-Dagbani ethnic group. There is also the need to intensify HIV testing education in other regions of Ghana. These efforts can increase the number of men who seek HIV testing services and also encourage men to protect themselves and also could be made to encourage their partners and other members in their households to test and know their HIV and AIDS status. This might be a means for those who might be HIV-positive to benefit from antiretroviral treatment and, in turn, reduce the number of new infections, thereby helping to achieve the UNAIDS' 90-90-90 targets.
Consent for Publication
This manuscript is an original work and has been done by the A-AS, and aware of its content and approved its submission. It is also important to mention that the manuscript has not been published elsewhere in part or in entirety and is not under consideration by another journal. The A-AS have given consent for this article to be submitted for publication in this Journal.
Data Availability Statement
The datasets generated for this study will not be made publicly available the author does not have the power to share them.
Author Contributions
A-AS conceived, analyzed the data, and drafted the manuscript.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
A-AS is grateful to MEASURE DHS for granting him access to the data. A-AS also acknowledge Mr. Ebenezer Agbaglo of the Department of English, University of Cape Coast, who thoroughly copy-edited this manuscript for language usage, spelling, and grammar.
References
1. Ghana Statistical Service (GSS) Ghana Health Service (GHS) ICF International. Ghana Demographic and Health Survey 2014. Rockville, MD: GSS, GHS, and ICF International (2015).
2. Levi J, Raymond A, Pozniak A, Vernazza P, Kohler P, Hill A. Can the UNAIDS 90-90-90 target be achieved? A systematic analysis of national HIV treatment cascades. Br Med J. (2016) 1:1–10. doi: 10.1136/bmjgh-2015-000010
3. Li J, Gilmour S, Zhang H, Koyanagi A, Shibuya K. The epidemiological impact and cost-effectiveness of HIV testing, antiretroviral treatment and harm reduction programs. Aids. (2012) 26:2069–78. doi: 10.1097/QAD.0b013e3283574e54
4. Kagee A, Swartz A, Swartz L. Theorising beyond the individual: adherence to antiretroviral therapy in resource-constrained societies. J Health Psychol. (2014) 19:103–9. doi: 10.1177/1359105313500247
5. World Health Organization. Service Delivery Approaches to HIV Testing and Consellingn (HTC): A Strategic HTC Programme Framework. Geneva: WHO (2012).
6. Woodring JV, Kruszon-Moran D, Oster AM, McQuillan GM. Did CDC's 2006 revised HIV testing recommendations make a difference? Evaluation of HIV testing in the US household population, 2003–2010. J Acquir Immune Defic Syndr. (2014) 67:331–40. doi: 10.1097/QAI.0000000000000303
7. UNAIDS. HIV/AIDS JUNPo: 90–90-90—An Ambitious Treatment Target to Help End the AIDS Epidemic. Geneva: UNAIDS (2014).
8. Jürgensen M, Tuba M, Fylkesnes K, Blystad A. The burden of knowing: balancing benefits and barriers in HIV testing decisions. a qualitative study from Zambia. BMC Health Serv Res. (2012) 12:2–9. doi: 10.1186/1472-6963-12-2
9. De Allegri M, Agier I, Tiendrebeogo J, Louis VR, Yé M, Mueller O, et al. Factors affecting the uptake of HIV testing among men: a mixed-methods study in rural burkina faso. PLoS ONE. (2015) 10:1–15. doi: 10.1371/journal.pone.0130216
10. Bwambale FM, Ssali SN, Byaruhanga S, Kalyango JN, Karamagi CA. Voluntary HIV counselling and testing among men in rural western Uganda: implications for HIV prevention. BMC Public Health. (2008) 8:263–76. doi: 10.1186/1471-2458-8-263
11. Mandiwa C, Namondwe B. Uptake and correlates of HIV testing among men in Malawi: evidence from a national population–based household survey. BMC Health Serv Res. 19:203–14. doi: 10.1186/s12913-019-4031-3
12. Makusha T, Mabaso M, Richter L, Desmond C, Jooste S, Simbayi L. Trends in HIV testing and associated factors among men in South Africa: evidence from 2005, 2008 and 2012 national population-based household surveys. Public Health. (2017) 143:1–7. doi: 10.1016/j.puhe.2016.10.017
13. Koku EF. Desire for, and uptake of HIV tests by Ghanaian women: the relevance of community level stigma. J Community Health. (2011) 36:289–99. doi: 10.1007/s10900-010-9310-1
14. Nyarko SH, Sparks C. Levels and determinants of HIV testing uptake among Ghanaian men. Afr J AIDS Res. (2020) 19:40–7. doi: 10.2989/16085906.2019.1679851
15. Katz DA, Kiarie JN, John-Stewart GC, Richardson BA, John FN, Farquhar C. Male perspectives on incorporating men into antenatal HIV counseling and testing. PloS ONE. (2009) 4:e7602. doi: 10.1371/journal.pone.0007602
16. Skovdal M, Campbell C, Madanhire C, Mupambireyi Z, Nyamukapa C, Gregson S. Masculinity as a barrier to men's use of HIV services in Zimbabwe. Global Health. (2011) 7:13–19. doi: 10.1186/1744-8603-7-13
17. Vaidya V, Partha G, Karmakar M. Gender differences in utilization of preventive care services in the United States. J Women's Health. (2012) 21:140–5. doi: 10.1089/jwh.2011.2876
18. Conserve DF, Alemu D, Yamanis T, Maman S, Kajula L. “He told me to check my health”: a qualitative exploration of social network influence on men's HIV testing behavior and HIV self-testing willingness in Tanzania. Am J Men's Health. (2018) 12:1185–96. doi: 10.1177/1557988318777674
19. Tenkorang EY, Owusu GA. Correlates of HIV testing among women in Ghana: some evidence from the demographic and health surveys. AIDS Care. (2010) 22:296–307. doi: 10.1080/09540120903193716
20. Kwapong GD, Boateng D, Agyei-Baffour P, Addy EA. Health service barriers to HIV testing and counseling among pregnant women attending Antenatal Clinic; a cross-sectional study. BMC Health Serv Res. (2014) 14:267–74. doi: 10.1186/1472-6963-14-267
21. Baiden F, Remes P, Baiden R, Williams J, Hodgson A, Boelaert M, et al. Voluntary counseling and HIV testing for pregnant women in the Kassena-Nankana district of northern Ghana: is couple counseling the way forward? AIDS Care. (2005) 17:648–57. doi: 10.1080/09540120412331319688
22. Kushwaha S, Lalani Y, Maina G, Ogunbajo A, Wilton L, Agyarko-Poku T, et al. “But the moment they find out that you are MSM…”: a qualitative investigation of HIV prevention experiences among men who have sex with men (MSM) in Ghana's health care system. BMC Public Health. (2017) 17:770–81. doi: 10.1186/s12889-017-4799-1
23. Darteh EK, Amo-Adjei J, Awusabo-Asare K. Correlates of HIV testing among young people in Ghana. J HIV/AIDS Soc Serv. (2014) 13:219–33. doi: 10.1080/15381501.2012.748583
24. Conserve D, Sevilla L, Mbwambo J, King G. Determinants of previous HIV testing and knowledge of partner's HIV status among men attending a voluntary counseling and testing clinic in Dar es Salaam, Tanzania. Am J Men's Health. (2013) 7:450–60. doi: 10.1177/1557988312468146
25. Kirakoya-Samadoulougou F, Jean K, Maheu-Giroux M. Uptake of HIV testing in Burkina Faso: an assessment of individual and community-level determinants. BMC Public Health. (2017) 17:486–94. doi: 10.1186/s12889-017-4417-2
26. Hensen B, Lewis JJ, Schaap A, Tembo M, Vera-Hernández M, Mutale W, et al. Frequency of HIV-testing and factors associated with multiple lifetime HIV-testing among a rural population of Zambian men. BMC Public Health. (2015) 15:960–7. doi: 10.1186/s12889-015-2259-3
27. Molla G, Huruy A, Mussie A, Wondowosen T. Factors associated with HIV counseling and testing among males and females in Ethiopia: evidence from ethiopian demographic and health survey data. J AIDS Clin Res. (2015) 6:1–17. doi: 10.4172/2155-6113.1000429
28. Kumi-Kyereme A, Seidu AA, Darteh EKM. Factors contributing to challenges in accessing sexual and reproductive health services among young people with disabilities in Ghana. Global Soc Welfare. (2020) 5:1–10. doi: 10.1007/s40609-020-00169-1
29. Andersen R, Newman JF. Societal and individual determinants of medical care utilization in the United States. Milbank Mem Fund Q Health Soc. (1973) 51:95–124. doi: 10.2307/3349613
30. Gelberg L, Andersen RM, Leake BD. The Behavioral model for vulnerable populations: application to medical care use and outcomes for homeless people. Health Serv Res. (2000) 34:1273–85.
31. Azfredrick EC. Using anderson's model of health service utilization to examine use of services by adolescent girls in south-eastern Nigeria. Int J Adolesc Youth. (2016) 21:523–9. doi: 10.1080/02673843.2015.1124790
32. Lo CC, Runnels RC, Cheng TC. Racial/ethnic differences in HIV testing: an application of the health services utilization model. SAGE Open Med. (2018) 6:2050312118783414. doi: 10.1177/2050312118783414
33. Andrews B. Determining the patterns and uptake of HIV testing among young women in trinidad and tobago: implications for HIV/AIDS policy. Sex Transm Infect. (2013) 89 (Suppl. 1):A292 doi: 10.1136/sextrans-2013-051184.0909
34. Wigfall LT, Richter DL, Torres ME, Annang L, Lindley LL, Swayampakala K, et al. HIV testing among midlife women in the deep south: an analysis of the 2008 behavioral risk factor surveillance system survey data. J Women's Health. (2011) 20:933–41. doi: 10.1089/jwh.2010.2096
35. Wright PB, Booth BM, Curran GM, Borders TF, Ounpraseuth ST, Stewart KE. Correlates of HIV testing among rural African American cocaine users. Res Nurs Health. (2014) 37:466–77. doi: 10.1002/nur.21629
36. Saint-Jean G, Metsch L, Gomez-Marin O, Pierre C, Jeanty Y, Rodriguez A, et al. Use of HIV primary care by HIV-positive Haitian immigrants in Miami, Florida. AIDS Care. (2011) 23:486–93. doi: 10.1080/09540121.2010.516339
37. Conserve DF, Iwelunmor J, Whembolua GL, Sofolahan-Oladeinde Y, Teti M, Surkan PJ. Factors associated with HIV testing among men in Haiti: results from the 2012 demographic and health survey. Am J Men's Health. (2017) 11:1322–30. doi: 10.1177/1557988316635247
38. Wilson K, Law M, Eyles J, Elliott S, Jerrett M, Moffat T, et al. Meeting health need, accessing health care: the role of neighbourhood. Health Place. (2005) 11:367–77. doi: 10.1016/j.healthplace.2004.05.004
39. Andersen RM. National health surveys and the behavioral model of health services use. Med Care. (2008) 46:647–53. doi: 10.1097/MLR.0b013e31817a835d
40. Obermeyer CM, Neuman M, Hardon A, Desclaux A, Wanyenze R, Ky-Zerbo O, et al. Socio-economic determinants of HIV testing and counselling: a comparative study in four African countries. Trop Med Int Health. (2013) 18:1110–8. doi: 10.1111/tmi.12155
41. Detsis M, Tsioutis C, Karageorgos SA, Sideroglou T, Hatzakis A, Mylonakis E. Factors associated with HIV testing and HIV treatment adherence: a systematic review. Curr Pharm Des. (2017) 23:2568–78. doi: 10.2174/1381612823666170329125820
42. Peltzer K, Matseke G. Determinants of HIV testing among young people aged 18–24 years in South Africa. Afr Health Sci. (2013) 13:1012–20. doi: 10.4314/ahs.v13i4.22
43. Brima N, Burns F, Fakoya I, Kargbo B, Conteh S, Copas A. Factors associated with HIV prevalence and HIV testing in Sierra Leone: findings from the 2008 demographic health survey. PloS ONE. (2015) 10:1–13. doi: 10.1371/journal.pone.0137055
44. Teklehaimanot HD, Teklehaimanot A, Yohannes M, Biratu D. Factors influencing the uptake of voluntary HIV counseling and testing in rural Ethiopia: a cross sectional study. BMC Public Health. (2016) 16:239–46. doi: 10.1186/s12889-016-2918-z
45. Trinitapoli J, Regnerus MD. Religion and HIV risk behaviors among married men: initial results from a study in rural Sub-Saharan Africa. J Sci Study Relig. (2006) 45:505–28. doi: 10.1111/j.1468-5906.2006.00325.x
46. Sambisa W, Curtis S, Mishra V. AIDS stigma as an obstacle to uptake of HIV testing: evidence from a Zimbabwean national population-based survey. AIDS Care. (2010) 22:170–86. doi: 10.1080/09540120903038374
47. Faulkner DM, Maynard-Tucker G. Strategy Recommendations and Planning Framework for HIV/AIDS Behavior Change Communication Activities Funded by USAID/Haiti (English version) (2002). Available online at: http://pdf.usaid.gov/pdf_docs/Pnacs914.pdf.
48. Mbago MC. Socio-demographic correlates of desire for HIV testing in Tanzania. Sex Health. 1:13–21. doi: 10.1071/SH03010
49. Agha S. Factors associated with HIV testing and condom use in Mozambique: implications for programs. Reprod Health. (2012) 9:20–7. doi: 10.1186/1742-4755-9-20
50. Ziraba AK, Madise NJ, Kimani JK, Oti S, Mgomella G, Matilu M, et al. Determinants for HIV testing and counselling in Nairobi urban informal settlements. BMC Public Health. (2011) 11:663–79. doi: 10.1186/1471-2458-11-663
51. Rennie S, Mupenda B. Ethics of mandatory premarital HIV testing in Africa: the case of Goma, Democratic Republic of Congo. Dev World Bioeth. (2008) 8:126–37. doi: 10.1111/j.1471-8847.2007.00199.x
52. Iwelunmor J, Sofolahan-Oladeinde Y, Airhihenbuwa CO. Sociocultural factors influencing HIV disclosure among men in South Africa. Am J Men's Health. (2014) 9:193–200. doi: 10.1177/1557988314535235
Keywords: men, Ghana, Anderson model, HIV/AIDS, health service utilization, global health, public health, model
Citation: Seidu A-A (2020) Using Anderson's Model of Health Service Utilization to Assess the Use of HIV Testing Services by Sexually Active Men in Ghana. Front. Public Health 8:512. doi: 10.3389/fpubh.2020.00512
Received: 08 July 2019; Accepted: 07 August 2020;
Published: 15 September 2020.
Edited by:
Olivier Vandenberg, Laboratoire Hospitalier Universitaire de Bruxelles (LHUB-ULB), BelgiumReviewed by:
Ana Afonso, University of São Paulo, BrazilSunny Oteikwu Ochigbo, University of Calabar, Nigeria
Copyright © 2020 Seidu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdul-Aziz Seidu, YWJkdWwtYXppei5zZWlkdSYjeDAwMDQwO3N0dS51Y2MuZWR1Lmdo
 Abdul-Aziz Seidu
Abdul-Aziz Seidu